ABSTRACT
Reductions in all-cause otitis media (OM) following widespread pneumococcal conjugate vaccine use have plateaued. Granular burden of disease data are needed to guide evaluation and implementation of new measures targeting OM prevention. We conducted a retrospective study to assess the incidence and treatment costs of OM, tympanostomy tube placement (TTP), and hearing loss in children aged <5 years in the United States (US). OM episodes and TTP between 2016 and 2017 were identified in IBM MarketScan Commercial Claims and Encounters, Medicare Supplemental and Coordination of Benefits, and Multi-Medicaid databases using diagnosis codes (ICD-10). The incidence rate per 100,000 person-years (IR) of OM in <5-year-olds was 62,726 in Commercial/Medicare databases and 55,874 in Medicaid. IRs peaked at 9–<12 months (115,552 and 110,960, respectively). Approximately 5% and 4% of OM episodes in the respective databases had TTP (IR 3233 and 2404). Around 2% of children with OM had hearing loss (IR 1468 and 1109, respectively), of whom 41% had TTP. We estimated that there were 11.1 million OM episodes in 2020 costing USD 4.8 billion. 243,618 children were estimated to have OM with hearing loss at a direct total cost of USD 637 million, or 13% of the overall cost. The clinical and economic burden attributable to OM in the US was high in children aged <5 years during the study period. Novel approaches are needed to improve and broaden vaccine-induced protection against OM and its complications. The study results could guide policymakers considering age-specific interventions to reduce the OM burden.
KEYWORDS: Cost, ear infection, epidemiology, hearing loss, Incidence, otitis media, pneumococcal conjugate vaccine, tympanostomy tube placement, vaccine
Plain Language Summary
What is the context?
Pneumococcal vaccines administered to young infants can prevent some, but not all cases of middle ear infection (otitis media).
We assessed the burden of middle ear infection in children aged less than 5 years in the United States (US) and estimated the cost to the healthcare system.
This study also looked at the incidence of middle ear infection with hearing loss and the costs associated with its treatment.
What is new?
We estimated the burden of hearing loss in children with otitis media.
The study showed that middle ear infection is still a very common disease in young children, especially between 9 and 12 months of age.
Most children with middle ear infection are treated as outpatients. Around 4% have a ventilation tube inserted and around 2% have middle ear infection with hearing loss.
We estimated that the cost of treating middle ear infection in the US is USD 4.8 billion.
What is the impact?
Middle ear infection remains a common disease in young children.
New methods to prevent middle ear infection need to be given early in life because disease rates start to increase from the age of 6 months.
Introduction
Otitis media (OM) is one of the most common infections in children under 5 years of age. Prior to the availability of pneumococcal conjugate vaccines (PCVs), 80% of children in the United States (US) experienced at least 1 episode of OM by the age of 3 years, and around 46% experienced multiple episodes.1,2 The introduction of PCV into universal childhood immunization programs, as well as changes in diagnostic criteria for acute OM, had marked impacts on the epidemiology of OM, characterized by a decrease in the incidence and frequency of OM, and a change in the distribution of pathogens causing OM.3,4 In a prospective study in the post-PCV years between 2006 and 2016, the percentage of US children experiencing at least 1 episode of OM by age 3 years had reduced to 60%, and those experiencing multiple episodes to 24%.3 The incidence of OM in children under 2 years of age declined from 1170.1 per 1000 person-years in the pre-PCV7 (7-valent PCV) period (1998–1999), to 768.8 per 1000 person-years in the late PCV13 (13-valent PCV) period (2014–2018).5 The incidence rate of OM-related surgical procedures such as myringotomy and tympanostomy tube placement (TTP) also declined.5 Non-typeable Haemophilus influenzae (NTHi) emerged as the most commonly identified pathogen, present in 34% of OM cases and as many as 54% of cases of severe OM.4,6 Streptococcus pneumoniae continues to be an important pathogen in OM in children but are dominated by non-PCV13 types and high levels of antimicrobial resistance.4 Moraxella catarrhalis continues to cause a substantial proportion of OM cases (around 15% in one study).4
Despite initial gains, the post-PCV incidence of OM appears to have plateaued, and the burden of disease and associated healthcare resource consumption due to OM remain high.5,7,8 OM risk factors and the incidence of OM-related complications such as tympanic membrane perforation, otorrhea, and acute mastoiditis, have not changed in the post-PCV period, and OM remains a key reason for prescribing antimicrobials in children.3,5,7 Mean direct medical costs associated with OM are estimated to be approximately USD 4.4 billion annually.7,8 However, these estimates do not include information on indirect costs or the socioeconomic costs of parental work absenteeism and the impact of complications of OM such as temporary or long-term hearing loss that may require surgical reconstruction and/or hearing aids.9
We conducted a literature review on the disease burden and direct medical costs associated with OM.3,5,7,8 To our knowledge, current research does not account for the burden and cost associated with hearing loss following OM. Hearing loss represents one of the main complications associated with OM, with chronic OM accounting for >50% of the global burden of hearing impairment.9 Chronic OM is a heterogeneous condition defined by persistent inflammation of the middle ear and/or mastoid cavity. Furthermore, incidence rates often lack granularity in terms of age breakdown, making it difficult to understand where peak incidences occur. To address this data gap, we evaluated the incidence of OM episodes by age group, as well as the costs associated with OM, TTP, and hearing loss in children less than 5 years of age in the US.
Patients and methods
Data source
This study used the IBM MarketScan Commercial Claims and Encounters (Commercial) database, the IBM MarketScan Medicare Supplemental and Coordination of Benefits (Medicare) database, and the IBM MarketScan Multi-Medicaid (Medicaid) database.10 The IBM MarketScan Research Databases include de-identified healthcare claims data at the individual level, encompassing clinical utilization, expenditures, insurance enrollment, and plan benefits.8,11 These data cover inpatient, outpatient, prescription drug, and carve-out services for a large population, including individuals and their dependents covered under employer-provided commercial insurance in the US. The database is recognized as representative of the US population covered by employer-provided health insurance and is extensively utilized to assess the impact and healthcare utilization patterns associated with various illnesses in the country.8
The Commercial database contains data from active employees, early retirees, and dependents insured by employer sponsored plans. It covers approximately 40 million beneficiaries and is representative of the commercially insured population of the US. The Medicare database holds data from Medicare-eligible retirees with employer-sponsored Medicare Supplemental plans. The Commercial and Medicare databases were merged and analyzed together to maintain continuity in the follow-up of subjects switching from a commercially insured status to a Medicare-insured status. Data from the Medicaid database were analyzed separately.
Study design
We conducted a retrospective, observational, descriptive study using MarketScan data to estimate the incidence of OM, OM with TPP, and OM with hearing loss, and the associated costs (Figure 1). The study comprised children less than 5 years of age who were continuously enrolled in the healthcare plan during the study period (2016–2017; or from at least 1 month after birth until December 31, 2017, if born after January 01, 2016). These study years were selected as being prior to the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic and to provide sufficient follow-up time for the evaluation of costs associated with hearing loss.
Figure 1.
Study design figure.
HL, hearing loss; OM, otitis media; TTP, tympanostomy tube placement.
This study complied with all applicable laws regarding subject privacy. No direct subject contact or primary collection of individual human subject data occurred, and all study results are aggregated. Therefore, informed consent and ethics committee approval were not required.
Study outcomes
OM episodes
OM claims were identified from January 1, 2016, until December 31, 2017, using International Classification of Diseases 10th Revision (ICD-10) codes (Table S1) from inpatient and outpatient databases merged to prevent duplication of cases. Calculations were based on OM overall (ICD-10), without any split according to OM subtypes. Two successive OM claims were considered the same episode if there were up to 30 days between consecutive record dates. The episode end date corresponded to the date of the last claim associated with the same episode. Episodes with claims from both outpatient and inpatient settings were considered hospitalized episodes and were not included in the analysis of outpatient episodes. Inpatient episodes were those with a primary diagnosis of OM.
OM episode with TTP
Claims for TTP were extracted from outpatient and inpatient databases using ICD-10 codes (Table S1). TTP was linked to a specific OM episode if it occurred between the start date and within 30 days after the end date of an OM episode. In the event of multiple TTP procedures, only the first event was counted.
OM episode with hearing loss
Hearing loss was defined as a claim using ICD-10 codes H90X (Conductive and sensorineural hearing loss and all subcategories) recorded between the start of the first OM episode until 6 months after the end date of the last OM episode (up to June 30, 2018). Patients were assumed to experience only 1 diagnosis of hearing loss during the whole study period.
Costs
Costs were expressed in US dollars and evaluated from the payer’s perspective inflated to year 2020 using a consumer price index for medical care.12 Four sub-costs were considered for each OM episode; diagnosis, treatment (medication, hospitalization), TTP, and hearing loss, and combined to obtain the total medical costs. OM episodes paid with capitated reimbursement schemes and episodes with total missing or negative costs were removed from the cost analysis. Treatment costs for hearing loss were followed up for 1 year after the hearing loss diagnosis.
Statistical analysis
Analyses performed in this study were descriptive. Incidence rates (per 100,000 person-years) of OM episodes were calculated as the number of episodes occurring during the study period (2016–2017) divided by the number of years at risk. Periods during which a patient had OM were removed from the time at risk. Incidence rates were computed separately for inpatient, outpatient, and total OM episodes, OM with/without TPP, and OM with hearing loss with/without TPP. As the exact date of birth was not available, age was imputed based on assumptions using procedure codes related to birth, or enrollment dates (provided in the supplement).
Costs were summarized using the number of non-capitated episodes during the study period and presented as mean cost by episode with standard deviation (SD). All costs associated with OM claims were considered; claims with TTP treatment with no OM diagnosis were also counted provided that they occurred within 30 days after an OM diagnosis. Treatment costs for hearing loss were followed up for 1 year after the hearing loss diagnosis and associated with the first OM episode with hearing loss claim.
Results were extrapolated to the entire US population assuming that 50% of all children are covered under Commercial/Medicare plans, 40% are covered under Medicaid, and that 10% of children are uninsured.13 The total cost was weighted by the proportion of children covered under Commercial/Medicare and Medicaid, respectively, and adjusted to year 2020.
All analyses were performed using SAS version 9.04.
Results
Incidence rate of OM
The incidence rate of OM in children under 5 between 2016 and 2017 was 62,726 per 100,000 person-years in the Commercial/Medicare databases and 55,874 per 100,000 person-years in the Medicaid database (Figure 2). OM incidence rates were highest between the ages of 6 and 18 months, peaking in both databases at 9–<12 months of age (115,552 and 110,960 per 100,000 person-years, respectively) (Table S2).
Figure 2.
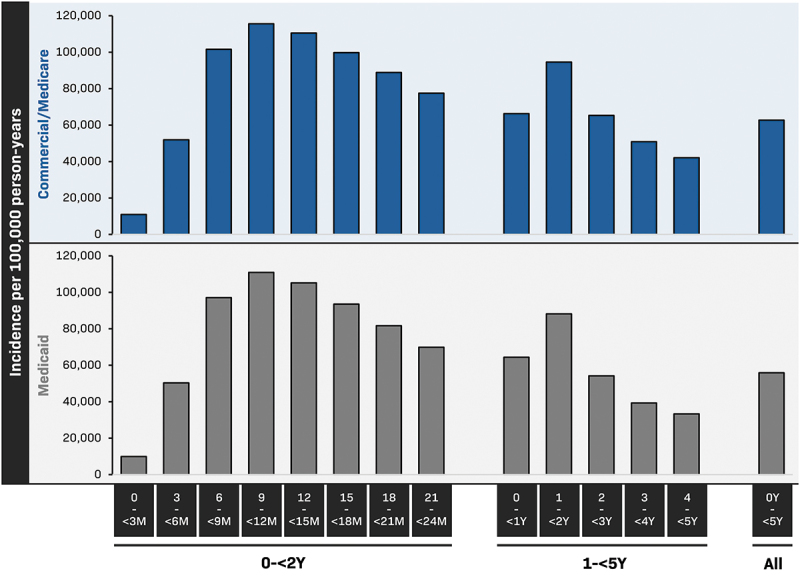
Incidence rates of otitis media in children less than 5 years of age (Marketscan, 2016–2017).
Commercial, Commercial Claims and Encounters; m, months; y, years. Data are tabulated in Table S2.
The vast majority of OM episodes were managed as outpatients with incidence rates that were close to overall rates (Table 1). The incidence rate of hospitalized OM in children less than 5 years of age was 11.2 per 100,000 person-years in the Commercial/Medicare databases and 16.7 per 100,000 person-years in the Medicaid database. The incidence of hospitalized OM was highest under the age of 2 years (19.7 per 100,000 person-years in Commercial/Medicare and 28.8 per 100,000 person-years in Medicaid in 0–<1-year-olds; 21.3 and 27.1 per 100,000 person-years, respectively in 1–<2-year-olds) and decreased with increasing age.
Table 1.
Incidence rate of otitis media episodes by treatment setting per 100,000 person-years (Marketscan 2016–2017, Commercial/Medicare (a) and Medicaid (b)), regardless of hearing loss.
| Age class (years) | Total | Outpatient | Inpatient |
|---|---|---|---|
| a) | |||
| Commercial/Medicare | |||
| 0-<1 | 66,282.6 | 66,262.9 | 19.7 |
| 1-<2 | 94,589.0 | 94,567.8 | 21.3 |
| 2-<3 | 65,311.2 | 65,306.0 | 5.2 |
| 3-<4 | 50,857.3 | 50,851.0 | 6.3 |
| 4-<5 | 42,071.8 | 42,066.9 | 5.0 |
| All (0-<5) | 62,725.9 | 62,714.7 | 11.2 |
| b) | |||
| Medicaid | |||
| 0-<1 | 64,403.7 | 64,374.9 | 28.8 |
| 1-<2 | 88,164.8 | 88,137.7 | 27.1 |
| 2-<3 | 54,204.1 | 54,191.5 | 12.6 |
| 3-<4 | 39,292.1 | 39,285.0 | 7.1 |
| 4-<5 | 33,318.4 | 33,312.2 | 6.1 |
| All (0-<5) | 55,874.2 | 55,857.5 | 16.7 |
Commercial, Commercial Claims and Encounters.
Incidence rate of OM with and without TTP (regardless of hearing loss)
Approximately 5% of OM episodes in children under 5 years of age in the Commercial/Medicare databases and 4% in the Medicaid database required TTP, with incidence rates of 3233 per 100,000 person-years and 2404 per 100,000 person-years in the respective databases (Table 2). Incidence rates were highest in children aged between 1 and 2 years (6090 per 100,000 person-years and 4611 per 100,000 person-years, respectively). A majority of OM followed by TTP occurred in the outpatient setting. The incidence rate of inpatient OM followed by TTP was highest between the ages of 1 and 2 years (9.2 per 100,000 person-years and 8.3 per 100,000 person-years in the respective databases).
Table 2.
Incidence rate of otitis media (OM) episodes with and without tympanostomy tube placement (TTP) and regardless of hearing loss per 100,000 person-years (MarketScan, 2016–2017, Commercial/Medicare (a) and Medicaid (b)).
| OM episodes with TTP |
OM episodes without TTP |
Overall incidence | |||||
|---|---|---|---|---|---|---|---|
| Age class (years) | Total | Outpatient | Inpatient | Total | Outpatient | Inpatient | |
| a) | |||||||
| Commercial/Medicare | |||||||
| 0-<1 | 4278.0 | 4273.1 | 4.9 | 62,004.6 | 61,989.8 | 14.8 | 66,282.6 |
| 1-<2 | 6089.9 | 6080.7 | 9.2 | 88,499.1 | 88,487.1 | 12.0 | 94,589.0 |
| 2-<3 | 3010.7 | 3009.2 | 1.5 | 62,300.5 | 62,296.8 | 3.7 | 65,311.2 |
| 3-<4 | 1876.7 | 1874.6 | 2.1 | 48,980.6 | 48,976.4 | 4.2 | 50,857.3 |
| 4-<5 | 1379.8 | 1378.2 | 1.7 | 40,692.0 | 40,688.7 | 3.3 | 42,071.8 |
| All (0-<5) | 3233.2 | 3229.5 | 3.7 | 59,492.7 | 59,485.2 | 7.5 | 62,725.9 |
| b) | |||||||
| Medicaid | |||||||
| 0-<1 | 2209.7 | 2205.8 | 3.9 | 62,193.9 | 62,169.1 | 24.9 | 64,403.7 |
| 1-<2 | 4611.0 | 4602.7 | 8.3 | 83,553.9 | 83,535.0 | 18.8 | 88,164.8 |
| 2-<3 | 2467.4 | 2464.6 | 2.9 | 51,736.7 | 51,726.9 | 9.7 | 54,204.1 |
| 3-<4 | 1592.6 | 1590.8 | 1.8 | 37,699.5 | 37,694.2 | 5.3 | 39,292.1 |
| 4-<5 | 1274.0 | 1271.7 | 2.3 | 32,044.4 | 32,040.5 | 3.9 | 33,318.4 |
| All (0-<5) | 2404.5 | 2400.7 | 3.8 | 53,469.7 | 53,456.8 | 12.9 | 55,874.2 |
Commercial, Commercial Claims and Encounters.
Incidence rate of OM with and without hearing loss
Around 2% of OM episodes in children under 5 years of age in each database had a claim related to hearing loss during the study. The incidence of OM with hearing loss in children less than 5 years of age was 1468 per 100,000 person-years in the Commercial/Medicare databases and 1109 per 100,000 person-years in the Medicaid database (Table 3). The incidence of OM with hearing loss was highest between the ages of 1 and 2 years (2110 and 1656 per 100,000 person-years, respectively). Among children with OM and hearing loss, 41% had TTP.
Table 3.
Incidence rate of otitis media (OM) episodes with and without hearing loss, and with or without tympanostomy tube placement (TTP) per 100,000 person-years (Marketscan, 2016–June 2018, Commercial/Medicare (a) and Medicaid (b)).
| OM episodes with hearing loss |
OM episodes without hearing loss |
Overall incidence | |||||
|---|---|---|---|---|---|---|---|
| Age class (years) | Total | Without TTP | With TTP | Total | Without TTP | With TTP | |
| a) | |||||||
| Commercial/Medicare | |||||||
| 0-<1 | 1514.4 | 709.0 | 805.3 | 64,768.2 | 61,295.6 | 3472.7 | 66,282.6 |
| 1-<2 | 2110.1 | 1066.1 | 1044.0 | 92,478.9 | 87,433.0 | 5045.9 | 94,589.0 |
| 2-<3 | 1340.5 | 845.4 | 495.1 | 63,970.8 | 61,455.1 | 2515.6 | 65,311.2 |
| 3-<4 | 1261.0 | 868.9 | 392.1 | 49,596.3 | 48,111.7 | 1484.6 | 50,857.3 |
| 4-<5 | 1205.7 | 886.1 | 319.7 | 40,866.1 | 39,805.9 | 1060.2 | 42,071.8 |
| All (0-<5) | 1468.2 | 870.7 | 597.5 | 61,257.7 | 58,622.0 | 2635.7 | 62,725.9 |
| b) | |||||||
| Medicaid | |||||||
| 0-<1 | 951.8 | 491.9 | 459.9 | 63,451.9 | 61,702.0 | 1749.9 | 64,403.7 |
| 1-<2 | 1655.9 | 863.5 | 792.4 | 86,508.9 | 82,690.4 | 3818.6 | 88,164.8 |
| 2-<3 | 1121.1 | 694.4 | 426.6 | 53,083.0 | 51,042.2 | 2040.8 | 54,204.1 |
| 3-<4 | 931.4 | 614.8 | 316.6 | 38,360.7 | 37,084.7 | 1276.0 | 39,292.1 |
| 4-<5 | 935.6 | 624.5 | 311.1 | 32,382.8 | 31,419.9 | 962.9 | 33,318.4 |
| All (0-<5) | 1109.0 | 650.4 | 458.6 | 54,765.2 | 52,819.3 | 1945.9 | 55,874.2 |
Commercial, Commercial Claims and Encounters.
Cost of OM episodes
The mean cost associated with any OM episode was $532 in the Commercial/Medicare databases and $294 in the Medicaid database (Table 4). For OM without TPP, the mean cost was $269, or approximately 50% lower than the overall cost in the Commercial/Medicare databases, and $207 or 30% lower in the Medicaid database. Mean costs were markedly higher for inpatient OM ($15,243 and $7089, respectively).
Table 4.
Mean (standard deviation) costs of otitis media (OM) episodes in patients who underwent tympanostomy tube placement (TTP), and in patients with post-otitis hearing loss (HL).
| Commercial/Medicare |
Medicaid |
|
|---|---|---|
| All OM | ||
| Any OM episode | $532 (1562) | $294 (695) |
| Any outpatient OM episode | $529 (1541) | $292 (648) |
| Any inpatient OM episode | $15,243 (12,553) | $7089 (13,053) |
| Any OM episode with TTP | $5349 (3726) | $2273 (1978) |
| Outpatient OM episode with TTP | $5329 (3654) | $2253 (1645) |
| Inpatient OM episode with TTP | $23,037 (12,963) | $16,756 (26,065) |
| Any OM episode without TTP | $269 (689) | $207 (392) |
| Outpatient OM episode without TTP | $267 (667) | $206 (379) |
| Inpatient OM episode without TTP | $11,346 (10,395) | $4673 (4534) |
| OM in patients with or without HL | ||
| Any OM episode with HL | $3062 (4099) | $1872 (2136) |
| OM with HL with TTP | $5846 (3822) | $2638 (1970) |
| OM with HL without TTP | $1107 (3013) | $1242 (2059) |
| Any OM episode without HL | $473 (1396) | $256 (568) |
| OM without HL with TTP | $5239 (3695) | $2149 (1966) |
| OM without HL without TTP | $256 (583) | $193 (288) |
| Outpatient OM without HL or TTP | $255 (558) | $192 (271) |
| In patient OM without HL or TTP | $11,309 (10,522) | $4629 (4562) |
Commercial, Commercial Claims and Encounters.
The mean cost of OM with hearing loss was $3062 in the Commercial/Medicare databases and $1872 in the Medicaid database, compared to $473 and $256, respectively, for OM without hearing loss. OM with hearing loss and TPP incurred mean costs of $5846 and $2638, respectively, versus mean costs of OM and TTP (without hearing loss) of $5239 and $2149.
Overall economic burden due to OM in the US
By extrapolating these data to the whole of the US, we estimate that there were 11,111,084 OM episodes in children less than 5 years of age in 2020, at a total direct medical cost of $4.8 billion after cost adjustment. Of this, 49% ($2.4 billion) were costs associated with the outpatient management of apparently uncomplicated OM (without hearing loss or TTP) (Figure 3). There were 243,618 children estimated to have OM with hearing loss in 2020, at a direct total cost of $637 million, contributing 13% to the overall cost. The total cost of 439,860 estimated episodes of OM with TTP (without hearing loss) was $1.8 billion. While the mean cost of hospitalized OM was high, the relative infrequency of hospitalized OM (1751 cases estimated in 2020) contributed minimally to the total cost burden ($13 million).
Figure 3.
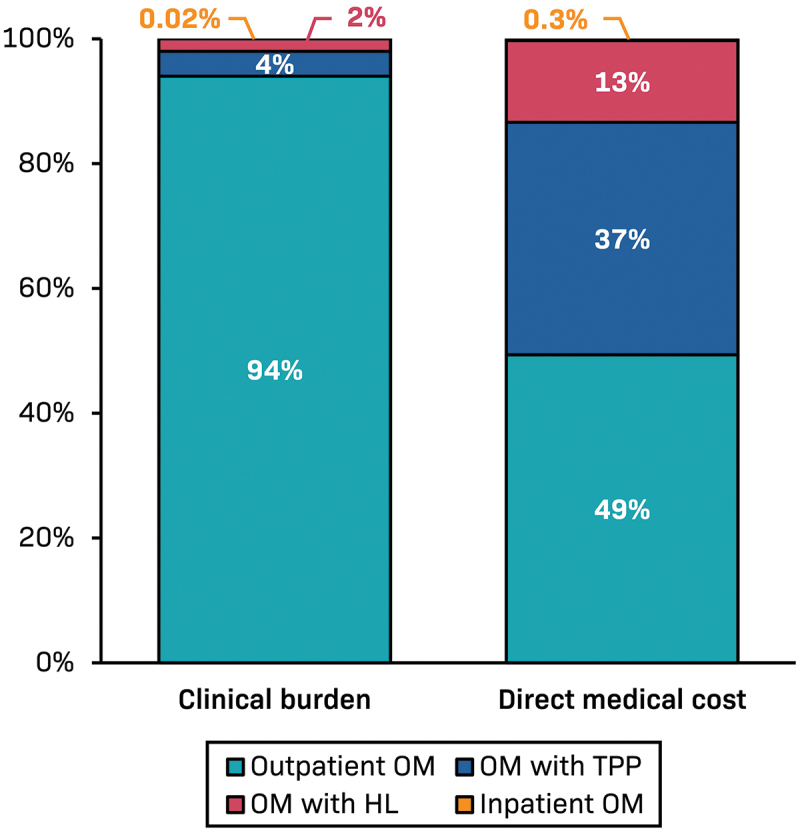
Estimate of the distribution of total medical costs associated with otitis media in the United States in 2020.
HL, hearing loss; OM, otitis media; TTP, tympanostomy tube placement, Outpatient OM, OM with TPP, and inpatient OM are OM without HL. OM with HL – almost all were treated as outpatients and around 40% required TTP.
Discussion
Our study confirms an ongoing substantial disease burden of OM in children less than 5 years of age and provides additional insights into the incidence of OM across age groups. Uniquely, we also estimated the burden of hearing loss in children with OM and the associated medical costs, which to our knowledge, has not been reported previously.
The estimated incidence rate of OM in children less than 5 years of age was 62,726 and 55,874 per 100,000 person-years (Commercial/Medicare and Medicaid databases, respectively). Disease incidence peaked at 9–<12 months of age, highlighting the need for early prophylactic intervention during infancy, prior to the rapid upscale in incidence that starts at 6 months of age. We estimate that the total medical costs associated with OM in children under 5 years of age in the US was $4.8 billion in 2020. Half of this amount was incurred by uncomplicated OM managed as an outpatient, and a further 37% was incurred by patients with OM who underwent TTP. A total of 2% of children with OM had an ICD-10 diagnosis code for hearing loss. However, the treatment of OM with hearing loss accounted for 13% of the total costs incurred, pointing to high resource use by this subgroup. Although we could not confirm a cause and effect relationship between OM and hearing loss, we assumed causality between claims for OM and hearing loss, an approach supported by data showing that OM causes 64% of hearing loss in children.14,15 The costs incurred by the management of hearing loss in our study may be underestimated because of truncation of follow-up after 1 year, and because only 1 diagnosis of hearing loss was included per child.
Our study contributes to a picture of the OM burden in the US along with other studies that together highlight different aspects of this burden. Hu et al., 2022, Tong et al. 2018, and Suaya et al. 2018 have all recently published studies reporting on the OM clinical and/or cost burden using different databases (MarketScan, National Disease & Therapeutic Index), age groups of interest (up to age 18 years), and outcome variables (such as acute otitis media, complications, prescriptions).5,7,8 Although not directly comparable, the studies are aligned in concluding that the burden of OM or acute OM and associated costs, remain high across all age groups despite overall reductions in the incidence rate of OM observed in the post-PCV period. All studies point to a very high burden in the first years of life, and annual costs to the health system of $4–5 billion. The true cost is likely to be considerably higher, given that none of the studies captured all potential associated costs, such as hearing loss which was only captured in the present study. A recent systematic review highlighted significant variability in the incidence rates of acute OM, ranging from 630 cases per 100,000 person years in Iceland (children aged 3 to <7 years in 2011–2013) to 62,400 in the Netherlands (children aged 6–12 months in 2008–2014).16 Similarly, there was a high variability in the economic burden estimates across European countries, with the United Kingdom showing a remarkably low cost per OM episode. This variability might be attributed to differences in the definitions of OM, the age groups studied, calculation methods, and different healthcare systems, among other factors. Despite this variability, these data underscore the persistent burden of pneumococcal OM, as demonstrated in the current study.
Nonetheless, the clinical and economic burden of OM has decreased following the introduction of PCV.3,4 This burden appears higher than other pediatric respiratory diseases, such as respiratory syncytial virus (RSV), for which prophylactic interventions have only recently become available.17 In the US, RSV treatment for children costs $709.6 million annually,18 compared to $4.8 billion for OM (as demonstrated in this study). Of note, these figures should also be considered in light of the multi-pathogenic nature of OM, involving multiple bacterial and viral etiologies. Direct costs for RSV are driven mainly by hospitalizations (66.5%) and emergency visits (23.9%), while costs related to outpatient care are relatively low (9.6%).18 Among children with RSV-related illnesses, 3% are hospitalized, 25% are treated in emergency departments, and 73% are treated by pediatric practices.19 Potential limitations of our study relate to the representativeness of the databases and the nonrandom selection of those enrolled. Large employers may be over-represented in Commercial, and the Medicaid data are based on a convenience sample from 9 to 12 states. Clinical data such as clinical symptoms, disease severity, and reasons for clinical decision-making are not captured in claims databases, preventing assessment of etiology or causality. Moreover, we assumed a 30-day interval between individual OM episodes but this could not be clinically confirmed. Finally, the seasonal respiratory virus activity over the 2 years of the study may have influenced the results. This study aimed to assess direct medical costs from the healthcare payer perspective; however, future research should also consider incorporating indirect costs and employing appropriate economic models for a comprehensive evaluation of the economic burden of OM.
In conclusion, our study highlights that the clinical and economic burden attributable to OM in the US continues to be substantial in American children under 5 years. PCVs provide only modest efficacy against mucosal infections such as OM, moreover other bacterial and viral pathogens cause OM; thus, novel and broad preventive approaches are needed to improve protection against OM.20 This is the first study of its kind to provide detailed information on the age-specific incidence of OM and incidence of hearing loss and TTP among the US pediatric population utilizing robust methodology and a large sample size. The study results could potentially guide policymakers considering age-specific interventions to reduce OM. Potential policy measures include improved vaccination strategies, such as the development of next-generation PCV vaccines covering additional serotypes and vaccines targeting pathogens associated with OM, such as RSV, NTHi, and Moraxella catarrhalis.
Supplementary Material
Acknowledgments
The authors thank Ekkehard Beck for his critical discussion about the study and Konstantina Chatzikonstantinidou for her programming support.
The authors also thank Business & Decision Life Sciences Medical Communication Service Center for editorial assistance and manuscript coordination, on behalf of GSK. Dr Joanne Wolter (independent, on behalf of GSK) provided writing support.
Biography
Lilia Ben Debba is Global vaccines value evidence lead within VEO (Value Evidence and Outcomes) department of GSK. She is a pharmacist with a master’s degree in health economics and market access from Paris Dauphine university. She also has an inter-university diploma in statistics applied to medicine and epidemiology from the university of Pierre et Marie Curie (UPMC) in Paris. She worked for several pharmaceutical companies at both local and global level including Boehringer-Ingelheim and Sanofi. She is interested in real-world evidence data and cost-effectiveness analysis.
Funding Statement
GSK funded this study [VEO-000264] and was involved in all stages of study conduct, including analysis of the data. GSK also took in charge all costs associated with the development and publication of this manuscript.
Disclosure statement
DD, LT, and MS are employed by GSK and LT and MS hold financial equities in GSK. LBD is a consultant for GSK. GL was employed by Business and Decision at the time of the study and is now employed by GSK. All authors declare no other financial or non-financial relationships and activities.
Authors’ contributions
All authors participated in the design and/or implementation and/or analysis, interpretation of the study and the development of this manuscript. All authors had full access to the data and gave final approval before submission.
Data availability statement
The data that support the findings of this study are available from IBM MarketScan Databases, but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Aggregated data are, however, available from the corresponding author upon reasonable request and with permission from IBM MarketScan Databases.
List of abbreviations
- Commercial
Commercial Claims and Encounters
- HL
hearing loss
- ICD-10
International Classification of Diseases 10th Revision
- NTHi
Non-typeable Haemophilus influenzae
- OM
Otitis media
- PCV
pneumococcal conjugate vaccine
- PCV7
7-valent pneumococcal conjugate vaccine
- PCV13
13-valent pneumococcal conjugate vaccine
- TTP
Tympanostomy tube placement
- US
United States
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2024.2409510
References
- 1.Klein JO. The burden of otitis media. Vaccine. 2000;19 Suppl 1:S2–8. doi: 10.1016/s0264-410x(00)00271-1. [DOI] [PubMed] [Google Scholar]
- 2.Teele DW, Klein JO, Rosner B. Epidemiology of otitis media during the first seven years of life in children in Greater Boston: a prospective, cohort study. J Infect Dis. 1989;160(1):83–94. doi: 10.1093/infdis/160.1.83. [DOI] [PubMed] [Google Scholar]
- 3.Kaur R, Morris M, Pichichero ME. Epidemiology of acute otitis media in the postpneumococcal conjugate vaccine era. Pediatrics. 2017;140:e20170181. doi: 10.1542/peds.2017-0181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaur R, Fuji N, Pichichero ME. Dynamic changes in otopathogens colonizing the nasopharynx and causing acute otitis media in children after 13-valent (PCV13) pneumococcal conjugate vaccination during 2015–2019. Eur J Clin Microbiol Infect Dis. 2022;41(1):37–44. doi: 10.1007/s10096-021-04324-0. [DOI] [PubMed] [Google Scholar]
- 5.Hu T, Done N, Petigara T, Mohanty S, Song Y, Liu Q, Lemus-Wirtz E, Signorovitch J, Sarpong E, Weiss T, et al. Incidence of acute otitis media in children in the United States before and after the introduction of 7- and 13-valent pneumococcal conjugate vaccines during 1998–2018. BMC Infect Dis. 2022;22(1):294. doi: 10.1186/s12879-022-07275-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Klein A, Tamir SO, Sorek N, Hanun G, Yeshayahu Y, Marom T. Increase in Haemophilus influenzae detection in 13-valent pneumococcal conjugate vaccine immunized children with acute otitis media. Pediatr Infect Dis J. 2022;41(8):678–680. doi: 10.1097/INF.0000000000003561. [DOI] [PubMed] [Google Scholar]
- 7.Suaya JA, Gessner BD, Fung S, Vuocolo S, Scaife J, Swerdlow DL, Isturiz RE, Arguedas AG. Acute otitis media, antimicrobial prescriptions, and medical expenses among children in the United States during 2011–2016. Vaccine. 2018;36(49):7479–7486. doi: 10.1016/j.vaccine.2018.10.060. [DOI] [PubMed] [Google Scholar]
- 8.Tong S, Amand C, Kieffer A, Kyaw MH. Trends in healthcare utilization and costs associated with acute otitis media in the United States during 2008–2014. BMC Health Serv Res. 2018;18(1):318. doi: 10.1186/s12913-018-3139-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Backous D, Choi BY, Jaramillo R, Kong K, Lenarz T, Ray J, Thakar A, Hol MKS. Hearing rehabilitation of patients with chronic otitis media: a discussion of current state of knowledge and research priorities. J Int Adv Otol. 2022;18(4):365–370. doi: 10.5152/iao.2022.21428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hansen L. The Truven health MarketScan databases for life sciences researchers. Ann Arbor: Truven Health Analytics; 2017. [Google Scholar]
- 11.Butler AM, Nickel KB, Overman RA, Brookhart MA. IBM MarketScan research databases. In: Sturkenboom M, Schink T. editors. Databases for pharmacoepidemiological research. Springer series on epidemiology and public health. Cham: Springer; doi: 10.1007/978-3-030-51455-6_20. [DOI] [Google Scholar]
- 12.Consumer price index values for medical care from the bureau of labor statistics. [accessed 2024 Jul 15]. https://www.in2013dollars.com/Medical-care/price-inflation.
- 13.Keisler-Starkey K, Bunch L. Current population reports, P60-274. Health insurance coverage in the United States: 2020. Washington (DC): U.S. Government Publishing Office; 2021. Sep [accessed 2024 Jul 15]. https://www.census.gov/library/publications/2022/demo/p60-278.html. [Google Scholar]
- 14.Haile LM, Kamenov K, Briant PS, Orji AU, Steinmetz JD, Abdoli A, Abdollahi M, Abu-Gharbieh E, Afshin A, Ahmed H, et al. 2019 Hearing loss prevalence and years lived with disability, 1990–2019: findings from the global burden of disease study 2019. Lancet. 2021;397(10278):996–1009. doi: 10.1016/S0140-6736(21)00516-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.World Health Organization . Chronic suppurative otitis media: burden of illness and management options. Geneve: World Health Organization; 2004. [accessed 2024 Jul 15]. https://iris.who.int/handle/10665/42941. [Google Scholar]
- 16.Ricci Conesa H, Skröder H, Norton N, Bencina G, Tsoumani E, Subha ST. Clinical and economic burden of acute otitis media caused by Streptococcus pneumoniae in European children, after widespread use of PCVs–A systematic literature review of published evidence. PLOS ONE. 2024. 2. 19(4):e0297098. doi: 10.1371/journal.pone.0297098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Long SS. Maternal/Pediatric respiratory syncytial virus (RSV) work group. ACIP General meeting. 2023. Centers for Disease Control and Prevention. National Center for Immunization and Respiratory Diseases. [accessed 2024 Jul 15]. https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2023-09-22/01-RSV-Mat-Ped-Long-508.pdf.
- 18.Bowser DM, Rowlands KR, Hariharan D, Gervasio RM, Buckley L, Halasa-Rappel Y, Glaser EL, Nelson CB, Shepard DS. Cost of respiratory syncytial virus infections in US infants: systematic literature review and analysis. J Infect Dis. 2022;226(Suppl 2):225–235. doi: 10.1093/infdis/jiac172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hall CB, Weinberg GA, Iwane MK, Blumkin AK, Edwards KM, Staat MA, Auinger P, Griffin MR, Poehling KA, Erdman D, et al. The burden of respiratory syncytial virus infection in young children. N Engl J Med. 2009;360(6):588–598. doi: 10.1056/NEJMoa0804877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Poolman JT. Expanding the role of bacterial vaccines into life-course vaccination strategies and prevention of antimicrobial-resistant infections. NPJ Vaccines. 2020;5:84. doi: 10.1038/s41541-020-00232-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from IBM MarketScan Databases, but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Aggregated data are, however, available from the corresponding author upon reasonable request and with permission from IBM MarketScan Databases.


