Abstract
Chronic kidney disease (CKD) poses a significant global health challenge, projected to become one of the leading causes of death by 2040. Current treatments primarily manage complications and slow progression, highlighting the urgent need for personalized therapies targeting the disease-causing genes. Our increased understanding of the underlying genomic changes that lead to kidney diseases coupled with recent successful gene therapies targeting specific kidney cells have turned gene therapy and genome editing into a promising therapeutic approach for treating kidney disease. This review paper reflects on different delivery routes and systems that can be exploited to target specific kidney cells and the ways that gene therapy can be used to improve kidney health.
Keywords: gene therapy, kidney disease, targeted gene delivery, viral vectors, nanodelivery systems
Graphical abstract

Welsh and colleagues report that progress in kidney gene therapy has been slow compared to other organs, but recent advancements using AAV and nanoparticles show promise. Although clinical application is pending, they suggest that refining delivery methods could soon enable effective treatments for kidney diseases, with AAV targeting podocytes expected to lead the way in glomerular disease.
Introduction
Incidence, prevalence, morbidity, mortality, and economic burden of genetic kidney diseases
Chronic kidney disease (CKD) affects a growing number of people worldwide and it is estimated that it will become the fifth highest cause of death by 2040.1 A wide range of disease-causing genetic variants are associated with this condition.2 These variants can be identified in 10% of adults and 20% of children with CKD.3 At current estimates, CKD costs the National Health Service (NHS) £1.79 billion annually, and this excludes the expenses associated with the treatments for end-stage kidney disease (ESKD), including dialysis and transplantation. It is projected that the total cost will significantly increase and could rise to £7.8 billion due to the increasing prevalence and associated costs of CKD stages 3–5.4 ESKD is reached at stage V CKD, with an estimated glomerular filtration rate below 15 mL per minute per 1.73 m2 body surface area.5 At present, renal replacement therapies, including long-term dialysis and transplantation, are used for treating ESKD.6
Limitations of current therapies
Current treatments seldom target the underlying disease.7 Interventions in kidney disease currently aim to manage the complications of CKD, such as high blood pressure, anemia, electrolyte imbalance, and mineral bone disease, or they aim to slow disease progression by reducing albuminuria and addressing cardiovascular risk factors such as hyperlipidemia.8 There are currently few targeted therapies for monogenic kidney disease where the pathogenesis of disease is within the kidney. Therefore, there is an unmet need for novel personalized therapies that target the underlying causes of genetic kidney disease.
The solution
Introduction to gene therapy
In gene therapy, therapeutic or preventive effects in a wide range of diseases are achieved through gene augmentation, gene editing, or the modulation of gene expression (e.g., gene silencing).9 Gene augmentation is defined as the introduction of a transgene, which is mostly applicable for treating monogenic disorders, cancer molecular chemotherapy and immunopotentiation, and introducing tolerance against transplant rejection without immunosuppressive drugs.10 In gene augmentation, gene improvements are obtained by introducing a wild-type allele into cells that have the mutant allele, which can have therapeutic effects.11 For example, autosomal dominant polycystic kidney disease (ADPKD) is a common renal monogenic disorder caused by mutations in the PKD1 or PKD2 genes, which encode the polycystin-1 and -2 proteins (PC1 and PC2), respectively. Studies have shown that low levels of polycystin protein in haploinsufficient ADPKD models are associated with vascular changes and more susceptibility toward renal damage through renal ischemia/reperfusion injury.12,13 Additionally, Piontek et al. have shown that inactivation of PKD1 in mouse models is associated with the development of cystic kidneys.14 Therefore, one possible solution for this issue is to provide cells with a wild-type allele of PKD1 (Figure 1A) to increase the protein level. The size of the PKD1 gene presents a significant limitation for gene augmentation studies. PKD1, responsible for encoding polycystin-1, a protein implicated in ADPKD, spans over 50 kb and includes 46 exons. This large size poses a challenge for current gene therapy vectors, such as adeno-associated viruses (AAVs), with a packaging capacity of around 4.7 kb. Consequently, delivering the entire PKD1 gene using AAV vectors is not feasible. Alternative strategies, such as using dual or multiple vector systems, must be developed and optimized, but these approaches can complicate the delivery process and reduce efficiency. Additionally, the large size increases the complexity of producing and purifying the therapeutic gene, further complicating clinical applications. Therefore, the considerable size of the PKD1 gene is a significant hurdle that must be addressed to advance gene augmentation therapies for ADPKD.15 Meanwhile, gene augmentation does not work for gain-of-function mutations and will be difficult to implement in polygenic and polyallelic diseases since the current viral vectors have a limited genetic payload. Potential strategies to overcome these challenges are targeted gene repair (via precise gene edits or small-fragment homologous replacement or exon skipping) using either naked DNA or RNA delivered via viral or non-viral vectors.10 The other form of gene therapy is gene expression modulation, which can be used for targeting relevant signaling pathways in non-monogenic or monogenic diseases.10,16 In this review, we focus on gene augmentation and modulation of gene expression.
Figure 1.
Gene augmentation for ADPKD treatment and delivery methods
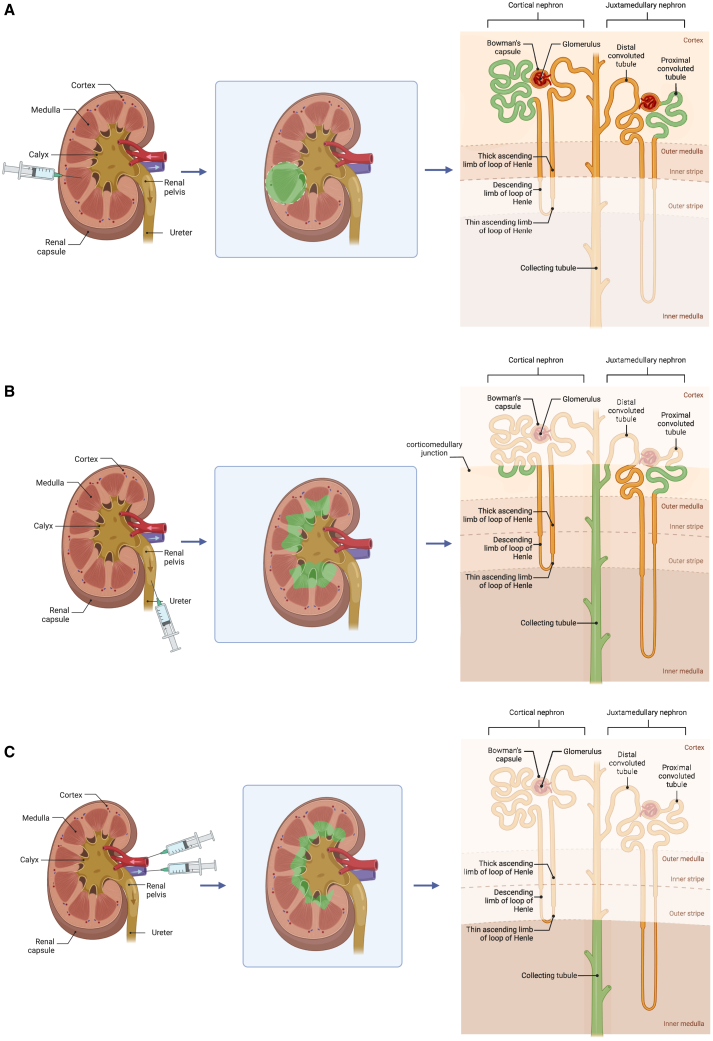
(A) The potential of gene augmentation in treating ADPKD caused by mutation in PKD1 allele. In ADPKD, PKD1 haploinsufficiency (a condition when there is only one functional copy of a gene instead of the usual two in diploid organisms) leads to a reduced amount or altered function of the polycystin-1 protein. Low polycystin-1 levels disrupt normal cellular processes and lead to the formation of cysts in the kidneys. Delivering a functional copy of the PKD1 gene into the cells of the affected individual can increase levels of polycystin-1 protein, compensating for the haploinsufficiency. (B) Different routes for kidney gene delivery. These routes include (A) subcapsular injection, (B) direct injection into the renal pelvis, (C) infusion into the renal artery, (D) retrograde infusion into the renal vein, (E) retrograde infusion into the ureter, and (F) local injection.
In recent years, there has been significant progress in using gene therapy for treating various diseases, including neuromuscular disorders, cancer, and blindness. For example, AAV has been used to treat an inherited retinal dystrophy (Leber congenital amaurosis) caused by mutations in the RPE65 gene. The direct injection of the functional version of the gene loaded in AAV vectors into the eye had promising results, with patients restoring their vision and maintaining the improved vision for 5 years.17 In cancer immunotherapy reprogramming T cells (chimeric antigen receptor [CAR] T cells) to kill lymphoblasts has been effective in treating refractory acute lymphoblastic leukemia, which results from excessive production of lymphoblasts due to mutations in lymphoid progenitors. This work has resulted in an 80% remission rate.17 Currently, gene therapy does not only aim to correct defect genes, but it might be used to regulate gene expression. It has been successful in saving the lives of children born with aromatic L-amino acid decarboxylase (AADC) deficiency, with the delivery of AAVs encoding human aromatic L-amino acid decarboxylase to the bilateral substantia nigra and ventral tegmental area, improving the motor function through increasing dopamine and serotonin levels in cerebrospinal fluid.18
Gene therapy is divided into two groups, germline gene therapy and somatic cell gene therapy, with the former being hereditary in contrast to the latter.11 Germline genomic modifications do present significant safety and ethical concerns, and there are many unknowns that need to be addressed.19 While germline gene therapy remains controversial and is not widely supported, somatic gene therapy is the currently accepted and practiced approach. Gene modifications can be performed either ex vivo, by correcting the cells removed from patients’ body and re-infusing them back; in vivo, by systemic administration of the therapeutic vectors into the blood circulation or the cerebrospinal fluid; or in situ, by the administration of the gene product to a specific site.
Generally, there are six major steps in gene augmentation therapy:
-
(1)
Identification of the mutant allele
-
(2)
Cloning of the identical healthy gene, known as the transgene
-
(3)
Loading the appropriate vector with the transgene
-
(4)
Vector reaching the target cells
-
(5)
Delivery of the genetic cargo into the nucleus of the cell
-
(6)
Expression of the healthy gene and correction of the phenotype20
Recent improvements in genotype-phenotype correlation
Over the past few years, there have been significant improvements in genotype-phenotype correlation; large-scale genomic studies have helped identify numerous genetic variants associated with specific phenotypes,21 and functional genomics has helped in the understanding of the functional implications of these genetic variations.22 These, coupled with integration of the data obtained from multi-Omics studies, have substantially helped to bridge the genotype-phenotype gap.23 For example, polygenic risk scores (PRSs), a statistical tool that combines information from multiple genetic variants, can help estimate an individual’s genetic predisposition to a particular disease and be informative at various stages of the disease trajectory.24
Increased understanding of the genetics of kidney diseases and the potential for kidney gene therapy
Table 1 lists the genes involved in the development of various kidney diseases.25,26,27,28 Renal disorders can be either monogenic or polygenic. The tightness of the genotype-phenotype correlation determines the predictive value and the penetrance of the mutations. Monogenic recessive diseases have the tightest correlation, followed by monogenic dominant and polygenic diseases, respectively.28 Recently, it has been shown that gene therapy in the kidney is possible. Ding et al. successfully demonstrated that gene therapy targeted specifically to the kidney podocyte can be effective in treating kidney disease.29 NPHS2, which encodes podocin, is the most frequently mutated gene in childhood-onset steroid-resistant nephrotic syndrome. The approach involved the administration of a functional copy of NPHS2, under the control of a podocyte-specific promotor, packaged into an AAV vector delivered via tail vein injection. Expression of this transgene improved the kidney phenotype of Nphs2 conditional-knockout mice and mice with a patient-relevant mutation.29 These results open the door to treat different genetic causes of kidney diseases. While promising, there are still challenges that need to be addressed.30 For instance, even though AAVs have relatively low immunogenicity31 and have been successful in many clinical trials,32 their small genetic cargo (4.6 kb) limits the number of diseases that can be targeted using this delivery system given that some of the mutated genes in kidney diseases are considerable larger than this. Meanwhile, although AAVs are relatively low in immunogenicity compared to the other viral vectors, lethal immunotoxicity has been observed in some cases, particularly at high doses, and, therefore, they are not completely free from immune responses.33 Factors affecting AAV immunogenicity include pre-existing immunity, innate and adaptive immune responses, capsid proteins, vector dosage, and the route of administration. Strategies to mitigate these responses involve using less immunogenic serotypes, engineering capsids to evade detection, employing immunosuppressive treatments, and developing novel delivery methods. Despite these immunogenic concerns, AAV remains a valuable tool in gene therapy.34
Table 1.
A comprehensive list of genes playing major roles in the development of various monogenic kidney diseases and syndromes
| Disease | Type | Inheritance | Gene |
|---|---|---|---|
| Adenine phosphoribosyl transferase deficiency | – | AR | adenine phosphoribosyl transferase 5 |
| Alport syndrome | – | AR | collagen type IV alpha 4 chain |
| collagen type IV alpha 3 chain | |||
| XD | collagen type IV alpha 5 chain | ||
| with leiomyomatosis | XD | collagen type IV alpha 6 chain | |
| Bardet-Biedl syndrome types 1–12 | – | AR | Bardet-Biedl syndrome 1, Bardet-Biedl syndrome 12 |
| Bartter syndrome types 1–4 | – | AR | solute carrier family 12 member 1, chloride voltage-gated channel Kb, potassium inwardly rectifying channel subfamily J member 1, barttin CLCNK type accessory subunit beta |
| Benign familial hematuria | – | AD | collagen type IV alpha 4 chain |
| Branchio-oto-renal syndrome | – | AD | EYA transcriptional coactivator and phosphatase 1, myogenin, SIX homeobox 1, SIX homeobox 5 |
| Congenital abnormalities of the kidney and urinary tract | – | AD | Forkhead box C1 |
| Cystinosis | – | AR | cystinosin, lysosomal cystine transporter |
| Cystinuria | type 1 | AR | solute carrier family 3 member 1, solute carrier family 3 member 1 |
| none type 1 | solute carrier family 7 member 9 | ||
| Dent disease | – | XR | chloride voltage-gated channel 5 |
| Denys-Drash syndrome, Frasier syndrome | – | AD | WT1 transcription factor |
| Diabetes insipidus, nephrogenic | – | XD | arginine vasopressin receptor 2, V2 antidiuretic hormone receptor type 2 |
| AR | aquaporin 2 | ||
| Distal renal tubular acidosis | distal renal tubular acidosis | AR | ATPase H+ transporting V1 subunit B1, ATPase H+ transporting V0 subunit a4 |
| type I | AD | solute carrier family 4 member 1 | |
| Distal renal tubular acidosis | – | AD | solute carrier family 4 member 1, the replication and transcription activator |
| Fabry disease | – | XR | galactosidase alpha |
| Familial hypocalciuric hypercalcemia | – | AD | collagen type IV alpha 3 chain |
| Fraser syndrome | – | AR | Fraser extracellular matrix complex subunit 1, Fraser extracellular matrix complex subunit 2 |
| Gitelman syndrome | – | AR | solute carrier family 12 member 3 |
| Glomerulopathy with fibronection deposits | – | AD | fibronectin 1 |
| Gordon syndrome (PHA type 2) | – | AD | WNK lysine deficient protein kinase 4 and 1 |
| HDR syndrome | – | AD | GATA binding protein 3 |
| Hemolytic uremic syndrome, atypical | – | AR | complement factor H 1 and 3 major capsid protein, ADAM metallopeptidase with thrombospondin type 1 motif 13 |
| Hypomagnesemia | – | AR | claudin 16 |
| AD | FXYD domain containing ion transport regulator 2, FXYD domain containing ion transport regulator 2 | ||
| Hypophosphatemia rickets | – | XR | CLCN5 |
| Hypophosphatemic rickets | – | XD-AR | phosphate regulating endopeptidase X-linked, solute carrier family 34 member 3, fibroblast growth factor 23, dentin matrix acidic phosphoprotein 1 |
| Juvenile nephronophthisis | – | AR | NPHP1 |
| Kallman syndrome | – | AD | Kallmann syndrome 1 |
| Liddle syndrome | – | AD | sodium channel epithelial 1 subunit beta and gamma |
| Lowe syndrome | – | XR | oxidized low-density lipoprotein receptor 1, inositol polyphosphate-5-phosphatase J |
| Lysinuric protein intolerance | – | AD | NHERF family PDZ scaffold protein 1, NHERF family PDZ scaffold protein 1 |
| MKS | – | AR | MKS transition zone complex subunit 1, transmembrane protein 67 |
| Medullary cystic kidney disease | – | AD | UMOD |
| Multicystic renal dysplasia | – | AD | cell division cycle 5 like, upstream transcription factor 2, c-fos interacting |
| Nail-Patella syndrome | – | AD | LIM homeobox transcription factor 1 beta |
| Nephrolithiasis | – | XR | chloride voltage-gated channel 5 |
| Nephronophthisis types 1–9 | – | AR | nephrocystin 1-NIMA related kinase 8 |
| Papillary renal cell carcinoma | – | AD | MET proto-oncogene, receptor tyrosine kinase |
| Pierson syndrome | – | AR | laminin subunit beta 2 |
| PKD | type 1 | AD | polycystic 1, transient receptor potential channel interacting |
| type 2 | AD | polycystic 2, transient receptor potential cation channel | |
| – | AR | PKHD1 ciliary IPT domain containing fibrocystin/polyductin, polycystic kidney disease 3 | |
| Primary hyperoxaluria | type 1 | AR | alanine-glyoxylate aminotransferase |
| type 2 | AD | glyoxylate and hydroxypyruvate reductase | |
| Proximal renal tubular acidosis | – | AR | carbonic anhydrase 2, solute carrier family 4 member 4 |
| Pseudohypoaldosteronism type 1 | – | AR | ligand dependent nuclear receptor corepressor like |
| – | AD | sodium channel epithelial 1 subunit alpha, beta, and gamma | |
| Renal agenesis | – | AD | ret proto-oncogene, uroplakin 3A |
| Renal coloboma syndrome | – | AD | paired box 2 |
| Renal cysts and diabetes syndrome, GCKD | – | AD | TCF2/HNF1B (HNF1 homeobox B) |
| Renal glucosuria | – | AR | solute carrier family 5 member 2, solute carrier family 5 member 2, solute carrier family 5 member 1 |
| Renal hypodysplasia | – | AD | bone morphogenetic protein 4, SIX homeobox 2 |
| Schimke immuno-osseous dystrophy | – | AR | SWI/SNF related, matrix associated, actin dependent regulator of chromatin, subfamily a like 1 |
| SeSAME syndrome | – | AR | potassium inwardly rectifying channel subfamily J member 1 |
| Split-hand/split-foot malformation | – | AD | bone morphogenetic protein 7; distal-less homeobox 5; distal-less homeobox 5; tumor protein p63 |
| SRNS | congenital | AR | NPHS1 adhesion molecule, nephrin |
| type 2 | AR | NPHS2 stomatin family member, podocin | |
| type 3 | AR | phospholipase C epsilon 1 | |
| type 4 | AR or AD | CD2-associated protein | |
| adult onset | – | – | |
| with mitochondrial disorders | AR | coenzyme Q2, polyprenyltransferase, decaprenyl diphosphate synthase subunit 2, mitochondrially encoded tRNA leucine 1 | |
| with lysosomal disorders | AR | scavenger receptor class B member 2 | |
| Townes-Brocks syndrome | – | AD | Spalt-like transcription factor 1 |
| Tuberous sclerosis types 1 and 2 | – | AD | TSC complex subunit 1, TSC complex subunit 2 |
| Vesicoureteral reflux grade2 | – | AD | roundabout guidance receptor 2, slit guidance ligand 2 |
| von Hippel-Lindau disease | – | AD | VHL tumor suppressor |
| Wilms-tumor-aniridia syndrome | – | AD | WT1 transcription factor |
| Xanthinuria | – | AR | xanthine dehydrogenase |
MKS, Meckel-Gruber syndrome.
Gene therapy for kidney diseases
The unique structure of the mammalian kidney is commensurate with its filtration and reabsorption roles. Filtration happens in the glomerulus of each nephron, where podocytes form slit diaphragms with diameters of only 10 nm.35 The large number of highly specialized cell types in the kidney36 makes cell-specific delivery more challenging. One of the determining factors in successful gene therapy is choosing the right gene delivery system.37 Apart from the naked delivery of the genetic materials (summarized in Table 2), either viral or non-viral vectors can be used as carriers to facilitate gene delivery; the applications, limitations, and advantages of each of these vectors have been discussed previously.20,38,39 The type of delivery vehicle, whether it be viral, non-viral,40 or viral-like particles,41 contributes significantly to its targeting properties. Selecting the right gene delivery route (Figure 1B) also plays an important role in targeting, meaning different compartments of the kidney, namely glomerular, tubular, vascular, and interstitial cells, are transfected, when they are administered through the renal artery, retrograde infusion via the renal vein or the ureter, or direct injection into the parenchyma or pelvis (Table 3).37,42 For instance, Gusella et al. have shown that delivering the same lentiviral vector by various routes of administration leads to different transduction patterns in the kidney (Figure 2).43 Therefore, the type of kidney disorder plays an important role in choosing the suitable vector and administration route (Figure 3A). In rodents, systemic delivery through tail vein injection, even though less invasive, leads to the accumulation of the genetic material mostly in the liver, which is clinically not ideal. Other routes (Figure 1B) are more invasive but result in higher doses of particles delivered to the kidney. For example, in an attempt to circumvent toxicity in the liver, Woodard et al. have suggested direct injection into renal pelvis; however, using this route, the transgene expression is not cell-type specific and is observed in patches across the kidney.42 Alternate delivery routes, such as lymphatics and the use of hydrodynamic pressure to improve penetration, have been suggested but not yet explored adequately.35
Table 2.
Different nucleic acid structures used for naked gene therapy
| Type of nucleic acid | Detail | Previous success in kidney gene therapy |
|---|---|---|
| miRs and antagomirs |
|
|
| LNAs |
|
|
| ASOs |
|
|
| saRNA |
|
|
| shRNAs |
|
|
| siRNA |
|
|
| Aptamer |
|
|
| circular RNAs |
|
|
AGE, advanced glycation end products; RAGE, advanced glycation end products receptor; LNA, locked nucleic acid.
Table 3.
Different delivery routes and their main target sites
| Delivery route | Target cells |
|---|---|
| Intravenous injection | Kupffer cells in the liver (98%) |
| Intravenous injection with liver bypass | lung, intestinal wall, and renal glomeruli |
| Renal artery infusion | kidney: proximal tubule cells, endothelial and epithelial cells, and cortical interstitium (endothelial cell transfection can be improved by modifying the AV fiber protein and making it targeted for the αv integrins of the vascular endothelium75) |
| Retrograde infusion into the pyelic cavity of the kidney using catheter | tubular cells in the papilla and medulla |
| Perfusing kidney ex vivo for up to 12 h | kidney: 85% of the glomeruli |
| Perfusing kidney in vivo for up to 2 h | kidney: 85% of the glomeruli |
| Slow renal artery infusion (up to 15 min) | liver (93.3% ± 11.5% of hepatocytes), spleen, kidney (51.7% ± 20.2% glomeruli) |
| Intra-renal-ureteral route | distal tubular pyelic epithelial cells |
| Intraparenchymal injection | kidney cortex and medulla (13.30% ± 5.30%), liver (8.66% ± 4.99%), spleen (4.73% ± 1.82%) |
| Subcapsular injection | entire kidney parenchyma (mainly to glomeruli) |
Figure 2.
The main delivery site lentiviral particles in mouse kidney can differ based on the chosen delivery route
With regard to the local intraparenchymal injection (A), the distribution of the components is limited to the area around the injection track, and within this area the transgene expression is predominantly detected in the proximal convoluted tubules located in the cortex and outer medulla. Compared to the renal vein and artery injection (C), administration through renal ureter (B) encompasses a larger area, with the former being confined to the inner medulla and the latter expanding to the outer medulla and corticomedullary junction. Green areas show the main target of the delivery route in the nephron structure.
Figure 3.
Optimizing kidney gene therapy based on disorder and delivery method
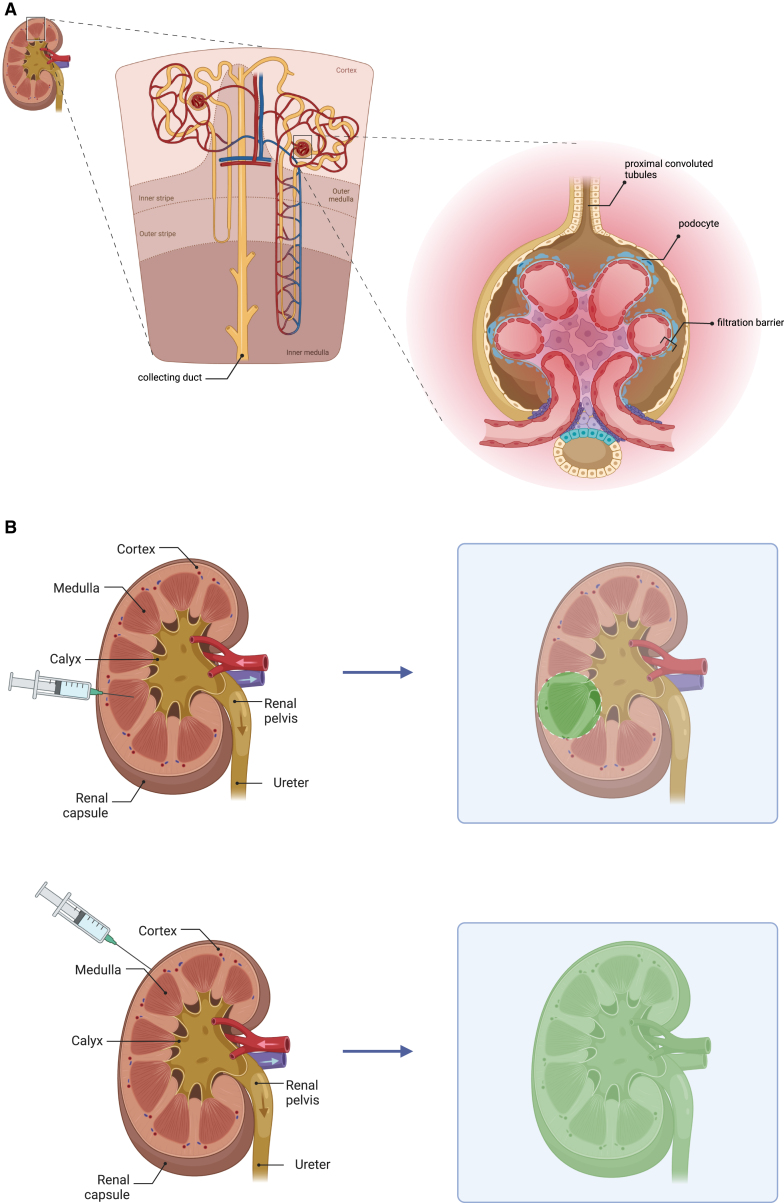
(A) The type of kidney disorder plays an important role in choosing the suitable vector and administration route; for example, if the tubule abnormality is the cause of disease development, given that collecting ducts come together and make the renal pelvis, injection can be made through the renal pelvis to circumvent the filtration that happens in the glomerular filtration barrier and that reduces the delivery efficiency. (B) Subcapsular injection is a better alternative for having the injected material diffused throughout the kidney parenchyma; with regard to the subcapsular (above) and local (below) delivery of the genes, while the latter can lead to the accumulation of the components in the area near the injection site, which can cause high drug deposition exposing the organ to toxic concentrations. The former is associated with the sustained release of the delivered agents preventing tissue injury and improving the therapeutic efficiency.
It is worth mentioning that, by introducing various modifications in the delivery routes shown in Figure 1B, the expression pattern can change; For example, the functional improvement in kidney after intraparenchymal injection of COL-PGE2 matrix (prostaglandin E2 that is covalently crosslinked to collagen matrix) is limited to the area of injection as the injected material does not diffuse throughout the kidney. To improve this, Chen et al. have shown that subcapsular injection can be a better alternative route, delivering components to the whole kidney parenchyma and restoring the function of the kidney at a higher level (Figure 3B).76 Another example is that, by decreasing the infusion rate of Ad5 into the renal artery of rats from 1–2 to 0.1 mL/min, the main site of delivery changes from proximal tubules to glomeruli (Figure 4).77,78
Figure 4.
Impact of infusion rate into the renal artery on delivery target: Faster rates direct to proximal tubules, while slower rates favor glomerular delivery
The rate of infusion into the renal artery of rats plays an important role in determining the main delivery site; although infusion with a flow rate of 1–2 min/mL targets the proximal tubules with no transfection in the glomeruli, a slow infusion leads to the most components delivered to the glomeruli.
How to target the therapy
Naked systems and their targeting limitations
The nucleic acids used for gene therapy can have different forms, such as microRNA (miR) mimics and antagomirs, small activating RNA (saRNA), short hairpin RNA (shRNA), small interfering RNA (siRNA), antisense oligonucleotide (ASO), aptamers, and circular RNAs. Nucleic acid-based gene therapy has had promising results in animal models and has shown great potential to be used in the clinic. For example, given that the upregulation of K-Ras is one of the key drivers of renal fibrosis, subcutaneous administration of K-Ras ASOs in a mouse model of chronic folic acid nephropathy was associated with 50% reduction in the interstitial fibrosis and prevented renal dysfunction.79 Systemic delivery of splice-blocking ASO’s in a mouse model of CEP290-associated Joubert syndrome led to mutated exon skipping and the production of a nearly full-length protein. As the intronic mutation disrupts the CEP290 transcription, skipping this exon restored the normal transcription and ameliorated the kidney pathology.80
As seen in Table 2, naked delivery of these structures has resulted in promising results; however, there are issues with the direct administration of genetic material (DNA or RNA) without the use of delivery vehicles or vectors, including low cellular uptake, nucleic acid degradation, lack of targeting, immune response activation, and rapid clearance.81,82 Given the success of gene therapy and the fact that 20%–30% of CKD is caused by monogenic variants, there is a need to fill the gap and overcome this issue. One way around this issue is kidney-specific targeting by conjugating the drugs with small peptides highly specific to different kidney cells, such as (KKEEE)3K for proximal tubule cells.83 A conjugated siRNA therapy, lumasiran, targeted to the liver, has been successfully used in patients with primary hyperoxaluria type I. Lumasiran is conjugated to a targeting ligand, N-acetylgalactosamine (GalNac), which facilitates hepatic uptake of the therapy.84 Apart from conjugating drugs with targeting molecules and using viral and non-viral vectors, vector cells and nanocarrier provide another opportunity for a targeted kidney-specific gene delivery; this is discussed further later in this paper.
Vector design and serotypes to target different tissues
Researchers have focused on developing different platforms for targeted gene delivery to kidney cells. The different types of platforms designed for delivering genetic materials can be categorized into three main groups, namely viral vectors, non-viral vectors, and cell vectors.
Viral vectors
The most popular viral vectors used in kidney gene therapy are adenovirus (AV), lentivirus, and AAV. AV, a non-enveloped virus with nucleocapsid and linear, double-stranded DNA, has many desirable properties as a gene delivery system, including its well-defined biology, high transfection efficiency, genetic stability, and simple large-scale production.85 Between the different AV serotypes, AV5 has been widely used for kidney transfection. When using AV particles, based on the chosen delivery route, different cell types will be targeted, as reviewed previously (Table 2).
AV was first demonstrated as a potential successful delivery tool for gene therapy of kidney diseases in 1994, when two different routes of administration, renal artery perfusion and ureteral retrograde infusion, were tried for the introduction of β-galactosidase into quiescent renal cells of adult Wistar rates. While renal artery perfusion targeted the proximal tubular cells more efficiently, the ureteral retrograde infusion mostly transduced medullary and papillary epithelial cells.78 Aortic injection of adenoviral-microsphere complexes in Sprague-Dawley rats was also shown as an efficient route for glomerulonephritis gene therapy, transducing up to 16% of glomeruli with sustained trans-protein activity lasting for about 21 days.86 However, although less invasive, this platform has several drawbacks such as the transient activity of the transgene, extra-renal transduction, and the possibility of glomerular ischemia resulting from insoluble microspheres. These can be overcome by using biodegradable microparticles, using less immunogenic viral vectors, and selective injection into the renal artery, respectively.87
Gene transfection of renal glomeruli is challenging as systemic administration of AV particles is followed by rapid clearance from the blood circulation by the liver, and this hinders high transfection efficiency in glomerular cells. In 2002, Xuehai et al. showed that an efficient glomerular transfection needs not only sufficient time of exposure but also an adequate number of viral particles and, by considering these two parameters, high transfection levels in the glomerulus can be obtained. To achieve this, they developed two techniques, named portal clamping and prolonged renal infusion, to solve this issue. The portal clamping technique revealed that, by clamping the portal vein and hepatic artery and bypassing liver circulation, circulating viral vectors can be maintained at a high level, which results in increased exposure time for glomerular cells. In the other technique, the rat aorta and above the renal artery and superior mesenteric artery were clamped off and then the viral solution was slowly injected through the superior mesenteric artery.88
Adenoviral vectors have also been examined as a tool for aquaporin gene replacement in aquaporin 1 knockout mice, which causes polyuria and a urinary concentrating defect. To do so, adenoviral vectors encoding aquaporin 1 were intravenously infused into aquaporin 1 null mice, resulting in the transfection of many proximal tubules and micro vessels. In contrast to untreated mice, the treated group had improved concentrating ability and were able to lose less weight when subjected to water deprivation.89 This therapeutic approach is improved by making the adenoviral vectors targeted. The integration of a high-affinity peptide (that binds to the αv-integrin, which is generally expressed in the vascular endothelium) into the viral fiber protein resulted in not only enhanced transgene expression in the vascular endothelial cells in vitro but also a novel distribution of the transgene to the renal cortex and the subcapsular region in vivo.90 However, there are several challenges and concerns associated with gene therapy using AV vectors; apart from their immunogenicity, which can reduce the effectiveness of the treatment and even cause severe adverse reactions in some patients, AV vectors can provoke inflammatory responses in the target tissues that cause tissue damage or unwanted side effects. These vectors can also potentially recombine with wild-type adenoviruses within the packaging cell lines or in vivo and make the vector replication competent, imposing a risk to the patient’s health.91 The transient nature of gene expression with AV vectors (AV vectors do not integrate into the host genome and remain episomal, which will be lost as cells divide, leading to a reduction in the expression of the therapeutic gene) can be advantageous in terms of low risk for insertional mutagenesis and minimizing long-term risks, but this means that they are less suitable for treating chronic or long-term diseases.92
Lentiviruses belong to the retrovirus family, which are enveloped, spherical, and single-stranded RNA viruses. Lentiviral-based kidney gene therapy has resulted in promising results; one in vitro study showed that mesangial cell transduction with lentiviral vectors carrying anti-collagen type I shRNA could stably and continuously inhibit Col1 expression at both the mRNA and protein levels, which is desirable given the close relation between increased collagen type I and renal fibrosis. Also, Col I shRNA arrested the cells at two different points of the cell cycle: (1) S phase, which proved its suppressive effects on mesangial cell proliferation and hyperplasia; and (2) G2/M phase with an increase in apoptosis rate, representing the mild cytotoxicity of lentivirus. The injection of this lentiviral construct into the lower pole of the left kidney at several sites has led to transgene expression around the glomerulus of renal cortex at injection sites but not in the liver.93 Before using lentiviruses on a clinical scale, there are some points that need to be taken into consideration. One of the key challenges with lentivirus-based gene therapy is the need to scale up production processes while ensuring the environmental stability of lentiviruses. Also, enhancing productivity and purification techniques is crucial to meet the growing demand. Improving the functionality of lentiviral vectors for difficult-to-transduce target cells, such as exploring pseudotyping (modifying the viral envelope to enhance transduction) strategies, and rigorous product characterization are important to explore. Additionally, the establishment of a reference standard, advanced analytics, and an emphasis on industrialization with cost-effectiveness and regulatory compliance are vital to navigate the complexities of this evolving field.94,95 More importantly, there are worrying risks associated with the random integration of lentiviruses into the genome, which is associated with high chances of oncogenesis; one clinical trial on retrovirus-based gene therapy of patients with X-linked severe combined immunodeficiency showed that the high therapeutic potential of this treatment (nine patients out of 10) is hampered by high risk of developing T cell leukemia in four out of nine patients due to insertional mutagenesis.96 Apart from activating oncogenes and deactivating tumor suppressor genes, depending on where the virus integrates, it can also lead to non-malignant clonal expansion.97 Recent studies have focused on tackling this issue by making the viral integration more targeted toward a genomic safe harbor such as ribosomal DNA (rDNA; includes all the 400–600 copies of the ribosomal RNA genes within the cells), which is located far from the oncogenic protein-encoding genes. This can be achieved using a modified lentivirus that carries both the transgene as well as an integration-targeted enzyme, leading to over 250 times more frequent integration into rRNA.98
The leading platform for gene delivery is AAV, which is a small non-enveloped virus with a capsid that is 22 nm in diameter. As discussed above, AAV gene therapy has recently been used successfully to target the kidney podocyte.29 One of the main advantages of using AAVs over other viruses is the low risk of insertional mutagenesis as they do not integrate into the genome. AAV vectors have different serotypes which can be either naturally occurring (AAV 1–13) or artificially engineered. The AAV’s genome is composed of rep and cap genes flanked by two inverted terminal repeats (ITRs); the cap gene encodes three different proteins called virion protein 1, 2, and 3. Variations in the cap gene lead to different AAV serotypes with distinct tissue tropism. However, capsid studies in mice often do not translate well to humans due to species-specific differences in receptor expression and immune responses. This highlights the challenge of viral vector retargeting for gene therapy, necessitating extensive testing in multiple models to ensure efficacy and safety in humans.99 Of different AAV serotypes, AAV2, AAV4, AAV8, AAV9, AAV9, AAV10, and AAV11 have previously shown varying levels of tropism for renal tissue.100 Capsid proteins are involved in the process of cell entry by recognizing the surface receptor and inducing the endocytosis, which leads to the viral DNA entry and the expression of the transgenes within the cells. Therefore, they play a key role in determining transduction efficiency.101 The pattern of gene expression after delivering AAV (serotype 2) vectors through either intraparenchymal102 or intrarenal arteria infusion103 has been shown to be predominantly limited to tubular cells with no glomerular transduction. By engineering novel hybrid AAV vectors, higher transduction efficiency, limited tropism toward specific cells, and less immunogenicity can be achieved.100 Using an AAV barcode sequence, Furushot et al. identified six AAV capsids that enhance the transduction efficiency in mouse kidney after being injected through the renal vein or pelvis.104 Evaluating the transduction profiles of various pseudotyped AAVs can also help identifying novel pseudotypes that preferentially transduce particular kidney cells; for instance, Ikeda et al. have reported a synthetic AAV called Anc80, which can efficiently transduce kidney stroma and mesangial cells, and this can be harnessed for treating kidney fibrosis given that its gene-therapy-based treatment requires transduction of myofibroblast progenitors.105 In another approach, nanobodies, which are the single immunoglobulin variable domains of heavy chain antibodies, can be inserted into a surface loop of the AAV capsid to improve the specificity of transduction in the targeted cells.106 However, as explained earlier, transducing kidney is very challenging and, regardless of what viral tropism is being used, with the systemic delivery of AAVs, the transduction in the kidney is not efficient.105 Therefore, optimizing the capsid protein should be coupled with choosing a proper delivery route to achieve higher efficiencies.
Using direct injection into kidney, the expression of the gene is observed in the tubular epithelial cells in the S3 segment of the proximal tubule and intercalated cells in the collecting duct but not in glomeruli or in the intrarenal vasculature. The same is seen with intraparenchymal injection, with the expression of the transgene being limited to epithelial cells. However, the transduction efficiency of renal cells with AAV in primary culture is relatively low; to tackle this issue, pre-treatment of the cells with pharmaceutical enhancing agents such as hydroxyurea, cisplatin, and etoposide is suggested, which can enhance the transfection efficiency up to 20%. It is worth mentioning that this chemically induced enhancement depends on the cell lineage and whether the cell system is primary culture or transformed.107 Using these pre-treatments for recombinant AAV5 vectors, transfection efficiency in both primary culture and in vivo was similar, although it was limited by the localization of virus strictly to the injection site.107 AAV has been successful in treating cold-induced hypertension (CIH), which is followed by renal damage in rats. CIH is closely associated with the overactivity of the renin-angiotensin-aldosterone system (RAAS) and the common drugs that are used for controlling hypertension are spironolactone and eplerenone, which block the mineralocorticoid receptor (MR), a key component of RAAS pathway; however, these drugs are not only short-lasting but associated with side effects. In one study, AAV2, due to its high affinity toward renal tubule epithelial cells, was selected and engineered to carry an shRNA for MR, showing that this novel platform could effectively silence MR expression for up to 20 h and control CIH.108
Targeting viral-based gene therapies to the correct cells is important; this can be achieved by (1) surface modification, by conjugating the viral particles with the ligands or antibodies that specifically bind to receptors on the surface of the target cells106; (2) using local administration routes such as injection through renal artery109; (3) pseudotyping or modifying the viral envelope proteins to change the virus’s tropism; and110 (4) using cell-specific promoters, such as the nephrin promoter for podocyte cells, to achieve the expression of the gene solely in the target cells.29 Limited cargo capacity is a challenge when in comes to viral-based gene therapies. One way to overcome this issue is using the recently designed baculovirus, which can potentially deliver DNA cargos exceeding 100 kb.111
Non-viral vectors for gene delivery
Although promising, there are some limitations with viral gene therapy delivery systems, such as triggering immune responses in the body and the chance of retroviral insertional mutagenesis, which reduces the effectiveness of the treatment and poses safety concerns. Meanwhile, the limited carrying capacity of viral delivery systems excludes the possibility of treating many genetic diseases using gene therapy.
Among non-viral vectors for gene delivery, cationic polymers, including polyethyleneimine (PEI), poly(L-lysine) (PLL), poly[2-(dimethylamino)ethyl methacrylate] (PDMAEM), poly(amidoamine) (PAMAM), and chitosan (CS), have gained in popularity and can be considered as promising alternatives to viral vectors. This is mainly because of their safety, cost-effectiveness, tunable physicochemical properties, long-term stability for storage and reconstitution, and unlimited gene packaging capacity.112 For instance, the formulation of siRNAs with a specific chitosan-based delivery system helps the biodistribution of the drugs and circulation time in vivo,113,114,115 with particles tending to accumulate in the inflamed kidney.116 With the purpose of knocking down the expression of the water channel AQP1, chitosan/siRNA particles can be injected via the tail vein, resulting in more than a 40% reduction in AQP1 expression both at the mRNA and protein level compared to control siRNA. Interestingly, this gene knockdown is limited to the proximal tubular epithelial cells and no apparent changes are detected in the thin limbs of Henle’s loop.117
To inhibit mesangial cell proliferation, one of the key characteristics of glomerulonephritis, E2F decoy oligodeoxynucleotide, as a competitive inhibitor, can be delivered to the kidney via cationic multilamellar vesicle (MLV; consists of several unilamellar vesicles inside one another) liposomes.118 These liposomes have little cytotoxicity, high affinity to the cells as they are positively charged, and high encapsulation efficiency, all of these allowing a high level of either transient or stable gene expression.119 Considering that the inhibition of just one mitogen is not enough to control mesangial cell proliferation, E2F, a transcription factor that regulates the expression of several cell-cycle regulators, is a promising target and has been proved to successfully inhibit mesangial cell proliferation both in vitro and in vivo.118
However, as liposomes do not fuse well with the cultured cells, their fusion efficiency is low; this issue can be improved by using virus particles of hemagglutinating virus of Japan (HVJ). The use of HVJ liposomes (HVJ liposomes are lipids combined with HVJ viral envelopes) coupled with the co-introduction of an exogen with high-mobility group 1 (HMG1) has been proved to significantly enhance the introduction of DNA into the cell nucleus.120 Based on these findings, a new platform for gene delivery to the kidney was developed in which plasmid DNA and HMG1 are co-encapsulated into liposomes, which are then mixed with HVJ to form HVJ-liposome complexes. Four days after these complexes were injected into adult rat kidney via the renal artery, transient glomerular-specific expression of the reporter gene was detected.121 This platform was then used for the delivery of an antisense deoxyoligonucleotide for transforming growth factor (TGF)-β1, the upregulation of which plays a key role in the pathogenesis of glomerulonephritis. In transfected kidneys, significant reduction in the expression of TGF-β1, both at the mRNA and protein level, with subsequent inhibition of the extracellular matrix (ECM) (ECM is a three-dimensional network of proteins and carbohydrates that provides structural support; influences cellular behavior; and plays a crucial role in the integrity, function, and regeneration of tissues and organs in multicellular organisms) expansion (ECM expansion in kidney is related to kidney injury as it disrupts normal kidney structure and function, impairs filtration and blood flow, and promotes inflammation and scarring, ultimately contributing to kidney damage) was confirmed, highlighting the therapeutic effects of this approach on glomerular diseases.122
Taking advantage of HVJ liposomes, the delivery of transcription factor decoy (TFD) is also possible. TFDs inhibit gene expression by preventing transcription factors binding to their target gene promoter.123 For instance, considering the role of abnormal mesangial cell proliferation in the pathogenesis of many glomerular diseases, a double-stranded decoy oligonucleotide targeting E2F, the transcription factor for several cell-cycle regulatory genes, was examined for its potential therapeutic applications. After renal artery administration in vivo, while the naked TFD only localized in tubular epithelial cells, the oligonucleotides packed into HVJ liposomes transfected 30% of the glomeruli as well, reducing the glomerular histological injury from 32% to 19%.124 E2F cooperates with the transcription factor Sp1 through a cis-acting mechanism to regulate the cell cycle. Delivering resistant ring-Sp1 decoy oligonucleotide using the same HVJ liposomes via retrograde renal vein administration into the Unilateral Ureteral Obstruction (UUO) models resulted in the inhibition of ECM accumulation in the interstitial areas of the targeted kidney.125
PEI is a cationic polymer and a gold standard among non-viral delivery systems. The transfection efficiency of various forms of the polymer with different molecular mass and chemical structure (branched or linear) varies considerably. Among different derivatives, the alanyl derivative of 25-kDa PEI and dodecyl derivative of 2-kDa PEI have several times higher transfection efficiency in comparison to the non-modified 25-kDa PEI and were non-toxic.126 Also, the low cytotoxicity of 5-kDa polymer allows the use of a higher nitrogen-to-phosphate (N/P) ratio of 6.7, and this results in higher transfection efficiency in the cell lines.127 To avoid the cytotoxicity of high-molecular-weight PEI while taking advantage of its high transfection efficiency, the use of poly ester amine (PEA) synthesized from glyceroldimethacrylate and low-molecular-weight (LMW) PEI was suggested. PEA was then conjugated to a kidney-targeted peptide and formed PEA/PEP copolymers. To transfer hepatocyte growth factor (HGF) as an inhibitor of renal fibrosis through promoting tubular repair, PEA/PEP/DNA complexes were intravenously injected into the tail vein of UUO-model rats, which results in improvements in kidney function and reduction of collagen accumulation.128
Dendrimers are nano-sized synthetic macromolecules with an inner and an outer shell that can condense nucleic acid and prevent endosomal and nuclear degradation. Among dendrimers, PLL and PAMAM are the two commonly used for gene delivery. There are problems associated with dendrimer-based gene delivery systems that should be overcome; these include low transfection efficiency and low cellular specificity. There are different adjustments, such as surface modification with amino acid, protein or peptide, carbohydrate, polymer, or lipid, that can be made to give these delivery systems desirable properties and these have been discussed before.129
Different modifications have been suggested to improve the use of PLL as a DNA delivery system; these include the addition of dextran chain to improve solubility and thermal stability, glycosylation to decrease toxicity, and covalent linkage to polyethylene glycol (PEG) to increase transfection efficiency.130 Intravenous administration of PLL-modified iron oxide nanoparticles bound to exogenous DNA plasmid containing the reporter gene EGFP-C2 has successfully transfected kidney, lung, spleen, and brain.131 However, the clinical applications of PLL-based delivery systems for kidney gene therapy have not been explored sufficiently yet. The third-generation amine-terminated poly(amidoamine) (NH2-PAMAM) dendrimers had the highest accumulation in the kidney and are small enough to pass through the glomerular barrier. In 2012, these NH2-PAMAM-G3 dendrimers were bound to megalin internalizing receptor for the delivery of a specific sunitinib analogue, 17864, into the renal proximal tubular cells. This analogue was conjugated to dendrimers via universal linkage system (ULS) linkers. The internalization was examined based on anti-tyrosine kinase activity and compared to sunitinib, showing that 17864-ULS-NH2-PAMAM-G3 had significantly higher inhibitory effects. Meanwhile, 15 min after being intravenously administered, 13% of the injected dose was accumulated in the kidney, which makes it promising for a targeted treatment.132 Interestingly, L-serine-modified polyamidoamine dendrimers have shown great potential as a renal-targeting delivery system, with successful accumulation of up to 82% of the drugs in the kidney.133,134 Conjugation of different generations of PAMAM dendrimers (G2-G4) with cyclodextrins (CyD) (α, β, and γ) improves the transfection efficiency of this delivery system, with α-CyD-PAMAM-G2 conjugates having the highest efficiency.135,136 Complexes of α-CyD-PAMAM-G3 with pDNA encoding luciferase with the charge ratio of 2:4 were studied as a gene delivery tool. These complexes were intravenously injected into BALB/c mouse models with the results confirming successful cell transfection in liver, kidney, lung, and spleen in vivo with insignificant cytotoxicity.137 Also, glycosylation has been used to make different delivery systems targeted to specific cell groups. Twelve hours after mannosylated α-CyD-PAMAM-G2 dendrimers (DSM 3.3) bearing pDNA encoding luciferase had been intravenously injected, even though liver, spleen, lung, heart, and kidney were all transfected, a significant preference for kidney accumulation was reported.138 However, to our knowledge, despite these promising results as a kidney delivery system, there has not been sufficient research to date on the potential of dendrimer as a gene therapy tool specifically for kidney.
Nanocarriers
A nanocarrier is a nanoscale vehicle used to deliver drugs or other therapeutic agents to specific cells or tissues in the body. Given the advantages that nanocarriers offer, including cost-effectiveness, size, flexibility, and low immunogenicity,139 many of these components have been used for producing nanoparticles targeting specific kidney cells. Using a nanodelivery system can potentially be a promising strategy to circumvent the cytotoxicity resulting from the accumulation of the injected components in the liver after systemic administration140; the intravenous injection of mesoscale nanoparticles made of poly(lactic-co-glycolic acid) (PLGA) conjugated to PEG in mouse models has shown efficient localization in the kidney with only minor delivery to the liver and no injury in any organs.141
For example, as a novel targeted delivery system to glomerular mesangial cells, polycationic cyclodextrin nanoparticles containing siRNA (siRNA/CDP-NP) were suggested. To this purpose, firstly, cyclodextrin-containing polymer was assembled with the siRNA through electrostatic forces. The cyclodextrin components of these nanoparticles were then conjugated with 5-kDa PEG molecules that are covalently linked to adamantane (AD). Finally, by attaching mannose and transferrin as targeting ligands to the distal end of the AD-PEG molecules, mesangial cell uptake was improved.142 In 2010, PEG-PLL copolymer-based nanocarriers loaded with mitogen-activated protein kinase 1 (MAPK1) siRNAs were examined as a delivery system to the glomeruli in the murine lupus nephritis model. Compared to HVJ envelope vectors or liposomes, which are above 200 nm in diameter, these 10- to 20-nm-sized complexes penetrate the endothelial fenestration, which are about 70–100 nm in size, while not being able to pass through the 4-nm-sized pores in the basement membrane. This allows a delivery to mesangial cells but not to podocytes and hence is a great candidate for the treatment of glomerulonephritis.143
PEI-coated superparamagnetic Fe3O4 nanoparticles were developed as a system for multiple-gene delivery into porcine kidney cells. In this delivery system, Fe3O4 nanoparticles are coated with positively charged PEI, electrostatically absorbing the negatively charged DNA. Due to the superparamagnetic component, the transfection efficiency can be promoted using external magnetic force. To clarify, the magnetic force results in accelerated targeting and sedimentation of the gene on the cell surface and faster transfection, which yields increased endocytosis and gene expression on the magnetofected cells.144 Megalin is an endocytic receptor that is highly expressed in proximal tubular epithelial cells, and there is strong affinity between polymyxin B and megalin. To develop a kidney-targeted gene delivery system, carboxyalkylated PEI was covalently coupled to polymyxin B through an amidation reaction to form megalin-targeted nanoplexes. The modified-polymyxin-PEI/DNA nanoplexes had higher transfection efficiency both in vitro and in vivo in comparison to unmodified counterparts and could efficiently target the megalin-expressing kidney cells.145
Recently, another targeted gene delivery system to vimentin-expressing cells of the kidney, including podocytes and tubules, has been developed. Sorbitol diacrylate-crosslinked PEI (PS) was prepared and conjugated with chitobionic acid, the targeting moiety to the vimentin receptor. Due to the presence of easily degradable ester linkages in PS and hydroxyl groups in polysorbitol, as well as using LMW PEI, the designed delivery system had low cytotoxicity both in vivo and in vitro. Besides PS facilitating cellular intake and improving transfection efficiency, animal experiments confirmed the accumulation of nanoplexes around inner medulla and outer cortical regions where tubules, glomeruli, and peritubular capillaries are located.146
PEI nanoparticles have also been used for the delivery of miR-146 with the purpose of treating renal fibrosis by targeting the TGF-β1-Smad signaling pathway and inflammation in the kidney. In contrast to the naked miR-146, PEI-miR-146 nanoparticles, when intravenously injected via tail vein of mouse model of renal fibrosis, could significantly increase miR-146a expression and inhibit renal fibrosis area with minimal immunotoxicity.147 Using nanodelivery systems, it is also possible to simultaneously inhibit two important inflammation pathways, namely p38 MAPK and nuclear factor (NF)-κB.
In 2020, a glomerulus-targeting liposomal siRNA delivery loaded with both p38α MAPK and p65 siRNAs was designed. To this purpose, firstly, anionic siRNAs were complexed with positively charged PEI, providing protection against siRNA degradation when being loaded into PEG-modified liposomal carriers. The negatively charged liposomal nanoparticles then became weakly cationic through the surface modification with octa-arginine (R8), which enhanced cellular uptake. In in vivo experiments in a mouse immunoglobulin A nephropathy model, the designed nanocarriers were successful in reaching mesangial cells and endothelial cells and inhibiting the two pathways and IgA nephropathy (IgAN) relief in mice with negligible toxicity.148
Vector cells
The vector cells used for kidney gene therapy can mainly be divided into mesangial vector system, modified macrophages, and monocytes. The preparation of vector cells has several main steps: firstly, cells are propagated from isolated glomeruli, then the desired genetic changes are introduced, and finally the cells are transferred back into glomeruli through renal circulation.149 Considering the size of capillaries and cultured mesangial cells, 5–25 μm and 15–25 μm, respectively, after being injected into the rat kidney via renal artery, genetically engineered mesangial cells are entrapped within the glomerular capillaries.
In 1994, to enhance the transfection efficiency, pre-treatment with monoclonal antibody 1-22-3, which targets Thy 1-associated molecule on the surface of mesangial cells, was used to induce transient mesangiolysis. This damage stimulated specific replication of the transferred reporter cells, leading to 7- to 12-fold higher level of gene expression, which lasted for 8 weeks instead of 4 (4 weeks for the control group without mesangial damage).150 Without anti-Thy 1 antibody pre-treatment and by using renal artery as the delivery route, about 90% of vector cells were delivered to the glomerular capillary with only less than 10% being detected in the mesangial area. In this experiment, the duration of transgene expression was much longer in the in vivo system compared to ex vivo system.151
In 2005, the Decorin (DCN) gene, a proteoglycan that can bind to and neutralize TGF-β1, was transferred into mesangial cell vectors; injecting the DCN-expressing vector cells into rat antithymocyte serum glomeruli through the renal artery resulted in a reduction in the expression of TGF-β1. This was followed by a subsequent reduction in the expression of fibronectin and collagen IV, two key components of ECM, indicating the potential of the vectors for treating TGF-β1-induced fibrotic diseases.152 Sun et al. used PEI-DCN nanocomplexes to transfect cultured mesangial cells. Transfected cells were then injected into the left renal artery of rat anti-Thy1.1 glomerulonephritis models, with the results showing an increase in the expression of DCN in the mesangium and the glomerular capillary.153
Mesangial cell vectors have also been used to deliver interleukin (IL)-1 receptor antagonist (IL-1ra), considering that IL-1 is one of the key pathogenic mediators of glomerulonephritis. The established vector cells were injected into the glomeruli of adult male Sprague-Dawley rats through the renal circulation, with results showing a significant number of vectors populated in glomeruli secreting IL-1ra. Therefore, these vectors can help overcoming the short half-life of IL-1ra and undesirable side effects of IL-1ra systemic delivery, which is currently used as a therapeutic approach in treating different diseases.154
As for modified monocytes, in 1998, mononuclear cells positive for CD11b and CD18 were developed from the bone marrow cells of DBA/2 mice and examined as a site-specific gene delivery into inflamed glomeruli. Intravenous administration of these vehicles followed by intraperitoneal injection of lipopolysaccharide (LPS) resulted in the accumulation of vehicles in the glomerulus. In detail, LPS induced the expression of the intercellular adhesion molecule 1 (ICAM-1), which is a ligand for CD11b and CD18. Transduction of these vehicle cells with glucocerebrosidase gene led to 3.2-fold increase in the expression in isolated glomeruli, confirming the benefits of using these vehicles as a renal-targeting gene delivery tool.155
Genetically modified macrophages have been used for delivering IL-4, IL-10, IL-1 ra, and TGF-β to the kidney. Even though the injection of the cell vectors expressing TGF-β did not promote renal injury,156 macrophages bearing different anti-inflammatory cytokine genes could significantly improve renal function. By infusion of IL-1ra-transduced macrophage-based vectors into mouse models of anti-GBM glomerulonephritis, significant suppression in the progression of renal injury through the inhibition of IL-1β was observed. Interestingly, vehicle cells were recruited into the glomerulus only after nephritis induction through antibody injection and only an insignificant number of cells was found in the glomerulus of uninjected animals.157
In a similar study, adenovirally transduced vector cells expressing IL-1ra were injected through the tail vein of mice with UUO, and this reduced the expression of ICAM-1 and macrophage infiltration attenuating interstitial fibrosis in UUO kidneys.158 The injection of Ad-IL4-transfected macrophages into the renal artery was followed by a 75% reduction in the level of albuminuria in rats with nephrotoxic nephritis (NTN), a macrophage-dependent form of glomerular inflammation. It worth mentioning that the number of ED1-positive macrophages has decreased not only in the injected kidney but also in the contralateral kidney,159 implying that the inhibitory effects on inflammation are also exerted on distant kidney.156 This contralateral effect was also observed in ad-IL-10-transduced macrophages, which efficiently localized in the inflamed glomeruli of NTN mouse models after being injected into the renal artery of rats and significantly inhibited the inflammation.160 Interestingly, injection of macrophages transduced with an inert gene still attenuated renal injury in the inflamed glomeruli. One possible explanation is that the competition between pathogenic and transduced macrophages for adhesion sites might prevent infiltration of pathogenic macrophages.156
The applications of kidney gene delivery are not limited to only therapeutic purposes
Aside from the therapeutic applications mentioned above, gene delivery to kidney cells has also been used to study the role of a specific gene. For example, in 2002, to elucidate the role of c-myc overexpression in renal cystogenesis and the development of polycystic kidney disease (PKD), a c-myc antisense oligomer (ASO) was delivered to mice (C57BL/6J-cpk/cpk) that overexpress c-myc mRNA and develop PKD and finally die of renal failure. The findings revealed that ASO treatment had an inhibitory effect on cystic renal enlargement and development of renal failure.161
In a similar study, the direct role of two different growth factors, TGF-β and platelet-derived growth factor (PDGF), in the pathogenesis of glomerulosclerosis was examined using the in vivo HVJ-liposome transfection method. These findings revealed that, although the introduction of both TGF-β and PDGF stimulates glomerulosclerosis, the former performs its role through extracellular matrix expansion with the latter involved in cell proliferation.162 Moreover, the role of sustained activation of YAP in the reduction of renal function and interstitial fibrosis after the acute phase of acute kidney injury (AKI) was proved using an adenoviral-based delivery system for knocking down the YAP in the ischemia-reperfusion (IR)-induced AKI to CKD animal model. In detail, the overactivation of YAP, one of the main components of Hippo pathway, is associated with an increase in the expression of profibrotic factors, TGF-β and connective tissue growth factor (CTGF), triggering interstitial fibrosis. Using the same delivery system and KLF4 shRNA expression AV, it was shown that, for the upregulation of YAP after IR induction, the reprogramming factor KLF4 is essential.163
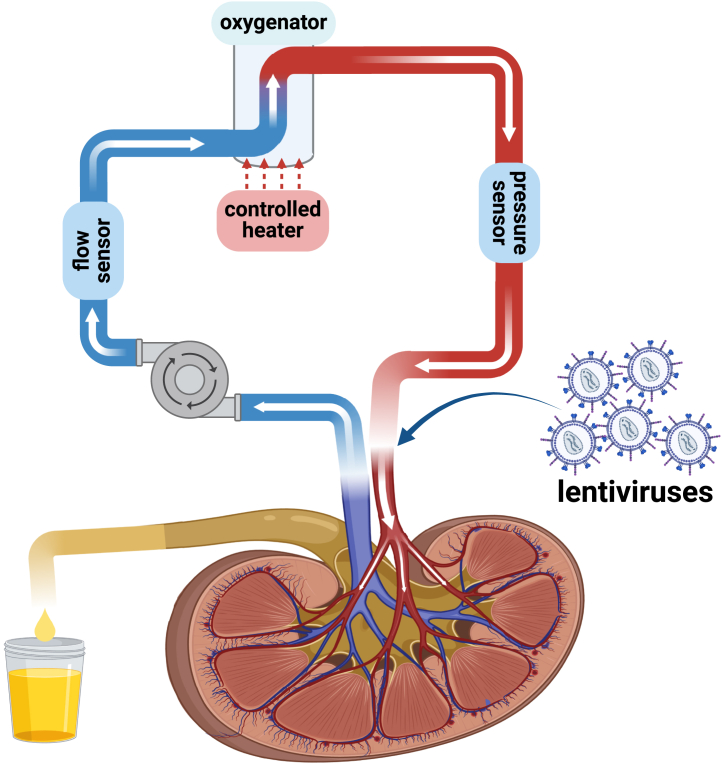
Gene delivery is also a promising approach to overcome the obstacles in transplantation such as organ shortage, chronic and acute rejection, delayed graft function, and death with graft function resulting from prolonged immunosuppressive treatments. Using gene delivery vectors, cells and organs can be treated ex vivo and this opens ways for local production of immunosuppressive molecules instead of long-term systemic immunosuppression. As the gene delivery is ex vivo, there is a lower probability for toxicity, immunogenicity, and systemic transduction.164 For example, with the purpose of prolonging the survival of rat kidney transplant recipients, lentiviral-based short hairpin RNA interference (shRNAi) therapy was suggested. IL-2 and interferon-gamma (IFN-gamma) have an important role in transplant rejection and their expression is controlled by split- and hairy-related protein-2 (SHARP-2). Using this SHARP-2 silencing construct, not only a 100% transfection efficiency was achieved in natural killer cell line but, in in vivo experiment, effective SHARP-2 gene silencing effect was followed by lower expression of both of the cytokines, resulting in a significantly higher survival time in comparison to the untreated rat models.165 In a similar study, by connecting the renal artery and vein to a perfusion system (Figure 5) (flow rate, 9–12 mL/min; pressure, 80–95 mm Hg; under sub-normothermic conditions [32°C]; oxygen saturation, 65%–70%), lentiviral vectors encoding shRNA were delivered to the kidney and successfully and stably downregulated levels of major histocompatibility complex (MHC) class I and II transcripts, the antigens involved in allogenic rejection after transplantation.166
Figure 5.
Generic perfusion system for pre-transplant kidney preservation and ex vivo gene therapy to enhance graft survival and reduce renal pathologies
A generic perfusion system used before kidney transplantation to preserve organ quality; this system can be used to do ex vivo organ gene therapy aiming to reduce renal pathologies and improve graft survival. The key components of this system are a glass heat exchanger, an oxygenator, a roller pump, and a circulating water bath.
To establish a link between animal model experiments and clinical applications and to examine whether gene delivery to allogenic kidney can be beneficial for dealing with posttransplant dysfunction, an AV-based molecular conjugate can be used as a gene delivery tool to intact isolated human kidney. Twelve hours after injecting AV-polylysine-β-galactosidase particles through renal artery, localized mRNA expression of the transgene was observed in tubular epithelial cells.167 Gene delivery has also been proved to be an efficient non-germline approach for establishing genetically engineered mouse models (GEMMs). In contrast to the traditional germline-based approaches, the in vivo sustained genetic manipulation of animal models is faster, cheaper, and more versatile, allowing the study of multiple genes and high-throughput functional genetic screens.168
In 1996, vector cells were used to study whether locally produced growth factors can summon macrophages and exacerbate renal injury. To deliver cytokines specifically to kidney, Naito et al. transfected renal tubular epithelial cells (TECs) with retroviral vectors encoding macrophage growth factors and then implanted them under the renal capsule of recipient mice models. This replacement resulted in an increase in the cytokines in the circulation followed by accumulation of macrophages in strains with lpr mutation but not in nonautoimmune hosts.169
In another study, to produce mouse models of kidney disease through non-germline methods, novel lentiviral-based vectors were designed and directly injected into the renal parenchyma using ultrasound guidance. With the von Hippel-Lindau (VHL) and Tsc1 genes being the target genes, two different approaches for gene knockdown, namely somatic recombination of floxed alleles and delivering shRNA, were selected. This lentiviral system was an effective renal tubular gene delivery tool for both delivering Cre-recombinase and shRNA, knocking down the expression of proteins only in cortical tubular epithelium and not in the glomeruli or medulla for up to 1 year after injection.168
Prospects for the future
Gene therapy for the kidney has been challenging, and progress has been slow compared to other organs such as the eye, liver, neuromuscular system, and in cancer. There have been promising developments in kidney delivery using AAV and nanoparticles. As described above, there has been significant progress in delivering therapeutic genes targeted to different kidney cells using different delivery systems or routes. It has been shown that AAV-mediated gene therapy can be delivered to the kidney to different cell types using a variety of routes, although with relatively low transduction efficiencies. Despite this, AAV has been used to successfully rescue the kidney function in a genetic mouse model of monogenic glomerular disease,29 and ASOs have been used successfully for a monogenic tubular disease80 and kidney fibrosis.79 However, these promising advancements in kidney gene therapy have mostly been demonstrated in rodent models, but translating these findings to large animal models and clinical applications poses several challenges. For instance, gene delivery efficiency in rodents may not translate directly to larger animals or humans due to differences in organ size, blood flow, and anatomy.170 Also, rodents have different immune responses compared to larger animals and humans, and the immunogenicity of vectors can lead to immune-mediated clearance, reduced transgene expression, or adverse reactions in humans.170 Potential toxicity needs thorough evaluation in long-term large-animal studies,170 and achieving sufficient transduction efficiency in target kidney cells is harder in larger organisms, requiring optimized vector designs. Finally, clinical translation involves regulatory hurdles, scalable manufacturing, and comprehensive trials to ensure efficacy and safety.171
However, although there have been promising results from basic research into kidney gene therapy, to date these have not been transferred to clinical practice. Within a few years, AAV-based podocyte targeting is likely to be the first clinical application in glomerular disease. Refining the delivery methods as well as gene delivery systems will expand the kidney gene therapy toolbox and will be key to improving the success of translating these gene therapies into clinical use.
Acknowledgments
None.
Author contributions
N.T. wrote the manuscript with the assistance of G.I.W. and C.M. All authors helped revise the manuscript and approved the final version.
Declaration of interests
G.I.W. and M.A.S. have consultancy agreements with Purespring Therapeutics.
References
- 1.Kovesdy C.P. Epidemiology of chronic kidney disease: an update 2022. Kidney Int. Suppl. (2011) 2022;12:7–11. doi: 10.1016/j.kisu.2021.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.May C.J., Chesor M., Hunter S.E., Hayes B., Barr R., Roberts T., Barrington F.A., Farmer L., Ni L., Jackson M., et al. Podocyte protease activated receptor 1 stimulation in mice produces focal segmental glomerulosclerosis mirroring human disease signaling events. Kidney Int. 2023;104:265–278. doi: 10.1016/j.kint.2023.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bleyer A.J., Westemeyer M., Xie J., Bloom M.S., Brossart K., Eckel J.J., Jones F., Molnar M.Z., Kotzker W., Anand P., et al. Genetic Etiologies for Chronic Kidney Disease Revealed through Next-Generation Renal Gene Panel. Am. J. Nephrol. 2022;53:297–306. doi: 10.1159/000522226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Powis S. 2023. Kidney disease: A UK public health emergency. [Google Scholar]
- 5.Hall Y.N., Chertow G.M. End stage renal disease. BMJ Clin. Evid. 2007;2007:2002. [PMC free article] [PubMed] [Google Scholar]
- 6.Abecassis M., Bartlett S.T., Collins A.J., Davis C.L., Delmonico F.L., Friedewald J.J., Hays R., Howard A., Jones E., Leichtman A.B., et al. Kidney transplantation as primary therapy for end-stage renal disease: a National Kidney Foundation/Kidney Disease Outcomes Quality Initiative (NKF/KDOQITM) conference. Clin. J. Am. Soc. Nephrol. 2008;3:471–480. doi: 10.2215/cjn.05021107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Frascà G.M., Sandrini S., Cosmai L., Porta C., Asch W., Santoni M., Salviani C., D’Errico A., Malvi D., Balestra E., Gallieni M. Renal cancer in kidney transplanted patients. J. Nephrol. 2015;28:659–668. doi: 10.1007/s40620-015-0219-8. [DOI] [PubMed] [Google Scholar]
- 8.Chronic kidney disease . Treating Chronic Kidney Disease. NHS inform; 2022. [Google Scholar]
- 9.Tavakolidakhrabadi N., Aulicino F., May C.J., Saleem M.A., Berger I., Welsh G.I. Genome editing and kidney health. Clin. Kidney J. 2024;17:sfae119. doi: 10.1093/ckj/sfae119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hanss B., Bruggeman L.A. Applications of gene therapy to kidney disease. Curr. Opin. Nephrol. Hypertens. 2003;12:439–445. doi: 10.1097/00041552-200307000-00013. [DOI] [PubMed] [Google Scholar]
- 11.Gonçalves G.A.R., Paiva R.D.M.A. Gene therapy: advances, challenges and perspectives. Einstein (Sao Paulo, Brazil) 2017;15:369–375. doi: 10.1590/s1679-45082017rb4024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bastos A.P., Piontek K., Silva A.M., Martini D., Menezes L.F., Fonseca J.M., Fonseca I.I., Germino G.G., Onuchic L.F. Pkd1 haploinsufficiency increases renal damage and induces microcyst formation following ischemia/reperfusion. J. Am. Soc. Nephrol. 2009;20:2389–2402. doi: 10.1681/asn.2008040435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bergmann C., Guay-Woodford L.M., Harris P.C., Horie S., Peters D.J.M., Torres V.E. Polycystic kidney disease. Nat. Rev. Dis. Primers. 2018;4:50. doi: 10.1038/s41572-018-0047-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Piontek K., Menezes L.F., Garcia-Gonzalez M.A., Huso D.L., Germino G.G. A critical developmental switch defines the kinetics of kidney cyst formation after loss of Pkd1. Nat. Med. 2007;13:1490–1495. doi: 10.1038/nm1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.WareJoncas Z., Campbell J.M., Martínez-Gálvez G., Gendron W.A.C., Barry M.A., Harris P.C., Sussman C.R., Ekker S.C. Precision gene editing technology and applications in nephrology. Nat. Rev. Nephrol. 2018;14:663–677. doi: 10.1038/s41581-018-0047-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rittiner J., Cumaran M., Malhotra S., Kantor B. Therapeutic modulation of gene expression in the disease state: Treatment strategies and approaches for the development of next-generation of the epigenetic drugs. Front. Bioeng. Biotechnol. 2022;10:1035543. doi: 10.3389/fbioe.2022.1035543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Daley J. Four Success Stories in Gene Therapy. Sci. Am. 2021;325 doi: 10.1038/scientificamerican112021-6hGSvdZt8Peew8oYe1NPc7. [DOI] [PubMed] [Google Scholar]
- 18.Pearson T.S., Gupta N., San Sebastian W., Imamura-Ching J., Viehoever A., Grijalvo-Perez A., Fay A.J., Seth N., Lundy S.M., Seo Y., et al. Gene therapy for aromatic L-amino acid decarboxylase deficiency by MR-guided direct delivery of AAV2-AADC to midbrain dopaminergic neurons. Nat. Commun. 2021;12:4251. doi: 10.1038/s41467-021-24524-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rubeis G., Steger F. Risks and benefits of human germline genome editing: An ethical analysis. Asian Bioeth. Rev. 2018;10:133–141. doi: 10.1007/s41649-018-0056-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ramamoorth M., Narvekar A. Non viral vectors in gene therapy- an overview. J. Clin. Diagn. Res. 2015;9:GE01–GE06. doi: 10.7860/jcdr/2015/10443.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bierzynska A., McCarthy H.J., Soderquest K., Sen E.S., Colby E., Ding W.Y., Nabhan M.M., Kerecuk L., Hegde S., Hughes D., et al. Genomic and clinical profiling of a national nephrotic syndrome cohort advocates a precision medicine approach to disease management. Kidney Int. 2017;91:937–947. doi: 10.1016/j.kint.2016.10.013. [DOI] [PubMed] [Google Scholar]
- 22.Przybyla L., Gilbert L.A. A new era in functional genomics screens. Nat. Rev. Genet. 2022;23:89–103. doi: 10.1038/s41576-021-00409-w. [DOI] [PubMed] [Google Scholar]
- 23.Subramanian I., Verma S., Kumar S., Jere A., Anamika K. Multi-omics Data Integration, Interpretation, and Its Application. Bioinform. Biol. Insights. 2020;14 doi: 10.1177/1177932219899051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lewis C.M., Vassos E. Polygenic risk scores: from research tools to clinical instruments. Genome Med. 2020;12:44. doi: 10.1186/s13073-020-00742-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Salomon R., Saunier S., Niaudet P. Nephronophthisis. Pediatr. Nephrol. 2009;24:2333–2344. doi: 10.1007/s00467-008-0840-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Afzal M., Kathuria P. StatPearls Publishing; 2022. Familial Hypocalciuric Hypercalcemia. StatPearls. [PubMed] [Google Scholar]
- 27.Stavljenić-Rukavina A. 5. Hereditary Kidney Disorders. Ejifcc. 2009;20:33–40. [PMC free article] [PubMed] [Google Scholar]
- 28.Hildebrandt F. Genetic kidney diseases. Lancet (London, England) 2010;375:1287–1295. doi: 10.1016/s0140-6736(10)60236-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ding W.Y., Kuzmuk V., Hunter S., Lay A., Hayes B., Beesley M., Rollason R., Hurcombe J.A., Barrington F., Masson C., et al. Adeno-associated virus gene therapy prevents progression of kidney disease in genetic models of nephrotic syndrome. Sci. Transl. Med. 2023;15:eabc8226. doi: 10.1126/scitranslmed.abc8226. [DOI] [PubMed] [Google Scholar]
- 30.Peek J.L., Wilson M.H. Cell and gene therapy for kidney disease. Nat. Rev. Nephrol. 2023;19:451–462. doi: 10.1038/s41581-023-00702-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zaiss A.K., Muruve D.A. Immune responses to adeno-associated virus vectors. Curr. Gene Ther. 2005;5:323–331. doi: 10.2174/1566523054065039. [DOI] [PubMed] [Google Scholar]
- 32.He X., Urip B.A., Zhang Z., Ngan C.C., Feng B. Evolving AAV-delivered therapeutics towards ultimate cures. J. Mol. Med. 2021;99:593–617. doi: 10.1007/s00109-020-02034-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ertl H.C.J. Immunogenicity and toxicity of AAV gene therapy. Front. Immunol. 2022;13:975803. doi: 10.3389/fimmu.2022.975803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Arjomandnejad M., Dasgupta I., Flotte T.R., Keeler A.M. Immunogenicity of Recombinant Adeno-Associated Virus (AAV) Vectors for Gene Transfer. BioDrugs. 2023;37:311–329. doi: 10.1007/s40259-023-00585-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rubin J.D., Barry M.A. Improving Molecular Therapy in the Kidney. Mol. Diagn. Ther. 2020;24:375–396. doi: 10.1007/s40291-020-00467-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Balzer M.S., Rohacs T., Susztak K. How Many Cell Types Are in the Kidney and What Do They Do? Annu. Rev. Physiol. 2022;84:507–531. doi: 10.1146/annurev-physiol-052521-121841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tomasoni S., Benigni A. Gene therapy: how to target the kidney. Promises and pitfalls. Curr. Gene Ther. 2004;4:115–122. doi: 10.2174/1566523044578013. [DOI] [PubMed] [Google Scholar]
- 38.Bulcha J.T., Wang Y., Ma H., Tai P.W.L., Gao G. Viral vector platforms within the gene therapy landscape. Signal Transduct. Targeted Ther. 2021;6:53. doi: 10.1038/s41392-021-00487-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Scheller E.L., Krebsbach P.H. Gene therapy: design and prospects for craniofacial regeneration. J. Dent. Res. 2009;88:585–596. doi: 10.1177/0022034509337480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cheng Q., Wei T., Farbiak L., Johnson L.T., Dilliard S.A., Siegwart D.J. Selective organ targeting (SORT) nanoparticles for tissue-specific mRNA delivery and CRISPR–Cas gene editing. Nat. Nanotechnol. 2020;15:313–320. doi: 10.1038/s41565-020-0669-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Segel M., Lash B., Song J., Ladha A., Liu C.C., Jin X., Mekhedov S.L., Macrae R.K., Koonin E.V., Zhang F. Mammalian retrovirus-like protein PEG10 packages its own mRNA and can be pseudotyped for mRNA delivery. Science. 2021;373:882–889. doi: 10.1126/science.abg6155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Woodard L.E., Cheng J., Welch R.C., Williams F.M., Luo W., Gewin L.S., Wilson M.H. Kidney-specific transposon-mediated gene transfer in vivo. Sci. Rep. 2017;7:44904. doi: 10.1038/srep44904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gusella G.L., Fedorova E., Hanss B., Marras D., Klotman M.E., Klotman P.E. Lentiviral gene transduction of kidney. Hum. Gene Ther. 2002;13:407–414. doi: 10.1089/10430340252792530. [DOI] [PubMed] [Google Scholar]
- 44.Fu Y., Chen J., Huang Z. Recent progress in microRNA-based delivery systems for the treatment of human disease. ExRNA. 2019;1:24. doi: 10.1186/s41544-019-0024-y. [DOI] [Google Scholar]
- 45.Gangemi S., Tonacci A. AntagomiRs: A novel therapeutic strategy for challenging COVID-19 cytokine storm. Cytokine Growth Factor Rev. 2021;58:111–113. doi: 10.1016/j.cytogfr.2020.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li X.Y., Zhang K., Jiang Z.Y., Cai L.H. MiR-204/miR-211 downregulation contributes to candidemia-induced kidney injuries via derepression of Hmx1 expression. Life Sci. 2014;102:139–144. doi: 10.1016/j.lfs.2014.03.010. [DOI] [PubMed] [Google Scholar]
- 47.Wilflingseder J., Jelencsics K., Bergmeister H., Sunzenauer J., Regele H., Eskandary F., Reindl-Schwaighofer R., Kainz A., Oberbauer R. miR-182-5p Inhibition Ameliorates Ischemic Acute Kidney Injury. Am. J. Pathol. 2017;187:70–79. doi: 10.1016/j.ajpath.2016.09.011. [DOI] [PubMed] [Google Scholar]
- 48.Wei Q., Liu Y., Liu P., Hao J., Liang M., Mi Q.S., Chen J.K., Dong Z. MicroRNA-489 Induction by Hypoxia-Inducible Factor-1 Protects against Ischemic Kidney Injury. J. Am. Soc. Nephrol. 2016;27:2784–2796. doi: 10.1681/asn.2015080870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Luan J., Fu J., Wang D., Jiao C., Cui X., Chen C., Liu D., Zhang Y., Wang Y., Yuen P.S.T., et al. miR-150-Based RNA Interference Attenuates Tubulointerstitial Fibrosis through the SOCS1/JAK/STAT Pathway In Vivo and In Vitro. Mol. Ther. Nucleic Acids. 2020;22:871–884. doi: 10.1016/j.omtn.2020.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gomez I.G., MacKenna D.A., Johnson B.G., Kaimal V., Roach A.M., Ren S., Nakagawa N., Xin C., Newitt R., Pandya S., et al. Anti-microRNA-21 oligonucleotides prevent Alport nephropathy progression by stimulating metabolic pathways. J. Clin. Invest. 2015;125:141–156. doi: 10.1172/jci75852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lee E.C., Valencia T., Allerson C., Schairer A., Flaten A., Yheskel M., Kersjes K., Li J., Gatto S., Takhar M., et al. Discovery and preclinical evaluation of anti-miR-17 oligonucleotide RGLS4326 for the treatment of polycystic kidney disease. Nat. Commun. 2019;10:4148. doi: 10.1038/s41467-019-11918-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Putta S., Lanting L., Sun G., Lawson G., Kato M., Natarajan R. Inhibiting MicroRNA-192 Ameliorates Renal Fibrosis in Diabetic Nephropathy. J. Am. Soc. Nephrol. 2012;23:458–469. doi: 10.1681/asn.2011050485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ravichandran K., Ozkok A., Wang Q., Mullick A.E., Edelstein C.L. Antisense-mediated angiotensinogen inhibition slows polycystic kidney disease in mice with a targeted mutation in Pkd2. Am. J. Physiol. Renal Physiol. 2015;308:F349–F357. doi: 10.1152/ajprenal.00478.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ravichandran K., Zafar I., He Z., Doctor R.B., Moldovan R., Mullick A.E., Edelstein C.L. An mTOR anti-sense oligonucleotide decreases polycystic kidney disease in mice with a targeted mutation in Pkd2. Hum. Mol. Genet. 2014;23:4919–4931. doi: 10.1093/hmg/ddu208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kausch I., Jiang H., Brocks C., Bruderek K., Krüger S., Sczakiel G., Jocham D., Böhle A. Ki-67-directed antisense therapy in an orthotopic renal cell carcinoma model. Eur. Urol. 2004;46:118–124. doi: 10.1016/j.eururo.2004.03.016. discussion 124-115. [DOI] [PubMed] [Google Scholar]
- 56.Shi W., Siemann D.W. Inhibition of renal cell carcinoma angiogenesis and growth by antisense oligonucleotides targeting vascular endothelial growth factor. Br. J. Cancer. 2002;87:119–126. doi: 10.1038/sj.bjc.6600416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang J.H., Newbury L.J., Knisely A.S., Monia B., Hendry B.M., Sharpe C.C. Antisense knockdown of Kras inhibits fibrosis in a rat model of unilateral ureteric obstruction. Am. J. Pathol. 2012;180:82–90. doi: 10.1016/j.ajpath.2011.09.036. [DOI] [PubMed] [Google Scholar]
- 58.Guha M., Xu Z.G., Tung D., Lanting L., Natarajan R. Specific down-regulation of connective tissue growth factor attenuates progression of nephropathy in mouse models of type 1 and type 2 diabetes. Faseb. J. 2007;21:3355–3368. doi: 10.1096/fj.06-6713com. [DOI] [PubMed] [Google Scholar]
- 59.Ghanbarian H., Aghamiri S., Eftekhary M., Wagner N., Wagner K.D. Small Activating RNAs: Towards the Development of New Therapeutic Agents and Clinical Treatments. Cells. 2021;10:591. doi: 10.3390/cells10030591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zeng T., Duan X., Zhu W., Liu Y., Wu W., Zeng G. SaRNA-mediated activation of TRPV5 reduces renal calcium oxalate deposition in rat via decreasing urinary calcium excretion. Urolithiasis. 2018;46:271–278. doi: 10.1007/s00240-017-1004-z. [DOI] [PubMed] [Google Scholar]
- 61.Fujino T., Muhib S., Sato N., Hasebe N. Silencing of p53 RNA through transarterial delivery ameliorates renal tubular injury and downregulates GSK-3β expression after ischemia-reperfusion injury. Am. J. Physiol. Renal Physiol. 2013;305:F1617–F1627. doi: 10.1152/ajprenal.00279.2013. [DOI] [PubMed] [Google Scholar]
- 62.Moore C.B., Guthrie E.H., Huang M.T.H., Taxman D.J. Short hairpin RNA (shRNA): design, delivery, and assessment of gene knockdown. Methods Mol. Biol. 2010;629:141–158. doi: 10.1007/978-1-60761-657-3_10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Koutsilieri E., Rethwilm A., Scheller C. The therapeutic potential of siRNA in gene therapy of neurodegenerative disorders. J. Neural. Transm. 2007:43–49. doi: 10.1007/978-3-211-73574-9_7. [DOI] [PubMed] [Google Scholar]
- 64.Molitoris B.A., Dagher P.C., Sandoval R.M., Campos S.B., Ashush H., Fridman E., Brafman A., Faerman A., Atkinson S.J., Thompson J.D., et al. siRNA targeted to p53 attenuates ischemic and cisplatin-induced acute kidney injury. J. Am. Soc. Nephrol. 2009;20:1754–1764. doi: 10.1681/asn.2008111204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zheng X., Zang G., Jiang J., He W., Johnston N.J., Ling H., Chen R., Zhang X., Liu Y., Haig A., et al. Attenuating Ischemia-Reperfusion Injury in Kidney Transplantation by Perfusing Donor Organs With siRNA Cocktail Solution. Transplantation. 2016;100:743–752. doi: 10.1097/tp.0000000000000960. [DOI] [PubMed] [Google Scholar]
- 66.Narváez A., Guiteras R., Sola A., Manonelles A., Morote J., Torras J., Grinyó J.M., Cruzado J.M. siRNA-silencing of CD40 attenuates unilateral ureteral obstruction-induced kidney injury in mice. PLoS One. 2019;14:e0215232. doi: 10.1371/journal.pone.0215232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mao W., Wang K., Xu B., Zhang H., Sun S., Hu Q., Zhang L., Liu C., Chen S., Wu J., et al. ciRS-7 is a prognostic biomarker and potential gene therapy target for renal cell carcinoma. Mol. Cancer. 2021;20:142. doi: 10.1186/s12943-021-01443-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Que-Gewirth N.S., Sullenger B.A. Gene therapy progress and prospects: RNA aptamers. Gene Ther. 2007;14:283–291. doi: 10.1038/sj.gt.3302900. [DOI] [PubMed] [Google Scholar]
- 69.Song K.M., Lee S., Ban C. Aptamers and their biological applications. Sensors. 2012;12:612–631. doi: 10.3390/s120100612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Matsui T., Higashimoto Y., Nishino Y., Nakamura N., Fukami K., Yamagishi S.-I. RAGE-Aptamer Blocks the Development and Progression of Experimental Diabetic Nephropathy. Diabetes. 2017;66:1683–1695. doi: 10.2337/db16-1281. [DOI] [PubMed] [Google Scholar]
- 71.Taguchi K., Yamagishi S.I., Yokoro M., Ito S., Kodama G., Kaida Y., Nakayama Y., Ando R., Yamada-Obara N., Asanuma K., et al. RAGE-aptamer attenuates deoxycorticosterone acetate/salt-induced renal injury in mice. Sci. Rep. 2018;8:2686. doi: 10.1038/s41598-018-21176-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Um J.E., Park J.T., Nam B.Y., Lee J.P., Jung J.H., Kim Y., Kim S., Park J., Wu M., Han S.H., et al. Periostin-binding DNA aptamer treatment attenuates renal fibrosis under diabetic conditions. Sci. Rep. 2017;7:8490. doi: 10.1038/s41598-017-09238-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhang H., Wang Z., Xie L., Zhang Y., Deng T., Li J., Liu J., Xiong W., Zhang L., Zhang L., et al. Molecular Recognition and In-Vitro-Targeted Inhibition of Renal Cell Carcinoma Using a DNA Aptamer. Mol. Ther. Nucleic Acids. 2018;12:758–768. doi: 10.1016/j.omtn.2018.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhao X., Zhong Y., Wang X., Shen J., An W. Advances in Circular RNA and Its Applications. Int. J. Med. Sci. 2022;19:975–985. doi: 10.7150/ijms.71840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.McDonald G.A., Zhu G., Li Y., Kovesdi I., Wickham T.J., Sukhatme V.P. Efficient adenoviral gene transfer to kidney cortical vasculature utilizing a fiber modified vector. J. Gene Med. 1999;1:103–110. doi: 10.1002/(SICI)1521-2254(199903/04)1:2<103::AID-JGM16>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 76.Chen S., Huang H., Liu Y., Wang C., Chen X., Chang Y., Li Y., Guo Z., Han Z., Han Z.C., et al. Renal subcapsular delivery of PGE(2) promotes kidney repair by activating endogenous Sox9(+) stem cells. iScience. 2021;24:103243. doi: 10.1016/j.isci.2021.103243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ye X., Jerebtsova M., Liu X.-H., Li Z., Ray P.E. Adenovirus-mediated gene transfer to renal glomeruli in rodents. Kidney Int. 2002;61:S16–S23. doi: 10.1046/j.1523-1755.2002.0610s1016.x. [DOI] [PubMed] [Google Scholar]
- 78.Moullier P., Friedlander G., Calise D., Ronco P., Perricaudet M., Ferry N. Adenoviral-mediated gene transfer to renal tubular cells in vivo. Kidney Int. 1994;45:1220–1225. doi: 10.1038/ki.1994.162. [DOI] [PubMed] [Google Scholar]
- 79.Newbury L.J., Wang J.H., Hung G., Hendry B.M., Sharpe C.C. Inhibition of Kirsten-Ras reduces fibrosis and protects against renal dysfunction in a mouse model of chronic folic acid nephropathy. Sci. Rep. 2019;9:14010. doi: 10.1038/s41598-019-50422-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ramsbottom S.A., Molinari E., Srivastava S., Silberman F., Henry C., Alkanderi S., Devlin L.A., White K., Steel D.H., Saunier S., et al. Targeted exon skipping of a CEP290 mutation rescues Joubert syndrome phenotypes in vitro and in a murine model. Proc. Natl. Acad. Sci. USA. 2018;115:12489–12494. doi: 10.1073/pnas.1809432115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Niculescu A.G., Bîrcă A.C., Grumezescu A.M. New Applications of Lipid and Polymer-Based Nanoparticles for Nucleic Acids Delivery. Pharmaceutics. 2021;13:2053. doi: 10.3390/pharmaceutics13122053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Meng F., Wang J., Yeo Y. Nucleic acid and oligonucleotide delivery for activating innate immunity in cancer immunotherapy. J. Control. Release. 2022;345:586–600. doi: 10.1016/j.jconrel.2022.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wischnjow A., Sarko D., Janzer M., Kaufman C., Beijer B., Brings S., Haberkorn U., Larbig G., Kübelbeck A., Mier W. Renal Targeting: Peptide-Based Drug Delivery to Proximal Tubule Cells. Bioconjug. Chem. 2016;27:1050–1057. doi: 10.1021/acs.bioconjchem.6b00057. [DOI] [PubMed] [Google Scholar]
- 84.Garrelfs S.F., Frishberg Y., Hulton S.A., Koren M.J., O'Riordan W.D., Cochat P., Deschênes G., Shasha-Lavsky H., Saland J.M., Van't Hoff W.G., et al. Lumasiran, an RNAi Therapeutic for Primary Hyperoxaluria Type 1. N. Engl. J. Med. 2021;384:1216–1226. doi: 10.1056/NEJMoa2021712. [DOI] [PubMed] [Google Scholar]
- 85.Lee C.S., Bishop E.S., Zhang R., Yu X., Farina E.M., Yan S., Zhao C., Zheng Z., Shu Y., Wu X., et al. Adenovirus-mediated gene delivery: Potential applications for gene and cell-based therapies in the new era of personalized medicine. Genes Dis. 2017;4:43–63. doi: 10.1016/j.gendis.2017.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Nahman N.S., Sferra T.J., Kronenberger J., Urban K.E., Troike A.E., Johnson A., Holycross B.J., Nuovo G.J., Sedmak D.D. Microsphere-adenoviral complexes target and transduce the glomerulus in vivo. Kidney Int. 2000;58:1500–1510. doi: 10.1046/j.1523-1755.2000.00312.x. [DOI] [PubMed] [Google Scholar]
- 87.Bhatt U.Y., Sferra T.J., Johnson A., Williams C., Shirey K., Venema T., Nuovo G.J., Nahman N.S. Glomerular β-galactosidase expression following transduction with microsphere-adenoviral complexes. Kidney Int. 2002;61:S68–S72. doi: 10.1046/j.1523-1755.2002.0610s1068.x. [DOI] [PubMed] [Google Scholar]
- 88.Ye X., Jerebtsova M., Liu X.-H., Li Z., Ray P.E. Adenovirus-mediated gene transfer to renal glomeruli in rodents. Kidney Int. 2002;61:S16–S23. doi: 10.1046/j.1523-1755.2002.0610s1016.x. [DOI] [PubMed] [Google Scholar]
- 89.Verkman A.S., Yang B. Aquaporin gene delivery to kidney. Kidney Int. 2002;61:S120–S124. doi: 10.1046/j.1523-1755.2002.0610s1120.x. [DOI] [PubMed] [Google Scholar]
- 90.McDonald G.A. Targeted adenoviral gene transfer to the kidney. Kidney Int. 2002;61:S42–S46. doi: 10.1046/j.1523-1755.2002.0610s1042.x. [DOI] [PubMed] [Google Scholar]
- 91.Ghosh S., Brown A.M., Jenkins C., Campbell K. Viral Vector Systems for Gene Therapy: A Comprehensive Literature Review of Progress and Biosafety Challenges. Appl. Biosaf. 2020;25:7–18. doi: 10.1177/1535676019899502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Russell R.A., Vassaux G., Martin-Duque P., McClure M.O. Transient foamy virus vector production by adenovirus vectors. Gene Ther. 2004;11:310–316. doi: 10.1038/sj.gt.3302177. [DOI] [PubMed] [Google Scholar]
- 93.Zhou C., Shan Y., Zhao H., He P. Biological effects of lentivirus-mediated shRNA targeting collagen type I on the mesangial cells of rats. Ren. Fail. 2011;33:334–340. doi: 10.3109/0886022x.2011.559679. [DOI] [PubMed] [Google Scholar]
- 94.McCarron A., Donnelley M., McIntyre C., Parsons D. Challenges of up-scaling lentivirus production and processing. J. Biotechnol. 2016;240:23–30. doi: 10.1016/j.jbiotec.2016.10.016. [DOI] [PubMed] [Google Scholar]
- 95.Martínez-Molina E., Chocarro-Wrona C., Martínez-Moreno D., Marchal J.A., Boulaiz H. Large-Scale Production of Lentiviral Vectors: Current Perspectives and Challenges. Pharmaceutics. 2020;12:1051. doi: 10.3390/pharmaceutics12111051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hacein-Bey-Abina S., Garrigue A., Wang G.P., Soulier J., Lim A., Morillon E., Clappier E., Caccavelli L., Delabesse E., Beldjord K., et al. Insertional oncogenesis in 4 patients after retrovirus-mediated gene therapy of SCID-X1. J. Clin. Invest. 2008;118:3132–3142. doi: 10.1172/jci35700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Schlimgen R., Howard J., Wooley D., Thompson M., Baden L.R., Yang O.O., Christiani D.C., Mostoslavsky G., Diamond D.V., Duane E.G., et al. Risks Associated With Lentiviral Vector Exposures and Prevention Strategies. J. Occup. Environ. Med. 2016;58:1159–1166. doi: 10.1097/jom.0000000000000879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Schenkwein D., Afzal S., Nousiainen A., Schmidt M., Ylä-Herttuala S. Efficient Nuclease-Directed Integration of Lentivirus Vectors into the Human Ribosomal DNA Locus. Mol. Ther. 2020;28:1858–1875. doi: 10.1016/j.ymthe.2020.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Martino A.T., Markusic D.M. Immune Response Mechanisms against AAV Vectors in Animal Models. Mol. Ther. Methods Clin. Dev. 2020;17:198–208. doi: 10.1016/j.omtm.2019.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Issa S.S., Shaimardanova A.A., Solovyeva V.V., Rizvanov A.A. Various AAV Serotypes and Their Applications in Gene Therapy: An Overview. Cells. 2023;12:785. doi: 10.3390/cells12050785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Aschauer D.F., Kreuz S., Rumpel S. Analysis of transduction efficiency, tropism and axonal transport of AAV serotypes 1, 2, 5, 6, 8 and 9 in the mouse brain. PLoS One. 2013;8:e76310. doi: 10.1371/journal.pone.0076310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lipkowitz M.S., Hanss B., Tulchin N., Wilson P.D., Langer J.C., Ross M.D., Kurtzman G.J., Klotman P.E., Klotman M.E. Transduction of Renal Cells in Vitro and in Vivo by Adeno-Associated Virus Gene Therapy Vectors. J. Am. Soc. Nephrol. 1999;10:1908–1915. doi: 10.1681/ASN.V1091908. [DOI] [PubMed] [Google Scholar]
- 103.Chen S., Agarwal A., Glushakova O.Y., Jorgensen M.S., Salgar S.K., Poirier A., Flotte T.R., Croker B.P., Madsen K.M., Atkinson M.A., et al. Gene Delivery in Renal Tubular Epithelial Cells Using Recombinant Adeno-Associated Viral Vectors. J. Am. Soc. Nephrol. 2003;14:947–958. doi: 10.1097/01.ASN.0000057858.45649.F7. [DOI] [PubMed] [Google Scholar]
- 104.Furusho T., Adachi K., Galbraith-Liss M., Sairavi A., Das R., Nakai H. Enhancing gene transfer to renal tubules and podocytes by context-dependent selection of AAV capsids. bioRxiv. 2023 doi: 10.1101/2023.07.28.548760. Preprint at. [DOI] [Google Scholar]
- 105.Ikeda Y., Sun Z., Ru X., Vandenberghe L.H., Humphreys B.D. Efficient Gene Transfer to Kidney Mesenchymal Cells Using a Synthetic Adeno-Associated Viral Vector. J. Am. Soc. Nephrol. 2018;29:2287–2297. doi: 10.1681/asn.2018040426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Eichhoff A.M., Börner K., Albrecht B., Schäfer W., Baum N., Haag F., Körbelin J., Trepel M., Braren I., Grimm D., et al. Nanobody-Enhanced Targeting of AAV Gene Therapy Vectors. Mol. Ther. Methods Clin. Dev. 2019;15:211–220. doi: 10.1016/j.omtm.2019.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lipkowitz M.S., Hanss B., Tulchin N., Wilson P.D., Langer J.C., Ross M.D., Kurtzman G.J., Klotman P.E., Klotman M.E. Transduction of renal cells in vitro and in vivo by adeno-associated virus gene therapy vectors. J. Am. Soc. Nephrol. 1999;10:1908–1915. doi: 10.1681/asn.v1091908. [DOI] [PubMed] [Google Scholar]
- 108.Wang X., Skelley L., Cade R., Sun Z. AAV delivery of mineralocorticoid receptor shRNA prevents progression of cold-induced hypertension and attenuates renal damage. Gene Ther. 2006;13:1097–1103. doi: 10.1038/sj.gt.3302768. [DOI] [PubMed] [Google Scholar]
- 109.Shintaro F., Shigeru K., Mitsuru H., Koyo N. In: Novel Gene Therapy Approaches. Ming W., David G., editors. Rijeka: IntechOpen); 2013. Targeted Gene Delivery: Importance of Administration Routes. Ch. 1. [DOI] [Google Scholar]
- 110.Gutierrez-Guerrero A., Cosset F.L., Verhoeyen E. Lentiviral Vector Pseudotypes: Precious Tools to Improve Gene Modification of Hematopoietic Cells for Research and Gene Therapy. Viruses. 2020;12:1016. doi: 10.3390/v12091016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Aulicino F., Pelosse M., Toelzer C., Capin J., Ilegems E., Meysami P., Rollarson R., Berggren P.-O., Dillingham M.S., Schaffitzel C., et al. Highly efficient CRISPR-mediated large DNA docking and multiplexed prime editing using a single baculovirus. Nucleic Acids Res. 2022;50:7783–7799. doi: 10.1093/nar/gkac587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Bahadur K.C., Uludag H. 2016. PEI and its derivatives for gene therapy; pp. 29–54. [DOI] [Google Scholar]
- 113.Gao S., Dagnaes-Hansen F., Nielsen E.J.B., Wengel J., Besenbacher F., Howard K.A., Kjems J. The effect of chemical modification and nanoparticle formulation on stability and biodistribution of siRNA in mice. Mol. Ther. 2009;17:1225–1233. doi: 10.1038/mt.2009.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Laursen M.B., Pakula M.M., Gao S., Fluiter K., Mook O.R., Baas F., Langklaer N., Wengel S.L., Wengel J., Kjems J., Bramsen J.B. Utilization of unlocked nucleic acid (UNA) to enhance siRNA performance in vitro and in vivo. Mol. Biosyst. 2010;6:862–870. doi: 10.1039/b918869j. [DOI] [PubMed] [Google Scholar]
- 115.Schyth B.D., Bramsen J.B., Pakula M.M., Larashati S., Kjems J., Wengel J., Lorenzen N. In vivo screening of modified siRNAs for non-specific antiviral effect in a small fish model: number and localization in the strands are important. Nucleic Acids Res. 2012;40:4653–4665. doi: 10.1093/nar/gks033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Yang C., Nilsson L., Cheema M.U., Wang Y., Frøkiær J., Gao S., Kjems J., Nørregaard R. Chitosan/siRNA nanoparticles targeting cyclooxygenase type 2 attenuate unilateral ureteral obstruction-induced kidney injury in mice. Theranostics. 2015;5:110–123. doi: 10.7150/thno.9717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Gao S., Hein S., Dagnæs-Hansen F., Weyer K., Yang C., Nielsen R., Christensen E.I., Fenton R.A., Kjems J. Megalin-mediated specific uptake of chitosan/siRNA nanoparticles in mouse kidney proximal tubule epithelial cells enables AQP1 gene silencing. Theranostics. 2014;4:1039–1051. doi: 10.7150/thno.7866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Maeshima Y., Kashihara N., Yasuda T., Sugiyama H., Sekikawa T., Okamoto K., Kanao K., Watanabe Y., Kanwar Y.S., Makino H. Inhibition of mesangial cell proliferation by E2F decoy oligodeoxynucleotide in vitro and in vivo. J. Clin. Invest. 1998;101:2589–2597. doi: 10.1172/jci429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Yagi K., Noda H., Kurono M., Ohishi N. Efficient gene transfer with less cytotoxicity by means of cationic multilamellar liposomes. Biochem. Biophys. Res. Commun. 1993;196:1042–1048. doi: 10.1006/bbrc.1993.2356. [DOI] [PubMed] [Google Scholar]
- 120.Kaneda Y., Iwai K., Uchida T. Introduction and expression of the human insulin gene in adult rat liver. J. Biol. Chem. 1989;264:12126–12129. [PubMed] [Google Scholar]
- 121.Tomita N., Higaki J., Morishita R., Kato K., Mikami H., Kaneda Y., Ogihara T. Direct in vivo gene introduction into rat kidney. Biochem. Biophys. Res. Commun. 1992;186:129–134. doi: 10.1016/S0006-291X(05)80784-3. [DOI] [PubMed] [Google Scholar]
- 122.Akagi Y., Isaka Y., Arai M., Kaneko T., Takenaka M., Moriyama T., Kaneda Y., Ando A., Orita Y., Kamada T., et al. Inhibition of TGF-beta 1 expression by antisense oligonucleotides suppressed extracellular matrix accumulation in experimental glomerulonephritis. Kidney Int. 1996;50:148–155. doi: 10.1038/ki.1996.297. [DOI] [PubMed] [Google Scholar]
- 123.Hecker M., Wagner A.H. Transcription factor decoy technology: A therapeutic update. Biochem. Pharmacol. 2017;144:29–34. doi: 10.1016/j.bcp.2017.06.122. [DOI] [PubMed] [Google Scholar]
- 124.Tomita N., Kashihara N., Morishita R. Transcription factor decoy oligonucleotide-based therapeutic strategy for renal disease. Clin. Exp. Nephrol. 2007;11:7–17. doi: 10.1007/s10157-007-0459-6. [DOI] [PubMed] [Google Scholar]
- 125.Chae Y.M., Park K.K., Lee I.K., Kim J.K., Kim C.H., Chang Y.C. Ring-Sp1 decoy oligonucleotide effectively suppresses extracellular matrix gene expression and fibrosis of rat kidney induced by unilateral ureteral obstruction. Gene Ther. 2006;13:430–439. doi: 10.1038/sj.gt.3302696. [DOI] [PubMed] [Google Scholar]
- 126.Thomas M., Klibanov A.M. Enhancing polyethylenimine's delivery of plasmid DNA into mammalian cells. Proc. Natl. Acad. Sci. USA. 2002;99:14640–14645. doi: 10.1073/pnas.192581499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kunath K., von Harpe A., Fischer D., Petersen H., Bickel U., Voigt K., Kissel T. Low-molecular-weight polyethylenimine as a non-viral vector for DNA delivery: comparison of physicochemical properties, transfection efficiency and in vivo distribution with high-molecular-weight polyethylenimine. J. Control. Release. 2003;89:113–125. doi: 10.1016/s0168-3659(03)00076-2. [DOI] [PubMed] [Google Scholar]
- 128.Kim Y.K., Kwon J.T., Jiang H.L., Choi Y.J., Cho M.H., Cho C.S. Kidney-specific peptide-conjugated poly(ester amine) for the treatment of kidney fibrosis. J. Nanosci. Nanotechnol. 2012;12:5149–5154. doi: 10.1166/jnn.2012.6372. [DOI] [PubMed] [Google Scholar]
- 129.Rai D.B., Pooja D., Kulhari H. In: Pharmaceutical Applications of Dendrimers. Chauhan A., Kulhari H., editors. Elsevier; 2020. 9 - Dendrimers in gene delivery; pp. 211–231. [DOI] [Google Scholar]
- 130.Han S., Mahato R.I., Sung Y.K., Kim S.W. Development of Biomaterials for Gene Therapy. Mol. Ther. 2000;2:302–317. doi: 10.1006/mthe.2000.0142. [DOI] [PubMed] [Google Scholar]
- 131.Xiang J.J., Tang J.Q., Zhu S.G., Nie X.M., Lu H.B., Shen S.R., Li X.L., Tang K., Zhou M., Li G.Y. IONP-PLL: a novel non-viral vector for efficient gene delivery. J. Gene Med. 2003;5:803–817. doi: 10.1002/jgm.419. [DOI] [PubMed] [Google Scholar]
- 132.Dolman M.E.E.M., van Dorenmalen K.M.A., Pieters E.H.E., Sparidans R.W., Lacombe M., Szokol B., Orfi L., Kéri G., Bovenschen N., Storm G., et al. Dendrimer-based macromolecular conjugate for the kidney-directed delivery of a multitargeted sunitinib analogue. Macromol. Biosci. 2012;12:93–103. doi: 10.1002/mabi.201100277. [DOI] [PubMed] [Google Scholar]
- 133.Matsuura S., Katsumi H., Suzuki H., Hirai N., Hayashi H., Koshino K., Higuchi T., Yagi Y., Kimura H., Sakane T., Yamamoto A. l-Serine-modified polyamidoamine dendrimer as a highly potent renal targeting drug carrier. Proc. Natl. Acad. Sci. USA. 2018;115:10511–10516. doi: 10.1073/pnas.1808168115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Katsumi H., Takashima R., Suzuki H., Hirai N., Matsuura S., Kimura H., Morishita M., Yamamoto A. S-nitrosylated l-serine-modified dendrimer as a kidney-targeting nitric oxide donor for prevention of renal ischaemia/reperfusion injury. Free Radic. Res. 2020;54:841–847. doi: 10.1080/10715762.2019.1697437. [DOI] [PubMed] [Google Scholar]
- 135.Arima H., Motoyama K., Higashi T. Polyamidoamine Dendrimer Conjugates with Cyclodextrins as Novel Carriers for DNA, shRNA and siRNA. Pharmaceutics. 2012;4:130–148. doi: 10.3390/pharmaceutics4010130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kihara F., Arima H., Tsutsumi T., Hirayama F., Uekama K. Effects of structure of polyamidoamine dendrimer on gene transfer efficiency of the dendrimer conjugate with alpha-cyclodextrin. Bioconjug. Chem. 2002;13:1211–1219. doi: 10.1021/bc025557d. [DOI] [PubMed] [Google Scholar]
- 137.Kihara F., Arima H., Tsutsumi T., Hirayama F., Uekama K. In vitro and in vivo gene transfer by an optimized alpha-cyclodextrin conjugate with polyamidoamine dendrimer. Bioconjug. Chem. 2003;14:342–350. doi: 10.1021/bc025613a. [DOI] [PubMed] [Google Scholar]
- 138.Wada K., Arima H., Tsutsumi T., Chihara Y., Hattori K., Hirayama F., Uekama K. Improvement of gene delivery mediated by mannosylated dendrimer/α-cyclodextrin conjugates. J. Control. Release. 2005;104:397–413. doi: 10.1016/j.jconrel.2005.02.016. [DOI] [PubMed] [Google Scholar]
- 139.Peek J.L., Wilson M.H. Gene therapy for kidney disease: targeting cystinuria. Curr. Opin. Nephrol. Hypertens. 2022;31:175–179. doi: 10.1097/mnh.0000000000000768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Tavakoli N., Divsalar A., Haertlé T., Sawyer L., Saboury A.A., Muronetz V. Milk protein-based nanodelivery systems for the cancer treatment. J. Nanostruct. Chem. 2021;11:483–500. doi: 10.1007/s40097-021-00399-5. [DOI] [Google Scholar]
- 141.Williams R.M., Shah J., Tian H.S., Chen X., Geissmann F., Jaimes E.A., Heller D.A. Selective Nanoparticle Targeting of the Renal Tubules. Hypertension. 2018;71:87–94. doi: 10.1161/hypertensionaha.117.09843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Zuckerman J.E., Gale A., Wu P., Ma R., Davis M.E. siRNA delivery to the glomerular mesangium using polycationic cyclodextrin nanoparticles containing siRNA. Nucleic Acid Ther. 2015;25:53–64. doi: 10.1089/nat.2014.0505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Shimizu H., Hori Y., Kaname S., Yamada K., Nishiyama N., Matsumoto S., Miyata K., Oba M., Yamada A., Kataoka K., Fujita T. siRNA-based therapy ameliorates glomerulonephritis. J. Am. Soc. Nephrol. 2010;21:622–633. doi: 10.1681/asn.2009030295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Wang Y., Cui H., Li K., Sun C., Du W., Cui J., Zhao X., Chen W. A magnetic nanoparticle-based multiple-gene delivery system for transfection of porcine kidney cells. PLoS One. 2014;9:e102886. doi: 10.1371/journal.pone.0102886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Oroojalian F., Rezayan A.H., Mehrnejad F., Nia A.H., Shier W.T., Abnous K., Ramezani M. Efficient megalin targeted delivery to renal proximal tubular cells mediated by modified-polymyxin B-polyethylenimine based nano-gene-carriers. Mater. Sci. Eng. C Mater. Biol. Appl. 2017;79:770–782. doi: 10.1016/j.msec.2017.05.068. [DOI] [PubMed] [Google Scholar]
- 146.Mathew A.P., Uthaman S., Bae E.H., Lee J.Y., Park I.K. Vimentin Targeted Nano-gene Carrier for Treatment of Renal Diseases. J. Korean Med. Sci. 2021;36:e333. doi: 10.3346/jkms.2021.36.e333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Morishita Y., Imai T., Yoshizawa H., Watanabe M., Ishibashi K., Muto S., Nagata D. Delivery of microRNA-146a with polyethylenimine nanoparticles inhibits renal fibrosis in vivo. Int. J. Nanomed. 2015;10:3475–3488. doi: 10.2147/ijn.s82587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Wang Y., Wu Q., Wang J., Li L., Sun X., Zhang Z., Zhang L. Co-delivery of p38α MAPK and p65 siRNA by novel liposomal glomerulus-targeting nano carriers for effective immunoglobulin a nephropathy treatment. J. Control. Release. 2020;320:457–468. doi: 10.1016/j.jconrel.2020.01.024. [DOI] [PubMed] [Google Scholar]
- 149.Yokoo T., Kitamura M. Gene Transfer of Interleukin-1 Receptor Antagonist into the Renal Glomerulus via a Mesangial Cell Vector. Biochem. Biophys. Res. Commun. 1996;226:883–888. doi: 10.1006/bbrc.1996.1444. [DOI] [PubMed] [Google Scholar]
- 150.Kitamura M., Taylor S., Unwin R., Burton S., Shimizu F., Fine L.G. Gene transfer into the rat renal glomerulus via a mesangial cell vector: site-specific delivery, in situ amplification, and sustained expression of an exogenous gene in vivo. J. Clin. Invest. 1994;94:497–505. doi: 10.1172/jci117361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Kim H.J., Kim S.I., Yun I.J., Kwak J.H., Yu S.H. Gene Delivery into Rat Glomerulus Using a Mesangial Cell Vector. Mol. Cells. 2000;10:662–668. doi: 10.1007/s10059-000-0662-8. [DOI] [PubMed] [Google Scholar]
- 152.Huijun W., Long C., Zhigang Z., Feng J., Muyi G. Ex vivo transfer of the decorin gene into rat glomerulus via a mesangial cell vector suppressed extracellular matrix accumulation in experimental glomerulonephritis. Exp. Mol. Pathol. 2005;78:17–24. doi: 10.1016/j.yexmp.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 153.Sun J.Y., Sun Y., Wu H.J., Zhang H.X., Zhao Z.H., Chen Q., Zhang Z.G. Transgene therapy for rat anti-Thy1.1 glomerulonephritis via mesangial cell vector with a polyethylenimine/decorin nanocomplex. Nanoscale Res. Lett. 2012;7:451. doi: 10.1186/1556-276x-7-451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Yokoo T., Kitamura M. Gene transfer of interleukin-1 receptor antagonist into the renal glomerulus via a mesangial cell vector. Biochem. Biophys. Res. Commun. 1996;226:883–888. doi: 10.1006/bbrc.1996.1444. [DOI] [PubMed] [Google Scholar]
- 155.Yokoo T., Utsunomiya Y., Ohashi T., Imasawa T., Kogure T., Futagawa Y., Kawamura T., Eto Y., Hosoya T. Inflamed site-specific gene delivery using bone marrow-derived CD11b+CD18+ vehicle cells in mice. Hum. Gene Ther. 1998;9:1731–1738. doi: 10.1089/hum.1998.9.12-1731. [DOI] [PubMed] [Google Scholar]
- 156.Wilson H.M., Kluth D.C. Targeting genetically modified macrophages to the glomerulus. Nephron Exp. Nephrol. 2003;94:e113–e118. doi: 10.1159/000072494. [DOI] [PubMed] [Google Scholar]
- 157.Yokoo T., Ohashi T., Utsunomiya Y., Kojima H., Imasawa T., Kogure T., Hisada Y., Okabe M., Eto Y., Kawamura T., Hosoya T. Prophylaxis of antibody-induced acute glomerulonephritis with genetically modified bone marrow-derived vehicle cells. Hum. Gene Ther. 1999;10:2673–2678. doi: 10.1089/10430349950016717. [DOI] [PubMed] [Google Scholar]
- 158.Yamagishi H., Yokoo T., Imasawa T., Mitarai T., Kawamura T., Utsunomiya Y. Genetically Modified Bone Marrow-Derived Vehicle Cells Site Specifically Deliver an Anti-Inflammatory Cytokine to Inflamed Interstitium of Obstructive Nephropathy1. J. Immunol. 2001;166:609–616. doi: 10.4049/jimmunol.166.1.609. [DOI] [PubMed] [Google Scholar]
- 159.Kluth D.C., Ainslie C.V., Pearce W.P., Finlay S., Clarke D., Anegon I., Rees A.J. Macrophages Transfected with Adenovirus to Express IL-4 Reduce Inflammation in Experimental Glomerulonephritis1. J. Immunol. 2001;166:4728–4736. doi: 10.4049/jimmunol.166.7.4728. [DOI] [PubMed] [Google Scholar]
- 160.Wilson H.M., Stewart K.N., Brown P.A.J., Anegon I., Chettibi S., Rees A.J., Kluth D.C. Bone-marrow-derived macrophages genetically modified to produce IL-10 reduce injury in experimental glomerulonephritis. Mol. Ther. 2002;6:710–717. doi: 10.1006/mthe.2002.0802. [DOI] [PubMed] [Google Scholar]
- 161.Ricker J.L., Mata J.E., Iversen P.L., Gattone V.H. c-myc antisense oligonucleotide treatment ameliorates murine ARPKD. Kidney Int. 2002;61:S125–S131. doi: 10.1046/j.1523-1755.2002.0610s1125.x. [DOI] [PubMed] [Google Scholar]
- 162.Isaka Y., Fujiwara Y., Ueda N., Kaneda Y., Kamada T., Imai E. Glomerulosclerosis induced by in vivo transfection of transforming growth factor-beta or platelet-derived growth factor gene into the rat kidney. J. Clin. Invest. 1993;92:2597–2601. doi: 10.1172/jci116874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Xu D., Chen P.P., Zheng P.Q., Yin F., Cheng Q., Zhou Z.L., Xie H.Y., Li J.Y., Ni J.Y., Wang Y.Z., et al. KLF4 initiates sustained YAP activation to promote renal fibrosis in mice after ischemia-reperfusion kidney injury. Acta Pharmacol. Sin. 2021;42:436–450. doi: 10.1038/s41401-020-0463-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Akalin E., Bromberg J.S. Gene therapy and solid-organ transplantation. Kidney Int. 2002;61:S56–S60. doi: 10.1046/j.1523-1755.2002.0610s1056.x. [DOI] [PubMed] [Google Scholar]
- 165.Shou Z., Xiao H., Xu Y., Wang Y., Yang Y., Jiang H., Chen J., Yamada K., Miyamoto K. SHARP-2 gene silencing by lentiviral-based short hairpin RNA interference prolonged rat kidney transplant recipients' survival time. J. Int. Med. Res. 2009;37:766–778. doi: 10.1177/147323000903700320. [DOI] [PubMed] [Google Scholar]
- 166.Yuzefovych Y., Valdivia E., Rong S., Hack F., Rother T., Schmitz J., Bräsen J.H., Wedekind D., Moers C., Wenzel N., et al. Genetic Engineering of the Kidney to Permanently Silence MHC Transcripts During ex vivo Organ Perfusion. Front. Immunol. 2020;11:265. doi: 10.3389/fimmu.2020.00265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Zeigler S.T., Kerby J.D., Curiel D.T., Diethelm A.G., Thompson J.A. Molecular conjugate-mediated gene transfer into isolated human kidneys1. Transplantation. 1996;61:812–817. doi: 10.1097/00007890-199603150-00023. [DOI] [PubMed] [Google Scholar]
- 168.Espana-Agusti J., Tuveson D.A., Adams D.J., Matakidou A. A minimally invasive, lentiviral based method for the rapid and sustained genetic manipulation of renal tubules. Sci. Rep. 2015;5:11061. doi: 10.1038/srep11061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Naito T., Yokoyama H., Moore K.J., Dranoff G., Mulligan R.C., Kelley V.R. Macrophage growth factors introduced into the kidney initiate renal injury. Mol. Med. 1996;2:297–312. [PMC free article] [PubMed] [Google Scholar]
- 170.Casal M., Haskins M. Large animal models and gene therapy. Eur. J. Hum. Genet. 2006;14:266–272. doi: 10.1038/sj.ejhg.5201535. [DOI] [PubMed] [Google Scholar]
- 171.Lee N.K., Chang J.W. Manufacturing Cell and Gene Therapies: Challenges in Clinical Translation. Ann. Lab. Med. 2024;44:314–323. doi: 10.3343/alm.2023.0382. [DOI] [PMC free article] [PubMed] [Google Scholar]