Abstract
Background
British soldiers undergoing jungle training in Belize typically experience a relatively low risk of developing cutaneous leishmaniasis. However, an uncharacteristically large outbreak of cutaneous leishmaniasis occurred in 2022. This study aimed to determine the cumulative incidence of the disease and highlight potential shortcomings in personal protective measures to mitigate exposure to sand fly vector bites.
Methods
A retrospective analysis was conducted on medical records of cutaneous leishmaniasis cases between 2005 and 2022, as well as on questionnaire responses regarding personal protective measures administered to cutaneous leishmaniasis cases in 2022. Data were sourced from Defence Public Health Unit, Military Environmental Health Department and British Army Training Support Unit Belize.
Results
Eighty-one confirmed clinical cutaneous leishmaniasis cases were recorded between 2005 and 2022, with a substantial peak (38 cases) in 2022. Most cases occurred during the wet season. Pre-2022, the median cumulative incidence per 8-week deployment was 0.90 % (Q1–Q3: 0.34 %–1.34 %), with an annual variation of 0.2 % to 2.0 %. In 2022, the cumulative incidence spiked to 4.22 %, associated with a risk ratio of 5.3 (95 % C.I.s, 3.41, 8.16), and rising to a cumulative incidence of 7.3 % in a single unit of 450 men (33 cases) in late 2022. These values are significantly higher than the median cumulative incidence of all previous years, and to published reports for other cutaneous leishmaniasis -endemic regions. Troop responses identified limitations in the supply of optimal equipment, and in sand fly bite and leishmaniasis risk avoidance information provided by the pre-deployment health education programme. Compliance with health education advise was also suboptimal, with irregular use of insect repellents, protective clothing / head netting, and insecticide-treated hammocks.
Conclusions
The reasons behind the unusually high numbers of cutaneous leishmaniasis cases and cumulative incidence in 2022 remain unclear, emphasising the need to improve personal protective measures provision and implement a comprehensive health education programme for troops undergoing jungle training in Belize.
Keywords: Leishmania, Cutaneous leishmaniasis, Cumulative incidence, Risk practices, British soldiers, Jungle training, Belize
1. Introduction
Cutaneous leishmaniasis (CL) is the most common form of human leishmaniasis. Leishmania are protozoan parasites transmitted by infectious female Phlebotomine sand flies when blood-feeding on human hosts (WHO Expert Committee on the Control of the Leishmaniases, World Health Organization, 2022; Leishmaniasis, 2022). Clinical signs of CL predominantly presents as skin lesions, particularly ulcers, and can significantly impact an individual's quality of life (Peleva and Walker, 2020; Vares et al., 2013). CL is prevalent in the Americas, Mediterranean basin countries, sub-Saharan Africa, the Middle East, and central and south east Asia, amounting to an estimated annual incidence of 600,000 to 1 million cases worldwide (Leishmaniasis, 2022; Prevention CC for DC and. CDC - Leishmaniasis - Epidemiology and Risk Factors 2020, 2022). In the Americas, CL is endemic in 19 countries where 1,105,545 cases were reported between 2001 and 2021, with an average of 52,645 cases per year (Leishmaniasis - PAHO/WHO | Pan American Health Organization, 2023).
CL is endemic in Belize (WHO | World Health Organization, 2023), primarily due to infection with Leishmania (L) mexicana and L.(V.) braziliensis (Bailey, 2011; Strangways-Dixon and Lainson, 1962; Murphy and Bailey, 2024). Multiple species of forest-dwelling sand fly vectors are historically documented (Williams, 1970; Williams, 1965) and rodents are suspected to be the zoonotic reservoir hosts (Lainson and Strangways-Dixon, 1964; Lainson and Strangways-Dixon, 1963; Strangways-Dixon and Lainson, 1966; Disney, 1968). Current understanding of Leishmania transmission in Belize relies on historical accounts that documented CL prevalence in the local human population (Williams et al., 1965), the diversity and abundance of rodent (Williams, 1965; Disney, 1968; Williams et al., 1965; Disney, 1966) and sand fly (Williams, 1965; Lainson and Strangways-Dixon, 1964) species, their associated phenologies, ecotypes and sand fly biting behaviours (Williams, 1965; Lainson and Strangways-Dixon, 1964), and attempts to detect Leishmania infections to incriminate these species as reservoirs and vectors, respectively.
CL is commonly observed among soldiers who have returned from international deployments to CL endemic regions. Notable case numbers are associated with deployments to Iraq (Beiter et al., 2019; Frenck et al., 2005), Afghanistan (Beiter et al., 2019; Frenck et al., 2005; Cutaneous Leishmaniasis (Leishmania major Infection) in Dutch Troops Deployed in Northern Afghanistan: Epidemiology, Clinical Aspects, and Treatment - PMC, 2023), French Guiana (Henry et al., 2021; Berger et al., 2006), Brazil (American cutaneous leishmaniasis in military training unit localized in Zona da Mata of Pernambuco State, Brazil - ProQuest, 2023; American tegumentary leishmaniasis caused by Leishmania (Viannia) braziliensis in military training area of Zona da Mata in Pernambuco - ProQuest, 2023), Panama (Sanchez et al., 1992), Peru (Oré et al., 2015), and Belize (Bailey, 2011; Hepburn et al., 1993; van Thiel et al., 2011; Biddlestone et al., 1994), with attack rates ranging from 2.1 % to 25.2 %. The risk of sand fly bite exposure leading to infection has been attributed to several factors, including failure to adopt personal protective measures (PPMs) (Henry et al., 2021; Sanchez et al., 1992; Oré et al., 2015; van Thiel et al., 2011; Royer and Crowe, 2002; van Thiel et al., 2010; Vickery et al., 2008; Van Der Snoek et al., 2009; Gunathilaka et al., 2020; Bailey et al., 2012), participation in military training activities during periods of sand fly biting (Henry et al., 2021; Sanchez et al., 1992; Oré et al., 2015), and in marshy areas near streams or rivers (Royer and Crowe, 2002; Van Der Snoek et al., 2009), and operations in areas undergoing significant environmental changes including deforestation, illegal logging, and other land use alterations for agriculture (Henry et al., 2021; Oré et al., 2015).
Leishmaniasis usually impedes soldiers' ability to perform their duties, leading to increased sick leave, reduced combat readiness, and potential long-term health consequences. Furthermore, the need for diagnosis, treatment, and compensation claims further strain military resources. Between 1998 and 2021, >65 % of diagnosed CL cases in the military returning to the UK were recently deployed to Belize (formerly British Honduras) (Bailey, 2011), where 306 CL cases were recorded in British soldiers between 1978 and 1990 (Hepburn et al., 1993). During a more recent outbreak in Belize in 2022, 33 CL cases were confirmed in British soldiers participating in a single 8-week jungle exercise (MOD data – restricted access). In addition to military jungle training (JT), infections leading to CL in Belize are predominantly associated with forest activites including chewing-gum latex collection, and tourism (Bailey, 2011; Blaylock and Wortmann, 2012; Pollack et al., 2019; Vinetz and Soong, 2007).
Despite the serious impacts of CL, there is a notable gap in the current knowledge among military personnel concerning the epidemiology, incidence, and risk factors for exposure to potentially infectious sand fly vectors. To address these knowledge gaps, this study undertook a review of the cumulative incidence of CL cases arising from British military JT in Belize between 2005 and 2022, and conducted a preliminary investigation of compliance to provide and adopt personal protective measures (PPMs) to mitigate vector exposure during deployment.
2. Methods
2.1. Ethics statement
The study was conducted in accordance with the principles of the Declaration of Helsinki and all applicable laws and regulations governing research ethics. The study was a review of anonymised secondary data belonging to, and authorised, by the Ministry of Defence.
2.2. Study setting
British troops undergo JT centred around the British military base in the British Army Training Support Unit Belize (BATSUB). The training areas include Rio Bravo, Laguna Sector, Gallon Jug and Yalbac in the north west of the country; 1963 Line, Chiquibul (Guacamallo) and Mountain Pine Ridge in the south west; Baldy Beacon and Sibuan Gorge in the centre, and Manatee north in the east (Fig. 1).
Fig. 1.
British army training areas (red boxes) and BATSUB in Belize, Central America. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Abreviations: JT = jungle training; BATSUB = Britiah army training support unit.
These training grounds are characterised by tropical rainforests, predominantly consisting of primary and/or secondary jungle including hardwoods, palms, pines and various flowering plants. Belize experiences two distinct climatic seasons: a dry season from December to April and a wet season from May to November, with average temperatures ranging from 23 °C to 27 °C throughout the year. In the wet season, the southern part of the country receives an average monthly rainfall of 150 mm to 400 mm, while other areas receive less than 100 mm per month (Belize - Climatology | Climate Change Knowledge Portal, 2023; Belize - Climate | Britannica, 2023). Troops are deployed in all seasons.
2.3. Study population
The British military's JT program in Belize involves approximately 1000 soldiers each year comprising various UK units, being sent to BATSUB, where they undergo an 8-week program designed to train soldiers in the jungle environment. The programme comprises two weeks of acclimatisation and preparation in BATSUB, five weeks of uninterupted time living in the jungle undergoing training (jungle Ex phase) based at one of the indicated training areas (Fig. 1), followed by a week of rest and recuperation (R&R) housed back at BATSUB. Participating in these training exercises are ranks of junior non-commissioned officers (JNCO; Privates, Lance Corporals, and Corporals), senior non-commissioned officers (SNCO; Sergeants, Staff Sergeants, and Warrant Officers), and Officers. Soldiers should receive pre-deployment health education (PDHE) providing them with information regarding health risks in the deployment area, highlighting the importance of adopting and usage of PPMs and related hygiene practices. The goal is to equip soldiers with the knowledge and skills needed to maintain their well-being. Typically, PDHE is intended to be delivered by qualified military environmental health practitioners (EHPs); however, there are instances when it is delivered by combat medical technicians (CMTs).
2.4. Data collection and analyses
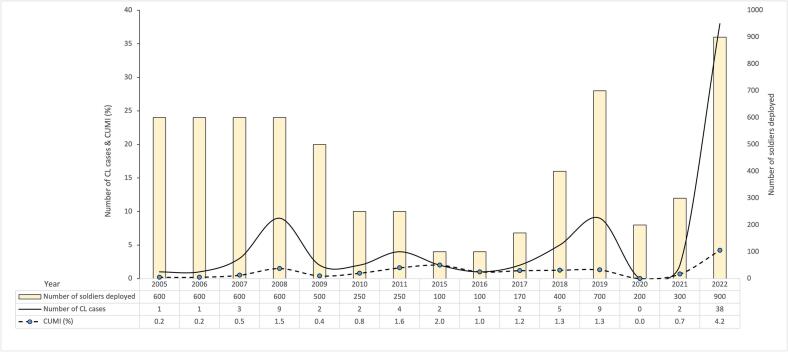
Anonymised medical records of clinically and laboratory confirmed CL cases among British soldiers who underwent JT in Belize between 2005 and 2022 were provided by the Defence Public Health Unit; the total numbers of British soldiers deployed (at risk) during specified periods of JT were provided by BATSUB. No duplicate/missing records were identified. Cases were laboratorily confirmed using both microscopy, culture and/or PCR. The cumulative incidence (CUMI) of CL was calculated by dividing the number of new CL cases during specified periods of training by the total number of troops deployed (all condiered at risk) at the beginning of the 8-week training period (Fig. 2, Fig. 3). The annual CUMI was calculated by aggregating annual total CL case and deployed troop numbers (Fig. 2).
Fig. 2.
Cumulative incidence (CUMI) of CL in British troops following JT in Belize.
Fig. 3.
The numbers of CL cases that reported to the medical treatment facility (MTF) (weeks 16 to 27 post deployment) and the epidemic curve as described by the British military environmental health department.
Abreviations: MTF = medical treatment facility.
To investigate provision and adoption of PPMs to mitigate sand fly vector bite exposure during JT, a retrospective analysis was conducted on questionnaire responses collected from a cohort of 22 soldiers with confirmed CL during the recent outbreak in 2022. The questionnaire captured demographic information including sex, age, military rank, previous endemic overseas postings, and forest training area where billeted. Data described the breadth and depth of received PDHE regarding vector-borne diseases including leishmaniasis, utilisation of PPMs, and the soldiers' attitudes and actual practices to mitigate exposure to biting insects.
Data were analysed using STATA v.18.
3. Results
3.1. Cumulative incidence of CL
Between 2005 and 2022, a total of 6304 British soldiers participated in JT in Belize. Among these individuals, there were 81 confirmed cases of CL, all of whom were males. The median age of the cases was 25 years (range: 19–40 years). The majority of the cases were JNCOs (85.2 %), in the army service (95.1 %), and deployed to Belize during the wet season (71.6 %) (Table 1). Due to the COVID-19 pandemic, only two units of limited numbers, were deployed in 2020 and 2021 (Table 2).
Table 1.
Demographic and deployment characteristics of the 81 confirmed CL cases.
| Characteristic | Sex |
Military Service |
Rank |
Season deployed |
||||
|---|---|---|---|---|---|---|---|---|
| Male | Army | Royal Navy | JNCO | SNCO | Ofr | Wet | Dry | |
| Number of individuals | 81 | 77 | 4 | 69 | 7 | 5 | 58 | 23 |
| % | 100 | 95.1 | 4.9 | 85.2 | 8.6 | 6.2 | 71.6 | 28.4 |
Abreviations: JNCO = junior non-commissioned officer; SNCO = senior non-commissioned officer; Ofr = officer.
Table 2.
Seasonal cumulative incidence (CUMI) of CL in British troop units deployed in an 8 – week jungle training in Belize between 2019 and 2022.
| Year | Number of units | Jungle training (JT) area | Month deployed | Season | Number of soldiers deployed | Number of confirmed CL cases | Cumulative incidence (CUMI) (%) |
|---|---|---|---|---|---|---|---|
| 2019 | 1st Unit | 1963 Lines | January | Dry | 100 | 0 | 0.0 |
| 2nd Unit | Chiquibul1 | April | Dry | 120 | 0 | 0.0 | |
| 3rd Unit | Gallon Jug1 and Yalbac | September | Wet | 150 | 0 | 0.0 | |
| 4th Unit | 1963 Lines | October | Wet | 330 | 9 | 2.7 | |
| 2020 | 1st Unit | 1963 Lines | April | Dry | 80 | 0 | 0.0 |
| 2nd Unit | Yalbac | September | Wet | 120 | 0 | 0.0 | |
| 2021 | 1st Unit | 1963 Lines | January | Dry | 120 | 0 | 0.0 |
| 2nd Unit | Gallon Jug1 and Yalbac | September | Wet | 180 | 2 | 1.1 | |
| 2022 | 1st Unit | Mountain Pine Ridge | January | Dry | 100 | 0 | 0.0 |
| 2nd Unit | 1963 Lines | April | Dry | 150 | 2 | 1.3 | |
| 3rd Unit | Mountain Pine Ridge & Chiquibul1 | September | Wet | 200 | 3 | 1.5 | |
| 4th Unit | Gallon Jug1 and Yalbac | October | Wet | 450 | 33 | 7.3 |
JT areas where historical accounts report Leishmania infections in sand flies (see text for literature sources).
The median CUMI of CL between 2005 and 2021 was 0.9 % (Q1–Q3: 0.34 %–1.34 %) with an annual variation of 0.2 %–2.0 % (Fig. 2). A positive upward trend in the CUMI with increasing year was detected, though this failed to reach statistical significance (Poisson regression: b = 0.05, z = 1.90, P = 0.058). In 2022, the proportion of troops that acquired CL significantly increased from 43/5370 pre-2022 to 38/900 (χ2 = 70.8, P < 0.0001), with a CUMI of 4.2 %. This represented a 5.3-fold increase in the relative risk of developing CL in 2022 (IRR = 5.3 [95 % C.I.s, 3.41, 8.16]), compared to the median CUMI for the years previous (z = 7.47, P < 0.0001).
Abreviations: CL = cutaneous leishmaniasis; CUMI = cumulative incidence.
Higher resolution data were available for 2019 to 2022 (Table 2). Usually, four units on average undergo JT in Belize per year, two during the dry season and two during the wet season. The vast majority of CL cases (95.9 %) during these years occurred among units that were deployed in the wet season. Most CL cases were associated with training in the 1963 Lines, Gallon Jug, and Yalbac JT areas, situated in the west of Belize (Fig. 1).
Comparing seasons of troop deployment, the CUMI was significantly higher in the wet season (47/1430) compared to in the dry season (2/670) (χ2 = 17.88, P < 0.001), even when excluding the unusually high number of CL cases in 2022 (11/780 vs 0/420) (χ2 = 5.98, P = 0.01) (Table 2).
3.2. The outbreak of 2022
In late 2022, an outbreak involved 33 confirmed CL cases in a single unit of 450 men (CUMI = 7.3 %) (Table 2). This unit underwent JT in the wet season in Gallon Jug and Yalbac, otherwise known as the Orange Walk District (Fig. 1), where past similar deployments, all in the wet season, resulted in 0/150 (2019) and 2/180 (2021) CL cases (Table 2). The 33 cases would have been exposed most likely during October 28th to December 10th during JT in 2021, and were initially documented from week 15 after their return to the UK, and continued to be confirmed up until week 26 (May 2022) (Fig. 3). The median incubation period is uncertain as the precise dates of exposure to sand fly bites and subsequent infections were not observed. Taken from the last day of potential exposure, prior to departure from Belize (in week 6), to the time of lesion onset by self-reporting to the Medical Treatment Facility (MTF), the median incubation period would be 6.5 weeks (Q1–Q3: 4–12 weeks; range: 1–20 weeks). Alternatively, if it is assumed that exposure and transmission occurred during the mid-training period on average (week 3), the equivalent median incubation period could be 11 weeks (range: 1–22 weeks).
3.3. Mitigating practises
All 33 confirmed cases of the 2022 outbreak were sent a questionnaire. Of these, 22 individuals responded (a response rate of 66.7 %). The demographic characteristics of the 22 cases were 100 % male, and aged 18–41 years old (most [40.9 %] 18–25 years old) (Table 3). The majority (68.2 %) were JNCOs and had no previous overseas experience. Six of the soldiers had a history of overseas deployments to a Leishmania-endemic country, including Iraq, Belize, and Kenya in 2020 and 2021. None of the soldiers had a documented history of leishmaniasis prior to the 2022 outbreak.
Table 3.
Demographic characteristics of 22 soldiers' responses to the questionnaire.
| Xtics | Age group |
Sex |
Rank |
History of overseas deployment to Leishmania EC |
Onset of symptoms post-deployment (weeks)1 |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 18 to 25 | 26 to 35 | 36+ | Male | JNCO | SNCO | Ofr | Yes | No | < 4 | 4 to 12 |
> 12 | |
| n | 9 | 7 | 6 | 22 | 15 | 4 | 3 | 6 | 16 | 5 | 13 | 4 |
| % | 40.9 | 31.8 | 27.3 | 100 | 68.2 | 18.2 | 13.6 | 27.3 | 72.7 | 22.7 | 59.1 | 18.2 |
Abreviations: Xtics = characteristics; n = frequency; % = percentage; JNCO = junior non-commissioned officer; SNCO = senior non-commissioned officer; Ofr = officer; EC = endemic country.
Self-reported first appearance of cutaneous lesions from last week of deployment.
3.3.1. Pre-deployment health education (PDHE)
The majority 18/22 (81.8 %) of respondants reported that they had received PDHE; one soldier did not, and three were unsure (Fig. 4). Fourteen (63.6 %) received PDHE from a CMT, and the remainder received from an EHP. These 18 soldiers reported receiving PDHE advice on PPMs including protective clothing and general methods to prevent insect bites. Only 6 (33.3 %) of the 18 soldiers were specifically informed about sand flies and leishmaniasis, 4/6 delivered by an EHP (Fig. 4).
Fig. 4.
CL case responses to a questionnaire on pre-deployment health education (PDHE) including awareness of sand flies and leishmaniasis. The PDHE brief was delivered either by a CMT or an EHP.
Abreviations: CMT = combat medical technician; EHP = environmental health practitioner.
3.3.2. Provision and use of personal protective measures (PPMs) to avoid insect bites
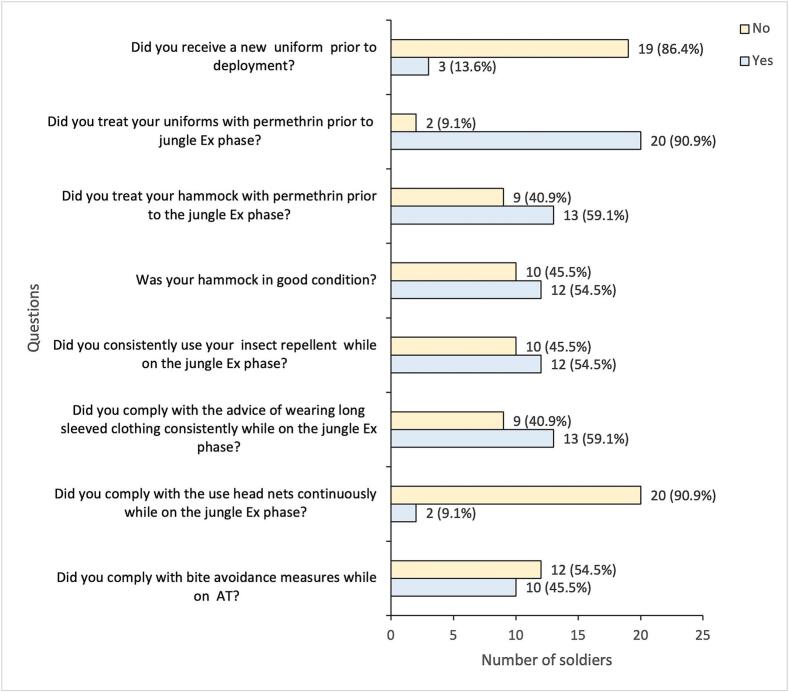
All soldiers received hammocks before the jungle exercise (Ex) phase, but 10 (45.5 %) reported the received hammocks were in poor condition (holes and/or tears) (Fig. 5). Most (90.9 %) of the soldiers treated their uniforms with Permapel (0.5 % permethrin in 300 ml) before the jungle Ex phase, whereas fewer (59.1 %) treated their hammocks. Three (13.6 %) soldiers requested new uniforms before their deployment (optional). All soldiers confirmed that they were provided with insect repellent (N,N-diethyl-meta-toluamide or DEET-34 %) and head nets, however, over 40 % and up to 90.9 % did not use their insect repellent, wear their head nets, or wear long sleeved clothing consistently during the jungle Ex phase. Post jungle Ex phase, while on (R&R), 54.5 % of the soldiers reported that they did not comply with these topical repellent or coverings (Fig. 5).
Fig. 5.
CL case responses to a questionnaire on the provision and compliance with personal protective measures (PPMs).
Abreviations: Ex = exercise; AT = adventurous training.
4. Discussion
The consequences of developing CL are not life-threatening, but at the same time are not trivial. Afflicted soldiers need to undergo lengthy treatments. This can be especially unpleasant particularly for infections with L. (V.) braziliensis, where prolonged/intensive treatments are necessary to clear the parasite in order to avoid serious disfigurement through the destruction of the naso-pharyngeal tissues, a condition known as mucocutaneous leishmaniasis, which often occurs only later in life. Second, the disease has financial implications to the armed forces. Chemotherapeutic treatments costs average £5000 per patient, and uncomplicated cases are typically out of service for 8–24 weeks. Compensation claims against the MOD can be substantial. Thus, increasing vigilance, adoption of socially acceptable PPMs, and evaluation of complimentary vector control methods, are undoubtedly justified.
Whilst transmission risk occurs all year round, greater risk of infection (>95 % of all CL cases and the CUMI) is experienced by troops deployed during the wet season compared to the dry season. The common sand fly species considered to be the important vectors in Belize include Lutzomyia (Lu.) panamensis, Lu. shannoni, Lu. cruciatus, and Lu. ovallesi (Williams, 1965). All of these vector species are traditionally considered forest species where leaf-litter, tree buttresses and hollows and animal burrows are potential adult diurnal resting sites (Williams, 1970). Information on the phenology of these sand fly species in Belize and elsewhere are scarce; many of the sand fly vectors in the Americas are more abundant during the wet season phases, whereas some are more dry-adapted, but spatio-temporal variations are reported (Dutari and Loaiza, 2014).
Though not based on exhaustive investigations, historical accounts report Leishmania infections in Lu. panamensis, Lu. shannoni, Lu. cruciatus, and Lu. ovallesi sand flies in Gallon Jug, Chiquibul, and Guacamallo (Williams, 1970; Williams, 1965) where soldiers conduct JT today (Fig. 1).
The majority of CL cases among British soldiers between 2019 and 2022 occurred following JT in Gallon Jug, Yalbac, and 1963 Lines, all situated in the west of Belize.
Belize lacks what is conventionally termed “primary” or “virgin” broadleaf forest, owing to historical land use practices by the ancient Maya peoples and various natural and human-induced disturbances like hurricanes, fires, and logging (Primack et al., 1998). Consequently, areas untouched since 1980 are designated as “mature forests” rather than “primary forests” (Voight et al., 2019). Although not as forested as the Toledo District in southern Belize (Voight et al., 2019), the western region of Belize is recognised for its mature forest, referred to locally as high bush (Garnham and Lewis, 1959). This region habours the main JT areas (Gallon jug, Yalbac and Laguna Sector in the northwest, and Guacamallo in the southwest). Other JT areas such as Chiquibul and Mountain Pine Ridge have been described as medium bush, and Sibun Gorge as low bush (Williams, 1965); all of which are mainly secondary forest. Hence it appears that sand fly vectors and Leishmania may be circulating in most if not all of these broadly characterised ecotypes (Table 2; Fig. 1).
The primary aetiological agents of CL in the region are L. (L.) mexicana and L. (V.) braziliensis, the former being more prevalent (Bailey, 2011; Strangways-Dixon and Lainson, 1962; Williams, 1970; Hepburn et al., 1993; van Thiel et al., 2011; Biddlestone et al., 1994). The traditional occupational risk of CL due to L. (L.) mexicana infection (giving rise to the name chiclero's ulcer) was predominant among collectors (“chicleros”) of chewing-gum latex (chiclé) from Sapodilla trees Manilkara zapota, conducted in forests during the wet season (Lainson, 1983). Beltran & Bustamente (1942) noted that most CL cases among 1506 inhabitants of 58 surveyed chiclé camps in neighbouring Mexico occurred during the wet season, with CL prevalences of 16.7 % and 2.2 % in males and females, respectively (Beltran and Bustamante, 1942). An epidemiological survey of 14 geographically independent farming communities located in forested areas (n = 1448 people), revealed a similar 10.8 % (18.1 % males, 3.7 % females) positive to L. (L.) mexicana skin test antigen which is indicative of exposure (Chalmers et al., 1968).
The 2022 military CL outbreak following training in western Belize represents a 5.3-fold (95 % C.I.s: 3.41, 8.16) increase in the relative risk of developing CL compared to previous years. This increase is unexpectedly high, considering that the 1 % CUMI is notably lower than the reported values in comparable military deployments in Belize (Dutch soldiers) (van Thiel et al., 2011), as well as the majority of values for French Guiana (French soldiers) (Henry et al., 2021) and Afghanistan (Dutch soldiers) (van Thiel et al., 2010), which share similar deployment characteristics (duration, training activities, and period).
The unsolved question is why did this outbreak occur? Plausible non-mutually exclusive reasons might include unusual climatic events promoting changes in sand fly vector and/or reservoir compositions or abundance affecting host-vector-parasite interactions; changes in troop susceptibility (e.g. host innate immunity or parasite virulence); and alterations in troop activities or behaviours which increase their exposure to infectious vector bites.
To initiate an investigation of the latter possiblity, the individuals afflicted during the 2022 outbreak in Unit 4 were asked for responses to a questionnaire covering the provision of, and compliance to, PPMs. The army provides PDHE through health briefings given to soldiers either prior to their deployment to Belize or immediately upon their arrival at BATSUB, and before the jungle Ex phase begins. The responses suggest that basic PDHE information was not effectively provided, and thus soldiers' knowledge about sand flies and the risk of leishmaniasis was likely inadequate. PDHE sessions highlighting leishmaniasis risks and emphasising the importance of PPMs have been suggested as an effective way of preventing CL in military personnel in endemic regions (Oré et al., 2015; Approach to Prevention of Infectious Diseases during Military Deployments | Clinical Infectious Diseases, 2023; Impact of Phlebotomine Sand Flies on U.S. Military Operations at Tallil Air Base, Iraq: 1. Background, Military Situation, and Development of a ‘Leishmaniasis Control Program”, 2023). In Colombia, knowledge about leishmaniasis was linked to an increased likelihood of soldiers adopting PPMs against insect bites (Gonzalez et al., 2017). Of course, knowledge about leishmaniasis alone does not guarantee the adoption and implementation of PPMs (Gonzalez et al., 2017). Failure to provide approriate protective equipment such as serviceable hammocks was reported. Notwithstanding, facilities were provided to reapply insect repellent (permethrin) to uniforms and hammocks prior to JT. Most of the soldiers (90.9 %) did apply this treatment to their uniforms, but a substantial number (40.9 %) failed to treat their hammocks. Studies indicate that reapplying topical permethrin to troop uniforms (Cebula et al., 2022) and to hammocks (Henry et al., 2021) can effectively reduce the risk of sand fly contact and leishmaniasis.
Inconsistent use of long-sleeved clothing, head nets and DEET insect repellent during jungle Ex phase was also reported. Not regularly using the provided head nets was said to be due to discomfort, reduced visibility, heat, and facial irritation, which are in line with reasons provided by soldiers in other deployments (Sanchez et al., 1992). Over 40 % of soldiers did not consistently use provisioned DEET repellents and wear long-sleeved clothing during the jungle Ex phase, which is likely due to similar reasons. In one study, only 5 % of soldiers regularly applied DEET repellent during training exercises (Vickery et al., 2008). The inconsistent use of DEET among military personnel is suggested to be a contributing factor to contracting leishmaniasis (Sanchez et al., 1992; van Thiel et al., 2011; Royer and Crowe, 2002; van Thiel et al., 2010; Vickery et al., 2008; Van Der Snoek et al., 2009; Gunathilaka et al., 2020; Cebula et al., 2022). The low acceptance of the army-issued 34 % DEET provided to troops in Belize is likely attributed not only to its reported unpleasant characteristics (repugnant smell, and accidential taste, and high viscosity) but also to a lack of awareness about vector-borne diseases. As commanders play a central role in establishing a culture of compliance and responsibility, command duty proves pivotal in promoting and enforcing the correct utilisation of protective measures among troops, as evidenced by a study of US troops deployed in various locations (Vickery et al., 2008).
We acknowledge that the small sample in this study, and absence of controls, limits our ability to quantify and attribute relative risk to provision and adoption of PDHE and PPMs. Nonetheless, we consider the current data sufficient to initiate focussed investigations to improve delivery of PDHE and uptake of PPMs. Thus, the following recommendations are currently indicated, that (i) soldiers should receive adequate PDHE, preferably from professionals, emphasising transmission biology, the potential consequences of infection, and PPM options; (ii) soldiers should be equipped with all necessary and serviceable protective equipment prior to jungle Ex phase; and (iii) commanders should encourage and even enforce compliance. Further research is recommended to evaluate in more detail the effectiveness of PDHE on compliance and against vector exposure. More acceptable formulations of DEET-based insect repellent could be considered.
5. Conclusions
CL, and the recent rise in case incidence, signifies a substantial burden to the British military. This preliminary study highlights the need for vigilance and preparedness e.g. mapping sand fly vector populations across training areas, to combat future CL outbreaks. Issues surround the provision and compliance to PPMs including the need for better communication and quality provisions are discussed.
CRediT authorship contribution statement
Ngwa Niba Rawlings: Conceptualization, Data curation, Formal analysis, Methodology, Software, Visualization, Writing – original draft, Writing – review & editing. Mark Bailey: Conceptualization, Funding acquisition, Project administration, Resources, Supervision, Writing – review & editing. Peter Craig: Conceptualization, Project administration, Supervision, Visualization, Writing – review & editing. Orin Courtenay: Conceptualization, Data curation, Formal analysis, Methodology, Project administration, Supervision, Validation, Writing – original draft, Writing – review & editing.
Declaration of competing interest
There are no conflicts of interest for any of the authors.
Acknowledgements
We thank the Defence Public Health Unit and Capt Hale Isra for providing information on CL cases, and BATSUB for providing information on number of troops deployed over the study period. We also thank the outbreak investigation team (WO2 M. Soffe, SSgt MT Fatchu, Sgt S. Ward and Sgt M Ellis) who provided valuable information on the CL outbreak. This study was supported by the Research and Clinical Innovation pillar of the Defence Medical Services (RE: 22/23.003) and The Drummond Foundation (Horne/211122).
References
- American cutaneous leishmaniasis in military training unit localized in Zona da Mata of Pernambuco State, Brazil - ProQuest 2023. https://www.proquest.com/openview/d01010e3e801699b05896f77ba85c1ef/1?pq-origsite=gscholar&cbl=1796426 Available from:
- American tegumentary leishmaniasis caused by Leishmania (Viannia) braziliensis in military training area of Zona da Mata in Pernambuco - ProQuest 2023. https://www.proquest.com/openview/af0e23e536638dda7eb7b4efde010c81/1?pq-origsite=gscholar&cbl=1796426 Available from: [DOI] [PubMed]
- Approach to Prevention of Infectious Diseases during Military Deployments | Clinical Infectious Diseases. Oxford Academic; 2023. https://academic.oup.com/cid/article/44/3/424/314476 Available from: [DOI] [PubMed] [Google Scholar]
- Bailey M.S. Cutaneous leishmaniasis in British troops following jungle training in Belize. Travel Med. Infect. Dis. 2011;9(5):253–254. doi: 10.1016/j.tmaid.2011.07.001. [DOI] [PubMed] [Google Scholar]
- Bailey M.S., Caddy A.J., McKinnon K.A., Fogg L.F., Roscoe M., Bailey J.W., et al. Outbreak of zoonotic cutaneous Leishmaniasis with local dissemination in Balkh, Afghanistan. J. R. Army Med. Corps. 2012;158(3):225–228. doi: 10.1136/jramc-158-03-16. [DOI] [PubMed] [Google Scholar]
- Beiter K.J., Wentlent Z.J., Hamouda A.R., Thomas B.N. Nonconventional opponents: a review of malaria and leishmaniasis among United States Armed Forces. PeerJ. 2019;7 doi: 10.7717/peerj.6313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belize - Climate | Britannica 2023. https://www.britannica.com/place/Belize/Climate Available from:
- Belize - Climatology | Climate Change Knowledge Portal 2023. https://climateknowledgeportal.worldbank.org/country/belize/climate-data-historical Available from:
- Beltran E., Bustamante M.E. Epidemiological notes on gum Collectors’ ulcer (American Leishmaniasis) in Mexico. Revista del Instituto de Salubridad y Enfermedades Tropicales. 1942;3(1):1–28. https://www.cabdirect.org/cabdirect/abstract/19432900611 [cited 2024 Jan 10]. Available from: [Google Scholar]
- Berger F., Romary P., Brachet D., Rapp C., Imbert P., Garrabé E., et al. Outbreak of cutaneous leishmaniasis in military population coming back from French Guiana. Rev. Epidemiol. Sante Publique. 2006;54(3):213–221. doi: 10.1016/s0398-7620(06)76717-7. [DOI] [PubMed] [Google Scholar]
- Biddlestone L.R., Hepburn N.C., McLaren K.M. A clinico-pathological study of cutaneous leishmaniasis in British troops from Belize. Trans. R. Soc. Trop. Med. Hyg. 1994;88(6):672–676. doi: 10.1016/0035-9203(94)90223-2. [DOI] [PubMed] [Google Scholar]
- Blaylock J.M., Wortmann G.W. A case report and literature review of ‘Chiclero’s ulcer’. Travel Med. Infect. Dis. 2012;10(5–6):275–278. doi: 10.1016/j.tmaid.2012.08.005. [DOI] [PubMed] [Google Scholar]
- Cebula B.R., Porter W.D., Wortmann G., Byrne W.R., Polhemus M., Billick K., et al. Epidemiology of Old World cutaneous Leishmaniasis among members of the U.S. Military. Open Forum Infectious Diseases. 2022;9(Supplement 2):S503. [Google Scholar]
- Chalmers A.H., Harris J.C., Swanton R.H., Thorley A.P. A survey of the distribution of dermal leishmaniasis in British Honduras. Trans. R. Soc. Trop. Med. Hyg. 1968;62(2):213–220. doi: 10.1016/0035-9203(68)90159-4. [DOI] [PubMed] [Google Scholar]
- Cutaneous Leishmaniasis (Leishmania major Infection) in Dutch Troops Deployed in Northern Afghanistan: Epidemiology, Clinical Aspects, and Treatment - PMC. 2023. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2990047/ Available from: [DOI] [PMC free article] [PubMed]
- Disney R.H.L. A trap for Phlebotomine sandflies attracted to rats. Bull. Entomol. Res. 1966;56(3):445–451. doi: 10.1017/s0007485300056510. [DOI] [PubMed] [Google Scholar]
- Disney R.H.L. The discovery of the vector of Leishmania mexicana. Trans. R. Soc. Trop. Med. Hyg. 1968;62(3):457. doi: 10.1016/0035-9203(68)90103-x. [DOI] [PubMed] [Google Scholar]
- Dutari L.C., Loaiza J.R. American Cutaneous Leishmaniasis in Panama: a historical review of entomological studies on anthropophilic Lutzomyia sand fly species. Parasit. Vectors. 2014;7(1):218. doi: 10.1186/1756-3305-7-218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frenck R.W., Frankart C., Putnam S.D., Sharp T.W., Riddle M.S., Monteville M.R., et al. Impact of illness and non-combat injury during operations IRAQI freedom and enduring freedom (Afghanistan) Am. J. Trop. Med. Hyg. 2005;73(4):713–719. [PubMed] [Google Scholar]
- Garnham P.C.C., Lewis D.J. Parasites of British Honduras with special reference to Leishmaniasis. Trans. R. Soc. Trop. Med. Hyg. 1959;53(1):12–35. doi: 10.1016/0035-9203(59)90080-x. [DOI] [PubMed] [Google Scholar]
- Gonzalez A.M., Solis-Soto M.T., Radon K. Leishmaniasis: who uses personal protection among military personnel in Colombia? Ann. Glob. Health. 2017;83(3–4):519–523. doi: 10.1016/j.aogh.2017.10.015. [DOI] [PubMed] [Google Scholar]
- Gunathilaka N., Semege S., Pathirana N., Manamperi N., Udayanga L., Wijesinghe H., et al. Prevalence of cutaneous leishmaniasis infection and clinico-epidemiological patterns among military personnel in Mullaitivu and Kilinochchi districts of the Northern Province, early war-torn areas in Sri Lanka. Parasit. Vectors. 2020;13(1):263. doi: 10.1186/s13071-020-04137-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry K., Mayet A., Hernandez M., Frechard G., Blanc P.A., Schmitt M., et al. Outbreak of Cutaneous Leishmaniasis among military personnel in French Guiana, 2020: clinical, phylogenetic, individual and environmental aspects. PLoS Negl. Trop. Dis. 2021;15(11) doi: 10.1371/journal.pntd.0009938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hepburn N.C., Tidman M.J., Hunter J.A.A. Cutaneous leishmaniasis in British troops from Belize. Br. J. Dermatol. 1993;128(1):63–68. doi: 10.1111/j.1365-2133.1993.tb00149.x. [DOI] [PubMed] [Google Scholar]
- Impact of Phlebotomine Sand Flies on U.S. Military Operations at Tallil Air Base, Iraq: 1. Background, Military Situation, and Development of a ‘Leishmaniasis Control Program’. Journal of Medical Entomology | Oxford Academic; 2023. https://academic.oup.com/jme/article/43/4/647/901944 Available from: [DOI] [PubMed] [Google Scholar]
- Lainson R. The American leishmaniases: some observations on their ecology and epidemiology. Trans. R. Soc. Trop. Med. Hyg. 1983;77(5):569–596. doi: 10.1016/0035-9203(83)90185-2. [DOI] [PubMed] [Google Scholar]
- Lainson R., Strangways-Dixon J. Leishmani Mexicana: the epidemiology of dermal leishmaniasis in british honduras. Trans. R. Soc. Trop. Med. Hyg. 1963;57:242–265. doi: 10.1016/0035-9203(63)90182-2. [DOI] [PubMed] [Google Scholar]
- Lainson R., Strangways-Dixon J. The epidemiology of dermal leishmaniasis in british honduras: II. Reservoir-hosts of Leishmania Mexicana among the forest rodents. Trans. R. Soc. Trop. Med. Hyg. 1964;58:136–153. doi: 10.1016/0035-9203(64)90003-3. [DOI] [PubMed] [Google Scholar]
- Leishmaniasis 2022. https://www.who.int/news-room/fact-sheets/detail/leishmaniasis Available from:
- Leishmaniasis - PAHO/WHO | Pan American Health Organization 2023. https://www.paho.org/en/topics/leishmaniasis Available from:
- Murphy R, Bailey MS. An Outbreak of Cutaneous Leishmaniasis from Belize. In press.
- Oré M., Sáenz E., Cabrera R., Sanchez J.F., Santos M.B.D.L., Lucas C.M., et al. Outbreak of cutaneous Leishmaniasis in Peruvian military personnel undertaking training activities in the Amazon Basin, 2010. Am. J. Trop. Med. Hyg. 2015;93(2):340–346. doi: 10.4269/ajtmh.15-0107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peleva E., Walker S.L. Cutaneous leishmaniasis and health-related quality of life in returning travellers to the UK. J. Travel Med. 2020;27(7):taaa188. doi: 10.1093/jtm/taaa188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollack K., Flowers R., Zlotoff B. Cutaneous Leishmaniasis in a boy from Belize. J. Pediatr. 2019;204 doi: 10.1016/j.jpeds.2018.08.057. 316–316.e1. [DOI] [PubMed] [Google Scholar]
- Prevention CC for DC and. CDC - Leishmaniasis - Epidemiology & Risk Factors 2020. 2022. https://www.cdc.gov/parasites/leishmaniasis/epi.html Available from:
- Primack R.B., Bray D., Galletti H.A., Ponciano I. Island Press; 1998. Timber, Tourists, and Temples. [Google Scholar]
- Royer M., Crowe M. American cutaneous leishmaniasis: a cluster of 3 cases during military training in Panama. Arch. Pathol. Lab Med. 2002;126(4):471–473. doi: 10.5858/2002-126-0471-ACL. [DOI] [PubMed] [Google Scholar]
- Sanchez J.L., Diniega B.M., Small J.W., Miller R.N., Andujar J.M., Weina P.J., et al. Epidemiologic investigation of an outbreak of cutaneous leishmaniasis in a defined geographic focus of transmission. Am. J. Trop. Med. Hyg. 1992;47(1):47–54. doi: 10.4269/ajtmh.1992.47.47. [DOI] [PubMed] [Google Scholar]
- Strangways-Dixon J., Lainson R. Dermal leishmaniasis in British Honduras: transmission of L. brasiliensis by Phlebotomus species. Br. Med. J. 1962;1(5274):297–299. doi: 10.1136/bmj.1.5274.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strangways-Dixon J., Lainson R. The epidemiology of dermal leishmansiasis in British Honduras. 3. The transmission of Leishmania mexicana to man by Phlebotomus pessoanus, with observations on the development of the parasite in different species of Phlebotomus. Trans. R. Soc. Trop. Med. Hyg. 1966;60(2):192–207. doi: 10.1016/0035-9203(66)90027-7. [DOI] [PubMed] [Google Scholar]
- Van Der Snoek E.M., Lammers A.M., Kortbeek L.M., Roelfsema J.H., Bart A., Jaspers C.A.J.J. Spontaneous cure of American cutaneous leishmaniasis due to Leishmania naiffi in two Dutch infantry soldiers. Clin. Exp. Dermatol. 2009;34(8):e889–e891. doi: 10.1111/j.1365-2230.2009.03658.x. [DOI] [PubMed] [Google Scholar]
- van Thiel P.P., Leenstra T., de Vries H.J., van der Sluis A., van Gool T., Krull A.C., et al. Cutaneous Leishmaniasis (Leishmania major infection) in Dutch troops deployed in northern Afghanistan: epidemiology, clinical aspects, and treatment. Am. J. Trop. Med. Hyg. 2010;83(6):1295–1300. doi: 10.4269/ajtmh.2010.10-0143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Thiel P.P.A.M., Zeegelaar J.E., van Gool T., Faber W.R., Kager P.A. Cutaneous leishmaniasis in three Dutch military cohorts following jungle training in Belize. Travel Med. Infect. Dis. 2011;9(3):153–160. doi: 10.1016/j.tmaid.2011.03.001. [DOI] [PubMed] [Google Scholar]
- Vares B., Mohseni M., Heshmatkhah A., Farjzadeh S., Safizadeh H., Shamsi-Meymandi S., et al. Quality of life in patients with cutaneous Leishmaniasis. Arch. Iran. Med. 2013;16(8) 0–0. [PubMed] [Google Scholar]
- Vickery J.P., Tribble D.R., Putnam S.D., McGraw T., Sanders J.W., Armstrong A.W., et al. Factors associated with the use of protective measures against vector-borne diseases among troops deployed to Iraq and Afghanistan. Mil. Med. 2008;173(11):1060–1067. doi: 10.7205/milmed.173.11.1060. [DOI] [PubMed] [Google Scholar]
- Vinetz J.M., Soong L. Leishmania mexicana infection of the eyelid in a traveler to Belize. Braz. J. Infect. Dis. 2007;11(1):149–152. doi: 10.1590/s1413-86702007000100030. [DOI] [PubMed] [Google Scholar]
- Voight C., Hernandez-Aguilar K., Garcia C., Gutierrez S. Predictive modeling of future Forest cover change patterns in southern Belize. Remote Sens. 2019;11(7):823. [Google Scholar]
- WHO | World Health Organization 2023. https://apps.who.int/neglected_diseases/ntddata/leishmaniasis/leishmaniasis.html Available from:
- WHO Expert Committee on the Control of the Leishmaniases, World Health Organization Control of the leishmaniases: report of a meeting of the WHO Expert Commitee on the Control of Leishmaniases, Geneva, 22-26 March 2010. Control de las leishmaniasis: informe de una reunión del Comité de Expertos de la OMS sobre el Control de las Leishmaniasis, Ginebra, 22 a 26 de marzo de 2010 [Internet]. 2010. 2022. https://apps.who.int/iris/handle/10665/44412 Available from:
- Williams P. Observations on the phlebotomine sandflies of British Honduras. Ann. Trop. Med. Parasitol. 1965;59(4):393–404. doi: 10.1080/00034983.1965.11686325. [DOI] [PubMed] [Google Scholar]
- Williams P. Phlebotomine sandflies and leishmaniasis in British honduras (Belize) Trans. R. Soc. Trop. Med. Hyg. 1970;64(3):317–363. doi: 10.1016/0035-9203(70)90171-9. [DOI] [PubMed] [Google Scholar]
- Williams P., Lewis D.J., Garnham P.C. On dermal leishmaniasis in british honduras. Trans. R. Soc. Trop. Med. Hyg. 1965;59:64–71. doi: 10.1016/0035-9203(65)90140-9. [DOI] [PubMed] [Google Scholar]