Abstract
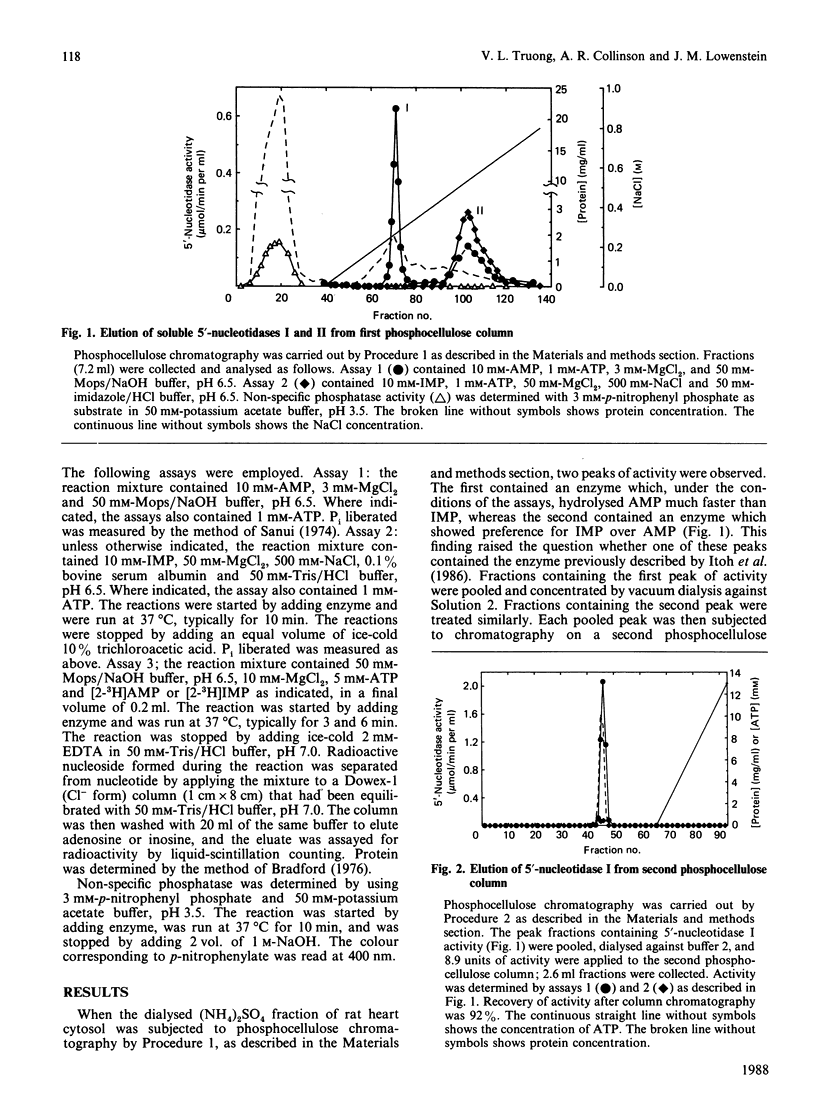
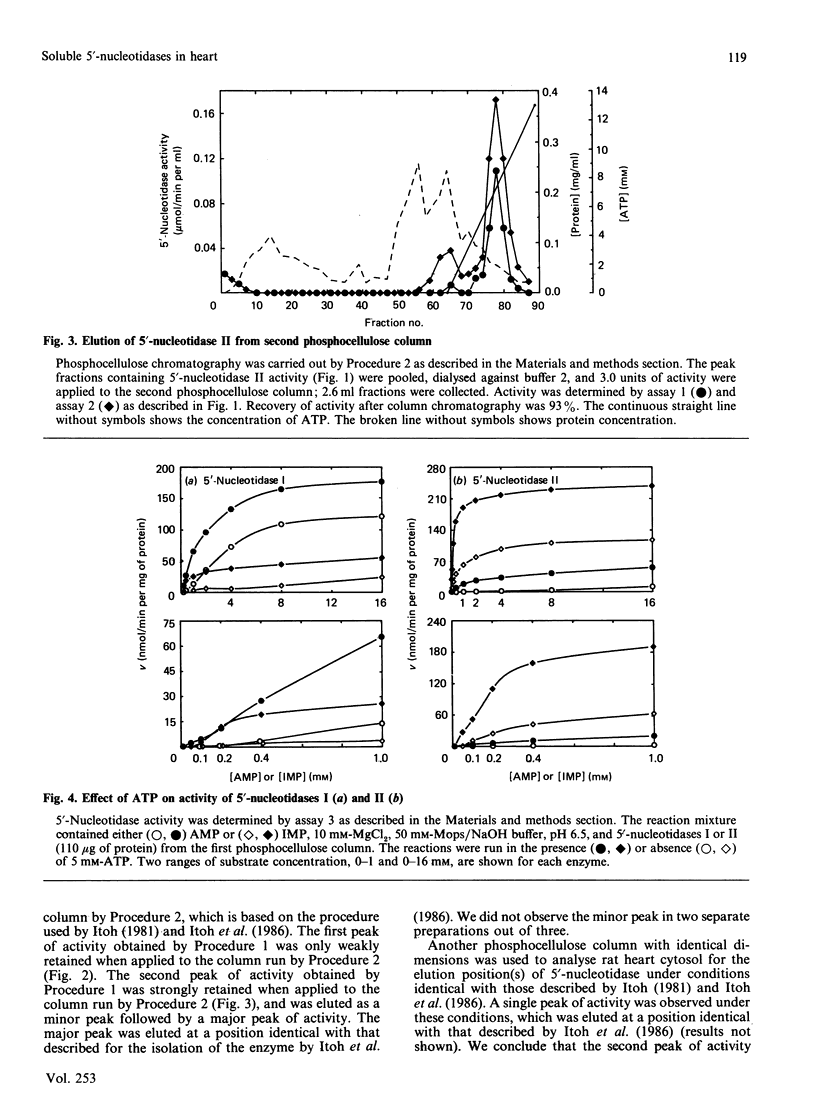
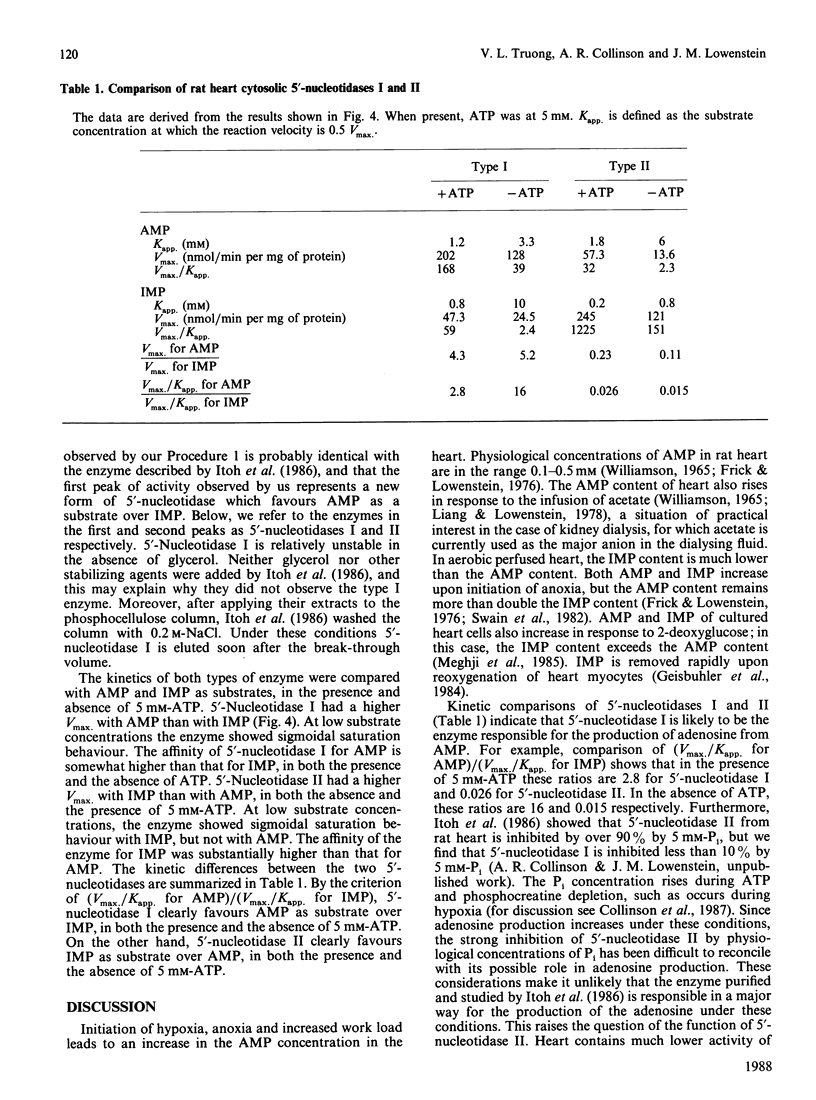
Chromatography of soluble proteins from rat heart on phosphocellulose columns separates two 5'-nucleotidases. The first to emerge from the column shows a preference for AMP over IMP as substrate, whereas the second shows a preference for IMP over AMP. The properties of the IMP-preferring enzyme, including the conditions under which it is eluted from phosphocellulose columns, show it to be the enzyme studied by Itoh, Oka & Ozasa [Biochem. J. (1986) 235, 847-851]. The kinetic properties of the AMP-preferring enzyme indicate that it is likely to be the enzyme responsible for the production of adenosine under conditions of hypoxia and increased work load, and with metabolic stresses such as a high load of acetate.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Burger R., Lowenstein J. M. Adenylate deaminase. 3. Regulation of deamination pathways in extracts of rat heart and lung. J Biol Chem. 1967 Nov 25;242(22):5281–5288. [PubMed] [Google Scholar]
- Frick G. P., Lowenstein J. M. Studies of 5'-nucleotidase in the perfused rat heart. Including measurements of the enzyme in perfused skeletal muscle and liver. J Biol Chem. 1976 Oct 25;251(20):6372–6378. [PubMed] [Google Scholar]
- Geisbuhler T., Altschuld R. A., Trewyn R. W., Ansel A. Z., Lamka K., Brierley G. P. Adenine nucleotide metabolism and compartmentalization in isolated adult rat heart cells. Circ Res. 1984 May;54(5):536–546. doi: 10.1161/01.res.54.5.536. [DOI] [PubMed] [Google Scholar]
- Gibson W. B., Drummond G. I. Properties of 5'-nucleotidase from avian heart. Biochemistry. 1972 Jan 18;11(2):223–229. doi: 10.1021/bi00752a013. [DOI] [PubMed] [Google Scholar]
- Itoh R., Oka J. Evidence for existence of a cytosol 5'-nucleotidase in chicken heart: comparison of some properties of heart and liver enzymes. Comp Biochem Physiol B. 1985;81(1):159–163. doi: 10.1016/0305-0491(85)90177-4. [DOI] [PubMed] [Google Scholar]
- Itoh R., Oka J., Ozasa H. Regulation of rat heart cytosol 5'-nucleotidase by adenylate energy charge. Biochem J. 1986 May 1;235(3):847–851. doi: 10.1042/bj2350847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh R. Purification and some properties of cytosol 5'-nucleotidase from rat liver. Biochim Biophys Acta. 1981 Feb 13;657(2):402–410. doi: 10.1016/0005-2744(81)90326-0. [DOI] [PubMed] [Google Scholar]
- Itoh R. Studies on some molecular properties of cytosol 5'-nucleotidase from rat liver. Biochim Biophys Acta. 1982 May 5;716(1):110–113. doi: 10.1016/0304-4165(82)90208-2. [DOI] [PubMed] [Google Scholar]
- Liang C. S., Lowenstein J. M. Metabolic control of the circulation. Effects of acetate and pyruvate. J Clin Invest. 1978 Nov;62(5):1029–1038. doi: 10.1172/JCI109207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madrid-Marina V., Fox I. H. Human placental cytoplasmic 5'-nucleotidase. Kinetic properties and inhibition. J Biol Chem. 1986 Jan 5;261(1):444–452. [PubMed] [Google Scholar]
- Madrid-Marina V., Fox I. H. Human placental cytoplasmic 5'-nucleotidase. Kinetic properties and inhibition. J Biol Chem. 1986 Jan 5;261(1):444–452. [PubMed] [Google Scholar]
- Meghji P., Holmquist C. A., Newby A. C. Adenosine formation and release from neonatal-rat heart cells in culture. Biochem J. 1985 Aug 1;229(3):799–805. doi: 10.1042/bj2290799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naito Y., Tsushima K. Cytosol 5'-nucleotidase from chicken liver. Purification and some properties. Biochim Biophys Acta. 1976 Jun 7;438(1):159–168. doi: 10.1016/0005-2744(76)90232-1. [DOI] [PubMed] [Google Scholar]
- Sanui H. Measurement of inorganic orthophosphate in biological materials: extraction properties of butyl acetate. Anal Biochem. 1974 Aug;60(2):489–504. doi: 10.1016/0003-2697(74)90259-0. [DOI] [PubMed] [Google Scholar]
- Schütz W., Schrader J., Gerlach E. Different sites of adenosine formation in the heart. Am J Physiol. 1981 Jun;240(6):H963–H970. doi: 10.1152/ajpheart.1981.240.6.H963. [DOI] [PubMed] [Google Scholar]
- Swain J. L., Sabina R. L., McHale P. A., Greenfield J. C., Jr, Holmes E. W. Prolonged myocardial nucleotide depletion after brief ischemia in the open-chest dog. Am J Physiol. 1982 May;242(5):H818–H826. doi: 10.1152/ajpheart.1982.242.5.H818. [DOI] [PubMed] [Google Scholar]
- WILLIAMSON J. R. GLYCOLYTIC CONTROL MECHANISMS. I. INHIBITION OF GLYCOLYSIS BY ACETATE AND PYRUVATE IN THE ISOLATED, PERFUSED RAT HEART. J Biol Chem. 1965 Jun;240:2308–2321. [PubMed] [Google Scholar]


