Abstract
Fruits of Elaeagnus angustifolia L. have been used as Uyghur medicine due to the properties of treating spleen and stomach weakness, indigestion, enteritis, diarrhea, lung heat, and cough. However, the anti-diarrhea mechanism was still not clear. This study explored the mechanism of E. angustifolia fruit alleviated diarrhea from the perspective of gut microbiota. Diarrhea model was established with Folium sennae in mice. Then, the levels of diarrhea rate and diarrhea index of mice were evaluated. Hematoxylin eosin (HE) staining was employed to detect pathological sections of colon tissue. 16S rRNA sequencing analysis was researched to confirm the gut microbiota in mice. Diversity and differential analysis were adopted to analyze the intestinal microflora. Furthermore, Gas chromatography-quadrupole time-of-flight tandem mass spectrometry (GC-Q-TOF-MS) was used to detect the concentrations of short chain fatty acids (SCFAs) in intestine. The high-dose group (3.2 g/kg) of E. angustifolia fruit could significantly reduce the diarrhea rate and diarrhea index of mice caused by Folium sennae (p < 0.01). We also found that E. angustifolia fruit enhanced the diversity of gut microbiota while ameliorating diarrhea. Alpha diversity revealed that the microbial composition of E. angustifolia fruit group tended to be more similar to that of the CON group (no significant difference at p < 0.05). E. angustifolia fruit also induced structural changes of gut microbiota in mice. In addition, the concentrations of SCFAs increased after administration of E. angustifolia fruit. This study demonstrated that E. angustifolia fruit could ameliorate diarrhea by regulating the composition and abundance of intestinal microbiota, together with the levels of SCFAs.
Keywords: Elaeagnus angustifolia L., Diarrhea, Intestinal microbiota, Short chain fatty acids, Mechanism
1. Introduction
Diarrhea is a common symptom in the gastrointestinal diseases, often with loose, watery, or more frequent soft excrement [1]. The study shows more than one billion people suffer diarrhea every year [2]. Diarrhea contains acute diarrhea and chronic diarrhea. Bacterial, viral, and protozoan infections are considered to be the main factors to cause diarrhea [3]. Moreover, infections of the stomach and intestines, changes in diet, anxiety also result in diarrhea. The treatment for diarrhea mainly involves stopping or changing the pathogenic antibiotics and maintaining sufficient hydration [4]. The common anti-diarrheal drugs are anticholinergic drugs as well as morphine and its derivatives [5]. Another more new drugs for treating diarrheal need to be discovered. Therefore, we focus on this point to select anti-diarrheal drugs.
Gut microbiota plays an important role in host health [1]. Bacteria, fungi, protozoa, and viruses composed of gut microbiota [6]. A growing number of studies have shown that diarrhea had a very close relationship to gut microbiota [7,8]. Diarrhea can lead to disturbances in the gut microbiota, such as an imbalance in the proportion of beneficial bacteria, a significant decrease in the number of beneficial bacteria, as well as an increase in harmful bacteria in the gut [9]. An increasing number of studies believed that Chinese medicines have the ability to regulate the composition of gut microbiota [10,11].
Short chain fatty acids (SCFAs) are fermentation products of microorganisms in the intestine, which play a crucial role in regulating intestinal function [12]. Different species of gut microbiota produce varying amounts of SCFAs. For example, Firmicutes mainly produce butyric acid, while Bacteroidetes produce acetic acid and propionic acid [13]. Usually acetic acid, butyric acid, propionic acid, isobutyric acid, isovaleric acid, valeric acid and caproic acid are included. Herin, the regulation of gut microbiota is of great significance for the prevention and treatment of diarrhea.
Elaeagnus angustifolia L., belongs to Elaeagnaceae family, mainly distributed in southwest of Asia, east of Europe [14]. It grows in arid and semiarid zones. In China, this plant mainly distributed in Gansu, Hebei, Henan, Liaoning, Nei Mongol, Ningxia, Qinghai, Shaanxi, Shanxi and Xinjiang [15]. Phytochemistry research showed that fruit of E. angustifolia contained various components, such as phenolic acid, caffeic acid, and flavonoids [14,16]. Furthermore, it also rich in protein, sugar and trace elements, and was applied in food [16,17]. On the other hand, its fruit is an important medicine in many countries, such as Xinjiang [18], and Iran [19], based on multiple properties, such as anti-inflammatory [20], and antioxidant [21]. It has been used for the treatment of spleen and stomach weakness, indigestion, enteritis, diarrhea, lung heat, and cough [18,22]. In addition, the flowers, bark, and gum of E. angustifolia can be used as medicine [23].
In China, fruit of Elaeagnus angustifolia L. also called “Shazao” (沙枣), is mainly used in Uyghur medicine to treat gastrointestinal diseases caused by diarrhea, and has definite therapeutic effects [17]. However, the mechanism of its anti-diarrhea has not been studied in terms of gut microbiota. Therefore, this article investigated the anti-diarrhea effect of E. angustifolia fruit through acute diarrhea model induced by Folium sennae in mice. Furthermore, we investigated its impact on the composition of intestinal microbiota, explored the mechanism of anti-diarrhea effect of its fruit, and provided a theoretical basis for the development of new anti-diarrhea drugs.
2. Materials and methods
2.1. Experimental materials and chemicals
E. angustifolia fruit was collected in Kashgar, Xinjiang Uygur Autonomous Region, China, which were authenticated by Professor Heng Wang from Shihezi University. Voucher samples (No: SZ5-1) were stored in Xinjiang Institute of Chinese Materia Medica and Ethnodrug. Folium sennae was purchased from Xinjiang Baicaotang Pharmacy.
Hematoxylin Eosin (HE) staining kit was supplied by Zhuhai Besso Biotechnology Co., Ltd. Mouse fecal DNA extraction kit, Quant iTTM dsDNA analysis kit, and Mag-bind soil DNA kit were produced by Omega (USA). NovaSeq 6000 SP Reagent Kit was produced by Illlumina (USA). Vazyme VAHTSTM DNA Clean Beads was purchased from Nanjing Nuoweizan Biotechnology Co., Ltd. ALL standard compounds (chlorogenic acid, caffeic acid, ferulic acid, and tannic acid) were provided by Chengdu Vicki Biotechnology Co., Ltd.
2.2. Preparation of E. angustifolia fruits extract and Folium sennae extract
E. angustifolia fruits extract (SZ) was prepared as following: E. angustifolia fruits weighed 100 g after drying, and then added water of 1000 mL, and decocted for 1 h. Later, filtered while hot, the filtrate was collected and concentrated to 1.0 g crude herb/mL, then stored at 4 °C for later use.
Folium sennae extract was prepared according to literature [24]. The extraction process was as follows: weighed 60 g of Folium sennae leaves, soaked them in 480 mL distilled water for 30 min. Then extracted for 10 min, filtered and prepared to an extract with concentration of 12.5 g/mL. Finally, Stored in a refrigerator at 4 °C until usage.
2.3. HPLC analysis of E. angustifolia fruits extract
Phenolic compounds (chlorogenic acid, caffeic acid, ferulic acid, and ellagic acid) were detected by High Performance Liquid Chromatography (HPLC, Agilent 1200, USA) according to standard substances. SZ was subjected on Symmetry C18 chromatographic column (4.6 × 150 mm, 5 μm, Waters) under a gradient elution at a flow rate of 1.0 mL/min. The gradient elution was composed of acetonitrile (A) and 0.2 % formic acid (B). The elution procedure as follows: 0–4 min, 4–7% A; 10–30 min, 7–8% A; 30–35 min, 8–10 % A; 35–65 min, 10–13 %; 65–80 min, 13–16 % A; and 80–100 min, 16–28 % A.
2.4. Construction of diarrhea model and administration of SZ
A total of 40 Specific pathogen free (SPF) grade Kunming mice aged 6 weeks, sex in half, weighing 18–22 g were purchased from Xinjiang Medical University Experimental Center. The animal experiments were performed in accordance with the Animal Ethics Committee of Xinjiang Institute of Chinese Materia Medica and Ethnodrug (experiment approval number: XJIMM-20230202).
Diarrhea model induced by Folium sennae was conducted according to Ref. [1]. Briefly, Mice were given 0.2 mL/10 g of Folium sennae extract for 3 days. The model was considered successful when the mice displayed loose stools of level 3. Fecal consistency was classified by four grades based on the area of contaminated filter paper or absorbent paper (cm) of fecal output [25]. The degree of loose stool of mice was divided as follows: Level 1 (less than 1.0), Level 2 (1–1.9), Level 3 (2–3), and Level 4 (greater than 3).
After the establishment of model, all the mice were divided into the healthy control group (CON), model group (MOD), and sample group (SZS) with 10 mice in each. From day 1–7, the CON group was given saline, while the others were given 0.2 mL/10 g of Folium sennae extract. After that, the CON and MOD groups were given normal saline. SZS group were administered with 3.2 g/kg of SZ.
2.5. Assessment of water content of feces, diarrhea rate and diarrhea index
All mice were evaluated by water content of mouse feces, diarrhea rate and diarrhea index within 4 h after administration [1]. Water content of feces: Collected and weighed the loose stool of mice, dried under a 100 °C constant temperature for 12 h, and then weighed its dry mass and calculated the water content of mouse feces.
| Water content of feces (%) = (wet mass of fresh feces - dry mass of feces) / wet mass of fresh feces × 100%. |
The diarrhea rate was calculated by number of mice with diarrhea/number of mice in the group × 100 %. The diarrhea index was gained from the diarrhea rate × the fecal grade.
2.6. Histological analysis
After experiment, all mice were sacrificed, and then collected colon tissues and intestine. HE stains was used to observe pathological features and intestinal mucosal degeneration.
2.7. Fecal DNA extraction and 16s rRNA sequencing
The colon samples were dissected, then collected colon feces aseptically. All feces were quenched in liquid nitrogen quickly and stored at −80 °C. Intestinal microbial DNA was extracted using DNA assay kit method and the genomic DNA was detected using fluorescence enzyme-linked immunosorbent assay according to the methods provided in literature [26]. Furthermore, Alpha biodiversity, including Chao1 index, Shannon index, Simpson index, Pielou_e index, Observated_species, Faith_pd index, Goods' coverage, and colony composition were executed [24,25].
2.8. Determination of short chain fatty acids
Short chain fatty acids (SCFAs), including acetic acid, butyric acid, propionic acid, isobutyric acid, isovaleric acid, valeric acid and caproic acid were recorded on Gas chromatography-quadrupole time-of-flight tandem mass spectrometry (GC-Q-TOF-MS). The sample pretreatment and measurement were conducted according to a previously published method [25].
2.9. Statistical analysis
Diarrhea pharmacodynamic experimental results were presented as the mean ± SD (standard deviation). QIIME software was adopted to analysis 16S rDNA Barcode sequence and operational taxonomic units (OTUs) cluster analysis. OTUs clustering analysis was measured after comparing OTU representation sequence with the Gene database. Species classification, annotation, and analysis of each OTU were analyzed. Significance analysis at p < 0.01 and p < 0.05 levels.
3. Results
3.1. Chemical analysis of SZ
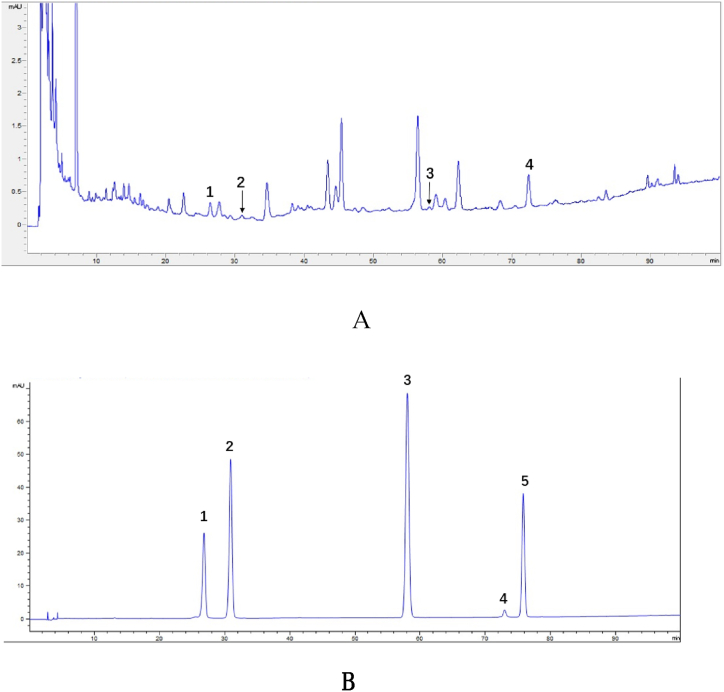
Based on HPLC chromatographic conditions, four constituents chlorogenic acid, caffeic acid, ferulic acid and ellagic acid in SZ were identified by comparing the retention times of individual peaks with those of standards. The HPLC separation effect of SZ was illustrated in Fig. 1.
Fig. 1.
The HPLC of SZ (A) and reference compounds (B).
1–Chlorogenic acid, 2–caffeic acid, 3–ferulic acid, 4–ellagic acid, 5- quercetin.
3.2. Effects of SZ on diarrhea model
As shown in Fig. 2, the fecal water content of mice in MOD group remarkably increased (p < 0.01) while comparing with CON group. This showed a dramatically decline after giving SZ (p < 0.05).
Fig. 2.
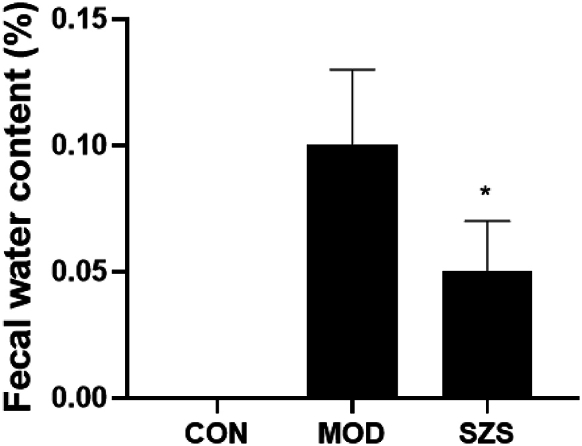
Fecal water content of mice (vs MOD group, ∗ p < 0.05).
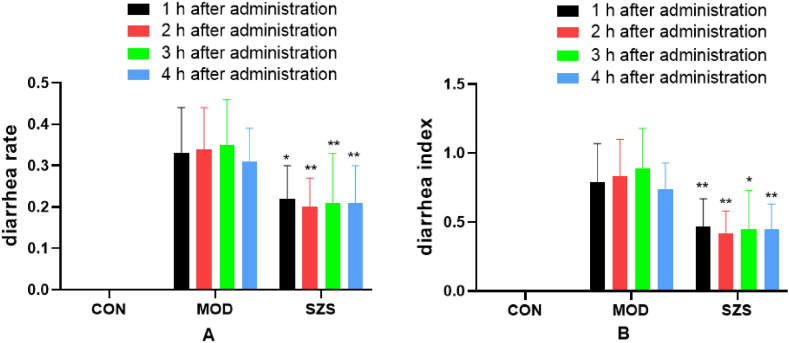
The diarrhea rate and diarrhea index of mice administrated with Folium sennae were significantly increased compared with CON group (p < 0.01) (Fig. 3), indicating that the model is well established. Compared with the MOD group, the diarrhea rate and diarrhea index of mice significantly decreased after administrated SZ (p < 0.01). The above results indicated that SZ had a significant anti-diarrheal effect on mice induced by Folium sennae.
Fig. 3.
The diarrhea rate (A) and diarrhea index (B) of mice (vs MOD group, ∗ p < 0.05, ∗∗p < 0.01).
3.3. Histopathologic analysis
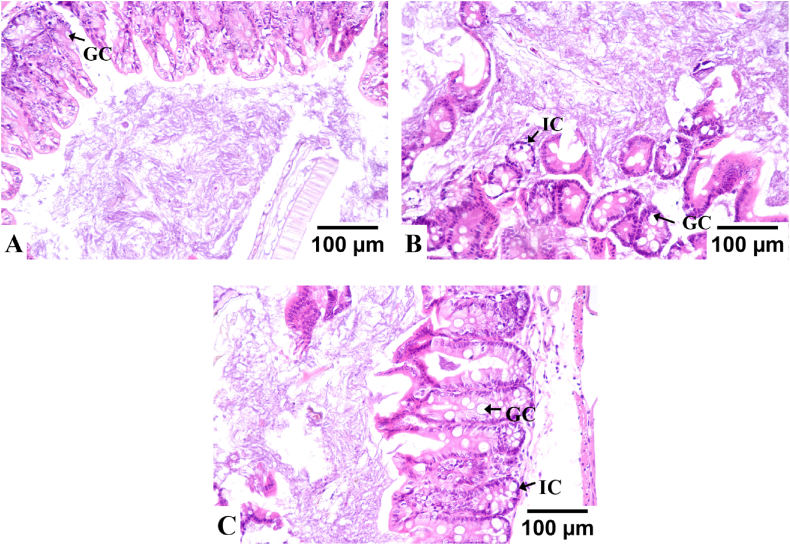
As shown in Fig. 4, the intestinal structure of CON group mice is normal, with clear and intact goblet cells and crypt edges visible, and no infiltration of inflammatory cells. The intestinal tissue of MOD group mice showed mucosal necrosis and degeneration accompanied by infiltration of inflammatory cells in the submucosa, with a large number of goblet cells disappearing and unclear crypt edges. Compared with MOD group, the intestinal tissue structure of the treatment group was basically normal, with slight proliferation of goblet cells and infiltration of mild inflammatory cells. It was concluded that the pathological changes in the intestinal tissue were improved after treating with SZ.
Fig. 4.
The influence of pathological changes of colon tissue (200X) (A-CON group; B- MOD group; C-SZS group; IC, inflammatory cell; GC, goblet cell).
3.4. Effects on intestinal microbiota
3.4.1. Analysis of microbial diversity
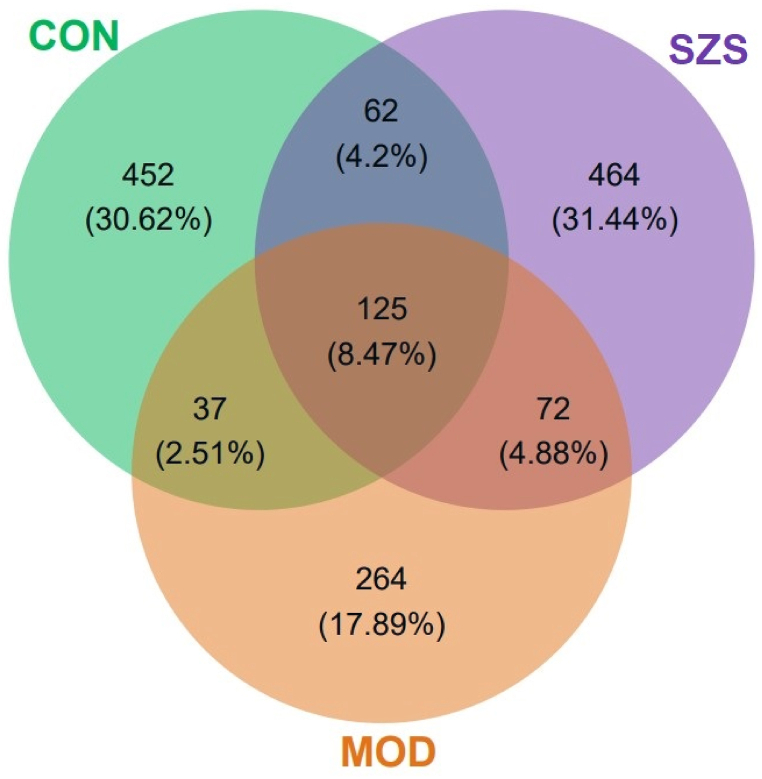
After 16s rRNA sequencing of fecal gut microbiota, we classified and evaluated the gut microbiota at phylum, class, order, family, genus and species levels. The veen plot of microbial OTUs in each group (Fig. 5) showed a total of 452, 264, 464 uniquely identified from CON, MOD, and SZS groups, respectively. The number of OTUs coincided all groups was 125. The number of coincided OTUs between the MOD and the CON group was 37. Similarly, the number of coincided OTUs between the SZS group and the MOD group, as well as the SZS group and the CON group was 72 and 62, respectively. The results indicated that the SZS group and CON group had higher similarity than that of MOD group.
Fig. 5.
Veen plot of gut microbiota in four groups.
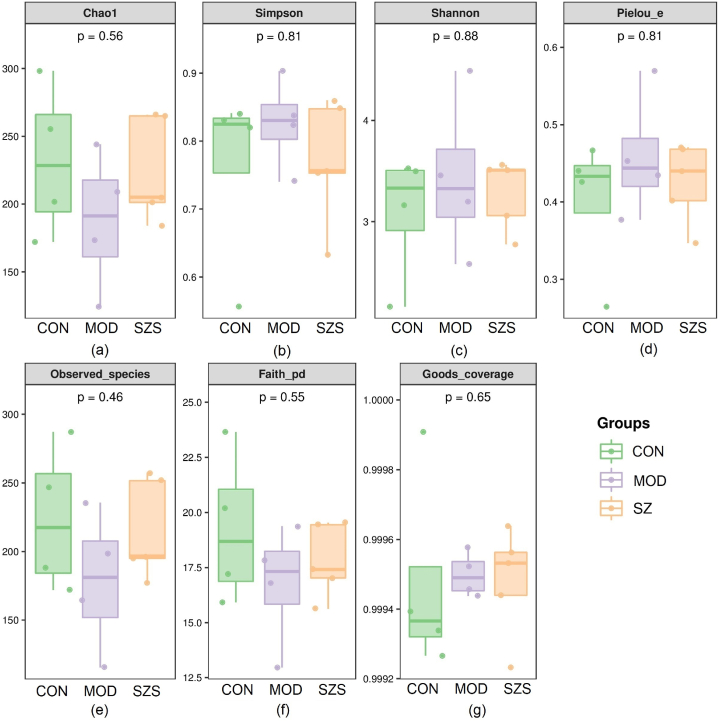
The alpha diversity index could reflect the changes in richness and diversity of microbial species [24]. Compared with the CON group (Fig. 6), the Chao1 index, Observated_species, and Faith_pd index of the MOD group showed significantly decrease (p < 0.05). While Shannon index, Simpson index, Pielou_e index and Good's coverage performed a notable enhancement (p < 0.05). After treating with SZ, all indexes demonstrated a significant rise (p < 0.05). It is worth noting that SZ group was more nearly to CON group, with no significant difference (p < 0.05). The above revealed that SZ could increase the diversity of gut microbiota, and recover the gut microenvironment.
Fig. 6.
Alpha diversity of gut microbiota in different groups. (a) Chao 1; (b) Simpson; (c) Shannon; (d) Pielou_e; (e) Observed_species; (f) Faith_pd; and (g) Goods_coverage
3.4.2. Analysis of microbial composition
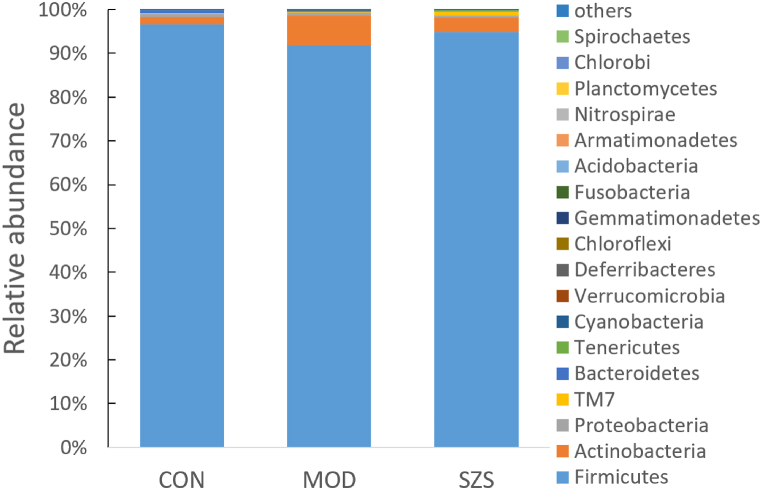
Further, we analyzed the differences in gut microbiota taxa and their relative abundance at phylum and genus levels. When compared to CON group, the proportion of Firmicutes and Deferribacteres was significantly decreased in MOD group (p < 0.01) at the phylum level (Fig. 7). Meanwhile, the relative abundance of Actinobacteria, TM7, Bacteroidetes, Cyanobacteria, and Verrucomicrobia displayed a significant increase (p < 0.01). The abundance of the above bacterial strains was restored after administrated with SZ.
Fig. 7.
Composition of gut microbiota in mice at the phylum level.
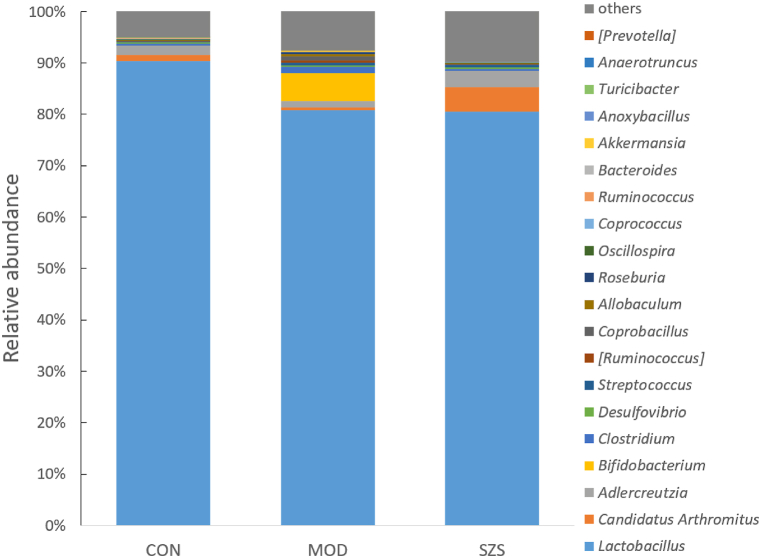
At the genus level (Fig. 8), results illustrated that the gut microbiota in MOD group was disturbed, harmful bacteria, such as Bifidobacterium, Clostridium prominently increased (p < 0.01). While beneficial ones, such as Lactobacillus, Adlercreutzia, reduced significantly (p < 0.01) while comparing to CON group. We also found that the composition of intestinal microbiota changed after given SZ. Especially, the proportion of Candidatus Arthromitus had a significant promote (p < 0.01). In addition, Lactobacillus showed a decrease at p < 0.05 level. The abundance of Bifidobacterium Clostridium, Allobaculum, Coprocillus, Roseburia, Streptococcus, and Ruminococcus was significantly reduced (p < 0.01). The results showed that SZ could reverse the changes in microbial composition, making the intestinal microbiota composition of mice with diarrhea close to that of normal healthy mice.
Fig. 8.
Composition of gut microbiota in mice at the gegus level.
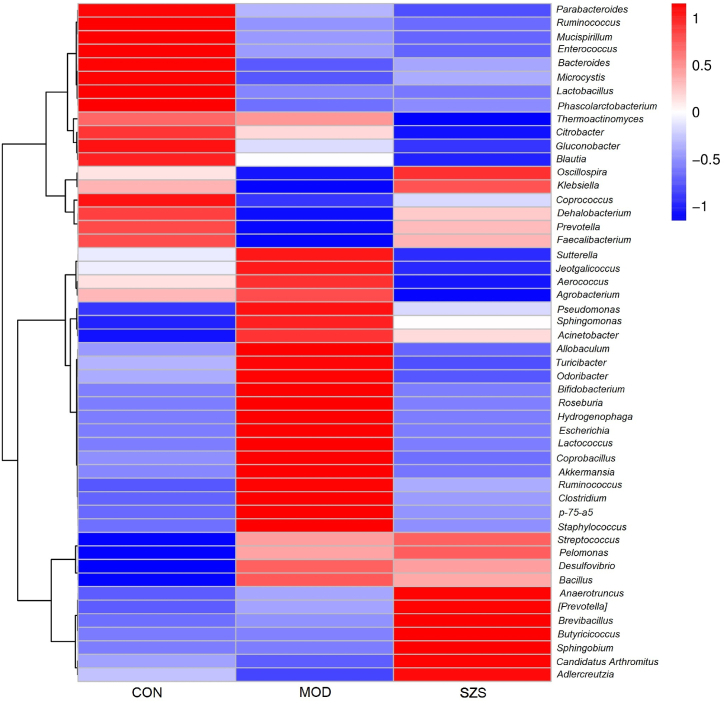
Furthermore, heatmap (Fig. 9) was applied to further explain the effects of SZ on the composition of gut microbiota in mice. The MOD group showed a significant increase in Sutterella, Aerococcus, Streptococcus, Bacillus, Acinetobacter, Turiciber, p-75-a5, Roseburia, Lactococcus, Clostridium, Staphylococcus, Allobaculum, Coprobacterius, Akkermansia, Bifidobacterium, Escherichia, Hydrogenophaga, Odoribacter, Ruminoccus, Pseudomonas and Sphingomonas. After SZ intervention, all strains except Streptococcus were decreased. At the same time, the ratio of Bacteroides, Dehalobacterium, Butyricicoccus, Pelomonas, Oscillospira, Anaerotruncus, [Prevotella], Candidatus, Arthromitus, Adlercreutzia, Microcystis, Coprococcus, Prevotella, Faecalibacterium, Lactobacillus, Klebsiella was increased. This hinted that SZ could improve the changes in microbial caused by Folium sennae.
Fig. 9.
Heatmap of composition of gut microbiota in mice at genus level.
3.4.3. Differential analysis of gut microbiota
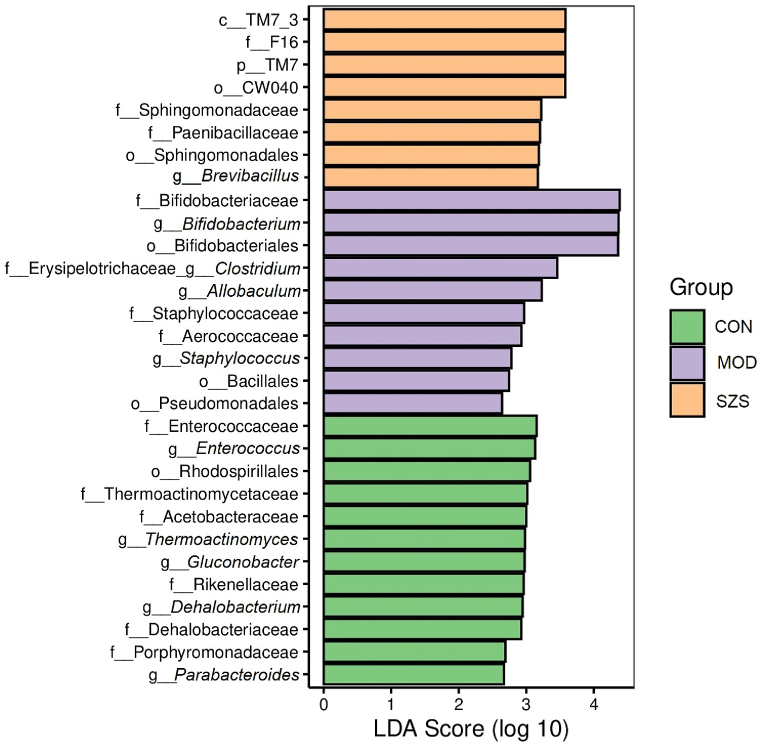
LDA distribution based on LESfe analysis were showed in Fig. 10. It showed that 4, 5, and 8 biomarkers were found in CON, MOD, and SZS groups, respectively (LDA ≥3). LDA scores also found that differential microbial communities of CON group were mainly composed of Thermoactinomyceteaceae, Enterococcaceae and Enterococcus (belonging to the Firmicutes phylum), as well as Rhodospirillales (belonging to the Proteobacteria phylum). The genus Staphylococcus, Clostridium, Allobaculum, and Bifidobacterium, along with the order Bifidobacteriales, and family Staphylococcaceae, Aerococcaceae, and Bifidobacteriaceae were high in MOD group. Spingomonadales, Spingomonadaceae, c-TM7-3, f-F16, P_TM7, o-CW040 and genus Brevibacilus were enriched by SZ administration. This further confirmed that SZ remedied diarrhea through regulating intestinal microbiota. Specifically, increasing beneficial bacteria and reducing harmful bacteria.
Fig. 10.
LDA scores of the four groups of mice.
3.4.4. short chain fatty acids in gut microbiota
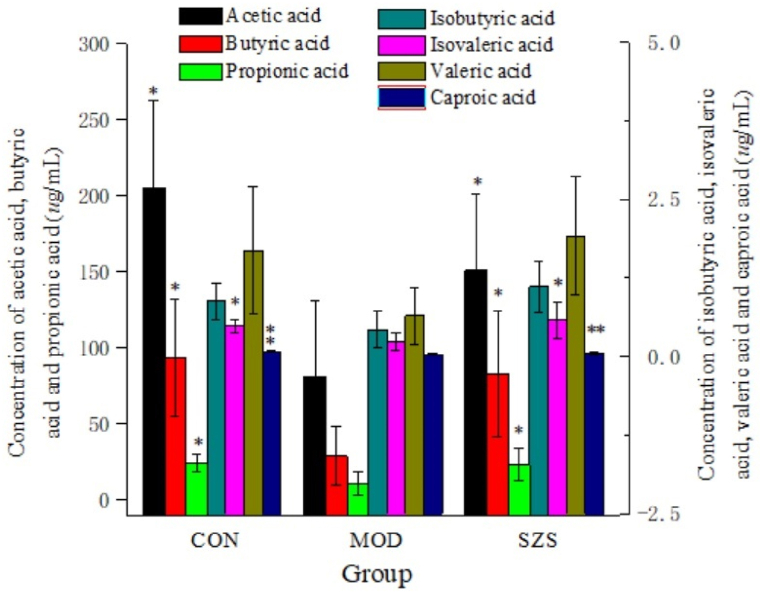
SCFAs production of cecum content were seen in Fig. 11. Compared with CON group, the concentration of all 7 products was decreased in MOD group. The levels of acetic acid, butyric acid, isovaleric acid, and propionic acid presented a significance at level 0.01 compared to the Mod group. While the levels of caproic acid decreased significantly (p < 0.05). Moreover, after administration of SZ, all 7 metabolites exhibited an elevation. It was worth noting that the SCFAs content in SZ group was comparable to that of CON group (no significant difference). This revealed that SZ could regulate the gut microbiota by adjusting the content of SCFAs.
Fig. 11.
Content of SCFAs in the colon (vs MOD group, ∗ p < 0.05, ∗∗p < 0.01).
4. Discussion
The pathogenesis of diarrhea is complex. Inflammation is considered as a major triggering factor. In addition, changes in gut microbiota and metabolic abnormalities in the body may also induce diarrhea [27]. As the main organ for digestion and absorption in the body, the intestine contains a large number of complex microbial communities. Changes in the diversity of gut microbiota may cause structural disorders and increase the risk of disease in the body [28]. The model mice was induced by Folium sennae in this study showed a significant disorder in the gut microbiota. This was consistent with literature reports [24]. The abundance of Bifidobacterium, Clostridium, Allobaculum, Coprocillus, Roseburia, Streptococcus, and Ruminococcus was significantly increased. While the ratios of Lactobacillus, Candidatus, Adlercreutzia, Arthromitus, Coprococcus, Bacteroides, and Oscillospira exibited a decline.
In recent years, an increasing number of studies have confirmed that gut microbiota is closely related to various diseases, and it has been clarified that affecting gut microbiota can improve various diseases [[29], [30], [31]]. Therefore, gut microbiota provides an important pathway for the study of the anti-diarrhea mechanisms of E. angustifolia fruit. This study found that SZ could upregulate the abundance of Lactobacillus, Blautia, Streptococcus, Allobaculum, and Oscillospira. A down regulation trend was found in the abundance of Prevotella, Bacteroides, Clostridium, Coprococcus, and Ruminococcus. A previously research reported the antibacterial, antifungal properties of E. angustifolia [32]. Our study indicated that SZ could reverse the composition changes in gut microbiota of mice caused by Folium sennae. The findings also demonstrated that SZ could inhibit diarrhea by killing harmful bacteria and increasing beneficial bacteria. This was similar to the repoted literature [33].
Short chain fatty acids (SCFAs), bile acids, methane, and tryptophan are important components of gut microbiota [34]. SCFAs are products formed by the fermentation of carbohydrates that cannot be digested by the human body by probiotics (such as lactic acid bacteria and bifidobacteria) in the intestine [29]. It has the functions of oxidative energy supply, regulating intestinal microbiota balance, improving intestinal function, regulating immune anti-tumor effects, regulating gene expression, and so on [35,36]. Our study showed that the metabolic products of SCFAs could recovery to normal levels.
Research shows a close relationship between gut microbiota and sex hormones [37]. The differences in longevity and incidence of diseases between males and females are related to gut microbiota. Unfortunately, this study did not observe and compare the effects of SZ on the gut microbiota of male and female mice. In future research, we will strengthen research in this area.
Folium sennae, a traditional Chinese medicine, has the effects of relieving heat and promoting stagnation, promoting bowel movements, and promoting diuresis [24]. The senosides and their active substances formed in the large intestine can reduce the absorption of water and electrolytes in the intestine. It is commonly used as a model drug for diarrhea, can result in intestinal microbiota diversity disorder [24]. Therefore, acute diarrhea model induced by Folium sennae was adopted.
Chlorogenic acid, caffeic acid, ferulic acid, and tannic acid are polyphenol. They display several important activities, such as anti-inflammation, antioxidant activity, antibacterial, and antiviral [38,39]. Our findings verified that polyphenols play an important role in diarrhea.
Fruit of E. angustifolia L. is a traditional Uyghur medicine in China, usually used for treating diarrhea. But excessive use can lead to constipation. Therefore, we should pay attention to its dosage when applying it. Tannins was one of the ingredients of E. angustifolia [32], which was considered as a substance for treating diarrhea [40]. This showed that E. angustifolia could suppress diarrhea by multi-component and multi-target.
5. Conclusions
In summary, the results of this study suggested SZ could alleviate the symptoms of intestinal microbiota imbalance induced by Folium sennae. Specifically, SZ may promote the secretion of acetic acid and butyric acid by regulating the relative abundance of Lactobacillus, Blautia, Streptococcus, Allobaculum, and Oscillospira in the gut microbiota of acute mice with diarrhea caused by Folium sennae. At the same time, we demonstrated that SZ could downregulate the proportion of Prevotella, Bacteroides, Clostridium, Coprococcus, and Ruminococcus, inhibiting the scale of propionic acid. In other words, SZ play the role of anti-diarrhea and keeping intestinal health through regulating the content of different SCFAs in gut metabolism. The study provides new ideas and directions for further elucidating the mechanism of SZ regulation in improving diarrhea. The pharmacological substances of SZ against diarrhea have not been explained clearly in our research, needing intensive study.
Declarations of ethics
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of Xinjiang Institute of Chinese Materia Medica and Ethnodrug (experiment approval number: XJIMM-20230202).
Data availability statement
The data includes in the Article.
CRediT authorship contribution statement
Xiatiguli Abulizi: Investigation, Funding acquisition. Ming-hui Shi: Software, Data curation. Yue-mei Jia: Software. Lei Xu: Software, Methodology. Lei-ling Shi: Writing – review & editing. Lan Pan: Writing – original draft, Supervision.
Declaration of competing interest
No conflict of interest exits in the submission of this manuscript “Elaeagnus angustifolia L. fruit alleviates diarrhea via regulating intestinal microbiota and short chain fatty acids”, and the manuscript is approved by all authors for publication and has not been published previously, or not under consideration for publication elsewhere, in whole or in part.
Acknowledgments
This work was supported by the Ethnic Minority Special Training Program of Xinjiang (2021D03008) and Basic Research Business Funding Project for Public Welfare Research Institutes of Xinjiang Autonomous (KY2023086).
Contributor Information
Xiatiguli Abulizi, Email: 369572138@qq.com.
Ming-hui Shi, Email: xjshmh@126.com.
Yue-mei Jia, Email: YYMM_2020@aliyun.com.
Lei Xu, Email: xulei19820218@126.com.
Lei-ling Shi, Email: shileiling@sina.com.
Lan Pan, Email: panlan_sc@126.com.
References
- 1.Lu J., Mao D., Li X., Ma Y., Luan Y., Cao Y., Luan Y. Changes of intestinal microflora diversity in diarrhea model of KM mice and effects of Psidium guajava L. as the treatment agent for diarrhea. J. Infect. Public Heal. 2020;13:16–26. doi: 10.1016/j.jiph.2019.04.015. [DOI] [PubMed] [Google Scholar]
- 2.Ferris A., Gaisinskaya P., Nandi N. Approach to diarrhea. Prim. Care Clin. Off. Pract. 2023;50:447–459. doi: 10.1016/j.pop.2023.03.010. [DOI] [PubMed] [Google Scholar]
- 3.Yin X., Gu X., Yin T., Wen H., Gao X., Zheng X. Study of enteropathogenic bacteria in children with acute diarrhoea aged from 7 to 10 years in Xuzhou, China. Microb. Pathog. 2016;91:41–45. doi: 10.1016/j.micpath.2015.11.027. [DOI] [PubMed] [Google Scholar]
- 4.Elseviers M.M., Van Camp Y., Nayaert S., Dure K., Annemans L., Tanghe A., Vermeersch S. Prevalence and management of antibiotic associated diarrhea in general hospitals. BMC Infect. Dis. 2015;15:129. doi: 10.1186/s12879-015-0869-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xu J., Liu C., Shi K., Sun X., Song C., Xu K., Liu Y. Atractyloside-A ameliorates spleen deficiency diarrhea by interfering with TLR4/MyD88/NF-κB signaling activation and regulating intestinal flora homeostasis. Int. Immunopharm. 2022;107 doi: 10.1016/j.intimp.2022.108679. [DOI] [PubMed] [Google Scholar]
- 6.Ryan M.J., Schloter M., Berg G., Kostic T., Kinkel L.L., Eversole K., Macklin J.A., Schelkle B., Kazou M., Sarand I., Singh B.K., Fischer D., Maguin E., Ferrocino I., Lima N., McClure R.S., Charles T.C., de Souza R.S.C., Kiran G.S., Krug H.L., Taffner J., Roume H., Selvin J., Smith D., Rybakova D., Sessitsch A. Development of microbiome biobanks-challenges and opportunities. Trends Microbiol. 2020;S0966–842X(20):30188. doi: 10.1016/j.tim.2020.06.009. 30188. [DOI] [PubMed] [Google Scholar]
- 7.Zhang C., Yu X., Cui Y., Wang H., Chen X., Ma X., Li H., Su J., Ma Z., Huang L. Shengjiang Xiexin decoction ameliorates antibiotic-associated diarrhea by altering the gut microbiota and intestinal metabolic homeostasis. Phytomedicine. 2023;113 doi: 10.1016/j.phymed.2023.154737. [DOI] [PubMed] [Google Scholar]
- 8.Xue H., Mei C.-F., Wang F.-Y., Tang X.-D. Relationship among Chinese herb polysaccharide (CHP), gut microbiota, and chronic diarrhea and impact of CHP on chronic diarrhea. Food Sci. Nutr. 2023;11:5837–5855. doi: 10.1002/fsn3.3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li Y., Xia S., Jiang X., Feng C., Gong S., Ma J., Fang Z., Yin J., Yin Y. Gut Microbiota and diarrhea: an updated review. Front. Cell. Infect. Microbiol. 2021;11 doi: 10.3389/fcimb.2021.625210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Qiao B., Xiao N., Deng N., Tan Z. Shenling Baizhu powder attenuates lard diet in a fatigued state-induced diarrhea via targeting microbial metabolites short chain fatty acids-mediated lipid metabolism. 3 Biotech. 2024;14:203. doi: 10.1007/s13205-024-04045-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.An X., Bao Q., Di S., Zhao Y., Zhao S., Zhang H., Lian F., Tong X. The interaction between the gut microbiota and herbal medicines. Biomed. Pharmacother. 2019;118 doi: 10.1016/i.biopha.2019.109252. [DOI] [PubMed] [Google Scholar]
- 12.Xia T., Liu C.S., Hu Y.N., Luo Z.Y., Chen F.L., Yuan L.X., Tan X.M. Coix seed polysaccharides alleviate type 2 diabetes mellitus via gut microbiota-derived short-chain fatty acids activation of IGF1/PI3K/AKT signaling. Food Res. Int. 2021;150 doi: 10.1016/j.foodres.2021.110717. [DOI] [PubMed] [Google Scholar]
- 13.Leth M.L., Ejby M., Workman C., Ewald D.A., Pedersen S.S., Sternberg C., Bahl M.I., Licht T.R., Aachmann F.L., Westereng B., Hachem M.A. Differential bacterial capture and transport preferences facilitate co-growth on dietary xylan in the human gut. Nat. Microbiol. 2018;3:570–580. doi: 10.1038/s41564-018-0132-8. [DOI] [PubMed] [Google Scholar]
- 14.Khadivi A., Mirheidari F., Moradi Y., Paryan S. Phenotypic variability of oleaster (Elaeagnus angustifolia L.) as revealed by morphological characteristics. Ind. Crops Prod. 2020;149 doi: 10.1016/j.indcrop.2020.112322. [DOI] [Google Scholar]
- 15.Editorial committee of the flora of China Flore ofChina. Science Press, Beijing. 1983;52(2):40. [Google Scholar]
- 16.Uzun A., Çelik B., Karadeniz T., Yilmaz K.U., Altintas C. Assessment of fruit characteristics and genetic variation among naturally growingwild fruit Elaeagnus angustifolia accessions. Turk. J. Agric. For. 2015;39:286–294. doi: 10.3906/tar-1408-88. [DOI] [Google Scholar]
- 17.Du H., Chen J., Tian S., Gu H., Li N., Sun Y., Ru J., Wang J. Extraction optimization, preliminary characterization and immunological activities in vitro of polysaccharides from Elaeagnus angustifolia L. pulp. Carbohydr. Polym. 2016;151:348–357. doi: 10.1016/j.carbpol.2016.05.068. [DOI] [PubMed] [Google Scholar]
- 18.State Administration of Traditional Chinese Medicine . Shanghai Science and Technology Press; Shanghai: 2005. Zhonghuabencao-Uygur Medicine; p. 183. 2. [Google Scholar]
- 19.Ahmadiani A., Hosseiny J., Semnanian S., Javan M., Saeedi F., Kamalinejad M., Saremi S. Antinociceptive and anti-inflammatory effects of Elaeagnus Angustifolia fruit extract. J. Ethnopharmacol. 2000;72:290–292. doi: 10.1016/s0378-8741(00)00222-1. [DOI] [PubMed] [Google Scholar]
- 20.Mamashli M., Nasseri S., Mohammadi Y., Ayati S., Zarban A. Anti-infammatory efects of N-Acetylcysteine and Elaeagnus Angustifolia extract on acute lung injury induced by λ-carrageenan in rat. Infammopharmacology. 2022;30:1759–1768. doi: 10.1007/s10787-022-01003-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen Q., Chen J., Du H., Li Q., Chen J., Zhang G., Liu H., Wang J. Structural characterization and antioxidant activities of polysaccharides extracted from the pulp of Elaeagnus Angustifolia L. Int. J. Mol. Sci. 2014;15:11446–11455. doi: 10.3390/ijms150711446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Konyalioglu S., Sogut O. Research on polyphenolic contents Elaeagnus angustifolia L. Free raddical bio. Méd. 2016;96:S63. doi: 10.1016/j.freeradbiomed.2016.04.137. [DOI] [Google Scholar]
- 23.Han J., Chen X., Liu W., Cui H., Yuan T. Triterpenoid saponin and lignan glycosides from the traditional medicine Elaeagnus angustifolia flowers and their cytotoxic activities. Molecules. 2020;25:462. doi: 10.3390/molecules25030462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang C., Shao H., Li D., Xiao N., Tan Z. Role of tryptophan-metabolizing microbiota in mice diarrhea caused by Folium sennae extracts. BMC Microbiol. 2020;20:185. doi: 10.1186/s12866-020-01864-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhu J., Yu L., Fan Y., Zhang H., Li F., Li X., Wei Y., Wang Z. Camelina sativa oil treatment alleviates castor oil-induced diarrhea in ICR mice by regulating intestinal flora composition. J. Evidence-Based Complementary Altern. Méd. 2022;2022 doi: 10.1155/2022/5394514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ma Z.J., Wang H.J., Ma X.J., Li Y., Yang H.J., Li H., Su J.R., Zhang C.E., Huang L.Q. Modulation of gut microbiota and intestinal barrier function during alleviation of antibiotic-associated diarrhea with rhizoma Zingiber officinale (ginger) extract. Food Funct. 2020;11:10839–10851. doi: 10.1039/d0fo01536a. [DOI] [PubMed] [Google Scholar]
- 27.Yu R., Zhou Q., Liu T., Liu P., Li H., Bian Y., Liu Z. Kaempferol relieves the DSS-induced chronic colitis in C57BL/6J mice, alleviates intestinal angiogenesis, and regulates colonic microflora structure. J. Funct.Foods. 2023;107 doi: 10.1016/j.jff.2023.105646. [DOI] [Google Scholar]
- 28.Guan F., Fu G., Ma Y., Zhou L., Li G., Sun C., Zhang T. Spirulina polysaccharide-based prebiotic foods preparations-a promising approach for modulating gut microbiota and improving health. J. Funct.Foods. 2024;116 doi: 10.1016/j.jff.2024.106158. [DOI] [Google Scholar]
- 29.Xu B., Yan Y., Huang J., Yin B., Pan Y., Ma L. Cortex Phellodendri extract's anti-diarrhea effect in mice related to its modification of gut microbiota. Biomed. Pharmacother. 2020;123 doi: 10.1016/j.biopha.2019.109720. [DOI] [PubMed] [Google Scholar]
- 30.Wang H., Zhou L., Zheng Q., Song Y., Huang W., Yang L., Xiong Y., Cai Z., Chen Y., Yuan J. Kai-Xin-San improves cognitive impairment in D-gal and Aβ25-35 induced AD rats by regulating gut microbiota and reducing neuronal damage. J. Ethnopharmacol. 2024;2024 doi: 10.1016/j.jep.2024.118161. [DOI] [PubMed] [Google Scholar]
- 31.Wang S., Li X., Zhang B., Li Y., Chen K., Qi H., Gao M., Rong J., Liu L., Wan Y., Dong X., Yan M., Ma L., Li P., Zhao T. Tangshen formula targets the gut microbiota to treat non-alcoholic fatty liver disease in HFD mice: a 16S rRNA and non-targeted metabolomics analyses. Biomed. Pharmacother. 2024;173 doi: 10.1016/j.biopha.2024.116405. [DOI] [PubMed] [Google Scholar]
- 32.Khan S.U., Khan A., Shah A.-H.A., Shah S.M., Hussain S., Ayaz M., Ayaz S. Heavy metals content, phytochemical composition, antimicrobial and insecticidal evaluation of Elaeagnus angustifolia. Toxicol. Ind. Health. 2013;32:154–161. doi: 10.1177/0748233713498459. [DOI] [PubMed] [Google Scholar]
- 33.Hamad N., EL-Sherry S., Abd-Elghaffar S., Abdul-Rahman M. Molecular detection and immune-profiling of circulating very virulent infectious bursal sisease in broiler farms in Egypt. Pak. Vet. J. 2022;42:316–321. [Google Scholar]
- 34.Silva Y.P., Bernardi A., Frozza R.L. The role of short-chain fatty acids grom gut microbiota in gut-brain communication. Front. Endocrinol. 2020;11:1–14. doi: 10.3389/fendo.2020.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen C., Liu C., Zhang K., Xue W. The role of gut microbiota and its metabolites short-chain fatty acids in food allergy. Food Sci. Hum. Wellness. 2023;12:702–710. doi: 10.1016/j.fshw.2022.09.003. [DOI] [Google Scholar]
- 36.Nabizadeh E., Sadeghi J., Rezaee M.A., Hamishehkar H., Hasani A., Kafil H.S., Sharifi Y., Asnaashari S., Kadkhoda H., Ghotaslou R. The profile of key gut microbiota members and short-chain fatty acids in patients with sepsis. Heliyon. 2023;9 doi: 10.1016/j.heliyon.2023.e17880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu Y., Peng X., Li X., Li D., Tan Z., Yu R. Sex hormones influences intestinal microbiota composition in mice. Front. Microbiol. 2022;13 doi: 10.3389/fmicb.2022.964847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhao M., Wang H., Yang B., Tao H. Identification of cyclodextrin inclusion complex of chlorogenic acid and its antimicrobial activity. Food Chem. 2010;120:1138–1142. doi: 10.1016/j.foodchem.2009.11.044. [DOI] [Google Scholar]
- 39.He Y., Sun Z., Bai J., Zhang Y., Qian Y., Zhao X., Chen S. Citrus peel polyphenols alleviate intestinal inflammation in mice with dextran sulfate sodium-induced acute colitis. Heliyon. 2023;9 doi: 10.1016/j.heliyon.2023.e18137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Würger G., McGaw L.J., Eloff J.N. Tannin content of leaf extracts of 53 trees used traditionally to treat diarrhoea is an important criterion in selecting species for further work. South Afr. J. Bot. 2014;90:114–117. doi: 10.1016/j.sajb.2013.11.003. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data includes in the Article.