Abstract
Background
Using neuroimaging techniques, growing evidence has suggested that the choroid plexus (CP) volume is enlarged in multiple neurodegenerative diseases, including amyotrophic lateral sclerosis (ALS). Notably, the CP has been suggested to play an important role in inflammation-induced CNS damage under disease conditions. However, to our knowledge, no study has investigated the relationships between peripheral inflammation and CP volume in sporadic ALS patients. Thus, in this study, we aimed to verify CP enlargement and explore its association with peripheral inflammation in vivo in independent ALS cohorts.
Methods
Based on structural MRI data, CP volume was measured using Gaussian mixture models and further manually corrected in two independent cohorts of sporadic ALS patients and healthy controls (HCs). Serum inflammatory protein levels were measured using a novel high-sensitivity Olink proximity extension assay (PEA) technique. Xtreme gradient boosting (XGBoost) was used to explore the contribution of peripheral inflammatory factors to CP enlargement. Then, partial correlation analyses were performed.
Results
CP volumes were significantly higher in ALS patients than in HCs in the independent cohorts. Compared with HCs, serum levels of CRP, IL-6, CXCL10, and 35 other inflammatory factors were significantly increased in ALS patients. Using the XGBoost approach, we established a model-based importance of features, and the top three predictors of CP volume in ALS patients were CRP, IL-6, and CXCL10 (with gains of 0.24, 0.18, and 0.15, respectively). Correlation analyses revealed that CRP, IL-6, and CXCL10 were significantly associated with CP volume in ALS patients (r = 0.462 ∼ 0.636, p < 0.001).
Conclusion
Our study is the first to reveal a consistent and replicable contribution of peripheral inflammation to CP enlargement in vivo in sporadic ALS patients. Given that CP enlargement has been recently detected in other brain diseases, these findings should consider extending to other disease conditions with a peripheral inflammatory component.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12987-024-00586-w.
Keywords: ALS, Choroid plexus, MRI, Inflammation
Background
To date, the aetiology of amyotrophic lateral sclerosis (ALS) remains unclear; however, interactions between genetic and environmental factors have been suggested to underpin disease susceptibility [1–5]. In most sporadic ALS patients, the main protein found in cytoplasmic inclusions is phosphorylated 43 kDa transactive response DNA-binding protein (TDP-43) [4]. In addition to TDP-43 pathology, other biological pathways, such as non-cell-autonomous mechanisms, blood-CSF barrier (BCSFB) damage and inflammation, are also likely involved in ALS pathology [6–13].
Recently, growing evidence has suggested that the choroid plexus (CP) may play a crucial role in the pathophysiology of multiple neurodegenerative conditions, such as Alzheimer’s disease (AD), Parkinson’s disease (PD), and frontotemporal dementia (FTD) [14–17]. It is well known that the CP is an epithelial–endothelial vascular structure responsible for the production of CSF and the establishment of the BCSFB, which prevents peripheral toxins, such as inflammatory proteins, from entering the central nervous system (CNS) [18–20]. Furthermore, the CP is thought to be an important part of the glymphatic system and is crucial for the clearance of waste from the CNS [18–20]. Thus, the CP may exert an important immunosurveillance function and is a key structure for peripheral inflammation and neuroinflammation crosstalk [18–20].
Specifically, some studies have demonstrated that peripheral inflammation can affect the CNS through the modulation of the CP barrier in mice and humans [21–23]. Moreover, based on structural MRI technique, the existence of CP dysfunction during peripheral inflammation has been detected in several brain diseases [21–23]. For example, Fleischer et al. recently suggested that peripheral inflammation might lead to an enlarged volume of the CP, and MRI-derived CP volume assessment is likely a promising biomarker of inflammatory activity in independent MS cohorts and in two experimental mouse models [21]. Moreover, Lizano et al. demonstrated that enlarged CP volumes can be detected across the psychosis spectrum and that CP enlargement is significantly correlated with worse cognition and higher levels of IL-6 [22]. In that study, they further suggested the involvement of the CP across the psychosis spectrum with a potential pathophysiological mechanism involving the neuroimmune axis [22]. Thus, these convergent evidence suggest that the CP may play an important role in inflammation-induced CNS damage in brain diseases [21–23].
In a large newly diagnosed cohort, using structural magnetic resonance imaging (MRI) techniques, we recently found that, similar to other neurodegenerative diseases, there is an observably enlargement of the CP volume in ALS patients than in healthy subjects [16]. We also found that CP enlargement is significantly related to motor disability and BCSFB damage in ALS patients [16]. However, as mentioned in our previous study, at present, very few studies have focused on CP alterations in ALS. Particularly, to our knowledge, no study has investigated the relationships between peripheral inflammation and CP volume in vivo in patients with ALS [24–26]. Notably, early peripheral inflammation and CP abnormalities can be detected in animal models of ALS, and one previous postmortem study demonstrated that the CP is inflamed in ALS patients [24–26]. Moreover, to date, replication neuroimaging findings regarding in vivo CP enlargement are still lacking in independent ALS cohorts, which may pose a significant barrier to preventing these previous findings from being applied and making truly meaningful advances [14–18].
Against this background, in the present study, we aimed to verify CP volume abnormalities and identify their associations with peripheral inflammation in independent ALS cohorts. Importantly, a novel high-throughput and high-sensitivity multiplex proteomic immunoassay technique, the proximity extension assay (PEA), was used to systematically assess serum inflammatory proteins in ALS patients and healthy controls (HCs)[27, 28, 29]. Moreover, Xtreme Gradient Boosting (XGBoost), has proven to be a powerful machine learning algorithm for classifications and predictions, which have been widely utilized in multiple disease conditions. XGBoost is an integrated method using gradient enhanced trees that can handle both high and sparse data, and the algorithm can generate accurate classifications and predictions even with small sample sizes [31]. Thus, XGBoost was used to assess the contribution of peripheral inflammatory factors to CP enlargement in sporadic ALS patients in this study. Finally, partial correlation analyses were used to further assess the relationships between CP volume and the serum inflammatory proteins selected by the machine learning algorithm in ALS patients.
Methods
Participants
In the present study, the criteria for including ALS patients were defined as follows: (1) patients who satisfied the Awaji criteria for probable or definite ALS; [33] and (2) patients who underwent clinical assessment, serum biomarker measurement, genetic testing and structural MRI scanning [35].
The criteria for excluding ALS patients were as follows: (1) patients who declined to participate; (2) patients unable to complete an MRI scan; and (3) patients with a diagnosis of FTD, which we opted to exclude since the occurrence rate of FTD is low (4.7%) among Chinese patients with sporadic ALS [34]. The diagnosis of FTD was based on the Rascovsky criteria; [34] (4) patients with other neuropsychiatric disorders; and (5) patients similar to those in previous studies, for whom we excluded patients with ALS presenting with any clinical evidence of acute infection or chronic inflammatory disorders [36]. Moreover, patients who needed nutritional or respiratory support were also excluded; [35] and (6) had a family history or carried a known mutation of ALS [35].
Finally, 82 ALS patients were included from April 2023 to April 2024 at Qilu Hospital, Cheeloo College of Medicine, Shandong University (Jinan, China). In addition, 53 age- and sex-matched HCs were recruited from the community and subjected to the same exclusion criteria as the ALS patients. The flow diagram of the inclusion process is shown in Fig. 1.
Fig. 1.
Flow diagram of the inclusion process. A. Original cohort. B. Replication cohort. Abbreviations: ALS = amyotrophic lateral sclerosis
Clinical assessments
The demographic and clinical information of the ALS patients, including age, sex, education level, family history, comorbid conditions, site of symptom onset, and duration of the disease (time from disease onset to diagnosis), was recorded [35]. The severity of the disease was measured using the Amyotrophic Lateral Sclerosis Functional Rating Scale-Revised (ALSFRS-R) [35]. Clinical staging was evaluated according to King’s clinical staging system. Additionally, levels of depression and anxiety were assessed via the Hamilton Depression Rating Scale (HDRS) and the Hamilton Anxiety Rating Scale (HARS), respectively [35].
Genetic testing
In the present study, similar to our previous study, we screened 32 ALS-related genes in ALS patients using whole exome sequencing (WES) [16]. The genetic testing revealed a total of 3 mutation carriers, and these 3 patients with ALS were excluded from further analysis.
MRI acquisition and CP volume calculation
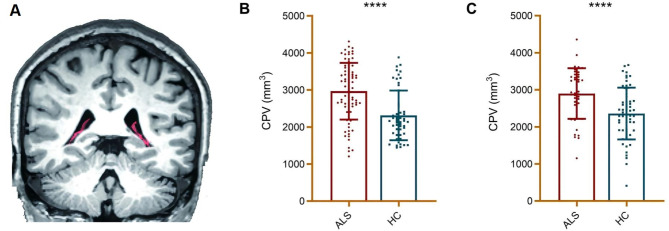
All MRI scans were performed on a 3.0 T magnetic resonance system (Prisma scanner, Siemens Medical Systems) with a 64 head coil, and similar to our previous study, the CP volume of the ALS patients and HCs was subsequently calculated in this study (see Supplemental material) [16]. A visual diagram of the choroid plexus is shown in Fig. 2A, and the specific segmentation process is described in detail in our previous study [16]. Then, we repeated analysis the ventricles volumes to exclude the possibility of CP-volumes measures interference with ventricles size.
Fig. 2.
The CP volume in ALS patients and HCs. A. Visualization of choroid plexus: The red is choroid plexus; B. Compared with HCs, a significantly higher CP volume was found in ALS patients in the original cohort. C. Compared with HCs, the CP volume was significantly higher in sporadic ALS patients in the replication cohort. Abbreviations: ALS = amyotrophic lateral sclerosis; CP = choroid plexus; HC = healthy control; CPV = choroid plexus volume
Serum inflammatory biomarker measurements
In this study, similar to previous studies, 92 serum inflammatory factor levels were assessed using Olink inflammatory panels based on proximity extension technology, and the serum protein levels were reported in NPX values (normalized protein expression levels) which are on a log2 scale [30].
Moreover, three promising inflammatory biomarkers involved in ALS, C-reactive protein (CRP), chitinase 1 (CHIT1), and chitinase-3–like protein 1 (CHI3L1, also known as YKL-40), were not included in the Olink inflammatory panel [13, 32]. Thus, similar to previous studies, serum CRP (Abcam, UK), CHIT1 (Abcam, UK), and YKL40 (R&D Systems, USA) levels were measured based on ELISA approach in this study [13, 32].
To ensure the rigor of data collection, all clinical measurements, neuropsychological assessments, and MRI scans were conducted within 2 days of serum sample collection by venipuncture.
Replication cohorts
In the present study, the relationships between peripheral inflammation and CP volume were verified in a spatially independent ALS cohort. The participants of the replication cohorts were included from April 2023 to April 2024 at Qilu Hospital (Qingdao), Cheeloo College of Medicine, Shandong University (Qingdao, China).
Subjected to the same inclusion and exclusion criteria with the cohort 1 (Jinan cohort), 45 sporadic patients with ALS and 60 HCs, that is, cohort 2 (Qingdao cohort), also underwent clinical assessment, serum biomarker measurement, genetic testing and structural MRI scanning (see Supplemental material) at Qilu Hospital (Qingdao).
Specifically, the serum inflammatory factors of participants in cohort 2 (Qingdao cohort) were measured using the PEA and ELISA techniques in the same batch, together with those in cohort 1 (Jinan cohort). The serum samples were collected following the same procedure in cohort 1 and cohort 2 [13, 32]. Then, the relationships between the serum inflammatory factors and CP volume were further verified in the sporadic ALS patients in cohort 2.
Importantly, the ID numbers of all participants were recorded to avoid duplication in the two cohorts. Moreover, all participants included in this study were not duplicated with the participants in our previous study.
Ethics approval
This study was approved by the Research Ethics Committee of Qilu Hospital, Shandong University. Participant information was collected only after all patients and HCs were made aware of the purpose of the study and provided written informed consent.
Statistical analysis
For the analysis of clinical data, continuous variables are reported as the means and standard deviations, and categorical variables are reported as frequencies and proportions. To compare continuous variables, Student’s t tests or analysis of variance (ANOVA) were utilized, with Mann-Whitney U tests applied when appropriate. Categorical variables were compared using chi-squared tests. Values of p < 0.05 indicated significance. The statistical analyses of the clinical data were conducted using SPSS version 20.0 (IBM Corp., Armonk, NY).
For CP volume, general linear models were used, and age, sex, and total intracranial volume (TIV) were used as covariates. The threshold for statistical significance was set at p < 0.05. Moreover, the significance of the effects of various inflammatory biomarkers on CP volume was predicted using XGBoost. Data processing was performed in R (version 4.4.0). The XGBoost package (version 0.90) was used for prediction. The parameters used in our analysis are as follows: nrounds = 1000, max depth = 5, eta = 0.2, gamma = 0.05, colsample bytree = 0.4, verbose = 1, subsample = 0.7. The relationships between CP volume and the top three serum inflammatory proteins selected by the machine learning algorithm were subsequently assessed using partial correlation analyses in ALS patients, and age, sex, and TIV were used as covariates.
Results
Demographic and clinical information
In this study, age or sex was not significantly different between the ALS patients and HCs in the original cohort. Compared with those in HCs, ECAS scores were significantly lower in ALS patients (p < 0.05). FBI scores were significantly increased in the ALS patients than in the HCs (p < 0.05). Moreover, the HDRS and HARS scores were significantly increased in the ALS patients than in the HCs (p < 0.05). The demographic and clinical data of the ALS patients and HCs in the original cohort is shown in Table 1.
Table 1.
Demographic and clinical information of the ALS patients and HCs in the original cohort
| ALS patients (n = 82) | HCs (n = 53) | P value | |
|---|---|---|---|
| Age (years) | 52.6 ± 10.5 | 52.1 ± 17.1 | 0.84 |
| Men/Women (n) | 53/29 | 33/20 | 0.46 |
| Education | 10.1 ± 3.4 | 9.9 ± 3.7 | 0.74 |
| ALS duration (month) | 20.5 ± 15.2 | - | - |
| ALSFRS-R score | 36.8 ± 7.6 | - | - |
| Bulbar ALS onset n, (%) | 21 (25.6) | ||
| King’s stage (n, 1/2/3/4) | 12/41/29/0 | - | - |
| HARS score | 11.5 ± 6.5 | 5.4 ± 6.8 | <0.01 |
| HDRS score | 7.6 ± 6.9 | 2.6 ± 3.9 | <0.01 |
Abbreviations: ALS = amyotrophic lateral sclerosis; HC = healthy control; ALFRS-R = ALS Functional Rating Scale-Revised; HARS = Hamilton Anxiety Rating Scale; HDRS = Hamilton Depression Rating Scale
Peripheral inflammatory factors in ALS patients
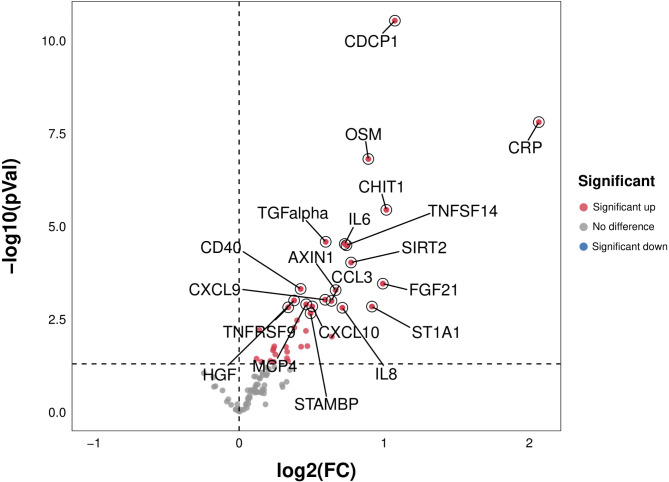
In the present study, in the original cohort, compared with HCs, we found significantly higher serum CRP, CHIT-1, interleukin 6 (IL-6), and other 35 inflammatory factor levels (p < 0.05) in ALS patients. The differences of peripheral inflammatory factors between the ALS patients and HCs in the original cohort are presented in Fig. 3.
Fig. 3.
The difference of peripheral inflammatory factors between ALS patients and HCs. Compared with HCs, serum CRP, CHIIT-1, IL-6, and other 35 inflammatory factor levels were significantly higher in ALS patients. Abbreviations: ALS = amyotrophic lateral sclerosis; CRP = C-reactive protein; IL-6 = interleukin 6; CHIT1 = chitinase 1; HC = healthy control
CP volume in ALS patients
In this study, in the original cohort, the CP volume was significantly increased in the ALS patients than in the HCs (p < 0.001). The degree of CP enlargement in the ALS patients in the original cohort is presented in Fig. 2B.
The contribution of peripheral inflammatory factors to the CP volume in ALS patients
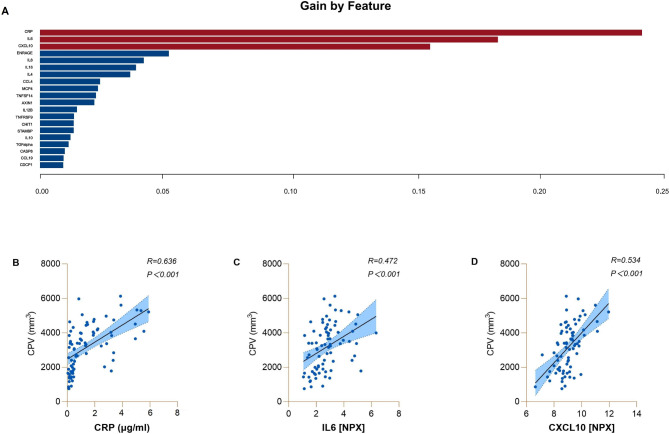
Based on the XGBoost approach, we established a model-based importance of features and sorted the importance of the predictors, and the top three predictors in the original cohort were CRP, IL-6, and C-X-C motif chemokine 10 (CXCL10) (with gains of 0.24, 0.18, and 0.15, respectively). Partial correlation analyses (age, sex, and TIV were used as covariates) revealed that CP volume was significantly associated with CRP (r = 0.636, p < 0.001), IL6 (r = 0.472, p < 0.001), and CXCL10 (r = 0.534, p < 0.001) levels in ALS patients. The relationships between serum inflammatory factors and CP volume in patients with ALS are presented in Fig. 4.
Fig. 4.
The contribution of serum inflammatory factors to CP volume in ALS patients. A. In the original cohort, based on the XGBoost approach, we established a model-based importance of features and sorted the importance of the predictors, and the top three predictors were CRP, IL-6, and CXCL10 in ALS patients. B. The relationships between serum CRP levels and CP volume in ALS patients. C. The relationships between serum IL6 levels and CP volume in ALS patients. D. The relationships between serum CXCL10 levels and CP volume in ALS patients. Abbreviations: ALS = amyotrophic lateral sclerosis; CRP = C-reactive protein; IL-6 = interleukin 6; CXCL10 = C-X-C motif chemokine 10; CPV = choroid plexus volume; NPX = normalized protein expression
Replication cohorts
In the replication cohort, no significant differences were found in age or sex between the ALS patients and HCs. The demographic and clinical data of the ALS patients and HCs in the replication cohort is shown in Table 2.
Table 2.
Demographic and clinical features of the ALS patients and HCs in the replication cohort
| ALS patients (n = 45) |
HCs (n = 60) |
P value | |
|---|---|---|---|
| Age (years) | 56.0 ± 7.5 | 57.6 ± 7.3 | 0.26 |
| Men/Women (n) | 23/22 | 29/31 | 0.46 |
| Education | 9.8 ± 3.2 | 10.1 ± 3.6 | 0.66 |
| ALS duration (month) | 12.0 ± 7.4 | - | - |
| Bulbar ALS onset n, (%) | 11 (24.4) | - | - |
| ALSFRS-R scores | 41.1 ± 3.4 | - | - |
| King’s stage (n, 1/2/3/4) | 16/21/8/0 | ||
| HARS scores | 7.7 ± 4.6 | 2.9 ± 3.1 | <0.01 |
| HDRS scores | 10.5 ± 4.5 | 2.1 ± 2.7 | <0.01 |
Abbreviations: ALS = amyotrophic lateral sclerosis; HC = healthy control; ALFRS-R = ALS Functional Rating Scale-Revised; HARS = Hamilton Anxiety Rating Scale; HDRS = Hamilton Depression Rating Scale
In the present study, in the replication cohort, the CP volume was significantly higher in sporadic ALS patients than in HCs (p < 0.001). The CP enlargement in the ALS patients in the replication cohort is presented in Fig. 2C.
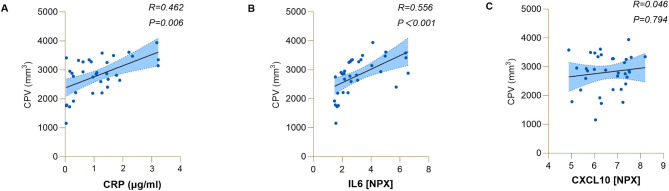
In the replication cohort, partial correlation analyses (age, sex, and TIV were used as covariates) revealed that CP volume was significantly associated with CRP (r = 0.462, p = 0.006) and IL-6 (r = 0.556, p < 0.001) in ALS patients (Fig. 5).
Fig. 5.
The relationships between serum inflammatory factors and CP volume in ALS patients in the replication cohort. A. The relationships between serum CRP levels and CP volume in ALS patients. B. The relationships between serum IL6 levels and CP volume in ALS patients. C. The relationships between serum CXCL10 levels and CP volume in ALS patients. Abbreviations: ALS = amyotrophic lateral sclerosis; CRP = C-reactive protein; IL-6 = interleukin 6; CXCL10 = C-X-C motif chemokine 10; CPV = choroid plexus volume; NPX = normalized protein expression
Discussion
To the best of our knowledge, this is the first study demonstrating a consistent and replicable contribution of peripheral inflammation to CP enlargement in vivo in sporadic ALS patients. Given that CP enlargement has recently been observed in other brain diseases, these findings may not be specific to ALS and might extend to other conditions with a peripheral inflammatory component.
In the present study, the first finding was that the CP volume is enlarged in independent ALS cohorts. This result was in line with our previous findings and with those of animal and neuropathological studies of ALS [16, 24–26]. Thus, these findings may provide firm evidence and suggest that in vivo CP enlargement is likely a consistent feature of sporadic patients with ALS [24–26].
Then, using the XGBoost approach, we found that three peripheral inflammatory factors, namely, serum CRP, IL-6, and CXCL10, may contribute to CP enlargement in sporadic ALS patients, which can account for 57% of the variance. Importantly, the CP volume was significantly associated with the serum CRP, IL6, and CXCL10 levels in sporadic ALS patients in the original cohort, and the CP volume was significantly related to the serum CRP and IL-6 levels in the replication cohort. Serum CRP and IL-6 are considered reliable markers of peripheral low-grade systemic inflammatory processes [36–38]. Recently, in a systematic review, Kharel et al. included a total of 2785 patients with ALS and 3446 HCs and confirmed that, compared with those in HCs, CRP levels were significantly increased in ALS patients and that serum CRP levels were clearly correlated with motor disability and disease progression [37]. In a longitudinal study, Lu et al. demonstrated that, compared with those in HCs, blood IL-6 levels were significantly increased in a large group of ALS patients [38]. Moreover, ALS patients with high serum CRP levels seem to be more responsive to anti-inflammatory drugs, such as NP001 and tocilizumab [13, 38]. Thus, these findings suggest that serum CRP and IL-6 may play important roles in ALS [36–38]. However, the pathophysiological pathways by which elevated CRP and IL-6 levels are involved in the neurodegenerative process in ALS patients are not fully understood [36–38].
As mentioned above, the CP is an important part of the glymphatic system and is crucial for CNS-periphery inflammation crosstalk [14–20]. Importantly, using structural MRI approaches, some previous studies reported that increased peripheral inflammatory factors, including CRP and IL-6, may contribute to CP enlargement in other brain diseases [21–23]. For example, Cao et al. recently demonstrated that greater CP volume was significantly correlated with greater low-grade inflammation in patients with bipolar II depression [39]. However, to our knowledge, no previous study has systematically assessed the relationships between peripheral inflammation and CP enlargement in vivo in ALS patients [16].
In this study, we first found a replicable relationship of serum CRP and IL-6 with CP volume in vivo in sporadic patients with ALS. Specifically, in a recent neuropathological investigation utilizing transcriptomic and ultrastructural analysis of BCSFB and CP changes in postmortem human tissues from ALS patients and nonneurologic disease controls, Saul et al. reported that immune activation can be detected in ALS patients’ CP, and a subset of ALS patients’ CP samples showed increased CRP levels compared with those of controls [24]. Moreover, Garbuzova-Davis suggested that inflammation mediated by systemic IL-6 might serve as an early external factor leading to endothelial cell death, which may also cause blood CNS barrier damage in ALS patients [40]. Thus, these studies might provide complementary evidence indicating that the CP may play a crucial role in the inflammatory process associated with ALS and that systemic inflammation may be one of the most potential contributors to CP abnormalities in ALS patients [22, 24, 40]. In addition, Rau et al. showed that subacute infection of coronavirus disease 2019 (COVID-19) was closely related to the increase of CP volume, and the increase of CP volume was closely related to inflammatory reactions such as IL-6 and peak counts of leukocytes [41]. In our study, all ALS patients and HCs did not have acute or subacute infection with COVID-19 to exclude this potential confounding factor.
Importantly, given that CP enlargement has recently been detected in other brain and inflammatory diseases, these results may not be specific to ALS and might extend to other conditions with a peripheral inflammatory component [14–18]. For example, Bonifacio et al. recently reported that serum levels of CRP were significantly correlated with CP volume and permeability in patients with Crohn’s disease and suggested that intestinal inflammation could affect the brain through the modulation of the CP vascular barrier [40]. Moreover, CXCL10 is a chemokine that responds to proinflammatory conditions. In this study, we found that the serum CXCL10 concentration was significantly related to CP volume in sporadic ALS patients in the original cohort. Unfortunately, this finding was not observed in the replication cohort, which may be partly due to the disease course of the patients in the replication cohort was earlier than that in the original cohort. However, to date, very few studies have explored the pathophysiological mechanisms of CXCL10 in ALS patients, and further studies should be conducted to confirm our viewpoints. Recently, growing evidence has suggested that elevated levels of CXCL10 are likely involved in the neurodegenerative process of other neurodegenerative diseases, including AD and MS [42]. Thus, further studies should be conducted to identify the potential relationships between CXCL10 and CP volume in these conditions in the future.
To date, there has been few research exploring the potential contributors to CP abnormalities in vivo in ALS and other disease conditions [7, 14–20]. Recently, Steinruecke et al. suggested that mitochondrial abnormalities, astrocyte damage and inflammation could play a role in BCNSB alterations associated with ALS [7]. Therefore, we propose that, beside inflammation, additional elements, such as mitochondrial dysfunction and astrocyte damage, might also contribute to CP abnormalities in ALS and other brain diseases [7]. However, as we mentioned above, it is essential to recognize that our understanding of the causal relationships between CP abnormalities and these mechanisms in ALS is currently scarce; further verification of these topics in the future is likely critical for advancing our understanding of the pathogenesis of ALS and other neurodegenerative diseases [7, 14–20].
The current study had some limitations. First, although our study was conducted in two independent cohorts, the present study remained a single centre study [16]. Thus, further population-based or multicenter studies are still needed to confirm our findings. Second, the cohorts were exclusively composed of individuals from the Chinese population; future studies should identify whether CP abnormalities may emerge in Caucasian and other racial groups of ALS patients.
Conclusion
Our study revealed a consistent and replicable contribution of peripheral inflammation to CP enlargement in vivo in sporadic ALS patients. We suggest that the CP is likely involved in the inflammatory process of ALS and that systemic inflammation is likely a contributor to CP abnormalities in ALS patients. Moreover, assessing CP volume is likely a noninvasive neuroimaging marker for monitoring anti-inflammatory therapeutic effects in ALS patients. Given that CP enlargement has been recently detected in other brain diseases, these results may not be specific to ALS and might extend to other conditions with a peripheral inflammatory component. Future studies are needed to confirm our findings.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We thank the ALS patients and healthy controls for participating in this study.
Abbreviations
- ALS
Amyotrophic lateral sclerosis
- BCSFB
Blood-CSF barrier
- CRP
C-reactive protein
- CP
Choroid plexus
- SOD1
Superoxide dismutase 1
- FUS
Fused in sarcoma
Author contributions
SWL: study concept, data acquisition, interpretation of the results, writing the first version of the manuscript. SJS: study concept, ALS diagnosis, FTD diagnosis, interpretation of the results, writing the final version of the manuscript. YJC: study concept, interpretation of the results, revising the manuscript. PFL: study concept, interpretation of the results, revising the manuscript. XSM and QGR: Image preprocessing, revising the manuscript. XHS, YY and BZ: data acquisition, revising the manuscript. CZY: ALS diagnosis, FTD diagnosis, revising the manuscript.
Funding
This work was supported by the Taishan Young Scholar Program (No. qnts202306347) and the Shandong Provincial Natural Science Foundation (No. ZR2024MH178).
Data availability
The anonymized data presented in this article are available at the request of a qualified investigator, after review by the corresponding author. Final approval will be granted by the Research Ethics Committee of the School of Medicine, Shandong University.
Declarations
Ethics approval and consent to participate
This study was approved by the Research Ethics Committee of the School of Medicine, Shandong University. Participant information was collected only after all patients and HCs had been made aware of the purpose of the study and provided written informed consent.
Consent for publication
All authors gave their consent for publication.
Competing interests
The authors declare no competing interests.
Publication history
None.
Statistical analysis
Statistical analysis was conducted by Dr. Xiaohan Sun, PhD, Department of Neurology, Qilu Hospital of Shandong University, Jinan, China
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Sujuan Sun, Yujing Chen, Pengfei Lin and Shuangwu Liu contributed equally to this work.
Contributor Information
Pengfei Lin, Email: lpfsdu@foxmail.com.
Shuangwu Liu, Email: whuliushuangwu@163.com.
References
- 1.Taylor JP, Brown RH Jr, Cleveland DW. Decoding ALS: from genes to mechanism. Nat 2016 Nov 10,539(7628):197–206. [DOI] [PMC free article] [PubMed]
- 2.Eisen A, Braak H, Del Tredici K et al. Cortical influences drive amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry 2017 Nov,88(11):917–24. [DOI] [PubMed]
- 3.Al-Chalabi A, Calvo A, Chio A, et al. Analysis of amyotrophic lateral sclerosis as a multistep process: a population-based modelling study. Lancet Neurol. 2014;13(11):1108–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Braak H, Brettschneider J, Ludolph AC, et al. Amyotrophic lateral sclerosis–a model of corticofugal axonal spread. Nat Rev Neurol. 2013 Dec;9(12):708–14. [DOI] [PMC free article] [PubMed]
- 5.Benatar M, Wuu J, McHutchison C, et al. Preventing amyotrophic lateral sclerosis: insights from pre-symptomatic neurodegenerative diseases. Brain. 2022;145(1):27–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Salmon K, Kiernan MC, Kim SH, et al. The importance of offering early genetic testing in everyone with amyotrophic lateral sclerosis. Brain. 2022;145(4):1207–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Steinruecke M, Lonergan RM, Selvaraj BT, Chandran S, Diaz-Castro B, Stavrou M. Blood-CNS barrier dysfunction in amyotrophic lateral sclerosis: proposed mechanisms and clinical implications. J Cereb Blood Flow Metab. 2023;43(5):642–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McCauley ME, Baloh RH. Inflammation in ALS/FTD pathogenesis. Acta Neuropathol. 2019;137(5):715–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Appel SH, Beers DR, Zhao W. Amyotrophic lateral sclerosis is a systemic disease: peripheral contributions to inflammation-mediated neurodegeneration. Curr Opin Neurol. 2021;34(5):765–72. [DOI] [PubMed] [Google Scholar]
- 10.Batty GD, Kivimäki M, Frank P, Gale CR, Wright L. Systemic inflammation and subsequent risk of amyotrophic lateral sclerosis: prospective cohort study. Brain Behav Immun. 2023;114:46–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xiao C, Gu X, Feng Y, Shen J. Two-sample mendelian randomization analysis of 91 circulating inflammatory protein levels and amyotrophic lateral sclerosis. Front Aging Neurosci. 2024;16:1367106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Staats KA, Borchelt DR, Tansey MG, Wymer J. Blood-based biomarkers of inflammation in amyotrophic lateral sclerosis. Mol Neurodegener. 2022;17(1):11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bowser R, An J, Mehta L, Chen J, Timmons J, Cudkowicz M, Paganoni S. Effect of sodium phenylbutyrate and taurursodiol on plasma concentrations of neuroinflammatory biomarkers in amyotrophic lateral sclerosis: results from the CENTAUR trial. J Neurol Neurosurg Psychiatry. 2023 Dec;2:jnnp–2023. [DOI] [PMC free article] [PubMed]
- 14.Choi JD, Moon Y, Kim HJ, Yim Y, Lee S, Moon WJ. Choroid Plexus volume and permeability at Brain MRI within the Alzheimer Disease Clinical Spectrum. Radiology. 2022;304(3):635–45. [DOI] [PubMed] [Google Scholar]
- 15.Tadayon E, Pascual-Leone A, Press D, Santarnecchi E. Alzheimer’s Disease Neuroimaging Initiative. Choroid plexus volume is associated with levels of CSF proteins: relevance for Alzheimer’s and Parkinson’s disease. Neurobiol Aging. 2020;89:108–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dai T, Lou J, Kong D, et al. Choroid plexus enlargement in amyotrophic lateral sclerosis patients and its correlation with clinical disability and blood-CSF barrier permeability. Fluids Barriers CNS. 2024;21(1):36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Margoni M, Gueye M, Meani A, et al. Choroid plexus enlargement in paediatric multiple sclerosis: clinical relevance and effect of sex. J Neurol Neurosurg Psychiatry. 2023;94(3):181–8. [DOI] [PubMed] [Google Scholar]
- 18.Solár P, Zamani A, Kubíčková L, Dubový P, Joukal M. Choroid plexus and the blood-cerebrospinal fluid barrier in disease. Fluids Barriers CNS. 2020;17(1):35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lun MP, Monuki ES, Lehtinen MK. Development and functions of the choroid plexus-cerebrospinal fluid system. Nat Rev Neurosci. 2015;16(8):445–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Municio C, Carrero L, Antequera D, Carro E. Choroid Plexus aquaporins in CSF Homeostasis and the Glymphatic System: their relevance for Alzheimer’s Disease. Int J Mol Sci. 2023;24(1):878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fleischer V, Gonzalez-Escamilla G, Ciolac D, et al. Translational value of choroid plexus imaging for tracking neuroinflammation in mice and humans. Proc Natl Acad Sci U S A. 2021;118(36):e2025000118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lizano P, Lutz O, Ling G, et al. Association of Choroid Plexus Enlargement with Cognitive, Inflammatory, and structural phenotypes across the psychosis spectrum. Am J Psychiatry. 2019;176(7):564–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carloni S, Bertocchi A, Mancinelli S, et al. Identification of a choroid plexus vascular barrier closing during intestinal inflammation. Science. 2021;374(6566):439–48. [DOI] [PubMed] [Google Scholar]
- 24.Saul J, Hutchins E, Reiman R, et al. Global alterations to the choroid plexus blood-CSF barrier in amyotrophic lateral sclerosis. Acta Neuropathol Commun. 2020;8(1):92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kunis G, Baruch K, Miller O, Schwartz M. Immunization with a myelin-derived Antigen activates the Brain’s Choroid Plexus for Recruitment of Immunoregulatory Cells to the CNS and attenuates Disease Progression in a mouse model of ALS. J Neurosci. 2015;35(16):6381–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smith R, Myers K, Ravits J, Bowser R. Amyotrophic lateral sclerosis: is the spinal fluid pathway involved in seeding and spread? Med Hypotheses. 2015;85(5):576–83. [DOI] [PubMed] [Google Scholar]
- 27.Hu Y, Cao C, Qin XY, Yu Y, Yuan J, Zhao Y, Cheng Y. Increased peripheral blood inflammatory cytokine levels in amyotrophic lateral sclerosis: a meta-analysis study. Sci Rep. 2017;7(1):9094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jiang Y, Zhou X, Ip FC, et al. Large-scale plasma proteomic profiling identifies a high-performance biomarker panel for Alzheimer’s disease screening and staging. Alzheimers Dement. 2022;18(1):88–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang J, Khademi M, Fugger L, et al. Inflammation-related plasma and CSF biomarkers for multiple sclerosis. Proc Natl Acad Sci U S A. 2020;117(23):12952–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pedersen CC, Ushakova A, Skogseth RE, et al. Inflammatory biomarkers in newly diagnosed patients with Parkinson Disease and related neurodegenerative disorders. Neurol Neuroimmunol Neuroinflamm. 2023;10(4):e200132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xue T, Liu W, Wang L, et al. Extracellular vesicle biomarkers for complement dysfunction in schizophrenia. Brain. 2024;147(3):1075–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thompson AG, Gray E, Bampton A, Raciborska D, Talbot K, Turner MR. CSF chitinase proteins in amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry. 2019;90(11):1215–20. [DOI] [PubMed] [Google Scholar]
- 33.de Carvalho M, Dengler R, Eisen A, et al. Electrodiagnostic criteria for diagnosis of ALS. Clin Neurophysiol. 2008;119(3):497–503. [DOI] [PubMed] [Google Scholar]
- 34.Rascovsky K, Hodges JR, Knopman D, et al. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain. 2011;134(Pt 9):2456–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu S, Sun X, Ren Q et al. Glymphatic dysfunction in patients with early-stage amyotrophic lateral sclerosis. Brain 2023 Aug 16:awad274. [DOI] [PubMed]
- 36.Lunetta C, Lizio A, Maestri E, et al. Serum C-Reactive protein as a prognostic biomarker in amyotrophic lateral sclerosis. JAMA Neurol. 2017;74(6):660–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kharel S, Ojha R, Preethish-Kumar V, Bhagat R. C-reactive protein levels in patients with amyotrophic lateral sclerosis: a systematic review. Brain Behav. 2022;12(3):e2532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lu CH, Allen K, Oei F, et al. Systemic inflammatory response and neuromuscular involvement in amyotrophic lateral sclerosis. Neurol Neuroimmunol Neuroinflamm. 2016;3(4):e244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cao Y, Lizano P, Deng G, et al. Brain-derived subgroups of bipolar II depression associate with inflammation and choroid plexus morphology. Psychiatry Clin Neurosci. 2023;77(11):613–21. [DOI] [PubMed] [Google Scholar]
- 40.Garbuzova-Davis S, Ehrhart J, Sanberg PR, Borlongan CV. Potential role of Humoral IL-6 cytokine in mediating pro-inflammatory endothelial cell response in amyotrophic lateral sclerosis. Int J Mol Sci. 2018;19(2):423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rau A, Gonzalez-Escamilla G, Schroeter N et al. Inflammation-Triggered Enlargement of Choroid Plexus in Subacute COVID-19 Patients with Neurological Symptoms. Ann Neurol. 2024 Jun 27. [DOI] [PubMed]
- 42.Bonifacio C, Savini G, Reca C, et al. The gut-brain axis: correlation of choroid plexus volume and permeability with inflammatory biomarkers in Crohn’s disease. Neurobiol Dis. 2024;192:106416. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The anonymized data presented in this article are available at the request of a qualified investigator, after review by the corresponding author. Final approval will be granted by the Research Ethics Committee of the School of Medicine, Shandong University.