Abstract
Vascular tissue engineering faces significant challenges in creating in vitro vascular disease models, implantable vascular grafts, and vascularized tissue/organ constructs due to limitations in manufacturing precision, structural complexity, replicating the composited architecture, and mimicking the mechanical properties of natural vessels. Light-based 3D bioprinting, leveraging the unique advantages of light including high resolution, rapid curing, multi-material adaptability, and tunable photochemistry, offers transformative solutions to these obstacles. With the emergence of diverse light-based 3D bioprinting techniques and innovative strategies, the advances in vascular tissue engineering have been significantly accelerated. This review provides an overview of the human vascular system and its physiological functions, followed by an in-depth discussion of advancements in light-based 3D bioprinting, including light-dominated and light-assisted techniques. We explore the application of these technologies in vascular tissue engineering for creating in vitro vascular disease models recapitulating key pathological features, implantable blood vessel grafts, and tissue analogs with the integration of capillary-like vasculatures. Finally, we provide readers with insights into the future perspectives of light-based 3D bioprinting to revolutionize vascular tissue engineering.
Keywords: Light-based 3D bioprinting, vascular tissue engineering, In vitro vascular disease model, Blood vessel graft, Vascularized tissue/organ graft
Graphical abstract
Graphical abstract of submitted review “Light-Based 3D Bioprinting Techniques for Illuminating the Advances of Vascular Tissue Engineering”.
1. Introduction
The human vascular system is crucial for maintaining physiological homeostasis, and performing essential functions such as transporting oxygen and nutrients, eliminating metabolic wastes, regulating blood pressure, and mediating immune responses [1]. Given the vital nature of these functions, any dysfunction within the vascular system can lead to severe, life-threatening conditions, posing significant healthcare burdens worldwide. For instance, cardiovascular diseases alone, such as heart attacks and strokes, were responsible for approximately 20 million deaths globally in 2023 [2]. This underscores the clinical need for artificial blood vessels, particularly small-diameter vascular grafts (caliber smaller than 6 mm), to bypass blockages [3,4]. Additionally, the severe shortage of available tissues and organs for transplantation highlights the critical challenge of effective vascularization. Cells fail to survive if they are more than 200 μm away from vascular support, emphasizing the necessity for integrated vasculature in artificial surrogates [[5], [6], [7]]. Moreover, the pathology of numerous vascular-related disorders, such as atherosclerosis, aneurysms, cancers, and Alzheimer's disease, remains poorly understood, further underlining the need for reliable vasculature-integrated disease models to explore the mechanisms regulating their onset and progression. These realities call for significant advancements and the convergence of multidisciplinary technologies, as well as the translation towards clinical applications.
Tissue engineering stands at the forefront of regenerative medicine, harnessing the synergistic potential of cells, scaffolds, and growth factors to reconstruct human tissue and organ equivalents [[8], [9], [10], [11], [12]]. These can replace injured natural counterparts or serve as in vitro platforms to study cellular responses to pathological stimuli [13,14]. Within this broad field, vascular tissue engineering (VTE) specifically targets the reconstruction of functional blood vessels and vascularized tissue or organ analogs, aiming to alleviate the burdens imposed by vascular diseases [15]. With the pioneering efforts, various technologies such as mold-casting [16,17], scaffold-based cell seeding [[18], [19], [20]], cell sheet assembly [[21], [22], [23]], decellularization [24,25], needle-templating [26,27], and microfluidic techniques [[28], [29], [30]] have significantly advanced the development of VTE. These methods have been widely employed to engineer vascular and vascularized constructs for regenerative medicine and to study the interactions between vasculature and surrounding tissues [[31], [32], [33]].
Despite technological advances, constructing vascular equivalents for VTE remains challenging. VTE can generally be categorized into three areas: in vitro vascular disease models [[34], [35], [36]], tissue-engineered blood vessels [[37], [38], [39]], and vascularized tissue/organ grafts [[40], [41], [42]]. Each category has specific requirements. For in vitro models, it is crucial to recreate functional and durable vascular tissues, such as functional endothelium and smooth muscle, and accurately mimic the pathological microenvironment, including abnormal hemodynamics, inflammation, hyperlipidemia, and hypoxia [[43], [44], [45], [46]]. Tissue-engineered blood vessels must exhibit physiological functions such as anticoagulation to prevent acute thrombosis and possess mechanical properties strong enough to resist robust pressures and prevent structural failures or stenosis [[47], [48], [49]]. The primary challenge in vascularizing artificial tissues and organs lies in rapidly constructing volumetric mimics with integrated, perfusable capillary-like channels to support cell survival [50,51]. This requires using multiple biomaterials to recreate heterogeneous tissue structures and precisely fabricating built-in microchannels. Therefore, the requirements for construction technology in VTE can be summarized as: (1) providing cell-friendly processes for functional tissue formation, (2) adapting to utilize multiple biomaterials for engineering tissue mimics with heterogeneous environments, (3) enabling precise construction of complex and fine architectures, (4) achieving rapid fabrication speeds for effective manufacturing, and (5) permitting flexible control of mechanical properties to secure clinical applicability.
3D bioprinting has been widely used to fabricate vascularized tissue constructs for disease modeling [[52], [53], [54]] and tissue regeneration [[55], [56], [57]]. In particular, light-based 3D bioprinting techniques, has emerged as a groundbreaking technology in this field. The utilization of light provides multiple essential advantages, making it an ideal tool in VTE. As a controllable energy source, light can be focused into ultrafine beams or digitally manipulated to produce pre-designed projections, enabling the construction of high-resolution and extremely complex architectures [58]. Moreover, relying on photochemical reactions, this technique adapts to a wide range of photocurable biomaterials by selecting light sources with wavelengths longer than ultraviolet (UV) light (>400 nm), which are more cell-friendly. The polymerization reaction typically occurs rapidly (several seconds to minutes) [59], allowing for the quick fabrication of complex cell-laden constructs. By controlling the degree of polymerization of photosensitive monomers, the mechanical strength of the constructs can be precisely harnessed, enabling the production of robust vascular structures [60]. Furthermore, light can also enhance other 3D bioprinting technologies by overcoming limitations such as poor bioink printability in extrusion-based and inkjet-based 3D printing. Owing to these advantages, a variety of light-based 3D bioprinting strategies have been developed to fabricate in vitro vascular disease models, tissue-engineered blood vessels, and vascularized tissue/organ grafts, addressing both medical and clinical challenges. Based on the roles of light in either directing or facilitating the 3D bioprinting process, the relevant techniques could be categorized into light-dominated and light-assisted approaches.
In this review, we focus on the advances and achievements in the current field of VTE, depending on light-based 3D bioprinting technology. We introduce light-dominated and light-assisted 3D bioprinting technologies, summarize the types and selection of light-based bioinks, and discuss their applications in creating in vitro vascular disease models, vascular grafts, and vascularized tissues/organs [61] (Fig. 1). Finally, we address the current challenges and offer future perspectives on leveraging this technology to further advance VTE. This comprehensive review highlights the transformative impact of light-based 3D bioprinting in VTE. By providing detailed insights into both the technological innovations and the practical applications, we aim to showcase the potential of this advanced manufacturing approach to meet clinical demands and overcome existing limitations in VTE. As we explore these developments, we also consider the broader implications for regenerative medicine, emphasizing how light-based bioprinting can play a pivotal role in creating more effective and sustainable healthcare solutions.
Fig. 1.
Schematic overview of the review scope. This figure was created using materials from Biorender. Reproduced with permission from Ref. [61]. Copyright 2018 Springer Nature.
2. Human vascular system and physiological functions
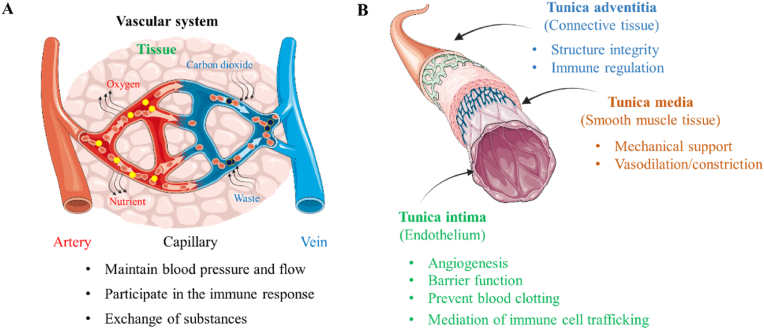
The human vascular system is comprised of three primary types of blood vessels: arteries, veins, and capillaries. Arteries and veins act as the main conduits for blood flow, analogous to highways, whereas capillaries function as smaller roads designed across the community, delivering oxygen and nutrients directly to cells in tissues and organs. Arteries, which sustain higher pressures, generally range from 3 mm to 30 mm in diameter, while veins vary from 2 mm to 25 mm. Capillaries, crucial for microcirculation, have a diameter of about 5–10 μm. The structure of these vessels is intricately designed to support their respective functions. Arteries and veins are composed of three distinct layers: the tunica intima, tunica media, and tunica adventitia. Capillaries, however, primarily consist of just the tunica intima, which simplifies the exchange of substances between the blood and surrounding tissues (Fig. 2A).
Fig. 2.
Human vascular system and functions. (A) Different types of vascular systems: arteries, veins and capillaries and the function of blood vessels. (B) The structure of each layer of the blood vessel and its corresponding function.
Central to the tunica intima is the endothelium, formed by a single layer of endothelial cells (ECs) that orchestrate several critical vascular functions (Fig. 2B). (1) Prevent blood clotting: ECs manage the coagulation system by regulating the expression of antithrombotic factors such as nitric oxide (NO), thrombomodulin, and prostacyclin, along with pro-coagulation factor binding sites on the cell surface [[62], [63], [64]]. (2) Barrier function: The intercellular junctions between ECs act as semi-permeable barriers, restricting the direct passage of large molecules while permitting small molecules, solutes, and ions to penetrate [65]. (3) Angiogenesis: Under conditions such as hypoxia, promoting the expression of genes that regulate angiogenesis, such as vascular endothelial growth factor (VEGF), which leads to the sprouting of new microvessels from endothelial cells, thereby enhancing the oxygen and nutrient supply to tissue cells [63,66,67]. This phenomenon typically occurs during the generation and repair of tissues and organs, such as in the regeneration following liver resection, as well as in disease progression, such as tumor growth. (4) Regulation of vascular tone: In response to stimuli such as angiotensin II, acetylcholine, and endothelin-1, ECs can release NO, which causes the relaxation or contraction of smooth muscle cells, thereby inducing vasodilation or vasoconstriction [68,69]. (5) Mediation of immune cell trafficking: ECs facilitate the migration of immune cells through the intercellular gaps, contributing to their localization and regulation during immune processes. Furthermore, the release of inflammatory and cellular factors can induce ECs contraction, increasing vascular permeability and facilitating the entry of immune cells into the tissue interstitium for immune responses [70,71]. Additionally, interactions between receptors on the surface of immune cells and ECs regulate the migration and activation of immune cells [66,72,73].
Located in the tunica media of arteries and veins, vascular smooth muscle cells (VSMCs) are circumferentially arranged and encased by densely packed elastic extracellular matrix (ECM) fibers composed of collagen proteins, proteoglycans, glycosaminoglycans, elastin proteins, fibronectin, laminin, and other glycoproteins [74,75]. VSMCs display multiple phenotypic characteristics in response to various physiological environments. Typically, these cells adopt a contractile phenotype in healthy blood vessels, characterized by an elongated, spindle-like morphology equipped with abundant contractile apparatus such as actin and myosin filaments and proteins. Under biochemical stimuli, such as calcium ions and angiotensin, contractile VSMCs regulate the wall pressure and diameter of blood vessels, controlling blood pressure [74]. In contrast, in response to vascular injury or pathological changes like inflammation or hyperlipidemia, VSMCs can transition to a synthetic phenotype capable of rapid proliferation and ECM deposition [76,77], thereby contributing to tissue repair, wound healing, and vascular remodeling [[78], [79], [80], [81]].
The tunica adventitia, primarily composed of loose connective tissue with collagen and fibronectin synthesized by fibroblasts, forms the outermost layer of the vessel [82]. This layer also contains progenitor cells, stem cells, pericytes, myofibroblasts, macrophages, and dendritic cells that respond to endothelial damage and undertake tasks such as immune cell transport and ECM component correction [83]. Therefore, the primary functions of the tunica adventitia include providing protection, support, and stability to the vascular structure, thereby maintaining normal vascular function and structural integrity, which has been shown in Table 1 (see Table 2).
Table 1.
The mechanical properties of natural blood vessel.
Table 2.
Light based 3D bioprinting technologies.
| Category | Technique/strategies | Explanations | Advantages | Limitations | Ref |
|---|---|---|---|---|---|
| Light-dominated 3D bioprinting | SLA | By using computer control, laser-induced photopolymerization achieves layer-by-layer controlled shaping on the resin. | high precision (25–300 μm), Rapid formation speed, | Unable to print multi-materials, Potential Damage of UV | [87,88] |
| DLP | DLP uses a digital light projector to rapidly cure entire layers of photosensitive resin. | Rapid formation speed, High printing resolution, Fabricate intricate structures, Print multi-materials, | Print volume limitation, Need support structures, minor distortions in printing, |
[89,90] | |
| VBP | The rapid volume construction of the whole structure which is realized by computer projection 3D model. | Rapid formation speed, No support structures, Wide selection of materials, Create smooth and accurate structures | Only suitable for transparent bio-inks, | [91] | |
| TPP | Using femtosecond pulsed lasers focused through a high numerical aperture objective lens to create a focal point within an uncured liquid photoresist. | Nanometer-scale manufacture, Low cell damage, | Difficult to create large-scale structures, Low printing efficiency (∼0.05 × 10−3 m2 min−1), Expensive, | [92,93] | |
| 3D bioprinting in vivo | Using specific wavelengths of light to penetrate human tissue allows for the direct bioprinting of constructs within the body. | Personalized treatment, Improve the fit of the implant, | Low photopolymerization efficiency, Light scattering limits the penetration depth, | [94] | |
| Light-assisted 3D bioprinting | pre-crosslinking printing | Crosslink the bioinks before printing. | Printing multi-material, Wide range of materials, increasing viscosity | Low printing accuracy | [95] |
| during- crosslinking printing | Crosslink the bioinks during printing. | Printing multi-material, Wide range of materials, | Low printing accuracy | [96] | |
| post- crosslinking printing | Crosslink the bioinks after printing. | Enhanced structural performance | Low printing accuracy | [97] |
Venous vessels, while structurally similar to arteries with their three-layered composition, differ significantly due to their role in guiding blood back to the heart. Since veins carry blood at a lower velocity and pressure, their walls are thinner compared to those of arteries. The middle layer of venous vessels contains fewer smooth muscle cells and elastic fibers, reflecting the lower pressure they must withstand. Of the three types of blood vessels, capillaries have the simplest structure, consisting of a single layer from endothelial cells, and lack a distinct media or outer membrane layer. The structure allows capillaries to facilitate the exchange of substances between blood and surrounding tissues [98]. For instance, capillaries lack the multi-layered structure characteristic of arteries and exhibit significant heterogeneity, including continuous, fenestrated, and discontinuous types, each demonstrating distinct barrier functions and mechanisms for substance passage through the vessel wall. Additionally, organ-specific endothelial subtype heterogeneity is also pertinent; for example, continuous capillaries in the brain feature tight junctions that restrict permeability, whereas discontinuous capillaries in the liver facilitate the exchange of nutrients and oxygen.
The function of the vascular system is achieved through the tight coordination between the three-layered structure of cells and the ECM in blood vessels. Interactions among vascular cells occur through mechanisms like paracrine signaling (involving the release of growth factors and cytokines), mechanotransducive signaling (involving shear stress and wall pressure), direct cell-cell contact (which synchronizes tissue repair or vascular tone regulation), and dynamic interactions with the ECM (involving degradation and synthesis) [[99], [100], [101]]. Understanding these functions and interactions between each vascular tissue component is crucial for engineering both physiological and pathological vascular equivalents. For instance, during the development of atherosclerosis, abnormal hemodynamics (e.g., turbulent flow, low-oscillating shear stress) or pro-inflammatory cytokines (e.g., tumor necrosis factor-alpha (TNF-alpha), interleukin-1L) can activate ECs, leading to the secretion of factors (e.g., transforming growth factor-beta, platelet-derived growth factor) that induce VSMC phenotypic transitions and relevant ECM remodeling [102,103]. This activation can decrease NO production, leading to increased vascular tone. Successfully replicating these complex interactions and environments is crucial for designing effective vascular grafts and in vitro disease models.
This integrated approach to understanding the vascular system not only highlights the complexity of the interactions at play but also the precise control required to maintain vascular health and function. Each layer of the vascular structure plays a specific role in responding to both physiological cues and pathological challenges. By harnessing this knowledge, researchers and clinicians can better design therapeutic strategies that address the underlying mechanisms of vascular diseases, leading to more effective treatments and improved patient outcomes. The ongoing exploration of these dynamic systems holds the potential to unlock new frontiers in VTE and regenerative medicine, paving the way for innovative solutions to some of the most pressing health issues related to the human vascular system.
3. Light-based 3D bioprinting technology
Advancing from conceptual designs to actual fabrications of vascular constructs for VTE presents significant technical hurdles, particularly when constructing complex anatomical structures, achieving precise mechanical properties, and integrating vascularized networks with multiple materials. Traditional methods often fall short in these areas, which critically limits the development of functional vascular grafts, accurate pathophysiological models, and fully vascularized tissues. Light-based 3D bioprinting technology, by harnessing the potential of high-energy and multi-bandwidth light, offers a sophisticated approach to construct high-resolution and complex structures, substantially advancing the field of VTE through both technological and material innovations. Based on whether light is directly involved in the printing process, we divided the light-based 3D bioprinting technology into light-dominated and light-assisted strategies.
3.1. Light-dominated 3D bioprinting
In the realm of tissue engineering, light-dominated 3D bioprinting has emerged as a crucial technique across various applications. By directly illuminating the photocurable bioink to build biological constructs, this technology facilitates rapid manufacturing, achieves microscale to nanoscale precision, and supports the formation of complex geometrical shapes using a diverse array of materials. Several representative light-dominated biofabrication techniques are pertinent to VTE, including stereolithography (SLA), digital Light Processing (DLP), volumetric bioprinting (VBP), two-photon polymerization (TPP), and printing in vivo will be explored.
3.1.1. Stereolithography
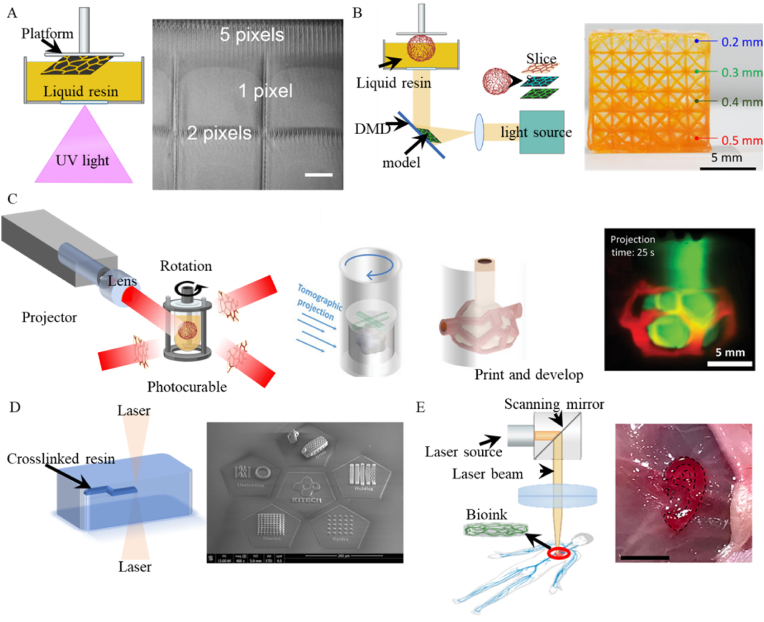
Pioneered by Hull and Arcadia in 1985 [104], SLA operates primarily through a computer-controlled laser that precisely cures liquid resin [105]. The process initiates with the model being horizontally sliced using 3D slicing software. The laser then traces the first layer, solidifying the resin from points to lines, thereby forming a complete cross-section. Subsequent layers are similarly crosslinked, each one building upon the last, until the programmed 3D structure is fully realized [106,107]. Known for its high precision, typically within 25–300 μm [108] (Fig. 3A), SLA features a rapid formation speed and produces surfaces with exceptional smoothness due to the continuous nature of the laser photopolymerization. Employing UV light, which has a shorter wavelength, SLA achieves the necessary high resolution and deep penetration for printing complex structures, although the high energy of UV light also poses challenges such as potential DNA damage to cells [94], necessitating post-process cell seeding.
Fig. 3.
Light-dominated 3D bioprinting techniques. (A) Schematic of SLA and the 3D structure made by SLA (scale bar 200 μm). Reproduced with permission from Ref. [108]. Copyright 2015 IOP Publishing Ltd. (B) Schematic of DLP and its applications. Reproduced with permission from Ref. [109]. Copyright 2021, The American Association. (C) Schematic of VBP and the vascular models made by VBP. Reproduced with permission from Ref. [110]. Copyright 2022 Wiley‐VCH. (D) Schematic of TPP and the SEM images of 3D microstructures made by TPP. Reproduced with permission from Ref. [111]. Copyright 2023 Springer Nature. (E). Schematic of printing in vivo and the ear models made by bioprinting in vivo. Reproduced with permission from Ref. [94]. Copyright 2020, The American Association.
3.1.2. Digital light processing
DLP technology, in contrast to SLA which employs point-by-point raster scanning with laser beams, utilizes two-dimensional photopolymerization. This process involves projecting the two-dimensional image of a desired pattern onto a photosensitive material using a Digital Micromirror Device (DMD) or Liquid Crystal Display [112,113]. This method significantly accelerates the production of complex structures, leveraging the high stability and fidelity of pattern reproduction enabled by the DMD. DLP achieves micrometer-level resolution and fine accuracy by exposing each layer of the sliced model to a broad plane of curing light, greatly enhancing printing speed compared to the point exposure used in SLA [114,115].
Despite its advantages, DLP's computational slicing process can lead to minor discrepancies between the intended and actual printed shapes [116]. This limitation, primarily due to light diffraction, often results in slight distortions of the final three-dimensional structures [117]. To address these issues of shape fidelity, researchers have implemented strategies such as reducing the layer height to mitigate the staircase effect, thus enhancing precision but at the cost of increased manufacturing time [118]. Additionally, enhancing DLP resolution through the rapid oscillation of micromirrors introduces grayscale modulation between off and on states, refining the detail captured in each layer. Johnson et al. demonstrated that using grayscale adjustments could maintain shape fidelity more effectively than simply reducing layer height, achieving accuracy within ±5 % of the desired dimensions [119].
Additionally, incorporating extra light absorbers into the photoinitiator system is another method to improve printing resolution. In DLP, the z-axis resolution is determined by the transparency of the printed structure. By adding additional light absorbers to the photoinitiator system, the light transmittance can be effectively reduced, thereby enhancing the resolution of the print [114,120]. He et al. [121] chemically modified curcumin with sodium bicarbonate to obtain a photoreaction inhibitor, which enabled full-pixel printing of gelatin methacryloyl (GelMA) using DLP. This approach resulted in a threefold increase in cell viability compared to pure GelMA, and successfully achieved the construction of high-fidelity, complex patterns. However, the addition of extra photoinitiators does not participate in the photopolymerization reaction, which can decrease the efficiency of the polymerization process, increase costs, and potentially lead to incomplete crosslinking of the structure.
The high-resolution capabilities of DLP are particularly beneficial for constructing complex biological structures. However, in the context of VTE, fabricating biomimetic blood vessels often requires the development of multi-layered, complex structures. Thus, advancing multi-material DLP technology has become essential. Ge et al. successfully utilized DLP to print composite structures by bonding acrylamide Poly (ethylene glycol) diacrylate (PEGDA) with various UV-curable polymers, which were then used to construct vascular stents, menisci, and sensors (Fig. 3B) [109]. Similarly, Yang et al. achieved significant advances by using DLP to print multi-component bioinks loaded with cells, enabling the fabrication of hollow channels suited for vascular applications [122]. These developments signify a substantial progression towards the construction of multi-layered vessels and the capability to create highly functional vascularized tissue and organ constructs.
Hence, the DLP technology, with its rapid fabrication capabilities and high-resolution outputs, holds substantial promise for the field of VTE. The ongoing advancements in multi-material printing and precision control continue to expand the potential applications of DLP, setting a foundation for future innovations in the creation of detailed, functional vascular tissues.
3.1.3. Volumetric bioprinting
While DLP technology has significantly improved fabrication efficiency over SLA, especially in terms of speed, the process of building layers sequentially can still be optimized, particularly when printing large volume constructs with complex structures. Volumetric printing addresses this need by enabling integrated three-dimensional forming, which drastically reduces manufacturing times and enhances efficiency [123]. First proposed by Levato's team in 2019 [124] and subsequently developed into Computed Axial Lithography (CAL) by Hayden K. Taylor's group in 2019 [125], VBP generates 3D models of target objects through computer simulations. Based on CT principles, it projects 2D slices of the 3D object onto a rotating print container. This enables the photocurable ink within the container to solidify from all directions simultaneously, allowing for the direct construction of the target 3D object in a single step. This method allows for rapid cross-linking of the entire volume simultaneously, thereby constructing the desired structure in a significantly shorter timeframe. The advent of CAL has been instrumental in advancing the capabilities of VBP, facilitating the production of structures with complex geometric shapes directly from digital models. Unlike traditional methods, VBP does not require support structures, simplifying the vascular construction process by reducing complexity and potential interference with the bioink's properties. Moreover, VBP's unique approach to simultaneous light curing during the construction process helps avoid the minor distortions typically associated with point-by-point or layer-by-layer fabrication.
A significant advantage of VBP is its ability to eliminate "stair-stepping" errors common in traditional additive manufacturing, allowing for the creation of smoother and more accurate structures. Enhancements to VBP technology have led to increased precision and complexity in manufactured structures. For instance, Loterie et al. [126] have improved the precision and speed of VBP by incorporating a feedback system. With this enhancement, they were able to achieve the fabrication of structures with 80 μm positive and 500 μm negative features within 30 s. In pursuit of further enhancing the printing resolution, Regely et al. [127] extended upon CAL by introducing feedback optimization, thus proposing the xylography technique. Through optimization via feedback systems, this technique achieves a tenfold improvement in resolution compared to CAL.
The rapid fabrication capabilities of VBP are also noteworthy, with the technology capable of completing entire layers in a single rotation cycle of the beam. This efficiency is evidenced in the production of complex human tissue models, such as living heart and meniscus structures, which were constructed in mere 45 s and 30 s, respectively [128]. The ear model constructed by Bernal et al. using volumetric bioprinting can be printed and completed within 22.7 s, far quicker than the hours typically required by other methods [124].
In recent years, the development of multi-material printing within VBP technology has been increasingly reported. Bernal et al. [129] designed a resin composed of gelatin and methacrylate to address the issue of light scattering caused by high cell concentrations. This multi-material composite resin enables volumetric printing with higher cell density, which they utilized to construct liver tissues with cellular activity and specific functionality. Similarly, Chansoria et al. [110] employed a specialized algorithm to achieve rapid multi-material construction of complex shapes. By integrating finite cell modeling within the algorithmic design framework and combining it with volumetric bioprinting, it is possible to manufacture complex structures such as bifurcated blood vessels and porous scaffolds (Fig. 3C). Another innovative approach to creating complex multi-material structures involves integrating extrusion-based 3D bioprinting with VBP [130]. This method facilitates the rapid creation of intricate structures in free form, incorporating multi-material and multi-cellular features. Such capabilities significantly enhance the applicability and versatility of volumetric bioprinting.
Additionally, VBP technology theoretically allows for the use of materials with extremely high/low viscosity since there is no need for point-by-point or layer-by-layer deposition to ensure its shape, which expands the range of material choices and possibilities in the VBP printing process. The decellularized extracellular matrix (dECM) used to manufacture heart models concentrations only 1 %, which was greatly lower than it used in extrusion 3D bioprinting [128]. Größbacher et al. [131] innovatively combined VBP and melt electrospinning writing (MEW) to create tubular structures with enhanced mechanical properties. Remarkably, despite the incorporation of opaque materials through MEW, the subsequent VBP process successfully produced the desired tubular structures. This breakthrough opens exciting possibilities for future VBP applications using opaque materials. Despite its groundbreaking potential and rapid adoption across various fields, including VTE, VBP faces certain limitations. The requirement for transparent bioinks, due to the nature of the light-based process, restricts the range of useable materials. Efforts to adapt VBP for use with non-transparent materials are ongoing and involve correcting for light scatter [132].
Additionally, the scale of objects producible by VBP is generally limited to the centimeter scale due to constraints in light intensity and penetration, presenting challenges that need to be addressed to fully realize the technology's potential in clinical applications [133].
3.1.4. Two-photon polymerization
While SLA, DLP, and VBP enhance precision and speed in 3D printing, they struggle to achieve nanometer-scale manufacturing accuracy. TPP stands out in this regard, offering the capability to fabricate structures with both arbitrary complexity and nanoscale precision, making it the most accurate of all 3D printing techniques [134]. TPP operates on the fundamental principle of using femtosecond pulsed lasers focused through a high numerical aperture objective lens to create a focal point within an uncured liquid photoresist. The incredibly short duration of these laser pulses, measured in femtoseconds, ensures that the region of light polymerization is exceedingly small, facilitating the achievement of nanoscale printing resolution [135,111] (Fig. 3D). In addition, TPP can get rid of the traditional process of layer by layer, which provides convenience for the construction of VTE.
In addition to constructing high-precision structures through printing methods, light can also be used in a subtractive way to fabricate high-precision structures in TPP. This approach typically involves using a focused laser to remove material within the hydrogel. For example, Brandenberg et al. [136] utilized photoablation to directly create micron-scale microfluidic channels within polyethylene glycol hydrogels. This method effectively reduces the complexity associated with conventional microchannel fabrication processes, while ensuring high precision and feasibility in the creation of complex structures. Heintz et al. [137] utilized laser-induced degradation to employ PEGDA hydrogels for photolithography, successfully fabricating microchannels on the scale of 3–8 μm. This advancement paves the way for the construction of vascularized tissues.
Moreover, the short duration of laser exposure in TPP minimizes thermal damage to cells, a critical advantage when working with live cells or sensitive biomaterials. This feature is particularly beneficial compared to UV-based DLP and SLA technologies, which can compromise cell viability due to longer exposure times and higher energy levels associated with UV light. The ability of TPP to preserve cell viability is underscored by studies like those conducted by Fei et al., who utilized TPP to construct three-dimensional microchannel networks [138,139]. This enabled dynamic analysis of molecules, subcellular structures, and cellular processes at the individual cell level, providing valuable insights into cellular behaviors within engineered tissues.
Furthermore, TPP's flexibility in using a variety of materials allows for the creation of diverse biological tissues and organ structures, expanding the possibilities for customized tissue engineering solutions. Despite its advanced capabilities, TPP's application is somewhat limited by the typically small build sizes it can produce, which restricts the rapid fabrication of large-volume tissues or organs. Additionally, the high cost of the specialized equipment and materials necessary for TPP poses a barrier to its widespread adoption in routine clinical or laboratory settings.
Overall, TPP represents a significant leap forward in the field of 3D bioprinting, especially for applications requiring ultra-high resolution and precision. Its development continues to open new avenues for the construction of highly detailed vascularized tissues and complex biomedical devices, potentially transforming approaches to tissue engineering and regenerative medicine.
3.1.5. 3D bioprinting in vivo
In addition to constructing complex biological structures in an ex vivo environment, light-based 3D bioprinting also extends to in situ or in vivo biofabrication, showcasing a pivotal advancement in medical technology. The ability of specific wavelengths of light to penetrate human tissue allows for the direct bioprinting of constructs within the body, paving a new way for clinical applications of 3D bioprinting [140]. This capability significantly enhances the potential for personalized medicine, enabling the precise placement of bioprinted tissues at targeted locations.
Despite the promising capabilities of printing in vivo, challenges related to the optical properties of human tissues remain. One such challenge is the scattering effect of light, which limits the penetration depth, particularly when using light of shorter wavelengths. This limitation has been circumvented in the work by Chen et al., who utilized near-infrared (NIR) light-dominated 3D printing to demonstrate the feasibility of printing in vivo [94] (Fig. 3E). The shape is constructed using DMD for printing, and then it is delivered inside the body through infrared radiation penetrating the skin, photopolymerization by nanoscale initiators. Similarly, Elvassore's team achieved in situ printing in mice by employing bio-orthogonal two-photon cycloaddition and crosslinking of polymers at wavelengths longer than 850 nm [141]. This approach avoids the generation of by-products and allows for precise localization through real-time observation with multiphoton microscopy, thereby ensuring accuracy in in vivo printing. Subsequent results demonstrated no adverse effects on the mice, and muscle regeneration at the printed sites was observed. Although their research confirmed the potential for conducting non-invasive surgical procedures through this technology, it also underscored the need for further development to overcome inherent limitations.
A critical aspect of advancing in vivo bioprinting involves enhancing the penetration capabilities of NIR light while maintaining efficient photopolymerization. The low energy characteristic of NIR light, while beneficial for deeper tissue penetration, results in reduced photopolymerization efficiency, posing a significant challenge [94]. Selecting and optimizing light sources that effectively balance photopolymerization efficiency with adequate tissue penetration depth is crucial for the practical application of this technology in medical procedures.
Moreover, safety concerns associated with printing in vivo also warrant thorough investigation. Specifically, the potential for residual monomers, which may remain after light-induced crosslinking polymerization, poses a risk to patient safety. Ensuring that these monomers do not cause harm to the human body is paramount, requiring rigorous testing and validation of bioprinting materials and methods used within clinical settings.
While bioprinting in vivo presents transformative potential for non-invasive and personalized medical treatments, its clinical adoption depends on overcoming significant technical challenges and ensuring the safety and efficacy of the bioprinted constructs. Continued research and development in this field are essential to unlock the full potential of bioprinting in vivo for future medical applications.
3.2. Light-assisted 3D bioprinting
Light serves a crucial role not just as a direct sculptor of structures but more as an enhancer and facilitator of the bioprinting process. This method differs markedly from traditional approaches like extrusion-based and inkjet-based bioprinting, which, although widely employed for constructing tissue or organ equivalents due to their high resolution and integrated forming capabilities, struggle with the use of protein-based natural hydrogels. These bioinks offer exceptional biocompatibility but present substantial challenges in terms of printability, shape fidelity, and mechanical strength, which significantly limit the construction of complex tissue architectures. Light-assisted strategies leverage light-induced polymerization to modify the rheological properties and mechanical strengths of bioinks on-demand, thereby overcoming some of the inherent limitations of conventional bioprinting methods. According to the stage that light is introduced, the light-assisted 3D bioprinting can be divided into pre-crosslinking printing, during-crosslinking printing, and post-crosslinking printing strategies (Fig. 4).
Fig. 4.
Light-assisted 3D bioprinting strategies. (A) (ⅰ) Schematic of pre-crosslinking printing of light-assisted 3D bioprinting. (ⅱ) The extrusion picture of pre-crosslinking material and printed structures. Reproduced with permission from Ref. [142]. Copyright 2024 Elsevier. (B) (ⅰ) Schematic of during-crosslinking printing. (ⅱ) The extrusion picture of during-crosslinking printing and the printed structures. Reproduced with permission from Ref. [96]. Copyright 2016 WILEY‐VCH. (C) (ⅰ) Schematic of post-crosslinking printing. (ⅱ) The bioprinted high strength tubes with various shapes. (ⅲ) Compressive modulus of bioinks after exposure to UV. Reproduced with permission from Ref. [97]. Copyright 2016 Elsevier.
Soft hydrogels, with their lower modulus, provide an environment conducive to cell survival, promoting cell spreading and migration. However, their poor shape fidelity poses significant challenges for precise printing and maintaining structure during the layer-by-layer manufacturing process. Photopolymerization, induced by light, can modify the viscosity and modulus of these bioinks before printing, enabling the creation of complex structures using soft materials. The challenge of fast polymerization kinetics and varying curing rates under different light conditions requires precise control to ensure successful outcomes. In a recent report, Agostinacchio et al. [142] proposed a method of UV pre-crosslinking of lap-mixed silk fibroin protein for in-situ 3D printing. This pre-crosslinking process only takes 40 s to complete (Fig. 4A). By using light-induced pre-crosslinking to increase the viscosity, this approach avoids nozzle clogging issues associated with enzymatic crosslinking of silk fibroin protein while achieving rapid crosslinking.
In addition to increasing viscosity, another advantage of pre-crosslinking technology is the ability to improve printing speed by precisely controlling the exposure dosage during the pre-crosslinking process. Despite the rapid manufacturing capabilities of DLP, the use of thinner slices can increase the overall printing time. Light crosslinking of bioinks in DLP can extend the fabrication time, leading to inefficiency. An effective method to enhance DLP printing efficiency is through pre-crosslinking of the bioink, which has been shown by Li et al. to improve printing speed. They achieved pre-crosslinking of GelMA without significant alteration in its rheological properties, ensuring the enhanced performance of GelMA in the printing process [95]. This approach eliminates the need for repetitive up-and-down movements during DLP printing, resulting in a 17-fold increase in printing efficiency.
Following the printing process, light-assisted techniques can also be employed to post-process and enhance the mechanical properties and structural stability of the fabricated constructs. Maintaining the fidelity of the printed shape is crucial for successful fabrication. Although various strategies have been proposed to preserve shape, such as temperature or ion-based approaches [143,144], these methods generally have limitations. Temperature-sensitive crosslinking requires a certain amount of time, during which the shape of the construct may change. Additionally, some temperature-sensitive crosslinking agents can potentially affect cell viability. Ion-based crosslinking methods can rapidly preserve shape but may introduce sacrificial materials that need to be removed afterward and can potentially damage cells. To overcome these limitations and minimize potential damage, strategies involving light-induced crosslinking have been proposed in bioprinting.
Numerous studies have demonstrated that using light for crosslinking during printing enables rapid shape fidelity [145]. Moreover, due to the fast-crosslinking speed (within a few seconds), even with exposure to UV light, cell viability can be maintained at over 90 % [146]. Ouyang et al. developed a method utilizing light penetration through photopermeable capillaries, establishing a relationship between capillary length, ink velocity, and polymerization time to achieve highly controllable ink cross-linking independent of viscosity [96] (Fig. 4B). This method facilitated the construction of lattices, hollow tubes, and macroscale tissue constructs, ensuring consistency across different materials while maintaining cell viability above 90 %. In addition to its rapid crosslinking capabilities, the in situ curing characteristic of this printing strategy makes it suitable for in vivo tissue repair. Li et al. recently achieved successful simulates the ossification center microenvironment by performing in situ light-induced crosslinking using bioink [145].
Existing hydrogels focus more on the biocompatibility properties of the material, which leads to problems of long-term stability and insufficient mechanical properties. Some strategies of shape construction through ion cross-linking still have the problem of shape dissipation in the later stage. Gao et al. [147] used a combination of dECM and alginate to construct biomimetic artificial blood vessels with excellent biocompatibility, allowing cells to form tissue and exhibit corresponding functionalities. However, this construct suffers from poor mechanical properties, making them unable to withstand blood vessel pressures and leading to vessel rupture. But the vascular constructs with high mechanical often exhibit suboptimal biocompatibility. To address these issues, light-assisted bioprinting techniques have been proposed by incorporating light into the aforementioned technologies. By applying light-induced crosslinking after the printing process, the mechanical properties, structural stability, and functionality of the printed constructs can be significantly improved. Jia et al. [97] utilized GelMA, PEGDA, and alginate to fabricate multi-layered perfusable vessels using a coaxial extrusion 3D bioprinting method. The initial shape stabilization was achieved through the ion crosslinking of alginate, while the subsequent light curing of GelMA and PEGDA ensured long-term stability and excellent mechanical strength of the multi-layered vessels (Fig. 4C). This combined approach not only maintained the structural integrity of the vessels under physiological conditions but also prevented the degradation of calcium alginate during long-term cultivation, providing a durable framework for vascular applications.
3.3. Photocurable bioinks
Photocurable bioinks, which contain photoinitiators, enable photopolymerization, transforming from a liquid to a solid structure upon exposure to UV or visible light. These bioinks consist of biocompatible biomaterials, known for promoting cell function and supporting the formation of mature organ tissues. Over decades, remarkable advancements in tissue engineering have centered on these biomaterials, which include hydrogels or bioinks known for their mild crosslinking capabilities, excellent biocompatibility, and tunable biochemical and biophysical properties [[148], [149], [150]]. Photocurable hydrogels, which operate by being exposed to light to perform functions required for various biomedical applications, have become integral in constructing VTE frameworks.
3.3.1. The crosslinking mechanism of photo-active bioink
The efficacy of photo-crosslinkable bioinks hinges on the presence of photoinitiators, compounds that absorb specific wavelengths of energy within the UV or visible light spectrum, thereby generating radicals or cations that initiate the polymerization and crosslinking of monomers. Photoinitiators are classified based on their mechanism into free-radical, cationic, and anionic photoinitiators. Free-radical photoinitiators can be further divided into Type I and Type II. Type I photoinitiators, like Lap and Irgacure 1173 [151,152], function by absorbing photons and undergoing self-cleavage to produce two free radicals. Conversely, Type II photoinitiators, such as Eosin-Y and camphorquinone [153], absorb energy under light exposure and abstract hydrogen ions from other compounds to generate free radicals. In contrast, cationic and anionic photoinitiators, upon light exposure, absorb energy and transition to an excited state. In this state, they can undergo photochemical reactions to generate cations or anions, respectively, and initiate chain polymerization reactions.
Various bioink systems may require specific types of photoinitiators to achieve optimal results. The choice of photoinitiators affects not just the curing speed and efficiency but also the mechanical properties and structural integrity of the final printed structure. Thus, understanding and selecting the appropriate photoinitiators are pivotal in advancing the applications of photocurable bioinks in tissue engineering, ensuring that the printed structures not only support cellular activities but also mimic the mechanical and biochemical cues of the native tissue environment.
The selection of a photoinitiators is critical and should consider factors such as absorption spectra, radical generation capability, stability, as well as the cytotoxicity potential towards cells. The efficiency of various photoinitiators differs based on their absorption principles and the wavelengths of light they absorb. For instance, photoinitiators like Lap and 1173 achieve higher efficiency under UV light conditions compared to when visible light is used for photo-crosslinking reactions. For rapid construction and ensuring shape fidelity in VTE, selecting a fast-acting photoinitiator system, such as the ruthenium (Ru)/sodium persulfate (SPS) system, is essential. Additionally, different bioink systems may require specific photoinitiator materials to achieve optimal results. Kim et al. [154] used RU/SPS to cross-linking dECM, which can be achieved in 5 s.
The table below presents commonly applied photoinitiators and their suitable photocurable bioink systems for light-based 3D bioprinting technique (Table 3).
Table 3.
Commercial photoinitiator and photocurable bioinks for 3D bioprinting.
| photoinitiator | λ(nm) | Bioinks | Technologies | Ref |
|---|---|---|---|---|
| Lithium phenyl-2,4,6-trimethylbenzoylphosphinate (LAP) | 365 | N-acryloyl glycinamide (NAGA)/nanoclay/GelMA | Post-bioprinting, SLA, VBP | [155] |
| Geltain/GelMA | [156] | |||
| GelMA/HAMA | [157] | |||
| GelMA | [129,130] | |||
| Irgacure 1173 | 365 | NAGA/poly(N-[tris(hydroxymethyl)methyl] acrylamide) | Post-bioprinting | [158] |
| RU/SPS | 450 | dECM | Post-bioprinting | [159] |
| Eosin-Y | LED tube | GelMA/poly (thioctic acid)/poly (acrylic acid)/amorphous calcium/phosphate | DLP, Post-bioprinting, SLA, TPP | [160] |
| 520 520 |
GelMA | [161] | ||
| PEGDA | [137,162] | |||
| Irgacure 2959 | 365 | GelMA/Hyaluronic Acid Methacrylate | During-bioprinting | [163] |
| PEGDA | [164] | |||
| Riboflavin | 400–500 | 2-hydroxyethyl methacrylate | Post-bioprinting | [165] |
| 4-(4,6-dimethoxy-1,3,5-triazin-2-yl)-4-methylmorpholinium chloride | 505 | hyaluronan | Post-bioprinting | [166] |
| up-conversion nanoparticle/lap | 980 | GelMA | 3D printing in vivo | [94] |
3.3.2. The selection of photocurable bioinks for VTE
For VTE, the selection of appropriate photocurable bioinks is critical due to the unique requirements of simulating the complex structure and function of natural blood vessels. Essential characteristics for these bioinks include biocompatibility, conducive cell interaction properties, structural stability, controlled degradation rates, and sufficient mechanical performance. In addition, bioinks utilized in light-based bioprinting technologies must meet specific criteria to facilitate the printing process effectively. These criteria include optimal viscosity for consistent extrusion and smooth flow through the printer nozzles when applied for light-assisted bioprinting strategies, excellent light transmittance to ensure effective depth and uniformity of light-induced cross-linking, and rapid photo-curing capabilities to maintain structural integrity during the construction process.
Despite extensive research and development, currently no single bioink formulation perfectly meets all these criteria. Therefore, the selection process often involves a strategic balance of trade-offs based on the specific functional requirements of the intended vascular application. For constructing microchannel structures, which are integral to nutrient and blood mimicry in tissue models, the focus often shifts toward bioinks that can achieve precise, long-term stability and support intricate architectural details. In such applications, it's common to introduce ECs post-printing, necessitating a bioink that not only supports high-resolution printing but also promotes cell adhesion and growth. Duda's team utilized SLA to achieve an organic integration of biofunctionality and printability by combining hyaluronic acid methacrylate and GelMA [157]. This approach allows for a precision of up to 100 μm. The inclusion of GelMA provides excellent biocompatibility, enabling ECs to grow well within the channels and achieve endothelialization.
In the context of vascular disease models or organ-on-chip systems, where functional vascularization is crucial, the priority shifts towards ensuring bioink biocompatibility and minimal cytotoxicity. These properties are essential to facilitate cell spreading, differentiation, and the expression of critical cellular functions within the engineered tissues. For instance, bioinks incorporating photoinitiators like RU/SPS within dECM materials enable rapid crosslinking in mere seconds, optimizing the fabrication process while preserving cell viability [159]. GelMA, an FDA approved material noted for its biocompatibility, is versatile for use with a variety of photoinitiators such as Irgacure 2959, lap, Eosin Y, and riboflavin, enhancing its application across different light-based bioprinting platforms [167,168].
Conversely, for applications requiring the construction of small-diameter blood vessels intended for implantation, where high mechanical strength is crucial to withstand physiological pressures, the selection criteria extend beyond biocompatibility to include robust mechanical properties. Materials like NAGA and PEGDA are often favored for their high mechanical strength. These are frequently combined with bioactive hydrogels like GelMA to enhance the biological functionality of the constructs. An illustrative example is the work by Ruan's team, which utilized a mixture of NAGA, nanoclay, and GelMA, achieving UV crosslinking with Lap to fabricate mechanically robust yet biologically active vascular constructs [155]. However, UV light is highly toxic to cells, particularly when prolonged exposure is required to achieve high mechanical performance. Therefore, using visible light for crosslinking is one approach to mitigate cell damage. Wang et al. [161] first proposed the use of Eosin Y for visible light (520 nm) crosslinking of GelMA ink loaded with cells. Remarkable mechanical properties (800 kPa) were achieved while ensuring cell viability. Additionally, different types or concentrations of bioinks may be chosen for different layers of the blood vessel to meet the specific growth requirements of different cell types.
The emergence of multi-material printing technologies has further broadened the possibilities for selecting and combining bioinks. This approach allows for the simultaneous use of multiple materials, each selected for their superior properties to meet the complex demands of VTE. A recent innovation involves the creation of double network hydrogels combining hyaluronic acid with poly propionamide, enhancing mechanical properties while maintaining high biocompatibility [169]. This method exemplifies how contemporary bioprinting techniques are advancing towards fabricating bioinks that effectively balance mechanical integrity with biological performance.
In short summary, the selection of photocurable bioinks for VTE is a nuanced process, dictated by the specific application. For disease models and vascularized systems, biocompatibility and low cytotoxicity are paramount to promote the tissue formation and functionalization. For implantable vascular constructs, the ability to reinforce the mechanical property and mimic the structural complexity of native vessels, as well as supporting varied cellular environments is crucial. Tailoring bioink compositions to match the mechanical, biological, and functional demands of different vascular applications is key to the success of VTE in both clinical and research settings.
4. Applications of light-based 3D bioprinting in vascular tissue engineering
Light-based 3D bioprinting techniques, due to their high biofabrication resolution, ability of balancing biological and physical properties to construct complex structures, and the flexibility of synergistically printing multiple materials, have revolutionized VTE. These advancements have facilitated the development of in vitro vascular disease models, implantable vascular grafts, and vascularized tissues/organ constructs.
4.1. In vitro vascular disease model
Vascular diseases, particularly cancer, cardiovascular diseases, neurodegenerative diseases, and immunological disease, along with their complications, account for over 50 % of global non-war-related deaths, posing a significant threat to human life. Despite ongoing research, the underlying mechanisms of most of these diseases remain incompletely understood, requesting novel platforms to overcome the limitations of the currently prevalent animal models and planar cell-culture models [170]. The in vitro disease models emerged as one of the most promising tools for understanding the pathology, by emulating the 3D microenvironments presenting in disease nidus using human cells. To recapitulate the key features of relevant diseases, the in vitro vascular disease models must mimic the composition, structure, and functionality of natural blood vessels, necessitating high cellular viability during the printing process, biocompatibility of the bioink, and excellent printability.
4.1.1. Light-dominated 3D bioprinting of vascular disease model
For the construction of models for certain vascular diseases, traditional 3D printing techniques such as filament-based printing and coaxial printing may not be capable of creating complex models. This requires the achievement of high precision and complex model construction. SLA and DLP have made contributions in this regard. Kaufmann et al. [171] utilized SLA to construct vascular models with specific structures for different patients, such as aneurysms. These models aid clinical practitioners in preoperative planning and simulation of surgical procedures. Guarino et al. [172] utilized DLP to construct intracranial aneurysm models for clinical anatomy to understand their structures. Existing aneurysm models primarily consist of cell-free resin, and by adding a solution containing fluorescent particles, the hemodynamics of the aneurysm can be simulated. Subsequent research can involve seeding cells in the model to achieve endothelialization, enabling the study of the pathophysiological mechanisms under the influence of blood flow dynamics and cellular interactions.
In diseases involving tumor migration, ischemic conditions, inflammation, diabetes, retinal disorders, and other conditions directly involving capillaries, the structure, morphology, and density of micro-vessels are directly linked to the occurrence and progression of the diseases. Therefore, the reconstruction of microvascular networks that possess physiological or pathological characteristics is essential for investigating their pathological mechanisms. Achieving this necessitates the high-precision construction of the structural and morphological features of target blood vessels. Technologies like SLA, DLP, and TPP have enabled the construction of vascular models with micron-scale precision, that resemble the dimensions of human capillary. For example, Qian et al. demonstrated the exceptional resolution of TPP by constructing microchannels on a microfluidic chip designed to mimic microvasculature, successfully fabricating channels with widths of just 10 μm and 20 μm [118]. These channels were then utilized to study the movement and metastatic abilities of tumor cells, illustrating TPP's potential for precise biomedical applications. These achievements demonstrated the capacity of light-based 3D bioprinting for constructing the pre-designed capillary-like microchannel networks built-in bulk hydrogel that mimics the ECM of tissues and organs. By seeding ECs onto the luminal surface of these interconnected channels, functionalized microvessels could be obtained [173], which resembles the healthy or abnormal capillary associated with relevant disorders, facilitating the revealing of underlying pathology.
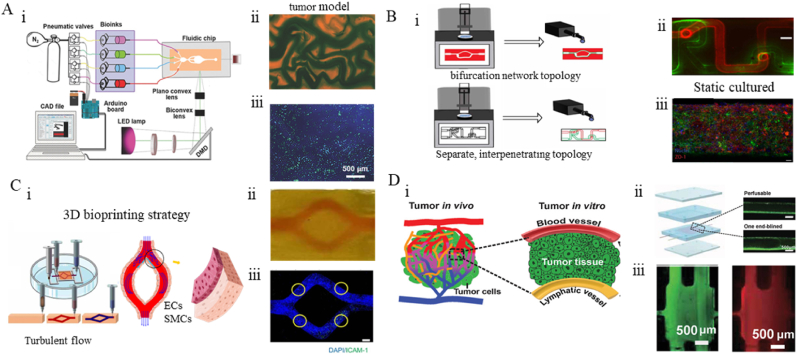
While the light-dominated 3D bioprinting exhibits unparalleled capacity for constructing high-precision construction vasculatures, the pathological models like cancer require not only the presence of blood vessels but also the surrounding tissues that might involve multiple types of cells. Light-based bioprinting excels in precisely controlling the positioning and proportions of different materials, enabling the fabrication of complex vascularized tissue structures to replicate the scenarios found in relevant disease. For instance, blood vessels are crucial for tumor cell metastasis and drug delivery. Investigating transport dynamics in tumor models with blood circulation systems is essential for revealing the pathological events and accurate drug screening. To establish the vascularized tumor models, Zhu et al. [174] utilized DLP technology to construct microvascular models of the liver with blood vessel channel widths ranging from 50 to 250 μm. They successfully achieved integration with the host vasculature. This tumor model, constructed using co-culture of multiple cell types, can serve as a platform for personalized drug screening. Miri et al. [175] combined microfluidic technology with DLP to fabricate a microvascular model of breast cancer with a resolution ranging from 20 to 30 μm. They utilized ECs implantation to simulate the generation of cancer (Fig. 5A). This model provides a valuable tool for investigating the mechanisms of tumor angiogenesis, tumor cell extravasation, and the effects of the tumor microenvironment on cancer behavior. Cui et al. [176] employed SLA to construct a three-dimensional cultured metastasis model to investigate the invasion of breast cancer cells into vascularized bone tissue. The cancer model successfully simulated the metastasis of breast cancer and its colonization on bone tissue. This metastasis model offers a valuable platform for studying the complex process of cancer spread and invasion. It enables researchers to investigate the interactions between cancer cells and the surrounding tissues, providing a deeper understanding of the molecular and cellular events involved in cancer metastasis. Such models contribute to the development of novel therapeutic strategies aimed at preventing or targeting the metastatic spread of cancer.
Fig. 5.
The in vitro vascular disease model constructed by light-based 3D bioprinting. (A) The tumor model made by DLP. (ⅰ) schematics of the setup of the tumor model. (ⅱ) Photographs of the tumor vascular model. (ⅲ) Bioprinted michigan cancer foundation-7 cells (blue) laden in the microvascular models of GelMA further seeded with ECs (green) in the channels. Reproduced with permission from Ref. [175]. Copyright 2018 WILEY‐VCH. (B) The BBB model made by DLP. (ⅰ). Schematics of the setup of the BBB models. (ⅱ) Photographs of the separate, interpenetrating topology. (ⅲ) Day 5 static cultured blood vessels (Scale Bar: 50 μm). Reproduced with permission from Ref. [177]. Copyright 2023 IOP Publishing. (C) The arterial model is made by extrusion 3D printing. (ⅰ). schematics of the setup of the arterial model and simulated hemodynamics. (ⅱ) Photograph showing the bifurcation structure. (ⅲ) EC dysfunction model (scale bar: 500 μm). Reproduced with permission from Ref. [159]. Copyright 2023 IOP Publishing. (D) The tumor-on-a-chip with a bioprinted blood and a lymphatic vessel pair (TOC-BBL). (ⅰ). Schematics of the setup of TOC-BBC. (ⅱ) Chip design and microchannel images therein. (ⅲ) FITC-dextran (green) and doxorubicin (red) transport in the TOC-BBL. Reproduced with permission from Ref. [178]. Copyright 2019 WILEY‐VCH.
Beyond the construction of disease models with capillary network as the primary element, the large blood vessels related disorders (e.g., atherosclerosis, aneurysm, artery dissection) can also be established. To create in vitro disease models of blood vessels, it is essential to replicate disease-inducing factors such as hemodynamic abnormalities, which play significant roles in conditions like atherosclerosis, aneurysms, and arterial dissections. These abnormalities are closely tied to the geometric shape of blood vessels. Normal vasculature experiences high-speed laminar flow, but bends, narrowings, and bifurcations can cause turbulent flow and low-speed oscillatory shear, leading to endothelial damage and pathological events. Zhang et al. employed DLP to create a hydrogel chip with a cross-section of 100 × 100 μm, featuring perfusable channels designed for microfluidic flow to simulate blood flow conditions within a vascular network [179].
Besides the hemodynamic changes, incorporating stimulating factors or other biomolecules into bioinks can be done to promote cell growth and mimic cell interactions in vascular models. Paone et al. [177] utilized DLP to construct a co-culture model of the blood-brain barrier (BBB) consisting of ECs and astrocytes. This model achieved complex topological structures and tunable biological activity. To simulate the interaction between ECs and astrocytes in the BBB model, multiple peptide motifs were mixed into the bioink to promote the attachment of astrocytes near the endothelialized vessels (Fig. 5B). This allowed for the investigation of the mechanical and biological effects of the endothelial-astrocyte interaction on the BBB model. Using DLP technology to construct the BBB model enables the fabrication of complex structures that support physiological flow rates.
4.1.2. Light-assisted 3D bioprinting of vascular disease model
On the other hand, with the presence of light, light-assisted 3D bioprinting was also enabled to establish vascularized tumor models. He's team combined sacrificial printing and coaxial extrusion, enabling the construction of disposable vascularized tissues [156]. The use of photopolymerizable GelMA enhanced the viability of these tissues during extended in vitro culture (>20 days). These tissues were successfully applied in the establishment of vascularized tumor models, where cancer cells migrated along the blood flow, laying the foundation for subsequent mechanistic interpretations.
The reconstruction of the multi-layered arterial structure is also of the greatest importance to recapitulate the essential events during the disease initiation and progression. For instance, the VSMCs plays an important role in the atherosclerotic plaque build-up, which is mainly mediated by the paracrine signaling with ECs. Hence, it is critical to consider both the geometry and anatomy recreation for constructing the in vitro arterial disease models. Pan et al. utilized light-assisted suspended bioprinting with Ru/SPS mixed into dECM to construct a biomimetic human arterial model (Fig. 5C) [159]. Ru/SPS enabled photocuring with visible light at 450 nm within 3 s, facilitating rapid construction. This light-assisted approach provided insights into the roles of cell-cell communication and hemodynamics in arterial diseases, laying the foundation for understanding disease mechanisms and complications.
The inevitable complexity of tissue construction requires the interaction of multiple materials. Light-based bioprinting excels in precisely controlling the positioning and proportions of different materials, enabling the design and fabrication of complex vascularized tissue structures. Zhang's team has constructed a tumor model with blood vessels and lymphatic vessels using coaxial printing [178]. By adjusting the composition of the bioink (GelMA, alginate, and PEGDA), they can regulate the vascular osmotic pressure, thereby achieving differential diffusion of anticancer drugs at different levels. This can improve the accuracy of anticancer drug screening (Fig. 5D). This advancement provides a valuable tool for integrating microvascular tissues into organ-on-chip platforms for drug screening purposes.
4.2. Implantable vascular graft
Different from in vitro models that only need to resist the pressure of perfused fluids to mimic dynamic signals, blood vessel grafts must endure the suture tension and burst pressure caused by pulsatile blood flow. Therefore, the primary challenge for tissue-engineered implantable vascular grafts is achieving the necessary mechanical strength. To meet this requirement, the robust and stable covalent bonding of photocurable polymers shows great potential. Various biocompatible materials, such as GelMA, acrylamide, PEGDA, and Polyvinyl Alcohol–Polyacrylamide, have been reported to engineer strong hydrogels [180].
4.2.1. Light-dominated 3D bioprinting of implantable vascular graft
In the realm of blood vessel graft, balancing the vessel's mechanical properties with its structural integrity, functionality, and long-term stability is a critical challenge. Current vascular grafts often struggle with issues related to mechanical performance, such as tensile strength and suture retention, which significantly impede their advancement. To address these challenges, Ye et al. [181] used DLP to construct high-precision bionic vein grafts using poly (vinyl alcohol)-based bioinks. This method allowed for the creation of vessels with precise valve structures. Performance enhancements were subsequently achieved through the incorporation of engineering the nanocrystalline domains and subsequent surface modification. As a result, they successfully developed venous vessels exhibiting highly desirable mechanical robustness, suture resistance (0.81 N), anti-swelling properties, thromboresistance, and long-term patency. Their vascular grafts were also demonstrated to support unidirectional venous flow in transplantation experiments.
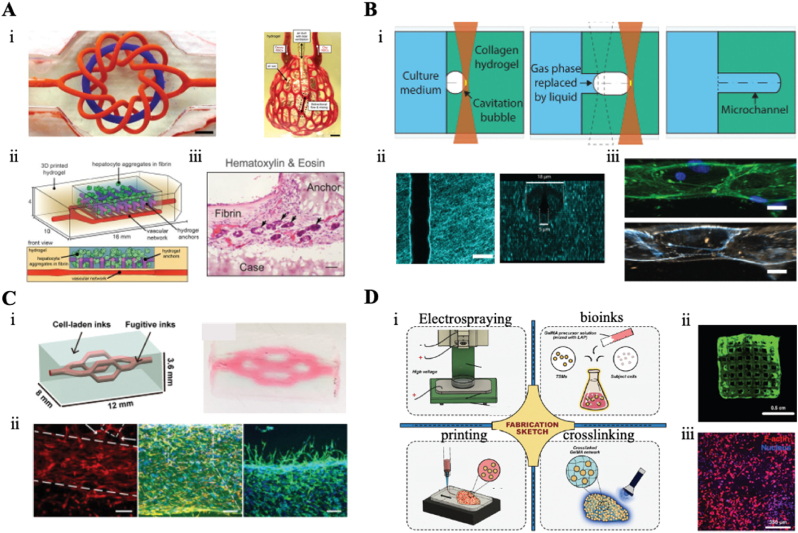
Compared to DLP, VBP enables the more rapid construction of hollow vascular structures. However, the bioinks used in VBP often prioritize biocompatibility over mechanical performance, leading to compromised strength. Größbacher et al. [131] innovatively combined MEW with VBP technology. They first used MEW to fabricate PCL vascular scaffolds with excellent mechanical properties, which were then incorporated into a volumetric printer to construct the vascular structures (Fig. 6A). This approach resulted in artificial blood vessels with enhanced mechanical performance compared to those made solely with bioinks. And the cell-laden vessels created using this method demonstrated high cell viability.
Fig. 6.
The vascular graft engineered by light-based 3D bioprinting. (A) The vascular graft with tunable architecture and echanics. (ⅰ). Schematic diagram of the blood vessel fabrication with MEW and VBP. (ⅱ). The photo of the blood vessel printed by VBP and the blood vessel printed by MEW combined with VBP (scale bar: 5 mm). (ⅲ) Lightsheet microscopy slices of the blood vessel model (scale bar: 1 mm). (ⅳ) Perpendicular cross-sectional fluorescence images of the three-layer printed blood vessel construct (scale bar: 500 μm). Reproduced with permission from Ref. [131]. Copyright 2023 Wiley‐VCH. (B) Construction of vessels with multiple bifurcation structure. (ⅰ). Schematic diagram of the construction of the bifurcation graft. (ⅱ). The size of the structure of multiple bifurcated vessels. (ⅲ) The photo of the structure of the multiple bifurcation vessel. (ⅳ) Fluorescent images of nuclei in the bifurcated blood vessel. Reproduced with permission from Ref. [132]. Copyright 2022 Wiley‐VCH. (C) The Fabrication of the high mechanical properties of the microtubes. (ⅰ). Schematic diagram of microtubule fabrication. (ⅱ). The photo of the microtubes. (ⅲ) The laser-scanning microscopy images of adhesion and live/dead ECs in the microtubes (scale bar: 200 μm). (ⅳ) The loading capacity of the printed microtubes. Reproduced with permission from Ref. [182]. Copyright 2020 Wiley‐VCH. (D) 3D bioprinting to construct implantable multi-branched graft. (ⅰ). Schematic diagram of the multi-branched graft fabrication. (ⅱ). Fluorescence images of ECs cultured in graft for 5 days (scale bar: 500 μm). (ⅲ) Observation of direct blood perfusion after implant. Reproduced with permission from Ref. [183]. Copyright 2023 Beijing Institute for Stem Cell and Regenerative Medicine and John Wiley & Sons.
Indeed, besides the fabrication of straight arterial vessels, there is also a significant clinical demand for complex-shaped arterial vessels, such as branching vessels, for transplantation. light-dominated 3D bioprinting offer distinct advantages in the creation of complex vascular grafts. VBP excels in rapidly fabricating intricate three-dimensional structures, making it particularly effective for building complex blood vessel graft. Rizzo et al. [184] developed a perfusable bifurcated vascular model with a resolution of 200 μm, achieving complete fabrication within 10–11 s. This rapid processing is highly advantageous for preserving cell viability during printing and reducing defects which caused by technologies. However, high concentrations of cells in bioinks can cause significant light scattering, which impedes volumetric bioprinting. Madrid-Wolff et al. [132] addressed this issue by employing scattering-corrected tomographic techniques to construct bifurcated vascular structures (Fig. 6B). By redistributing light through scattering correction, they avoided excessive polymerization and channel occlusion, ultimately producing a four-branch artificial blood vessel. The printed bifurcated vessels maintained high cell viability over subsequent days.
As clinical situations become more diverse, the demand for transplantable blood vessels extends beyond single straight tubes or bifurcated structures to complex vascular tissues and organs. There is an urgent need to construct complex vascular tissues with high biocompatibility. Additionally, the rising demand for personalized medicine requires the construction of artificial blood vessels tailored to individual patient characteristics and needs. This poses higher requirements on traditional bioprinting technologies. Mahjoubnia et al. [185] proposed a solution for bioprinting blood vessels using DLP technology based on personalized patient modeling. They demonstrated excellent mechanical properties and biocompatibility through vascular transplantation and performed patient-specific modeling based on scans of specific atrial appendages. These personalized atrial occluders were subjected to in vitro transplantation experiments, maintaining stability through multiple cycles. This innovation opens new possibilities for the development of personalized medical implants.
4.2.2. Light-assisted 3D bioprinting of implantable vascular graft
For implantable vascular graft, in addition to light-dominated 3D bioprinting, light-assisted 3D bioprinting have also played a crucial role in this area. A usually strategy for constructing artificial blood vessels involves directly integrating high-performance polymer materials. In a recent study, Liang et al. addressed this challenge by blending nanoclay, H-bonding monomer NAGA, and GelMA to formulate a UV-crosslinked bioink for microtube fabrication (Fig. 6C) [182]. NAGA enhances the mechanical properties of the material through the formation of dual hydrogen bonds during the crosslinking process. The incorporation of nanoclay improved the printability of the bioink, resulting in microtubes with a tensile strength of 22 MPa and favorable adhesion and growth of ECs. In addition to using materials with robust mechanical properties, another approach to enhance the mechanical performance of graft is through the construction of interpenetrating networks using multiple materials. In a recent study, a combination of GelMA and alginate was utilized as a bioink to fabricate vascular grafts [186]. The tensile strength of the interpenetrating network (0.8 MPa) was significantly higher than that of alginate alone at the same concentration (<0.01 MPa) or GelMA alone (0.01 MPa).
However, achieving functional vascular tissues requires more than just robust hydrogels. Constructing composite vascular grafts could be a viable solution. For instance, Gao et al. [147] used coaxial printing of dECM bioink to engineer small-diameter blood vessel grafts containing both functional endothelial and muscular cell layers, demonstrating in vivo efficacy in a living rat model. After three weeks of implantation, the blood vessels exhibited excellent patency, with intact endothelial and smooth muscle layers and integration with host tissue. Combining such approaches with NAGA could produce vascular grafts that possess both functionality and mechanical strength, leveraging the flexibility of coaxial printing technology. There have been reports on the utilization of interpenetrating networks and biocompatible materials for coaxial printing [97]. Zhou et al. [187] utilized a composite ink consisting of GelMA, PEGDA, and alginate to construct blood grafts through coaxial printing. The crosslinked networks formed by GelMA, PEGDA, and alginate significantly enhanced the mechanical properties of the blood vessels (1 MPa), while the cell-laden suspension within the ink provided space for cell growth through the degradation of alginate. The endothelial and smooth muscle cells could function stably within the vessel construct. Such blood vessels, combining mechanical performance and biological activity, hold promise as a potential choice for small-diameter vascular transplantation in the future.
For branching vessels, methods like coaxial bioprinting or rotational axis printing face challenges in achieving the construction of such intricate structures. Sequential suspension printing offers a solution by printing sacrificially bioink and middle layer bioink into a cell-laden suspension pool, utilizing the shear thinning properties of the bioink and the self-healing characteristics of the suspension pool bioink to create complex tubular structures. Wang et al. [183] utilized this approach to construct photopolymerized bifurcated vessels, which were subsequently implanted in rats (Fig. 6D). By combining multiple materials, the mechanical performance of branching vascular structures can withstand shear stress within the vessels. The construction method enables the fabrication of complex-shaped vessels while providing support for angiogenesis, offering an effective strategy for subsequent vascularized tissue engineering.
4.3. Vascularized tissue/organ graft
The vascular system plays a crucial role in maintaining homeostasis in the human body. In engineered tissues, as cells continuously grow and assemble, tissue density increases. Without a microvascular system in the tissue, oxygen deficiency can occur, leading to tissue necrosis. Therefore, vascularization is essential for tissue engineering and the successful construction of functional tissues [188]. Unlike vascular disease models, which focus on mimicking disease conditions, vascularized tissue engineering aims at constructing vascularized organs for future implantation, emphasizing the creation of large tissues and organs with integrated vascular networks.
4.3.1. Light-dominated 3D bioprinting of vascularized tissue/organ graft
Light-dominated 3D bioprinting is renowned for its ability to achieve rapid and high-precision structural fabrication. DLP, utilizing the opening and closing of its micro-mirrors, allows for precise shape fabrication at a microscale. Additionally, it achieves high-fidelity shape reproduction and resolution at a larger scale, which is crucial for vascularized tissue/organ construction. By employing shape design, DLP enables the fabrication of vascularized tissue/organ configurations. Grigoryan et al. [189] successfully demonstrated the construction of a vascular network using DLP technology (26.1 × 15.4 mm). Through model design, they achieved a composite design of axial and helical channels, which served as the basis for constructing artificial lung alveoli with externally surrounding blood vessels. These lung alveoli simulate respiration and facilitate substance exchange between the vascular system and the alveoli (Fig. 7A). This vascular system construction addresses the challenge of substance exchange in artificial organ manufacturing. Mazari-Arrighi et al. [190] utilized DLP to construct capillary networks with diameters ranging from 10 to 100 μm. Similarly, Orellano et al. [157] use SLA to construct the capillary networks with diameters ranging from 0.2 to 0.4 mm. This network was used for multi-layer assembly. which facilitates the development of highly complex and functional tissues.
Fig. 7.
The vascularized tissue/organ analogs constructed by light-based 3D bioprinting. (A) Construction of vascularized alveolar tissue. (ⅰ). Construction of alveolar vascular network. (ⅱ). Prevascularized hepatic hydrogel with ECs in the vascular network. (ⅲ) Confocal image of a fibrin gel containing hepatocyte aggregates (scale bar, 1 mm). Reproduced with permission from Ref. [189]. Copyright 2019 The American Association. (B) Microvascularized Tissue Models by Laser-Based Cavitation. (ⅰ). Schematic diagram of 3D laser cavitation forming of vascularized tissue model. (ⅱ) Microchannels created by laser-based cavitation. (ⅲ) Growth of ECs on microchannels (scale bar, 30 μm). Reproduced with permission from Ref. [191]. Copyright 2022 Wiley‐VCH. (C) 3D liver tissue model made by multi-material bioprinting. (ⅰ). Schematic and optical images of the model. (ⅱ) Fluorescent images of vascularized ECs (scale bar, 200 μm). Reproduced with permission from Ref. [192]. Copyright 2021 Wiley‐VCH. (D) Construction of centimeter-scale tissue based on light-based 3D bioprinting. (ⅰ). Schematic diagram of the construction of the centimeter-scale Tissue with Angiogenesis. (ⅱ) Images of 3D patterns printed by bioprinting. (ⅲ) F-actin and nucleus staining and 3D pattern of the structures. Reproduced with permission from Ref. [193]. Copyright 2022 Whioce Publishing.
Developing functional, vascularized tissues that accurately mimic human tissue is crucial for the transplantation of vascularized tissues. Printing within hydrogels that possess high cellular density similar to in vivo conditions presents greater challenges compared to seeding cells in acellular hydrogels post-printing. This is due to the need to prevent cellular damage caused by printing parameters, such as light exposure. Moreover, factors such as the photothermal effect, the free radical damage, the pH of bioinks, and the reduced cell viability over extended printing periods all pose significant challenges for cell-laden 3D bioprinting. Additionally, the presence of cells may lead to light scattering issues, which must also be addressed in cell-laden printing. While many reviews have detailed the impact of various printing parameters on cell printing [194,195], this review will focus specifically on the effects of light-related parameters on cell printing and potential solutions.
In light-based cell-laden bioprinting for constructing vascularized tissues, a major concern is how to mitigate light-induced damage to cells to preserve cellular viability as much as possible. A common approach is to investigate cellular activity under varying durations of ultraviolet light exposure to determine optimal photocrosslinking parameters. Jia et al. [97] demonstrated that cell-laden GelMA and PEGTA inks could maintain over 80 % viability when exposed to UV light for 30 s, and cell viability significantly declines after exposure to ultraviolet light for more than 30 s. Building on this approach, Byambaa et al. [196] developed bone tissues incorporating vascular networks using cell-laden GelMA hydrogels for vascularization and a separate GelMA hydrogel component to support bone formation. After 21 days of cultivation, the central angiogenic vessels facilitated the formation and maturation of the bone tissue constructs. This printing method paves the way for repairing bone tissues with multi-scale vascular networks. Despite the widespread use of UV light for rapid crosslinking in the bioprinting of vascularized tissues, concerns remain about the potential cellular damage and toxicity associated with UV exposure. Exploring materials with higher functional group conversion rates to reduce UV exposure time, as well as identifying materials suitable for visible light crosslinking, are promising strategies for addressing these issues [197,198].
In addition to the direct effects of light exposure, the photothermal effects induced by light should not be underestimated. TPP can rapidly construct intricate vascular networks with a resolution of less than 100 μm; however, the photothermal effects occurring during this process can severely compromise cellular viability and the integrity of the hydrogel system. To address this issue, Enrico et al. [191] proposed a method for in situ 3D imaging using femtosecond lasers to construct microvascular networks (Fig. 7B). This approach effectively avoids photothermal damage to cells and successfully creates channels with diameters ranging from 20 to 60 μm, by working directly on a cell-carrying hydrogel, eliminating the need for subsequent cell seeding in traditional manufacturing methods, which reduces operational complexity and facilitates the construction of vascularized tissue.
For vascularized tissues, achieving a high cell density is essential to mimic the composition of natural tissues. However, the mismatch in refractive index between cells and the surrounding biomaterials often leads to a decrease in resolution in DLP. To address this issue, You et al. [199] introduced iodixanol into the bioink, which reduced light scattering tenfold while having minimal impact on the cells. By incorporating iodixanol, they were able to adjust the refractive index of the ink, enabling the manufacturing of tissue-engineered vessels with a resolution of 50 μm. They successfully fabricated a vascularized tissue structure measuring 17 × 11 × 3.6 mm, with vessel channels ranging from 250 μm to 750 μm in diameter. Iodixanol can be applied to various cell-laden bioinks systems, making it widely applicable in light-dominated 3D printing.
4.3.2. Light-assisted 3D bioprinting of vascularized tissue/organ graft
In addition to light-dominated 3D bioprinting, light-assisted 3D bioprinting is another approach for constructing vascular tissues. By carefully selecting the materials and utilizing sacrificial materials, the combination of these techniques ensures the fidelity of the fabricated shapes. A variety of vascularized organs were constructed.
One common approach in 3D printing for constructing vascularized tissues is based on the method of suspended bioprinting. The self-healing properties of the suspension bath and the high controllability of the nozzle trajectory provide a feasible solution for building complex tissues. However, when it comes to constructing vascularized tissues using suspended printing, the major challenge lies in achieving a balance between elasticity and biocompatibility in the bioink. Lee et al. [200] addressed this challenge by selecting recombinant human tropoelastin as a highly biocompatible component in the bioink. They mixed this material with GelMA to perform suspended printing and construct a vascularized heart model. Biocompatibility assessments conducted in vitro and in vivo demonstrated the successful establishment of endothelial barriers and spontaneous beating functionality of cardiomyocytes. Additionally, the resulting construct exhibited minimal inflammatory response, indicating the feasibility of its application in cardiac tissue replacement. By utilizing suitable bioink compositions and optimizing the printing process, suspended printing technology holds promising potential in the fabrication of vascularized tissues, allowing for the creation of complex tissue structures with functional vasculature that can closely mimic natural tissues. Similarly, Liu et al. [192] utilized suspended printing to construct centimeter-sized vascularized liver tissue for the study of human tissue development and diseases. In addition to creating a functional vascular network, the printed liver tissue models allowed for the evaluation of drug metabolism and toxicity in a more physiologically relevant environment (Fig. 7C).
For centimeter-scale vascularized tissues, direct construction of micro-vessels using light-assisted 3D printing is challenging. However, He's team has made a creative breakthrough by proposing a method that involves the use of ECs-loaded gelatin microspheres printed within a GelMA hydrogel [193] (Fig. 7D). By manipulating temperature changes, the gelatin within the construct releases the encapsulated ECs, inducing the formation of a vascular network. This strategy has been successfully employed to construct centimeter-scale vascularized tissues. Endothelialization is a crucial step for vascularized tissues, as it plays a vital role in enabling their functionality. One of the challenges in 3D bioprinting is achieving the uniform distribution of ECs on the surface of the vascular network and facilitating rapid and effective endothelialization to ensure proper functionality. Ouyang et al. [201] successfully addressed this problem through in-situ endothelialization. They employed a strategy involving the sequential deposition of sacrificial ink and matrix ink loaded with ECs. After ink photocrosslinking, the sacrificial ink was removed, resulting in the formation of interconnected vascular networks. This manufacturing approach overcomes the issue of structural collapse commonly encountered in traditional 3D printing techniques. Moreover, it enables in-situ deposition and crosslinking of ECs, facilitating rapid endothelialization.
5. Conclusions and future directions
In the field of VTE, numerous techniques have emerged for developing in vitro vascular disease models, implantable vascular grafts, and vascularized tissue/organ constructs. However, most of these methods face limitations in creating complex structures, achieving necessary mechanical properties, and maintaining cell viability. Light-based 3D bioprinting, leveraging the unique advantages of light, such as high resolution, rapid curing, great material adaptability, and tunable photochemistry, offers promising solutions to these challenges. Current methods for constructing capillary-like microvessels primarily involve topological modeling or the addition of pro-angiogenic factors such as VEGF to promote the formation of microvessels and capillaries within larger vascular networks. While these approaches effectively overcome the resolution limits of existing fabrication techniques, the high costs and complex equipment required pose significant barriers to biological vascular construction of capillaries. Research on directly 3D printing capillary-like structures is still in its infancy. Recently, reports have highlighted the use of 3D printing technology to fabricate capillary channels directly. For example, Zhang Yu's team successfully achieved microvessels construction on the scale of tens of micrometers using coaxial printing technology [202]. Orellano et al. [157] utilized SLA to construct microvessels with diameters ranging from 0.2 to 0.4 mm, subsequently developing a vascular network through angiogenesis. However, manufacturing containers with inner diameters less than 50 μm remains a major challenge.
This review highlights the precision and effectiveness of light-based techniques such as SLA, DLP, and TPP in fabricating detailed and functional vascular networks. These advancements are pivotal for in vitro disease modeling, enabling comprehensive studies of disease mechanisms and facilitating drug screening processes. Additionally, we have discussed innovative strategies for implantable vascular grafts that balance mechanical strength and biocompatibility, including the use of composite materials and combined biofabrication techniques. For vascularized tissues, successful morphological construction is the first step towards application, while the perfusion of the vascular network and its transplantability are crucial factors determining its practical use in vascular tissue engineering. A number of the studies regarding the vascularized tissues cited in chapter 4 have achieved simple in vitro perfusion. However, significant progress is still needed to realize functional vascular networks through perfusion methods, such as pump or gravity-driven perfusion, to offer physiological signals (e.g., shear stress, pulsatile radial strain). Addressing these issues should be a key focus for the next steps in vascular network construction. Moreover, directly integrating vascularized tissue with the host remains a significant challenge in tissue engineering, which requests robust mechanical properties of the blood vessels at the anastomotic sites to resist the suture tension and burst pressure.
Despite the extensive applications of light-based 3D bioprinting in VTE, several areas require further exploration. For instance, existing light-based 3D bioprinting primarily utilizes UV and near-UV light (e.g., blue light at 450 nm), which still carries the risk of inducing significant DNA and cellular damage as irradiation times prolong [94]. Developing strategies using longer wavelengths of visible light for bioprinting to avoid cell damage, exploring relevant photoinitiators, and identifying suitable materials are crucial for the advancement of light-based bioprinting. Despite numerous photoinitiators (e.g., RU/SPS, Eosin-Y) demonstrating their capacity for facilitating light-based 3D bioprinting, limited reports have contributed to pushing the boundaries of VTE. Extending current photocurable bioinks and advancing relevant printing systems might help overcome this bottleneck.
Beyond the discussed achievements in this review, light-based 3D bioprinting could further assist the advancement of VTE in various ways. For example, in addition to constructing capillary networks, TPP can also be utilized for manufacturing micro/nanorobots [203,204], with potential applications in treating vascular diseases. By employing TPP to build venous microrobots and using driving forces such as magnetic fields, targeted thrombus removal can be achieved [205,206]. This area warrants further exploration to expand the applications of TPP in VTE.
While in vivo bioprinting of pre-designed constructs presents impressive technical progress, current strategies mainly rely on the penetration of long-wavelength light sources (e.g., infrared light) and the conversion of nanoscale initiators, which may lead to incomplete crosslinking of the bioink, posing potential adverse effects on the human body. Moreover, the limited penetration capability of infrared light (a few hundred micrometers) and the restricted options of bioinks and photoinitiators hinder further development. However, such an instructive concept has led to intriguing technical evolutions. Recent studies have proposed ultrasound-based in-body printing technology [207]. By constructing an acoustic bioink that heats and solidifies upon absorbing ultrasound pulses, the ink can be injected into the desired implantation site within the body. External probes emitting ultrasound waves can then solidify the acoustic ink into the desired shape, while the remaining ink can be extracted using a syringe. Compared to light-based in-body printing, ultrasound printing technology avoids light scattering issues and allows for the construction of large volumes of tissue/organs.
As a common challenge of 3D bioprinting artificial tissues/organs on the earth, the presence of gravity inevitably induces bioink flow after deposition, thereby causing structural collapse and resolution compromise. Although the emergence of suspension 3D bioprinting has helped to counteract the gravitational effects [[208], [209], [210], [211], [212]], innovative fabrication strategies are still required to fully unlock the potential of light-based 3D bioprinting for producing complex vascular structures. An attractive strategy is to perform the light-based 3D bioprinting process under microgravity or zero-gravity conditions. The microgravity could enable the levitation of seed cells, biomaterials, or bioinks, which protects them from detachment and precipitation, thereby remarkably facilitating the fabrication of sophisticated vascular structures with desired functionalities. In a pioneering study, the microgravity environment has been reported to enhance the printing fidelity of soft tissue constructs [213]. Besides facilitating the biofabrication process, microgravity 3D bioprinting in space is also beneficial for investigating specific healthcare issues related to blood vessels. Many space stressors exist, typically consistent microgravity and extraterrestrial cosmic radiation, which may lead to severe health risks and pathophysiological alterations of astronauts and voyagers in space [[214], [215], [216], [217]]. For example, microgravity has been reported to trigger various space diseases [[218], [219], [220], [221], [222], [223]]. Facing these rising problems, several reports have attempted 3D bioprinting in space, particularly under microgravity, for tissue engineering and regenerative medicine [[224], [225], [226], [227], [228], [229], [230], [231], [232], [233]]. In future, the tissue engineering blood vessels and vascularized tissues could be constructed under microgravity environment present in space [[234], [235], [236], [237]] or simulated on Earth [[238], [239], [240], [241]], thereby elucidating the effect of microgravity on human health during long-term spaceflight and space missions. Taken together, these efforts are envisioned to accelerate the development of advanced bioprinting strategies, gravity-free bioinks, and space medicine that cannot be developed on Earth [227,[242], [243], [244], [245]].
In addition to overcoming the limitations in biofabrication, convergence with other cutting-edge technologies is advisable for expanding the applications of light-based 3D bioprinting techniques in VTE. For example, constructing vascularized tissue/organs requires designing capillary-like systems to feed each residing cell, a challenge in personalized medicine. Advanced medical imaging, big data, and artificial intelligence can guide the production of blueprints for effective vascularization.
Lastly, as a multidisciplinary field, advancements in VTE cannot be achieved solely by researchers and scientists specialized in engineering and materials science but also require collaboration with medical experts. Without medical knowledge and experience, it is difficult for researchers to understand urgent clinical needs and issues (e.g., the pathology of relevant vascular diseases, requirements for medical implants). Therefore, building bridges between scientists and medical doctors is crucial for rapidly advancing VTE to better serve clinical research and address global health issues.
CRediT authorship contribution statement
Wei Li: Writing – original draft. Jinhua Li: Writing – review & editing. Chen Pan: Writing – original draft. Jae-Seong Lee: Data curation. Byoung Soo Kim: Writing – review & editing. Ge Gao: Supervision, Funding acquisition, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Funding: This research is supported by the Beijing Natural Science Foundation (7232344), the National Natural Science Foundation of China (32201126, 32311540144), the National Research Foundation of Korea (NRF) grant funded by the Korean Government (MSIT) (No. 2022R1A5A2027161), and Beijing Institute of Technology Teli Young Fellow Program (RCPT-20220029).
Contributor Information
Jinhua Li, Email: lijinhua@bit.edu.cn.
Byoung Soo Kim, Email: bskim7@pusan.ac.kr.
Ge Gao, Email: gaoge@bit.edu.cn.
Data availability
No data was used for the research described in the article.
References
- 1.Makode S., Maurya S., Niknam S.A., Mollocana-Lara E., Jaberi K., Faramarzi N., Tamayol A., Mortazavi M. Three dimensional (bio)printing of blood vessels: from vascularized tissues to functional arteries. Biofabrication. 2024;16(2) doi: 10.1088/1758-5090/ad22ed. [DOI] [PubMed] [Google Scholar]
- 2.Fazal F., Raghav S., Callanan A., Koutsos V., Radacsi N. Recent advancements in the bioprinting of vascular grafts. Biofabrication. 2021;13(3) doi: 10.1088/1758-5090/ac0963. [DOI] [PubMed] [Google Scholar]
- 3.Carrabba M., Madeddu P. Current strategies for the manufacture of small size tissue engineering vascular grafts. Front. Bioeng. Biotechnol. 2018;6(APR):41. doi: 10.3389/fbioe.2018.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zeng Z., Hu C., Liang Q., Tang L., Cheng D., Ruan C. Coaxial-printed small-diameter polyelectrolyte-based tubes with an electrostatic self-assembly of heparin and YIGSR peptide for antithrombogenicity and endothelialization. Bioact. Mater. 2021;6(6):1628–1638. doi: 10.1016/j.bioactmat.2020.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Parkhideh S., Calderon G.A., Janson K.D., Mukherjee S., Mai A.K., Doerfert M.D., Yao Z., Sazer D.W., Veiseh O. Perfusable cell-laden matrices to guide patterning of vascularization in vivo. Biomater. Sci. 2023;11(2):461–471. doi: 10.1039/d2bm01200f. [DOI] [PubMed] [Google Scholar]
- 6.Skylar-Scott M.A., Uzel S.G.M., Nam L.L., Ahrens J.H., Truby R.L., Damaraju S., Lewis J.A. Biomanufacturing of organ-specific tissues with high cellular density and embedded vascular channels. Sci. Adv. 2019;5(9) doi: 10.1126/sciadv.aaw2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vajda J., Milojević M., Maver U., Vihar B. Microvascular tissue engineering—a review. Biomedicines. 2021;9(6):589. doi: 10.3390/biomedicines9060589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kirillova A., Yeazel T.R., Asheghali D., Petersen S.R., Dort S., Gall K., Becker M.L. Fabrication of biomedical scaffolds using biodegradable polymers. Chem. Rev. 2021;121(18):11238–11304. doi: 10.1021/acs.chemrev.0c01200. [DOI] [PubMed] [Google Scholar]
- 9.Zhao H., Liu M., Zhang Y., Yin J., Pei R. Nanocomposite hydrogels for tissue engineering applications. Nanoscale. 2020;12(28):14976–14995. doi: 10.1039/d0nr03785k. [DOI] [PubMed] [Google Scholar]
- 10.Zhao Y., Song S., Ren X., Zhang J., Lin Q., Zhao Y. Supramolecular adhesive hydrogels for tissue engineering applications. Chem. Rev. 2022;122(6):5604–5640. doi: 10.1021/acs.chemrev.1c00815. [DOI] [PubMed] [Google Scholar]
- 11.Khademhosseini A., Langer R. A decade of progress in tissue engineering. Nat. Protoc. 2016;11(10):1775–1781. doi: 10.1038/nprot.2016.123. [DOI] [PubMed] [Google Scholar]
- 12.Li J., Wu C., Chu P.K., Gelinsky M. 3D printing of hydrogels: rational design strategies and emerging biomedical applications. Mater. Sci. Eng. R Rep. 2020;140 [Google Scholar]
- 13.Lee J., Park D., Seo Y., Chung J.J., Jung Y., Kim S.H. Organ-level functional 3D tissue constructs with complex compartments and their preclinical applications. Adv. Mater. 2020;32(51) doi: 10.1002/adma.202002096. [DOI] [PubMed] [Google Scholar]
- 14.Zhang B., Korolj A., Lai B.F.L., Radisic M. Advances in organ-on-a-chip engineering. Nat. Rev. Mater. 2018;3(8):257–278. [Google Scholar]
- 15.Li S., Sengupta D., Chien S. Vascular tissue engineering: from in vitro to in situ. WIREs Systems Biology and Medicine. 2014;6(1):61–76. doi: 10.1002/wsbm.1246. [DOI] [PubMed] [Google Scholar]
- 16.Boccafoschi F., Rajan N., Habermehl J., Mantovani D. Preparation and characterization of a scaffold for vascular tissue engineering by direct-assembling of collagen and cells in a cylindrical geometry. Macromol. Biosci. 2007;7(5):719–726. doi: 10.1002/mabi.200600242. [DOI] [PubMed] [Google Scholar]
- 17.Liu C.-S., Feng B.-W., He S.-R., Liu Y.-M., Chen L., Chen Y.-L., Yao Z.-Y., Jian M.-Q. Preparation and evaluation of a silk fibroin–polycaprolactone biodegradable biomimetic tracheal scaffold. J. Biomed. Mater. Res. B Appl. Biomater. 2022;110(6):1292–1305. doi: 10.1002/jbm.b.35000. [DOI] [PubMed] [Google Scholar]
- 18.Nerem R.M., Seliktar D. Vascular Tissue Engineering. 2001;3:225–243. doi: 10.1146/annurev.bioeng.3.1.225. 3, 2001. [DOI] [PubMed] [Google Scholar]
- 19.Masuda S., Shimizu T., Yamato M., Okano T. Cell sheet engineering for heart tissue repair. Adv. Drug Deliv. Rev. 2008;60(2):277–285. doi: 10.1016/j.addr.2007.08.031. [DOI] [PubMed] [Google Scholar]
- 20.Saito J., Kaneko M., Ishikawa Y., Yokoyama U. Challenges and possibilities of cell-based tissue-engineered vascular grafts. Cyborg Bionic Syst. 2021;2021 doi: 10.34133/2021/1532103. https://spj.science.org/doi/10.34133/2021/1532103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jang B.S., Cheon J.Y., Kim S.H., Park W.H. Small diameter vascular graft with fibroblast cells and electrospun poly (L-lactide-co-ε-caprolactone) scaffolds: cell Matrix Engineering. J. Biomater. Sci. Polym. Ed. 2018;29(7–9):942–959. doi: 10.1080/09205063.2017.1367635. [DOI] [PubMed] [Google Scholar]
- 22.Sakaguchi K., Shimizu T., Okano T. Construction of three-dimensional vascularized cardiac tissue with cell sheet engineering. J. Contr. Release. 2015;205:83–88. doi: 10.1016/j.jconrel.2014.12.016. [DOI] [PubMed] [Google Scholar]
- 23.Akiyama Y. Design of temperature-responsive cell culture surfaces for cell sheet engineering. Cyborg Bionic Syst. 2021;2021 doi: 10.34133/2021/5738457. https://spj.science.org/doi/10.34133/2021/5738457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sugimura Y., Chekhoeva A., Oyama K., Nakanishi S., Toshmatova M., Miyahara S., Barth M., Assmann A.K., Lichtenberg A., Assmann A., Akhyari P. Controlled autologous recellularization and inhibited degeneration of decellularized vascular implants by side-specific coating with stromal cell-derived factor 1α and fibronectin. Biomed. Mater. 2020;15(3) doi: 10.1088/1748-605X/ab54e3. [DOI] [PubMed] [Google Scholar]
- 25.Steffens D., Braghirolli D.I., Maurmann N., Pranke P. Update on the main use of biomaterials and techniques associated with tissue engineering. Drug Discov. Today. 2018;23(8):1474–1488. doi: 10.1016/j.drudis.2018.03.013. [DOI] [PubMed] [Google Scholar]
- 26.Paek J., Park S.E., Lu Q., Park K.-T., Cho M., Oh J.M., Kwon K.W., Yi Y.-s., Song J.W., Edelstein H.I., Ishibashi J., Yang W., Myerson J.W., Kiseleva R.Y., Aprelev P., Hood E.D., Stambolian D., Seale P., Muzykantov V.R., Huh D. Microphysiological engineering of self-assembled and perfusable microvascular beds for the production of vascularized three-dimensional human microtissues. ACS Nano. 2019;13(7):7627–7643. doi: 10.1021/acsnano.9b00686. [DOI] [PubMed] [Google Scholar]
- 27.Polacheck W.J., Kutys M.L., Yang J., Eyckmans J., Wu Y., Vasavada H., Hirschi K.K., Chen C.S. A non-canonical Notch complex regulates adherens junctions and vascular barrier function. Nature. 2017;552(7684):258–262. doi: 10.1038/nature24998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hasan A., Paul A., Vrana N.E., Zhao X., Memic A., Hwang Y.-S., Dokmeci M.R., Khademhosseini A. Microfluidic techniques for development of 3D vascularized tissue. Biomaterials. 2014;35(26):7308–7325. doi: 10.1016/j.biomaterials.2014.04.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li H., Shang Y., Zeng J., Matsusaki M. Technology for the formation of engineered microvascular network models and their biomedical applications. Nano Convergence. 2024;11(1):10. doi: 10.1186/s40580-024-00416-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sakaguchi K., Akimoto K., Takaira M., Tanaka R.-i., Shimizu T., Umezu S. Cell-based microfluidic device utilizing cell sheet technology. Cyborg Bionic Syst. 2022;2022 doi: 10.34133/2022/9758187. 9758187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Song H.H.G., Rumma R.T., Ozaki C.K., Edelman E.R., Chen C.S. Vascular tissue engineering: progress, challenges, and clinical promise. Cell Stem Cell. 2018;22(3):340–354. doi: 10.1016/j.stem.2018.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Seifu D.G., Purnama A., Mequanint K., Mantovani D. Small-diameter vascular tissue engineering. Nat. Rev. Cardiol. 2013;10(7):410–421. doi: 10.1038/nrcardio.2013.77. [DOI] [PubMed] [Google Scholar]
- 33.West-Livingston L., Lim J.W., Lee S.J. Translational tissue-engineered vascular grafts: from bench to bedside. Biomaterials. 2023;302 doi: 10.1016/j.biomaterials.2023.122322. [DOI] [PubMed] [Google Scholar]
- 34.Gao G., Park J.Y., Kim B.S., Jang J., Cho D.-W. Coaxial cell printing of freestanding, perfusable, and functional in vitro vascular models for recapitulation of native vascular endothelium pathophysiology. Adv. Healthcare Mater. 2018;7(23) doi: 10.1002/adhm.201801102. [DOI] [PubMed] [Google Scholar]
- 35.Lust S.T., Shanahan C.M., Shipley R.J., Lamata P., Gentleman E. Design considerations for engineering 3D models to study vascular pathologies in vitro. Acta Biomater. 2021;132:114–128. doi: 10.1016/j.actbio.2021.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cochrane A., Albers H.J., Passier R., Mummery C.L., van den Berg A., Orlova V.V., van der Meer A.D. Advanced in vitro models of vascular biology: human induced pluripotent stem cells and organ-on-chip technology. Adv. Drug Deliv. Rev. 2019;140:68–77. doi: 10.1016/j.addr.2018.06.007. [DOI] [PubMed] [Google Scholar]
- 37.Gupta P., Mandal B.B. Tissue-engineered vascular grafts: emerging trends and technologies. Adv. Funct. Mater. 2021;31(33) [Google Scholar]
- 38.Fleischer S., Tavakol D.N., Vunjak-Novakovic G. From arteries to capillaries: approaches to engineering human vasculature. Adv. Funct. Mater. 2020;30(37) doi: 10.1002/adfm.201910811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cai Q., Liao W., Xue F., Wang X., Zhou W., Li Y., Zeng W. Selection of different endothelialization modes and different seed cells for tissue-engineered vascular graft. Bioact. Mater. 2021;6(8):2557–2568. doi: 10.1016/j.bioactmat.2020.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang G., Mahadik B., Choi J.Y., Fisher J.P. Vascularization in tissue engineering: fundamentals and state-of-art. Prog. Biomed. Eng. 2020;2(1) doi: 10.1088/2516-1091/ab5637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Amirsadeghi A., Jafari A., Eggermont L.J., Hashemi S.-S., Bencherif S.A., Khorram M. Vascularization strategies for skin tissue engineering. Biomater. Sci. 2020;8(15):4073–4094. doi: 10.1039/d0bm00266f. [DOI] [PubMed] [Google Scholar]
- 42.Redenski I., Guo S., Machour M., Szklanny A., Landau S., Kaplan B., Lock R.I., Gabet Y., Egozi D., Vunjak-Novakovic G., Levenberg S. Engineered vascularized flaps, composed of polymeric soft tissue and live bone, repair complex tibial defects. Adv. Funct. Mater. 2021;31(44) [Google Scholar]
- 43.Wan H.-Y., Chen J.C.H., Xiao Q., Wong C.W., Yang B., Cao B., Tuan R.S., Nilsson S.K., Ho Y.-P., Raghunath M., Kamm R.D., Blocki A. Stabilization and improved functionality of three-dimensional perfusable microvascular networks in microfluidic devices under macromolecular crowding. Biomater. Res. 2023;27(1):32. doi: 10.1186/s40824-023-00375-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wan L., Flegle J., Ozdoganlar B., LeDuc P.R. Toward vasculature in skeletal muscle-on-a-chip through thermo-responsive sacrificial templates. Micromachines. 2020;11(10):907. doi: 10.3390/mi11100907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wan Z., Zhong A.X., Zhang S., Pavlou G., Coughlin M.F., Shelton S.E., Nguyen H.T., Lorch J.H., Barbie D.A., Kamm R.D. A robust method for perfusable microvascular network formation in vitro. Small Methods. 2022;6(6) doi: 10.1002/smtd.202200143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yin H., Wang Y., Liu N., Zhong S., Li L., Zhang Q., Liu Z., Yue T. Advances in the model structure of in vitro vascularized organ-on-a-chip. Cyborg Bionic Syst. 2024;5:107. [Google Scholar]
- 47.Rodríguez-Soto M.A., Polanía-Sandoval C.A., Aragón-Rivera A.M., Buitrago D., Ayala-Velásquez M., Velandia-Sánchez A., Peralta Peluffo G., Cruz J.C., Muñoz Camargo C., Camacho-Mackenzie J., Barrera-Carvajal J.G., Briceño J.C. Small diameter cell-free tissue-engineered vascular grafts: biomaterials and manufacture techniques to reach suitable mechanical properties. Polymers. 2022;14(17):3440. doi: 10.3390/polym14173440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li M.-X., Wei Q.-Q., Mo H.-L., Ren Y., Zhang W., Lu H.-J., Joung Y.K. Challenges and advances in materials and fabrication technologies of small-diameter vascular grafts. Biomater. Res. 2024;27(1):58. doi: 10.1186/s40824-023-00399-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Obiweluozor F.O., Emechebe G.A., Kim D.-W., Cho H.-J., Park C.H., Kim C.S., Jeong I.S. Considerations in the development of small-diameter vascular graft as an alternative for bypass and reconstructive surgeries: a review. Cardiovascular Engineering and Technology. 2020;11(5):495–521. doi: 10.1007/s13239-020-00482-y. [DOI] [PubMed] [Google Scholar]
- 50.Wang H., Liu X., Gu Q., Zheng X. Vascularized organ bioprinting: from strategy to paradigm. Cell Prolif. 2023;56(5) doi: 10.1111/cpr.13453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang B., Radisic M. Organ-level vascularization: the Mars mission of bioengineering. J. Thorac. Cardiovasc. Surg. 2020;159(5):2003–2007. doi: 10.1016/j.jtcvs.2019.08.128. [DOI] [PubMed] [Google Scholar]
- 52.Miri A.K., Khalilpour A., Cecen B., Maharjan S., Shin S.R., Khademhosseini A. Multiscale bioprinting of vascularized models. Biomaterials. 2019;198:204–216. doi: 10.1016/j.biomaterials.2018.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kim S.-J., Kim M.-G., Kim J., Jeon J.S., Park J., Yi H.-G. Bioprinting methods for fabricating in vitro tubular blood vessel models. Cyborg Bionic Syst. 2023;4:43. doi: 10.34133/cbsystems.0043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gold K.A., Saha B., Rajeeva Pandian N.K., Walther B.K., Palma J.A., Jo J., Cooke J.P., Jain A., Gaharwar A.K. 3D bioprinted multicellular vascular models. Adv. Healthcare Mater. 2021;10(21) doi: 10.1002/adhm.202101141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang D., Maharjan S., Kuang X., Wang Z., Mille L.S., Tao M., Yu P., Cao X., Lian L., Lv L., He J.J., Tang G., Yuk H., Ozaki C.K., Zhao X., Zhang Y.S. Microfluidic bioprinting of tough hydrogel-based vascular conduits for functional blood vessels. Sci. Adv. 2022;8(43) doi: 10.1126/sciadv.abq6900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Louis F., Piantino M., Liu H., Kang D.-H., Sowa Y., Kitano S., Matsusaki M. Bioprinted vascularized mature adipose tissue with collagen microfibers for soft tissue regeneration. Cyborg Bionic Syst. 2021;2021 doi: 10.34133/2021/1412542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cao X., Maharjan S., Ashfaq R., Shin J., Zhang Y.S. Bioprinting of small-diameter blood vessels. Engineering. 2021;7(6):832–844. [Google Scholar]
- 58.Chatani S., Kloxin C.J., Bowman C.N. The power of light in polymer science: photochemical processes to manipulate polymer formation, structure, and properties. Polym. Chem. 2014;5(7):2187–2201. [Google Scholar]
- 59.Corrigan N., Yeow J., Judzewitsch P., Xu J., Boyer C. Seeing the light: advancing materials chemistry through photopolymerization. Angew. Chem. Int. Ed. 2019;58(16):5170–5189. doi: 10.1002/anie.201805473. [DOI] [PubMed] [Google Scholar]
- 60.Boydston A.J., Cao B., Nelson A., Ono R.J., Saha A., Schwartz J.J., Thrasher C.J. Additive manufacturing with stimuli-responsive materials. J. Mater. Chem. A. 2018;6(42):20621–20645. [Google Scholar]
- 61.Chen D., Zheng X. Multi-material additive manufacturing of metamaterials with giant, tailorable negative Poisson's ratios. Sci. Rep. 2018;8(1):9139. doi: 10.1038/s41598-018-26980-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Krüger-Genge A., Blocki A., Franke R.-P., Jung F. Vascular endothelial cell biology: an update. Int. J. Mol. Sci. 2019;20(18):4411. doi: 10.3390/ijms20184411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Potente M., Gerhardt H., Carmeliet P. Basic and therapeutic aspects of angiogenesis. Cell. 2011;146(6):873–887. doi: 10.1016/j.cell.2011.08.039. [DOI] [PubMed] [Google Scholar]
- 64.Kawecki F., L'Heureux N. Current biofabrication methods for vascular tissue engineering and an introduction to biological textiles. Biofabrication. 2023;15(2) doi: 10.1088/1758-5090/acbf7a. [DOI] [PubMed] [Google Scholar]
- 65.Cahill P.A., Redmond E.M. Vascular endothelium – gatekeeper of vessel health. Atherosclerosis. 2016;248:97–109. doi: 10.1016/j.atherosclerosis.2016.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chistiakov D.A., Orekhov A.N., Bobryshev Y.V. Effects of shear stress on endothelial cells: go with the flow. Acta Physiol. 2017;219:382–408. doi: 10.1111/apha.12725. [DOI] [PubMed] [Google Scholar]
- 67.Wei H., Jiang H., Zhou Y., Xiao X., Zhou C., Ji X. Vascular endothelial cells: a fundamental approach for brain waste clearance. Brain. 2023;146(4):1299–1315. doi: 10.1093/brain/awac495. [DOI] [PubMed] [Google Scholar]
- 68.Loh Y.C., Tan C.S., Ch’ng Y.S., Yeap Z.Q., Ng C.H., Yam M.F. Overview of the microenvironment of vasculature in vascular tone regulation. Int. J. Mol. Sci. 2018;19(1):120. doi: 10.3390/ijms19010120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Garland C.J., Dora K.A. EDH: endothelium-dependent hyperpolarization and microvascular signalling. Acta Physiol. 2017;219(1):152–161. doi: 10.1111/apha.12649. [DOI] [PubMed] [Google Scholar]
- 70.Pober J.S., Tellides G. Participation of blood vessel cells in human adaptive immune responses. Trends Immunol. 2012;33(1):49–57. doi: 10.1016/j.it.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Amersfoort J., Eelen G., Carmeliet P. Immunomodulation by endothelial cells — partnering up with the immune system? Nat. Rev. Immunol. 2022;22(9):576–588. doi: 10.1038/s41577-022-00694-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hassoun P.M., Mouthon L., Barberà J.A., Eddahibi S., Flores S.C., Grimminger F., Jones P.L., Maitland M.L., Michelakis E.D., Morrell N.W., Newman J.H., Rabinovitch M., Schermuly R., Stenmark K.R., Voelkel N.F., Yuan J.X.J., Humbert M. Inflammation, growth factors, and pulmonary vascular remodeling. J. Am. Coll. Cardiol. 2009;54(1, Supplement):S10–S19. doi: 10.1016/j.jacc.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 73.Sun H.-J., Wu Z.-Y., Nie X.-W., Bian J.-S. Role of endothelial dysfunction in cardiovascular diseases: the link between inflammation and hydrogen sulfide. Front. Pharmacol. 2020;10:1568. doi: 10.3389/fphar.2019.01568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bevan J.A., Laher I. Pressure and flow-dependent vascular tone. Faseb. J. 1991;5(9):2267–2273. doi: 10.1096/fasebj.5.9.1860618. [DOI] [PubMed] [Google Scholar]
- 75.Theocharis A.D., Skandalis S.S., Gialeli C., Karamanos N.K. Extracellular matrix structure. Adv. Drug Deliv. Rev. 2016;97:4–27. doi: 10.1016/j.addr.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 76.Shaw A., Xu Q. Biomechanical stress-induced signaling in smooth muscle cells: an update. Curr. Vasc. Pharmacol. 2003;1(1):41–58. doi: 10.2174/1570161033386745. [DOI] [PubMed] [Google Scholar]
- 77.Webb R.C. Smooth muscle contraction and relaxation. Adv. Physiol. Educ. 2003;27(4):201–206. doi: 10.1152/advan.00025.2003. [DOI] [PubMed] [Google Scholar]
- 78.Rensen S.S.M., Doevendans P.A.F.M., van Eys G.J.J.M. Regulation and characteristics of vascular smooth muscle cell phenotypic diversity. Neth. Heart J. 2007;15(3):100–108. doi: 10.1007/BF03085963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Shi N., Mei X., Chen S.-Y. Smooth muscle cells in vascular remodeling. Arterioscler. Thromb. Vasc. Biol. 2019;39(12):e247–e252. doi: 10.1161/ATVBAHA.119.312581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Liu S., Lin Z. Vascular smooth muscle cells mechanosensitive regulators and vascular remodeling. J. Vasc. Res. 2021;59(2):90–113. doi: 10.1159/000519845. [DOI] [PubMed] [Google Scholar]
- 81.Bkaily G., Abou Abdallah N., Simon Y., Jazzar A., Jacques D. Vascular smooth muscle remodeling in health and disease. Can. J. Physiol. Pharmacol. 2020;99(2):171–178. doi: 10.1139/cjpp-2020-0399. [DOI] [PubMed] [Google Scholar]
- 82.Mazurek R., Dave J.M., Chandran R.R., Misra A., Sheikh A.Q., Greif D.M. In: Advances in Pharmacology. Khalil R.A., editor. Academic Press; 2017. Chapter eight - vascular cells in blood vessel wall development and disease; pp. 323–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Majesky M.W. Adventitia and perivascular cells. Arterioscler. Thromb. Vasc. Biol. 2015;35(8):e31–e35. doi: 10.1161/ATVBAHA.115.306088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Stekelenburg M., Rutten M.C.M., Snoeckx L.H.E.H., Baaijens F.P.T. Dynamic straining combined with fibrin gel cell seeding improves strength of tissue-engineered small-diameter vascular grafts. Tissue Eng. 2008;15(5):1081–1089. doi: 10.1089/ten.tea.2008.0183. [DOI] [PubMed] [Google Scholar]
- 85.Montini-Ballarin F., Calvo D., Caracciolo P.C., Rojo F., Frontini P.M., Abraham G.A., Guinea G.V. Mechanical behavior of bilayered small-diameter nanofibrous structures as biomimetic vascular grafts. J. Mech. Behav. Biomed. Mater. 2016;60:220–233. doi: 10.1016/j.jmbbm.2016.01.025. [DOI] [PubMed] [Google Scholar]
- 86.Awad N.K., Niu H., Ali U., Morsi Y.S., Lin T. Electrospun fibrous scaffolds for small-diameter blood vessels. A Review. 2018;8(1):15. doi: 10.3390/membranes8010015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Huang J., Qin Q., Wang J. A review of stereolithography: processes and systems. 2020;8(9):1138. [Google Scholar]
- 88.Skoog S.A., Goering P.L., Narayan R.J. Stereolithography in tissue engineering. J. Mater. Sci. Mater. Med. 2014;25(3):845–856. doi: 10.1007/s10856-013-5107-y. [DOI] [PubMed] [Google Scholar]
- 89.Zhang J., Hu Q., Wang S., Tao J., Gou M. Digital light processing based three-dimensional printing for medical applications. 2019;6(1) doi: 10.18063/ijb.v6i1.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lu Y., Mapili G., Suhali G., Chen S., Roy K. A digital micro-mirror device-based system for the microfabrication of complex, spatially patterned tissue engineering scaffolds. J. Biomed. Mater. Res. 2006;77A(2):396–405. doi: 10.1002/jbm.a.30601. [DOI] [PubMed] [Google Scholar]
- 91.Zhang X., Zhang X., Li Y., Zhang Y. Applications of light-based 3D bioprinting and photoactive biomaterials for tissue engineering. 2023;16(23):7461. doi: 10.3390/ma16237461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Moroni L., Boland T., Burdick J.A., De Maria C., Derby B., Forgacs G., Groll J., Li Q., Malda J., Mironov V.A., Mota C., Nakamura M., Shu W., Takeuchi S., Woodfield T.B.F., Xu T., Yoo J.J., Vozzi G. Biofabrication: a guide to technology and terminology. Trends Biotechnol. 2018;36(4):384–402. doi: 10.1016/j.tibtech.2017.10.015. [DOI] [PubMed] [Google Scholar]
- 93.Xing J.-F., Zheng M.-L., Duan X.-M. Two-photon polymerization microfabrication of hydrogels: an advanced 3D printing technology for tissue engineering and drug delivery. Chem. Soc. Rev. 2015;44(15):5031–5039. doi: 10.1039/c5cs00278h. [DOI] [PubMed] [Google Scholar]
- 94.Chen Y., Zhang J., Liu X., Wang S., Tao J., Huang Y., Wu W., Li Y., Zhou K., Wei X., Chen S., Li X., Xu X., Cardon L., Qian Z., Gou M. Noninvasive in vivo 3D bioprinting. Sci. Adv. 2020;6(23) doi: 10.1126/sciadv.aba7406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Li Y., Mao Q., Li X., Yin J., Wang Y., Fu J., Huang Y. High-fidelity and high-efficiency additive manufacturing using tunable pre-curing digital light processing. Addit. Manuf. 2019;30 [Google Scholar]
- 96.Ouyang L., Highley C.B., Sun W., Burdick J.A. A generalizable strategy for the 3D bioprinting of hydrogels from nonviscous photo-crosslinkable inks. Adv. Mater. 2017;29(8) doi: 10.1002/adma.201604983. [DOI] [PubMed] [Google Scholar]
- 97.Jia W., Gungor-Ozkerim P.S., Zhang Y.S., Yue K., Zhu K., Liu W., Pi Q., Byambaa B., Dokmeci M.R., Shin S.R., Khademhosseini A. Direct 3D bioprinting of perfusable vascular constructs using a blend bioink. Biomaterials. 2016;106:58–68. doi: 10.1016/j.biomaterials.2016.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Germain L., Rémy-Zolghadri M., Auger F. Tissue engineering of the vascular system: from capillaries to larger blood vessels. Med. Biol. Eng. Comput. 2000;38(2):232–240. [PubMed] [Google Scholar]
- 99.Rangrez A.Y., Massy Z.A., Metzinger-Le Meuth V., Metzinger L. miR-143 and miR-145. Circulation: Cardiovascular Genetics. 2011;4(2):197–205. doi: 10.1161/CIRCGENETICS.110.958702. [DOI] [PubMed] [Google Scholar]
- 100.Nerem R.M., Seliktar D. Vascular tissue engineering. Annu. Rev. Biomed. Eng. 2001;3:225–243. doi: 10.1146/annurev.bioeng.3.1.225. 3, 2001. [DOI] [PubMed] [Google Scholar]
- 101.Frismantiene A., Philippova M., Erne P., Resink T.J. Smooth muscle cell-driven vascular diseases and molecular mechanisms of VSMC plasticity. Cell. Signal. 2018;52:48–64. doi: 10.1016/j.cellsig.2018.08.019. [DOI] [PubMed] [Google Scholar]
- 102.Fatkhullina A.R., Peshkova I.O., Koltsova E.K. The role of cytokines in the development of atherosclerosis. Biochemistry (Moscow) 2016;81(11):1358–1370. doi: 10.1134/S0006297916110134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kumar A., Hung O.Y., Piccinelli M., Eshtehardi P., Corban M.T., Sternheim D., Yang B., Lefieux A., Molony D.S., Thompson E.W., Zeng W., Bouchi Y., Gupta S., Hosseini H., Raad M., Ko Y.-A., Liu C., McDaniel M.C., Gogas B.D., Douglas J.S., Quyyumi A.A., Giddens D.P., Veneziani A., Samady H. Low coronary wall shear stress is associated with severe endothelial dysfunction in patients with nonobstructive coronary artery disease. JACC Cardiovasc. Interv. 2018;11(20):2072–2080. doi: 10.1016/j.jcin.2018.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mauriello J., Maury R., Guillaneuf Y., Gigmes D. 3D/4D printing of polyurethanes by vat photopolymerization. Advanced Materials Technologies. 2023;8(23) [Google Scholar]
- 105.Li W., Wang M., Ma H., Chapa-Villarreal F.A., Lobo A.O., Zhang Y.S. Stereolithography apparatus and digital light processing-based 3D bioprinting for tissue fabrication. iScience. 2023;26(2) doi: 10.1016/j.isci.2023.106039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zhang X., Zhang K., Zhang L., Wang W., Li Y., He R. Additive manufacturing of cellular ceramic structures: from structure to structure–function integration. Mater. Des. 2022;215 [Google Scholar]
- 107.Rasaki S.A., Xiong D., Xiong S., Su F., Idrees M., Chen Z. Photopolymerization-based additive manufacturing of ceramics: a systematic review. Journal of Advanced Ceramics. 2021;10(3):442–471. [Google Scholar]
- 108.Wang Z., Abdulla R., Parker B., Samanipour R., Ghosh S., Kim K. A simple and high-resolution stereolithography-based 3D bioprinting system using visible light crosslinkable bioinks. Biofabrication. 2015;7(4) doi: 10.1088/1758-5090/7/4/045009. [DOI] [PubMed] [Google Scholar]
- 109.Ge Q., Chen Z., Cheng J., Zhang B., Zhang Y.-F., Li H., He X., Yuan C., Liu J., Magdassi S., Qu S. 3D printing of highly stretchable hydrogel with diverse UV curable polymers. Sci. Adv. 2021;7(2) doi: 10.1126/sciadv.aba4261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Chansoria P., Rütsche D., Wang A., Liu H., D'Angella D., Rizzo R., Hasenauer A., Weber P., Qiu W., Ibrahim N.B.M., Korshunova N., Qin X.-H., Zenobi-Wong M. Synergizing algorithmic design, photoclick chemistry and multi-material volumetric printing for accelerating complex shape engineering. Adv. Sci. 2023;10(26) doi: 10.1002/advs.202300912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ha C.W. Overcoming delamination in two-photon lithography for improving fabrication of 3D microstructures. Micro and Nano Systems Letters. 2023;11(1):8. [Google Scholar]
- 112.Perrucci F., Bertana V., Marasso S.L., Scordo G., Ferrero S., Pirri C.F., Cocuzza M., El-Tamer A., Hinze U., Chichkov B.N., Canavese G., Scaltrito L. Optimization of a suspended two photon polymerized microfluidic filtration system. Microelectron. Eng. 2018;195:95–100. [Google Scholar]
- 113.Maines E.M., Porwal M.K., Ellison C.J., Reineke T.M. Sustainable advances in SLA/DLP 3D printing materials and processes. Green Chem. 2021;23(18):6863–6897. [Google Scholar]
- 114.Ligon S.C., Liska R., Stampfl J., Gurr M., Mülhaupt R. Polymers for 3D printing and customized additive manufacturing. Chem. Rev. 2017;117(15):10212–10290. doi: 10.1021/acs.chemrev.7b00074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Gong J., Qian Y., Lu K., Zhu Z., Siow L., Zhang C., Zhou S., Gu T., Yin J., Yu M., Wang H., Yang H. Digital light processing (DLP) in tissue engineering: from promise to reality, and perspectives. Biomed. Mater. 2022;17(6) doi: 10.1088/1748-605X/ac96ba. [DOI] [PubMed] [Google Scholar]
- 116.Tumbleston J.R., Shirvanyants D., Ermoshkin N., Janusziewicz R., Johnson A.R., Kelly D., Chen K., Pinschmidt R., Rolland J.P., Ermoshkin A., Samulski E.T., DeSimone J.M. Continuous liquid interface production of 3D objects. Science. 2015;347(6228):1349–1352. doi: 10.1126/science.aaa2397. [DOI] [PubMed] [Google Scholar]
- 117.Park I.-B., Ha Y.-M., Kim M.-S., Kim H.-C., Lee S.-H. Three-dimensional grayscale for improving surface quality in projection microstereolithography. Int. J. Precis. Eng. Manuf. 2012;13(2):291–298. [Google Scholar]
- 118.Sun C., Fang N., Wu D.M., Zhang X. Projection micro-stereolithography using digital micro-mirror dynamic mask. Sensor Actuator Phys. 2005;121(1):113–120. [Google Scholar]
- 119.Johnson A.R., Procopio A.T. Low cost additive manufacturing of microneedle masters. 3D Printing in Medicine. 2019;5(1):2. doi: 10.1186/s41205-019-0039-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Saroia J., Yanen W., Wei Q., Zhang K., Lu T., Zhang B. A review on biocompatibility nature of hydrogels with 3D printing techniques, tissue engineering application and its future prospective. Bio-Design and Manufacturing. 2018;1(4):265–279. [Google Scholar]
- 121.He N., Wang X., Shi L., Li J., Mo L., Chen F., Huang Y., Liu H., Zhu X., Zhu W., Mao Y., Han X. Photoinhibiting via simultaneous photoabsorption and free-radical reaction for high-fidelity light-based bioprinting. Nat. Commun. 2023;14(1):3063. doi: 10.1038/s41467-023-38838-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Yang M., Chu L., Zhuang Y., Qi C., Meng S., Liu Z., Kong T. Multi-material digital light processing (DLP) bioprinting of heterogeneous hydrogel constructs with perfusable networks. Advanced Functional Materials n/ 2024;a(n/a) [Google Scholar]
- 123.Shusteff M., Browar A.E.M., Kelly B.E., Henriksson J., Weisgraber T.H., Panas R.M., Fang N.X., Spadaccini C.M. One-step volumetric additive manufacturing of complex polymer structures. Sci. Adv. 2017;3(12) doi: 10.1126/sciadv.aao5496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Bernal P.N., Delrot P., Loterie D., Li Y., Malda J., Moser C., Levato R. Volumetric bioprinting of complex living-tissue constructs within seconds. Adv. Mater. 2019;31(42) doi: 10.1002/adma.201904209. [DOI] [PubMed] [Google Scholar]
- 125.Kelly B.E., Bhattacharya I., Heidari H., Shusteff M., Spadaccini C.M., Taylor H.K. Volumetric additive manufacturing via tomographic reconstruction. Science. 2019;363(6431):1075–1079. doi: 10.1126/science.aau7114. [DOI] [PubMed] [Google Scholar]
- 126.Loterie D., Delrot P., Moser C. High-resolution tomographic volumetric additive manufacturing. Nat. Commun. 2020;11(1):852. doi: 10.1038/s41467-020-14630-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Regehly M., Garmshausen Y., Reuter M., König N.F., Israel E., Kelly D.P., Chou C.-Y., Koch K., Asfari B., Hecht S. Xolography for linear volumetric 3D printing. Nature. 2020;588(7839):620–624. doi: 10.1038/s41586-020-3029-7. [DOI] [PubMed] [Google Scholar]
- 128.Lian L., Xie M., Luo Z., Zhang Z., Maharjan S., Mu X., Garciamendez-Mijares C.E., Kuang X., Sahoo J.K., Tang G., Li G., Wang D., Guo J., González F.Z., Abril Manjarrez Rivera V., Cai L., Mei X., Kaplan D.L., Zhang Y.S. Rapid volumetric bioprinting of decellularized extracellular matrix bioinks. Advanced Materials n/ 2024;a(n/a) doi: 10.1002/adma.202304846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Bernal P.N., Bouwmeester M., Madrid-Wolff J., Falandt M., Florczak S., Rodriguez N.G., Li Y., Größbacher G., Samsom R.-A., van Wolferen M., van der Laan L.J.W., Delrot P., Loterie D., Malda J., Moser C., Spee B., Levato R. Volumetric bioprinting of organoids and optically tuned hydrogels to build liver-like metabolic biofactories. Adv. Mater. 2022;34(15) doi: 10.1002/adma.202110054. [DOI] [PubMed] [Google Scholar]
- 130.Ribezzi D., Gueye M., Florczak S., Dusi F., de Vos D., Manente F., Hierholzer A., Fussenegger M., Caiazzo M., Blunk T., Malda J., Levato R. Shaping synthetic multicellular and complex multimaterial tissues via embedded extrusion-volumetric printing of microgels. Adv. Mater. 2023;35(36) doi: 10.1002/adma.202301673. [DOI] [PubMed] [Google Scholar]
- 131.Größbacher G., Bartolf-Kopp M., Gergely C., Bernal P.N., Florczak S., de Ruijter M., Rodriguez N.G., Groll J., Malda J., Jungst T., Levato R. Volumetric printing across melt electrowritten scaffolds fabricates multi-material living constructs with tunable architecture and mechanics. Adv. Mater. 2023;35(32) doi: 10.1002/adma.202300756. [DOI] [PubMed] [Google Scholar]
- 132.Madrid-Wolff J., Boniface A., Loterie D., Delrot P., Moser C. Controlling light in scattering materials for volumetric additive manufacturing. Adv. Sci. 2022;9(22) doi: 10.1002/advs.202105144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Jing S., Lian L., Hou Y., Li Z., Zheng Z., Li G., Tang G., Xie G., Xie M. Advances in volumetric bioprinting. Biofabrication. 2024;16(1) doi: 10.1088/1758-5090/ad0978. [DOI] [PubMed] [Google Scholar]
- 134.Florea L., Blasco E., Mattoli V. New frontiers in materials and technologies for 3D two photon polymerization. Adv. Funct. Mater. 2023;33(39) [Google Scholar]
- 135.Wang H., Pan C.-F., Li C., Menghrajani K.S., Schmidt M.A., Li A., Fan F., Zhou Y., Zhang W., Wang H., Nair P.N.S., Chan J.Y.E., Mori T., Hu Y., Hu G., Maier S.A., Ren H., Duan H., Yang J.K.W. Two-photon polymerization lithography for imaging optics. Int. J. Extrem. Manuf. 2024;6(4) [Google Scholar]
- 136.Brandenberg N., Lutolf M.P. In situ patterning of microfluidic networks in 3D cell-laden hydrogels. Adv. Mater. 2016;28(34):7450–7456. doi: 10.1002/adma.201601099. [DOI] [PubMed] [Google Scholar]
- 137.Heintz K.A., Bregenzer M.E., Mantle J.L., Lee K.H., West J.L., Slater J.H. Fabrication of 3D biomimetic microfluidic networks in hydrogels. Adv. Healthcare Mater. 2016;5(17):2153–2160. doi: 10.1002/adhm.201600351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Fei P., Ding H., Duan Y., Wang X., Hu W., Wu P., Wei M., Peng Z., Gu Z., Chen W. Utility of TPP-manufactured biophysical restrictions to probe multiscale cellular dynamics. Bio-Design and Manufacturing. 2021;4(4):776–789. [Google Scholar]
- 139.Gehre C., Qiu W., Klaus Jäger P., Wang X., Marques F.C., Nelson B.J., Müller R., Qin X.-H. Guiding bone cell network formation in 3D via photosensitized two-photon ablation. Acta Biomater. 2024;174:141–152. doi: 10.1016/j.actbio.2023.11.042. [DOI] [PubMed] [Google Scholar]
- 140.Tang L. Printing in vivo. Nat. Methods. 2020;17(8):758. doi: 10.1038/s41592-020-0920-y. 758. [DOI] [PubMed] [Google Scholar]
- 141.Urciuolo A., Poli I., Brandolino L., Raffa P., Scattolini V., Laterza C., Giobbe G.G., Zambaiti E., Selmin G., Magnussen M., Brigo L., De Coppi P., Salmaso S., Giomo M., Elvassore N. Intravital three-dimensional bioprinting. Nat. Biomed. Eng. 2020;4(9):901–915. doi: 10.1038/s41551-020-0568-z. [DOI] [PubMed] [Google Scholar]
- 142.Agostinacchio F., Fitzpatrick V., Dirè S., Kaplan D.L., Motta A. Silk fibroin-based inks for in situ 3D printing using a double crosslinking process. Bioact. Mater. 2024;35:122–134. doi: 10.1016/j.bioactmat.2024.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Hockaday L.A., Kang K.H., Colangelo N.W., Cheung P.Y.C., Duan B., Malone E., Wu J., Girardi L.N., Bonassar L.J., Lipson H., Chu C.C., Butcher J.T. Rapid 3D printing of anatomically accurate and mechanically heterogeneous aortic valve hydrogel scaffolds. Biofabrication. 2012;4(3) doi: 10.1088/1758-5082/4/3/035005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Kesti M., Müller M., Becher J., Schnabelrauch M., D'Este M., Eglin D., Zenobi-Wong M. A versatile bioink for three-dimensional printing of cellular scaffolds based on thermally and photo-triggered tandem gelation. Acta Biomater. 2015;11:162–172. doi: 10.1016/j.actbio.2014.09.033. [DOI] [PubMed] [Google Scholar]
- 145.Li W., Miao W., Liu Y., Wang T., Zhang Y., Wang W., Lu D., Zhou X., Jiao X., Jia X., Lin Y., Li Y., He H., Mao Y., Ma Z., Li T., Wang J. Bioprinted constructs that mimic the ossification center microenvironment for targeted innervation in bone regeneration. Adv. Funct. Mater. 2022;32(9) [Google Scholar]
- 146.Ma X., Huang X., Wang A., Sun T., Tai R., Li J., Qiao Z., Zhao L., Zhang T., Zhao Y. In Situ injectable photo-crosslinking hydrogel with heterojunction nanoparticles for dual-channel synergistic disinfection and cutaneous regeneration in diabetic chronic wound healing. Nano Today. 2024;56 [Google Scholar]
- 147.Gao G., Kim H., Kim B.S., Kong J.S., Lee J.Y., Park B.W., Chae S., Kim J., Ban K., Jang J., Park H.-J., Cho D.-W. Tissue-engineering of vascular grafts containing endothelium and smooth-muscle using triple-coaxial cell printing. Appl. Phys. Rev. 2019;6(4) [Google Scholar]
- 148.Li J., Mo L., Lu C.-H., Fu T., Yang H.-H., Tan W. Functional nucleic acid-based hydrogels for bioanalytical and biomedical applications. Chem. Soc. Rev. 2016;45(5):1410–1431. doi: 10.1039/c5cs00586h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Kim B.S., Das S., Jang J., Cho D.-W. Decellularized extracellular matrix-based bioinks for engineering tissue- and organ-specific microenvironments. Chem. Rev. 2020;120(19):10608–10661. doi: 10.1021/acs.chemrev.9b00808. [DOI] [PubMed] [Google Scholar]
- 150.Choi J.R., Yong K.W., Choi J.Y., Cowie A.C. Recent advances in photo-crosslinkable hydrogels for biomedical applications. Biotechniques. 2019;66(1):40–53. doi: 10.2144/btn-2018-0083. [DOI] [PubMed] [Google Scholar]
- 151.Mendes-Felipe C., Oliveira J., Etxebarria I., Vilas-Vilela J.L., Lanceros-Mendez S. State-of-the-Art and future challenges of UV curable polymer-based smart materials for printing technologies. Advanced Materials Technologies. 2019;4(3) [Google Scholar]
- 152.Pahoff S., Meinert C., Bas O., Nguyen L., Klein T.J., Hutmacher D.W. Effect of gelatin source and photoinitiator type on chondrocyte redifferentiation in gelatin methacryloyl-based tissue-engineered cartilage constructs. J. Mater. Chem. B. 2019;7(10):1761–1772. doi: 10.1039/c8tb02607f. [DOI] [PubMed] [Google Scholar]
- 153.Sun R., Ruccolo S., Nascimento D.L., Qin Y., Hibbert N., Nocera D.G. Chemoselective bond activation by unidirectional and asynchronous PCET using ketone photoredox catalysts. Chem. Sci. 2023;14(47):13776–13782. doi: 10.1039/d3sc04362b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Kim H., Kang B., Cui X., Lee S.-H., Lee K., Cho D.-W., Hwang W., Woodfield T.B.F., Lim K.S., Jang J. Light-activated decellularized extracellular matrix-based bioinks for volumetric tissue analogs at the centimeter scale. Adv. Funct. Mater. 2021;31(32) [Google Scholar]
- 155.Yang J., Chen Z., Gao C., Liu J., Liu K., Wang X., Pan X., Wang G., Sang H., Pan H., Liu W., Ruan C. A mechanical-assisted post-bioprinting strategy for challenging bone defects repair. Nat. Commun. 2024;15(1):3565. doi: 10.1038/s41467-024-48023-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Shao L., Gao Q., Xie C., Fu J., Xiang M., He Y. Directly coaxial 3D bioprinting of large-scale vascularized tissue constructs. Biofabrication. 2020;12(3) doi: 10.1088/1758-5090/ab7e76. [DOI] [PubMed] [Google Scholar]
- 157.Orellano I., Thomas A., Herrera A., Brauer E., Wulsten D., Petersen A., Kloke L., Duda G.N. Engineering vascular self-assembly by controlled 3D-printed cell placement. Adv. Funct. Mater. 2022;32(52) [Google Scholar]
- 158.Gao F., Xu Z., Liang Q., Liu B., Li H., Wu Y., Zhang Y., Lin Z., Wu M., Ruan C., Liu W. Direct 3D printing of high strength biohybrid gradient hydrogel scaffolds for efficient repair of osteochondral defect. Adv. Funct. Mater. 2018;28(13) [Google Scholar]
- 159.Pan C., Xu J., Gao Q., Li W., Sun T., Lu J., Shi Q., Han Y., Gao G., Li J. Sequentially suspended 3D bioprinting of multiple-layered vascular models with tunable geometries for in vitro modeling of arterial disorders initiation. Biofabrication. 2023;15(4) doi: 10.1088/1758-5090/aceffa. [DOI] [PubMed] [Google Scholar]
- 160.Liang M., Wei D., Ren P., Xu L., Tao Y., Yang L., Jiao G., Zhang T., Serizawa T. A visible light cross-linked underwater hydrogel adhesive with biodegradation and hemostatic ability. Adv. Healthcare Mater. 2024;13(7) doi: 10.1002/adhm.202302538. [DOI] [PubMed] [Google Scholar]
- 161.Wang Z., Kumar H., Tian Z., Jin X., Holzman J.F., Menard F., Kim K. Visible light photoinitiation of cell-adhesive gelatin methacryloyl hydrogels for stereolithography 3D bioprinting. ACS Appl. Mater. Interfaces. 2018;10(32):26859–26869. doi: 10.1021/acsami.8b06607. [DOI] [PubMed] [Google Scholar]
- 162.Chen L., Kenkel S.M., Hsieh P.-H., Gryka M.C., Bhargava R. Freeform three-dimensionally printed microchannels via surface-initiated photopolymerization combined with sacrificial molding. ACS Appl. Mater. Interfaces. 2020;12(44):50105–50112. doi: 10.1021/acsami.0c12158. [DOI] [PubMed] [Google Scholar]
- 163.Lin Y.-C., Nixon E.J., Wang H.-Y., Dhawan U., Huang Y.-W., Huang S.-H., Jiang C.-P., Kuo Y.-J., Chung R.-J. Photocrosslinked gelatin methacryloyl (GelMA)/Hyaluronic acid methacryloyl (HAMA) composite scaffold using anthocyanidin as a photoinitiator for bone tissue regeneration. ACS Appl. Polym. Mater. 2023;5(8):6012–6021. [Google Scholar]
- 164.Han W.T., Jang T., Chen S., Chong L.S.H., Jung H.-D., Song J. Improved cell viability for large-scale biofabrication with photo-crosslinkable hydrogel systems through a dual-photoinitiator approach. Biomater. Sci. 2020;8(1):450–461. doi: 10.1039/c9bm01347d. [DOI] [PubMed] [Google Scholar]
- 165.Bertolotti S.G., Previtali C.M., Rufs A.M., Encinas M.V. Riboflavin/triethanolamine as photoinitiator system of vinyl polymerization. A mechanistic study by laser flash photolysis. Macromolecules. 1999;32(9):2920–2924. [Google Scholar]
- 166.Petta D., Grijpma D.W., Alini M., Eglin D., D'Este M. Three-dimensional printing of a tyramine hyaluronan derivative with double gelation mechanism for independent tuning of shear thinning and postprinting curing. ACS Biomater. Sci. Eng. 2018;4(8):3088–3098. doi: 10.1021/acsbiomaterials.8b00416. [DOI] [PubMed] [Google Scholar]
- 167.De Moor L., Smet J., Plovyt M., Bekaert B., Vercruysse C., Asadian M., De Geyter N., Van Vlierberghe S., Dubruel P., Declercq H. Engineering microvasculature by 3D bioprinting of prevascularized spheroids in photo-crosslinkable gelatin. Biofabrication. 2021;13(4) doi: 10.1088/1758-5090/ac24de. [DOI] [PubMed] [Google Scholar]
- 168.Man K., Barroso I.A., Brunet M.Y., Peacock B., Federici A.S., Hoey D.A., Cox S.C. Controlled release of epigenetically-enhanced extracellular vesicles from a GelMA/nanoclay composite hydrogel to promote bone repair. Int. J. Mol. Sci. 2022;23(2):832. doi: 10.3390/ijms23020832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Ren X., Zhang J., Yang F., Xu H., Guo G., Wang Y. Enzyme-immobilized surface-catalyzed cross-linking: creating multifunctional double network hydrogel coatings on diverse substrates. Advanced Functional Materials n/ 2024;a(n/a) [Google Scholar]
- 170.Ozawa N., Sato Y., Mori Y., Masuda H., Yamane M., Yamamoto Y., Shirai R., Watanabe R., Sato K., Mori Y., Hirano T., Watanabe T. Legumain promotes atherosclerotic vascular remodeling. Int. J. Mol. Sci. 2019;20(9):2195. doi: 10.3390/ijms20092195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Kaufmann R., Deutschmann M., Meissnitzer M., Scharinger B., Hergan K., Votsch A., Dinges C., Hecht S. Manufacturing flexible vascular models for cardiovascular surgery planning and endovascular procedure simulations: an approach to segmentation and post-processing with open-source software and end-user 3D printers. Int J Bioprint. 2023;9(2):669. doi: 10.18063/ijb.v9i2.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Guarino S., Marchese E., Ponticelli G.S., Scerrati A., Tagliaferri V., Trovalusci F. Additive manufacturing for neurosurgery: digital light processing of individualized patient-specific cerebral aneurysms. Materials. 2021;14(20):6057. doi: 10.3390/ma14206057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Song B., Wang C., Fan S., Zhang L., Zhang C., Xiong W., Hu Y., Chu J., Wu D., Li J. Rapid construction of 3D biomimetic capillary networks with complex morphology using dynamic holographic processing. Adv. Funct. Mater. 2024;34(1) Adv. Funct. Mater. 1/2024. [Google Scholar]
- 174.Zhu W., Qu X., Zhu J., Ma X., Patel S., Liu J., Wang P., Lai C.S.E., Gou M., Xu Y., Zhang K., Chen S. Direct 3D bioprinting of prevascularized tissue constructs with complex microarchitecture. Biomaterials. 2017;124:106–115. doi: 10.1016/j.biomaterials.2017.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Miri A.K., Nieto D., Iglesias L., Goodarzi Hosseinabadi H., Maharjan S., Ruiz-Esparza G.U., Khoshakhlagh P., Manbachi A., Dokmeci M.R., Chen S., Shin S.R., Zhang Y.S., Khademhosseini A. Microfluidics-enabled multimaterial maskless stereolithographic bioprinting. Adv. Mater. 2018;30(27) doi: 10.1002/adma.201800242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Cui H., Esworthy T., Zhou X., Hann S.Y., Glazer R.I., Li R., Zhang L.G. Engineering a novel 3D printed vascularized tissue model for investigating breast cancer metastasis to bone. Adv. Healthcare Mater. 2020;9(15) doi: 10.1002/adhm.201900924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Paone L.S., Benmassaoud M.M., Curran A., Vega S.L., Galie P.A. A 3D-printed blood-brain barrier model with tunable topology and cell-matrix interactions. Biofabrication. 2024;16(1) doi: 10.1088/1758-5090/ad0260. [DOI] [PubMed] [Google Scholar]
- 178.Cao X., Ashfaq R., Cheng F., Maharjan S., Li J., Ying G., Hassan S., Xiao H., Yue K., Zhang Y.S. A tumor-on-a-chip system with bioprinted blood and lymphatic vessel pair. Adv. Funct. Mater. 2019;29(31) doi: 10.1002/adfm.201807173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Zhang R., Larsen N.B. Stereolithographic hydrogel printing of 3D culture chips with biofunctionalized complex 3D perfusion networks. Lab Chip. 2017;17(24):4273–4282. doi: 10.1039/c7lc00926g. [DOI] [PubMed] [Google Scholar]
- 180.Wang X.-Q., Xie A.-Q., Cao P., Yang J., Ong W.L., Zhang K.-Q., Ho G.W. Structuring and shaping of mechanically robust and functional hydrogels toward wearable and implantable applications. Adv. Mater. 2024;36(23) doi: 10.1002/adma.202309952. [DOI] [PubMed] [Google Scholar]
- 181.Ye T., Chai M., Wang Z., Shao T., Liu J., Shi X. 3D-Printed hydrogels with engineered nanocrystalline domains as functional vascular constructs. ACS Nano. 2024;18:25765–25777. doi: 10.1021/acsnano.4c08359. [DOI] [PubMed] [Google Scholar]
- 182.Liang Q., Gao F., Zeng Z., Yang J., Wu M., Gao C., Cheng D., Pan H., Liu W., Ruan C. Coaxial scale-up printing of diameter-tunable biohybrid hydrogel microtubes with high strength, perfusability, and endothelialization. Adv. Funct. Mater. 2020;30(43) [Google Scholar]
- 183.Wang X., Liu X., Liu W., Liu Y., Li A., Qiu D., Zheng X., Gu Q. 3D bioprinting microgels to construct implantable vascular tissue. Cell Prolif. 2023;56(5) doi: 10.1111/cpr.13456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Rizzo R., Ruetsche D., Liu H., Zenobi-Wong M. Optimized photoclick (Bio)Resins for fast volumetric bioprinting. Adv. Mater. 2021;33(49) doi: 10.1002/adma.202102900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Mahjoubnia A., Cai D., Wu Y., King S.D., Torkian P., Chen A.C., Talaie R., Chen S.-Y., Lin J. Digital light 4D printing of bioresorbable shape memory elastomers for personalized biomedical implantation. Acta Biomater. 2024;177:165–177. doi: 10.1016/j.actbio.2024.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Ye M., Lu B., Zhang X., Li B., Xiong Z., Zhang T. Coaxial embedded printing of gelatin methacryloyl–alginate double network hydrogel for multilayer vascular tubes. Chin. J. Mech. Eng.: Additive Manufacturing Frontiers. 2022;1(2) [Google Scholar]
- 187.Zhou X., Nowicki M., Sun H., Hann S.Y., Cui H., Esworthy T., Lee J.D., Plesniak M., Zhang L.G. 3D bioprinting-tunable small-diameter blood vessels with biomimetic biphasic cell layers. ACS Appl. Mater. Interfaces. 2020;12(41):45904–45915. doi: 10.1021/acsami.0c14871. [DOI] [PubMed] [Google Scholar]
- 188.Li W., Yin Y., Zhou H., Fan Y., Yang Y., Gao Q., Li P., Gao G., Li J. Recent advances in electrospinning techniques for precise medicine. Cyborg Bionic Syst. 2024;5:101. doi: 10.34133/cbsystems.0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Grigoryan B., Paulsen S.J., Corbett D.C., Sazer D.W., Fortin C.L., Zaita A.J., Greenfield P.T., Calafat N.J., Gounley J.P., Ta A.H., Johansson F., Randles A., Rosenkrantz J.E., Louis-Rosenberg J.D., Galie P.A., Stevens K.R., Miller J.S. Multivascular networks and functional intravascular topologies within biocompatible hydrogels. Science. 2019;364(6439):458–464. doi: 10.1126/science.aav9750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Mazari-Arrighi E., Lépine M., Ayollo D., Faivre L., Larghero J., Chatelain F., Fuchs A. Self-organization of long-lasting human endothelial capillary-like networks guided by DLP bioprinting. Adv. Healthcare Mater. 2024;13 doi: 10.1002/adhm.202302830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Enrico A., Voulgaris D., Östmans R., Sundaravadivel N., Moutaux L., Cordier A., Niklaus F., Herland A., Stemme G. 3D microvascularized tissue models by laser-based cavitation molding of collagen. Adv. Mater. 2022;34(11) doi: 10.1002/adma.202109823. [DOI] [PubMed] [Google Scholar]
- 192.Liu X., Wang X., Zhang L., Sun L., Wang H., Zhao H., Zhang Z., Liu W., Huang Y., Ji S., Zhang J., Li K., Song B., Li C., Zhang H., Li S., Wang S., Zheng X., Gu Q. 3D liver tissue model with branched vascular networks by multimaterial bioprinting. Adv. Healthcare Mater. 2021;10(23) doi: 10.1002/adhm.202101405. [DOI] [PubMed] [Google Scholar]
- 193.Xie M., Sun Y., Wang J., Fu Z., Pan L., Chen Z., Fu J., He Y. Thermo-sensitive sacrificial microsphere-based bioink for centimeter-scale tissue with angiogenesis. Int J Bioprint. 2022;8(4):599. doi: 10.18063/ijb.v8i4.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Hull S.M., Brunel L.G., Heilshorn S.C. 3D bioprinting of cell-laden hydrogels for improved biological functionality. Adv. Mater. 2022;34(2) doi: 10.1002/adma.202103691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Matai I., Kaur G., Seyedsalehi A., McClinton A., Laurencin C.T. Progress in 3D bioprinting technology for tissue/organ regenerative engineering. Biomaterials. 2020;226 doi: 10.1016/j.biomaterials.2019.119536. [DOI] [PubMed] [Google Scholar]
- 196.Byambaa B., Annabi N., Yue K., Trujillo-de Santiago G., Alvarez M.M., Jia W., Kazemzadeh-Narbat M., Shin S.R., Tamayol A., Khademhosseini A. Bioprinted osteogenic and vasculogenic patterns for engineering 3D bone tissue. Adv. Healthcare Mater. 2017;6(16) doi: 10.1002/adhm.201700015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Fedorovich N.E., Oudshoorn M.H., van Geemen D., Hennink W.E., Alblas J., Dhert W.J.A. The effect of photopolymerization on stem cells embedded in hydrogels. Biomaterials. 2009;30(3):344–353. doi: 10.1016/j.biomaterials.2008.09.037. [DOI] [PubMed] [Google Scholar]
- 198.Colosi C., Shin S.R., Manoharan V., Massa S., Costantini M., Barbetta A., Dokmeci M.R., Dentini M., Khademhosseini A. Microfluidic bioprinting of heterogeneous 3D tissue constructs using low-viscosity bioink. Adv. Mater. 2016;28(4):677–684. doi: 10.1002/adma.201503310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.You S., Xiang Y., Hwang H.H., Berry D.B., Kiratitanaporn W., Guan J., Yao E., Tang M., Zhong Z., Ma X., Wangpraseurt D., Sun Y., Lu T.-y., Chen S. High cell density and high-resolution 3D bioprinting for fabricating vascularized tissues. Sci. Adv. 2023;9(8) doi: 10.1126/sciadv.ade7923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Lee S., Sani E.S., Spencer A.R., Guan Y., Weiss A.S., Annabi N. Human-recombinant-elastin-based bioinks for 3D bioprinting of vascularized soft tissues. Adv. Mater. 2020;32(45) doi: 10.1002/adma.202003915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Ouyang L., Armstrong J.P.K., Chen Q., Lin Y., Stevens M.M. Void-free 3D bioprinting for in situ endothelialization and microfluidic perfusion. Adv. Funct. Mater. 2020;30(1) doi: 10.1002/adfm.201908349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Tang G., Luo Z., Lian L., Guo J., Maharjan S., Garciamendez-Mijares C.E., Wang M., Li W., Zhang Z., Wang D., Xie M., Ravanbakhsh H., Zhou C., Kuang X., Hou Y., Yu X., Zhang Y.S. Liquid-embedded (bio)printing of alginate-free, standalone, ultrafine, and ultrathin-walled cannular structures. Proc. Natl. Acad. Sci. USA. 2023;120(7) doi: 10.1073/pnas.2206762120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Li J., Pumera M. 3D printing of functional microrobots. Chem. Soc. Rev. 2021;50(4):2794–2838. doi: 10.1039/d0cs01062f. [DOI] [PubMed] [Google Scholar]
- 204.Rajabasadi F., Schwarz L., Medina-Sánchez M., Schmidt O.G. 3D and 4D lithography of untethered microrobots. Prog. Mater. Sci. 2021;120 [Google Scholar]
- 205.Liang X., Zhao Y., Liu D., Deng Y., Arai T., Kojima M., Liu X. Magnetic microrobots fabricated by photopolymerization and assembly. Cyborg Bionic Syst. 2023;4 doi: 10.34133/cbsystems.0060. 0060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Liang X., Chen Z., Deng Y., Liu D., Liu X., Huang Q., Arai T. Field-controlled microrobots fabricated by photopolymerization. Cyborg Bionic Syst. 2023;4 doi: 10.34133/cbsystems.0009. https://spj.science.org/doi/10.34133/cbsystems.0009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Kuang X., Rong Q., Belal S., Vu T., López López A.M., Wang N., Arıcan M.O., Garciamendez-Mijares C.E., Chen M., Yao J., Zhang Y.S. Self-enhancing sono-inks enable deep-penetration acoustic volumetric printing. Science. 2023;382(6675):1148–1155. doi: 10.1126/science.adi1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Levato R., Dudaryeva O., Garciamendez-Mijares C.E., Kirkpatrick B.E., Rizzo R., Schimelman J., Anseth K.S., Chen S., Zenobi-Wong M., Zhang Y.S. Light-based vat-polymerization bioprinting. Nature Reviews Methods Primers. 2023;3(1):47. [Google Scholar]
- 209.McCormack A., Highley C.B., Leslie N.R., Melchels F.P.W. 3D printing in suspension baths: keeping the promises of bioprinting afloat. Trends Biotechnol. 2020;38(6):584–593. doi: 10.1016/j.tibtech.2019.12.020. [DOI] [PubMed] [Google Scholar]
- 210.Fang Y., Guo Y., Wu B., Liu Z., Ye M., Xu Y., Ji M., Chen L., Lu B., Nie K., Wang Z., Luo J., Zhang T., Sun W., Xiong Z. Expanding embedded 3D bioprinting capability for engineering complex organs with freeform vascular networks. Adv. Mater. 2023;35(22) doi: 10.1002/adma.202205082. [DOI] [PubMed] [Google Scholar]
- 211.Yu C., Schimelman J., Wang P., Miller K.L., Ma X., You S., Guan J., Sun B., Zhu W., Chen S. Photopolymerizable biomaterials and light-based 3D printing strategies for biomedical applications. Chem. Rev. 2020;120(19):10695–10743. doi: 10.1021/acs.chemrev.9b00810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Daly A.C., Prendergast M.E., Hughes A.J., Burdick J.A. Bioprinting for the Biologist, Cell. 2021;184(1):18–32. doi: 10.1016/j.cell.2020.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Mo X., Zhang Y., Wang Z., Zhou X., Zhang Z., Fang Y., Fan Z., Guo Y., Zhang T., Xiong Z. Satellite-based on-orbit printing of 3D tumor models. Adv. Mater. 2024;36(34) doi: 10.1002/adma.202309618. [DOI] [PubMed] [Google Scholar]
- 214.Wang Y., Qin P., Hong J., Li N., Zhang Y., Deng Y. Deep membrane proteome profiling of rat Hippocampus in simulated complex space environment by SWATH. Space: Sci. Technol. 2021;2021 [Google Scholar]
- 215.Li B., Li T., Han C., Liu Y., Zhong X., Cao Y., Deng Y. Potential of dragon's blood as a space radiation protectant especially on brain-liver bystander effect. Space: Sci. Technol. 2022;2022 [Google Scholar]
- 216.Mishra B., Luderer U. Reproductive hazards of space travel in women and men. Nat. Rev. Endocrinol. 2019;15(12):713–730. doi: 10.1038/s41574-019-0267-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Chua C.Y.X., Jimenez M., Mozneb M., Traverso G., Lugo R., Sharma A., Svendsen C.N., Wagner W.R., Langer R., Grattoni A. Advanced material technologies for space and terrestrial medicine. Nat. Rev. Mater. 2024:1–14. [Google Scholar]
- 218.Paulsen K., Thiel C., Timm J., Schmidt P.M., Huber K., Tauber S., Hemmersbach R., Seibt D., Kroll H., Grote K.-H., Zipp F., Schneider-Stock R., Cogoli A., Hilliger A., Engelmann F., Ullrich O. Microgravity-induced alterations in signal transduction in cells of the immune system. Acta Astronaut. 2010;67(9):1116–1125. [Google Scholar]
- 219.Afshinnekoo E., Scott R.T., MacKay M.J., Pariset E., Cekanaviciute E., Barker R., Gilroy S., Hassane D., Smith S.M., Zwart S.R., Nelman-Gonzalez M., Crucian B.E., Ponomarev S.A., Orlov O.I., Shiba D., Muratani M., Yamamoto M., Richards S.E., Vaishampayan P.A., Meydan C., Foox J., Myrrhe J., Istasse E., Singh N., Venkateswaran K., Keune J.A., Ray H.E., Basner M., Miller J., Vitaterna M.H., Taylor D.M., Wallace D., Rubins K., Bailey S.M., Grabham P., Costes S.V., Mason C.E., Beheshti A. Fundamental biological features of spaceflight: advancing the field to enable deep-space exploration. Cell. 2020;183(5):1162–1184. doi: 10.1016/j.cell.2020.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Yan R., Zhang Y., Li Y., Wang J., Bibi H., Deng Y.-L., Li Y. Dragon's blood protect rat blood-brain barrier dysfunction induced by simulated microgravity effect. Space: Sci. Technol. 2023;3:71. [Google Scholar]
- 221.Scotti M.M., Wilson B.K., Bubenik J.L., Yu F., Swanson M.S., Allen J.B. Spaceflight effects on human vascular smooth muscle cell phenotype and function. npj Microgravity. 2024;10(1):41. doi: 10.1038/s41526-024-00380-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Mao W., Huai Y., An L., Wang X., Ru K., Patil S., Zhang W., Ran F., Chen Z., Qian A. Microgravity inhibits cell proliferation and promotes senescence and apoptosis in embryonic stem cells, space. Sci. Technol. 2024;4:104. [Google Scholar]
- 223.Yin Y., Yang J., Gao G., Zhou H., Chi B., Yang H., Li J., Wang Y. Enhancing cell-scale performance via sustained release of varicella-zoster virus antigen from microneedle patch under simulated microgravity. Biomater. Sci. 2024;12:763–775. doi: 10.1039/d3bm01440a. [DOI] [PubMed] [Google Scholar]
- 224.Cubo-Mateo N., Podhajsky S., Knickmann D., Slenzka K., Ghidini T., Gelinsky M. Can 3D bioprinting be a key for exploratory missions and human settlements on the Moon and Mars? Biofabrication. 2020;12(4) doi: 10.1088/1758-5090/abb53a. [DOI] [PubMed] [Google Scholar]
- 225.Sun W., Starly B., Daly A.C., Burdick J.A., Groll J., Skeldon G., Shu W., Sakai Y., Shinohara M., Nishikawa M., Jang J., Cho D.-W., Nie M., Takeuchi S., Ostrovidov S., Khademhosseini A., Kamm R.D., Mironov V., Moroni L., Ozbolat I.T. The bioprinting roadmap. Biofabrication. 2020;12(2) doi: 10.1088/1758-5090/ab5158. [DOI] [PubMed] [Google Scholar]
- 226.Cubo-Mateo N., Gelinsky M. Wound and skin healing in space: the 3D bioprinting perspective. Front. Bioeng. Biotechnol. 2021;9 doi: 10.3389/fbioe.2021.720217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Sharma A., Clemens R.A., Garcia O., Taylor D.L., Wagner N.L., Shepard K.A., Gupta A., Malany S., Grodzinsky A.J., Kearns-Jonker M., Mair D.B., Kim D.-H., Roberts M.S., Loring J.F., Hu J., Warren L.E., Eenmaa S., Bozada J., Paljug E., Roth M., Taylor D.P., Rodrigue G., Cantini P., Smith A.W., Giulianotti M.A., Wagner W.R. Biomanufacturing in low Earth orbit for regenerative medicine. Stem Cell Rep. 2022;17(1):1–13. doi: 10.1016/j.stemcr.2021.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Silvani G., Bradbury P., Basirun C., Mehner C., Zalli D., Poole K., Chou J. Testing 3D printed biological platform for advancing simulated microgravity and space mechanobiology research. npj Microgravity. 2022;8(1):19. doi: 10.1038/s41526-022-00207-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Moroni L., Tabury K., Stenuit H., Grimm D., Baatout S., Mironov V. What can biofabrication do for space and what can space do for biofabrication? Trends Biotechnol. 2022;40(4):398–411. doi: 10.1016/j.tibtech.2021.08.008. [DOI] [PubMed] [Google Scholar]
- 230.Van Ombergen A., Chalupa-Gantner F., Chansoria P., Colosimo B.M., Costantini M., Domingos M., Dufour A., De Maria C., Groll J., Jungst T., Levato R., Malda J., Margarita A., Marquette C., Ovsianikov A., Petiot E., Read S., Surdo L., Swieszkowski W., Vozzi G., Windisch J., Zenobi-Wong M., Gelinsky M. 3D bioprinting in microgravity: opportunities, challenges, and possible applications in space. Adv. Healthcare Mater. 2023;12(23) doi: 10.1002/adhm.202300443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Windisch J., Reinhardt O., Duin S., Schütz K., Rodriguez N.J.N., Liu S., Lode A., Gelinsky M. Bioinks for space missions: the influence of long-term storage of alginate-methylcellulose-based bioinks on printability as well as cell viability and function. Adv. Healthcare Mater. 2023;12(23) doi: 10.1002/adhm.202300436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Majumder N., Ghosh S. 3D biofabrication and space: a ‘far-fetched dream’ or a ‘forthcoming reality’? Biotechnol. Adv. 2023;69 doi: 10.1016/j.biotechadv.2023.108273. [DOI] [PubMed] [Google Scholar]
- 233.Ren Z., Harriot A.D., Mair D.B., Chung M.K., Lee P.H.U., Kim D.-H. Biomanufacturing of 3D tissue constructs in microgravity and their applications in human pathophysiological studies. Adv. Healthcare Mater. 2023;12(23) doi: 10.1002/adhm.202300157. [DOI] [PubMed] [Google Scholar]
- 234.Wu X.-T., Yang X., Tian R., Li Y.-H., Wang C.-Y., Fan Y.-B., Sun L.-W. Cells respond to space microgravity through cytoskeleton reorganization. Faseb. J. 2022;36(2) doi: 10.1096/fj.202101140R. [DOI] [PubMed] [Google Scholar]
- 235.Ludtka C., Silberman J., Moore E., Allen J.B. Macrophages in microgravity: the impact of space on immune cells. npj Microgravity. 2021;7(1):13. doi: 10.1038/s41526-021-00141-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Juhl O.J., Buettmann E.G., Friedman M.A., DeNapoli R.C., Hoppock G.A., Donahue H.J. Update on the effects of microgravity on the musculoskeletal system. npj Microgravity. 2021;7(1):28. doi: 10.1038/s41526-021-00158-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Blaber E., Marçal H., Burns B.P. Bioastronautics: the influence of microgravity on astronaut health. Astrobiology. 2010;10(5):463–473. doi: 10.1089/ast.2009.0415. [DOI] [PubMed] [Google Scholar]
- 238.Wu F., Du H., Overbey E., Kim J., Makhijani P., Martin N., Lerner C.A., Nguyen K., Baechle J., Valentino T.R., Fuentealba M., Bartleson J.M., Halaweh H., Winer S., Meydan C., Garrett-Bakelman F., Sayed N., Melov S., Muratani M., Gerencser A.A., Kasler H.G., Beheshti A., Mason C.E., Furman D., Winer D.A. Single-cell analysis identifies conserved features of immune dysfunction in simulated microgravity and spaceflight. Nat. Commun. 2024;15(1):4795. doi: 10.1038/s41467-023-42013-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Caddy H.T., Kelsey L.J., Parker L.P., Green D.J., Doyle B.J. Modelling large scale artery haemodynamics from the heart to the eye in response to simulated microgravity. npj Microgravity. 2024;10(1):7. doi: 10.1038/s41526-024-00348-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Luo J., Yang J., Zhao J., Cui B., Cui Y., Tang S., Wang A., Chen Y., Wang J., Yan J., Wang G., Han H., Du J. Effects of short-term simulated microgravity on changes in extracellular space structure and substance diffusion and clearance. Acta Astronaut. 2024;215:405–414. [Google Scholar]
- 241.Li Y., Huang L., Iqbal J., Deng Y. Investigation on P-glycoprotein function and its interacting proteins under simulated microgravity. Space: Sci. Technol. 2021;2021 [Google Scholar]
- 242.Jemison M., Olabisi R. Biomaterials for human space exploration: a review of their untapped potential. Acta Biomater. 2021;128:77–99. doi: 10.1016/j.actbio.2021.04.033. [DOI] [PubMed] [Google Scholar]
- 243.Cinelli I., Russomano T. Advances in space medicine applied to pandemics on earth. Space: Sci. Technol. 2021;2021 [Google Scholar]
- 244.Cui Y., Liu W., Zhao S., Zhao Y., Dai J. Advances in microgravity directed tissue engineering. Adv. Healthcare Mater. 2023;12(23) doi: 10.1002/adhm.202202768. [DOI] [PubMed] [Google Scholar]
- 245.Banimohamad-Shotorbani B., Azizsoltani A., Khalaj Z., Rafiei-Baharloo M., Ghotaslou A., Fathi-karkan S. Exploring the frontier of space medicine: the nexus of bone regeneration and astronautic health in microgravity conditions. Microgravity Sci. Technol. 2024;36(5):51. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.