Abstract
Bladder tumors have a high mutational burden and tend to be responsive to immune therapies; however, response rates remain modest. To date, immunotherapy in bladder cancer has largely focused on enhancing T-cell immune responses in the bladder tumor microenvironment. It is anticipated that other immune cells, including innate lymphoid cells (ILC), which play an important role in bladder oncogenesis and tumor suppression, could be targeted to improve response to existing therapies. ILCs are classified into five groups: natural killer cells, ILC1s, ILC2s, ILC3s, and lymphoid tissue inducer cells. ILCs are pleiotropic and play dual and sometimes paradoxical roles in cancer development and progression. Here, a comprehensive discussion of the current knowledge and recent advancements in understanding the role of ILCs in bladder cancer is provided. We discuss the multifaceted roles that ILCs play in bladder immune surveillance, tumor protection, and immunopathology of bladder cancer. This review provides a rationale for targeting ILCs in bladder cancer, which is relevant for other solid tumors.
Background
Bladder tumors carry one of the highest known cancer mutational loads, which correlates positively with response to immunotherapy (1). Thus, the rationale for immunotherapy to treat bladder cancer is strong (1). Bacillus Calmette–Guérin (BCG) immune therapy remains the gold standard treatment for non–muscle-invasive bladder cancer (NMIBC; ref. 2), and over the last decade, immune checkpoint therapies have revolutionized the treatment of patients with more advanced bladder cancer. Several monoclonal antibodies targeting the programmed cell death ligand 1/programmed cell death protein 1 (PD-L1/PD-1) checkpoint axis (3) are now FDA-approved for bladder cancer (4). Despite the success of these immunotherapies, not all patients with bladder cancer respond to these immune therapies (5), highlighting the urgent need for novel next-generation immune therapeutic strategies. Even though T cells are key players in the regulation of immune responses in bladder cancer (6, 7), they do not function autonomously. From their activation to eliciting antitumor functions, T cells rely on the functions of different innate cells (8, 9); elucidation of the contributions of innate immune cells towards bladder cancer immunopathology is predicted to lead to novel therapeutic targets. One promising group of candidates is the innate lymphoid cell (ILC) family which has garnered attention in bladder cancer due to their critical functions in bladder cancer immune responses. Here, we review the pleiotropic functions of these ILCs in bladder cancer and discuss whether modifying and targeting these ILCs can unleash new possibilities for the future of bladder cancer immunotherapy.
Different subsets of ILCs
ILCs form a distinct branch of the innate immune system. Unlike T cells, their activation is not dependent on antigen presentation, but they express similar transcription factors and cytokines as their T-cell counterparts (10, 11). ILCs have developed in parallel with the adaptive immune system, working synergistically to maintain homeostasis and protect against infections and cancer development (11). Natural killer (NK) cells were the first identified members of the ILC family, initially discovered in the 1970s as non-T and non-B lymphoid cells, which directly kill target cells without prior immunization (12). Next came the identification of lymphoid tissue inducer (LTi) cells, which are critical for lymphoid tissue organogenesis during the embryonic stages (12, 13). However, it was not until the late 2000s that these cells were officially recognized and designated as a distinct cell population and these different but related cell types were grouped under a common umbrella, the ILC family (12). Currently, ILCs are divided into five major subsets based on their transcriptional regulation, adaptive immune counterparts, and secretory profiles (Fig. 1; Table 1). These subsets include NK cells, ILC1s, ILC2s, ILC3s, and LTi cells (14). Previously, NK cells and ILC1s were grouped as group 1 ILCs based on the expression of T-box transcription factor T-bet (Tbet), which drives the secretion of IFNγ—a hallmark of their T helper-1 (Th1)-like immune response (11, 12, 15). NK cells, however, also require the transcription factor Eomesodermin (Eomes) and secrete cytotoxic effector molecules such as perforin and granzyme (11, 12, 15). ILC2s are regulated by the transcription factor GATA-binding protein 3 (GATA3) and activated ILC2s secrete IL4, IL5, IL9, and IL13 (11, 12). Finally, ILC3s and LTi cells, are regulated by the transcription factor retinoic acid receptor-related orphan receptor (ROR)γt and produce IL22 and IL17 upon activation (refs. 10, 13; Box 1).
Figure 1.
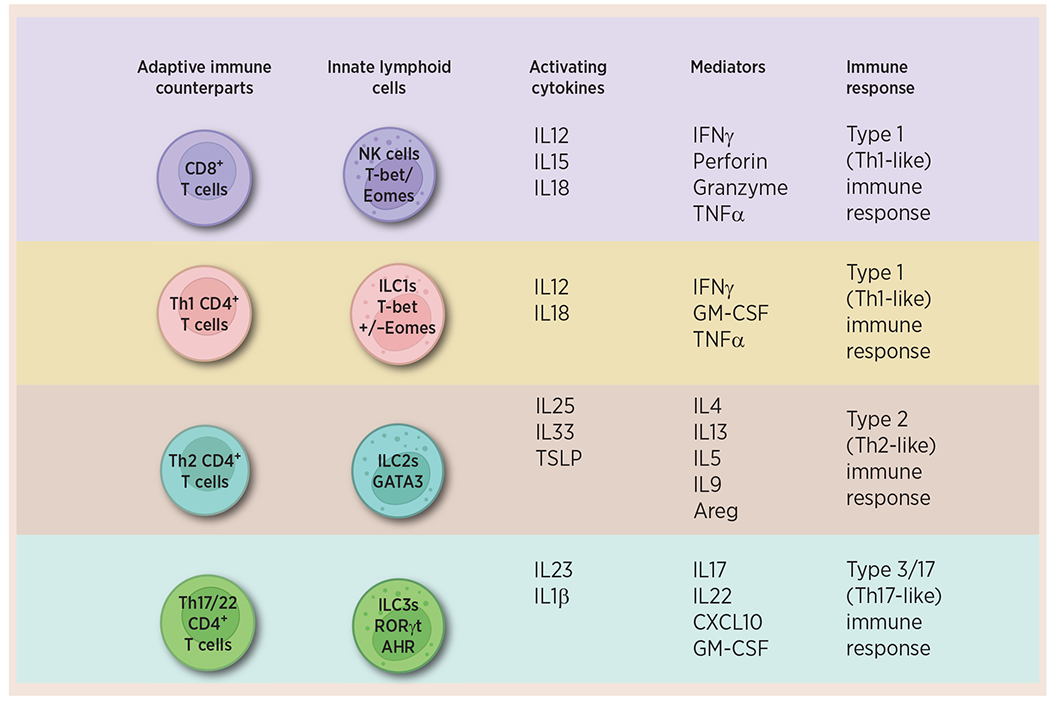
ILCs are the innate immune counterparts of the adaptive immune cells. The ILC family is classified into five groups, namely NK cells, ILC1s, ILC2s, ILC3s, and LTi cells, based on their unique developmental paths and functions. These subsets are primarily activated by different cytokines and produce distinct effector cytokines, thereby contributing to specific immune responses. Created with BioRender.com
Table 1.
Phenotypes and functions of ILCs in bladder cancer.
| ILC subset | Cytokines produced | Activating cytokines | Transcription factors | Role in bladder cancer |
|---|---|---|---|---|
| NK cells | IFNγ, perforin, granzyme B, TNFα | IL12, IL15, IL18 | T-bet, Eomesodermin (Eomes) | Dysfunctional NK cells with low expression of CD56 and activation markers accumulate in high-stage bladder tumors, suggesting the loss of NK cell-mediated protective functions. |
| ILC1 | IFNγ | IL12, IL15 | T-bet | ILC1s exhibit an exhausted phenotype, characterized by decreased cytotoxicity and increased exhaustion markers. |
| ILC2 | IL4, IL5, IL9, IL13 | IL25, IL33, TSLP | GATA-3, RORα | ILC2 secrete IL13 in the TME favoring the polarization of M2 macrophages and M-MDSCs. |
| ILC3 | CXCL10, IL22, IL17, GM-CSF | IL23, IL1β | RORγt, T-bet, AHR | ILC3s coexpress CD103 and CD69, markers of tissue-resident innate memory cells. ILC3s share significant phenotypic features with ILC1s suggesting potential plasticity and overlapping functions of these ILCs in bladder tumors. |
Abbreviations: T-bet, T-box transcription factor TBX21; GATA3, GATA binding protein 3; TSLP, Thymic stromal lymphopoietin; Areg, Amphiregulin; CXCL10, C-X-C motif chemokine ligand 10.
BOX 1: Key Concepts—ILCs.
ILCs have been often referred to as the innate counterparts of the T cells based on common progenitors, regulation by specific transcription factors, and secretion of cytokines.
ILCs are a highly diverse family of cells and adapt to a varied array of environmental cues in different microenvironments by their plasticity.
ILCs are involved in eliciting immune responses to viruses, bacteria, helminths, and cancer cells. They display pleiotropic and paradoxical functions including tissue repair, allergen-induced immunopathology, and oncogenesis.
ILCs are divided into five groups: NK cells, ILC1s, ILC2s, ILC3s, and LTi cells. NK cells and ILC1s are activated by IL12, IL15, and IL18 and secrete granzymes, perforin, IFNγ, and TNFα. Both NK cells and ILC1s require expression of transcription factor T-bet, while NK cells have an additional requirement for transcription factor Eomesodermin. ILC2s are dependent on transcription factors GATA3 and RORα; when activated by IL25, and IL33, these cells secrete IL4, IL15, IL9, and IL13. ILC3s and LTis are regulated by transcription factor RORγt, activated by IL1β and IL23, and can produce IL22 and IL17.
Among the ILCs, NK cells were identified first and remain the most researched subtype in cancer for their antitumor functions. Other ILC subtypes were initially thought to be mostly protumorigenic. However, recent studies have shown that the function of non-NK ILCs in cancer is more complicated and critical than originally assumed. As such, there is now potential to unveil novel immunotherapeutic agents by targeting this unique group of cells.
Origin of ILCs
The majority of the earlier studies investigating the development of ILCs have primarily relied on adult mouse bone marrow–derived hematopoietic stem cells or adult human bone marrow/blood progenitors as experimental models (16). However, specific ILC subsets, such as ILC3s and LTi cells, are already in the embryo prior to hematopoiesis (16–18). ILC precursors have been found early in development in the mouse fetal liver (19). Further, single-cell RNA sequencing (scRNA-seq) of human embryonic tissue has offered important insights into the temporal expression of ILC precursors; these studies have revealed that ILC precursors originate from various sources, including the fetal liver, thymus, and intestines (20, 21). The precise differentiation pathway of these embryonic ILCs, whether they arise from distinct progenitors separate from those in the bone marrow, remains uncertain. Nevertheless, it is commonly accepted that the final stages of development take place in the resident tissues (16, 19).
Through lineage-tracing studies, all ILCs are thought to develop from the lymphoid-primed multipotent progenitor (LMPP), which further differentiates into an IL7 receptor α–negative (IL7Rα−) common lymphoid progenitor (CLP; refs. 19, 22, 23; Fig. 2). From there, two distinct populations are derived: an early innate lymphoid progenitor (EILP), which is IL7Rα− and lacks or expresses low levels of inhibitor of DNA binding 2 (Id2); and IL7Rα+Id2+ α–lymphoid precursor (αLP; refs. 19, 22). Collectively, these cells are known as global innate lymphoid progenitors (19, 22), and they are further differentiated into an Id2+ common helper innate lymphoid progenitor (CHILP; refs. 19, 24). These progenitors are further subdivided into cells that lack promyelocytic leukemia zinc finger protein (PLZF), which differentiate into LTi and PLZF+PD-1+ expressing cells, which can differentiate into ILC1, 2, and 3 (19, 22). Of note, CHILPs have been traditionally thought to lack the ability to differentiate into NK cells, which have been shown to develop from early progenitors such as EILP (24). However, recent developmental studies in mice have demonstrated innate lymphoid common progenitors (ILCP), derived from CHILP, can also give rise to NK cells (22, 24). This specific population of progenitor cells harbors expression signatures of ILC1, ILC3, and NK cells (22).
Figure 2.
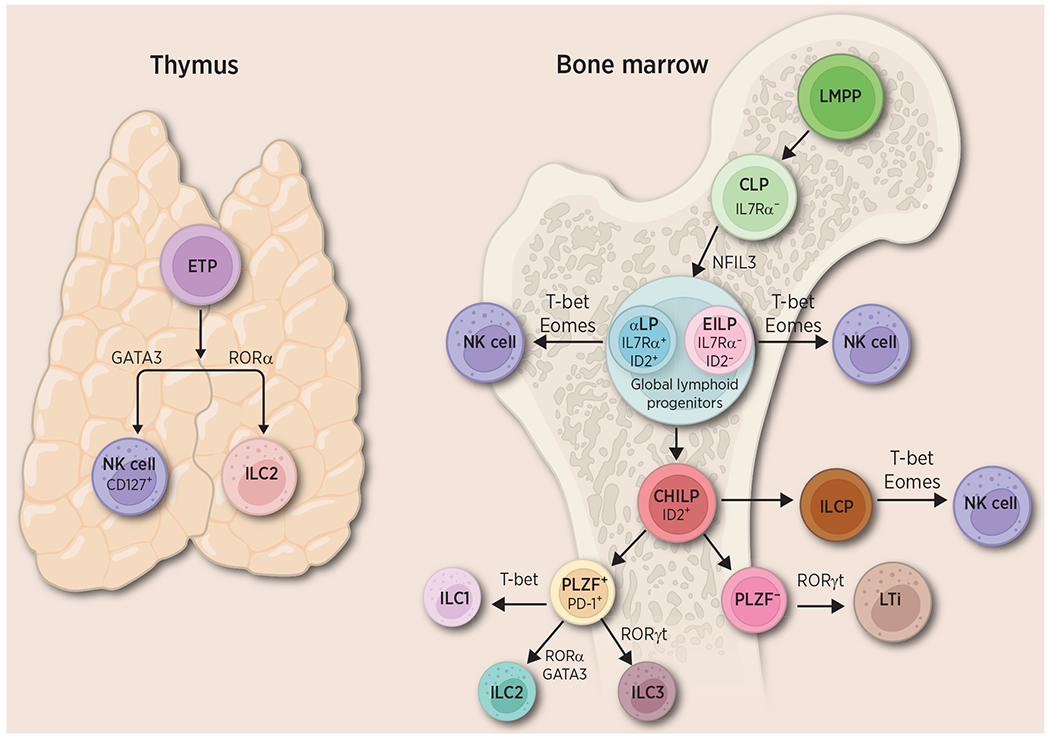
An overview of ILC development. ILCs in the bone marrow originate from the LMPP, which then differentiates into the CLP. From there, two distinct populations are derived: an EILP and a lymphoid progenitor (aLP), collectively known as global lymphoid progenitors. They are further differentiated into CHILP. These progenitors are further subdivided into cells that lack the PLZF−, which differentiate into LTi cells and PLZF+, PD-1+ cells, which differentiate into ILC1, 2 and 3. NK cells can differentiate from EILP and aLP directly, and from CHILP via ILCP. ETP in the thymus can give rise to ILC2 and NK cells, driven by RORα and GATA3, respectively. Created with BioRender.com. CHILP, common helper innate lymphoid progenitor; EILP, early innate lymphoid progenitor; EPT, early T-cell precursor; ILCP, innate lymphoid common progenitors.
Moreover, investigations into immune cell development in the thymus have identified a distinctive precursor that can give rise to certain ILCs (17, 25). In the thymus, ILC2s have been shown to develop alongside T cells from the early T-cell precursor (ETP), where expression of RORα mediates ILC2-favored differentiation while repressing differentiation of T cells (17). A unique subpopulation of NK cells is also derived from thymic ETP and is characterized by their cell-surface expression of IL7Rα (CD127), which is not expressed by conventional NK cells (25). Development of thymic NK cells requires GATA3, which is responsible for CD127 expression, another unique characteristic of thymic NK cells (25). The diversity within specific ILC subsets and their potential for plasticity may be largely dependent on the specific progenitor they originate from and the local tissue microenvironment they inhabit. While the progenitors and differentiation pattern of ILCs continue to be researched, the overlap in ILC development and maturation is possibly what allows for the plasticity and heterogenous nature of ILCs in the bladder and throughout various tissues in the body.
ILCs in bladder cancer
ILCs were first identified in blood and lymphoid tissues (20, 26). It is now evident, however, that they play important roles in cancer and that their function throughout the body is largely tissue-dependent (27). Recently, several studies investigating the role of ILCs in the bladder, have identified both tumor-promoting and tumor-suppressive effects of different ILC subsets in bladder cancer (Fig. 3; Box 2).
Figure 3.
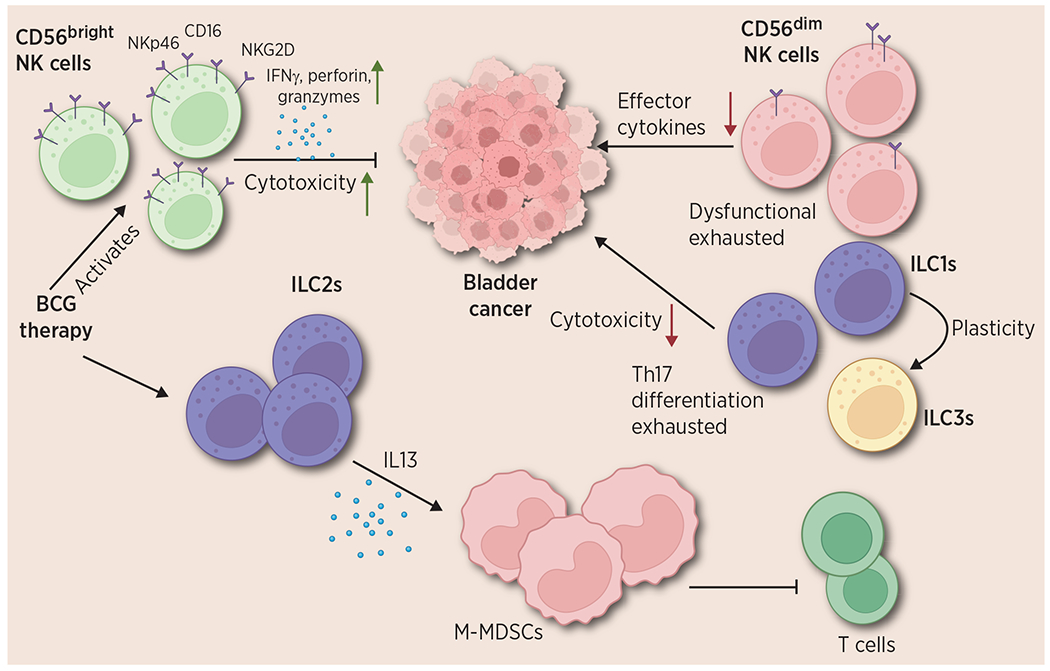
Functions of ILCs in bladder cancer. The different roles of NK cells, ILC1s, ILC2s, and ILC3s are depicted. Bladder intratumoral CD56bright NK cells show an activated phenotype with higher expression of NKp46, NKG2D, and other activation markers; they elicit antitumor functions via secretion of IFNγ, granzymes, and perforin. However, CD56dim NK cells in the tumor are dysfunctional and display an exhausted phenotype and their antitumor abilities are significantly decreased. ILC1s, also show lowered cytotoxicity and Th17 differentiation in bladder cancer. They display plasticity with ILC3s. ILC2s show a protumorigenic function in bladder cancer via IL13 mediated M-MDSCs differentiation which suppresses T-cell functions. Created with BioRender.com.
BOX 2: Major Highlights—ILCs in Bladder Cancer.
NK cells are one of the predominant tumor infiltrating lymphocytes ILCs in bladder tumors. However, NK cells derived from bladder cancer patients display an exhausted phenotype and expression of exhaustion molecules such as Tim3.
CD56dim subset of NK cells are dysfunctional in bladder tumors whereas the CD56bright NK cells are functional and have been correlated with a better prognosis in bladder cancer.
CD56bright NK cells are characterized by their higher cytotoxicity and higher production of effector cytokines compared with their CD56dim counterparts in bladder tumors. CD56bright subset is specifically stimulated by BCG treatment.
ILC1s are increased in high stage bladder tumors. Bladder intratumoral ILC1s display higher expression of exhaustion signals and Th17-like differentiation. ILC1 to ILC3 plasticity is observed in the bladder TME where the ILC1s showed reduced cytolytic activity.
Recruitment of ILC2s to the bladder tumors induces IL13 mediated differentiation of CD14+ monocytes to monocytic myeloid-derived suppressor cells (MDSC) and is associated with a skewing toward type 2 immunity and BCG failure.
Mechanisms of direct and indirect cytotoxicity by NK cells and ILC1s
NK cells and ILC1s mediate cytotoxicity through indirect and direct mechanisms by the secretion of IFNγ, TNFα, perforin, and granzymes (11, 12, 15), and they are primarily stimulated by IL12 and IL18 (11, 12, 28). NK cells recognize target cells through a complex combination of activation and inhibitory receptors (29). For example, during immune surveillance, killer immunoglobulin-like receptors (KIR) on NK cells bind to HLA class I molecules expressed by most healthy normal cells (30). This delivers an inhibitory signal to NK cells, preventing the cascade of NK cell–mediated cytotoxicity (self-tolerance) that would otherwise lead to autoimmunity (30). However, when a cell is stressed (i.e., infected or transformed), it loses its HLA class I expression (‘missing-self hypothesis’) and also expresses other NK-cell activation ligands, which bind to activation receptors, including natural cytotoxicity receptors (NCRs: NKp44, NKp46, NKp30), NK group 2 member D (NKG2D) and DNAX accessory molecule-1 (DNAM-1; ref. 31). The resultant NK-cell activation mediates cytotoxicity by the following mechanisms: (i) Direct cytotoxicity through induction of apoptosis and NK-cell degranulation, or the release of effector molecules (perforin and granzymes) into the immunologic synapse between the NK cells and target cells; (ii) Release of cytokines such as IFNγ and TNFα that can directly mediate cytotoxicity or activate other immune cells including CD8+ T cells, providing a mechanism by which the innate and adaptive immune systems collaborate in their response to tumorigenesis; (iii) Chemokine-mediated recruitment of dendritic cells, which assist in antigen presentation to tumor-specific T cells; (iv) Antibody-dependent cellular cytotoxicity (ADCC), whereby CD16 on NK cells binds to the Fc region of the antibody-coated target cell leading to NK-cell activation and degranulation (29, 32).
ILC1s are the innate counterpart to Th1 cells, previously described as non or minimally cytotoxic, although there has been mounting evidence demonstrating that there are subsets of cytotoxic ILC1s in humans and mice (12, 33, 34). ILC1s isolated from murine liver, salivary glands, and intestines differ in expression of Eomes, IFNγ production, and TRAIL (35). In humans, two phenotypic subsets of ILC1s were identified in the gastrointestinal tract, lamina propria ILC1s (LP ILC1s), which express high levels of CD127, and intraepithelial ILC1s (ieILC1), which express CD103 (36). Although both subsets express NKp46 and produce IFNγ, ieILC1s also express NKp44, CD56, and Eomes, which LP ILC1s lack (36, 37). Liver ILC1s are unique due to their cytotoxicity, specifically their ability to mediate TRAIL-induced cell death (38). Liver ILC1s also express CD56 and produce IFNγ, TNFα, and granulocyte-macrophage colony-stimulating factor (GM-CSF), but they lack the expression of Eomes and the ability to produce granzyme B and perforin, in contrast to NK cells (18). Further, Dadi and colleagues reported another type of ILC1s — “tumor-associated” ILC1s with granzyme B expression and similar functionality to NK cells (33). The role of these cells in immunosurveillance is dependent on the expression of IL15 (33). While there is no official classification of ILC1 subsets, there is a fair amount of diversity and plasticity owing to their developmental origins.
ILC1s and NK cells infiltrate bladder tumor tissues but display an exhausted phenotype
It is critical to understand the native composition of ILCs in the bladder to elucidate how they vary in the setting of infection or tumor invasion. Huang and colleagues used a murine model to determine the relative composition of ILCs in the normal genitourinary tract (10). Within the murine bladder, NK cells represent the largest population of ILCs and ~15% of all CD45+ cells. The increased presence of NK cells was reflected in a higher expression of Eomes in the bladder compared with ILCs isolated from the small intestine (10). ILC1s, defined as CD45+Lin−NK1.1−CD127+, represent less than 5% of the immune-cell population (10). The predominant presence of NK cells corroborates their role in cancer immunosurveillance, as they are the first to recognize non-MHC presenting transformed cells (33). We previously found that NK cells make up ~25% of the tumor-infiltrating immune cells in patients with bladder cancer, making them one of the predominant bladder tumor–infiltrating immune cells (39). However, despite the high percentage, most intratumoral NK cells were dysfunctional with low expression of CD56 and activation receptors, as well as low cytotoxicity and cytokine production. Our findings are supported by other studies that show that upon IL2/IL15 stimulation, NK cells derived from bladder cancer patients display reduced activation and cytotoxic effects compared with NK cells from healthy donors (31, 40). These NK cells also have a significantly higher expression of T-cell immunoglobulin mucin-3 (Tim-3)—a checkpoint molecule expressed by exhausted T cells and known to suppress CD4+ and CD8+ T cells in other solid malignancies (31, 41)—suggesting that inhibitory signals from the bladder tumor result in exhaustion of NK cells and may be a potential mechanism by which tumors escape immunosurveillance (41). In vitro murine models of bladder cancer have also demonstrated increased expression of Tim-3+PD-1+ NK cells particularly in tumors with MHC class I deficiency (41).
Prognostic relevance of NK cells and ILC1s in bladder cancer
NK cells have been classically divided into two subsets based on five distinct developmental stages, corresponding to their ability to secrete cytokines and mediate cytotoxicity (39, 42, 43). The “immature” CD56brightCD16− subset represents ~10% of circulating NK cells and is characterized by a robust ability to secrete IFNγ (42, 43). In contrast, the CD56dimCD16+ subset is known as the main mediator for cytotoxicity based on the presence of perforin and granzyme (42, 43). Analysis of the intratumoral NK-cell population, however, has demonstrated that this classification is not fixed (39). NK cells isolated from human bladder tumors have a predominance of the CD56dim population, and the CD56bright population shows greater cytotoxicity and secretion of IFNγ and TNFα compared with their CD56dim counterparts (37, 39). We found CD56bright NK cells correlate with improved overall and disease-free survival in our local cohort of patients with bladder cancer (39). We also found that Killer cell lectin-like receptor subfamily F member 1 (KLRF1) is preferentially expressed on CD56bright NK cells and is a surrogate marker of CD56bright NK cells in predicting functional NK cells (44). The high expression of KLRF1 is also associated with improved overall and cancer-specific survival in The Cancer Genome Atlas cohort of patients with bladder cancer (44). On the other hand, expression of the inhibitory KIR receptor KIR2DL5 correlates with poor oncologic outcomes independent of cancer stage or grade (45, 46).
Other investigations into bladder NK cells have yielded a potential immunomodulatory target— sialic acid–binding immunoglobulin-like lectins (Siglecs). Elevated expression of Siglec 6 and Siglec7 on circulating NK cells has been linked to poor outcomes and reduced overall survival in bladder cancer patients (47). As expected, in cases where the ligands for Siglecs are absent on tumor cells, NK cells exhibit enhanced production of IFNγ, TNFα, and CD107a (47). Defects in cytotoxicity and IFNγ production have also been demonstrated in intratumoral Tim+PD-1+ NK cells isolated from patients with bladder cancer (41).
Unlike NK cells, ILC1s have not been studied extensively in the context of bladder cancer. We reported that ILC1s represented a heterogeneous group of bladder intratumoral immune cells and were associated with higher-stage disease (15). Expression of RORγt and IL17 in bladder ILC1s correlates with advanced bladder cancer, possibly resulting from the transdifferentiation of CD127+ ILC1s to NKp44+ ILC3s (15). The decrease in functionality of ILC1s was due to the loss of expression of IFNγ and GzmB (15). ILC1s in bladder cancer are mostly protective when these cells can maintain a functional cytotoxic phenotype, however, within the tumor microenvironment (TME) they are susceptible to exhaustion and differentiation that can promote tumorigenesis and predict disease outcomes.
ILC2s induce tumor-suppressive type 2 immune response in bladder cancer
ILC2s are defined as CRTH2+CD127+ cells and are the innate counterparts of CD4+ Th2 cells (48). Apart from the expression of GATA3, another notable characteristic of ILC2s is the expression of the transcription factor RORα. Besides its role in ILC2 development, RORα has been demonstrated to bind to genes associated with the immunosuppressive functions of ILC2s, including Il13, Il5, and Arg1. This suggests that RORα is involved in regulating functional aspects of ILC2s beyond their development (17, 27, 49). ILC2s mainly reside in mucosal tissue, mesenteric lymph nodes, and the spleen and play an immunoregulatory role through the activation of M2-macrophages and M-MDSCs (48, 50, 51). Polarization of M2 macrophages and M-MDSCs has been shown to promote an immunosuppressive TME and suppress the cytotoxic abilities of innate and adaptive cytotoxic cells (48, 52, 53). Under specific conditions, such as hypoxia, ILC2s have demonstrated the capacity for alternative activation, leading to a shift in their cytokine secretion profile from IL13 to IL10. These molecularly distinct subsets of ILC2s express regulatory markers and exhibit an IL10+ regulatory phenotype, akin to regulatory T cells (Treg) and have been referred to as ILC210 (51, 54); these ILC2s are characterized by their expression of suppression of tumorigenicity (ST2), cytotoxic T lymphocyte-associated protein 4 (CTLA-4), CD25, and CD73 (52, 54, 55).
Higher frequencies of ILC2s are associated with bladder cancer progression and poor prognosis due to the shift to a type 2 immune response. Increased infiltration of ILC2s into the bladder is mediated by the expression of prostaglandin D2, chemokine ligand 2 (CCL2), and CCL4 (48). ILC2 infiltration in bladder tumors leads to the production of IL13, which mediates the recruitment and differentiation of CD14+ monocytes and is linked with failed response to standard immune therapy (48). Accumulation of ILC2s also induces the differentiation of other immune cells, further leading to an immunosuppressive TME (48, 54). Studies in other malignancy models have shown other ways in which ILC2s contribute to an immunosuppressive TME. ILC2s exposed to hypoxic environments can express high levels of tumor-promoting molecules such as TGFβ and VEGF (54). 73% of bladder tumors express hypoxic markers, and although hypoxia-mediated ILC2 activation has not been studied in bladder cancer, the hypoxic environment in the bladder increases M-MDSC infiltration and correlates with tumor progression and poor prognosis (52, 53). Further investigation of ILC2s in this hypoxia-mediated immunosuppressive axis in bladder cancer is warranted.
ILC3s and LTis display plasticity with other ILCs in bladder cancer
LTis and ILC3s, characterized by expression of the transcription factors RORγt and aryl hydrocarbon receptor (AHR), produce IL17, GM-CSF, and IL22 following stimulation by IL23 or IL1β (56–60). ILC3s, the innate counterpart to Th17 cells, are further subdivided into NCR+CD127+ ILC3s and NCR− CD127+ ILC3s (15, 57). NCR+ ILC3s have also been shown to coexpress CD56, although, unlike NK cells, they lack the expression of perforin and granzymes (57). The function and phenotype of ILC3s are highly heterogenous and tissue-specific, which has made definitive characterization within these two subsets difficult, however, it has been observed that IL22 production is higher in NCR+ILC3s in response to IL23 stimulation (61). Furthermore, expression of the NCR NKp44 in NCR+ ILC3 induces a potent inflammatory response via the production of TNFα, IL2, and IL8 upon activation by NKp44 ligands, and this has been observed in cancer models including models of non–small cell lung cancer (61, 62).
In a murine model, bladder ILC3s were shown to express higher levels of CCR6 compared with small intestine resident ILC3s, a characteristic of LTi-like ILC3s, which have been previously found in the murine gut and secondary lymphoid tissue (10, 13, 60). Elevated CCR6 expression is also observed in intratumoral bladder ILC3s (15). In addition, these cells express CD69 and CD103, which have been associated with tissue-resident innate memory cells (15). It remains unclear whether these cells utilize CCR6 and CCL20 to recruit other immune cells to the bladder TME, similar to their Th17 counterparts. This CCR6/CCL20 axis is further discussed later in the manuscript. While ILC3s have not been extensively studied in bladder cancer it is known that bladder tumors express both NKp44 ligands as well as IL23 (63, 64), making them an attractive target for further investigation.
Role of ILCs in Bladder Cancer Treatment
ILCs regulate the response to BCG immune therapy
Intravesical immunotherapy with BCG is the standard of care for intermediate and high-risk NMIBC (65). It is superior to other intravesical chemotherapy agents in reducing the recurrence and progression of high-grade tumors, especially carcinoma in situ (66). Despite being known to elicit a localized immune response, the exact mechanism(s) of action of BCG is unknown and failure rates are between 30% to 40% (67). NK cells have been purported to be central to the immune response since the adoption of BCG into clinical practice in the 1990s (68). Early in vitro studies of patient-derived lymphocytes and NK cells showed enhanced NK cell–mediated tumor cytotoxicity following pretreatment with BCG (69). These indiscriminately termed “lymphocyte activated” or “BCG-activated killer cells” were later identified as perforin-producing CD56+CD3− cells, suggesting a critical role of NK cells in BCG response (70). Even though the exact antitumor mechanism of BCG remains elusive, it is characterized by an inflammatory response wherein immune cells are recruited, including IL12- and TNFα-producing monocytes, which in turn stimulate NK-cell activation (48, 71). However, this early distinction of NK cells as CD56+CD3− is not sufficient to distinguish them from certain types of ILC1s, which share a similar phenotype.
In vivo experiments using orthotopic bladder tumors in mice showed improved survival with BCG treatment and the treatment effects were abolished in NK cell–deficient mice compared with wild-type mice (72), showing the requirement of NK cells in BCG efficacy. An in-depth analysis of the immune response to BCG has also highlighted the plasticity of NK cells (73). BCG has been shown to preferentially stimulate the CD56bright subpopulation in vitro, resulting in increased proliferation and IFNγ production (43, 74). These BCG-activated CD56bright NK cells are unique in their high expression of CD16 and KIRs, and increased NK-cell degranulation (73, 74). It was suggested that this cytotoxic CD56bright population may arise from CD56dim cells in the presence of BCG, although no lineage tracing was conducted to delineate a specific precursor of origin of these cells (74). Similar to the in vitro observations, CD56bright cells isolated from patients treated with BCG show higher levels of CD16 compared with Mitomycin C treated patients, suggesting a possible BCG-specific effect on the NK cells (74).
Tumors express ligands for both activating and inhibitory NK-cell receptors (45, 75, 76). In one study, bladder tumors were tested for ligands to the NK cell–activating receptors NKp44, NKp30, and NKp46 by IHC pre- and post-BCG treatment in a small, single cohort of patients with high-risk NMIBC (76). Patients who responded to BCG had a significantly higher tumoral expression of NK-cell markers pretreatment compared with non-responders (76). Even though these findings need to be validated in larger cohorts, they demonstrate the importance of recruitment and activation of NK cells in the setting of BCG treatment. Moreover, preclinical studies also show that priming using percutaneous BCG improves the response to intravesical BCG treatment; priming with BCG also leads to the activation of innate cells including NK cells (77). Resistance of certain bladder tumors to NK cell–mediated cytotoxicity has been linked with failure to respond to intravesical BCG (68, 77).
In contrast to NK cells, the ability of ILC2s to activate immunosuppressive immune cells has been linked with the failure of BCG treatment in NMIBC (48). Elevated levels of polarized ILC2s in peripheral blood have been found to correlate with elevated levels of M-MDSCs in the urine of patients post-BCG treatment; a high ratio of these M-MDSCs to CD8+ T cells was then linked to BCG failure and cancer progression and recurrence (48, 50). BCG also induces the infiltration of CCR4 expressing CRTH2+ ILC2s into tumors by increasing the expression of the CCR4 ligand (48, 78). The ILC2-rich TME creates an environment that further attracts IL13Rα1 expressing monocytes and favors the differentiation of CD14+ monocytes into M-MDSCs, characterized by increased levels of ARG1, iNOS, and CCAAT/enhancer binding proteinβ (48); this increases their suppressive ability against CD4+ and CD8+ T cells through the increased production of IL13 (48).
Therapeutic efficacy of ILCs in bladder cancer
Strategies tailored to enhance the immune response are one mechanism by which investigators are addressing the limitations of BCG and immune checkpoint inhibitor (ICI) therapy. Unfortunately, due to limited data on other ILCs, most clinical trials in bladder cancer have just focused on NK cells (Table 2). Several recent NK-cell trials in cancer, especially in leukemias, involve the adoptive transfer of NK cells or the transfer of NK cells engineered to express a chimeric antigen receptor that help to target the NK cells specifically to the tumor cells (79). However, in bladder cancer, most of the trials have included agents to activate NK-cell functionality. IL-based therapies exploit the enhancement of proliferation and effector functions of NK and T cells (80, 81). MDNA11 is a long-acting IL2 derivative with preferential binding for the IL2Rβ (CD122) found on NK cells and CD8+ T cells, which bypasses the activation of immunosuppressive Tregs through interactions with CD25 (also known as IL2Rα; refs. 81, 82). A Beta-only IL2 Immunotherapy (ABILIFY) is an ongoing phase I/II study that is using MDNA11 as a single agent or in combination with ICIs in bladder cancer (82). Similarly, IL15 has been shown to enhance the effector functions of NK and T cells in in vitro and preclinical studies (80, 83, 84). ALT-803, an IL15 superagonist, is an IL15N72D/IL15Rα-Fc complex featuring a mutated IL15 with increased biological activity fused to an IgG1 Fc to allow for cis and trans binding between immune cells and tumor cells (84, 85). QUILT-3.032 is a phase II/III trial that has yielded promising and durable responses with ALT-803 in combination with BCG for patients with BCG-refractory high-risk NMIBC (86).
Table 2.
NK cell–related bladder cancer clinical trials.
| Drug | Target | Clinical Setting | Phase | Trial ID |
|---|---|---|---|---|
| UGN-201 | TLR7 | NMIBC/MIBC undergoing cystectomy | Phase I | NCT05055050 |
| N-803 | IL15 | NMIBC | Phase Ib/IIb | NCT02138734 |
| N-803 | IL15 | BCG unresponsive NMIBC | Phase II/III | NCT03022825 |
| EG-70 | IL2/RIG-I | BCG unresponsive NMIBC | Phase I/II | NCT04752722 |
| SEA-TGT | TIGIT | Refractory/metastatic solid tumor | Phase I | NCT02326168 |
| MDNA11 | IL2 | Unresectable/metastatic solid tumor | Phase I/II | NCT05086692 |
| NKTR-214 (± nivolumab) | IL2 | Cisplatin ineligible, locally advanced/metastatic bladder cancer | Phase II | NCT03785925 |
| ST-067 | IL18 | Refractory/progressive solid tumor | Phase I/II | NCT04787042 |
| IK-175 (± nivolumab) | AHR | Refractory, unresectable, progressive disease | Phase I | NCT04200963 |
| S0-C101 (± pembrolizumab) | IL15 | Refractory/metastatic solid tumors | Phase I | NCT04234113 |
| DF1001–001 (± nivolumab or paclitaxel) | NK cell activation | Locally advanced/metastatic solid tumors | Phase I/II | NCT04143711 |
| Lirilumab + nivolumab (vs. nivolumab) | KIR | Cisplatin-ineligible MIBC | Phase Ib | NCT03532451 |
| D-CIK (+ anti-PD-1) | NK cell activation | Refractory advanced solid tumors | Phase I/II | NCT02886897 |
| CIK | NK cell activation | Stage I-III bladder cancer | Phase II | NCT02489890 |
| M7824/M9241 + SBRT | PD-1, TGFβ, IL12 | Advanced or metastatic non-prostate GU cancer | Phase I | NCT04235777 |
Abbreviations: CIK, cytokine-induced killer cells; D-CIK, dendritic cells and cytokine-induced killer cells; RIG-I, retinoic acid-inducible gene I; TIGIT, T cell immunoreceptor with Ig and ITIM domains.
Other immunomodulatory regimens have targeted activating and/or inhibitory receptors on NK cells. Toll-like receptor 7 (TLR7) is a single-strand RNA–sensing pattern recognition receptor that can stimulate inflammatory cytokine production through activation of the transcription factor NFκβ and IRF7 (87). The TLR7 pathway has been implicated in BCG treatment and TLR7 stimulation in immune cells following BCG instillation results in increased apoptosis of urothelial cancer cells (88). The TLR7 agonist, imiquimod, is an FDA-approved drug for human papillomavirus treatment and has been used to treat Basal cell carcinoma (89, 90). Clinical trials with reformulated imiquimod in NMIBC have been performed due to the immunomodulatory mechanism of imiquimod on NK-cell activation. In vivo tumor treatment with the TLR agonist R848 shows increased activation of NK cells and CD8+ T cells by activating dendritic cells in a murine model, with an overall skewing towards a Th1 response with elevated levels of IFNγ+CD4+ T cells and the absence of Th2 and Th17 cells (91). Lirilumab is a monoclonal antibody against the inhibitor KIR KIR2DL that enhances NK cell–mediated ADCC (92). Clinical studies with lirilumab in hematologic and solid malignancies show that while the drug is tolerable, its efficacy as a single agent is limited (92, 93), but it shows promise in combination with ICIs in bladder cancer. SEA-TGT is a monoclonal antibody against T cell immunoreceptors with Ig and ITIM domains (TIGIT), found on T cells and NK cells (94), and it is currently being studied in combination with ICIs in advanced bladder cancer. A phase II/III trial with DF1001, a small molecule that facilitates directed binding to Her2+ tumors and activates NK cells is being studied in combination with ICI and paclitaxel (95) in bladder cancer.
Finally, two trials, in China, are currently testing the efficacy of the adoptive transfer of cytokine-induced killer (CIK) cells, whereby autologous peripheral blood mononuclear cells (PBMC) are stimulated to induce NK- and T-cell activation prior to infusion into patients (96, 97). One study will infuse CIKs in combination with chemotherapy in patients with muscle-invasive disease and the other will transfer autologous PBMCs after culturing them with IL2, IFNγ, IL1α, anti-CD3, and anti–PD-1 to increase the activation of NK cells and T cells in patients with metastatic and locally advanced bladder cancer (29).
What Does the Future Hold for ILCs in Bladder Cancer? Mechanistic and Therapeutic Implications
The past decade has witnessed a new horizon in the understanding of bladder tumor–infiltrating immune cells, enhanced by the discovery of ILCs. This recent explosion in studies has resulted in the identification of novel tumor suppressive and pro-tumorigenic contributions by different ILCs in bladder cancer, revolutionizing the bladder innate immunity field. While this has been exciting in terms of the prospects of new prognostic and therapeutic strategies, a lot remains unknown and there is an urgent need for further research to delineate the role of ILCs in the bladder TME. One challenge in the field is the controversy surrounding the markers used to define each ILC subset. While certain markers have been proposed as defining features for specific ILC subsets, their expression can be variable and context-dependent. The immune responses of ILCs exhibit intricate diversity and transitions across different cellular states, even within a single cell type. For example, in a recent study by Alkon and colleagues (98), scRNA-seq revealed the presence of a heterogeneous group of ILC2s in skin samples. Interestingly, ILCs also showed substantial lineage infidelity with co-expression of genes associated with both type 2 and type 3/17 immunity under pathologic conditions (atopic dermatitis; ref. 98). This was also observed to a lesser extent in healthy skin and in ILCs from skin explant cultures and peripheral blood (98). Another study used scRNA-seq, single-cell assay for transposase-accessible chromatin using sequencing, and in vivo fate mapping to confirm a dense transcriptional continuum of ILCs, persisting even under normal conditions in skin ILCs (99). During psoriatic inflammation, this continuum changes to a mixed subset resembling both ILC3s and ILC2s—ILC3-like cells, but expressing cytokines typically associated with ILC2s. These findings indicate that skin ILC responses can dynamically alter under disease conditions, potentially contributing to pathologic remodeling (99). Further, integrative inference of ILCs using single-cell genomics has revealed unique intermediary cytotoxic subsets of ILC2s and NK cells in hepatocellular carcinoma (100). Hence, to thoroughly assess the ILC heterogeneity during bladder cancer development, it is essential to utilize new multi-omics techniques such as proteomics, single-cell transcriptomic studies, ultrahigh multispectral spatial imaging, along with lineage tracing and epigenetic analyses to correctly identify individual ILC subsets, their location, and interactions with other cells in the bladder tumor tissues.
With the growing body of knowledge of the contributions of ILCs to bladder cancer, we need to translate these into clinically relevant tools that can aid in diagnosis and treatment. ILCs have many of the same cell-surface and immunoregulatory markers, including exhaustion and checkpoint molecules as the T cells (i.e., PD-1, Tim-3, and Siglecs), which suggests that some of the standard immune therapies designed to target T cells may be also affecting the ILCs, and this may contribute to the success and failure of these treatment strategies in bladder cancer (101, 102). This highlights the need for in vivo and in vitro studies using genetic manipulations and other experimental tools that modulate the ILC subsets without affecting adaptive immune cells; this will help us delineate the functions of each ILC subset and how they interact with adaptive immunity in bladder cancer. Such studies will help us understand how to implement ILC-related therapies to improve bladder tumor immunity and therapy. They will also delineate the effect of the TME and identify factors (cytokines and chemokines) that modulate ILC plasticity, heterogeneity, and their functions in bladder cancer, opening avenues for novel targeting strategies.
What is intriguing about ILCs in bladder cancer is that we already know that bladder tumors are infiltrated with these cells, in fact, NK cells are one of the predominant bladder tumor–infiltrating immune cells. However, these cells are either dysfunctional or undergoing tumor-promoting differentiation, which gives way to exciting new strategies for activating these cells and unlocking their cytotoxicity. For example, a combination of cisplatin and an inhibitor of the epigenetic regulator, Enhancer of Zeste Homolog 2 (EZH2), has shown therapeutic effects in invasive bladder cancer through NK-cell responses (103). Features of exhaustion of these NK cells can be rescued with intratumoral IL21, representing a potential avenue for therapeutic intervention (41, 104). Another promising target is androgen receptor blockade, which has been shown to decrease PD-L1 expression on tumor cells and results in increased NK-cell cytotoxicity in vitro (105). NK-cell activity is also increased upon blockade of exhaustion molecules such as Tim-3 and TIGIT in bladder cancer murine models and primary human cells (101, 106, 107), which demonstrates promising opportunities for clinical translation.
Adoptive transfer of NK cells has been largely investigated for leukemia, although this method shows potential in bladder cancer as well. Adoptive transfer of healthy donor-derived, IL2/IL15-activated NK cells showed therapeutic potential in a humanized orthotopic murine model of chemoresistant bladder cancer (31). However, peripheral blood–derived NK cells from patients with high-grade bladder cancer lacked similar efficacy, probably due to exhaustion, supporting our previous findings (31). In that case, supplementation with an agent that activates dysfunctional NK cells during the autologous transfer may be needed. Intravesical transfer of NK cells may also be investigated to test whether it improves NK-cell function as was previously observed with bladder intravesical instillation of γδ T cells, another type of innate immune lymphocyte (108). However, before substantially investing in NK cell adoptive transfer studies, it needs to be delineated whether it is possible to reactivate dysfunctional NK cells in bladder tumors by intravesical instillation of novel drugs including small molecule agonists or targeting antibodies (41, 109). The approval of PD-1/PD-L1-specific antibodies, which function by overcoming T-cell exhaustion via blockade of PD-1/PD-L1 signaling, has been a huge success for immune therapy in bladder cancer (4). However, one of the clinical roadblocks is the low or no response to these therapies in a substantial percentage of patients. Several studies have now shown that ILCs, especially NK cells, play a critical role in anti–PD-1/PD-L1 therapy (102) and can complement the functions of T cells, particularly against MHC-low tumors that display reduced antigenicity or are resistant to T cells. One study showed NK cells induce ADCC response in response to anti–PD-L1 and mediate cytotoxic activity against PD-L1+ tumors (110). Interestingly, Dong and colleagues observed that anti–PD-L1 also promotes NK-cell activity against PD-L1− tumors by directly stimulating PD-L1+ NK cells through the p38 pathway (111). As a result, the combination of anti–PD-1/PD-L1 therapy with the adoptive transfer of autologous or allogeneic NK cells and agents that induce recruitment, persistence, and activation of NK cells holds exciting opportunities for bladder cancer immune therapy. However, the function of NK cells in anti–PD-/PD-L1 therapy is still not completely understood and requires future investigations to substantiate these findings and translate them into the clinical setting.
ILC1s also modulate bladder cancer progression, however, in contrast to NK cells, they are mostly tissue-resident; ILC1s with similar phenotypes differ in their functions depending on their distribution and are highly context-dependent. Another roadblock in deciphering the exact function of ILC1s in cancer is the plasticity between NK cells and ILC1s with the presence of an intermediate population expressing both transcription factors and cell-surface receptors of NK cells and ILC1s such as Eomes and CD49a (112). In a murine model, expression of tumor-associated intermediate ILC1s was driven by TGFβ signaling and resulted in reduced immunosurveillance (112). So together with investigating the functions and phenotypes of bladder tumor–infiltrating ILC1s, it will be important to study the cues and factors that promote these transitions of ILC1s in bladder cancer. Th17-like ILC1s have been associated with higher-stage bladder cancer, suggesting that ILC1s can also be pro-tumorigenic in this setting. As such, methods to restore their antitumor functions and induce their transdifferentiation into their more cytotoxic counterparts in bladder tumors may hold therapeutic relevance (15). However, more in-depth characterization of bladder ILC1s is needed before developing immunotherapeutic agents targeting their function.
Finally, the ILC2/IL13 axis mediating the recruitment of M-MDSCs has been linked with increased recurrence, BCG failure, and immune suppression in the bladder TME; strategies to block ILC2s or initiate alternative ILC polarization may be useful in sensitizing tumor cells to BCG therapy (48). On the basis of our current comprehensive overview of published studies, it is evident that research into non-NK ILCs remains highly limited in bladder cancer, and detailed future studies are required to delineate the function and therapeutic relevance of this critical family of innate immune cells in bladder cancer.
Conclusions
ILCs play critical roles, both pro-tumorigenic and tumor-suppressive, in bladder cancer. However, there are still substantial gaps in our understanding of these cells and their functions in bladder cancer. To advance our knowledge and develop novel ILC-targeting immunotherapies, it is essential to develop a comprehensive understanding of the numerous mechanisms by which ILCs regulate bladder cancer development and progression. The success of ICIs targeting PD-1/PD-L1 and CTLA-4 underscores the potential of regulating immune pathways to enhance the antitumor immune responses in bladder cancer. In a similar vein, blocking or activating immune pathways that modulate ILC functions could offer alternative treatment strategies, especially for bladder cancer resistant to standard immunotherapies. Drawing lessons from other malignancies with similar molecular characteristics, such as high tumor mutation burden and common molecular subtypes, can provide valuable insights (113). For example, in melanoma, where ICIs have been highly successful, ILC2s have a dual function by two distinct mechanisms. IL33-activated ILC2s exert a tumor-suppressive function by recruiting eosinophils, which although they promote the infiltration of T cells into the TME (114, 115), also support lung metastases by inducing NK-cell dysfunction (116). Given the accumulation of ILC2s in bladder tumor tissues, exploring the IL33/ILC2/eosinophil axis in localized and metastatic bladder cancer could be of interest. Furthermore, expression of PD-1 has been detected in tumor-infiltrating ILC2s in melanoma (117), and it was found to hinder the tumor-suppressive functions of ILC2s. This observation suggests that ILC2s may play a role in modulating the effectiveness of ICI therapy in bladder cancer as well. In the context of melanoma, a combination approach involving IL33-mediated activation of ILC2s and PD-1 blockade demonstrated antitumor efficacy (117). Although previous studies have predominantly focused on the tumor-promoting role of ILC2s in bladder cancer, elucidating the different functions of ILC2s in bladder cancer could prove valuable in the development of novel immune therapies. In addition, recent studies in triple-negative breast cancer (TNBC) and lung cancer have revealed that the drug cisplatin, commonly used in muscle-invasive bladder cancer, promotes immune-cell infiltration in “cold” tumors, particularly ILC3 infiltration, by upregulating IL1β expression. This, in turn, induces tumor cells to produce CCL20, recruiting CCR6+ ILC3s that generate CXCL10, facilitating the recruitment of CD4+ and CD8+ T cells to the TME (118). These findings in TNBC and lung cancer models warrant further investigation of the role of CCR6+ILC3s in bladder cancer, especially in cisplatin-resistant bladder cancer. In summary, there is an urgent requirement for further research to unravel the intricate mechanisms of ILC-mediated immunity in bladder cancer. This will enable the synergistic utilization of both innate and adaptive immunity in the development of combination therapies and unlock the potential for transformative immunotherapies in bladder cancer.
Acknowledgments
This study is funded by The Mays Family Cancer Center at University of Texas Health San Antonio (P30 CA054174), The Roger L. and Laura D. Zeller Charitable Foundation Chair in Urologic Cancer, The Glenda and Gary Woods Distinguished Chair in GU Oncology, The Max & Minnie Tomerlin Voelcker Fund, CDMRP CA170270/P1P2, Bladder Cancer Advocacy Network (BCAN), Research Training Award (RP170345) from the Cancer Prevention & Research Institute of Texas, MSTP Program (NIH T32GM113896), Mays Cancer Center P30 Cancer Center Support Grant (NCI; CA054174), and Long School of Medicine at UTHSCSA and the Institute for the Integration of Medicine and Science.
Authors’ Disclosures
R.S. Svatek reports personal fees from CG Oncology; other support from Japanese BCG Laboratories; and other support from Merck outside the submitted work. No disclosures were reported by the other authors.
References
- 1.Topalian SL, Taube JM, Anders RA, Pardoll DM. Mechanism-driven biomarkers to guide immune checkpoint blockade in cancer therapy. Nat Rev Cancer 2016;16:275–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mukherjee N, Julian E, Torrelles JB, Svatek RS. Effects of Mycobacterium bovis Calmette et Guérin (BCG) in oncotherapy: bladder cancer and beyond. Vaccine 2021;39:7332–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mukherjee N, Svatek R. Cancer immune therapy: prognostic significance and implications for therapy of PD-1 in BCG-relapsing bladder cancer. Ann Surg Oncol 2018;25:2498–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stenehjem DD, Tran D, Nkrumah MA, Gupta S. PD-1/PD-L1 inhibitors for the treatment of advanced urothelial bladder cancer. Onco Targets Ther 2018;11:5973–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mukherjee N, Svatek RS, Mansour AM. Role of immunotherapy in bacillus Calmette-Guerin–unresponsive non–muscle-invasive bladder cancer. Urol Oncol 2018;36:103–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oh DY, Kwek SS, Raju SS, Li T, McCarthy E, Chow E, et al. Intratumoral CD4 (+) T cells mediate antitumor cytotoxicity in human bladder cancer. Cell 2020;181:1612–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu J, Abraham SN. The roles of T cells in bladder pathologies. Trends Immunol 2021;42:248–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schuster IS, Coudert JD, Andoniou CE, Degli-Esposti MA. “Natural Regulators”: NK cells as modulators of T-cell immunity. Front Immunol 2016;7:235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crome SQ, Ohashi PS. Immunoregulatory functions of innate lymphoid cells. J Immunother Cancer 2018;6:121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang J, Fu L, Huang J, Zhao J, Zhang X, Wang W, et al. Group 3 innate lymphoid cells protect the host from the uropathogenic Escherichia coli infection in the bladder. Adv Sci 2022;9:2103303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Artis D, Spits H. The biology of innate lymphoid cells. Nature 2015;517:293–301. [DOI] [PubMed] [Google Scholar]
- 12.Vivier E, van de Pavert SA, Cooper MD, Belz GT. The evolution of innate lymphoid cells. Nat Immunol 2016;17:790–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Takatori H, Kanno Y, Watford WT, Tato CM, Weiss G, Ivanov II, et al. Lymphoid tissue inducer–like cells are an innate source of IL17 and IL22. J Exp Med 2009;206:35–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vivier E, Artis D, Colonna M, Diefenbach A, Di Santo JP, Eberl G, et al. Innate lymphoid cells: 10 years on. Cell 2018;174:1054–66. [DOI] [PubMed] [Google Scholar]
- 15.Mukherjee N, Ji N, Tan X, Lin CL, Rios E, Chen CL, et al. Bladder tumor ILC1s undergo Th17-like differentiation in human bladder cancer. Cancer Med 2021;10:7101–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.DeLozier-Blanchet CD, Guenin R. Cytogenetics of ring chromosome 7. Clin Genet 1984;25:84–5. [DOI] [PubMed] [Google Scholar]
- 17.Ferreira ACF, Szeto ACH, Heycock MWD, Clark PA, Walker JA, Crisp A, et al. RORα is a critical checkpoint for T cell and ILC2 commitment in the embryonic thymus. Nat Immunol 2021;22:166–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krämer B, Nalin AP, Ma F, Eickhoff S, Lutz P, Leonardelli S, et al. Single-cell RNA sequencing identifies a population of human liver-type ILC1s. Cell Rep 2023;42:111937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sheikh A, Abraham N. Interleukin-7 receptor alpha in innate lymphoid cells: more than a marker. Front Immunol 2019;10:2897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lim AI, Li Y, Lopez-Lastra S, Stadhouders R, Paul F, Casrouge A, et al. Systemic human ILC precursors provide a substrate for tissue ILC differentiation. Cell 2017;168:1086–100. [DOI] [PubMed] [Google Scholar]
- 21.Liu C, Gong Y, Zhang H, Yang H, Zeng Y, Bian Z, et al. Delineating spatiotemporal and hierarchical development of human fetal innate lymphoid cells. Cell Res 2021;31:1106–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Walker JA, Clark PA, Crisp A, Barlow JL, Szeto A, Ferreira ACF, et al. Polychromic reporter mice reveal unappreciated innate lymphoid cell progenitor heterogeneity and elusive ILC3 progenitors in bone marrow. Immunity 2019;51:104–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Possot C, Schmutz S, Chea S, Boucontet L, Louise A, Cumano A, et al. Notch signaling is necessary for adult, but not fetal, development of RORγt+ innate lymphoid cells. Nat Immunol 2011;12:949–58. [DOI] [PubMed] [Google Scholar]
- 24.Xu W, Cherrier DE, Chea S, Vosshenrich C, Serafini N, Petit M, et al. An Id2RFP-reporter mouse redefines innate lymphoid cell precursor potentials. Immunity 2019;50:1054–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hashemi E, Malarkannan S. Tissue-resident NK cells: development, maturation, and clinical relevance. Cancers 2020;12:1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mjösberg J, Mazzurana L. ILC-poiesis: making tissue ILCs from blood. Immunity 2017;46:344–6. [DOI] [PubMed] [Google Scholar]
- 27.Chung DC, Jacquelot N, Ghaedi M, Warner K, Ohashi PS. Innate lymphoid cells: role in immune regulation and cancer. Cancers 2022;14:2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ebbo M, Crinier A, Vely F, Vivier E. Innate lymphoid cells: major players in inflammatory diseases. Nat Rev Immunol 2017;17:665–78. [DOI] [PubMed] [Google Scholar]
- 29.Bhardwaj N, Farkas AM, Gul Z, Sfakianos JP. Harnessing natural killer cell function for genitourinary cancers. Urol Clin North Am 2020;47:433–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Duygu B, Olieslagers TI, Groeneweg M, Voorter CEM, Wieten L. HLA class I molecules as immune checkpoints for NK cell alloreactivity and anti-viral immunity in kidney transplantation. Front Immunol 2021;12:680480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ferreira-Teixeira M, Paiva-Oliveira D, Parada B, Alves V, Sousa V, Chijioke O, et al. Natural killer cell-based adoptive immunotherapy eradicates and drives differentiation of chemoresistant bladder cancer stem-like cells. BMC Med 2016;14:163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Böttcher JP, Bonavita E, Chakravarty P, Blees H, Cabeza-Cabrerizo M, Sammicheli S, et al. NK cells stimulate recruitment of cDC1 into the tumor microenvironment promoting cancer immune control. Cell 2018;172:1022–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dadi S, Chhangawala S, Whitlock BM, Franklin RA, Luo CT, Oh SA, et al. Cancer immunosurveillance by tissue-resident innate lymphoid cells and innate-like T cells. Cell 2016;164:365–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Simoni Y, Fehlings M, Kløverpris HN, Mcgovern N, Koo S-L, Loh CY, et al. Human innate lymphoid cell subsets possess tissue-type based heterogeneity in phenotype and frequency. Immunity 2017;46:148–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fuchs A. ILC1s in tissue inflammation and infection. Front Immunol 2016;7:104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fuchs A, Vermi W, Lee JS, Lonardi S, Gilfillan S, Newberry RD, et al. Intraepithelial Type 1 innate lymphoid cells are a unique subset of IL12- and IL15-responsive IFN-γ-producing cells. Immunity 2013;38:769–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bernink JH, Krabbendam L, Germar K, de Jong E, Gronke K, Kofoed-Nielsen M, et al. Interleukin-12 and −23 control plasticity of CD127+ Group 1 and Group 3 innate lymphoid cells in the intestinal lamina propria. Immunity 2015;43:146–60. [DOI] [PubMed] [Google Scholar]
- 38.Daussy C, Faure F, Mayol K, Viel S, Gasteiger G, Charrier E, et al. T-bet and Eomes instruct the development of two distinct natural killer cell lineages in the liver and in the bone marrow. J Exp Med 2014;211:563–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mukherjee N, Ji N, Hurez V, Curiel TJ, Montgomery MO, Braun AJ, et al. Intratumoral CD56bright natural killer cells are associated with improved survival in bladder cancer. Oncotarget 2018;9:36492–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang W, Feng H, Chen Q, Lu X, Ge J. The functional potency of natural killer cells in response to IL2/ IL15/ IL21 stimulation is limited by a concurrent upregulation of Tim-3 in bladder cancer. Exp Cell Res 2018;372:92–8. [DOI] [PubMed] [Google Scholar]
- 41.Seo H, Jeon I, Kim BS, Park M, Bae EA, Song B, et al. IL21-mediated reversal of NK cell exhaustion facilitates antitumor immunity in MHC class I-deficient tumors. Nat Commun 2017;8:15776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nagler A, Lanier LL, Cwirla S, Phillips JH. Comparative studies of human FcRIII-positive and negative natural killer cells. J Immunol 1989;143:3183–91. [PubMed] [Google Scholar]
- 43.Batoni G, Esin S, Favilli F, Pardini M, Bottai D, Maisetta G, et al. Human CD56bright and CD56dim natural killer cell subsets respond differentially to direct stimulation with Mycobacterium bovis bacillus Calmette-Guérin. Scand J Immunol 2005;62:498–506. [DOI] [PubMed] [Google Scholar]
- 44.Mukherjee N, Ji N, Tan X, Chen CL, Noel ODV, Rodriguez-Padron M, et al. KLRF1, a novel marker of CD56. Cancer Med 2022;12:8970–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Guillamón CF, Gimeno L, Server G, Martínez-Sánchez MV, Escudero JF, López-Cubillana P, et al. Immunological risk stratification of bladder cancer based on peripheral blood natural killer cell biomarkers. Eur Urol Oncol 2021;4:246–55. [DOI] [PubMed] [Google Scholar]
- 46.Ren X, Peng M, Xing P, Wei Y, Galbo PM, Corrigan D, et al. Blockade of the immunosuppressive KIR2DL5/PVR pathway elicits potent human NK cell–mediated antitumor immunity. J Clin Invest 2022;132:e163620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Benmerzoug S, Chevalier MF, Villier L, Nguyen S, Cesson V, Schneider AK, et al. Siglec-7 may limit natural killer cell-mediated antitumor responses in bladder cancer patients. Eur Urol Open Sci 2021;34:79–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chevalier MF, Trabanelli S, Racle J, Salomé B, Cesson V, Gharbi D, et al. ILC2-modulated T cell-to-MDSC balance is associated with bladder cancer recurrence. J Clin Invest 2017;127:2916–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ghaedi M, Ohashi PS. ILC transdifferentiation: roles in cancer progression. Cell Res 2020;30:562–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Herbert DB, Douglas B, Zullo K. Group 2 Innate Lymphoid Cells (ILC2): Type 2 Immunity and Helminth Immunity. Int J Mol Sci 2019;20:2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Seehus CR, Kadavallore A, Torre BDL, Yeckes AR, Wang Y, Tang J, et al. Alternative activation generates IL10 producing type 2 innate lymphoid cells. Nat Commun 2017;8:1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang H, Ye Y-L, Li M-X, Ye S-B, Huang W-R, Cai T-T, et al. CXCL2/MIF-CXCR2 signaling promotes the recruitment of myeloid-derived suppressor cells and is correlated with prognosis in bladder cancer. Oncogene 2017;36:2095–104. [DOI] [PubMed] [Google Scholar]
- 53.Lodhi T, Song YP, West C, Hoskin P, Choudhury A. Hypoxia and its modification in bladder cancer: current and future perspectives. Clin Oncol (R Coll Radiol) 2021;33:376–90. [DOI] [PubMed] [Google Scholar]
- 54.Ye L, Jin K, Liao Z, Xiao Z, Xu H, Lin X, et al. Hypoxia-reprogrammed regulatory group 2 innate lymphoid cells promote immunosuppression in pancreatic cancer. EBioMedicine 2022;79:104016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Griesenauer B, Paczesny S. The ST2/ IL33 axis in immune cells during inflammatory diseases. Front Immunol 2017;8:475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Castillo-González R, Valle-Noguera A, Gomez-Sánchez MJ, Xia P, Cruz-Adalia A. Innate lymphoid cells type 3 in cancer. Front Immunol 2022;13:1033252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rethacker L, Boy M, Bisio V, Roussin F, Denizeau J, Vincent-Salomon A, et al. Innate lymphoid cells: NK and cytotoxic ILC3 subsets infiltrate metastatic breast cancer lymph nodes. OncoImmunology 2022;11:2057396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yuan X, Rasul F, Nashan B, Sun C. Innate lymphoid cells and cancer: role in tumor progression and inhibition. Eur J Immunol 2021;51:2188–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Riding AM, Loudon KW, Guo A, Ferdinand JR, Lok LSC, Richoz N, et al. Group 3 innate lymphocytes make a distinct contribution to type 17 immunity in bladder defence. iScience 2022;25:104660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Li S, Bostick JW, Zhou L. Regulation of innate lymphoid cells by Aryl hydrocarbon receptor. Front Immunol 2017;8:1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Carrega P, Loiacono F, Di CE, Scaramuccia A, Mora M, Conte R, et al. NCR+ILC3 concentrate in human lung cancer and associate with intratumoral lymphoid structures. Nat Commun 2015;6:8280. [DOI] [PubMed] [Google Scholar]
- 62.Glatzer T, Killig M, Meisig J, Ommert I, Luetke-Eversloh M, Babic M, et al. RORγt+ innate lymphoid cells acquire a proinflammatory program upon engagement of the activating receptor NKp44. Immunity 2013;38:1223–35. [DOI] [PubMed] [Google Scholar]
- 63.Baychelier F, Sennepin A, Ermonval M, Dorgham K, Debré P, Vieillard V. Identification of a cellular ligand for the natural cytotoxicity receptor NKp44. Blood 2013;122:2935–42. [DOI] [PubMed] [Google Scholar]
- 64.Siegler JJ, Correia MP, Hofman T, Prager I, Birgin E, Rahbari NN, et al. Human ILC3 exert TRAIL-mediated cytotoxicity towards cancer cells. Front Immunol 2022;13:742571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Woldu SL, Bagrodia A, Lotan Y. Guideline of guidelines: non–muscle-invasive bladder cancer. BJU Int 2017;119:371–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Veeratterapillay R, Heer R, Johnson MI, Persad R, Bach C. High-risk non–muscle-invasive bladder cancer—therapy options during intravesical BCG shortage. Curr Urol Rep 2016;17:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zlotta AR, Fleshner NE, Jewett MA. The management of BCG failure in non–muscle-invasive bladder cancer: an update. Can Urol Assoc J 2013;3:199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yazici G, Gokmen E, Kose MG, Cetin B, Arslan B, Ozalevli M, et al. The use of natural killer cell activity and PPD test in the prediction of results in intravesical BCG treatment of patients with non–muscle-invasive bladder cancer. Int Urol Nephrol 2023;55:301–8. [DOI] [PubMed] [Google Scholar]
- 69.Mizutani Y, Yoshida O. In vitro enhancement of natural killer cell activity by BCG and the antagonistic inhibition of the susceptibility of K562 cells to lysis by peripheral blood lymphocytes in patients with urinary bladder tumor. Int J Urol 1994;1:49–56. [DOI] [PubMed] [Google Scholar]
- 70.Brandau S, Suttmann H, Riemensberger J, Seitzer U, Arnold J, Durek C, et al. Perforin-mediated lysis of tumor cells by Mycobacterium bovis bacillus Calmette-Guérin–activated killer cells. Clin Cancer Res 2000;6:3729–38. [PubMed] [Google Scholar]
- 71.Suttmann H, Jacobsen M, Reiss K, Jocham D, Böhle A, Brandau S. Mechanisms of bacillus calmette-guerin mediated natural killer cell activation. J Urol 2004;172:1490–5. [DOI] [PubMed] [Google Scholar]
- 72.Brandau S, Böhle A. Activation of natural killer cells by bacillus Calmette-Guérin. Eur Urol 2001;39:518–24. [DOI] [PubMed] [Google Scholar]
- 73.Esteso G, Felgueres MJ, García-Jiménez Á, Reyburn-Valés C, Benguría A, Vázquez E, et al. BCG-activation of leukocytes is sufficient for the generation of donor-independent innate antitumor NK and γδ T-cells that can be further expanded. Oncoimmunology 2023;12:2160094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.García-Cuesta EM, Esteso G, Ashiru O, López-Cobo S, Álvarez-Maestro M, Linares A, et al. Characterization of a human antitumoral NK cell population expanded after BCG treatment of leukocytes. Oncoimmunology 2017;6:e1293212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Garcia-Cuesta EM, Lopez-Cobo S, Alvarez-Maestro M, Esteso G, Romera-Cardenas G, Rey M, et al. NKG2D is a key receptor for recognition of bladder cancer cells by IL2-activated NK cells and BCG promotes NK cell activation. Front Immunol 2015;6:284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yutkin V, Pode D, Pikarsky E, Mandelboim O. The expression level of ligands for natural killer cell receptors predicts response to bacillus Calmette-Guerin therapy: a pilot study. J Urol 2007;178:2660–4. [DOI] [PubMed] [Google Scholar]
- 77.Ji N, Mukherjee N, Morales EE, Tomasini ME, Hurez V, Curiel TJ, et al. Percutaneous BCG enhances innate effector antitumor cytotoxicity during treatment of bladder cancer: a translational clinical trial. Oncoimmunology 2019;8:1614857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Xue L, Salimi M, Panse I, Mjösberg JM, Mckenzie ANJ, Spits H, et al. Prostaglandin D2 activates group 2 innate lymphoid cells through chemoattractant receptor-homologous molecule expressed on TH2 cells. J Allergy Clin Immunol 2014;133:1184–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Huang R, Wen Q, Zhang X. CAR-NK cell therapy for hematological malignancies: recent updates from ASH 2022. J Hematol Oncol 2023;16:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kansler ER, Dadi S, Krishna C, Nixon BG, Stamatiades EG, Liu M, et al. Author correction: cytotoxic innate lymphoid cells sense cancer cell-expressed interleukin-15 to suppress human and murine malignancies. Nat Immunol 2022;23:1285. [DOI] [PubMed] [Google Scholar]
- 81.Reyes RM, Zhang C, Deng Y, Ji N, Mukherjee N, Padron AS, et al. CD122-targeted interleukin-2 and alphaPD-L1 treat bladder cancer and melanoma via distinct mechanisms, including CD122-driven natural killer cell maturation. Oncoimmunology 2021;10:2006529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Merchant R, Galligan C, Munegowda MA, Pearce LB, Lloyd P, Smith P, et al. Fine-tuned long-acting interleukin-2 superkine potentiates durable immune responses in mice and non-human primate. J Immunother Cancer 2022;10:e003155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Miller JS, Morishima C, McNeel DG, Patel MR, Kohrt HEK, Thompson JA, et al. A first-in-human Phase I study of subcutaneous outpatient recombinant human IL15 (rhIL15) in adults with advanced solid tumors. Clin Cancer Res 2018;24:1525–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Furuya H, Chan OTM, Pagano I, Zhu C, Kim N, Peres R, et al. Effectiveness of two different dose administration regimens of an IL15 superagonist complex (ALT-803) in an orthotopic bladder cancer mouse model. J Transl Med 2019;17:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wrangle JM, Velcheti V, Patel MR, Garrett-Mayer E, Hill EG, Ravenel JG, et al. ALT-803, an IL15 superagonist, in combination with nivolumab in patients with metastatic non–small cell lung cancer: a non-randomized, open-label, phase Ib trial. Lancet Oncol 2018;19:694–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chamie K. QUILT-3.032: A multicenter clinical trial of intravesical bacillus Calmette-Guerin (BCG) in combination with ALT-803 (N-803) in patients with BCG unresponsive high grade non–muscle-invasive bladder cancer. 2017. [Google Scholar]
- 87.Sun Y, Reddy P. Immune biology of allogeneic hematopoietic stem cell transplantation. Socié G, Blazar BR, editors: Academic Press; 2013. [Google Scholar]
- 88.Yu DS, Wu CL, Ping SY, Keng C, Shen KH. Bacille Calmette-Guerin can induce cellular apoptosis of urothelial cancer directly through Toll-like receptor 7 activation. Kaohsiung J Med Sci 2015;31:391–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Skinner RB. Imiquimod. Dermatol Clin 2003;21:291–300. [DOI] [PubMed] [Google Scholar]
- 90.Singh M, Kaur M. Eyelid Basal cell carcinoma treated with Imiquimod 5% cream monotherapy. Orbit 2023:1. [DOI] [PubMed] [Google Scholar]
- 91.Hotz C, Treinies M, Mottas I, Rötzer LC, Oberson A, Spagnuolo L, et al. Reprogramming of TLR7 signaling enhances antitumor NK and cytotoxic T cell responses. OncoImmunology 2016;5:e1232219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Vey N, Karlin L, Sadot-Lebouvier S, Broussais F, Berton-Rigaud D, Rey J, et al. A phase I study of lirilumab (antibody against killer immunoglobulin-like receptor antibody KIR2D; IPH2102) in patients with solid tumors and hematologic malignancies. Oncotarget 2018;9:17675–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Carlsten M, Korde N, Kotecha R, Reger R, Bor S, Kazandjian D, et al. Checkpoint inhibition of KIR2D with the monoclonal antibody IPH2101 induces contraction and hyporesponsiveness of NK cells in patients with myeloma. Clin Cancer Res 2016;22:5211–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ge Z, Peppelenbosch MP, Sprengers D, Kwekkeboom J. TIGIT, the next step towards successful combination immune checkpoint therapy in cancer. Front Immunol 2021;12:699895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.ClinicalTrials.gov [database on the Internet]. National Library of Medicine. [updated 2022. Dec 6; cited 2023 Mar 2]. Unique ID: NCT04143711. Available from: https://clinicaltrials.gov/search?term=NCT04143711. [Google Scholar]
- 96.ClinicalTrials.gov [database on the Internet]. National Library of Medicine. [cited 2023 Mar 2]. Available from: https://clinicaltrials.gov/search?term=NCT02489890. [Google Scholar]
- 97.ClinicalTrials.gov [database on the Internet]. National Library of Medicine. [cited 2023 Mar 2]. Available from: https://clinicaltrials.gov/search?term=NCT02886897. [Google Scholar]
- 98.Alkon N, Bauer WM, Krausgruber T, Goh I, Griss J, Nguyen V, et al. Single-cell analysis reveals innate lymphoid cell lineage infidelity in atopic dermatitis. J Allergy Clin Immunol 2022;149:624–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bielecki P, Riesenfeld SJ, Hutter JC, Torlai Triglia E, Kowalczyk MS, Ricardo-Gonzalez RR, et al. Skin-resident innate lymphoid cells converge on a pathogenic effector state. Nature 2021;592:128–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Song P, Cao K, Mao Y, Ai S, Sun F, Hu Q, et al. Tissue specific imprinting on innate lymphoid cells during homeostasis and disease process revealed by integrative inference of single-cell transcriptomics. Front Immunol 2023;14:1127413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhang Q, Bi J, Zheng X, Chen Y, Wang H, Wu W, et al. Blockade of the checkpoint receptor TIGIT prevents NK cell exhaustion and elicits potent antitumor immunity. Nat Immunol 2018;19:723–32. [DOI] [PubMed] [Google Scholar]
- 102.Bai R, Cui J. Burgeoning exploration of the role of natural killer cells in anti–PD-1/PD-L1 therapy. Front Immunol 2022;13:886931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ramakrishnan S, Granger V, Rak M, Hu Q, Attwood K, Aquila L, et al. Inhibition of EZH2 induces NK cell–mediated differentiation and death in muscle-invasive bladder cancer. Cell Death Differ 2019;26:2100–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Seo H, Kim BS, Bae EA, Min BS, Han YD, Shin SJ, et al. IL21 therapy combined with PD-1 and Tim-3 blockade provides enhanced NK cell antitumor activity against MHC class I-deficient tumors. Cancer Immunol Res 2018;6:685–95. [DOI] [PubMed] [Google Scholar]
- 105.Liu Q, You B, Meng J, Huang CP, Dong G, Wang R, et al. Targeting the androgen receptor to enhance NK cell killing efficacy in bladder cancer by modulating ADAR2/circ_0001005/PD-L1 signaling. Cancer Gene Ther 2022;29:1988–2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.da Silva IP, Gallois A, Jimenez-Baranda S, Khan S, Anderson AC, Kuchroo VK, et al. Reversal of NK-cell exhaustion in advanced melanoma by Tim-3 blockade. Cancer Immunol Res 2014;2:410–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ndhlovu LC, Lopez-Verges S, Barbour JD, Jones RB, Jha AR, Long BR, et al. Tim-3 marks human natural killer cell maturation and suppresses cell-mediated cytotoxicity. Blood 2012;119:3734–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Yuasa T, Sato K, Ashihara E, Takeuchi M, Maita S, Tsuchiya N, et al. Intravesical administration of gammadelta T cells successfully prevents the growth of bladder cancer in the murine model. Cancer Immunol Immunother 2009;58:493–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bald T, Krummel MF, Smyth MJ, Barry KC. The NK cell–cancer cycle: advances and new challenges in NK cell–based immunotherapies. Nat Immunol 2020;21:835–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Park JE, Kim SE, Keam B, Park HR, Kim S, Kim M, et al. Antitumor effects of NK cells and anti–PD-L1 antibody with antibody-dependent cellular cytotoxicity in PD-L1-positive cancer cell lines. J Immunother Cancer 2020;8:e000873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Dong W, Wu X, Ma S, Wang Y, Nalin AP, Zhu Z, et al. The mechanism of anti–PD-L1 antibody efficacy against PD-L1-negative tumors identifies NK cells expressing PD-L1 as a cytolytic effector. Cancer Discov 2019;9:1422–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Gao Y, Souza-Fonseca-Guimaraes F, Bald T, Ng SS, Young A, Ngiow SF, et al. Tumor immunoevasion by the conversion of effector NK cells into type 1 innate lymphoid cells. Nat Immunol 2017;18:1004–15. [DOI] [PubMed] [Google Scholar]
- 113.Damrauer JS, Hoadley KA, Chism DD, Fan C, Tiganelli CJ, Wobker SE, et al. Intrinsic subtypes of high-grade bladder cancer reflect the hallmarks of breast cancer biology. Proc Natl Acad Sci USA 2014;111:3110–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Carretero R, Sektioglu IM, Garbi N, Salgado OC, Beckhove P, Hammerling GJ. Eosinophils orchestrate cancer rejection by normalizing tumor vessels and enhancing infiltration of CD8(+) T cells. Nat Immunol 2015;16:609–17. [DOI] [PubMed] [Google Scholar]
- 115.Reichman H, Itan M, Rozenberg P, Yarmolovski T, Brazowski E, Varol C, et al. Activated eosinophils exert antitumorigenic activities in colorectal cancer. Cancer Immunol Res 2019;7:388–400. [DOI] [PubMed] [Google Scholar]
- 116.Schuijs MJ, Png S, Richard AC, Tsyben A, Hamm G, Stockis J, et al. ILC2-driven innate immune checkpoint mechanism antagonizes NK cell antimetastatic function in the lung. Nat Immunol 2020;21:998–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Jacquelot N, Seillet C, Wang M, Pizzolla A, Liao Y, Hediyeh-Zadeh S, et al. Blockade of the co-inhibitory molecule PD-1 unleashes ILC2-dependent antitumor immunity in melanoma. Nat Immunol 2021;22:851–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Bruchard M, Geindreau M, Perrichet A, Truntzer C, Ballot E, Boidot R, et al. Recruitment and activation of type 3 innate lymphoid cells promote antitumor immune responses. Nat Immunol 2022;23:262–74. [DOI] [PubMed] [Google Scholar]


