Abstract
Although many recombinant adenovirus vectors (rAd) have been developed, especially by using group C adenoviruses, to transfer and express genes, such rAd do not readily infect B-cell lines due to the lack of the coxsackievirus-adenovirus receptor. Bispecific antibodies have been used in different cell systems to facilitate entry of rAd into otherwise nonpermissive cells. Bispecific antibody is synthesized by covalently linking two monoclonal antibodies with distinct specificities. It has been shown that lymphoproliferative tumors commonly express the cell surface protein CD70, while this receptor is normally expressed on only a small subset of highly activated B cells and T cells. We therefore investigated whether a bispecific antibody with specificities for the adenovirus fiber protein and CD70 can facilitate rAd entry and subsequent expression of rAd-encoded genes in CD70-positive B cells. We found high CD70 expression on Epstein-Barr virus (EBV)-transformed lymphoblastoid cell lines (LCLs), as well as some, but not all, Burkitt lymphoma (BL) lines. We show here that rAd encoding green fluorescent protein (Ad-GFP) infects EBV-transformed LCLs and a CD70-positive BL line 10- to 20-fold more efficiently in the presence of the CD70-fiber bispecific antibody. In contrast, the bispecific antibody does not enhance Ad-GFP infection in CD70-deficient BL cells. Using the CD70-fiber bispecific antibody, we increased the ability of rAd vectors encoding the EBV immediate-early proteins BZLF1 and BRLF1 to induce the lytic form of EBV infection in LCLs. These results indicate that the CD70-fiber bispecific antibody can enhance rAd infection of CD70-positive B cells and suggest the use of this vector to explore EBV-positive LCLs.
The consistent presence of the Epstein-Barr virus (EBV) genome in certain malignancies, particularly its nearly universal presence in AIDS-related central nervous system lymphomas (4, 6, 29) and nasopharyngeal carcinomas (31), suggests that EBV itself could serve as a target for the preferential killing of tumor cells using gene delivery methods. Although the use of adenovirus vectors expressing EBV-specific toxins could potentially be useful for treating EBV-positive epithelial cell tumors, EBV-associated B-cell tumors are unlikely to be susceptible to conventional adenovirus-mediated delivery. The recently identified adenovirus receptor for serogroups 2 and 5, the coxsackievirus-adenovirus receptor (CAR), is not expressed in most hematologic cell lines (19, 28, 44), and consequently, adenovirus delivery to most B-cell lines is extremely inefficient. Nevertheless, other advantageous aspects of recombinant adenovirus vectors (rAd), including the extremely high achievable titers and the large size (10 kb) of the gene inserts tolerated, continue to make rAd the most attractive currently available gene delivery vectors. Therefore, there has been intense interest in modifying rAd to improve their delivery into hematopoietic cell types.
Bispecific antibodies (BsAb) are covalently linked antibodies with distinct specificities (40). BsAb can extend a virus's normal tropism by using specific antibodies to the virus's receptor (the fiber protein, in the case of adenovirus [41]) and an alternate cellular ligand. For example, the delivery of rAd with a FLAG epitope-modified adenovirus fiber protein to T cells was shown to be greatly enhanced when a bispecific antibody directed against the FLAG epitope and the T-cell-specific CD3 cell surface receptor (48) was used. The method has been applied to other virus-cell surface ligand systems (5, 7, 49).
In this study, we have investigated the use of an anti-CD70–antifiber BsAb to enhance adenovirus delivery to CD70-positive B-cell lines. CD70 expression is usually limited to a small subset of highly activated B and T cells (42). In contrast, EBV-immortalized B cells (lymphoblastoid cell lines [LCLs]) routinely express CD70 (42), as do a number of EBV-positive, as well as EBV-negative, B-cell lymphomas (17, 27). Expression of CD70 (which has been identified as the CD27 ligand) on T cells appears to have a physiological role in inducing CD27+ B cells to proliferate and differentiate into plasma cells (2), while on B cells, CD70 seems to have a costimulatory effect upon T cells (3). Interestingly, although CD70 expression in vivo is usually limited to a few highly activated B cells and T cells, CD70 is also expressed in EBV-positive nasopharyngeal carcinomas (1, 31). Thus, CD70 expression could potentially serve as a relatively specific marker with which to direct adenovirus vectors to many EBV-infected cells and/or LCLs.
Here we show that BsAb directed against the CD70 receptor and the adenovirus fiber protein (BsAb-CD70-fiber) significantly enhance adenovirus delivery to CD70-positive, but not CD70-negative, cell lines. By using this technique, we can effectively transfer genes to EBV-positive LCLs, including genes that induce the EBV lytic cycle. Our group has previously shown that adenovirus vectors expressing the EBV immediate-early (IE) protein BZLF1 or BRLF1 can induce the lytic form of EBV infection in EBV-positive tumors in vivo, thereby resulting in specific killing of tumor cells (47). However, by using methods including lipofection and electroporation, we have been previously unable to demonstrate that BRLF1 expression in LCLs induces lytic EBV infection (50), perhaps due to inefficient delivery of BRLF1. Using BsAb-CD70-fiber, we now demonstrate that the EBV BRLF1 IE protein (as well as the BZLF1 protein) induces the lytic form of EBV infection in LCLs. Thus, use of the CD70-fiber bispecific antibody may be a useful approach by which to enhance the delivery of rAd to CD70-positive cell lines.
MATERIALS AND METHODS
Cell lines.
LCLs were obtained by transforming primary human B cells from different donors with the B958 strain of EBV. Raji, Jijoye, P3HR1, and Akata cells are derived from various EBV-positive Burkitt lymphomas (BLs). DG75 cells are an EBV-negative BL cell line. A9 cells are a murine epithelioid fibroblast cell line. The culture medium used was RPMI medium (for all suspension cells) or Dulbecco modified Eagle medium (for A9 cells) with 10% fetal bovine serum at 37°C with 4% CO2 and 100% humidity.
Adenovirus vectors.
The recombinant adenoviruses used in this study have been previously developed and described (47). In brief, the relevant gene was cloned into an adenovirus shuttle vector, and Cre-Lox-mediated recombination between the shuttle vector and a replication-deficient type 5 adenovirus genome with E1 and E3 deleted resulted in the recombinant adenovirus. Expression of each gene of interest is driven by the cytomegalovirus IE promoter. Recombinant adenoviruses were propagated in 293 cells by using initial stocks provided by the UNC Gene Therapy Core facility.
BsAb.
The anti-CD70 monoclonal antibody (MAb) Ki-24 used to construct the anti-CD70–antifiber BsAb was produced from a hybridoma cell line that was a gift from H. Stein. The anti-CD70-antifiber BsAb were made, as previously described, by using succinimidyl-3-(2-pyridyldithiol)-propionate (SPDP) as a cross-linking agent (40, 49). Briefly, a fourfold molar excess of SPDP was added to 0.5 mg each of the neutralizing 7H11 antifiber knob MAb and the Ki-24 anti-CD70 MAb in borate-buffered solution (BBS), pH 8.5. After 30 min at room temperature, the pH of Ki-24 was lowered by addition of 0.1 volume of 1 M sodium acetate, pH 4.5, and 1 mg of solid dithiothreitol was added. After 5 min at room temperature, the reduced Ki-24 was passed over a Pharmacia PD10 disposable Sephadex G-25 column in BBS and added to the SPDP-treated antifiber MAb. Cross-linking was allowed to proceed for 1 h at room temperature, and the protein was concentrated to 0.3 ml and fractionated on an HR 10/30 Superose 12 column (Pharmacia) in BBS. Monomeric immunoglobulin G (approximately 50%) was discarded, and the cross-linked material was pooled. It consisted of about 50% dimer, and the rest was higher-molecular-weight aggregates. The final BsAb product had a concentration of 0.063 μg/μl and was estimated to contain 1.2 × 1011 molecules per μl.
BsAb-adenovirus infection.
To infect cells with rAd, 106 cells were washed three times in Dulbecco modified Eagle medium-F12 medium (prechilled to 4°C) and incubated for 30 min on ice. One million cells per sample were collected in a 15-ml conical tube, and the cells were then pelleted in a centrifuge. The pellets were then resuspended in 100 μl of medium in the presence or absence of BsAb (0.63 μg/ml unless otherwise indicated) and incubated on ice for 1.5 to 2 h with manual resuspension every 10 min. All samples were then washed three times with medium, pelleted, resuspended in 100 μl with a given rAd (multiplicity of infection [MOI] of 20:1 unless otherwise indicated) or plain medium, and incubated on ice for 1.5 to 2 h as before. Finally, the cells were resuspended in 1.4 ml of RPMI medium with 10% fetal bovine serum and cultured for 24 to 48 h before further analysis. In some experiments, the BsAb was, instead, preincubated with virus at 4°C for 1 h and then further incubated with cells for 1 h at 4°C before culturing for 24 to 48 h in 1.4 ml of RPMI medium.
FACS.
Cells to be examined for green fluorescent protein-associated fluorescence were trypsinized, if adherent, and then washed three times with phosphate buffered saline (PBS) with 1% (wt/vol) bovine serum albumin. Finally, they were resuspended in 0.5 to 1 ml of PBS and analyzed for relative fluorescence using a Becton-Dickinson FACScan flow cytometer. Cells to be studied for the presence of CD70 were fixed using ice-cold 60% acetone for 10 min and then washed three times with PBS-bovine serum albumin. Cells were next incubated in a 1:100 dilution of the Ki-24 MAb at room temperature for 1 h and rewashed three times. The fluorescein isothiocyanate-conjugated secondary antibody was applied for 1 h at room temperature in the dark, and cells were washed three more times. Finally, the cells were resuspended in 0.5 to 1 ml of PBS and analyzed by fluorescence-activated flow cytometry (FACS).
To quantitate CD70 receptor expression, a 1:100 dilution of anti-CDw70 (Pharmingen) and an equal concentration of an isotype control were used for FACS. A 1:100 dilution of fluorescein isothiocyanate-conjugated goat anti-mouse immunoglobulin G (Sigma) was used for immunofluorescence and FACS studies.
Western blot analysis.
Tissue culture samples were collected and washed in PBS three times. Cells were resuspended in 40 to 80 μl of lysis buffer (0.25 M NaCl, 0.1% NaPO4, 0.05 M HEPES, 0.005 EDTA) containing fresh 1× protease inhibitor (Complete; Boehringer) and rapidly freeze-thawed three times. Cellular debris was removed by centrifugation in a tabletop microcentrifuge at 14,930 × g for 15 min at 4°C, and the protein concentration was determined by a Bradford assay. Between 40 and 100 μg of total cellular protein was loaded onto a 10% denaturing polyacrylamide gel, and immunoblot analysis was subsequently performed using anti-BZLF1 and anti-BRLF1 (Argenene) antibodies (both were used at a 1:100 dilution) or anti-BMRF1 (Capricorn, Scarborough, Maine) (also used at a 1:100 dilution) and the ECL detection kit (Amersham Pharmacia).
RESULTS
CD70 is expressed on a variety of EBV-positive B-cell lines.
To examine whether CD70 is expressed on a variety of different EBV-positive B-cell lines, we used FACS. The levels of cell surface CD70 expression on three different EBV-transformed B-cell LCLs (LCL-1, LCL-2, and LCL-3), four different EBV-positive BL cell lines (Jijoye, Raji, P3HR1, and Akata), and an EBV-negative BL cell line (DG75) were determined by comparing the level of fluorescence of cells stained with a MAb to CD70 with that of cells stained with an isotype control antibody (Fig. 1). A9 cells (a murine epithelioid fibroblast line) served as a negative control in these experiments.
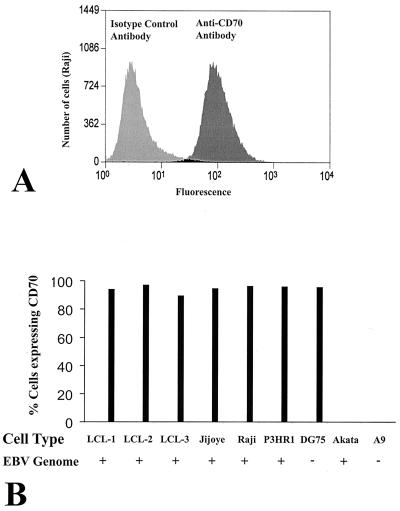
FIG. 1.
Expression of CD70 on LCLs and BLs. The presence of surface CD70 in various cell lines was quantitated by FACS. (A) The level of fluorescence of Raji cells stained with a CD70 antibody versus that of Raji cells stained with an isotype control antibody is shown. (B) A bar graph of the percentage of CD70-positive stained cells is shown using three LCLs (LCL-1, -2, and -3), three type III latency EBV-positive BL lines (Jijoye, Raji, and P3HR1), an EBV-negative BL line (DG75), a type I latency EBV-positive BL line (Akata), and a murine epithelioid fibroblast line (A9).
As shown in Fig. 1, LCLs derived from three different individuals each expressed a high level of CD70. Of the EBV-positive BL cell lines tested, three (Raji, Jijoye, and P3HR1) expressed high levels of CD70, although one (Akata) expressed essentially no CD70. The EBV-negative DG75 BL cell line also expressed a high level of CD70. As expected, A9 cells stained negative for CD70.
Interestingly, each of the EBV-positive cell lines shown to express a high level of the CD70 receptor has type III EBV latency (in which nine different EBV genes are expressed) (36). In contrast, Akata cells, which have type I latency (characterized by viral gene expression restricted to expression of the EBNA 1, BamHI-A, and EBER transcripts [24, 36]), have no CD70 expression. This suggests that neither type I EBV infection nor c-myc translocation (which occurs in all BLs) is sufficient to induce CD70 activation.
BsAb-CD70-fiber targets adenovirus vector-BsAb to CD70+ cells.
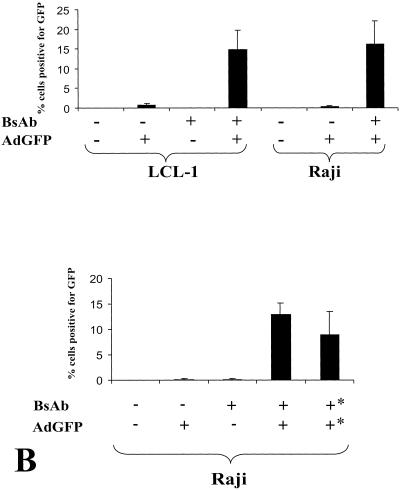
We next examined whether BsAb containing antibodies directed against CD70 and the adenovirus fiber protein (BsAb-CD70-fiber) increases the efficiency of rAd infection in CD70-positive cell lines. A rAd expressing the green fluorescent protein (AdGFP) under the control of the cytomegalovirus IE promoter was used to infect LCLs (LCL-1 line) and the CD70-positive Raji BL line in the presence or absence of BsAb. Adenovirus infection of cells was assessed by quantitating the level of green fluorescence using flow cytometry (Fig. 2). At an MOI of 20, AdGFP did not efficiently infect either LCL-1 or Raji cells, although at this MOI, AdGFP infected virtually 100% of HeLa cells (data not shown). Preincubation of cells with BsAb alone, in the absence of AdGFP, did not increase the percentage of fluorescent B cells. However, when either Raji or LCL-1 cells were preincubated with the combination of BsAb and AdGFP, a significant percentage of the cells (10 to 20%) were fluorescent (Fig. 2A). Preincubation of the adenovirus with BsAb and subsequent infection of the cells also resulted in improved transduction of CD70 cells (Fig. 2B). These results indicate that BsAb-CD70-fiber increases adenovirus infectivity in CD70-positive B-cell lines 10- to 20-fold.
FIG. 2.
BsAb increase adenovirus delivery to CD70+ cells. (A) LCL-1 or Raji cells (106) were pretreated with either medium alone or CD70-fiber BsAb (0.6 μg/ml) for 2 h on ice. Cells were then treated with AdGFP (a recombinant adenovirus vector that expresses the green fluorescent protein) at an MOI of 20 for 2 h. The cells were washed and cultured for 2 days in a 24-well plate. Cells were then examined by FACS for green fluorescence. (B) Raji cells were first exposed to BsAb and then washed and subsequently incubated with AdGFP (as for panel A) (first four columns), or alternatively, BsAb were first preincubated with AdGFP on ice for 1 h and this mixture was then incubated with the cells (results shown in the last column labeled with asterisks).
BsAb-CD70-fiber does not target adenovirus vector to CD70− cells.
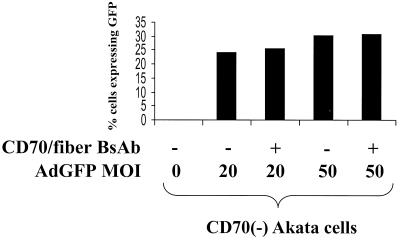
If the BsAb-CD70-fiber targets rAd to CD70, then adenovirus gene transfer into CD70-negative cell lines should not be augmented by BsAb. To confirm that this is the case, we compared the levels of infectivity of AdGFP in a CD70-negative BL line (Akata) in the presence and absence of BsAb-CD70-fiber. Although Akata cells are intrinsically more susceptible to adenovirus transduction than either Raji or LCL-1 cells in the absence of BsAb, the addition of BsAb-CD70-fiber did not increase the level of AdGFP transduction in Akata cells at an MOI of either 20 or 50 (Fig. 3). Therefore, BsAb-CD70-fiber does not enhance adenovirus transduction in B-cell lines lacking CD70.
FIG. 3.
CD70-fiber BsAb do not enhance AdGFP infection in CD70− cells. Akata cells, with or without CD70-fiber BsAb (0.6 μg/ml), were infected with different MOIs of AdGFP. Green fluorescence of cells was quantitated by FACS.
Dose-response relationship of BsAb-CD70-fiber.
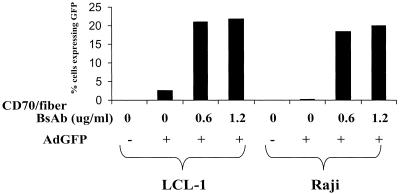
Although BsAb-CD70-fiber increased rAd delivery to LCL-1 and Raji cells 10- to 20-fold, we were still unable to transduce the majority of cells using rAd at an MOI of 20 (Fig. 2). The relative inefficiency of BsAb-CD70-fiber rAd delivery may reflect the fact that there are only 250 high-affinity CD70 receptors on EBV-transformed lymphoblasts (14), versus 3,000 to 10,000 CARs on HeLa cells (11, 18, 32). In an effort to increase the number of cells transduced, we examined the effect of raising the concentration of BsAb from 0.6 to 1.2 μg/ml upon AdGFP transduction into LCL-1 and Raji cells (Fig. 4). Increasing the concentration of BsAb from 0.6 to 1.2 μg/ml produced only a small increase in the percentage of AdGFP-infected cells. Since we estimated that a BsAb dose of 0.6 μg/ml already contains over 100 molecules of BsAb per CD70 receptor, as well as over 100 molecules of BsAb per adenovirus particle, the failure of an increased dose of BsAb to enhance the infectivity of CD70-positive cells is not particularly surprising. Higher doses of BsAb were associated with increasing levels of cytotoxicity in the absence of rAd. In addition, increasing the AdGFP MOI to 500 did not significantly enhance AdGFP transduction of Raji cells (data not shown). Thus, the inability to infect all Raji and LCL-1 cells using the CD70-fiber bispecific antibody cannot readily be overcome by simply increasing the concentration of bispecific antibody or adenovirus vector and may, instead, reflect a level of expression of the CD70 receptor inadequate to mediate adenovirus entry into a portion of these cells. We cannot completely exclude the possibility that not all antibody-virus complexes are infectious due to steric hindrance.
FIG. 4.
Dose-response relationship of CD70-fiber BsAb and AdGFP entry. LCL-1 and Raji cells were exposed to various concentrations of CD70-fiber BsAb, infected with AdGFP, and incubated for 2 days. The percentage of cells expressing GFP was quantitated by FACS.
CD70 specificity in BsAb is required for enhanced transduction of LCL-1 cells.
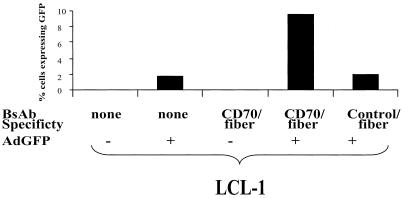
If BsAb-CD70-fiber functions as expected, then we would anticipate that BsAb containing the adenovirus fiber antibody linked to an irrelevant antibody (directed against the influenza virus hemagglutinin [HA] epitope) would not be able to mediate adenovirus entry into CD70-positive cell lines. As shown in Fig. 5, this prediction is indeed the case. The bispecific HA-fiber antibody did not enhance transduction of the AdGFP vector into LCL-1 cells, while the CD70-fiber BsAb did show enhancement of gene transfer. In contrast, the HA-fiber BsAb does increase adenovirus entry into cells constitutively expressing an HA-tagged receptor (Ray Pickles, unpublished data). Thus, as expected, the ability of BsAb-CD70-fiber to mediate adenovirus entry into B cells requires both the CD70 receptor and the CD70 component of the antibody.
FIG. 5.
CD70 specificity of BsAb is required for enhanced infection of LCL-1 cells. LCL-1 cells were exposed to no BsAb, CD70-fiber BsAb, or negative control (HA-fiber) BsAb. Each sample was then washed and exposed to either medium alone or AdGFP. The percentage of cells expressing GFP was quantitated by FACS after 2 days of incubation.
BsAb-CD70-fiber enhances delivery of EBV IE genes by adenovirus vectors into LCLs.
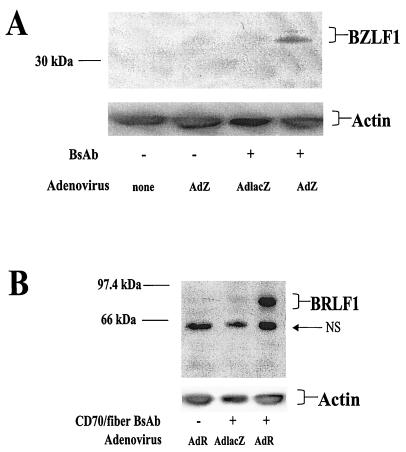
A major interest of our laboratory has been the development of gene delivery methods by which to induce EBV-dependent cell killing (23). However, we have previously been unable to efficiently deliver genes into LCLs by using a variety of methods (electroporation, lipofection, or adenovirus delivery). We thus examined whether BsAb-CD70-fiber can enhance the delivery of rAd vectors encoding the EBV IE proteins BZLF1 and BRLF1 into EBV-positive LCLs. LCL-1 cells were infected with the adenovirus-BZLF1 (AdZ) (Fig. 6A) or adenovirus-BRLF1 (AdR) (Fig. 6B) vector in the presence or absence of bispecific CD70-fiber antibody. As a control, an adenovirus vector expressing the β-galactosidase gene (AdLacZ) was also used to infect LCL-1 cells in the presence of bispecific antibody. The levels of BZLF1 and BRLF1 expression were quantitated by immunoblot analysis of infected cells. Both the BZLF1 and BRLF1 rAd vectors produced substantially more protein in LCL-1 cells in the presence of BsAb. Actin staining of the immunoblots confirmed equal protein loading. Thus, BsAb-CD70-fiber can be used to allow BZLF1 and BRLF1 delivery to EBV-positive LCLs in vitro.
FIG. 6.
CD70-fiber BsAb enhance AdZ and AdR entry into LCL-1 cells. (A) LCL-1 cells, with or without CD70-fiber BsAb, were either mock infected, infected with AdZ, or infected with AdLacZ. All infections were done at an MOI of 20. After 2 days, immunoblot assays were performed on extracts from each sample, followed by staining with a MAb against BZLF1. Staining for β-actin was used to ensure equal sample loading. (B) LCLs, with or without CD70-fiber BsAb, were mock infected, infected with AdR, or infected with AdLacZ. After 2 days, immunoblot assays were performed on extracts from each sample, followed by staining with a MAb against BRLF1 or actin. NS indicates a nonspecific band.
BsAb-CD70-fiber-mediated expression of BZLF1 or BRLF1 induces lytic EBV infection in LCLs.
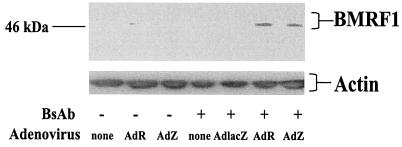
Although it is clear that both BZLF1 and BRLF1 can induce the lytic form of EBV infection in certain BL lines (8, 33, 34, 47), as well as in EBV-positive epithelial cell lines (50), the ability of BZLF1 and BRLF1 to induce the lytic form of EBV replication in LCLs has been somewhat controversial (8, 50), perhaps due to the lack of an efficient system of delivery to these cells. We previously reported that transfected BRLF1 did not induce the lytic form of EBV replication in two different LCL lines, but these negative results could potentially have resulted from insufficient transfection efficiency (50). We therefore used BsAb-CD70-fiber to reexamine the effect of BZLF1 and BRLF1 expression in LCLs. As shown in Fig. 7, both the AdZ and AdR vectors induced expression of the lytic EBV protein BMRF1 in the presence, but not in the absence, of BsAb-CD70-fiber, whereas the control AdLacZ combined with BsAb had no effect. These results thus clearly show that both BZLF1 and BRLF1 can induce the lytic form of EBV infection in LCLs. Furthermore, the inclusion of the bispecific CD70-fiber antibody was required for BMRF1 expression.
FIG. 7.
BsAb-enabled expression of BZLF1 and BRLF1 induces BMRF1 in LCLs. LCL-1 cells, with or without CD70-fiber BsAb (0.6 μg/ml), were mock infected or infected with AdR, AdZ, or AdLacZ at an MOI of 20. After 2 days, immunoblot assays were performed on extracts from each sample, followed by staining with a MAb against the early lytic EBV protein BMRF1. Staining for β-actin was used to ensure equal sample loading.
DISCUSSION
Adenovirus vectors have many attractive features (such as very high viral titers and the accommodation of large gene inserts) that are not currently available when other vectors are used. However, many hematopoietic cell types are not readily infectible by conventional adenovirus vectors, primarily due to insufficient CAR expression (28). In this study, we have created BsAb directed against the adenovirus fiber protein and a cellular receptor, CD70, which is expressed on highly activated B cells and T cells. We demonstrated that these BsAb specifically and significantly enhance adenovirus infection of CD70-positive, but not CD70-negative, B-cell lines. Thus, this method should prove useful for specifically increasing delivery of adenovirus vectors to CD70-positive cells.
The primary receptor of the fiber protein for adenovirus serotypes 2 and 5 has been identified (CAR) (37, 44). The CAR protein interacts with the knob domain of the adenovirus fiber protein to mediate initial attachment of the virus to the cell (38). Adenovirus internalization is also significantly enhanced by the presence of αvβ3/5 integrins, which interact with the virus penton base (20, 30). EBV infection of B cells has been reported to increase αv integrin expression (21), as well as increase the efficiency of adenovirus infection (22). Nevertheless, because the CAR protein is not present in lymphocyte lineages, EBV-positive LCLs still remain highly resistant to adenovirus infection. As shown in this report, the use of a conventional adenovirus vector at an MOI of 20 to 50 results in gene delivery to, at most, 1 to 2% of EBV-positive LCLs, in contrast to essentially 100% delivery to a variety of CAR-positive epithelial lines.
A variety of methods are currently being tested as ways to increase the delivery of adenovirus vectors to lymphocytes. Modification of the fiber protein has been used successfully to broaden adenovirus tropism to alternative cellular receptors (13), and a similar approach could potentially be used to target adenovirus infection to a lymphocyte-specific receptor. Alternately, Curiel et al. have shown that adenovirus particles can transduce plasmid DNA that has been coupled to the capsid exterior into LCLs, transducing up to 60% of cells; however, an MOI of 1,000 to 3,000 was required for this level of efficiency (10). Other studies have likewise required MOIs of 100 to 500 in order to gain entry in LCLs (21, 22). By using another approach, an adenovirus type 3-pseudotyped vector has been shown to infect LCLs 10 times more efficiently than a control vector pseudotyped with adenovirus type 5 (the serotype used in the prototype adenovirus vectors [46]). When 5,000 to 50,000 particles per cell were used, serotype 3-derived vectors could transduce genes into 20 to 50% of LCLs (46). Nevertheless, adenovirus type 3-derived vectors also deliver genes to non-LCLs as well (43) and thus cannot be used to specifically deliver toxic genes to lymphoid cells.
We report here the first attempt to target the cellular CD70 receptor for specific gene delivery. The CD70 receptor could potentially be used to induce relatively specific killing of malignant lymphocytes, since CD70 is highly expressed in a number of hematologic malignancies (15, 27, 35, 45) but is normally expressed on only a small subset of highly activated B and T lymphocytes and is not detectably expressed in resting or memory lymphoid compartments or other tissue types (26). In addition, CD70-specific gene delivery could potentially be used to regulate undesirable immune responses, for example, to prevent organ transplant rejection.
A significant advantage of the BsAb approach is that adenovirus tropism can be redirected away from the normal target cells and toward a specific cell type of interest. Adenovirus vectors with a specific cellular tropism would be particularly useful for limiting the toxicity of vectors that are intentionally designed to kill cells. In addition, the toxicity of adenovirus transduction to liver cells (which are CD70 negative [42]), even in the absence of toxic gene inserts, could be minimized. However, the bispecific-antibody approach cannot prevent adenovirus delivery to the natural cellular receptor (CAR) unless the fiber protein is completely bound by the bispecific antibody. In this study, we were unable to prevent adenovirus transduction to CD70-negative cells using the CD70-fiber bispecific antibody. However, the use of higher concentrations of the CD70-fiber bispecific antibody could potentially prevent infection through the natural CAR.
Although we were able to increase adenovirus delivery to CD70-positive B-cell lines 10- to 20-fold by using BsAb-CD70-fiber, we nevertheless were still unable to infect the majority of cells. It is not clear from the present study what limits the rate of transduction. The amount of BsAb does not appear to be limiting, given that we used in excess of 100 molecules per CD70 receptor, and increased BsAb did not increase transduction efficiency. Variable stoichiometry between the individual particles within our current BsAb preparation may sterically decrease the efficiency of binding. More likely, the amount of surface CD70 expression, which is considerably less than the level of CAR found on rAd-infectible cells (11, 14, 18, 32), may be limiting. Alternatively, subsequent receptor internalization may be inadequate in the majority of cells to support adenovirus entry.
In the case of EBV-associated tumors, our results suggest that some, but not all, EBV-positive malignancies express CD70. There are at least three different patterns of EBV gene expression associated with malignancy (types I, II, and III), with type I malignancies expressing the fewest EBV genes and type III expressing the most. We found that all cell lines containing the type III pattern of EBV gene expression also showed CD70 expression, whereas the one cell line with type I infection (the BL line Akata) did not express CD70. Similar results have been reported by others (39). This suggests that neither the EBV genes expressed in type I infection (characterized by viral expression restricted to EBNA-1, EBERs, and BamHI-A RNAs) nor c-myc translocation is sufficient to induce CD70. EBV-positive BLs in patients commonly manifest type I, rather than type III, infection and rarely express the CD70 receptor (17). Nevertheless, EBV-positive malignancies which commonly have type III infection (such as AIDS-associated lymphomas [12], primary central nervous system lymphoma [16, 25], and posttransplant lymphoproliferative disease [9]) have been shown to express the CD70 receptor in vivo. In addition, at least one type of malignancy associated with type II EBV infection (nasopharyngeal carcinoma) expresses CD70 as well (31). This strongly suggests that the EBV LMP-1 or LMP-2 protein is responsible for CD70 induction (since these viral proteins are expressed in type II and III, but not type I, latency). In addition, the finding that nasopharyngeal carcinomas express CD70 suggests that EBV type II latent infection induces CD70 expression on a cell type(s) which normally expresses no CD70.
Our ability to increase adenovirus delivery to CD70-positive B-cell lines 10- to 20-fold by using the CD70-fiber bispecific antibody has already proved highly useful, since these cells are normally resistant to adenovirus-mediated delivery. For example, by using the BsAb technology, we were able to examine the effect of adenovirus vectors expressing the EBV IE proteins BZLF1 and BRLF1 in LCLs. Our laboratory had previously shown induction of the lytic form of EBV infection in LCLs by BZLF1 but not BRLF1 (delivered by electroporation) (50), while another laboratory found the converse by using a vaccinia virus vector to deliver BZLF1 and BRLF1 (8). We show here that efficient delivery of the gene for either BZLF1 or BRLF1 into LCLs (which was only achieved with adenovirus vectors by using the BsAb approach) induces the lytic form of EBV infection. This approach could potentially be useful for inducing specific killing of EBV-positive lymphoblastoid malignancies.
ACKNOWLEGMENTS
We thank Harald Stein for providing the Ki-24 antibody and hybridoma and Veerle Laurysens at the Center for Transgene Technology and Gene Therapy at the Catholic University of Leuven in Belgium for purification of the 7H11 MAb. Thanks to the UNC Gene Therapy Center for construction of AdGFP, AdZ, AdR, and AdLacZ and to Amy E. Mauser and Jennifer J. Swenson for assistance with the preparation of AdZ, AdR, and AdLacZ.
This work was supported by NIH grants R01 CA 66519 and 5-T32-AI07151-21.
REFERENCES
- 1.Agathanggelou A, Niedobitek G, Chen R, Nicholls J, Yin W, Young L S. Expression of immune regulatory molecules in Epstein-Barr virus-associated nasopharyngeal carcinomas with prominent lymphoid stroma. Evidence for a functional interaction between epithelial tumor cells and infiltrating lymphoid cells. Am J Pathol. 1995;147:1152–1160. [PMC free article] [PubMed] [Google Scholar]
- 2.Agematsu K, Hokibara S, Nagumo H, Shinozaki K, Yamada S, Komiyama A. Plasma cell generation from B-lymphocytes via CD27/CD70 interaction. Leukemia Lymphoma. 1999;35:219–225. doi: 10.3109/10428199909145724. [DOI] [PubMed] [Google Scholar]
- 3.Akiba H, Oshima H, Takeda K, Atsuta M, Nakano H, Nakajima A, Nohara C, Yagita H, Okumura K. CD28-independent costimulation of T cells by OX40 ligand and CD70 on activated B cells. J Immunol. 1999;162:7058–7066. [PubMed] [Google Scholar]
- 4.Auperin I, Mikolt J, Oksenhendler E, Thiebaut J B, Brunet M, Dupont B, Morinet F. Primary central nervous system malignant non-Hodgkin's lymphomas from HIV-infected and noninfected patients: expression of cellular surface proteins and Epstein-Barr viral markers. Neuropathol Appl Neurobiol. 1994;20:243–252. doi: 10.1111/j.1365-2990.1994.tb00966.x. [DOI] [PubMed] [Google Scholar]
- 5.Bartlett J S, Kleinschmidt J, Boucher R C, Samulski R J. Targeted adeno-associated virus vector transduction of nonpermissive cells mediated by a bispecific F(ab′γ)2 antibody. Nat Biotechnol. 1999;17:181–186. doi: 10.1038/6185. [DOI] [PubMed] [Google Scholar]
- 6.Bashir R, Luka J, Cheloha K, Chamberlain M, Hochberg F. Expression of Epstein-Barr virus proteins in primary CNS lymphoma in AIDS patients. Neurology. 1993;43:2358–2362. doi: 10.1212/wnl.43.11.2358. [DOI] [PubMed] [Google Scholar]
- 7.Blackwell J L, Miller C R, Douglas J T, Li H, Peters G E, Carroll W R, Peters G E, Strong T V, Curiel D T. Retargeting to EGFR enhances adenovirus infection efficiency of squamous cell carcinoma. Arch Otolaryngol–Head Neck Surg. 1999;125:856–863. doi: 10.1001/archotol.125.8.856. [DOI] [PubMed] [Google Scholar]
- 8.Bogedain C, Alliger P, Schwarzmann F, Marschall M, Wolf H, Jilg W. Different activation of Epstein-Barr virus immediate-early and early genes in Burkitt lymphoma cells and lymphoblastoid cell lines. J Virol. 1994;68:1200–1203. doi: 10.1128/jvi.68.2.1200-1203.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brink A A, Dukers D F, van den Brule A J, Oudejans J J, Middeldorp J M, Meijer C J, Jiwa M. Presence of Epstein-Barr virus latency type III at the single cell level in post-transplantation lymphoproliferative disorders and AIDS related lymphomas. J Clin Pathol. 1997;50:911–918. doi: 10.1136/jcp.50.11.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Curiel T J, Cook D R, Bogedain C, Jilg W, Harrison G S, Cotten M, Curiel D T, Wagner E. Efficient foreign gene expression in Epstein-Barr virus-transformed human B-cells. Virology. 1994;198:577–585. doi: 10.1006/viro.1994.1069. [DOI] [PubMed] [Google Scholar]
- 11.Defer C, Belin M T, Caillet-Boudin M L, Boulanger P. Human adenovirus-host cell interactions: comparative study with members of subgroups B and C. J Virol. 1990;64:3661–3673. doi: 10.1128/jvi.64.8.3661-3673.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Diebold J, Raphael M, Prevot S, Audouin J. Lymphomas associated with HIV infection. Cancer Surv. 1997;30:263–293. [PubMed] [Google Scholar]
- 13.Dmitriev I, Krasnykh V, Miller C R, Wang M, Kashentseva E, Mikheeva G, Belousova N, Curiel D T. An adenovirus vector with genetically modified fibers demonstrates expanded tropism via utilization of a coxsackievirus and adenovirus receptor-independent cell entry mechanism. J Virol. 1998;72:9706–9713. doi: 10.1128/jvi.72.12.9706-9713.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goodwin R G, Alderson M R, Smith C A, Armitage R J, VandenBos T, Jerzy R, Tough T W, Schoenborn M A, Davis-Smith T, Hennen K. Molecular and biological characterization of a ligand for CD27 defines a new family of cytokines with homology to tumor necrosis factor. Cell. 1993;73:447–456. doi: 10.1016/0092-8674(93)90133-b. [DOI] [PubMed] [Google Scholar]
- 15.Gruss H J, Kadin M E. Pathophysiology of Hodgkin's disease: functional and molecular aspects. Baillieres Clin Haematol. 1996;9:417–446. doi: 10.1016/s0950-3536(96)80019-9. . (Review.) [DOI] [PubMed] [Google Scholar]
- 16.Guterman K S, Hair L S, Morgello S. Epstein-Barr virus and AIDS-related primary central nervous system lymphoma. Viral detection by immunohistochemistry, RNA in situ hybridization, and polymerase chain reaction. Clin Neuropathol. 1996;15:79–86. [PubMed] [Google Scholar]
- 17.Hamilton-Dutoit S J, Rea D, Raphael M, Sandvej K, Delecluse H J, Gisselbrecht C, Marelle L, van Krieken H J, Pallesen G. Epstein-Barr virus-latent gene expression and tumor cell phenotype in acquired immunodeficiency syndrome-related non-Hodgkin's lymphoma. Correlation of lymphoma phenotype with three distinct patterns of viral latency. Am J Pathol. 1993;143:1072–1085. [PMC free article] [PubMed] [Google Scholar]
- 18.Henry L J, Xia D, Wilke M E, Deisenhofer J, Gerard R D. Characterization of the knob domain of the adenovirus type 5 fiber protein expressed in Escherichia coli. J Virol. 1994;68:5239–5246. doi: 10.1128/jvi.68.8.5239-5246.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang R M, Olsson M, Kallin A, Pettersson U, Totterman T H. Efficient adenovirus-mediated gene transduction of normal and leukemic hematopoietic cells. Gene Ther. 1997;4:1093–1099. doi: 10.1038/sj.gt.3300499. [DOI] [PubMed] [Google Scholar]
- 20.Huang S, Kamata T, Takada Y, Ruggeri Z M, Nemerow G R. Adenovirus interaction with distinct integrins mediates separate events in cell entry and gene delivery to hematopoietic cells. J Virol. 1996;70:4502–4508. doi: 10.1128/jvi.70.7.4502-4508.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang S, Stupack D, Liu A, Cheresh D, Nemerow G R. Cell growth and matrix invasion of EBV-immortalized human B lymphocytes is regulated by expression of alpha(v) integrins. Oncogene. 2000;19:1915–1923. doi: 10.1038/sj.onc.1203509. [DOI] [PubMed] [Google Scholar]
- 22.Huang S, Stupack D, Mathias P, Wang Y, Nemerow G. Growth arrest of Epstein-Barr virus immortalized B lymphocytes by adenovirus-delivered ribozymes. Proc Natl Acad Sci USA. 1997;94:8156–8161. doi: 10.1073/pnas.94.15.8156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kenney S, Ge J Q, Westphal E M, Olsen J. Gene therapy strategies for treating Epstein-Barr virus-associated lymphomas: comparison of two different Epstein-Barr virus-based vectors. Hum Gene Ther. 1998;9:1131–1141. doi: 10.1089/hum.1998.9.8-1131. [DOI] [PubMed] [Google Scholar]
- 24.Komano J, Sugiura M, Takada K. Epstein-Barr virus contributes to the malignant phenotype and to apoptosis resistance in Burkitt's lymphoma cell line Akata. J Virol. 1998;72:9150–9156. doi: 10.1128/jvi.72.11.9150-9156.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Larocca L M, Capello D, Rinelli A, Nori S, Antinori A, Gloghini A, Cingolani A, Migliazza A, Saglio G, Cammilleri-Broet S, Raphael M, Carbone A, Gaidano G. The molecular and phenotypic profile of primary central nervous system lymphoma identifies distinct categories of the disease and is consistent with histogenetic derivation from germinal center-related B cells. Blood. 1998;92:1011–1019. [PubMed] [Google Scholar]
- 26.Lens S M, de Jong R, Hintzen R Q, Koopman G, van Lier R A, van Oers R H. CD27-CD70 interaction: unravelling its implication in normal and neoplastic B-cell growth. Leukemia Lymphoma. 1995;18:51–59. doi: 10.3109/10428199509064922. [DOI] [PubMed] [Google Scholar]
- 27.Lens S M, Drillenburg P, den Drijver B F, van Schijndel G, Pals S T, van Lier R A, van Oers M H. Aberrant expression and reverse signalling of CD70 on malignant B cells. Br J Haematol. 1999;106:491–503. doi: 10.1046/j.1365-2141.1999.01573.x. [DOI] [PubMed] [Google Scholar]
- 28.Leon R P, Hedlund T, Meech S J, Li S, Schaack J, Hunger S P, Duke R C, DeGregori J. Adenoviral-mediated gene transfer in lymphocytes. Proc Natl Acad Sci USA. 1998;95:13159–13164. doi: 10.1073/pnas.95.22.13159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.MacMahon E M, Glass J D, Hayward S D, Mann R B, Becker P S, Charache P, McArthur J C, Ambinder R F. Epstein-Barr virus in AIDS-related primary central nervous system lymphoma. Lancet. 1991;338:969–973. doi: 10.1016/0140-6736(91)91837-k. [DOI] [PubMed] [Google Scholar]
- 30.Nemerow G R, Stewart P L. Role of alpha(v) integrins in adenovirus cell entry and gene delivery. Microbiol Mol Biol Rev. 1999;63:725–734. doi: 10.1128/mmbr.63.3.725-734.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Niedobitek G, Young L S, Sam C K, Brooks L, Prasad U, Rickinson A B. Expression of Epstein-Barr virus genes and of lymphocyte activation molecules in undifferentiated nasopharyngeal carcinomas. Am J Pathol. 1992;140:879–887. [PMC free article] [PubMed] [Google Scholar]
- 32.Philipson L, Lonberg-Holm K, Pettersson U. Virus-receptor interaction in an adenovirus system. J Virol. 1968;2:1064–1075. doi: 10.1128/jvi.2.10.1064-1075.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ragoczy T, Heston L, Miller G. The Epstein-Barr virus Rta protein activates lytic cycle genes and can disrupt latency in B lymphocytes. J Virol. 1998;72:7978–7984. doi: 10.1128/jvi.72.10.7978-7984.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ragoczy T, Miller G. Role of the Epstein-Barr virus RTA protein in activation of distinct classes of viral lytic cycle genes. J Virol. 1999;73:9858–9866. doi: 10.1128/jvi.73.12.9858-9866.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ranheim E A, Cantwell M J, Kipps T J. Expression of CD27 and its ligand, CD70, on chronic lymphocytic leukemia B cells. Blood. 1995;85:3556–3565. [PubMed] [Google Scholar]
- 36.Rickinson A B, Kieff E. Epstein-Barr virus. In: Fields B N, Knipe D M, Howley P M, editors. Fields virology. Philadelphia, Pa: Raven Publishers; 1996. pp. 2397–2446. [Google Scholar]
- 37.Roelvink P W, Lizonova A, Lee J G, Li Y, Bergelson J M, Finberg R W, Brough D E, Kovesdi I, Wickham T J. The coxsackievirus-adenovirus receptor protein can function as a cellular attachment protein for adenovirus serotypes from subgroups A, C, D, E, and F. J Virol. 1998;72:7909–7915. doi: 10.1128/jvi.72.10.7909-7915.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Roelvink P W, Mi L G, Einfeld D A, Kovesdi I, Wickham T J. Identification of a conserved receptor-binding site on the fiber proteins of CAR-recognizing Adenoviridae. Science. 1999;286:1568–1571. doi: 10.1126/science.286.5444.1568. [DOI] [PubMed] [Google Scholar]
- 39.Rowe M, Rowe D T, Gregory C D, Young L S, Farrell P J, Rupani H, Rickinson A B. Differences in B cell growth phenotype reflect novel patterns of Epstein-Barr virus latent gene expression in Burkitt's lymphoma cells. EMBO J. 1987;6:2743–2751. doi: 10.1002/j.1460-2075.1987.tb02568.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Segal D M, Bast B J E G. Production of bispecific antibodies. In: Coligan J E, Kruisbeek A M, Marguiles D H, Shevach E M, Strober W, editors. Current protocols in immunology. New York, N.Y: John Wiley & Sons, Inc.; 1995. pp. 12.13.11–12.13.16. [Google Scholar]
- 41.Shenk T. Adenoviridae: the viruses and their replication. In: Fields B N, Knipe D M, Howley P M, editors. Fields virology. Philadelphia, Pa: Raven Publishers; 1996. pp. 2111–2171. [Google Scholar]
- 42.Stein H, Ferszt A, Dallenbach F, Dienemann D, Rentrop O, Hock H, Diamantstein T. CDw70 mAb A109 (Ki-24): expression by reactive and neoplastic lymphoid cells. 1989. pp. 446–455. p. 495–499. In W. Knapp et al. (ed.), Leucocyte typing IV. Oxford University Press, New York, N.Y. [Google Scholar]
- 43.Stevenson S C, Rollence M, Marshall-Neff J, McClelland A. Selective targeting of human cells by a chimeric adenovirus vector containing a modified fiber protein. J Virol. 1997;71:4782–4790. doi: 10.1128/jvi.71.6.4782-4790.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tomko R P, Xu R, Philipson L. HCAR and MCAR: the human and mouse cellular receptors for subgroup C adenoviruses and group B coxsackieviruses. Proc Natl Acad Sci USA. 1997;94:3352–3356. doi: 10.1073/pnas.94.7.3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Trentin L, Zambello R, Sancetta R, Facco M, Cerutti A, Perin A, Siviero M, Basso U, Bortolin M, Adami F, Agostini C, Semenzato G. B lymphocytes from patients with chronic lymphoproliferative disorders are equipped with different costimulatory molecules. Cancer Res. 1997;57:4940–4947. [PubMed] [Google Scholar]
- 46.Von Seggern D J, Huang S, Fleck S K, Stevenson S C, Nemerow G R. Adenovirus vector pseudotyping in fiber-expressing cell lines: improved transduction of Epstein-Barr virus-transformed B cells. J Virol. 2000;74:354–362. doi: 10.1128/jvi.74.1.354-362.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Westphal E M, Mauser A, Swenson J, Davis M G, Talarico C L, Kenney S C. Induction of lytic Epstein-Barr virus (EBV) infection in EBV-associated malignancies using adenovirus vectors in vitro and in vivo. Cancer Res. 1999;59:1485–1491. [PubMed] [Google Scholar]
- 48.Wickham T J, Lee G M, Titus J A, Sconocchia G, Bakacs T, Kovesdi I, Segal D M. Targeted adenovirus-mediated gene delivery to T cells via CD3. J Virol. 1997;71:7663–7669. doi: 10.1128/jvi.71.10.7663-7669.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wickham T J, Segal D M, Roelvink P W, Carrion M E, Lizonova A, Lee G M, Kovesdi I. Targeted adenovirus gene transfer to endothelial and smooth muscle cells by using bispecific antibodies. J Virol. 1996;70:6831–6838. doi: 10.1128/jvi.70.10.6831-6838.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zalani S, Holley-Guthrie E, Kenney S. Epstein-Barr viral latency is disrupted by the immediate-early BRLF1 protein through a cell-specific mechanism. Proc Natl Acad Sci USA. 1996;93:9194–9199. doi: 10.1073/pnas.93.17.9194. [DOI] [PMC free article] [PubMed] [Google Scholar]