Abstract
Background
Laparoscopic surgery is associated with a lower morbidity than open surgery. No recent data compared kidney cancer surgery in the French population using the National Health Insurance database (PMSI-MCO).
Aims
We explore and compare the surgical morbidity rates between laparoscopic and open laparotomy for kidney cancer.
Methods
The initial length of stay and complications parameters during the three postoperative months were described for renal cancer in every French center in 2018. We compared Relative Risks (RR [95% CI]) between laparoscopic and open surgery for both radical and partial nephrectomy.
Results
Among 8,162 patients, 3,525 had a radical nephrectomy, 978 open, 2,547 laparoscopic surgeries; 4,637 patients had partial nephrectomies, 1,778 open 2,859 laparoscopic surgeries. For radical surgery, the most common complications were urinary infections (7.8%), acute renal failure (8.9%), sepsis (8.4%), bleeding (9.3%), and postoperative anemia (5.9%); the RR for laparoscopic versus open surgery were respectively 0.68 [0.54;0.86], 0.71 [0.57;0.88], 0.69 [0.55;0.86], 0.83 [0.66;1.03], 0.56 [0.43;0.73]. For partial nephrectomies, the most common complications were urinary infections (7.7%), bleeding (11.6%), and postoperative anemia (5.8%), with RR of 0.71 [0.58;0.87], 0.61 [0.52;0.71], and 0.64 [0.51;0.81]. The mean length of stay was 7.7 for open radical nephrectomy, 6.3 for laparoscopic radical nephrectomy, 7.5 for open partial nephrectomy, and 5 for laparoscopic partial nephrectomy.
Conclusions
The laparoscopic approach had fewer postoperative complications and a shorter length of stay than open surgery for partial and radical nephrectomy. The PMSI analysis provided an exhaustive description of surgical practice for kidney cancer and surgical complications in France.
Clinical trial number
Not applicable.
Keywords: Complications, Kidney, Laparoscopy, Neoplasia, Surgery
Background
Kidney cancer is the world’s fourteenth most common solid tumor [1]. Historically, kidney tumors were managed by removing the cancer in open surgery [2]. Surgical excision of the tumor or the entire kidney in non-metastatic patients remains the standard of care [3]. In large data series, overall complications range from 12 to 36% [4–8], regardless of the surgical approach.
Surgical complications delay hospital discharge and increase the expense of care. Over time, renal surgery techniques for cancer have been improved from the first description of open radical nephrectomy by Robson in 1963 [2]. In 1991, Clayman et al. described laparoscopic radical nephrectomy [9], and Winfield performed the first laparoscopic partial nephrectomy for benign disease in 1992 [10]. Mastering laparoscopy led urologists to prefer this approach when possible, lowering the risk of complications for the same oncology outcomes [3, 4]. Nevertheless, these studies are conducted mainly in expert centers; to our knowledge, no study has ever been conducted in an entire country population for one year about open and laparoscopic approaches detailing all types of rehospitalization-related complications.
In this context, the use of the French National Health Insurance Database, PMSI MCO (Programme de Médicalisation des Systèmes d’Information - Médecine, Chirurgie, Obstétrique), is particularly relevant. This centralized medico-economic database covers all private and public hospital activities in France and is the basis for hospital billing and reimbursement. It contains comprehensive and coded information on pathologies, treatments, and complications for each patient’s hospitalization since 1991 for every public center and 1997 for every private center. In contrast, other countries like Germany or the United States only include in-hospital patients based on their healthcare insurance. The PMSI MCO database is not only exhaustive and reliable but also provides a standardized format for data collection, enabling robust and large-scale analyses of clinical outcomes across various healthcare settings.
Hospitalizations in the PMSI are classified under a “Groupe Homogène de Malades” (GHM), which is akin to the American model of Diagnosis-Related Groups (DRGs). Both the GHM and DRG systems categorize hospital stays into clinically similar groups and expect them to use the same level of hospital resources, providing detailed medical and cost information essential for billing purposes. GHS in PMSI further allows for a systematic approach to evaluating hospital performance and patient outcomes, adding another layer of utility for research purposes.
For an observational study focusing on surgical outcomes, the PMSI MCO offers an invaluable data source to document and compare the morbidity associated with radical and partial kidney cancer surgeries performed via open and laparoscopic approaches. By leveraging this rich dataset, which includes variables such as surgical techniques, complication rates, and GHS classifications, we can conduct an in-depth analysis of the entire French population scale in real-life settings. This approach allows for a more comprehensive understanding of surgical outcomes outside specialized centers, ultimately contributing to better informing patients preoperatively and guiding clinical decision-making.
Materials et methods
Population
We included all adult patients (> 20 years) operated between January 1 and December 31, 2018, with newly diagnosed renal tumors (International Classification of Diseases (ICD)-10, code: C64) and hospitalized for surgical treatment in all French public or private centers. From the PMSI-MCO, we worked with the “Classification commune des actes médicaux” (CCAM), a complementary coding list for therapeutic acts included in the database, to define four groups: open radical nephrectomy (ORN), laparoscopic radical nephrectomy (LRN), open partial nephrectomy (OPN), and laparoscopic partial nephrectomy (LPN), (Table 1). Robotic-assisted laparoscopy was not separated from other laparoscopies because no distinct code was available in 2018.
Table 1.
CIM-10 corresponding codes for complications and CCAM corresponding codes for surgeries
| Complications | code CIM-10 |
| Wound complication | L022, K432, T8138, T8130, S308 |
| Urinary infection | N10, N410, N390 |
| Pulmonary infection | J150-159, J180-189 |
| Acute renal failure | N17, N990, R392 |
| Venous thrombosis | I80, I26 |
| Bowel occlusion | K913, K560 |
| Sepsis | A40, A41, R65, R572 |
| Peritonitis | K65 |
| Renal abscess | N151 |
| Bleeding | S3700, T810, R571 |
| Postoperative anemia | D500, D62 |
| Pneumothorax | S2760 |
| Renal fistula | N288 |
| False aneurysm | I722 |
| Type of surgery | CCAM codes |
| Laparoscopic radical nephrectomy | JAFC006, JAFC010, JAFC019 |
| Laparoscopic partial nephrectomy | JAFC005 |
| Open radical nephrectomy | JAFA023, JAFA002, JAFA010, JAFA009, JAFA029 |
| Open partial nephrectomy | JAFA019, JAFA030, JAFA008, JAFA024 |
The exclusion criteria were morbid surgeries such as cava thrombectomy, thoracophrenolaparotomy or binephrectomy, metastatic patients, and simple nephrectomy. In addition, nephrectomy associated with ureterectomy was also excluded, as it was upper tract urothelial carcinoma.
Morbidity evaluation
Morbidity was determined with complications related to surgery: wound complication, urinary infection, pulmonary infection, acute renal failure, bowel occlusion, sepsis, peritonitis, renal abscess, bleeding, postoperative anemia, pneumothorax, renal fistula, false aneurysm as well as venous thrombosis. The initial hospital length of stay was also sorted by surgery type.
Wound complications were defined as wound infections and eventrations; anemia as hemoglobin levels lower than the standard for age and sex (men < 140 g/L, women < 130 g/L); bleeding as any hemorrhage or hematoma following surgery; sepsis as inflammatory syndrome from any cause; acute renal failure was coded according to KDIGO criteria [11] independently of the severity; venous thrombosis as deep venous thrombosis or pulmonary embolism.
Statistical analysis
Complications associated with the two surgical procedures are described by percentages and compared between laparoscopic and open nephrectomy by relative risks (RR) and their 95%CIs. Fisher exact p values were calculated for complications with zero frequencies when RRs could not be calculated. Statistical analyses used SAS, version 9.4 (SAS Institute Inc., Cary, NC, USA).
Results
In 2018, 8,162 patients underwent renal surgery for kidney cancer in France. We identified 3,525 (43%) radical nephrectomies, with 2,547 (72%) LRN and 978 ORN, and 4,637 (57%) partial nephrectomies, with 2,859 (62%) LPN and 1,778 OPN. Initial hospital stay was shorter for LPN, with a mean length of stay of 5 days and 9.7 days for ORN (Table 2).
Table 2.
Time of hospital stay by type of surgery
| Type of surgery | ORN | LRN | OPN | LPN |
|---|---|---|---|---|
| Mean length of stay | 9.7 | 6.3 | 7.5 | 5 |
Radical nephrectomy
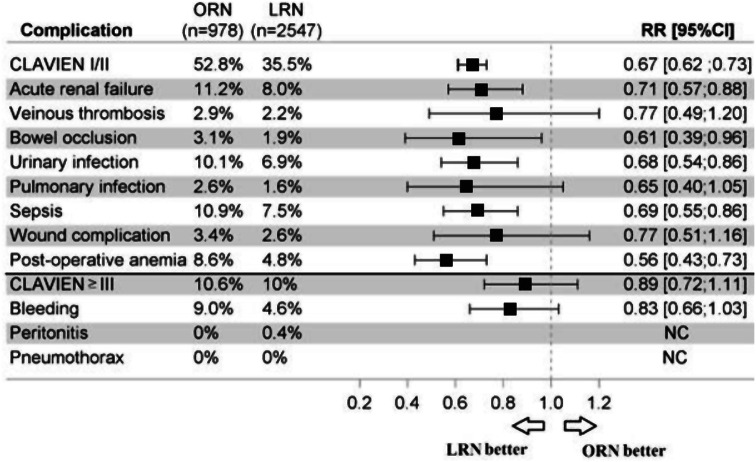
The most frequent complications were urinary infections (7.8%, 6.9%, and 10.1%, respectively for overall, LRN, and ORN), acute renal failure (8.9%: 8.0%, 11.2%), sepsis (8.4%: 7.5%, 10.9%), bleeding (9.3%: 8.8%, 10.6%) and post-operative anemia (5.9%: 4.8%, 8.6%) (Fig. 1).
Fig. 1.
Complications following open radical nephrectomy and laparoscopic radical nephrectomy in France in 2018. ORN: Open radical nephrectomy; LRN: Laparoscopic radical nephrectomy; RR[95%CI]: 95% Confidence intervalle Relative risk
The risk of complications with LRN was lower than with ORN (p < 0.05): for urinary infections with RR = 0.68 [0.54;0.86]; acute renal failure, 0.71 [0.57; 0.88]; bowel occlusions, 0.61 [0.39; 0.96]; sepsis, 0.69 [0.55; 0.86]; postoperative anemia, 0.56 [0.43; 0.73]; with a trend for bleeding 0.83 [0.66; 1.03] (Fig. 1).
Peritonitis was more frequent with LRN than ORN, with 18 (0.7%) versus zero cases (p = 0.006).
Partial nephrectomy
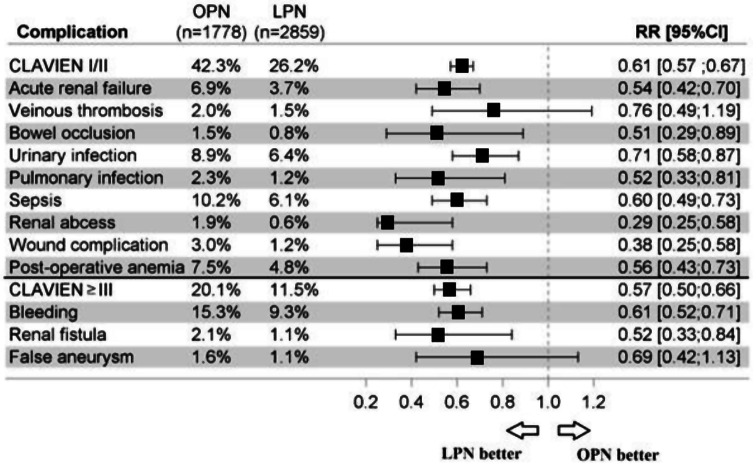
The predominant complications were urinary infection (7.4%: 8.9%, 6.4% overall and for OPN, LPN), acute renal failure (4.9%: 6.9%, 3.7%), sepsis (7.7%: 10.2%, 6.1%), bleeding (11.6%: 15.3%, 9,3%), and postoperative anemia (5.8%: 7.5%, 4.8%) (Fig. 2). All complications with LPN were lower than with OPN (p < 0.05) except for false aneurysms with RRs 0.69 [0.42; 1.13] and venous thrombosis 0.76 [0.49; 1.19]. Pneumothorax was more frequent in OPN, with zero cases in LPN (p < 0.001).
Fig. 2.
Complications following partial nephrectomy open partial nephrectomy and laparoscopic partial nephrectomy in France in 2018. OPN: Open partial nephrectomy; LPN: Laparoscopic partial nephrectomy; RR [95%CI]: 95% confidence interval Relative risk
We found no peritonitis in patients undergoing a partial nephrectomy.
Discussion
This manuscript provides data from daily surgical practice in France for the 3-month postoperative complication rates for non-metastatic cancer renal surgery. Literature often came from leading expert centers where laparoscopic surgeries are well-mastered. As our data include all renal surgeries in reference centers, community hospitals, and public and private practices, we provide real-life in-hospital postoperative complication rates.
We confirmed that the laparoscopic approach decreased morbidity and length of stay compared to open surgery for radical and partial nephrectomy in renal tumors. For radical and partial nephrectomies, laparoscopy provided statistically significant lower complication rates for urinary infection, acute renal failure, bowel occlusion, sepsis, and postoperative anemia.
Regarding bleeding, results are quite heterogeneous in the literature, with 1.14 to 11.9% for LRN and 2.09 to 19% for ORN [6, 12–14]. For partial nephrectomies, the literature shows bleeding and anemia from 5.8 to 9% for laparoscopy and 2 to 12.7% for open surgeries [4–6, 12]. Stang and Buchel reported that for partial nephrectomies, laparoscopy, and open combined, 18.5% had bleeding or anemia. For ORN and LRN, the rates were 19.0% and 11.9%, a statistically significant difference with an RR of 0.69 (0.61–0.78) [15]. Our incidences were similar, with 11.6% for partial nephrectomy and 10.6% and 8.8% for ORN and LRN, respectively, with a lower incidence for laparoscopy than open surgery. Comparisons between studies should be made carefully as postoperative anemia and bleeding definitions have variable definitions and are often confused.
Renal failure varied from 1.9 to 14% in prolonged ischemia with eGFR < 45mL/min for partial nephrectomy and up to 35% for radical nephrectomy [16, 17]. We recorded 8.9% of renal failure in patients undergoing radical nephrectomy and 4.9% for partial nephrectomy. Our results showed that LRN and LPN patients had better postoperative renal function than those treated with open nephrectomy. In contrast, no difference was found in clinical studies for open vs. laparoscopic partial nephrectomy [4, 5]. However, renal failure was coded in the ICD-10 as all acute renal failure stages with only one code. Furthermore, the literature’s criteria and the failure cutoffs were very heterogeneous.
Peritonitis occurred only with LRN (0.7%) in our study and was more frequent than open surgery (p = 0.006). We hypothesized that bowel wounds may be overlooked during laparoscopy when trocars or Veress needles are introduced in the peritoneal cavity, followed by a coagulator [18].
Patients who underwent a laparoscopic approach were less exposed to urinary infections in the French population. The literature prevalence of urinary infection was around 0.6 to 11.8% [6, 19] for radical nephrectomy and 0.5 to 7.9% [6, 15, 19, 20] for partial nephrectomy, and these are similar to our findings of 7.8% and 7.4% respectively, without accounting for laparoscopic or open approaches.
There is no equivalent in the literature between the rate of sepsis as defined in our study and that of the literature. The different types of infections and inflammatory syndromes are poorly defined. However, these complications should not be neglected and are statistically significantly more frequent in open surgery in our data.
Compared to the literature, the overall rate of pneumothorax was low, with 1–4.6% [6, 15, 17, 21, 22] for partial nephrectomy and 0.76–2.6% [14, 17] for radical nephrectomy. Our study recorded just 18 such complications only in OPN, statistically significantly higher than for LPN with zero recorded (p < 0.001).
Jordan et al. showed statistically significant differences in venous thrombosis between open and laparoscopic approaches, 2.0% vs. 0.8% p < 0.001 [23]. The rates were not dissimilar to our study, with an overall rate of 2%. However, the rates did not differ between open and laparoscopic surgeries.
According to the surgical approach for partial nephrectomy, we found a similar frequency of a false anevrysm and renal fistula. This result was relevant to the literature, showing no differences [5, 15, 22–24]. Nevertheless,
Conversely, wound complications, pulmonary infection, bowel occlusion, renal abscess, and renal fistula were less frequent in LPN than in OPN, with statistically significant differences, following the literature [4, 5, 15, 20, 24, 25] (Table 3).
Table 3.
Comparison between complications of partial and radical nephrectomy between PMSI-MCO in 2018 and other studies
| Complications | French PMSI data | Other studies data |
|---|---|---|
| Veinous thrombosis | ||
| Laparoscopic PN | 1.5% | 1.7% [20] |
| Open PN | 2% | 1.7-2% [6, 20] |
| RN* | 2.2–2.9% | 1.1% [20] |
| Bleeding | ||
| Laparoscopic PN | 9.3% | 5.8-9% [5, 6, 12, 15, 20] |
| Open PN | 15.3% | 2-12.7% [4–6, 12, 15, 20] |
| Laparoscopic RN | 8.8 | 1.14–11.9% [6, 12–14] |
| Open RN | 10.6% | 2.09-19% [6, 12–14] |
| False aneurysm | ||
| Laparoscopic | 1.1% | 0.42–3.6% [4, 24, 26, 30] |
| Open | 1.6% | 0.06–5.5% [4, 24, 26, 30] |
| Urinary fistula | ||
| Laparoscopic | 1.1% | 0.37-4% [5, 15, 20, 26] |
| Open | 2.1% | 0.87–4.3% [5, 15, 20, 24] |
| Urinary infections* | ||
| PN | 6.4–8.9% | 0.51–7.9% [6, 19, 20, 24] |
| RN | 6.9–10.1% | 0.57–11.8% [6, 15, 19, 20] |
| Wound complications | ||
| Laparoscopic PN | 1.2% | 0.53–0.8% [15, 20, 26] |
| Open PN | 3% | 1-3.21% [6, 15, 20, 24, 26] |
| Laparoscopic RN | 2.6% | 0% [6] |
| Open RN | 3.4% | 1.33% [6] |
| Occlusions | ||
| Laparoscopic PN | 0.8% | 0-0.8% [6, 20, 24] |
| Open PN | 1.5% | 0.8–2.1% [6, 20, 24] |
| Laparoscopic RN | 1.9% | 0% [6] |
| Open RN | 3.1% | 0.57% [6] |
| Peritonitis* | ||
| PN | 0% | 0.5% [17] |
| RN | 0-0.4% | 1.3% [17] |
| Pulmonary infection* | ||
| PN | 1.2–2.3% | 0.79–4.3% [17, 24, 26] |
| RN | 1.6–2.6% | 0.76–2.6% [17, 20] |
PN: Partial nephrectomy; RN: Radical nephrectomy; * laparoscopy and open approaches not separated
Our analysis has a few limitations. In France in 2018, 72% of radical nephrectomies and 62% of partial nephrectomies were performed by laparoscopy. Robotic assistance can explain the rate of LPNs as it is not accessible in all centers. We could not individualize robotic and classical laparoscopy in our data as the code was the same in 2018. This robotic subset was identified only after 2019. Bic et al. showed a slight difference in favor of the robot, but it was not statistically significant [26]. Moreover, recent recommendations favoring kidney preservation at all costs have led to more complex tumors being operated on robotically rather than laparoscopically, potentially increasing complication rates and introducing a recruitment bias that shifts the statistics in favor of laparoscopy.
The declarative nature of the PMSI database may lead to an underestimation of the number of complications. However, its use primarily for billing purposes could also lead to over-reporting. Refund rules are standard for all hospitals, so we have assumed these biases do not affect the type of surgery being compared. The large sample size limits the risk of these biases significantly impacting the overall results. It is also important to note that the PMSI database does not include outpatient care, underestimating complications that do not require hospitalization.
Given the limited or no information on epidemiologic characteristics of the different patient groups, such as comorbidity and tumor parameters, we conducted a statistical analysis of the relative risk to confirm or refute the results. Also, data on the conversion rate of partial to radical nephrectomy were unavailable because they were already considered radical nephrectomy in the national health insurance database. Clavien-Dindo classification is probably less precise as we don’t have information about surgical revision and may be overestimated.
Despite these clarifications, our method was retrospective and declarative, based on the hospital’s information about the complications encountered by each patient to obtain reimbursement for the care provided. Fair reporting is therefore encouraged in entering data into the database. Other medical specialties have already published studies showing that the data from the PMSI-MCO are reliable with an excellent predictive value [27–29], which offers a great overall picture of various complication rates.
A closer examination of high- and low-volume centers would be helpful to determine a center effect. However, adjusting for tumor complexity and patient comorbidities would be necessary, as low-volume centers operate on the most uncomplicated cases. Unfortunately, this type of detail is not yet available.
PMSI data provide valuable information on the complications associated with different surgical approaches, whether laparoscopic or open. For low-volume centers, these results provide an overview of the risks and benefits associated with each method, which can be better anticipated and managed. Clinicians can also evaluate treatment options based on their experience and the tools available in their facility, better inform patients, and improve shared decision-making in line with patient preferences.
Conclusions
Exploitation of the French national health insurance data showed a decrease in length of stay and postoperative laparoscopic partial and radical kidney surgery complications for urinary infection, acute renal failure, bowel occlusion, sepsis, and postoperative anemia.
Acknowledgements
We thank Mr. Bruno Sarfati from REAL CONSULTING DATA, 11-19 rue de la Vanne SOPARQ Batiment C, 92 120 Montrouge France, for collecting data from PMSI-MCO database.
Abbreviations
- ICD-10
International classification disease, 10th edition
- PMSI-MCO
Programme de médicalisation des systèmes d’information – Médecine chirurgie obstétrique.
- LRN
Laparoscopic radical nephrectomy
- OPN
Open partial nephrectomy
- LPN
Laparoscopic partial nephrectomy
- KDIGO
Kidney disease improving global outcomes
- RR
Relative risk
- eGFR
Estimated Glomerular fonction rate
Author contributions
Protocol/project development: P.E., G.P., C.M. Data collection: G.P. Data analysis: J.S. Manuscript writing: G.P., B.B., J.H., C.M. Critical revision of the manuscript: B.B., P.E., J.S., J.H.
Funding
None.
Data availability
Data were collected retrospectively from ATIH (Agence technique de l’information sur l’hospitalisation) with the aknowledgements of Mr Bruno Sarfati from REAL CONSULTING DATA https://www.atih.sante.fr/https://www.scansante.fr/.
Declarations
Ethics approval and consent to participate
This Study has been evaluated and approved by the Ethics Committee of Nancy CHRU Hospital, represented by Mr Yves Martinet, and this research has been carried out in accordance to current French and European ethical standards, as well as The Code of Ethics of the World Medical Association. Furthermore, the Ethics Committee of Nancy CHRU Hospital has waived the need for informed consent.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A. Global Cancer statistics 2020: GLOBOCAN estimates of incidence and Mortality Worldwide for 36 cancers in 185 countries. CA Cancer J Clin Mai. 2021;71(3):209–49. [DOI] [PubMed] [Google Scholar]
- 2.Robson CJ. Radical nephrectomy for renal cell carcinoma. J Urol janv. 1963;89:37–42. [DOI] [PubMed] [Google Scholar]
- 3.Ljungberg B, Albiges L, Abu-Ghanem Y, Bedke J, Capitanio U, Dabestani S. European Association of Urology Guidelines on Renal Cell Carcinoma: the 2022 Update. Eur Urol oct. 2022;82(4):399–410. [DOI] [PubMed] [Google Scholar]
- 4.French Comittee of Urologic Oncology (CCAFU), Peyronnet B, Seisen T, Oger E, Vaessen C, Grassano Y. Comparison of 1800 robotic and open partial nephrectomies for renal tumors. Ann Surg Oncol déc. 2016;23(13):4277–83. [DOI] [PubMed] [Google Scholar]
- 5.Gill IS, Kavoussi LR, Lane BR, Blute ML, Babineau D, Colombo JR. Comparison of 1,800 laparoscopic and open partial nephrectomies for single renal tumors. J Urol Juill. 2007;178(1):41–6. [DOI] [PubMed] [Google Scholar]
- 6.Xu H, Ding Q, Jiang H. wen. Fewer complications after laparoscopic nephrectomy as compared to the open procedure with the modified Clavien classification system - a retrospective analysis from Southern China. World J Surg Oncol. 31 juill 2014;12:242. [DOI] [PMC free article] [PubMed]
- 7.Lee H, Lee CU, Yoo JH, Sung HH, Jeong BC, Jeon SS, et al. Comparisons of oncological outcomes and perioperative complications between laparoscopic and open radical nephrectomies in patients with clinical T2 renal cell carcinoma (≥ 7 cm). PLoS ONE. 2018;13(1):e0191786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Permpongkosol S, Link RE, Su LM, Romero FR, Bagga HS, Pavlovich CP. Complications of 2,775 urological laparoscopic procedures: 1993 to 2005. J Urol févr. 2007;177(2):580–5. [DOI] [PubMed] [Google Scholar]
- 9.Clayman RV, Kavoussi LR, Soper NJ, Dierks SM, Meretyk S, Darcy MD. and al. Laparoscopic Nephrectomy: Initial Case Report. J Urol [Internet]. févr 2017 [cité 4 août 2019];197(2S). Disponible sur: http://www.jurology.com/doi/10.1016/j.juro.2016.10.074 [DOI] [PubMed]
- 10.Winfield HN, Donovan JF, Godet AS, Clayman RV. Laparoscopic partial nephrectomy: initial case report for Benign Disease. J Endourol déc. 1993;7(6):521–6. [DOI] [PubMed] [Google Scholar]
- 11.Notice. Kidney Int Suppl mars. 2012;2(1):1. [Google Scholar]
- 12.Vricella GJ, Finelli A, Alibhai SMH, Ponsky LE, Abouassaly R. The true risk of blood transfusion after nephrectomy for renal masses: a population-based study. BJU Int. 2013;111(8):1294–300. [DOI] [PubMed] [Google Scholar]
- 13.Stang A, Büchel C. Renal surgery for kidney cancer in Germany 2005–2006: length of stay, risk of postoperative complications and in-hospital death. BMC Urol 12 sept. 2014;14:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gratzke C, Seitz M, Bayrle F, Schlenker B, Bastian PJ, Haseke N. Quality of life and perioperative outcomes after retroperitoneoscopic radical nephrectomy (RN), open RN and nephron-sparing surgery in patients with renal cell carcinoma. BJU Int. 2009;104(4):470–5. [DOI] [PubMed] [Google Scholar]
- 15.Marszalek M, Meixl H, Polajnar M, Rauchenwald M, Jeschke K, Madersbacher S. Laparoscopic and open partial nephrectomy: a matched-pair comparison of 200 patients. Eur Urol Mai. 2009;55(5):1171–8. [DOI] [PubMed] [Google Scholar]
- 16.Lane BR, Fergany AF, Weight CJ, Campbell SC. Renal functional outcomes after partial nephrectomy with extended ischemic intervals are Better Than after Radical Nephrectomy. J Urol oct. 2010;184(4):1286–90. [DOI] [PubMed] [Google Scholar]
- 17.Tomaszewski JJ, Uzzo RG, Kutikov A, Hrebinko K, Mehrazin R, Corcoran A. Assessing the burden of complications following surgery for clinically localized kidney cancer by age and co-morbidity status. Urol Avr. 2014;83(4):843–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van der Voort M, Heijnsdijk EAM, Gouma DJ. Bowel injury as a complication of laparoscopy. Br J Surg 1 oct. 2004;91(10):1253–8. [DOI] [PubMed] [Google Scholar]
- 19.An JY, Ball MW, Gorin MA, Hong JJ, Johnson MH, Pavlovich CP. Partial vs radical nephrectomy for T1-T2 renal masses in the Elderly: comparison of complications, renal function, and oncologic outcomes. Urol févr. 2017;100:151–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pignot G, Méjean A, Bernhard JC, Bigot P, Timsit MO, Ferriere JM. The use of partial nephrectomy: results from a contemporary national prospective multicenter study. World J Urol janv. 2015;33(1):33–40. [DOI] [PubMed] [Google Scholar]
- 21.White VM, Marco DJT, Bolton D, Papa N, Neale RE, Coory M. Age at diagnosis and the surgical management of small renal carcinomas: findings from a cross-sectional population-based study. BJU Int. 2018;122(S5):50–61. [DOI] [PubMed] [Google Scholar]
- 22.Porpiglia F. and al. Partial nephrectomy in clinical T1b renal tumors: Multicenter comparative study of Open, Laparoscopic and Robot-assisted Approach (the RECORd Project). Urol 2016 Mar:89:45–51. [DOI] [PubMed]
- 23.Jordan BJ, Matulewicz RS, Trihn B, Kundu S. Venous thromboembolism after nephrectomy: incidence, timing and associated risk factors from a national multi-institutional database. World J Urol Nov. 2017;35(11):1713–9. [DOI] [PubMed] [Google Scholar]
- 24.Guglielmetti GB, Dos Anjos GC, Sawczyn G, Rodrigues G, Cardili L, Cordeiro MD, et al. A prospective, randomized trial comparing the outcomes of Open vs laparoscopic partial nephrectomy. J Urol août. 2022;208(2):259–67. [DOI] [PubMed] [Google Scholar]
- 25.Erlich T, Abu-Ghanem Y, Ramon J, Mor Y, Rosenzweig B, Dotan Z. Postoperative urinary leakage following partial nephrectomy for renal Mass: risk factors and a proposed algorithm for the diagnosis and management. Scand J Surg juin. 2017;106(2):139–44. [DOI] [PubMed] [Google Scholar]
- 26.Bic A, Mazeaud C, Salleron J, Bannay A, Balkau B, Larose C, et al. Complications after partial nephrectomy: robotics overcomes open surgery and laparoscopy: the PMSI French national database. BMC Urol 15 sept. 2023;23(1):146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Prat M, Derumeaux H, Sailler L, Lapeyre-Mestre M, Moulis G. Positive predictive values of peripheral arterial and venous thrombosis codes in French hospital database. Fundam Clin Pharmacol févr. 2018;32(1):108–13. [DOI] [PubMed] [Google Scholar]
- 28.Sahli L, Lapeyre-Mestre M, Derumeaux H, Moulis G. Positive predictive values of selected hospital discharge diagnoses to identify infections responsible for hospitalization in the French national hospital database: PPV of hospitalization for infection codes in France. Pharmacoepidemiol Drug Saf Juill. 2016;25(7):785–9. [DOI] [PubMed] [Google Scholar]
- 29.Champeaux C, Weller J, Katsahian S. Epidemiology of meningiomas. A nationwide study of surgically treated tumours on French medico-administrative data. Cancer Epidemiol févr. 2019;58:63–70. [DOI] [PubMed] [Google Scholar]
- 30.Jain S, Nyirenda T, Yates J, Munver R. Incidence of renal artery pseudoaneurysm following Open and minimally invasive partial nephrectomy: a systematic review and comparative analysis. J Urol Mai. 2013;189(5):1643–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data were collected retrospectively from ATIH (Agence technique de l’information sur l’hospitalisation) with the aknowledgements of Mr Bruno Sarfati from REAL CONSULTING DATA https://www.atih.sante.fr/https://www.scansante.fr/.