Abstract
Aims
This study aims to examine cases identified with endometrial polyp and carcinoma originating from polyps in patients presenting with gynaecological problems, and to highlight the significance of risk factors contributing to malignancy.
Materials and methods
The study comprised 203 patients who visited our clinic between January 2019 and 2024 with various gynaecological problems and were identified with endometrial polyps after a clinical, radiographic, and laboratory assessment. We retrospectively analysed data from 191 benign endometrial polyps and hyperplasia without atypia and 12 patients with endometrial polyps and underlying endometrial hyperplasia with atypia and/or endometrial carcinoma, diagnosed histopathologically after hysteroscopic resection, retrieved from our hospital's electronic archive system.
Two hundred three participants were tested in the study, with 191 classifieds with benign tumours and 12 diagnosed with malignant tumours and atypical endometrial hyperplasia (premalignant). Cases were chosen according on consistent criteria for age, BMI, gravida, parity, abortion, educational level, smoking habits, operation history, and co-morbidities. After determining the sample size for the malignant group, patients from the control group were selected to be included in the study. Initially, patients with similar age and BMI distributions were included into the study. Next, the cases were analysed for similarities in gravida, parity, and abortion parameters, and those that matched were chosen. Following this step, the educational status was compared for resemblance, and examples with matching educational status were chosen. Consequently, the study covered a total of 34 patients, with 12 identified with malignant tumours and atypical endometrial hyperplasia (premalignant) and 22 with benign tumours.
Two groups of cases were diagnosed with endometrial polyp, and risk factors that may cause the development of endometrial polyp and underlying carcinoma: age, gravida, parity, abortion, education level, smoking, previous operation history, comorbidity, gynaecological complaints, fasting blood sugar, CRP values, haemoglobin, and haematocrit were evaluated in terms of endometrial polyp sizes, endometrial thickness level, and endometrial polyp localization. By examining the pathological risk factors of these cases, particularly during the premenopausal period, the goal is to predict endometrial cancer, the most prevalent gynaecological cancer in women, along with its antecedents, and implement preventive measures proactively.
Results
Age, BMI, gravida, parity, number of abortions, educational status, smoking status, operation history, co-morbidity, and complaint variables did not exhibit a statistically significant difference between the groups (p > 0.05). It was revealed that the FBG level, CRP level, Polyp length and Endometrial thickness level of the malignant group were statistically significantly higher than the benign group (p < 0.01) (p < 0.05). Upon analysing the FBG distribution among groups, it is noted that the ODDS ratio is 10.20 for FBG values of 122.5 and above (95% CI: 1.97 – 52.78). Upon analysing the CRP distribution by groups, it is noted that the ODDS ratio is 231 for CRP values of 9.7 and above (95% CI: 13.15 – 4058.67). Upon analysing the distribution of Polyp length based on groups, it was determined that the ODDS ratio is 13.5 for Polyp lengths of 2.25 and above (95% CI: 2.47 – 73.71). Upon analysing the distribution of EM thickness based on groups, it is shown that the ODDS ratio is 5.25 for EM thicknesses of 11 and above (95% CI: 1.09 – 25.21).
Conclusion
Endometrial polyps are common benign growths that are typically not seen as cancer precursors but may be linked to cancer in people with advanced age. It is vital to remember that in cases of endometrial polyps, variables such as increasing polyp length, endometrial thickness, fasting glucose level, and elevated CRP levels are significant risk factors for the development of cancer associated with polyps.
Keywords: Endometrial polyp, Endometrial carcinoma, Endometrial thickness, Glucose, CRP, Polyp length
Introduction
Polypoid structures that form within the endometrial cavity are local lesions that contain endometrial glandular, stromal, and vascular components. They continue to expand with stalked or wide surfaces covered with endometrial epithelium. Endometrial basal layer cells are said to have localised hyperplasic regions and induce menstrual cycle problems, but they do not respond well to progesterone treatment [1]. Although its prevalence in women of all age’s ranges from 16 to 34%, it rises in perimenopausal and postmenopausal women [2]. Although it is unclear how and why endometrial polyps form, it is believed that they form as a result of oestrogen exposure, particularly when they are associated with endometrial hyperplasia. Endometrial polyps are caused by hormonal variables, obesity, late menopause, hormone replacement treatment, polycystic ovarian syndrome (PCOS), and tamoxifen usage, with obesity being the most significant risk factor [3].
Polypoid formations form as a result of excessive endometrial epithelial proliferation and can be histopathologically identified as atrophic, hyperplastic, or malignant tumoral lesions. If the morphological alterations of endometrial polyps continue, 0.8–4.8% may develop into malignant lesions, particularly polyps [4, 5]. However, current research indicates that endometrial polyps have the biological, morphological, and genetic characteristics of normal endometrium and that there is no difference in the risk of developing malignant lesions between endometrial polyps and normal endometrial tissue [6].
The primary diagnostic criteria for endometrial polyps are clinical symptoms and the patient's history, with transvaginal ultrasonography being the initial procedure employed. Saline infusion sonography can disclose more sonographic aspects of the lesion in the endometrial cavity, as well as the polyp size, location, and other diseases in the uterine cavity. However, hysteroscopy remains the gold standard for diagnosing endometrial polyps. The size, number, placement, origin, and tissue alterations of all lesions in the endometrial cavity may be seen. Furthermore, by analysing the tissue samples, benign, pre-malignant, and malignant cellular alterations may be clearly identified [7].
The frequency of malignant tumour formation as a result of endometrial polyps may differ depending on the people in society. Many studies report these rates as ranging from 0 to 12.9% [8]. When we look at the risk factors for cancer, we see advanced age, postmenopausal status, infertility, continuation of treatment-resistant vaginal bleeding, polyp size, location, increased endometrial thickness, obesity, hypertension, diabetes, metabolic syndrome positive cases, polycystic ovary syndrome, breast cancer and its history, Tamoxifen-like selective oestrogen receptor modulator drug use, and chronic inflammatory processes [9–13].
Hyperplastic and carcinomatous alterations in endometrial polyps can emerge as a result of pathophysiological changes in the endometrial tissue. As a result, our study intends to identify the risk variables that play a crucial role in carcinogenesis and to take the appropriate measures for instances including these risk factors by addressing them in light of the literature.
Materials and methods
The information of the patients who applied to our clinic for gynaecological complaints between January 2019 and 2024, who were diagnosed with endometrial polyp after clinical examination, radiological and laboratory evaluation, and whose histopathological diagnosis was made after hysteroscopic resection, and who had endometrial hyperplasia with atypia and endometrial carcinoma, were examined retrospectively from the electronic archive system after obtaining approval. Patients must be over the age of 18, have a histological diagnosis of endometrial polyp, endometrial hyperplasia with atypia, and/or endometrial cancer, and have given their consent and participated in the study. The study excluded those that received persistent endometrial sampling without hysteroscopy due to abnormal uterine bleeding or another reason and were later identified with endometrial polyps. Furthermore, cases who do not want to participate in the study, cases with second primary cancer, cases whose information cannot be fully accessed, cases who have previously received chemotherapy and/or radiotherapy, cases with breast cancer or a history of it, those using tamoxifen-like selective oestrogen receptor or aromatase inhibitors, those receiving steroid treatment, those taking hormone replacement therapy, those with diabetes mellitus, patients with haematological and rheumatological diseases, and patients with signs and symptoms of serious infection.
Two hundred three participants were tested in the study, with 191 classifieds with benign tumours and 12 diagnosed with malignant tumours and atypical endometrial hyperplasia (premalignant). Cases were chosen according on similar criteria for age, BMI, gravida, parity, abortion, educational level, smoking habits, operation history, and co-morbidity variables. The study chose cases from the control group according to the determined number of samples in the malignant group. Initially, patients with similar age and BMI distributions were incorporated into the study. The gravida, parity, and abortion parameters of the cases were compared to identify similarities, and cases with similar parameters were chosen. Following this phase, the educational status was assessed for similarity, and cases with similar educational status were chosen. Consequently, the study covered a total of 34 individuals, with 12 identified with malignant tumours and atypical endometrial hyperplasia (premalignant) and 22 with benign tumours.
Two groups of cases were diagnosed with endometrial polyp and in terms of risk factors that may cause the development of endometrial polyp and underlying carcinoma: age, gravida, parity, abortion, education level, smoking, previous operation history, comorbidity, gynaecological complaints, fasting blood sugar, CRP values, haemoglobin, haematocrit, endometrial polyp sizes, endometrial thickness level and endometrial polyp localization were evaluated.
Cases with clinical symptoms and signs were evaluated hysteroscopically in the gynaecology outpatient clinic after a transvaginal examination revealed a thickening of the uterine cavity with a contrast structure different from the surrounding endometrial tissue. Saline infusion sonography was performed after suspicion of endometrial polyp, and the diagnosis of endometrial polyp was better established. Polyp size, determined as the maximum diameter of the endometrial polyp, was obtained from preoperative ultrasound reports. The endometrial thickness, determined as the maximum diameter of the endometrial thickness, was obtained from preoperative ultrasound reports measured from the area where the endometrium double wall thickness was present, where there was no polyp lesion. Following the identification of an endometrial polyp and while in the proliferative phase of the menstrual cycle, the cervical canal, internal cervical os, uterine cavity, and both tubal ostia were plainly visible during hysteroscopic examination. All samples sent to pathology were examined by the same histopathologist at the pathological laboratory. By histopathological examination, endometrial polyp and endometrial curettage materials, regular/irregular proliferative endometrium, and secretory endometrium lesions, which are benign endometrial pathologies, and hyperplasia with/without atypia and cases diagnosed with direct carcinoma, were evaluated for malignant-benign epithelial changes.
Before hysteroscopic resection treatment, all patients had routine hemogram and biochemistry testing. Blood samples were collected from the forearm's peripheral venous vessels. For Fasting Blood Glucose (FBG) (mg/dl) values, blood samples were collected from patients between 7:00 and 7:30 in the morning following an 8-h fast, without any caloric intake, to prevent interference from the circadian cycle. FBG measurements will be conducted using the glucose oxidase technique. The CRP (C-reactive protein) (mg/L) levels were measured in all patients before to treatment.
Patients identified with premalignant and malignant endometrial cancer were referred to the gynaecological oncology centre for required surgery and further treatments in our study.
Statistical reviews
During the examination of the study's results, statistical analysis was conducted using the IBM SPSS Statistics 22.0 programme. The Kolmogorov–Smirnov test was used to assess the parameters' the suitability for normal distribution while examining the study's data. Descriptive statistical methods such as mean and standard deviation were utilised. Student's t-test was employed to compare regularly distributed data in two groups, while the Mann–Whitney U test was used for non-normally distributed values. Qualitative data was compared using Chi-Square test and Fisher's Exact test. ROC analysis was conducted to establish the cut-off point. The significance was assessed at p < 0.05 level.
Results
Out of 203 cases in the study, 191 were benign and 12 were malignant and atypical endometrial hyperplasia (premalignant). Cases with similar features in age, BMI, gravida, parity, abortion, educational level, smoking, operation history, and co-morbidity variables were analysed. Cases with similar age and then BMI distributions were included in the study. From these cases, those with similar gravida, parity, and abortion parameters were chosen. From the recently obtained group, patients with similar educational status were chosen. Finally, cases with similar co-morbidity were chosen. The study proceeded with 34 patients in all, consisting of 12 premalignant and malignant cases, and 22 benign cases.
Age, BMI, gravida, parity, number of abortions, educational status, smoking status, operation history, co-morbidity, and complaint variables do not exhibit a statistically significant difference among the groups (p > 0.05) (Table 1).
Table 1.
Analysis of clinical and demographic data of the cases
| Benign | Malignant | p-value | ||
|---|---|---|---|---|
| Mean ± SD (median) | Mean ± SD (median) | |||
| Age (year) | 45.14 ± 9.0 | 48.92 ± 13.63 | 10.338 | |
| BMI (kg/m2) | 29.08 ± 6.03 | 29.45 ± 4.82 | 10.855 | |
| Gravida | 2.86 ± 2.27 (2.5) | 1.92 ± 1.51 (2) | 20.292 | |
| Parity | 2.27 ± 1.83 (2) | 1.08 ± 0.90 (1) | 20.068 | |
| n; % | n; % | |||
| Abortion | 0 | 13; 59.1% | 5; 41.7% | 30.622 |
| 1 | 5; 22.7% | 4; 33.3% | ||
| ≥ 2 | 4; 18.2% | 3; 25% | ||
| Education | Primary school | 10; 45.5% | 2; 16.7% | |
| High school | 10; 45.5% | 6; 50% | 30.108 | |
| University | 2; 9.1% | 4; 33.3% | ||
| Smoke | No | 16; 72.7% | 10; 83.3% | 40.681 |
| Yes | 6; 27.3% | 2; 16.7% | ||
|
Operation History |
Not | 17; 77.3% | 7; 58.3% | 40.271 |
| Yes | 5; 22.7% | 5; 41.7% | ||
| Co-morbidity | No | 11; 50% | 2; 16.7% | 40.075 |
| Yes | 11; 50% | 10; 83.3% | ||
| Complain | Pain | 3; 19.6% | 0; 0% | 30.304 |
| A. uterine Bleeding | 3; 13.6% | 5; 41.7% | ||
| Infertility | 11; 50% | 2; 16.7% | ||
| Asemptomatic | 2; 9.1% | 0; 0% | ||
| Postmenopausal V. Bleeding | 2; 9.1% | 5; 41.7% | ||
1Student t test
2Mann-Whitney U test
3Ki-Kare test
4Exact test
The fasting blood glucose (FBG) level in the malignant group was statistically significantly higher than the benign group (p < 0.01). The CRP level in the malignant group was statistically significantly higher than the benign group (p < 0.01). There is no statistically significant difference in haemoglobin levels across the groups (p > 0.05). There is no statistically significant difference in haematocrit levels across the groups (p > 0.05). The polyp length of the malignant group was statistically significantly higher than the benign group (p < 0.01. The endometrial thickness in the malignant group was statistically significantly higher than the benign group (p < 0.05). There was no statistically significant difference in polyp locations between the groups, (p > 0.05) (Table 2).
Table 2.
Evaluation of laboratory results between groups
| Benign | Malignant | p-value | ||
|---|---|---|---|---|
| Mean ± SD (median) | Mean ± SD (median) | |||
| FBG (mg/dl) | 102.82 ± 18.41 | 130.67 ± 12.54 | 10.001** | |
| CRP (mg/L) | 4.68 ± 2.50 (4.05) | 16.69 ± 5.74 (17.6) | 20.001** | |
| Hgb | 11.08 ± 1.81 | 10.88 ± 1.06 | 10.731 | |
| Htc | 33.75 ± 5.14 (33.15) | 32.08 ± 2.54 (32.2) | 20.261 | |
| Polyp (cm) | 1.98 ± 1.08 (1.7) | 3.11 ± 1.12 (3.35) | 20.005** | |
| Em thickness (mm) | 10.82 ± 2.89 (10) | 14.42 ± 4.70 (14) | 20.025* | |
| n; % | n; % | |||
| Polyp Localization | Isthmus | 1; 4.5% | 1; 8.3% | 30.833 |
| Corpus | 9; 40.9% | 6; 50% | ||
| Fundal | 8; 36.4% | 4; 33.3% | ||
| Not | 4; 18.2% | 1; 8.3% |
1Student t test
2Mann-Whitney U test
3Ki-Kare test
*p < 0.05
**p < 0.01
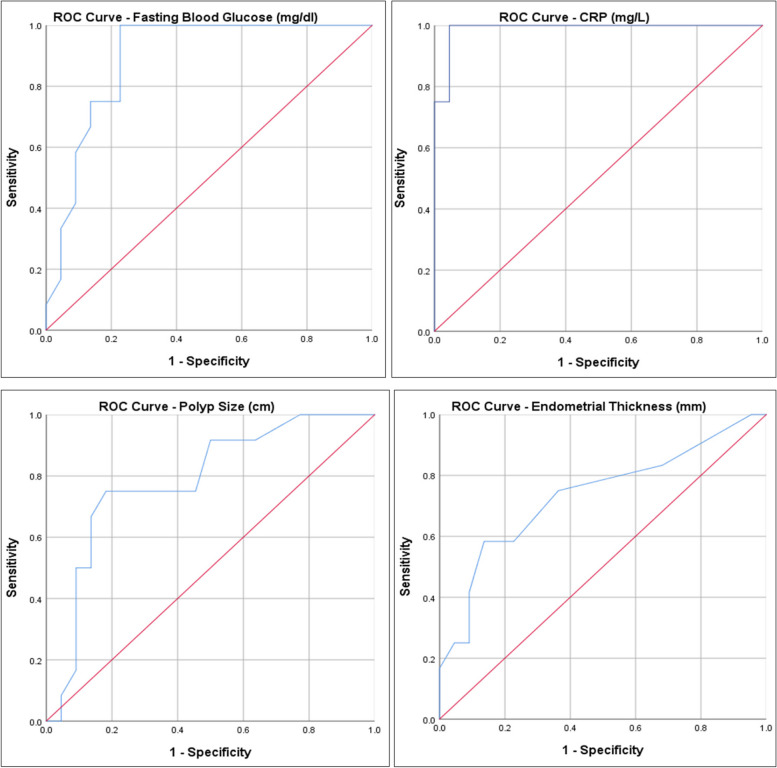
The cut-off value for fasting blood glucose (FBG) to predict malignancy was determined at 122.5, with a sensitivity of 75% and a specificity of 77%. As a result of the ROC analysis, the area under the curve was determined to be 0.892 (95% CI: 0.784–1.000).
The cut-off value for CRP to predict malignancy was determined as 9.7, with a sensitivity of 92% and a specificity of 96%. As a result of the ROC analysis, the area under the curve was determined to be 0.989 (95% CI: 0.962–1.000).
The cut-off value for polyp length to predict malignancy was determined at 2.25 cm, with a sensitivity of 75% and a specificity of 82%. As a result of the ROC analysis, the area under the curve was determined to be 0.786 (95% CI: 0.623–0.949).
The cut-off value for Endometrial thickness to predict malignancy was determined as 11 mm, with a sensitivity of 75% and a specificity of 64%. As a result of the ROC analysis, the area under the curve was determined to be 0.735 (95% CI: 0.547–0.923) (Table 3) (Fig. 1).
Table 3.
Cut-Off Value and ROC Analysis for FBG, CRP, Polyp Size and EM Thickness
| Cut-off Point | Sensitivity | Specifity | AUC | %95 CI | p-value | |
|---|---|---|---|---|---|---|
| FBG (mg/dl) | 122.5 | 75% | 77% | 0.892 | 0.784 – 1.000 | 0.001** |
| CRP (mg/L) | 9.70 | 92% | 96% | 0.989 | 0.962 – 1.000 | 0.001** |
| Polyp size (cm) | 2.25 | 75% | 82% | 0.786 | 0.623 – 0.949 | 0.007** |
| EM thickness (mm) | 11 | 75% | 64% | 0.735 | 0.547 – 0.923 | 0.025* |
*p < 0.05
**p < 0.01
Fig. 1.
ROC curve for FBG, CRP, Polyp size and Endometrial thickness
Upon analysing the FBG distribution by groups, it is seen that the ODDS ratio is 10.20 for FBG values of 122.5 and higher, with a 95% (95% CI: 1.97 – 52.78).
Upon analysing the CRP distribution by groups, it is noted that the ODDS ratio is 231 for CRP values of 9.7 and above (95% CI: 13.15 – 4058.67).
Upon analysing the Polyp length distribution by groups, it is observed that the ODDS ratio is 13.5 for Polyp lengths of 2.25 and above (95% CI: 2.47 – 73.71). Upon examining the EM thickness distribution by groups, it is observed that the ODDS ratio is 5.25 for EM thicknesses of 11 and above, (95% CI: 1.09 – 25.21) (Table 4).
Table 4.
Risk Ratios for FBG, CRP, Polyp Size and EM Thickness
| Benign | Malignant |
ODDS Ratio (%95 CI Lower—Upper) |
||
|---|---|---|---|---|
| n; % | n; % | |||
| FBG (mg/dl) | < 122.5 | 17; 77.3% | 3; 25% | 10.20 (1.97 – 52.78) |
| ≥ 122.5 | 5; 22.7% | 9; 75% | ||
| CRP (mg/L) | < 9.7 | 21; 95.5% | 1; 8.3% | 231.00 (13.15 – 4058.67) |
| ≥ 9.7 | 1; 4.5% | 11; 91.7% | ||
| Polyp size (cm) | < 2.25 | 18; 81.8% | 3; 25% | 13.50 (2.47 – 73.71) |
| ≥ 2.25 | 4; 18.2% | 9; 75% | ||
| EM thick. (mm) | < 11 | 14; 63.6% | 3; 25% | 5.25 (1.09 – 25.21) |
| ≥ 11 | 8; 36.4% | 9; 75% |
Exact test was used
Discussion
When we look at the general results of our study; After the histopathological examination of 203 cases diagnosed with endometrial polyps after hysteroscopic resection, 3 cases were diagnosed as endometrial carcinoma on the basis of endometrial polyps (2 cases as endometrioid type adenocarcinoma on the basis of polyps, 1 case as serous endometrial carcinoma on the basis of polyps) and 9 cases were diagnosed as hyperplasia with atypia on the basis of polyps. Furthermore, 17 out of the remaining 191 cases exhibited hyperplasia without atypia on the basis of endometrial polyps, while the remaining 174 cases were diagnosed as benign endometrial polyps. To identify risk factors for carcinogenesis, two homogeneous groups were established by initially matching patients based on age and BMI, followed by matching similar cases in terms of gravida, parity, abortions, and then those with same comorbidities. A study comparing 22 benign endometrial polyp groups with 12 malignant and premalignant endometrial polyp groups found a high risk of developing carcinoma in cases with fasting blood sugar (FBG) levels of 122.5 and above, CRP levels of 9.7 and above, polyp lengths of 2.25 cm and above, and endometrial thickness of 11 mm and above.
The risk of pre-malignant and malignant cancer in endometrial polyps differs among various studies. It is thought that the diagnostic methods used especially in the diagnosis of endometrial polyps are not standardized and the high diagnosis rates with random endometrial sampling are also due to deficiencies in preoperative patient evaluation [5, 9, 14]. We included cases suspected of endometrial polyps after clinical and ultrasonographic evaluation, as well as cases where the diagnosis was confirmed through hysteroscopic examination and sample analysis, to showcase diagnostic accuracy and malignancy risk.
The most significant risk factors for carcinomatous alterations in endometrial polyps include advanced age, postmenopausal status, and atypical vaginal bleeding symptoms [12, 13, 15]. Increasing age is well recognised as the most important risk factor for the development of endometrial polyps and any borderline or malignant lesions that arise from them. The likelihood of acquiring lesions is around 7.2% in those aged 25–65, while it rises to almost 30% in those aged 65 and above. Endometrial polyp-like benign lesions are less common in women of reproductive age due to the fact that most of them are shed and disappear with menstrual cycles [4, 16].
The probability of developing premalignant or malignant carcinoma from endometrial polyps is reported to be 10 times greater in postmenopausal and symptomatic patients compared to asymptomatic and premenopausal cases [9, 10, 17].
It is stated that malignant endometrial lesions can arise from endometrial polyps. Serous epithelial endometrial carcinomas are more common in individuals over 65 years old, while endometrioid type endometrial carcinomas are more common in the 45–65 age group. These lesions originating from polyps are typically well-differentiated [18].
Diabetes mellitus in women with metabolic syndrome is linked to endometrial polyps that have the potential to become cancerous. It is uncertain if elevated BMI, diabetes, obesity, high CRP levels, and advanced age together or independently provide a danger, which remains a topic of controversy [19].
The impact of elevated glucose levels on the development of gynaecological cancer is still being determined. Glucose is essential for cells. In both in vivo and in vitro studies, the Hexokinase 2 enzyme becomes active in high glucose conditions, leading to cell proliferation and the formation of tumour cells. Tumour cells are activated by hypoxia-inducible factor (HIF) rather than mitochondrial oxidative phosphorylation, which leads to glycolysis as a result of the expression of glycolytic enzymes. Elevated glucose levels speed up the proliferation of potential carcinogenesis cells that have the potential to develop into cancer because they are used for energy. High glucose levels lead to a rise in carrier proteins on the cell membrane and excessive proliferation of cells as a consequence of increased glycosylation. Increased glucose levels result in excessive proliferation of pluripotent stem cells, enhanced migration ability, and invasion potential. High glucose levels also lead to increased proliferation of pluripotent stem cells. The increased glucose in carcinogenesis is believed to play a function through the mechanisms where insulin and insulin-like growth factors inhibit apoptosis and promote fast progression in tumour cells with high glucose levels. It is understood that factors associated with glucose metabolism also contribute to the development of cancer [20, 21]. Upon reviewing the literature, a correlation has been established between the levels of glucose or glycaemic index and several types of cancer including colorectal, breast, stomach, ovarian, endometrial, and cervical cancer [22–24].
After homogenising the two groups based on similar clinical and demographic characteristics, the malignant group had higher fasting blood sugar values compared to the other group (130.67 ± 12.54 versus 102.82 ± 18.41), with a statistically significant difference observed between them (p:0.001). The cut-off value for predicting malignancy based on fasting blood sugar was determined to be 122.5 mg/dl. The sensitivity value was 75%, specificity value was 77%, and the area under the curve from ROC analysis was 0.892 (95% CI: 0.784–1.000), suggesting a reliable confidence interval for the study's power. The study found that the relative risk of developing carcinoma due to endometrial polyps was 10.20 times higher in cases with fasting blood sugar levels above 122.5 mg/dl (95% CI: 1.97–52.78). but the confidence interval was found to be very wide, which may be attributed to the small number of cases in both groups. Because higher fasting glucose levels are critical in the development of cancer, we may predict that this confidence interval will expand to more suitable values as the number of cases grows.
C-reactive protein (CRP), an acute phase reactant, is extensively produced in liver hepatocytes and plays a crucial role in acute and chronic inflammation. CRP has been demonstrated to have a significant part in the systemic inflammatory response and has shown effectiveness at a crucial stage of carcinogenesis, such as in cell death. As the inflammatory response intensifies, cell DNA damage happens, angiogenesis rises, and cell apoptosis is inhibited, leading to cell proliferation and the stimulation of carcinogenesis [25, 26]. Many proinflammatory cytokines such as IL-1, IL-6, tumour necrosis factor-alpha, interferon-gamma, and tumour growth factor elevate CRP levels, promoting survival, growth, mutation, proliferation, differentiation, and migration in tumour cells [27]. It has also been shown that serum CRP increases in parallel with carcinogenesis as a reaction of natural immunity [25]. Elevated CRP levels are linked to higher serum vascular endothelial growth factor (VEGF) levels, which can promote neo angiogenesis and invasion by inducing immunosuppression in the tumour microenvironment [28, 29]. Research on the involvement of CRP, an inflammatory marker, in the development and prognosis of human malignancies has risen as the understanding of inflammation and carcinogenesis progresses. Analysis of existing literature reveals that elevated CRP levels are associated with a poor prognosis in gynaecological malignancies [ 24, 28, 29].
Our study indicated that CRP levels were significantly higher in the malignant group (16.69 ± 5.74) compared to the benign group (4.68 ± 2.50), with a statistically significant difference between the two groups (p:0.001). The cut-off value for elevated CRP levels to indicate malignancy was found to be 9.7, with a sensitivity of 92% and a specificity of 96%. In ROC analysis, the area under the curve is 0.989 (95% CI: 0.962–1.000), and this narrow confidence interval shows the power of our study to indicate malignancy in terms of CRP value. The analysis revealed that the probability of developing cancer from an endometrial polyp was 231 times higher when the CRP value was 9.7 or above (95% CI: 13.15–4058.67). The confidence interval was determined to be very broad. This scenario is due to the low number of cases in both groups. As the number of cases rises, the confidence interval for CRP levels, which is crucial in malignancy development, will become more accurate.
Upon reviewing the literature, it is seen that there is a lack of clear consistency in the articles reporting the correlation between polyp size and location and the progression to malignancy, particularly in asymptomatic cases of endometrial polyps [14, 15, 30–33]. In a separate study, more than one endometrial polyp was detected at the same time in 29.2% of endometrial polyp cases, and it was revealed that the number of polyps and polyp lengths had no relationship with the increase in malignancy or the presenting symptoms of the patients [34]. In another study, 1152 patients underwent ultrasonographic evaluation, revealing a notable increase in the probability of premalignant and malignant lesions in cases with an average estimated endometrial polyp diameter of 18 mm or higher [17]. Yet, in a different study, it was noted that the risk of premalignant and malignant lesions did not rise in correlation with the size of the biggest maximum endometrial polyp diameter. The variation in findings might be attributed to discrepancies in measuring units (such as length or volume) or measurement instruments utilised to assess polyp length in some studies.
While some studies show that endometrial polyps in asymptomatic premenopausal women with a length of 1 or 1.5 cm or less have a high likelihood of spontaneous regression over time and only require monitoring. However, other studies indicate that larger polyps than 1.5 cm in older, postmenopausal women may be associated with malignancy. Because of the high risk, it is also suggested that it be respected and histopathologically examined [9, 35]. Study has shown that there is no link between assessing polyp lengths and increased blood flow in Doppler ultrasonographic examination for endometrial polyps and the risk of developing malignancy. Histopathological examination is considered more crucial in this context [36]. The malignancy risk was 0.1% in asymptomatic premenopausal patient group with endometrial polyps and roughly 1% in postmenopausal patients with abnormal uterine bleeding. A significant difference was seen (p < 0.01). When a multivariate study was conducted on the relationship between polyp length and malignancy, it was shown that endometrial polyps measuring 18 mm and larger were 6.9 times more likely to be associated with malignancy and atypical hyperplasia (95% CI = 2.2–21.4). Additionally, in the same study, malignancy was reported by histopathological examination in the case of a single 40 mm endometrial polyp in the asymptomatic group [17]. In a separate study, a threshold measurement of 15 mm is deemed hazardous in relation to the development of malignancy [9]. In a different study, it was reported that the risk of malignancy was 4.9% in cases where the size of an endometrial polyp was 2–3 cm. However, the risk of malignancy decreased as the length of the polyp increased [14].
,The most comprehensive reviewer study in the literature included a total of 35,345 cases. The prevalence of malignant endometrial polyp was 2.73%. It was 4.93% in postmenopausal group and 1.12% in premenopausal group. The risk of malignancy was 5.14% in symptomatic cases and 1.89% in asymptomatic cases. These rates were found to be statistically significantly higher between the two groups (p < 0.0001). Researchers have generally observed that, especially in prospective studies, there is a higher risk of malignancy due to clearer and more stringent diagnostic criteria, and that heterogeneity rates are high between studies. As a result, it is stated that the risk of malignancy is high in the postmenopausal group and in cases with symptomatic bleeding, and that resection is a suitable therapeutic choice, independent of polyp length and other risk factors [37].
Most polyps in both the benign and premalignant/malignant groups ranged from 0.8 to 5.3 cm and were mostly found in the uterine corpus in our study. While the average polyp length was 3.11 ± 1.12 cm in the group that developed malignancy on the basis of endometrial polyps, it was 1.98 ± 1.08 cm in benign polyps. A statistically significant difference in polyp lengths between the two groups was identified (p:0.005). The cut-off value for polyp lengths to indicate malignancy was set at 2.25 cm, with a sensitivity of 75% and a specificity of 82%. The ROC analysis yielded an area under the curve of 0.786 (95% CI: 0.623–0.949), and this narrow confidence interval shows the power of the study. Upon analysing the distribution of polyp lengths across different groups, it is seen that the relative risk of malignancy associated with endometrial polyps is 13.5 times higher in cases where the polyp length is 2.25 cm or larger (95% CI: 2.47–73.71). However, the confidence interval was determined to be broad. The condition can be attributed to the low number of cases in both groups. As polyp lengths rise, the risk of developing malignancy also increases. Therefore, the confidence interval will become more accurate as the number of cases increases.
Ultrasound is the most common method of diagnosing endometrial polyps. Ultrasonography is the best diagnostic method, particularly for abnormal cases that are either isolated or associated with increased endometrial thickness. It is advisable to conduct additional examinations, particularly histopathological examination, to exclude the presence of malignancy in asymptomatic cases with endometrial thickness exceeding 11 mm, increased vascularity, heterogeneous structure, and endometrial wall structure with endometrial fluid [38]. International gynaecology associations do not recommend histological examination for malignancy in the cases of asymptomatic premenopausal and postmenopausal cases. even if reference values for endometrial thickness evaluation are unclear. They did not provide a definitive suggestion regarding the conservative approach in asymptomatic postmenopausal cases [39, 40]. However, some groups suggest removing and examining endometrial polyps in asymptomatic postmenopausal cases, including characteristics such as endometrial thickness, polyp length, location, and other risk factors to assess the risk of malignancy. Clinicians are recommended to conduct an assessment using transvaginal ultrasonography in order to identify cervical stenosis, particularly in asymptomatic postmenopausal patients, where clinical symptoms such as discharge and bleeding may not be apparent. When devising treatment strategies, clinicians should consider similar risk factors including endometrial thickness, polyp length, and location and the treatment should be planned according to the patient [41, 42].
The retrospective ultrasonographic study, conducted by many sonographers with different specialties and including a small number of patients, did not allow for a definitive correlation to be made between endometrial thickness and the risk of malignancy [43]. The study assessed risk factors for malignancy by analysing endometrial thickness and polyp length using ultrasonography in patients presenting with abnormal uterine bleeding. The identified cut-off value for endometrial thickness was 11.5 mm. The specified cut-off value showed a sensitivity of 53.8% and specificity of 85.8%, with positive predictive value of 24.6% and negative predictive value of 95.6%. The study found that the detection rates of malignancy were low when the endometrial thickness cut-off value was set at 11.5 mm. Hence hysteroscopic examination is considered the most valuable instrument for diagnosing and detecting malignancy [44].
The average endometrial thickness was 14.42 ± 4.70 mm in the group with malignancy on the basis of endometrial polyps and 10.82 ± 2.89 mm in the benign group. A statistically significant difference in endometrial thickness was observed between the two groups (p:0.025). The cut-off value for endometrial thickness to predict malignancy was established at 11 mm, with a sensitivity of 75% and a specificity of 64%. The ROC analysis yielded an area under the curve of 0.735 (95% CI: 0.547–0.923), this narrow confidence interval shows the power of the study. When endometrial thickness distribution is 11 mm or greater, the relative risk of malignancy associated with endometrial polyps is 5.25 times higher (95% CI: 1.09–25.21) compared to other groups. However, the confidence interval was determined to be broad. The condition can be attributed to the low number of cases in both groups. As the number of cases grows, the confidence interval will become more suitable due to the significance of increasing endometrial thickness in malignancy development.
There have been recent studies that have revealed malignancy risk factors using artificial intelligence technology using hysteroscopic images of endometrial pathologies. As it presents a novel approach using deep learning (DL) to detect and classify endometrial pathologies from hysteroscopic images. The study highlights the potential of DL models in enhancing diagnostic accuracy, especially when combined with clinical factors. Despite the preliminary nature of the findings and the modest improvement in diagnostic performance, the research underscores the importance of integrating artificial intelligence in gynecological diagnostics, which could be relevant to the discussion of identifying malignancy risk factors in endometrial polyps [45].
Another study analyzing endometrial cancer cases provides a comprehensive review of the current challenges and controversies in the treatment of endometrial cancer (EC). It highlights that EC is the most common malignancy of the female genital tract, with surgery being the cornerstone of treatment. The study discusses various aspects such as risk factors, including obesity and hormonal imbalances, diagnostic approaches like hysteroscopy and transvaginal ultrasonography, and the controversies surrounding the role of lymphadenectomy and radiotherapy in intermediate-risk cases. Additionally, it explores fertility-sparing treatments and the increasing role of chemotherapy in advanced stages. The review underscores the need for more research and clearer guidelines, especially regarding the use of radiotherapy and the management of early-stage EC with fertility-preserving intentions [46].
Due to the retrospective nature of our study, bias may naturally arise in patient selection, given the study's limitations. Furthermore, the study's shortcomings include being conducted at a single centre and having a rather small sample size.
A further limitation is the absence of randomised controlled studies on this topic. The novelty of our study being the first on this subject demonstrates its robustness.
Endometrial polyps are often occurring growths that are not typically viewed as cancer precursors, however, with advancement of age and the onset of menopause, they may become associated with malignancy. It is crucial to remember that the risk for malignancy is high in endometrial polyps, particularly in cases of big size, increased endometrial thickness, high fasting glucose levels, and elevated CRP. Management of patients diagnosed with endometrial polyp should be individualised and carefully considered, taking into consideration the patient's age, menopausal state, symptoms, and clinical and laboratory risk factors.
Authors’ contributions
Conception and design of the research: Cetin F, Birge O. Acquisition of data: Kayar I, Birge O. Analysis and interpretation of the data: Goc G, Cetin F. Statistical analysis: Birge O, Kayar I. Writing of the manuscript: Cetin F, Birge O. Critical revision of the manuscript for intellectual content: Goc G, Kayar I. All authors read and approved the final draft.
Funding
Not applicable.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Osmaniye Provincial Health Ethics Committee of the Ministry of Health of the Republic of Turkey (Approval Date & No: 30.05.2023 & E-774.99–216604593).). Written informed consent was obtained from all participants.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Berek JS, Novak E. Berek & Novak’s Gynecology. 14th ed. Philadelphia: Lippincott Williams & Wilkins; 2007. [Google Scholar]
- 2.Sherman ME, Mazur MT, Kurman RJ. Benign diseases of the endometrium Kurman RJ (Ed), Blaustein’s pathology of the female genital tract. New York: Springer-Verlag; 2002. p. 421–6. [Google Scholar]
- 3.Shan W, Ning C, Luo X, Zhou Q, Gu C, Zhang Z, Chen X. Hyperinsulinemia is associated with endometrial hyperplasia and disordered proliferative endometrium: a prospective cross-sectional study. Gynecol Oncol. 2014;132(3):606–10. [DOI] [PubMed] [Google Scholar]
- 4.Hileeto D, Fadare O, Martel M, Zheng W. Age dependent association of endometrial polyps with increased risk of cancer involvement. World J Surg Oncol. 2005;3:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Savelli L, Iacco De, P,Santini D, et al. Histopathologic features and risk factors for benignity, hyperplasia, and cancer in endometrial polyps Am J Obstet Gynecol. 2003;188:927–31. [DOI] [PubMed] [Google Scholar]
- 6.Perri T, Rahimi K, Ramanakumar AV, Wou K, Pilavdzic D, Franco EL, Gotlieb WH, Ferenczy A. Are endometrial polyps true cancer precursors? Am J Obstet Gynecol. 2010;203(3):232.e1-6. [DOI] [PubMed] [Google Scholar]
- 7.American Association of Gynecologic Laparoscopists. AAGL practice report: practice guidelines for the diagnosis and management of endometrial polyps. J Minim Invasive Gynecol. 2012;19(1):3–10. [DOI] [PubMed] [Google Scholar]
- 8.Lieng M, Istre O, Qvigstad E. Treatment of endometrial polyps: a systematic review. Acta Obstet Gynecol Scand. 2010;89:992–1002. [DOI] [PubMed] [Google Scholar]
- 9.Ben-Arie A, Goldchmit C, Laviv Y, Levy R, Caspi B, Huszar M, Dgani R, Hagay Z. The malignant potential of endometrial polyps. Eur J Obstet Gynecol Reprod Biol. 2004;115(2):206–10. [DOI] [PubMed] [Google Scholar]
- 10.Yela DA, Ribeiro CM, Laguna C, Benetti P. Malignancy risk in Brazilian women with endometrial polyps. J Gynecol Surg. 2016;32(4):226–9. [Google Scholar]
- 11.Machtinger R, Korach J, Padoa A, et al. Transvaginal ultrasound and diagnostic hysteroscopy as a predictor of endometrial polyps: risk factors for premalignancy and malignancy. Int J Gynecol Cancer. 2005;15:325–8. [DOI] [PubMed] [Google Scholar]
- 12.Costa-Paiva L, Godoy CE, Antunes A, et al. Risk of malignancy in endometrial polyps in premenopausal and postmenopausal women according to clinicopathologic characteristics. Menopause. 2011;18:1278–82. [DOI] [PubMed] [Google Scholar]
- 13.Antunes A, Costa-Paiva L, Arthuso M, et al. Endometrial polyps in pre- and postmenopausal women: factors associated with malignancy. Maturitas. 2007;57:415–21. [DOI] [PubMed] [Google Scholar]
- 14.Fernández-Parra J, Rodríguez Oliver A, López Criado S, ParrillaFernández F, Montoya VF. Hysteroscopic evaluation of endometrial polyps. Int J Gynaecol Obstet. 2006;95(2):144–8. [DOI] [PubMed] [Google Scholar]
- 15.Namazov A, Gemer O, Ben-Arie A, Israeli O, Bart O, Saphier O, Mahler N, Kapustian V, Silberstein T. Endometrial Polyp Size and the Risk of Malignancy in Asymptomatic Postmenopausal Women. J Obstet Gynaecol Can. 2019;41(7):912–5. [DOI] [PubMed] [Google Scholar]
- 16.Tabrizi AD, Vahedi A, Esmaily HA. Malignant endometrial polyps: Report of two cases and review of literature with emphasize on recent advances. J Res Med Sci. 2011;16:574–9. [PMC free article] [PubMed] [Google Scholar]
- 17.Ferrazzi E, Zupi E, Leone FP, Savelli L, Omodei U, Moscarini M, Barbieri M, Cammareri G, Capobianco G, Cicinelli E, Coccia ME, Donarini G, Fiore S, Litta P, Sideri M, Solima E, Spazzini D, Testa AC, Vignali M. How often are endometrial polyps malignant in asymptomatic postmenopausal women? A multicenter study. Am J Obstet Gynecol. 2009;200(3):235.e1-6. [DOI] [PubMed] [Google Scholar]
- 18.Scully RE, Bonfiglio TA, Kurman RJ. World Health Organization International histologic classification of tumors. Histologic typing of female genital tract tumors. Berlin, Germany: Springer- Verlag; 1994. [Google Scholar]
- 19.Nappi L, Indraccolo U, Di Spiezio Sardo A, Gentile G, Palombino K, Castaldi MA, Spinelli M, Greco P. Are diabetes, hypertension, and obesity independent risk factors for endometrial polyps? J Minim Invasive Gynecol. 2009;16(2):157–62. [DOI] [PubMed] [Google Scholar]
- 20.Ryu TY, Park J, Scherer PE. Hyperglycemia as a risk factor for cancer progression. Diabetes Metab J. 2014;38(5):330–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mueckler M, Thorens B. The SLC2 (GLUT) family of membrane transporters. Mol Aspects Med. 2013;34(2–3):121–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mulholland HG, Murray LJ, Cardwell CR, Cantwell MM. Dietary glycaemic index, glycaemic load and endometrial and ovarian cancer risk: a systematic review and meta-analysis. Br J Cancer. 2008;99(3):434–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Silvera SA, Rohan TE, Jain M, Terry PD, Howe GR, Miller AB. Glycaemic index, glycaemic load and risk of endometrial cancer: a prospective cohort study. Public Health Nutr. 2005;8:912–9. [DOI] [PubMed] [Google Scholar]
- 24.Nie D, Zhang L, Wang C, Guo Q, Mao X. A high Glasgow prognostic score (GPS) or modified Glasgow prognostic score (mGPS) predicts poor prognosis in gynecologic cancers: a systematic review and meta-analysis. Arch Gynecol Obstet. 2020;301(6):1543–51. [DOI] [PubMed] [Google Scholar]
- 25.Black S, Kushner I, Samols D. C-reactive protein. J Biol Chem. 2004;279:48487–90. [DOI] [PubMed] [Google Scholar]
- 26.Fleming JS, Beaugie CR, Haviv I, Chenevix-Trench G, Tan OL. Incessant ovulation, inflammation and epithelial ovarian carcinogenesis: revisiting old hypotheses. Mol Cell Endocrinol. 2006;247:4–21. [DOI] [PubMed] [Google Scholar]
- 27.Elliott RL, Blobe GC. Role of transforming growth factor Beta in human cancer. J Clin Oncol. 2005;23:2078–93. [DOI] [PubMed] [Google Scholar]
- 28.Barsoum IB, Smallwood CA, Siemens DR, Graham CH. A mechanism of hypoxia-mediated escape from adaptive immunity in cancer cells. Cancer Res. 2014;74:665–74. [DOI] [PubMed] [Google Scholar]
- 29.Nozoe T, Korenaga D, Futatsugi M, Saeki H, Maehara Y, Sugimachi K. Immunohistochemical expression of C-reactive protein in squamous cell carcinoma of the esophagus-significance as a tumor marker. Cancer Lett. 2003;192:89–95. [DOI] [PubMed] [Google Scholar]
- 30.Wang JH, Zhao J, Lin J. Opportunities and risk factors for premalignant and malignant transformation of endometrial polyps: management strategies. J Minim Invasive Gynecol. 2010;17(1):53–8. [DOI] [PubMed] [Google Scholar]
- 31.Godoy CE Jr, Antunes A Jr, Morais SS, Pinto-Neto AM, Costa-Paiva L. Accuracy of sonography and hysteroscopy in the diagnosis of premalignant and malignant polyps in postmenopausal women. Rev Bras Ginecol Obstet. 2013;35(6):243–8. [DOI] [PubMed] [Google Scholar]
- 32.Gregoriou O, Konidaris S, Vrachnis N, et al. Clinical parameters linked with malignancy in endometrial polyps. Climacteric. 2009;12:454–8. [DOI] [PubMed] [Google Scholar]
- 33.Shushan A, Revel A, Rojansky N. How often are endometrial polyps malignant? Gynecol Obstet Invest. 2004;58(4):212–5. [DOI] [PubMed] [Google Scholar]
- 34.Hassa H, Tekin B, Senses T, Kaya M, Karatas A. Are the site, diameter, and number of endometrial polyps related with symptomatology? Am J Obstet Gynecol. 2006;194(3):718–21. [DOI] [PubMed] [Google Scholar]
- 35.DeWaay DJ, Syrop CH, Nygaard IE, Davis WA, Van Voorhis BJ. Natural history of uterine polyps and leiomyoma. Obstet Gynecol. 2002;100:3–9. [DOI] [PubMed] [Google Scholar]
- 36.Goldstein SR, Monteagudo A, Popiolek D, Maybery P, TimorTristch I. Evaluation of endometrial polyps. Am J Obstet Gynecol. 2002;186:669–74. [DOI] [PubMed] [Google Scholar]
- 37.Uglietti A, Buggio L, Farella M, Chiaffarino F, Dridi D, Vercellini P, Parazzini F. The risk of malignancy in uterine polyps: A systematic review and meta-analysis. Eur J Obstet Gynecol Reprod Biol. 2019;237:48–56. [DOI] [PubMed] [Google Scholar]
- 38.Wolfman WN. 249-Asymptomatic Endometrial Thickening. J Obstet Gynaecol Can. 2018;40(5):e367–77. [DOI] [PubMed] [Google Scholar]
- 39.Colombo N, Creutzberg C, Amant F, Bosse T, González-Martín A, Ledermann J, Marth C, Nout R, Querleu D, Mirza MR, Sessa C. ESMO-ESGO-ESTRO Endometrial Consensus Conference Working Group. ESMO-ESGO-ESTRO Consensus Conference on Endometrial Cancer: diagnosis, treatment and follow-up. Ann Oncol. 2016;27(1):16–41. [DOI] [PubMed] [Google Scholar]
- 40.ACOG Committee Opinion No. 734: The Role of Transvaginal Ultrasonography in Evaluating the Endometrium of Women With Postmenopausal Bleeding. Obstet Gynecol. 2018;131:e124–9. [DOI] [PubMed] [Google Scholar]
- 41.Wolfman W, Leyland N, Heywood M, Singh SS, Rittenberg DA, Soucy R, Allaire C, Awadalla A, Best C, Dunn S, Leroux N, Potestio F, Senikas V, Wallace S, Menzies R. Society of Obstetricians and Gynaecologists of Canada. Asymptomatic endometrial thickening. J Obstet Gynaecol Can. 2010;32(10):990–9. [DOI] [PubMed] [Google Scholar]
- 42.Ludwin A, Lindheim SR, Booth R, Ludwin I. Removal of uterine polyps: clinical management and surgical approach. Climacteric. 2020;23(4):388–96. [DOI] [PubMed] [Google Scholar]
- 43.Bel S, Billard C, Godet J, Viviani V, Akladios C, Host A, Faller E, Boisrame T, Hummel M, Baldauf JJ, Lecointre L, Garbin O. Risk of malignancy on suspicion of polyps in menopausal women. Eur J Obstet Gynecol Reprod Biol. 2017;216:138–42. [DOI] [PubMed] [Google Scholar]
- 44.Cavkaytar S, Kokanali MK, Ceran U, Topcu HO, Sirvan L, Doganay M. Roles of sonography and hysteroscopy in the detection of premalignant and malignant polyps in women presenting with postmenopausal bleeding and thickened endometrium. Asian Pac J Cancer Prev. 2014;15(13):5355–8. [DOI] [PubMed] [Google Scholar]
- 45.Raimondo D, Raffone A, Salucci P, Raimondo I, Capobianco G, Galatolo FA, Cimino MGCA, Travaglino A, Maletta M, Ferla S, Virgilio A, Neola D, Casadio P, Seracchioli R. Detection and Classification of Hysteroscopic Images Using Deep Learning. Cancers (Basel). 2024;16(7):1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Masciullo V, Amadio G, Lo Russo D, Raimondo I, Giordano A, Scambia G. Controversies in the management of endometrial cancer. Obstet Gynecol Int. 2010;2010:638165. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.