Abstract
Background
Endoscopic submucosal dissection (ESD) has revolutionized the treatment of early stage gastrointestinal cancers. However, ESD can be associated with increased postprocedural pain and higher complication rates. This systematic review and meta-analysis evaluated the efficacy and safety of local anesthesia.
Methods
A comprehensive search was conducted to identify relevant randomized controlled trials investigating the effect of local anesthesia in ESD procedures. The Cochrane risk of bias tool for randomized trials was used to assess study quality. A meta-analysis was performed using Review Manager 5.4, with summary measures expressed as pooled odds ratios (OR) or mean differences with corresponding 95% confidence intervals (CI).
Results
Four randomized controlled trials with 296 patients undergoing ESD procedures were included. The use of local anesthesia did not significantly impact procedural time (mean difference = −2.05, 95% CI = −9.29, 5.18, I2 = 30%, P = 0.58). Lastly, the use of local anesthesia did not increase the risk of bleeding or other adverse events (P > 0.05) and decreased the incidence of bradycardia (OR = 0.16, 95% CI = 0.03, 0.95; I2 = 0%; P = 0.04).
Conclusion
Our study found that the use of local anesthesia did not significantly affect the procedural time of ESD. However, it effectively reduced postoperative pain in some trials with no risk of increased incidence of adverse events.
Keywords: Bupivacaine, endoscopic submucosal dissection, intraoperative pain, lidocaine, local anesthesia
Endoscopic submucosal dissection (ESD) is a pioneering endoscopic method initially devised for the treatment of early stage gastric neoplastic lesions. To date, ESD has been established as an efficient method that achieves en bloc and R0 resection regardless of the size of the lesion, not only for early gastric cancerous lesions but also for lesions located in the colon or esophagus.1 This procedure is associated with higher rates of complications than endoscopic mucosal resection, including bleeding, perforation, and abdominal pain. Despite its longer procedure time and higher perforation rate, ESD resulted in higher en bloc resection and curative rates compared with endoscopic mucosal resection, and most ESD perforations are successfully managed by conservative endoscopic treatment.2
The theory behind using local anesthetics to decrease procedure time in ESD relates to their ability to provide targeted analgesia, thereby allowing for a more efficient sedation and expedited recovery. By locally blocking nerve impulses at the site of the resection, local anesthetics may effectively reduce pain sensation, facilitating early discharge of the patients and less use of post-ESD analgesia. There are different approaches to use of local anesthesia during ESD procedure. Two of the included trials have used it by mixing the local anesthetic agent with the injected submucosal cushioning fluid.3,4 Another approach is by injecting the local anesthetic around the lesion (at the oral and anal sides).5 Kim et al injected the local anesthetic directly in the cautery ulcer base.6 The variety of approaches may have influenced the wide range of doses used in these trials, which ranged from 20 to 200 mg of either lidocaine or bupivacaine. This may have led to lower risk of adverse events compared to controls. Patients under local anesthesia are more likely to remain stable throughout the procedure, enabling endoscopists to maintain focus and accuracy during intricate dissections.7,8 Eventually, this will lead to a lower risk of inadvertent tissue trauma or procedural errors.
Additionally, local anesthesia could reduce the need for general anesthesia altogether and may eliminate potential time-consuming processes such as induction, airway intubation, recovery, and monitoring associated with these modalities. Therefore, the strategic use of local anesthetics not only ensures patient comfort but also contributes to streamlining ESD procedures with shorter procedural time and recovery.9
METHODS
Search strategy and data extraction
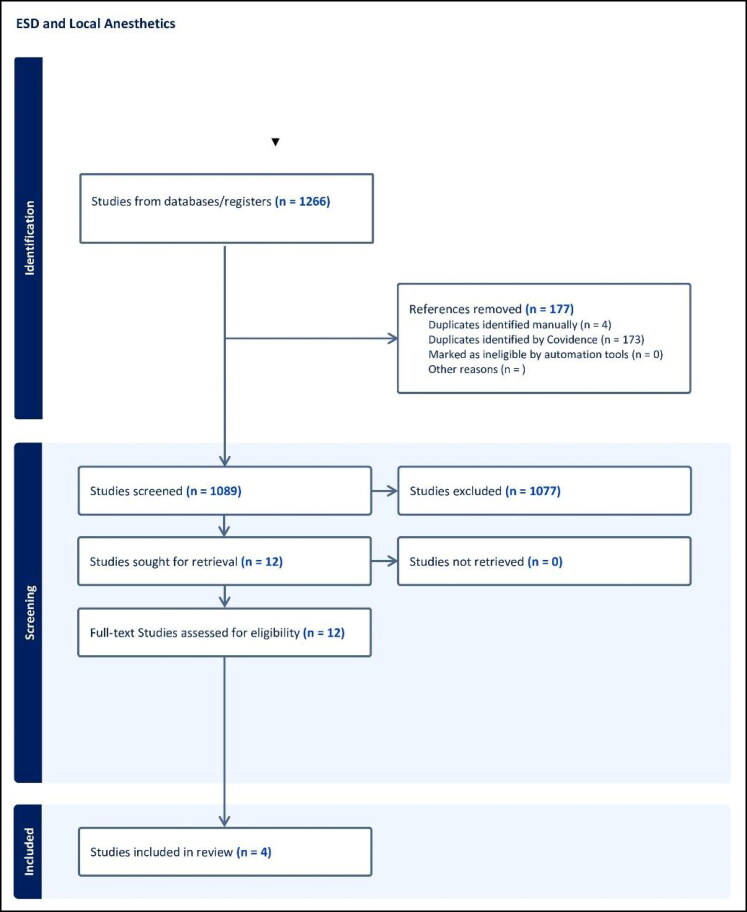
A systematic search of relevant literature was conducted across multiple databases, including Embase, Scopus, Web of Science, Medline/PubMed, and Cochrane, from their inception to February 28, 2024. The search strategy utilized Boolean operators to combine terms related to the population, intervention, and outcomes of interest. The following search strategy was employed: (“endoscopic submucosal dissection” OR “ESD” OR “submucosal dissection”) AND (“local anesthesia” OR “lidocaine” OR “local injection” OR “local lidocaine”). The search strategy aimed to identify studies investigating the use of local anesthetic agents, particularly lidocaine, during ESD procedures. Our research adhered to the recommended guidelines for reporting systematic reviews and meta-analyses. Furthermore, we conducted the review and meta-analysis following the Cochrane criteria and the PRISMA checklist.10,11 Figure 1 shows the PRISMA flow diagram of this systematic process.
Figure 1.
PRISMA flow diagram of our search.
Two independent reviewers screened titles, abstracts, and full-text articles for inclusion based on predefined eligibility criteria. Any disagreements were resolved through discussion or consultation with a third reviewer. Data extraction was conducted independently by two coauthors using a standardized data extraction form, with discrepancies resolved through consensus. Extracted data included study characteristics, patient demographics, details of the intervention and comparator, and outcomes of interest.
Inclusion criteria and study outcomes
Studies eligible for inclusion in this meta-analysis were those focusing on patients undergoing ESD procedures in the gastrointestinal tract. The intervention of interest was the use of local anesthetic agents, such as lidocaine, administered at the site of endoscopic resection through any means. Comparators included interventions without local anesthesia, including a placebo or no injection. The primary outcome of interest was the efficacy of using local anesthetics on ESD procedural time. Secondary outcomes included the degree of pain on the short-form McGill pain (SFMP) scale experienced by patients at different time intervals after the ESD procedure, as well as adverse events (bleeding, hypotension, hypoxemia, and bradycardia). Total SFMP questionnaire scores range from 0 to 45, with higher scores indicating higher intensity of pain. Adverse events were defined as the incidence of these events during the ESD procedure or the hospital stay afterwards.
Only randomized controlled trials (RCTs) were considered eligible for inclusion, without restrictions on publication date. Non-English studies and those lacking adequate translation were excluded to prevent potential misinterpretation of findings. Additionally, case reports, editorials, letters, or conference abstracts without full-text availability were excluded. Animal studies or those conducted on nonhuman subjects were also excluded. Studies with inadequate reporting of the primary outcome, insufficient follow-up duration, or incomplete follow-up data were excluded to ensure the reliability of outcome assessments.
Risk of bias assessment
The risk of bias and methodological quality of the included studies were assessed independently by two authors. The Cochrane risk of bias tool for randomized trials, version 2, was employed for RCTs. Any discrepancies were resolved through discussion or consultation with a third reviewer.12
Statistical analysis
A meta-analysis was conducted using Review Manager 5.4 (Cochrane Collaboration, Copenhagen, The Nordic Cochrane Centre). Given the anticipated heterogeneity in study designs and populations, a random effects model was utilized. Summary measures were expressed as pooled odds ratios (OR) with corresponding 95% confidence intervals (CI) for proportional variables and mean differences with corresponding 95% CIs for continuous variables. Statistical significance was set at a P value <0.05. Heterogeneity was assessed using the I2 statistic, with an I2 value of ≥50% indicating significant heterogeneity.
RESULTS
Four studies were included in this meta-analysis. All included studies were RCTs and were conducted in Japan and South Korea. We included only the studies written in English. We pooled a total of 1266 studies from the four databases and excluded 177 duplicate studies. Abstract and title screening eliminated 1077, and the rest13 were full-text screened, leaving four studies eligible to be included according to our strategy. The characteristics of the four studies are displayed in Table 1.
Table 1.
Characteristics of the studies included in the review
| Variable | Ijiri (2020)5 | Jung (2019)3 | Kim (2014)6 | Yoshizaki (2022)4 | |
|---|---|---|---|---|---|
| Title | The efficacy of the submucosal injection of lidocaine during endoscopic submucosal dissection for colorectal neoplasms: a multicenter randomized controlled study | Efficacy of submucosal bupivacaine injection for pain relief after endoscopic submucosal dissection: a multicenter, prospective, randomized controlled, and double-blind trial | The efficacy of topical bupivacaine and triamcinolone acetonide injection in the relief of pain after endoscopic submucosal dissection for gastric neoplasia: a randomized double-blind, placebo-controlled trial | Efficacy of lidocaine injection method for esophageal endoscopic submucosal dissection: single-center, double-blind, randomized controlled trial | |
| Country | Japan | Korea | Korea | Japan | |
| Study design | RCT | RCT | RCT | RCT | |
| Number of cases | 51 | 40 | 32 | 25 | |
| Number of controls | 37 | 43 | 32 | 25 | |
| Local anesthesia | Lidocaine | Bupivacaine | Bupivacaine | Lidocaine | |
| Age mean (SD) | Cases | 69.2 (11.9) | 65.9 (10.7) | 55.4 (10.9) | 70.28 (15.72) |
| Controls | 70.8 (8.2) | 59.6 (12.9) | 57.7 (10.9) | 67.92 (13.36) | |
| Sex, male n. (%) | Cases | 30 (59) | 30 (75) | 18 (56.25) | 21 (84) |
| Controls | 19 (51) | 30 (69.8) | 17 (53.13) | 22 (88) | |
| Locations of lesions | Colorectal | Stomach | Stomach | Esophagus | |
Quality of included studies
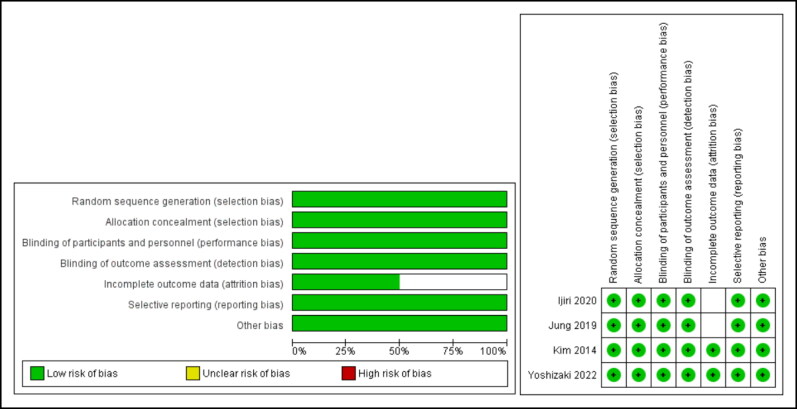
Quality assessment of included studies was determined using the Cochrane RoB 2 tool for clinical trials. Two studies had a total low risk of bias and the other two had unclear risk of bias. Details on the quality of papers across each domain are shown in Figure 2.
Figure 2.
Risk of bias for included studies across different domains.
Meta-analysis results
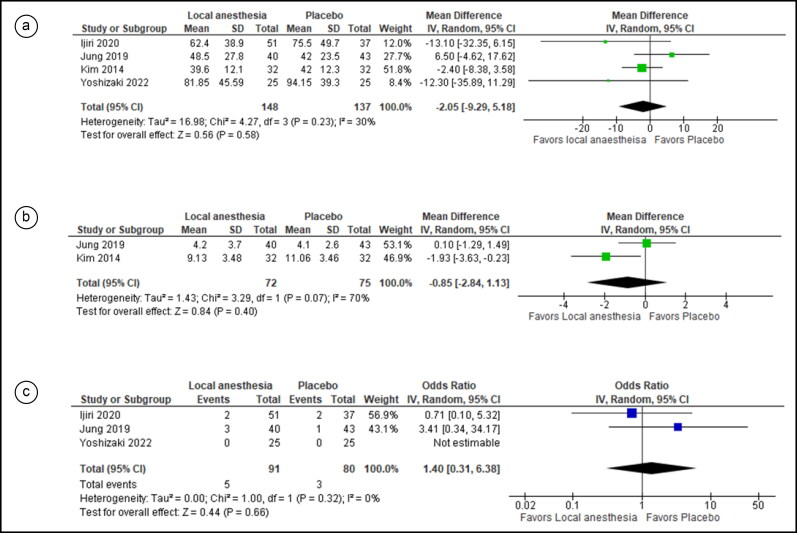
Four studies were included in the efficacy analysis, and there was no statistically significant difference in procedural time between the two groups (MD = −2.05, 95% CI = −9.29, 5.18; I2 =30%; P = 0.58). (Figure 3a). Two studies were pooled in the analysis of pain assessment, revealing no statistically significant difference between the two groups (MD = −0.85; 95% CI = −2.84, 1.13; I2 = 70%; P = 0.40) (Figure 3b).
Figure 3.
Forest plot for comparison of three outcomes between local anesthetic–injected vs saline-injected participants: (a) procedural time, (b) pain score, and (c) incidence of bleeding events.
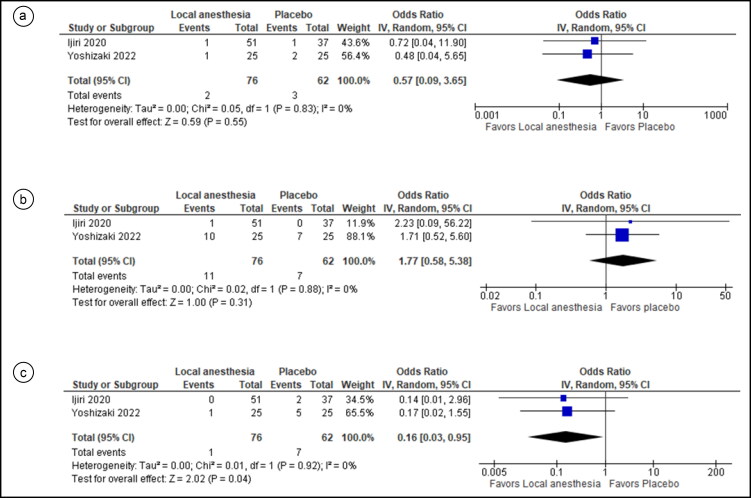
For safety outcomes, two studies were pooled for bleeding events and revealed no statistically significant difference between the two groups (OR = 1.40, 95% CI = 0.31, 6.38; I2= 0%; P = 0.66) (Figure 3c). Two studies were pooled in the analysis without statistically significant differences in the odds of hypotension in both groups (OR = 0.57, 95% CI = 0.09, 3.65; I2 = 0%; P = 0.55) (Figure 4a). Two studies were pooled evaluating hypoxemia without a statistically significant difference in incidence between the two groups (OR = 1.77, 95% CI = 0.58, 5.38; I2 = 0%; P = 0.31) (Figure 4b). Two studies were pooled regarding bradycardia incidence. Results showed a statistically significant difference in the incidence of this event between the two groups, with lower odds of bradycardia in the local anesthesia group (OR = 0.16, 95% CI = 0.03, 0.95; I2 = 0%; P = 0.04) (Figure 4c).
Figure 4.
Forest plot for comparison of the incidence of three outcomes between local anesthetic–injected vs saline-injected participants: (a) hypotension events, (b) hypoxemia events, and (c) bradycardia events.
DISCUSSION
ESD plays an increasing role in the en bloc removal of advanced gastrointestinal cancers. ESD techniques involve the injection of fluid to create a submucosal cushion, leading to safe removal of the epithelial lesions. However, the submucosa underneath is dissected using a specific tool called an ESD knife.14 This technique has some adverse effects that should be avoided and monitored, like perforation, postoperative bleeding, and intraoperative and postoperative pain.13 In our review, the main aspect was procedural time which, in our theory, may be affected by intraoperative pain management. Intraoperative pain during ESD is generally managed in various ways. Several approaches have been adopted to determine the best pain management method to use during or after ESD procedures.6,15,16 In these studies, postoperative pain management was generally facilitated by an intraoperative nerve-blocking agent, like bupivacaine, or through other ways, like a proton pump inhibitor postoperatively. Pain is concerning not only after the procedure but also during the procedure, as pain can lead to patient movement and cause interruption. It is worth noting that the local anesthetic agents used in the four studies included in our systematic review were bupivacaine and lidocaine, which are nerve-blocking agents. Of these, three studies have reported pain outcomes at consistent time intervals.
One drawback is that the studies used different scales to measure pain. This is a limitation of our study and led to considerable variation in results, with a high level of heterogeneity even after merging the two scales. This may have rendered the pain outcome to not be significant through the different time intervals reported. Three different scales were used in the three studies. Jung et al reported pain with a visual analog scale for pain (VAS) and SFMP scores, Kim et al reported SFMP and Pupillary Pain Index (PPI) scores, and Yoshizaki reported postoperative pain incidence as a categorical yes or no question without using any pain assessment questionnaire. VAS scores of 1 to 3 mean mild pain; 4 to 6, moderate pain; and 7 to 10, severe pain. PPI scores were derived from a Likert-type scale and ranged from 0 to 5 (0 = none, 1 = mild, 2 = discomforting, 3 = distressing, 4 = horrible, 5 = excruciating). VAS scores reported by Jung et al were not significantly different between the two groups. Regarding PPI scores, Kim et al reported significantly lower scores in the bupivacaine group and the bupivacaine plus triamcinolone group than the placebo group at 6 hours postprocedure. Scores were significantly lower only in the bupivacaine–triamcinolone group than the placebo group at 12 hours postprocedure.
The non–statistically significant findings in studies comparing the effectiveness of different local anesthesia modalities in ESD procedures could be attributed to the utilization of monitored anesthesia care in the operating room. Monitored anesthesia care involves the administration of sedatives and analgesics to achieve moderate to deep sedation while allowing patients to maintain protective reflexes, obviating the need for airway intubation and mechanical ventilation.10 This might result in the standardization of sedation levels, enhance the patient experience, and promote the role of sedation in pain perception.17
Safety aspects of using local anesthesia and the assessment of its impact on adverse events and procedural complications during ESD are critical aspects to consider when evaluating its use in clinical practice. Bleeding is a potential concern during ESD.7 Our findings indicated that the use of local anesthesia during ESD did not increase the risk of bleeding. Hypotension, hypoxemia, and bradycardia are known risks associated with endoscopic procedures done under general anesthesia or sedation.5 As for the safety of local anesthesia compared to placebo, it was comparable to placebo except for bradycardia, which had a lower incidence in the local anesthesia group.
Our results showed significant heterogenicity in pain outcomes after ESD, likely due to differences in patient pain perception, operator techniques, and the doses of local anesthetics used as well as nonstandard pain evaluation scales.15 Our study indicated that local anesthesia in ESD is safe, with no significant difference in any adverse event compared to saline, and it may even reduce bradycardia, indicating a potential protective effect against this adverse event. To enhance the standardization and efficacy of pain management in ESD, future research should focus on large-scale double-blinded RCTs utilizing standardized pain assessment tools like the VAS and uniform dosing protocols for local anesthetics. Moreover, direct comparisons between different local anesthetic agents are crucial to determine their relative potency in providing pain relief during and after ESD procedures. It is also recommended that longer-term pain assessments be conducted to accurately evaluate the sustained effectiveness of pain relief after ESD.
In conclusion, our study demonstrates that the utilization of local anesthetic did not have a significant impact on the procedure time of ESD. Nevertheless, it successfully alleviated postoperative pain in several trials, while the variability in pain evaluation methods hindered the ability to reach conclusive findings. Importantly, the use of local anesthetics did not elevate the likelihood of bleeding or other unfavorable occurrences and even diminished the occurrence of bradycardia. The results of our study emphasize the significance of pain management in enhancing procedural and patient outcomes after ESD. Although local anesthesia is generally considered safe and advantageous, further study is necessary to provide standardized techniques for assessing pain and determining the appropriate dosage of local anesthetics.
Disclosure statement/Funding
The authors report no funding or conflicts of interest.
References
- 1.Tziatzios G, Ebigbo A, Gölder SK, Probst A, Messmann H.. Methods that assist traction during endoscopic submucosal dissection of superficial gastrointestinal cancers: a systematic literature review. Clin Endosc. 2020;53(3):286–301. doi: 10.5946/ce.2019.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saito Y, Fukuzawa M, Matsuda T, et al. Clinical outcome of endoscopic submucosal dissection versus endoscopic mucosal resection of large colorectal tumors as determined by curative resection. Surg Endosc. 2010;24(2):343–352. doi: 10.1007/s00464-009-0562-8. [DOI] [PubMed] [Google Scholar]
- 3.Jung JH, Jang HJ, Bang CS, Baik GH, Park SW.. Efficacy of submucosal bupivacaine injection for pain relief after endoscopic submucosal dissection: a multicenter, prospective, randomized controlled, and double-blind trial. Medicine. 2019;98(17):e15360. doi: 10.1097/MD.0000000000015360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yoshizaki T, Matsumoto M, Sako T, Kodama Y, Okada A.. Efficacy of lidocaine injection method for esophageal endoscopic submucosal dissection: single-center, double-blind, randomized controlled trial. Surg Endosc. 2023;37(3):1962–1969. doi: 10.1007/s00464-022-09716-7. [DOI] [PubMed] [Google Scholar]
- 5.Ijiri M, Sasaki T, Fujiya M, et al. The efficacy of the submucosal injection of lidocaine during endoscopic submucosal dissection for colorectal neoplasms: a multicenter randomized controlled study. Surg Endosc. 2021;35(9):5225–‐5230. doi: 10.1007/s00464-020-08017-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim B, Lee H, Chung H, et al. The efficacy of topical bupivacaine and triamcinolone acetonide injection in the relief of pain after endoscopic submucosal dissection for gastric neoplasia: a randomized double-blind, placebo-controlled trial. Surg Endosc. 2015;29(3):714–722. doi: 10.1007/s00464-014-3730-4. [DOI] [PubMed] [Google Scholar]
- 7.Oka S, Tanaka S, Kaneko I, et al. Advantage of endoscopic submucosal dissection compared with EMR for early gastric cancer. Gastrointest Endosc. 2006;64(6):877–883. doi: 10.1016/j.gie.2006.03.932. [DOI] [PubMed] [Google Scholar]
- 8.Chang J-I, Kim TJ, Hwang NY, et al. Clinical outcomes and adverse events of gastric endoscopic submucosal dissection of the mid to upper stomach under general anesthesia and monitored anesthetic care. Clin Endosc. 2022;55(1):77–85. doi: 10.5946/ce.2021.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yoshizaki T, Obata D, Ueda C, et al. Feasibility of the lidocaine injection method during esophageal endoscopic submucosal dissection. JGH Open. 2020;4(2):251–255. doi: 10.1002/jgh3.12257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moher D, Liberati A, Tetzlaff J, Altman DG.. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cumpston M, Li T, Page MJ, et al. Updated guidance for trusted systematic reviews: a new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst Rev. 2019;10(10):Ed000142. doi: 10.1002/14651858.ED000142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 13.Han S, Hsu A, Wassef WY.. An update in the endoscopic management of gastric cancer. Curr Opin Gastroenterol. 2016;32(6):492–500. doi: 10.1097/MOG.0000000000000318. [DOI] [PubMed] [Google Scholar]
- 14.Rashid MU, Alomari M, Afraz S, Erim T.. EMR and ESD: indications, techniques and results. Surg Oncol. 2022;43:101742. doi: 10.1016/j.suronc.2022.101742. [DOI] [PubMed] [Google Scholar]
- 15.Jung DH, Youn YH, Kim JH, Park H.. Factors influencing development of pain after gastric endoscopic submucosal dissection: a randomized controlled trial. Endoscopy. 2015;47(12):1119–1123. doi: 10.1055/s-0034-1392537. [DOI] [PubMed] [Google Scholar]
- 16.Kim JE, Shin CS, Lee YC, Lee HS, Ban M, Kim SY.. Beneficial effect of intravenous magnesium during endoscopic submucosal dissection for gastric neoplasm. Surg Endosc. 2015;29(12):3795–3802. doi: 10.1007/s00464-015-4514-1. [DOI] [PubMed] [Google Scholar]
- 17.Kim N, Yoo YC, Lee SK, Kim H, Ju HM, Min KT.. Comparison of the efficacy and safety of sedation between dexmedetomidine-remifentanil and propofol-remifentanil during endoscopic submucosal dissection. World J Gastroenterol. 2015;21(12):3671–3678. doi: 10.3748/wjg.v21.i12.3671. [DOI] [PMC free article] [PubMed] [Google Scholar]