Abstract
Background
It is challenging to correctly identify and diagnose breast nonmass lesions. This study aimed to explore the multimodal ultrasound features associated with malignant breast nonmass lesions (NMLs), and evaluate their combined diagnostic performance.
Methods
This retrospective analysis was conducted on 573 breast NMLs, including 309 were benign and 264 were malignant, their multimodal ultrasound features (B-mode, color Doppler and strain elastography) were assessed by two experienced radiologists. Univariate and multivariate logistic regression analysises were used to explore multimodal ultrasound features associated with malignancy, and a nomogram was developed. Diagnostic performance and clinical utility were evaluated and validated by the receiver operating characteristic (ROC) curve, calibration curve and decision curve in the training and validation cohorts.
Results
Multimodal ultrasound features including linear (odds ratio [OR] = 4.69) or segmental distribution (OR = 7.67), posterior shadowing (OR = 3.14), calcification (OR = 7.40), hypovascularity (OR = 0.38), elasticity scored 4 (OR = 7.00) and 5 (OR = 15.77) were independent factors associated with malignant breast NMLs. The nomogram based on these features exhibited diagnostic performance in the training and validation cohorts were comparable to that of experienced radiologists, with superior specificity (89.4%, 89.5% vs. 81.2%) and positive predictive value (PPV) (89.2%, 90.4% vs. 82.4%). The nomogram also demonstrated good calibration in both training and validation cohorts (all P > 0.05). Decision curve analysis indicated that interventions guided by the nomogram would be beneficial across a wide range of threshold probabilities (0.05-1 in the training cohort and 0.05–0.93 in the validation cohort).
Conclusions
The combined use of linear or segmental distribution, posterior shadowing, calcification, hypervascularity and high elasticity score, displayed as a nomogram, demonstrated satisfied diagnostic performance for malignant breast NMLs, which may contribute to the imaging interpretation and clinical management of tumors.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12880-024-01462-7.
Keywords: Multimodal ultrasound, Breast, Nonmass lesion
Introduction
With the advancement of ultrasound technology and the widespread application of ultrasound in clinical practice, we often encounter lesions that do not meet the criteria for breast masses, known as nonmass lesions (NMLs), which are often associated with mammographic abnormalities such as asymmetries or suspicious calcifications, as well as nonmass enhancement on MRI [1–3]. Mammography is the gold standard for detecting breast microcalcifications, but the sensitivity limited especially in women with dense breasts, as well as by other challenges and potential problems including radiation exposure and poor reproducibility. MRI showed high sensitivity for nonmass enhancement, but the accompanying high false positive results may lead to unnecessary treatment, which was not recommended for breast disease screening. In recent years, there has been an increase in recognition and understanding of nonmass findings on ultrasound, and there is possible to introduce them as a distinct category in the upcoming sixth edition of the Breast Imaging Reporting and Data System (BI-RADS) [4]. The incidence of breast NMLs detected during screening ultrasound ranges from 1.0 to 5.3% [5, 6], with malignancy accounting for 10–54%, underscoring the importance of accurate identification and diagnosis of these lesions [7, 8].
Reports on ultrasound-detected NMLs are increasing, its definition, features and outcomes have been investigated in multiple studies [5, 6, 9–12]. Based on a review of relevant literature and pertinent clinical expertise, the widely recognized description of NML is discrete areas with altered echotexture that different from the normal breast tissue, lacking specific shape and margin on ultrasound [9, 13–15]. The proposed major features of breast NMLs are distribution and echo pattern, associated features including calcification, architectural distortion, duct changes, posterior features, small cysts, vascularity and elasticity [9, 13–16]. Various studies have assessed NMLs based on their distribution and/or some of the associated features, results showed the average positive predictive value (PPV) ranged from 7.4 to 83.9% [5, 10–12, 17, 18]. With the development and application of multimodal ultrasound, it has been reported to improve the diagnostic performace for malignant NMLs, especially in specificity, from 29 to 77.4% [17, 19, 20]. Higher specificities (69.0-90.5%) have reported in the combination with color Doppler ultrasound and/or shear-wave elastography features in BI-RADS category 4a NMLs detected on 2D ultrasound, without significant loss in sensitivities (97.3–100%) [19].
In our breast ultrasound diagnostic center, we routinely utilize B-mode ultrasound, color Doppler, and strain elastography techniques for each patient, providing comprehensive data for analysis. To our knowledge, this study represents the first retrospective evaluation of breast NMLs based on proposed features and descriptors, aiming to investigate the ultrasound features associated with malignancy and assess their combined diagnostic performance and clinical utility.
Materials and methods
Patients
The institutional review board of our institution approved this retrospective study, and requirement for individual informed consent was waived. From January 2020 to March 2023, 1537 consecutive women underwent breast ultrasound examination at our institution’s Breast Ultrasound Diagnostic Center, and were described or reported as NMLs [9, 13–15]. The inclusion criteria were: (1) completed multimodal ultrasound, and were confirmed as nonmass lesions after retrospective analyzed by 2 experienced radiologists; (2) no previous surgery history or current neoadjuvant chemotherapy; (3) ultimately confirmed by ultrasound guided core needle biopsy or surgical pathology. The exclusion criteria were: (1) failed to complete multimodal ultrasound examinations, or the image quality was unsatisfied; (2) pregnant or breastfeeding women; (3) The final pathological results have not been confirmed. All included lesions were randomly assigned to a training and validation cohort in a 7:3 ratio. Flowchart for patient selection is shown in Fig. S1.
Ultrasound examinations
Breast ultrasound examinations were conducted using an Acuson S2000 machine equipped with a 5–14 MHz linear array transducer (Siemens Medical Solutions, Mountain View). All patients concurrent underwent B-mode ultrasound, color Doppler, and strain elastography examinations by experienced radiologists. Patients were positioned supine with elevated arms to ensure complete exposure of the area under examination. The ultrasound device was set with pre-established configurations for breast scanning, and adjustments were made to the depth and gain according to the specific characteristics of the breast tissue, focusing on the region of interest (ROI). For each identified breast lesion, at least two orthogonal B-mode images were saved, and the lesion dimensions were accurately measured. Vascular flow signals were then assessed, with the initial velocity setting for color Doppler set between 3 and 4 cm/s. Further adjustments to the velocity range, color scanning area, and gain were made based on the observed vascular pattern. Once an optimal B-mode image was obtained, the system was switched to strain elastography mode, ensuring that the selected ROI included various tissue types and that the lesion comprised at least one-quarter of the ROI. The probe was consistently kept perpendicular to the skin surface without applying additional pressure, and the rigidity of the lesion was considered assessable when the quality factor reached or exceeded 50.
Image analysis
Two radiologists (L.F. and L.X with 7 and 18 years of breast ultrasound experience, respectively) retrospectively examined the B-mode ultrasound, color Doppler, and strain elastography images without knowledge of the pathological results. They recorded and evaluated the location, number, size, distribution, internal echo, and associated features such as calcifications, architectural distortion, duct changes, posterior features, small cysts, vascularity and elasticity. Subsequently, a BI-RADS category was assigned to each NML mainly based on the distribution and associated features. For purely hypoechoic area with focal distribution or small cysts were recommend a category 3, and the lesions with the other associated features should be assigned to category 4 or 5. For the cases of disagreement, the two radiologists would engage in a detailed discussion to reach a mutual consensus on the final interpretation of the results.
The vascularity of the NMLs were assessed based on the Adler classification [21]: level 0 indicated no blood flow; level 1 showed minimal flow, with 1 to 2 punctate or slender rod-shaped flows, not exceeding half the lesion’s diameter; level 2 indicated moderate flow, with 3 to 4 punctate flows or a longer flow traversing the lesion, reaching or exceeding half its diameter; level 3 represented substantial flow, visible as ≥ 5 punctate flows or 2 longer flows penetrating the lesion, each reaching or surpassing half its diameter in length. In this study, level 0 and 1 were classified as hypovascularity, while levels 2 and 3 were considered hypervascularity.
A five-point scoring system was utilized in strain elastography to assess the elasticity of lesions [22]: scored 1 indicated soft lesion; scored 2 indicated the lesion has both soft and hard components; scored 3 indicated the lesion is relatively stiff and smaller on the elastogram than on B-mode; scored 4 indicated the lesion is stiff and the same size as B-mode; scored 5 indicated the lesion is stiff and larger than B-mode. Scored 1 to 3 typically suggest benign lesions, while scored 4 and 5 are indicative of malignant lesions.
Data and statistical analysis
Data processing and analysis were performed using R version 4.3.0 (2023-04-21), along with Storm Statistical Platform (www.medsta.cn/software). Chi-square test and the t-test were used to evaluate the consistency of various factors in the training and validation cohort. Univariate and multivariate logistic regression analysises were used to explore the multimodal ultrasound features associated with malignant breast NMLs, and a nomogram was developed based on the significant features. Receiver operating characteristic (ROC) curve was constructed to evaluate the diagnostic performance of the nomogram and radiologists. The area under the curve (AUC), accuracy, sensitivity, specificity, PPV and negative predictive value (NPV) were calculated. Calibration curve analysis was used to explore the consistency between the predicted and true values. Decision curve analysis was used to calculate the net benefit from the models at different threshold probabilities. P < 0.05 indicated a statistically significant difference.
Results
Patient characteristics
A total of 548 female patients (range: 16–86 years, average age: 46.42 ± 13.07 years) with 573 breast NMLs were finally included in the study. 309 (53.9%) were benign and 264 (46.1%) were malignant, of which 163 were diagnosed by ultrasound guided core needle biopsy and 410 were confirmed by surgical pathology, details of the results are shown in Table S1. Patient characteristics were comparable between the training and validation cohorts (all P > 0.05, Table 1).
Table 1.
Patient characteristics in the training and validation cohorts
| Variables | Total (n = 573) |
Training cohort (n = 401) |
Validation cohort (n = 172) |
Statistic | P value |
|---|---|---|---|---|---|
| Age, Mean ± SD | 46.42 ± 13.07 | 46.14 ± 13.09 | 47.07 ± 13.03 | t = 0.78 | 0.437 |
| BI-RADS, n(%) | χ² = 0.24 | 0.993 | |||
| 3 | 73 (12.74) | 50 (12.47) | 23 (13.37) | ||
| 4 A | 210 (36.65) | 146 (36.41) | 64 (37.21) | ||
| 4B | 164 (28.62) | 115 (28.68) | 49 (28.49) | ||
| 4 C | 90 (15.71) | 64 (15.96) | 26 (15.12) | ||
| 5 | 36 (6.28) | 26 (6.48) | 10 (5.81) | ||
| Max Diameter, n(%) | χ²=1.95 | 0.377 | |||
| < 2 | 267 (46.60) | 180 (44.89) | 87 (50.58) | ||
| 2–5 | 204 (35.60) | 145 (36.16) | 59 (34.30) | ||
| > 5 | 102 (17.80) | 76 (18.95) | 26 (15.12) | ||
| Distribution, n(%) | χ²=1.17 | 0.760 | |||
| focal | 446 (77.84) | 308 (76.81) | 138 (80.23) | ||
| linear | 19 (3.32) | 15 (3.74) | 4 (2.33) | ||
| regional | 69 (12.04) | 50 (12.47) | 19 (11.05) | ||
| segmental | 39 (6.81) | 28 (6.98) | 11 (6.40) | ||
| Echo Pattern, n(%) | χ²=0.43 | 0.805 | |||
| hyperechoic | 9 (1.57) | 6 (1.50) | 3 (1.74) | ||
| Hypoechoic | 520 (90.75) | 366 (91.27) | 154 (89.53) | ||
| mixed | 44 (7.68) | 29 (7.23) | 15 (8.72) | ||
| Small Cysts, n(%) | χ²=0.05 | 0.822 | |||
| no | 548 (95.64) | 383 (95.51) | 165 (95.93) | ||
| yes | 25 (4.36) | 18 (4.49) | 7 (4.07) | ||
| Posterior Shadowing, n(%) | χ²=2.79 | 0.095 | |||
| no | 488 (85.17) | 335 (83.54) | 153 (88.95) | ||
| yes | 85 (14.83) | 66 (16.46) | 19 (11.05) | ||
| Calcifcation, n(%) | χ²=0.32 | 0.573 | |||
| no | 330 (57.59) | 234 (58.35) | 96 (55.81) | ||
| yes | 243 (42.41) | 167 (41.65) | 76 (44.19) | ||
| Architectural Distortion, n(%) | χ²=0.03 | 0.859 | |||
| no | 370 (64.57) | 258 (64.34) | 112 (65.12) | ||
| yes | 203 (35.43) | 143 (35.66) | 60 (34.88) | ||
| Duct Change, n(%) | χ²=0.32 | 0.572 | |||
| no | 496 (86.56) | 345 (86.03) | 151 (87.79) | ||
| yes | 77 (13.44) | 56 (13.97) | 21 (12.21) | ||
| Vascularity, n(%) | χ²=1.01 | 0.315 | |||
| hypervascularity | 328 (57.24) | 235 (58.60) | 93 (54.07) | ||
| hypovascularity | 245 (42.76) | 166 (41.40) | 79 (45.93) | ||
| Elasticity Score, n(%) | χ²=1.14 | 0.564 | |||
| ≤ 3 | 318 (55.50) | 220 (54.86) | 98 (56.98) | ||
| 4 | 182 (31.76) | 126 (31.42) | 56 (32.56) | ||
| 5 | 73 (12.74) | 55 (13.72) | 18 (10.47) | ||
| Result, n(%) | χ²=0.35 | 0.553 | |||
| benign | 309 (53.93) | 213 (53.12) | 96 (55.81) | ||
| malignant | 264 (46.07) | 188 (46.88) | 76 (44.19) |
BI-RADS: Breast Imaging Reporting and Data System, t: t-test, χ²: Chi-square test, SD: standard deviation
Multimodal ultrasound features associated with malignant NMLs
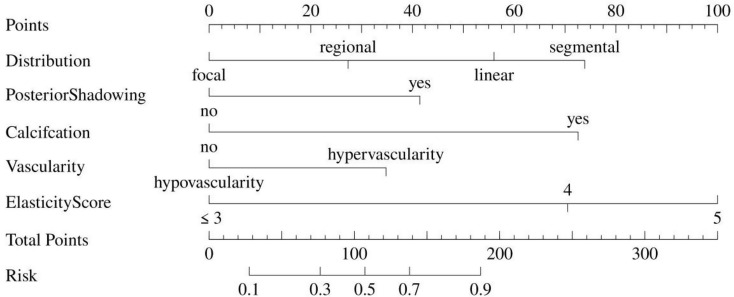
In the univariate logistic regression analysis, various ultrasound features such as regional or segmental distribution, posterior shadowing, calcification, architectural distortion, duct change, vascularity and elasticity scored 4 and 5 were found to be significantly associated with malignant breast NMLs (all P < 0.05, as shown in Table 2). Subsequent multivariate logistic regression analysis further confirmed that linear (odds ratio [OR] = 4.69, P = 0.021) or segmental distribution (OR = 7.67, P = 0.020), posterior shadowing (OR = 3.14, P = 0.006), calcification (OR = 7.40, P < 0.001), hypovascularity (OR = 0.38, P = 0.003), elasticity scored 4 (OR = 7.00, P < 0.001) and 5 (OR = 15.77, P < 0.001) in strain elastography were independent factors associated with malignant NMLs (Table 2). A nomogram incorporating these features was developed and presented in Fig. 1. Each ultrasound feature corresponds to a specific quantitative value, and the cumulative points for each NML correspond to a corresponding malignant risk value.
Table 2.
Results of univariate and multivariate logistic regression analysis between ultrasound features and malignant breast nonmass lesions
| Variables | Univariate logistic regression analysis | Multivariate logistic regression analysis | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| β | S.E | Z | P | OR (95%CI) |
β | S.E | Z | P | OR (95%CI) |
|
| Distribution | ||||||||||
| focal | 1.00 (Reference) |
1.00 (Reference) |
||||||||
| linear | 0.61 | 0.53 | 1.15 | 0.250 |
1.84 (0.65–5.21) |
1.55 | 0.67 | 2.31 | 0.021 |
4.69 (1.26–17.42) |
| regional | 1.42 | 0.34 | 4.23 | < 0.001 |
4.14 (2.14–8.00) |
0.75 | 0.45 | 1.69 | 0.090 |
2.13 (0.89–5.09) |
| segmental | 3.04 | 0.74 | 4.09 | < 0.001 |
20.93 (4.88–89.81) |
2.04 | 0.87 | 2.33 | 0.020 |
7.67 (1.39–42.46) |
| Echo Pattern | ||||||||||
| hyperechoic | 1.00 (Reference) | |||||||||
| Hypoechoic | -0.09 | 0.82 | -0.11 | 0.915 |
0.92 (0.18–4.60) |
|||||
| mixed | -0.64 | 0.91 | -0.71 | 0.478 |
0.53 (0.09–3.10) |
|||||
| Small Cysts | ||||||||||
| no | 1.00 (Reference) | |||||||||
| yes | -0.59 | 0.51 | -1.16 | 0.245 |
0.55 (0.20–1.50) |
|||||
| Posterior Shadowing | ||||||||||
| no | 1.00 (Reference) |
1.00 (Reference) |
||||||||
| yes | 0.98 | 0.28 | 3.44 | < 0.001 |
2.65 (1.52–4.62) |
1.14 | 0.41 | 2.78 | 0.006 |
3.14 (1.40–7.03) |
| Calcifcation | ||||||||||
| no | 1.00 (Reference) |
1.00 (Reference) |
||||||||
| yes | 2.42 | 0.24 | 10.02 | < 0.001 |
11.30 (7.03–18.16) |
2 | 0.31 | 6.42 | < 0.001 |
7.40 (4.02–13.64) |
| Architectural Distortion | ||||||||||
| no | 1.00 (Reference) | |||||||||
| yes | 0.57 | 0.21 | 2.7 | 0.007 |
1.76 (1.17–2.66) |
|||||
| Duct Change | ||||||||||
| no | 1.00 (Reference) | |||||||||
| yes | 0.74 | 0.3 | 2.49 | 0.013 |
2.09 (1.17–3.74) |
|||||
| Vascularity | ||||||||||
| hypervascularity | 1.00 (Reference) |
1.00 (Reference) |
||||||||
| hypovascularity | -2.17 | 0.24 | -8.95 | < 0.001 |
0.11 (0.07–0.18) |
-0.96 | 0.32 | -3.02 | 0.003 |
0.38 (0.21–0.71) |
| Elasticity Score | ||||||||||
| ≤ 3 | 1.00 (Reference) |
1.00 (Reference) |
||||||||
| 4 | 2.41 | 0.26 | 9.14 | < 0.001 |
11.11 (6.63–18.62) |
1.95 | 0.33 | 5.93 | < 0.001 |
7.00 (3.68–13.31) |
| 5 | 3.26 | 0.44 | 7.45 | < 0.001 |
25.94 (11.01–61.11) |
2.76 | 0.51 | 5.45 | < 0.001 |
15.77 (5.85–42.52) |
OR: Odds Ratio, CI: Confidence Interval
Fig. 1.
A nomogram was developed in the training cohort by integrating multiple ultrasound features. The points assigned to each feature were established by projecting a vertical line onto the points’ axis, 56 points in linear distribution, 74 points in segmental distribution, 42 points in posterior shadowing, 72 points in calcification, 35 points in hypervascularity, 70 points in elasticity scored 4 and 100 points in scored 5, and the total points for each NML has a corresponding malignant risk value
Performance of the nomogram based on the associated multimodal ultrasound features
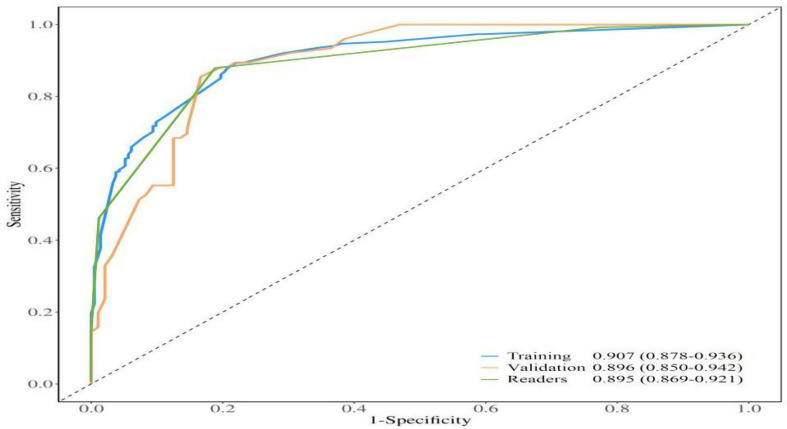
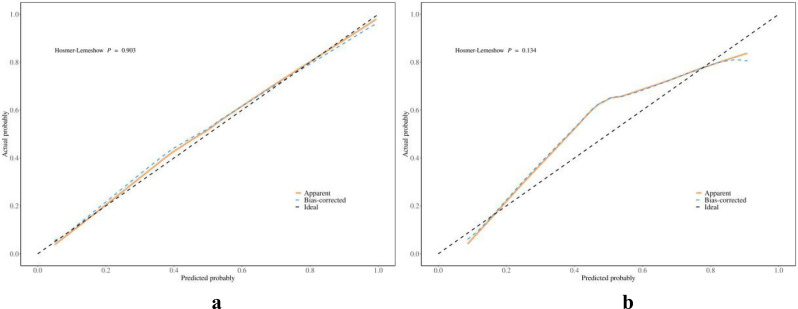
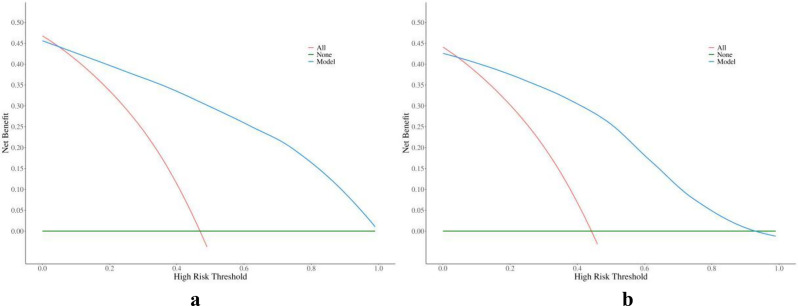
ROC curves of the nomogram and radiologists to diagnose malignant breast nonmass lesions are depicted in Fig. 2, with corresponding values for AUC, accuracy, sensitivity, specificity, PPV, and NPV provided in Table 3. Which showed the diagnostic performance of the nomogram based on the multimodal ultrasound features was satisfactory both in the training and validation cohorts, comparable to that of experienced radiologists. Calibration curves in Fig. 3 demonstrated good calibration in both cohorts. Decision curves in Fig. 4 indicated that interventions guided by the nomogram would be beneficial across a wide range of threshold probabilities (0.05-1 in the training cohort and 0.05–0.93 in the validation cohort). Representative cases are illustrated in Figs. 5 and 6.
Fig. 2.
Receiver operating characteristic curves of the readers and the nomogram in the training cohort and validation cohort. Performance of the nomogram in the two cohorts were comparable to that of the experienced radiologists
Table 3.
The diagnostic performances among the readers and the nomogram
| AUC (95%CI) | Accuracy | Sensitivity | Specificity | PPV | NPV | cut-off value | |
|---|---|---|---|---|---|---|---|
| Training cohort | 0.907 (0.878–0.936) | 83.3% | 77.9% | 89.4% | 89.2% | 78.1% | 0.343 |
| Validation cohort | 0.896 (0.850–0.942) | 83.1% | 78.1% | 89.5% | 90.4% | 76.4% | 0.343 |
| Readers | 0.895 (0.869–0.921) | 84.3% | 87.9% | 81.2% | 82.4% | 87.0% | BI-RADS 4A |
AUC: Area Under the Curve, CI: Confidence Interval, PPV: Positive Predictive Value, NPV: Negative Predictive Value
Fig. 3.
The calibration curve of the nomogram in the training (a) and validation (b) cohort. The bias-corrected calibration curve was used to evaluate the predictive ability of the model, with a closer resemblance to the ideal curve indicating better performance. Results demonstrated good calibration in both cohorts
Fig. 4.
The decision curve of the nomogram in the training (a) and validation (b) cohort. Three curves shows the benefit of intervening on patients based on the prediction model (blue), intervening all patients (red) or no patients (green), respectively. Take the intersection of the blue curve with the red curve as the starting point and the intersection with the green curve as the end point, within which patients would benefit from intervention according to the model. Results showed that interventions guided by the nomogram would be beneficial across a wide range of threshold probabilities (0.05-1 in the training cohort and 0.05–0.93 in the validation cohort)
Fig. 5.
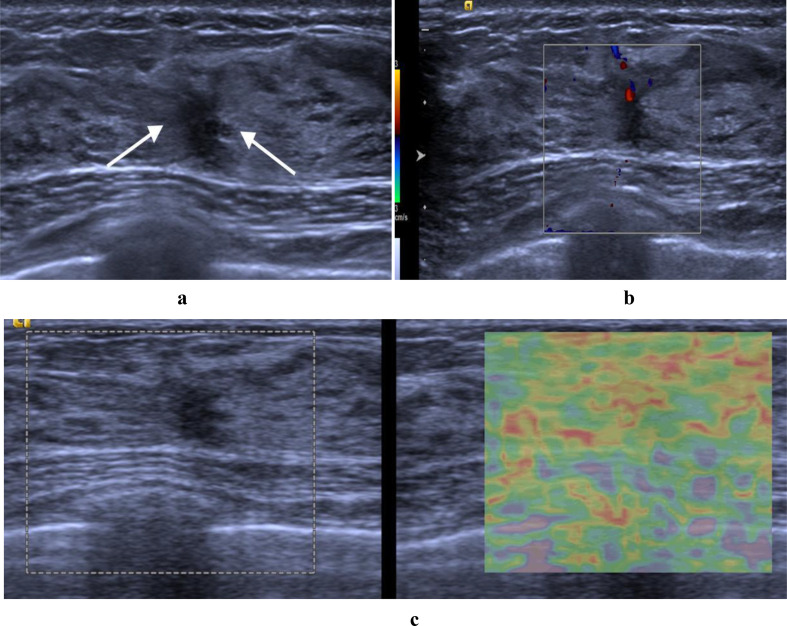
The multimodal ultrasound features of a benign nonmass lesion attributed to adenosis were validated through surgical pathological confirmation. (a) hypoechoic area with focal distribution and architectural distortion on B-mode ultrasound (arrows) (b) hypervascularity (level 2 in Adler classification) on color Doppler (c) scored 3 on strain elastography. Assessed as BI-RADS category 4B by the two radiologists. Total points were 35 on the nomogram (35 points in hypervascularity), resulting in a malignant risk value less than the cut-off value (0.343), suggesting that unnecessary interventions could be avoided
Fig. 6.
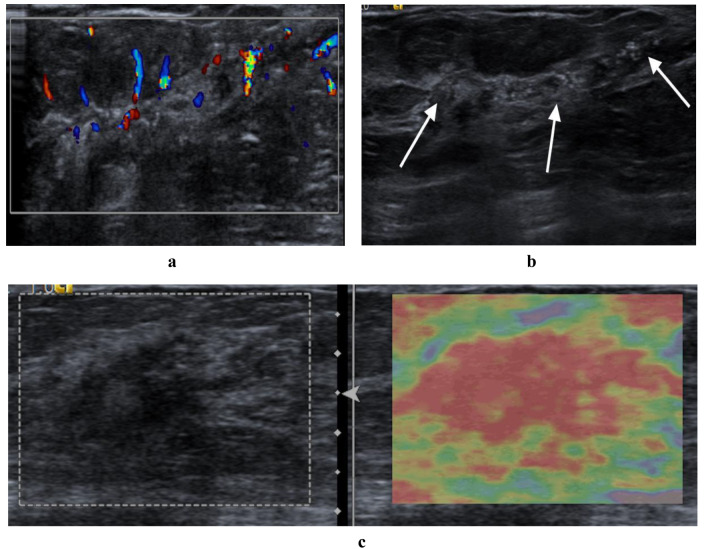
The multimodal ultrasound features of a malignant nonmass lesion attributed to invasive breast cancer (grade III, invasive micropapillary carcinoma) were verified through surgical pathological confirmation. (a) hypoechoic area with segmental distribution and multiple calcifications on B-mode ultrasound (arrows) (b) hypervascularity (level 3 in Adler classification) on color Doppler (c) scored 4 on strain elastography. Assessed as BI-RADS category 4 C by the two radiologists. Total points were 251 on the nomogram (74 points in segmental distribution, 72 points in calcification, 35 points in hypervascularity, 70 points in elasticity scored 4), resulting in a malignant risk value exceeding 0.9, in which intervention was required
Discussion
In this study, multimodal ultrasound features including linear or segmental distribution, posterior shadowing, calcification, vascularity, high elasticity scored 4 and 5 were identified as significant factors associated with malignant breast NMLs. The nomogram’s performance utilizing these features was found to be comparable to that of experienced radiologists, and exhibiting superior specificity and PPV. Furthermore, the nomogram displayed good calibration and satisfactory clinical utility both in the training and validation cohorts.
Since the introduction of breast NMLs on ultrasound, several studies have presented findings on the utilization of various ultrasound techniques either independently or in conjunction for assessing NMLs [23–28]. These studies have demonstrated varying degrees of enhancement in diagnostic accuracy and specificity of biopsy decisions. Nevertheless, a consistent characterization and classification system for NMLs on ultrasound have been lacking in these studies, potentially resulting in discrepancies in interpretation and assessment among different radiologists. For instance, Ko et al. categorized NMLs into four types and assigned BI-RADS categories based on their PPVs, thereby establishing a dependable reference for stratifying NML risk [11]. However, this system exhibited a higher malignancy incidence (10-79%), which could lead to an increase in unnecessary biopsies. Another classification system, developed by Park et al., based on suspicious ultrasound findings or in combination with mammographic features, aimed to aid in the interpretation and management of breast NMLs [10]. The results indicated that this system significantly improved diagnostic performance among radiologists, with an AUC ranging from 0.951 to 0.956, and specificity increasing from 49.3 to 76.8%. Choi et al. further outlined a standardized interpretation algorithm flowchart for BI-RADS classification of NMLs using ultrasound features, this approach has demonstrated a reduction in false-positive rates on NML management in their clinical practice [10, 14, 15, 29]. However, the efficacy of this approach requires further validation across diverse institutions and scenarios. Recently, nomograms have emerged as valuable tools for establishing intuitive relationships between evaluation variables, offering quantitative and personalized methods for predicting cancer risk [30–32]. In our study, we have presented a quantitative association between multimodal ultrasound features and malignant breast NMLs, with the nomogram based on these features exhibiting satisfactory performance.
In a prior study involving 229 cases, a nomogram was developed using patient age, clinical symptoms, and ultrasound features to predict malignant NMLs in the Asian population, demonstrating favorable diagnostic accuracy and clinical utility [33]. Of which, the meaningful ultrasound features encompass orientation, echo patterns, calcification, and vascularity graded by Adler’s classification. But in our study, we only focused on the ultrasound features for the following reasons: ① the contentious relationship between breast cancer and age [34, 35], ② the majority of the participants in this research exhibit clinical manifestations, and this ultrasound examination is mostly used for diagnostic purposes, ③ multiple ultrasound descriptions of breast NMLs have been documented in existing literature, warranting further investigation and discussion. The current study incorporated proposed features and descriptors of NMLs, along with a larger sample size. Ultimately, our results exhibited higher AUC, sensitivity, and specificity compared to previous studies, indicating that the nomogram model has excellent diagnostic performance for malignant breast NMLs, and comparable to the performance achieved by experienced radiologists using BI-RADS categories. It is worth mentioning that it is important to acknowledge the considerable dependence of radiologists on their experience when characterizing and assessing breast NMLs.
In order to enhance the utility of multimodal ultrasound features in clinical practise, a nomogram was developed. This involved assigning numerical values to each individual ultrasound feature, with the total points for each NML corresponding to a specific malignant risk value for use in subsequent clinical decision-making. Of which, the elasticity scores emerged as the most influential feature in predicting malignancy in breast NMLs. The strain elasticity scores demonstrated a notable specificity of 93.8% for evaluating NMLs when the threshold was set between 3 and 4, as reported in a previous study [24]. In our study, scored 4 and 5 exhibited the highest ORs (7.00, 15.77) and points (70, 100), we concluded that when the elastography suggests that a stiff NMLs will increase our confidence in the diagnosis of malignant lesions. The linear and segmental distribution patterns detected on ultrasound were found to be indicative of lesions within ducts and branches, often suggestive of ductal carcinoma in situ (DCIS) or suspicious multifocal breast cancer [36]. Compared to a previous study reporting an OR of 3.65 [10], the current study revealed higher ORs of 4.69 and 7.67 for linear and segmental distributions, with corresponding nomogram point values of 56 and 74, which further confirmed the risk correlation between linear and segmental distribution and malignancy. Additionally, the presence of calcifications on ultrasound was identified as a significant risk factor for breast cancer, which have been reported to be more than three times more likely to be malignant [5, 17, 37]. This was associated with an OR of 7.40 and assigned 72 points in predicting malignant NMLs in the present study. The presence of posterior shadowing on ultrasound imaging may indicate pathological alterations that stimulate the proliferation of connective tissue, leading to attenuation of the ultrasound beam [38]. This phenomenon can be observed in both benign and malignant lesions. Our findings revealed an OR of 3.14 and 42 points for malignant NMLs. Internal vascularity within a focal isoechoic or hypoechoic area was noted to aid in identifying NMLs, with PPVs of hypervascularity for malignant NMLs ranging from 27.5 to 90.5% [17]. In our research, over half (57.2%, 328/573) of the NMLs were found to associated with hypervascularity, and 67.7% of these were subsequently verified as malignant. Architectural distortion and duct changes are common features in breast NMLs, and more frequent in malignant lesions [1, 39, 40]. These findings indicated a statistically significant association with malignancy in the univariate logistic regression analysis, however, it was not observed in the multivariate logistic regression analysis. Considering the potential overlap of features between benign and malignant lesions in NMLs, pathological findings in this study may offer insights into this scenario. It is noteworthy that benign lesions such as adenosis, mastitis, and intraductal papillomas have been significantly identified in NML cases exhibiting structural distortion or ductal changes in our study. Multi-center studies are warranted to be carried out in the future.
The present study had some limitations. Firstly, it was a retrospective, single center study with only internal validation of the retrospective data, further investigation is necessary to assess the predictive precision of the nomogram for ultrasound NMLs and its practical application through additional multi-center external validation and prospective research studies. Secondly, all cases included in this study were confirmed by biopsy or surgical pathological results, potentially introducing bias in patient selection. Thirdly, the institution where the study was conducted serves as a breast tumor diagnosis center with a substantial number of referral patients, utilizing ultrasound examinations primarily for diagnostic purposes. Given the relatively high proportion of malignant breast NMLs in this setting, the findings may not be directly generalizable to screen all populations. Lastly, breast NML is still a relatively new concept that highly depends on the experience of the radiologists. The promotion, acceptance, and diagnostic efficacy of this concept in different institutions still need further research.
Conclusions
Breast nonmass lesions with linear or segmental distribution, posterior shadowing, calcification, hypervascularity and high elasticity score were associated with malignancy. The combined use of these multimodal ultrasound features displayed as a nomogram, demonstrated satisfied diagnostic performance for malignant breast NMLs, which may contribute to the imaging interpretation and clinical management of tumors.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
None.
Abbreviations
- NML
Nonmass lesion
- BI-RADS
Breast Imaging Reporting and Data System
- PPV
Positive predictive value
- ROI
Region of interest
- ROC
Receiver operating characteristic
- AUC
Area under the curve
- NPV
Negative predictive value
- OR
Odds ratio
- DCIS
Ductal carcinoma in situ
Author contributions
Li-Fang Yu: Writing-original draft; Review and editing. Luo-Xi Zhu: Funding acquisition; Review and editing. Chao-Chao Dai: Editing; Images collection. Xiao-Jing Xu: Funding acquisition; Data curation. Yan-Juan Tan and Hong-Ju Yan: Data analysis and images collection. Ling-Yun Bao: Conceptualization; Review and editing. All authors reviewed and approved the final manuscript.
Funding
This work was supported by funds from Hangzhou Biomedical and Health Industry Development Support Special Projects (2022WJC241 and 2022WJC247).
Data availability
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
This study was carried out in accordance with the Declaration of Helsinki and approved by the Medical ethics committee of Hangzhou First people’s Hospital. Written informed consent was waived by the IRB due to the retrospective nature of the study.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Clinical trial number
Not applicable.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Giess CS, Chesebro AL, Chikarmane SA. Ultrasound features of mammographic developing asymmetries and correlation with histopathologic findings. AJR Am J Roentgenol. 2018;210(1):W29–38. [DOI] [PubMed] [Google Scholar]
- 2.Coskun Bilge A, Demir PI, Aydin H, et al. Dynamic contrastenhanced breast magnetic resonance imaging findings that affect the magnetic resonance-directed ultrasound correlation of nonmass enhancement lesions: a single-center retrospective study. Br J Radiol. 2022;95(1132):20210832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coffey K, Sung J, Comstock C. Utility of targeted ultrasound to predict malignancy among lesions detected on contrast-enhanced digital mammography. AJR Am J Roentgenol. 2021;217(3):595–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leung JWT. Nonmass Descriptor at Breast US to Expand Clinical Utility. J Breast Imaging. 2024;6(1):99–101. [DOI] [PubMed] [Google Scholar]
- 5.Kim SJ, Park YM, Jung HK. Nonmasslike lesions on breast sonography: comparison between benign and malignant lesions. J Ultrasound Med. 2014;33(3):421–30. [DOI] [PubMed] [Google Scholar]
- 6.Lee J, Lee JH, Baik S, et al. Non-mass lesions on screening breast ultrasound. Med Ultrason. 2016;18(4):446–51. [DOI] [PubMed] [Google Scholar]
- 7.Yamaguchi R, Watanabe H, Mihara Y et al. Histopathology of non-mass-like breast lesions on ultrasound. J Med Ultrason (2001). 2023;50(3):375–80. [DOI] [PMC free article] [PubMed]
- 8.Uematsu T. Non-mass lesions on breast ultrasound: why does not the ACR BI-RADS breast ultrasound lexicon add the terminology? J Med Ultrason (2001). 2023;50(3):341–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Choe J, Chikarmane SA, Giess CS. Nonmass findings at Breast US: definition, classifications, and Differential diagnosis. Radiographics. 2020;40(2):326–35. [DOI] [PubMed] [Google Scholar]
- 10.Park KW, Park S, Shon I, et al. Non-mass lesions detected by breast US: stratification of cancer risk for clinical management. Eur Radiol. 2021;31(3):1693–706. [DOI] [PubMed] [Google Scholar]
- 11.Ko KH, Hsu HH, Yu JC, et al. Non-mass-like breast lesions at ultrasonography: feature analysis and BI-RADS assessment. Eur J Radiol. 2015;84(1):77–85. [DOI] [PubMed] [Google Scholar]
- 12.Wang ZL, Li N, Li M, et al. Non-mass-like lesions on breast ultrasound: classification and correlation with histology. Radiol Med. 2015;120(10):905–10. [DOI] [PubMed] [Google Scholar]
- 13.DeMartini WB, Destounis SV, Eby PR et al. BI-RADS update: the edition formerly known as fifth. Proceedings of the 2023 SBI Breast Imaging Symposium; 2023 May 4–7; National Harbor, MD, USA: Society of Breast Imaging p.9.
- 14.Choi JS, Tsunoda H, Moon WK. Nonmass lesions on breast US: An International Perspective on Clinical Use and outcomes. J Breast Imaging. 2024;6(1):86–98. [DOI] [PubMed] [Google Scholar]
- 15.Tsunoda H, Moon WK. Beyond BI-RADS: Nonmass Abnormalities on breast ultrasound. Korean J Radiol. 2024;25(2):134–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ito T, Ueno E, Endo T et al. The Japan society of ultrasonics in medicine guidelines on non-mass abnormalities of the breast. J Med Ultrason (2001). 2023;50(3):331–9. [DOI] [PMC free article] [PubMed]
- 17.Choi JS, Han BK, Ko EY, et al. Additional diagnostic value of shear-wave elastography and color Doppler US for evaluation of breast non-mass lesions detected at B-mode US. Eur Radiol. 2016;26(10):3542–9. [DOI] [PubMed] [Google Scholar]
- 18.Lin M, Wu S. Ultrasound classification of non-mass breast lesions following BI-RADS presents high positive predictive value. PLoS ONE. 2022;17(11):e0278299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li L, Zhou X, Zhao X, et al. B-Mode Ultrasound combined with Color Doppler and Strain Elastography in the diagnosis of non-mass breast lesions: a prospective study. Ultrasound Med Biol. 2017;43(11):2582–90. [DOI] [PubMed] [Google Scholar]
- 20.Zhang W, Xiao X, Xu X, et al. Non-mass breast lesions on Ultrasound: Feature Exploration and Multimode Ultrasonic diagnosis. Ultrasound Med Biol. 2018;44(8):1703–11. [DOI] [PubMed] [Google Scholar]
- 21.Adler DD, Carson PL, Rubin JM, et al. Doppler ultrasound color flow imaging in the study of breast cancer: preliminary findings. Ultrasound Med Biol. 1990;16(6):553–9. [DOI] [PubMed] [Google Scholar]
- 22.Barr RG. Sonographic breast elastography: a primer. J Ultrasound Med. 2012;31(5):773–83. [DOI] [PubMed] [Google Scholar]
- 23.Wang ZL, Li Y, Wan WB, et al. Shear-Wave Elastography: could it be helpful for the diagnosis of non-mass-like breast lesions? Ultrasound Med Biol. 2017;43(1):83–90. [DOI] [PubMed] [Google Scholar]
- 24.Qu XX, Song Y, Zhang YH, et al. Value of Ultrasonic Elastography and Conventional Ultrasonography in the Differential diagnosis of non-mass-like breast lesions. Ultrasound Med Biol. 2019;45(6):1358–66. [DOI] [PubMed] [Google Scholar]
- 25.Liu W, Zong M, Gong HY, et al. Comparison of diagnostic efficacy between contrast-enhanced Ultrasound and DCE-MRI for Mass- and non-mass-like enhancement types in breast lesions. Cancer Manag Res. 2020;12:13567–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang J, Cai L, Pan X, et al. Comparison and risk factors analysis of multiple breast cancer screening methods in the evaluation of breast non-mass-like lesions. BMC Med Imaging. 2022;22(1):202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li J, Liu Y, Li Y, et al. Comparison of diagnostic potential of shear wave elastography between breast mass lesions and non-mass-like lesions. Eur J Radiol. 2023;158:110609. [DOI] [PubMed] [Google Scholar]
- 28.Zhang J, Cai L, Chen L, et al. Re-evaluation of high-risk breast mammography lesions by target ultrasound and ABUS of breast non-mass-like lesions. BMC Med Imaging. 2021;21(1):156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kwon MR, Choi JS, Lee MY, et al. Screening outcomes of Supplemental Automated breast US in Asian women with dense and nondense breasts. Radiology. 2023;307(4):e222435. [DOI] [PubMed] [Google Scholar]
- 30.Luo WQ, Huang QX, Huang XW, et al. Predicting breast Cancer in breast imaging reporting and Data System (BI-RADS) Ultrasound Category 4 or 5 lesions: a Nomogram combining Radiomics and BI-RADS. Sci Rep. 2019;9(1):11921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xiong L, Chen H, Tang X, et al. Ultrasound-based Radiomics Analysis for Predicting Disease-Free survival of invasive breast Cancer. Front Oncol. 2021;11:621993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jiang M, Li CL, Luo XM, et al. Ultrasound-based deep learning radiomics in the assessment of pathological complete response to neoadjuvant chemotherapy in locally advanced breast cancer. Eur J Cancer. 2021;147:95–105. [DOI] [PubMed] [Google Scholar]
- 33.Lin X, Zhuang S, Yang S, et al. Development and internal validation of a conventional ultrasound-based nomogram for predicting malignant nonmasslike breast lesions. Quant Imaging Med Surg. 2022;12(12):5452–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jackson B, Gondara E, Speers L. Does age affect outcome with breast cancer? Breast. 2023;70:25–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Coughlin SS. Epidemiology of breast Cancer in women. Adv Exp Med Biol. 2019;1152:9–29. [DOI] [PubMed] [Google Scholar]
- 36.Greenwood HI, Heller SL, Kim S, et al. Ductal carcinoma in situ of the breasts: review of MR imaging features. Radiographics. 2013;33(6):1569–88. [DOI] [PubMed] [Google Scholar]
- 37.Li Y, Cao J, Zhou Y, et al. Mammographic casting-type calcification is an independent prognostic factor in invasive breast cancer. Sci Rep. 2019;9(1):10544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Porter AJ, Evans EB, Foxcroft LM, et al. Mammographic and ultrasound features of invasive lobular carcinoma of the breast. J Med Imaging Radiat Oncol. 2014;58(1):1–10. [DOI] [PubMed] [Google Scholar]
- 39.Zheng FY, Yan LX, Huang BJ, et al. Comparison of retraction phenomenon and BI-RADS-US descriptors in differentiating benign and malignant breast masses using an automated breast volume scanner. Eur J Radiol. 2015;84(11):2123–9. [DOI] [PubMed] [Google Scholar]
- 40.Japan Association of Breast and Thyroid Sonology. Guideline for breast ultrasound: management and diagnosis. Tokyo, Japan: Nankodo; 2004. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.