Abstract
Background
Dietary patterns and lifestyle factors can influence the intensity of systemic inflammation and, consequently, the development and progression of coronary artery calcification (CAC). This study aimed to explore the relationship between the inflammatory potentials of diet and lifestyle, as captured by novel dietary and lifestyle inflammation scores (DIS and LIS), with CAC incidence and progression.
Methods
We analyzed data on 5949 Black and White men and women ≥ 45 years old participating in the Multi-Ethnic Study of Atherosclerosis (MESA) cohort. Baseline data on diet and lifestyle factors were collected from 2000 to 2002 and used to construct the DIS and LIS, which reflect the overall inflammatory potential of diet and lifestyle. Cox proportional hazard regression was used to calculate the hazard ratios (HR) and 95% confidence intervals (95% CI) for CAC incidence and progression across quartiles of DIS and LIS, adjusting for potential confounders.
Results
Over a median follow-up of 8.0 years, among 2638 participants with zero CAC score at baseline, 977 individuals developed positive scores, and 1681 out of 2561 participants showed CAC progression. For individuals in the highest (more pro-inflammatory) compared to the lowest (more anti-inflammatory) quartiles of the LIS, the multivariable-adjusted HR for CAC incidence was 1.35 (95% CI, 1.10–1.65; P trend < 0.002). This association was stronger among younger adults aged < 60 years compared to those aged ≥ 60 years, with respective values of 1.76 (1.34–2.30) and 1.02 (0.78–1.35) (P interaction < 0.001). However, the LIS was not significantly associated with the progression of existing CAC. Among the components of the LIS, a body mas index (BMI) ≥ 25 kg/m2 and current smoking were significant predictors for the incidence and progression of CAC, respectively. No significant association was found between DIS and CAC incidence and progression.
Conclusions
Lifestyle factors, through their impact on systemic inflammation, may be associated with a higher risk of CAC incidence in middle and late adulthood.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12937-024-01028-x.
Keywords: Diet, Lifestyle, Inflammation, Score, Coronary artery calcium, Adult
Introduction
Cardiovascular disease (CVD) is still a primary cause of morbidity and mortality worldwide [1], with atherosclerosis playing a central role in its pathogenesis [2]. Coronary artery calcification (CAC), is known as a surrogate for clinically significant atherosclerosis and an independent predictor of future coronary heart disease. CAC score, as detected by computed tomography (CT) imaging, is a measure of the amount of calcium in the walls of the coronary arteries [3]. Studies have shown that a greater CAC score is associated with a greater risk of heart disease [4], cause-specific cardiovascular mortality, and all-cause mortality in both younger and older adults [4].
Inflammation is not only important for the progression of atherosclerosis but is an important trigger for vascular calcification [5]. Studies have demonstrated that inflammation of the arteries precedes the development of arterial calcification [6]. Therefore, addressing inflammation may be an important aspect of preventing CVD. It has been observed that many chronic inflammatory diseases originate or have their development promoted by lifestyle factors such as diet, exercise, stress, and sleep [6]. Research findings indicate that adherence to a healthy dietary pattern such as Mediterranean diets, high-fiber diets, and diets high in fruits, vegetables, and other plant foods is independently associated with lower inflammation [7]. However, Western diets with imbalanced dietary components have been linked to pro-inflammatory effects [8], potentially leading to various inflammatory diseases [9]. Various dietary inflammation scores, such as the dietary inflammatory index (DII) and empirical dietary inflammatory pattern (EDIP) [10], have been created to assess the overall influence of dietary factors on systemic inflammation. While both the DII and the EDIP have been linked to CVD in numerous studies [11], certain limitations have been proposed for them in prior research, suggesting the need for further investigation and refinement. Limitations of these indices encompass issues with reproducibility, generalizability, assumptions, and for the DII, a predominant emphasis on nutrients [12, 13]. Recently, Byrd et al. developed and validated the novel, dietary inflammation score (DIS) and lifestyle inflammation score (LIS), which exhibited a stronger association with inflammation biomarkers compared to the DII and EDIP [13]. The associations of these new scores have been examined with various non-communicable diseases [14–19], but their correlation with CAC scores has not yet been investigated. Accordingly, this study investigated the separate associations of the DIS and the LIS with the incidence and progression of CAC among middle-aged and older adults who participated in the prospective Multi-Ethnic Study of Atherosclerosis (MESA) cohort.
Methods
The data for this study were obtained from The Biologic Specimen and Data Repository Information Coordinating Center (BioLINCC), which can be accessed at https://biolincc.nhlbi.nih.gov/.
Study design and study population
The MESA, initiated in 2000, is a prospective cohort study that followed a diverse group of individuals across six US cities: Baltimore, MD; Chicago, IL; Los Angeles, CA; New York, NY; St. Paul, MN; and Winston-Salem, NC. All participants were free of CVD at baseline, were not on active cancer treatment, and underwent CAC scans at baseline. More information on the study design of MESA has been previously published [20]. Institutional review boards at each city reviewed and approved examination protocols, and written informed consent was obtained from all study participants at each examination.
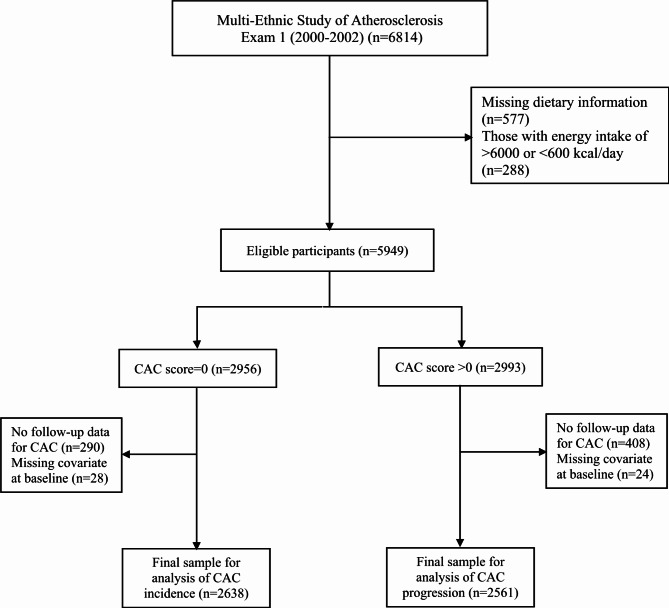
For this study, we initially included 6814 participants aged 45 to 84 years old at the baseline examination (2000–2002). Subsequently, we excluded 577 participants due to missing dietary information and 288 individuals with implausible energy intakes (< 600 or > 6000 kcal/day). Among the remaining 5949 participants, we selected 2956 individuals with a baseline CAC score of 0, and excluded 28 individuals with missing covariate data and 290 individuals without follow-up data until the end of the study (December 2012), resulting in 2638 (1011 men) individuals for the analysis of CAC incidence. For the analysis of CAC progression, we included 2993 participants from the initial 5949 individuals who had a CAC score > 0 at baseline, and excluded 408 individuals without follow-up data and 24 individuals with missing covariate data at recruitment, leaving us a final study sample of 2561 individuals (Fig. 1).
Fig. 1.
Flow chart of the participants included in the study CAC: coronary artery calcium
Data collection
Examination data and covariates
At baseline, information on demographics, medical history, family history, tobacco use, and alcohol consumption was collected by interviewer and self-administered questionnaires. Height and weight were measured to calculate body mass index (BMI) as weight (kg)/height (m2). Three readings of resting blood pressure were taken, with the average of the last two used for analysis. Laboratory measurements for fasting plasma glucose (FPG), total cholesterol (TC), and high-density lipoprotein cholesterol (HDL-C) were obtained using standard protocols. The participants’ use of blood pressure, cholesterol, Nonsteroidal anti-inflammatory drugs (NSAIDs), and diabetes medications were assessed through a questionnaire and verified with the actual medications [20]. Smoking was classified as never, former, or current smoker. Alcohol consumption was measured based on consumption of alcoholic drinks per week and was classified as never, moderate, and heavy for the analysis. A semi-quantitative questionnaire adapted from the Cross-Cultural Activity Participation Study [21] was used to evaluate physical activity in metabolic equivalent of task (METs). Physical activity was calculated by adding up the minutes per week of intentional activities, such as walking, sports, dance, and conditioning (e.g. aerobics, bicycling, running, jogging, rowing, swimming, judo, karate), and then multiplying the sum by the activity’s individual MET value. Education was categorized as (i) high school degree or lower; (ii) some college, technical school, associate degree, or bachelor’s degree; and (iii) graduate degree or professional school. A positive family history of coronary heart disease (CHD) was defined as the presence of a heart attack in a parent, sibling, or child. Diabetes was defined as having a FPG level of ≥ 7.0 mmol/l, or using insulin or oral hypoglycemic medications. Hypertension was defined as having a systolic blood pressure (SBP) of ≥ 130 mmHg, or a diastolic blood pressure (DBP) of ≥ 80 mmHg, or the use of antihypertensive medications.
Outcome ascertainment
After the baseline examination (2000–2002), participants attended up to 4 follow-up examinations: Exam 2 (2002–2004), Exam 3 (2004–2005), Exam 4 (2005–2007), and Exam 5 (2010–2012) [20]. At baseline and follow-ups coronary calcium was measured with cardiac-gated electron-beam computed tomography (CT) or a multi-detector CT depending on the study site [20]. CAC scores were determined by Agatston method, which was calculated by multiplying the area of each calcified lesion by a factor related to the maximum plaque attenuation; and then summing the scores across all lesions within a given artery and across all arteries (left main, left anterior descending, circumflex, and right coronary) to obtain the total calcium score. Each participant was scanned twice, with a mean score used for analysis [20]. All scans were phantom-adjusted and were analyzed independently by two trained radiologists at a central reading center. Rescan agreement was high using both electron-beam CT and multidetector CT scanning [22]. In this study, CAC incidence was defined as the development of CAC in individuals with baseline CAC = 0, and CAC progression was defined as an annualized change of > 10 Agatston units at follow-up among those with 0 < CAC ≤ 100 at baseline; and an annualized percent change (annualized change in CAC score divided by the baseline CAC score) > 10% among those with CAC > 100 Agatston units at baseline [23].
Dietary assessments
The MESA study consists of two dietary assessments performed at baseline (2000–2002) and in Exam 5 (2010–2012), respectively. For this study, we utilized dietary data from the initial exam (2000–2002). Dietary assessment was assessed with a modified Block-style 120-item food-frequency questionnaire (FFQ) [24]. Consumption frequency and serving size of each food or beverage were quantified. The serving sizes were quantified as small, medium, or large, with corresponding weights (g) imputed according to National Health and Nutrition Examination Survey (NHANES) data. Total energy intake was calculated by summing energy from all foods. The frequencies of alcohol and supplemental micronutrient intakes were also quantified.
DIS and LIS calculations
The DIS developed by Byrd et al. [13] was used to determine the inflammatory scores of each participant based on their baseline dietary data. DIS has 19 components including leafy greens and cruciferous vegetables, legumes, refined grains, and starchy vegetables, apples and berries, deep yellow or orange vegetables and fruit, tomatoes, other fruits, and real fruit juices, other vegetables, added sugars, red and organ meats, processed meats, fish, poultry, high-fat dairy, low-fat dairy and tea, nuts, other fats, and supplements. The weights for each component were calculated based on the strengths of their associations with the inflammation biomarker [13]. To calculate DIS, we standardized the daily intake (serving size) of each food group by sex to a mean of 0 and SD of 1.0. Supplemental micronutrients were ranked into tertiles based on sex-specific distribution. Then, the values were multiplied by their weight, and the weighted components were summed.
The LIS has four components including BMI, physical activity, smoking status, and alcohol consumption in categorical form. To calculate the LIS score, a dummy variable was created for each component category. Each category was then multiplied by its respective weight, and the weighted values for each participant were summed to calculate their LIS [13].
Statistical analysis
All analyses were conducted using R version 4.2.1. All statistical tests were two-sided, and P values < 0.05 were considered statistically significant. Follow-up time was defined as the time from baseline exam to incident outcome (CAC incidence/progression). We categorized participants into LIS and sex-specific DIS quartiles at baseline. The baseline characteristics of study participants were described using mean (standard deviation (SD)) and frequency (%), and comparisons were made using analysis of variance (ANOVA) and chi-square test.
We used Cox proportional hazard (Cox PH) regression models to investigate the association between DIS and LIS scores, considered as both continuous and categorical variables (quartiles), and the incidence and progression of CAC during the follow-up. The first quartile of each DIS and LIS score was considered the reference group. Model 1 was adjusted for age, sex, and race. For assessing the relation between the DIS and outcomes, Model 2 was additionally adjusted for BMI (continuous), educational level (less than high school/ high school, some college, graduate degree or professional school), family history of CHD, physical activity (as tertiles of MET), smoking (never, former, current), alcohol consumption (never, former, current), and total energy intake (continuous). In Model 3, additional adjustments were made for confounders in Model 2, as well as HDL-C, TC, lipid-lowering medications, NSAID, diabetes, and hypertension at baseline. Regarding the associations of the LIS and outcomes, Model 2 was further adjusted for educational level, family history of CHD, total energy intake, and also former smoker (yes/no), since it is not included in the LIS [25]. Before making further adjustments in Model 3, we created equally weighted DIS versions by assigning positive or negative equal weights to dietary components. Then, in Model 3, we made additional adjustments for confounders in Model 2, HDL-C, TC, lipid-lowering medications, NSAID, diabetes, and hypertension at baseline, as well as equally weighted DIS (to reduce model size) [25]. The linear trend of DIS/LIS in categorical form was evaluated by incorporating the medians of DIS and LIS quartiles into the multivariable Cox PH models (Model 3) as a continuous variable.
We fitted Cox PH models with restricted cubic splines (RCS) to statistically evaluate the potential non-linear relationship between DIS/LIS and the outcomes. The number of knots in the models was determined based on the minimum Akaike Information Criterion (AIC). In this study, the optimal number of knots was found to be 5, located at the 5th, 25th, 50th, 75th, and 95th percentiles of the DIS/LIS. The Wald Chi-square test was employed to assess the nonlinearity hypothesis in the RCS analyses. The Cox models were adjusted for all confounders, as previously mentioned.
Subgroup analysis
We assessed the effect of participant characteristics (age, sex, race, BMI category, diabetes, and hypertension) that could potentially modify the DIS-or LIS-outcome associations. We calculated the P-value for interaction terms using the likelihood ratio test in the multivariable analysis. To address the issue of multiple testing across subgroups, the Bonferroni correction method was implemented (P value 0.05/6 = 0.0083).
Furthermore, we investigated the associations of each LIS component with the study outcomes. To ensure that the relationships between BMI, physical activity level, alcohol use, and smoking status with the incidence and progression of CAC were not confounded by other LIS components, all lifestyle components of LIS were included simultaneously in the Cox models, along with the following additional variables: age, sex, race, education, family history of CHD, total energy intake, equally weighted DIS, HDL-C, TC, lipid-lowering medication, NSAIDs, hypertension, and diabetes.
Results
The baseline characteristics of individuals with a baseline CAC of 0, categorized by DIS and LIS quartiles, are presented in Table 1. Individuals in the highest DIS quartiles, compared to those in the lowest quartiles, were more likely to have lower levels of formal education, be current smokers, be nondrinkers, and were less likely to be diabetic and to use lipid-lowering medication. Additionally, individuals in the highest DIS quartiles were younger, and had lower MET value, total carbohydrate, calcium, and protein intake, but had higher BMI, DBP, total energy, and fat intake.
Table 1.
Baseline characteristics of individuals with baseline CAC of 0 (n = 2638), by quartiles of DIS and LIS; MESA cohort (2000–2012)
| DIS* | LIS* | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Q1 (n = 658) |
Q2 (n = 660) |
Q3 (n = 660) |
Q4 (n = 660) |
P Value | Q1 (n = 697) |
Q2 (n = 780) |
Q3 (n = 486) |
Q4 (n = 675) |
P Value | |
| Score range | -11.3, -1.4 | -1.4, 0.06 | 0.03, 1.46 | 1.4, 13.2 | - | -1.1, 0.00 | 0.05, 0.7 | 0.7, 1.3 | 1.4, 2.7 | - |
| Age (year) (mean, SD) | 58.8 (8.9) | 58.3 (9.2) | 57.8 (9.2) | 56.1 (8.6) | < 0.001 | 58.2 (9.6) | 58.1 (9.2) | 56.9 (8.5) | 57.5 (8.6) | 0.043 |
| Male (%) | 38.3 | 38.3 | 38.3 | 38.3 | 1.000 | 36.9 | 30.0 | 60.7 | 33.3 | < 0.001 |
| Race/ethnicity (%) | < 0.001 | < 0.001 | ||||||||
| White | 45.7 | 35.8 | 32.9 | 31.1 | 43.2 | 38.5 | 32.9 | 29.3 | ||
| Chinese | 12.0 | 13.5 | 18.5 | 4.7 | 30.0 | 9.1 | 6.0 | 1.8 | ||
| Black | 22.8 | 27.4 | 23.5 | 41.1 | 15.1 | 28.3 | 34.4 | 39.1 | ||
| Hispanic | 19.5 | 23.3 | 25.2 | 23.2 | 11.8 | 24.1 | 26.7 | 29.8 | ||
| Education (%) | < 0.001 | < 0.001 | ||||||||
| Less than high school/ High school | 21.7 | 30.2 | 38.8 | 36.5 | 26.1 | 32.3 | 30.5 | 38.1 | ||
| Some college | 49.5 | 48.0 | 44.5 | 51.8 | 48.4 | 47.1 | 50.4 | 48.9 | ||
| Graduate degree or professional school | 28.7 | 21.8 | 16.7 | 11.7 | 25.5 | 20.6 | 19.1 | 13.0 | ||
| Current smoker (%) | 8.1 | 10.8 | 11.7 | 20.3 | < 0.001 | 1.4 | 11.7 | 15.0 | 24.0 | < 0.001 |
| Alcohol consumption (%) | 0.030 | < 0.001 | ||||||||
| Never | 18.2 | 21.8 | 24.1 | 17.6 | 26.3 | 21.7 | 12.6 | 18.7 | ||
| Former | 21.6 | 20.3 | 20.5 | 24.5 | 19.1 | 18.7 | 24.7 | 25.8 | ||
| Current | 60.2 | 57.9 | 55.5 | 57.9 | 54.7 | 59.6 | 62.8 | 55.6 | ||
| †Physical activity (MET.min/wk) (mean, SD) | 747.3 (1703.1) | 520.5 (1334.2) | 363.1 (1032.5) | 293.5 (770.6) | < 0.001 | 642.7 (1666.6) | 599.9 (1229.4) | 587.4 (1234.7) | 99.4 (632.1) | < 0.001 |
| BMI (kg/m2) (mean, SD) | 27.4 (5.2) | 28.0 (5.3) | 27.5 (5.1) | 29.8 (5.9) | < 0.001 | 22.6 (1.9) | 26.7 (2.3) | 30.2 (4.5) | 34.0 (4.5) | < 0.001 |
| SBP (mm Hg) (mean, SD) | 122.1 (20.8) | 121.7 (19.9) | 120.5 (19.4) | 122.5 (19.1) | 0.291 | 115.7 (19.4) | 122.0 (20.1) | 123.5 (18.4) | 126.2 (19.6) | < 0.001 |
| DBP (mm Hg) (mean, SD) | 70.8 (10.4) | 70.6 (10.1) | 70.9 (9.9) | 72.2 (10.1) | 0.018 | 68.8 (10.1) | 71.0 (9.8) | 73.5 (10.6) | 72.0 (9.7) | < 0.001 |
| HDL-C (mmol/L) (mean, SD) | 1.4 (0.4) | 1.3 (0.4) | 1.3 (0.3) | 1.3 (0.3) | 0.001 | 1.5 (0.4) | 1.4 (0.3) | 1.2 (0.3) | 1.4 (0.4) | < 0.001 |
| TC (mmol/L) (mean, SD) | 4.9 (0.8) | 5.0 (0.8) | 5.0 (0.9) | 5.0 (0.9) | 0.051 | 4.9 (0.8) | 5.1 (0.8) | 4.9 (0.8) | 4.9 (0.9) | 0.168 |
| FPG (mmol/L) (mean, SD) | 5.1 (1.3) | 5.2 (1.4) | 5.1 (1.3) | 5.1 (1.2) | 0.474 | 4.8 (1.0) | 5.0 (1.1) | 5.3 (1.4) | 5.5 (1.7) | < 0.001 |
| Positive family history of CHD (%) | 34.7 | 33.2 | 35.6 | 37.0 | 0.530 | 30.3 | 34.4 | 36.4 | 40.0 | 0.002 |
| Diabetes (%) | 7.3 | 10.5 | 7.9 | 6.5 | 0.050 | 3.9 | 5.3 | 10.1 | 14.1 | < 0.001 |
| Hypertension (%) | 47.6 | 47.9 | 47.7 | 49.2 | 0.926 | 34.7 | 48.5 | 55.1 | 56.4 | < 0.001 |
| Lipid lowering medication (%) | 10.6 | 11.5 | 10.9 | 8.9 | 0.464 | 7.6 | 11.7 | 11.7 | 11.3 | 0.036 |
| Antihypertensive medications (%) | 27.4 | 29.4 | 26.4 | 27.1 | 0.645 | 17.5 | 26.8 | 30.5 | 36.7 | < 0.001 |
| Insulin or oral hypoglycemic medications (%) | 5.6 | 6.5 | 5.8 | 4.1 | 0.269 | 2.9 | 3.8 | 6.8 | 9.2 | < 0.001 |
| Anti-inflammatory drugs (%) | 40.1 | 37.7 | 33.8 | 37.1 | 0.123 | 30.7 | 38.5 | 37.4 | 42.2 | < 0.001 |
| Energy (Kcal/day) (mean, SD) | 1570.7 (726.8) | 1496.5 (678.2) | 1438.3 (679.1) | 1878.2 (914.8) | < 0.001 | 1417.7 (638.0) | 1521.3 (722.4) | 1764.5 (845.2) | 1744.8 (852.6) | < 0.001 |
| Total protein (%Kcal/day) (mean, SD) | 16.7 (3.6) | 16.5 (3.3) | 15.8 (3.1) | 14.3 (2.8) | < 0.001 | 16.3 (3.5) | 15.9 (3.4) | 15.6 (3.1) | 15.5 (3.2) | < 0.001 |
| Total carbohydrate (%Kcal/day) (mean, SD) | 54.8 (9.1) | 53.0 (8.8) | 52.7 (8.7) | 50.0 (8.3) | < 0.001 | 54.1 (8.8) | 53.1 (9.2) | 52.1 (8.2) | 50.8 (8.7) | < 0.001 |
| Total fat (%Kcal/day) (mean, SD) | 28.8 (6.6) | 30.5 (6.5) | 31.3 (6.6) | 35.1 (6.5) | < 0.001 | 30.1 (6.7) | 30.9 (7.2) | 31.8 (6.4) | 33.2 (6.8) | < 0.001 |
| Total calcium (mg/day) (mean, SD) | 1448.1 (1863.5) | 1619.3 (8583.2) | 975.2 (920.7) | 909.1 (677.1) | 0.006 | 1477.1 (7908.9) | 1288.1 (2808.1) | 1076.8 (1118.6) | 1048.6 (1526.5) | 0.262 |
CAC: coronary artery calcification; DIS: dietary inflammation score; LIS: lifestyle inflammation score; MET: metabolic equivalent of task; BMI: body mass index; SBP: systolic blood pressure; DBP: diastolic blood pressure; HDL-C: high-density lipoprotein cholesterol; TC: total cholesterol; FPG: fasting plasma glucose; CHD: coronary heart disease; SD: standard deviation; MESA: Multi-Ethnic Study of Atherosclerosis
*Participants were categorized into LIS and sex-specific DIS quartiles at baseline
†Conditioning physical activity such as walking, sports, dance, and conditioning (e.g. aerobics, bicycling, running, jogging, rowing, swimming, judo, karate)
Analysis of the baseline characteristics of participants based on the LIS quartiles showed that those in the highest LIS quartile were younger, less likely to be male, had a lower academic education, were more likely to have a positive family history of CHD, and be current smokers, drinkers, and to have diabetes and hypertension. Additionally, they were more likely to use lipid-lowering, antihypertensive, NSAIDs, and hypoglycemic medications. On average, participants in the highest LIS quartiles had higher levels of BMI, SBP, DBP, FPG, total energy, and fat intake, but lower levels of MET value, HDL-C, total protein, and carbohydrate intake. The baseline characteristics of individuals with a baseline CAC > 0, by DIS and LIS quartiles, are presented in Table 2. Overall, the aforementioned patterns were found for the characteristics of individuals with CAC > 0 based on DIS and LIS.
Table 2.
Baseline characteristics of individuals with baseline CAC > 0 (n = 2561), by quartiles of DIS and LIS; MESA cohort (2000–2012)
| DIS* | LIS* | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Q1 (n = 639) |
Q2 (n = 641) |
Q3 (n = 641) |
Q4 (n = 640) |
P Value | Q1 (n = 639) |
Q2 (n = 479) |
Q3 (n = 762) |
Q4 (n = 681) |
P Value | |
| Score range | -12.78, -1.48 | -1.5, 0.08 | 0.06, 1.57 | 1.52, 10.71 | - | -1.07, 0.05 | 0.09, 0.73 | 0.89, 1.31 | 1.39, 2.67 | - |
| Age (year) (mean, SD) | 67.0 (9.1) | 66.7 (9.4) | 65.4 (9.9) | 64.7 (9.3) | < 0.001 | 67.5 (9.6) | 66.3 (9.3) | 66.2 (9.2) | 63.9 (9.4) | < 0.001 |
| Male (%) | 59.6 | 59.6 | 59.6 | 59.7 | 1.00 | 61.3 | 70.6 | 60.1 | 49.8 | < 0.001 |
| Race/ethnicity (%) | < 0.001 | < 0.001 | ||||||||
| White | 58.4 | 46.2 | 39.3 | 42.5 | 47.3 | 54.9 | 46.2 | 40.5 | ||
| Chinese | 8.8 | 13.4 | 17.5 | 8.1 | 27.1 | 8.8 | 10.2 | 1.9 | ||
| Black | 16.3 | 18.6 | 21.4 | 28.9 | 13.0 | 20.0 | 22.3 | 28.8 | ||
| Hispanics | 16.6 | 21.8 | 21.8 | 20.5 | 12.7 | 16.3 | 21.3 | 28.8 | ||
| Education (%) | < 0.001 | < 0.001 | ||||||||
| Less than high school/ High school | 24.9 | 32.9 | 41.2 | 45.0 | 31.3 | 29.2 | 36.6 | 44.5 | ||
| Some college | 45.2 | 48.2 | 42.3 | 43.4 | 46.2 | 44.1 | 45.8 | 42.9 | ||
| Graduate degree or professional school | 29.9 | 18.9 | 16.5 | 11.6 | 22.5 | 26.7 | 17.6 | 12.6 | ||
| Current smoker (%) | 6.4 | 9.7 | 10.8 | 20.3 | < 0.001 | 2.3 | 16.9 | 4.9 | 24.8 | < 0.001 |
| Alcohol consumption (%) | < 0.001 | < 0.001 | ||||||||
| Never | 15.5 | 17.2 | 21.2 | 16.9 | 21.6 | 13.8 | 18.2 | 16.2 | ||
| Former | 20.0 | 22.5 | 25.0 | 28.1 | 20.0 | 17.3 | 25.5 | 30.4 | ||
| Current | 64.5 | 60.4 | 53.8 | 55.0 | 58.4 | 68.9 | 56.3 | 53.5 | ||
| †Physical activity (MET.min/wk) (mean, SD) | 791.2 (1838.5) | 536.1 (1131.5) | 448.7 (1187.8) | 335.5 (966.6) | < 0.001 | 651.1 (1412.8) | 1161.1 (1972.5) | 437.7 (1088.3) | 67.3 (486.3) | < 0.001 |
| BMI (kg/m2) (mean, SD) | 27.8 (4.7) | 27.9 (4.7) | 28.2 (5.2) | 29.2 (5.5) | < 0.001 | 23.1 (1.9) | 26.6 (2.6) | 29.3 (3.7) | 33.2 (4.5) | < 0.001 |
| SBP (mm Hg) (mean, SD) | 128.6 (20.1) | 130.7 (21.9) | 131.1 (21.1) | 129.3 (21.6) | 0.123 | 127.1 (22.5) | 127.8 (19.7) | 131.4 (20.85) | 132.4 (21.0) | < 0.001 |
| DBP (mm Hg) (mean, SD) | 71.8 (9.9) | 72.3 (10.4) | 73.5 (9.7) | 72.8 (10.3) | 0.021 | 71.1 (9.9) | 72.5 (9.5) | 73.3 (10.3) | 73.2 (10.3) | < 0.001 |
| HDL-C (mmol/L) (mean, SD) | 1.3 (0.3) | 1.2 (0.4) | 1.2 (0.3) | 1.3 (0.4) | 0.005 | 1.4 (0.4) | 1.3 (0.3) | 1.2 (0.3) | 1.2 (0.3) | < 0.001 |
| TC (mmol/L) (mean, SD) | 4.9 (0.9) | 5.0 (0.9) | 4.9 (0.8) | 5.1 (0.9) | 0.222 | 5.1 (0.9) | 4.9 (0.8) | 5.0 (0.9) | 5.0 (0.9) | 0.119 |
| FPG (mmol/L) (mean, SD) | 5.4 (1.6) | 5.5 (1.7) | 5.6 (1.7) | 5.5 (1.6) | 0.262 | 5.2 (1.7) | 5.3 (1.4) | 5.5 (1.4) | 5.9 (2.0) | < 0.001 |
| Positive family history of CHD (%) | 47.9 | 45.2 | 42.9 | 44.7 | 0.348 | 37.1 | 49.9 | 45.9 | 48.6 | < 0.001 |
| Diabetes (%) | 12.8 | 14.7 | 15.6 | 16.2 | 0.340 | 9.5 | 9.8 | 15.4 | 22.8 | < 0.001 |
| Hypertension (%) | 68.1 | 71.0 | 69.1 | 67.3 | 0.525 | 59.2 | 66.4 | 72.4 | 75.8 | < 0.001 |
| Lipid lowering medication (%) | 23.3 | 26.5 | 20.4 | 18.6 | 0.004 | 17.5 | 22.3 | 24.9 | 23.5 | 0.007 |
| Antihypertensive medications (%) | 45.2 | 48.0 | 42.0 | 44.5 | 0.182 | 33.2 | 43.4 | 48.3 | 53.3 | < 0.001 |
| Insulin or oral hypoglycemic medications (%) | 9.5 | 10.6 | 11.2 | 11.2 | 0.732 | 6.1 | 6.9 | 12.2 | 15.9 | < 0.001 |
| Anti-inflammatory drugs (%) | 48.8 | 46.8 | 40.7 | 40.5 | 0.003 | 39.3 | 43.0 | 45.4 | 48.3 | 0.009 |
| Energy (Kcal/day) (mean, SD) | 1544.2 (716.4) | 1409.5 (629.1) | 1388.4 (630.8) | 1822.5 (833.6) | < 0.001 | 1410.5 (637.3) | 1497.2 (654.7) | 1556.3 (742.2) | 1677.3 (812.1) | < 0.001 |
| Total protein (%Kcal/day) (mean, SD) | 16.8 (3.3) | 16.2 (3.12) | 15.8 (3.2) | 14.5 (2.9) | < 0.001 | 15.9 (3.2) | 15.7 (3.2) | 16.0 (3.2) | 15.6 (3.2) | 0.048 |
| Total carbohydrate (%Kcal/day) (mean, SD) | 55.1 (9.1) | 52.9 (8.7) | 52.8 (8.5) | 49.8 (8.4) | < 0.001 | 54.6 (8.4) | 52.1 (8.7) | 52.5 (9.0) | 51.3 (9.1) | < 0.001 |
| Total fat (%Kcal/day) (mean, SD) | 28.3 (6.7) | 30.3 (6.6) | 30.8 (6.3) | 34.8 (6.9) | < 0.001 | 29.3 (6.9) | 30.4 (6.9) | 31.5 (7.2) | 32.3 (7.0) | < 0.001 |
| Total calcium (mg/day) (mean, SD) | 1474.9 (5626.0) | 1151.3 (2127.1) | 893.3 (777.5) | 929.4 (733.9) | 0.002 | 1294.1 (5638.3) | 976.3 (876.5) | 1005.3 (733.1) | 1156.1 (2050.4) | 0.233 |
CAC: coronary artery calcification; DIS: dietary inflammation score; LIS: lifestyle inflammation score; MET: metabolic equivalent of task (METs); BMI: body mass index; SBP: systolic blood pressure; DBP: diastolic blood pressure; HDL-C: high-density lipoprotein cholesterol; TC: total cholesterol; FPG: fasting plasma glucose; CHD: coronary heart disease; SD: standard deviation; MESA: Multi-Ethnic Study of Atherosclerosis
*Participants were categorized into LIS and sex-specific DIS quartiles at baseline
†Conditioning physical activity such as walking, sports, dance, and conditioning (e.g. aerobics, bicycling, running, jogging, rowing, swimming, judo, karate)
Supplementary Tables 1 and 2 show the dietary inflammatory components among individuals with a baseline CAC of 0 and CAC > 0, respectively, categorized by quartiles of DIS. On average, in both groups, participants in the highest DIS quartiles had lower levels of all components except for red and organ meats, processed meats, added sugars, high-fat dairy, other fats, refined grains and starchy vegetables.
During a median follow-up of 9.0 years (interquartile range (IQR): 5.0–10.0), out of the 2638 participants with CAC = 0 at baseline, 977 individuals (432 men) developed CAC. The incidence rate (95% confidence interval (CI)) of CAC development was 68.8 (64.4–73.0) per 1000 person-years. Furthermore, among the 2561 participants with baseline CAC > 0, over a median follow-up of 8.0 years (IQR: 4.0–9.0), 1687 participants (1045 men) showed CAC progression, with an incidence rate of 210.5 (200.6-220.7) per 1000 person-years. Supplementary Tables 3 and 4 present incidence rates (95% CI) by quartiles of LIS and DIS. For both CAC incidence and progression, the incidence rates increased among those in the highest (most proinflammatory) relative to the lowest LIS quartiles. However, there were no consistent, clear patterns of differences in incidence rates by DIS quartiles.
The associations of the DIS and LIS with CAC incident and progression are presented in Tables 3 and 4. There was a direct association between LIS and CAC incidence; each one-point increase in LIS was associated with a 15% higher risk (HR: 1.15; 95% CI: 1.04–1.27) for CAC incidence in the fully adjusted model. When LIS was analyzed in quartile categories, compared with the subjects in quartile 1, those in quartile 4 had a significantly increased risk (1.67; 1.38–2.03) of developing CAC in model 1. These associations were attenuated but remained significant in Model 2 (1.62; 1.33–1.96) after controlling for additional confounders including education, former smoker, family history of CHD, and total energy intake, and in Model 3 (1.35; 1.10–1.65; P-trend = 0.002) after further adjusting for equally weighted DIS, HDL-C, TC, lipid-lowering medication, NSAIDs, hypertension, and diabetes.
Table 3.
Associations between DIS and LIS and CAC incidence; MESA cohort (2000–2012)
| DIS | LIS | |||||||
|---|---|---|---|---|---|---|---|---|
|
Model 1
HR (95% CI) |
Model 2
*
HR (95% CI) |
Model 3
†
HR (95% CI) |
Model 1
HR (95% CI) |
Model 2
‡
HR (95% CI) |
Model 3
§
HR (95% CI) |
|||
| Score as continuous | 1.00 (0.97–1.03) | 0.98 (0.95-1.00) | 0.98 (0.96–1.01) | 1.29 (1.17–1.42) | 1.26 (1.15–1.39) | 1.15 (1.04–1.27) | ||
| Quartiles | No. cases/event | No. cases/event | ||||||
| Q1 | 658/256 | Reference | Reference | Reference | 697/209 | Reference | Reference | Reference |
| Q2 | 660/248 | 1.04 (0.87–1.24) | 1.02 (0.85–1.21) | 0.98 (0.82–1.17) | 780/278 | 1.21 (1.00-1.46) | 1.21 (1.00-1.46) | 1.10 (0.91–1.32) |
| Q3 | 660/227 | 0.97 (0.81–1.16) | 0.92 (0.77–1.11) | 0.90 (0.75–1.08) | 486/196 | 1.47 (1.20–1.81) | 1.44 (1.17–1.77) | 1.21 (0.98–1.50) |
| Q4 | 660/246 | 1.02 (0.85–1.22) | 0.88 (0.73–1.05) | 0.87 (0.73–1.05) | 675/294 | 1.67 (1.38–2.03) | 1.62 (1.33–1.96) | 1.35 (1.10–1.65) |
| P for trend | 0.110 | 0.002 | ||||||
Model 1: adjusted for age (continuous), sex, and race (White, Chinese, Black, Hispanic)
*Model 2: adjusted for model 1 and education (less than high school/ high school, some college, graduate degree or professional school), smoking (never, former, current), alcohol consumption (never, former, current), physical activity level (as tertiles of MET), BMI (continuous), family history of CHD (yes/no) and total energy intake (continuous)
†Model 3: adjusted for model 2 and HDL-C (continuous), TC (continuous), lipid-lowering medication (yes/no), NSAIDs (yes/no), hypertension (yes/no), and diabetes (yes/no)
‡Model 2: adjusted for model 1 and education, former smoker (yes/no), family history of CHD, and total energy intake
§Model 3: adjusted for model 2‡ and equally weighted DIS (continuous), HDL-C, TC, lipid-lowering medication, NSAIDs, hypertension, and diabetes
Table 4.
Associations between DIS and LIS and CAC progression; MESA cohort (2000–2012)
| DIS | LIS | |||||||
|---|---|---|---|---|---|---|---|---|
| Model 1 HR (95% CI) |
Model 2* HR (95% CI) |
Model 3† HR (95% CI) |
Model 1 HR (95% CI) |
Model 2‡ HR (95% CI) |
Model 3§ HR (95% CI) |
|||
| Score as continuous | 0.99 (0.98–1.01) | 0.99 (0.97–1.01) | 0.99 (0.97–1.01) | 1.09 (1.02–1.17) | 1.08 (1.01–1.17) | 1.02 (0.95–1.11) | ||
| Quartiles | No. cases/events | No. cases/events | ||||||
| Q1 | 639/428 | Reference | Reference | Reference | 639/402 | Reference | Reference | Reference |
| Q2 | 641/414 | 0.97 (0.85–1.11) | 0.97 (0.85–1.11) | 0.97 (0.85–1.12) | 479/315 | 1.05 (0.90–1.22) | 1.05 (0.90–1.22) | 1.02 (0.87–1.18) |
| Q3 | 641/431 | 1.03 (0.90–1.18) | 1.04 (0.90–1.19) | 1.05 (0.91–1.20) | 762/504 | 1.09 (0.95–1.24) | 1.08 (0.94–1.23) | 1.00 (0.86–1.15) |
| Q4 | 640/414 | 0.96 (0.84–1.10) | 0.92 (0.80–1.06) | 0.94 (0.82–1.09) | 681/466 | 1.22 (1.06–1.41) | 1.21 (1.04–1.40) | 1.08 (0.93–1.26) |
| P for trend | 0.665 | 0.386 | ||||||
Model 1: adjusted for age (continuous), sex, and race (White, Chinese, Black, Hispanic)
*Model 2: adjusted for model 1 and education (less than high school/ high school, some college, graduate degree or professional school), smoking (never, former, current), alcohol consumption (never, former, current), physical activity level (as tertiles of MET), BMI (continuous), family history of CHD (yes/no) and total energy intake (continuous)
†Model 3: adjusted for model 2 and HDL-C (continuous), TC (continuous), lipid-lowering medication (yes/no), NSAIDs (yes/no), hypertension (yes/no) and diabetes (yes/no)
‡Model 2: adjusted for model 1 and education, former smoker (yes/no), family history of CHD, and total energy intake
§Model 3: adjusted for model 2‡ and equally weighted DIS (continuous), HDL-C, TC, lipid-lowering medication, NSAIDs, hypertension, and diabetes
We found that each one-point increase in LIS was associated with a 9% higher risk (1.09; 1.02–1.17) for CAC progression in Model 1, and an 8% higher risk (1.08; 1.01–1.17) in Model 2. However, the associations became non-significant (1.02; 0.95–1.11) in the fully adjusted model (Model 3). Similar associations were observed when quartiles of the LIS were used; we found about a 20% higher risk for CAC progression for those in the highest LIS quartile compared with the first LIS quartile in models 1 and 2. However, after adjusting for all confounders in the study, the risk became non-significant, with a value of 1.08 (0.93–1.26). We found no significant associations between DIS and CAC incidence and progression, even in model 1.
Although the RCS plots in Supplementary Figs. 1 and 2 show a curvilinear relationship between DIS/LIS and the study outcomes, the p-value for non-linearity was not statistically significant (P > 0.05), indicating that the relationship between DIS/LIS and the outcomes did not significantly deviate from linearity. However, significant linear association was observed solely for the relationship between LIS and CAC incidence, as previously stated (Table 3).
The results of the subgroup analysis are presented in Supplementary Tables 5 and 6. A significant interaction between the LIS (as quartiles) and age was found for CAC incidence (P-interaction < 0.001). Among younger participants (aged < 60 years), those in the highest quartile had a 76% higher risk (1.76; 1.34–2.30) for CAC incidence than those in the first quartile after controlling for all confounders (Supplementary Table 5).
Table 5 presents the associations of the individual components of the LIS and the incidence and progression of CAC. Individuals classified as overweight and obese, compared to those with normal weight, showed a 20% (1.20; 1.01–1.43) and 38% (1.38; 1.14–1.67) higher risk for CAC incidence, respectively. Further, current relative to never smokers had a 38% (1.38; 1.17–1.62) higher risk for CAC progression.
Table 5.
Associations of the individual components of the LIS and the incidence and progression of CAC; MESA cohort (2000–2012)
| CAC Incidence n = 2638 |
CAC Progression n = 2561 |
|||
|---|---|---|---|---|
| No. cases | Adjusted HR (95% CI)* | No. cases | Adjusted HR (95% CI)* | |
| Lifestyle factor | ||||
| Body mass index † | ||||
| Normal | 797 | Reference | 711 | |
| Overweight | 1017 | 1.20 (1.01–1.43) | 1046 | 1.08 (0.95–1.23) |
| Obese | 824 | 1.38 (1.14–1.67) | 804 | 1.10 (0.94–1.27) |
| Physical activity level ‡ | ||||
| Tertile 1 | 591 | Reference | 1631 | Reference |
| Tertile 2 | 1195 | 1.04 (0.82–1.31) | 67 | 0.76 (0.55–1.06) |
| Tertile 3 | 852 | 0.98 (0.80–1.20) | 863 | 1.05 (0.95–1.17) |
| Alcohol use § | ||||
| Non-drinker | 2349 | Reference | 2170 | Reference |
| Moderate | 160 | 1.17 (0.90–1.51) | 235 | 1.11 (0.94–1.31) |
| Heavy | 129 | 0.93 (0.68–1.27) | 156 | 0.87 (0.71–1.09) |
| Smoking status | ||||
| Never smoker | 1469 | Reference | 1153 | Reference |
| Past smoker | 834 | 1.10 (0.95–1.27) | 1106 | 1.08 (0.97–1.21) |
| Current smoker | 335 | 1.16 (0.94–1.43) | 302 | 1.38 (1.17–1.62) |
CAC: coronary artery calcification; DIS: dietary inflammation score; LIS: lifestyle inflammation score; MET: metabolic equivalent of task; BMI: body mass index; HDL-C: high-density lipoprotein cholesterol; TC: total cholesterol; CHD: coronary heart disease; HR: hazard ratio; CI: confidence interval; MESA: Multi-Ethnic Study of Atherosclerosis
*All lifestyle components were included in the Cox models and additionally included: age (continuous), sex, race ((White, Chinese, Black, Hispanic), education (less than high school/ high school, some college, graduate degree or professional school),
family history of CHD (yes/no), total energy intake (kcal/day), equally weighted DIS (continuous), HDL-C (continuous), TC (continuous), lipid-lowering medication (yes/no), hypertension (yes/no) and diabetes (yes/no)
† Normal BMI: 18.5–24.99 kg/m2; Overweight BMI: 25–29.99 kg/m2; Obese BMI: ≥ 30 kg/m2
‡ Physical activity was measured in MET and then ranked into tertiles.
§ Moderate drinker: 1 drink/wk for women, 1–2 drinks/wk for men; heavy drinker: > 1 drink/wk for women, > 2 drinks/wk drinks for men
Discussion
In this multi-ethnic, community-based cohort study, a higher LIS was found to be associated with a higher risk of CAC incidence, with a more pronounced effect observed among younger adults aged under 60 years. However, no significant relation was observed between DIS and CAC incidence and progression risk. An analysis of LIS components showed that overweight and obese individuals, compared to those with normal weight, had a higher risk for CAC incidence. Additionally, current smokers demonstrated a higher risk of CAC progression, compared to never smokers.
Despite numerous studies on dietary intakes and patterns in relation to CAC [26–29], the association between an inflammatory diet, characterized by scoring methods such as the DIS/ DII, and the risk of CAC incidence and progression has not been extensively investigated. To the best of our knowledge, this study is the first to explore the association between DIS with the incidence and progression of CAC.
In the present cohort, no significant association was found between DIS and CAC incidence or progression. Comparing the results of the current study with previous research is challenging due to the lack of similar studies in this specific field. Nonetheless, the previous studies investigating the association between DIS and various diseases and conditions have produced conflicting results. For instance, in the cardiovascular field, a study conducted by Troeschel et al. [14], found no significant association between DIS with CVD mortality among Black and White American men and women. In contrast, Li et al. [19], in the prospective Iowa Women’s Health Study, found that women in the highest relative to the lowest DIS quintiles had a 12% higher risk for CVD mortality.
In non-cardiovascular conditions, a higher score of DIS has been associated with an increased risk of all-cause and cancer mortality [14], nonalcoholic fatty liver diseases [15], and metabolic syndrome [16]. However, it has not been associated with diabetes [17] and chronic kidney disease [18].
The lack of an association between DIS and CAC incidence and progression in our study may be attributed to several factors. One key factor is that DIS provides an overall dietary score derived from a combination of pro-inflammatory and anti-inflammatory components present in the diet [13], without indicating which specific components were consumed more or less frequently. Consequently, individuals with the same DIS score may have different patterns of anti-inflammatory and pro-inflammatory food intake. The potential interaction of these opposing foods may attenuate their prediction abilities for CAC incidence or progression. For example, in our study, individuals in the higher DIS quartiles had relatively similar intakes of certain anti-inflammatory foods, such as legumes, poultry, and fish, which could mitigate or neutralize the effects of other pro-inflammatory foods, such as added sugars [13]. Another possibility for the absence of an association between DIS and outcomes in our study could be that individuals in the upper quartiles of DIS may not have reached a sufficient DIS level to cause inflammation. As there is no established cut-off or threshold for DIS that triggers inflammation, further studies are warranted to confirm our findings. Another factor could be the use of improper cooking and food preservation methods, which may lead to increased oxidative stress and inflammation [30]. For instance, frying vegetables can diminish their antioxidant and anti-inflammatory properties [31]. Additionally, using various tools for gathering dietary information may account for the inconsistencies observed across studies. Different questionnaires may vary in their comprehensiveness, the types of foods included, and the way they capture dietary patterns [32]. For example, Byrd et al. [13] used a self-administered, 109-food item, Block 98 FFQ (NutritionQuest) for developing the DIS. However, the MESA utilized a modified Block-style 120-item FFQ [24]. Another reason may be related to the complexity of inflammatory pathways and the specific biomarkers involved in CAC development [33], which may not have been fully captured by the DIS. For example, the DIS was developed in relation to four biomarkers, including high-sensitivity C-reactive protein (hs-CRP); however, the specific relationship between hs-CRP and CAC scores is not consistently aligned across different studies [34, 35]. Furthermore, additional factors such as genetic variations [36] and environmental impact [37] could influence the development and progression of CAC independently of dietary factors, which were not considered in our study.
Moreover, some studies investigating the association between DIS and various diseases and conditions have not measured alcohol intake and supplement use [15, 16, 18], potentially overlooking important dietary factors that could influence the outcomes and further complicate comparisons. Further research considering these complexities and interactions can enhance our understanding of the intricate relationship between dietary inflammatory patterns and CAC outcomes.
The research on lifestyle factors and CAC has shown a significant relationship between smoking, alcohol consumption, obesity, physical activity, and CAC, independent of other important confounders. Studies have indicated that these factors, individually, play a role in the prevalence, incidence, or progression of CAC [17, 29, 35, 36]. However, the combined impact of these lifestyle factors, weighted based on their contributions to inflammation, on CAC has not been studied.
In this study, a pro-inflammatory lifestyle as assessed using the LIS was significantly associated with an increased risk of CAC incidence, even after adjusting for important confounders, specifically, equally weighted DIS.
Although the relationship between LIS and CAC has not yet been explored, most studies examining this index have found a significant association between LIS and multiple diseases and health conditions in the cardiovascular [14, 19] and non-CVD fields [16–18].
In this study, the stronger association between LIS and the incidence of CAC, compared to DIS, may be attributed to the fact that each component of the LIS is known as an independent pro-inflammatory risk factor [38–41]. Consequently, the cumulative effects of these lifestyle-related factors on inflammation may elevate the risk of CAC, compared to DIS.
In subgroup analysis, we identified that the impact of the LIS on CAC incidence was stronger among younger adults aged under 60 years. This may be due to the aging process which plays a significant role in vascular aging, cellular senescence, and structural changes, all of which contribute to the progression of arterial calcification independent of lifestyle factors [42].
In our study, the analysis of each individual component of LIS revealed that overweight and obese individuals had a higher risk of CAC incidence compared to those with normal weight, even in the presence of other lifestyle components of LIS, such as physical activity, smoking, and alcohol use. The results of previous studies regarding the association between BMI and CAC incidence are controversial. While some studies suggest that elevated BMI is not a significant predictor of CAC incidence [43], others indicate that increased BMI level, but not obesity and overweight, is a risk factor for CAC development [44].
Interestingly, in the current study, the adjusted effect of obesity alone was found to be comparable to the effect of the LIS (a collection of four lifestyle factors) in predicting CAC incidence (HR of 1.38 vs. 1.36). This indicates that obesity, as a single factor, carries a similar predictive effect as the combined influence of multiple lifestyle factors when assessing the risk of developing CAC. As demonstrated in the Byrd et al. study [13], among the different components of diet and lifestyle, the greatest weight in the association with inflammation is attributed to obesity (1.57) and then to overweight (0.89).
In this study, the significant association between LIS and CAC progression was no longer significant once additional factors including equally weighted DIS, HDL-C, TC, lipid-lowering medication, NSAIDs, hypertension, and diabetes, were considered. This suggests that these additional factors may directly impact CAC progression more than the LIS. A Brazilian longitudinal study revealed that among various risk factors, only hypertension, diabetes, hypertriglyceridemia, and metabolic syndrome were significant predictors of CAC progression [44].
Analysis of each component of LIS in our study showed that current smokers had a higher risk of CAC progression, compared to non-smokers, even after adjusting for all confounders. Smoking has been consistently associated with the presence and progression of CAC [45]. One study indicated that moderate and heavy smokers tend to have higher CAC scores when compared to non-smokers and light smokers. Moreover, the duration and intensity of smoking, quantified in pack-years and years of smoking, were closely linked to the progression of CAC [45].
In this study, the effect of smoking individually was found to be stronger on the progression of CAC compared to the combined effect of all components in the LIS. This discrepancy may be due to interactions between the LIS components that dilute the overall effect when combined. Additionally, the specific inflammatory pathways associated with CAC progression may not align entirely with the inflammatory markers typically considered in the LIS. Therefore, the weights of the components in the LIS may not be appropriate for predicting the progression of CAC.
Strengths and limitations of the study
Our study has several strengths. The current study is the first to examine the association of the novel DIS and LIS with the risk of CAC incidence and progression in middle and late adulthood. Second, the study was conducted using a prospective study design and included a large and racially diverse study population, enhancing the findings’ generalizability. Finally, the MESA study implemented rigorous, standardized data collection procedures and extensive quality control measures to ensure the accuracy and reliability of its data collection processes.
Our findings should be considered in context with our study’s limitations. First, data on dietary patterns, food consumption, and lifestyle habits were self-reported and may be subject to recall bias. Second, despite adjusting for major confounding variables in the analysis, there may still be residual or unmeasured confounders whose effects cannot be entirely ruled out. Third, we did not consider changes in the nutrient composition of foods and temporal changes in DIS and LIS. Finally, our study results may not be generalizable to adults < 45 years.
Conclusions
In summary, our findings, suggest that a combination of lifestyle factors, including physical activity, obesity, alcohol, and tobacco use, with higher inflammatory potentials, may be associated with a higher incidence of CAC. Reducing inflammation, through lifestyle interventions, could potentially reduce the risk for CAC development. Nonetheless, our analysis did not reveal a significant assosiation between a higher DIS and the risk of CAC. Further epidemiological research is needed to investigate the potential inflammatory impacts of dietary patterns and their combinations on the development of CAC, as well as the underlying mechanisms involved.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We express our gratitude to the MESA Study staff and participants for their invaluable contributions. We extend our sincere appreciation to Dr. Pantea Nazeri for her valuable consultation on our manuscript.
Abbreviations
- CAC
Coronary artery calcification
- DIS
Dietary inflammation score
- LIS
Lifestyle inflammation score
- MESA
Multi-Ethnic Study of Atherosclerosis
- HR
Hazard ratio
- 95% CI
95% confidence interval
- CVD
Cardiovascular disease
- CT
Computed tomography
- DII
Dietary inflammatory index
- EDIP
Empirical dietary inflammatory pattern
- BioLINCC
Biologic Specimen and Data Repository Information Coordinating Center
- FFQ
Food-frequency questionnaire
- BMI
Body mass index
- FPG
Fasting plasma glucose
- TC
Total cholesterol
- HDL-C
High-density lipoprotein cholesterol
- METs
Metabolic equivalent of task
- CHD
Coronary heart disease
- SBP
Systolic blood pressure
- DBP
Diastolic blood pressure
- NHANES
National Health and Nutrition Examination Survey
- SD
Standard deviation
- ANOVA
By analysis of variance
- IQR
Interquartile range
- LCD
Low-carbohydrate diet
- hs-CRP
High-sensitivity C-reactive protein
Author contributions
F.H. and A.R. contributed to conceptualizing the study and design; A.R. analyzed and interpreted the data; A.R. wrote the initial manuscript. P.H. contributed to the interpretation of results and discussion. All authors reviewed the manuscript and provided final approval of the manuscript.
Funding
None declared.
Data availability
The data used for this study were obtained from The Biologic Specimen and Data Repository Information Coordinating Center (BioLINCC) is accessed at https://biolincc.nhlbi.nih.gov/.
Declarations
Ethics approval and consent to participate
Institutional review boards at each site reviewed and approved examination protocols, and written informed consent was obtained from all study participants at each examination. Approval for undertaking the current project was also obtained from the Research Institute for Endocrine Sciences, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Amini M, Zayeri F, Salehi M. Trend analysis of cardiovascular disease mortality, incidence, and mortality-to-incidence ratio: results from global burden of disease study 2017. BMC Public Health. 2021;21(1):401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Frąk W, Wojtasińska A. Pathophysiology of Cardiovascular diseases: New insights into Molecular mechanisms of atherosclerosis, arterial hypertension, and coronary artery disease. Biomedicines. 2022;10(8):1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Onnis C, Virmani R. Coronary artery calcification: current concepts and clinical implications. Circulation. 2024;149(3):251–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peng AW, Dardari ZA, Blumenthal RS, Dzaye O, Obisesan OH, Iftekhar Uddin SM, et al. Very high coronary artery calcium (≥ 1000) and Association with Cardiovascular Disease Events, non-cardiovascular Disease outcomes, and mortality: results from MESA. Circulation. 2021;143(16):1571–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee H-Y, Lim S, Park S. Role of inflammation in arterial calcification. Korean Circulation J. 2021;51(2):114–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cai X, Tintut Y. A potential New Link between inflammation and vascular calcification. J Am Heart Association. 2023;12(1):e028358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smidowicz A, Regula J. Effect of nutritional status and dietary patterns on human serum C-reactive protein and interleukin-6 concentrations. Adv Nutr. 2015;6(6):738–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang YB, Page AJ, Gill TK, Melaku YA. Association of dietary and nutrient patterns with systemic inflammation in community dwelling adults. Front Nutr. 2022;9:977029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mozaffarian D. Dietary and policy priorities for cardiovascular disease, diabetes, and obesity: a comprehensive review. Circulation. 2016;133(2):187–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tabung FK, Smith-Warner SA, Chavarro JE, Wu K, Fuchs CS, Hu FB, et al. Development and validation of an empirical Dietary Inflammatory Index. J Nutr. 2016;146(8):1560–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Farazi M, Jayedi A, Shab-Bidar S. Dietary inflammatory index and the risk of non-communicable chronic disease and mortality: an umbrella review of meta-analyses of observational studies. Crit Rev Food Sci Nutr. 2023;63(1):57–66. [DOI] [PubMed] [Google Scholar]
- 12.Hébert JR, Shivappa N, Wirth MD, Hussey JR, Hurley TG. Perspective: the Dietary Inflammatory Index (DII)—lessons learned, improvements made, and future directions. Adv Nutr. 2019;10(2):185–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Byrd DA, Judd SE, Flanders WD, Hartman TJ, Fedirko V, Bostick RM. Development and validation of novel dietary and lifestyle inflammation scores. J Nutr. 2019;149(12):2206–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Troeschel AN, Byrd DA, Judd S, Flanders WD, Bostick RM. Associations of dietary and lifestyle inflammation scores with mortality due to CVD, cancer, and all causes among black and white American men and women. Br J Nutr. 2023;129(3):523–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Farhadnejad H, Tehrani AN, Jahromi MK, Teymoori F, Mokhtari E, Salehi-Sahlabadi A, et al. The association between dietary inflammation scores and non-alcoholic fatty liver diseases in Iranian adults. BMC Gastroenterol. 2022;22(1):267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Farhadnejad H, Parastouei K, Rostami H, Mirmiran P, Azizi F. Dietary and lifestyle inflammatory scores are associated with increased risk of metabolic syndrome in Iranian adults. Diabetol Metab Syndr. 2021;13:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Teymoori F, Farhadnejad H, Mokhtari E, Sohouli MH, Moslehi N, Mirmiran P, et al. Dietary and lifestyle inflammatory scores and risk of incident diabetes: a prospective cohort among participants of Tehran lipid and glucose study. BMC Public Health. 2021;21:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Farhadnejad H, Teymoori F, Jahromi MK, Mokhtari E, Asghari G, Mirmiran P, et al. High dietary and lifestyle inflammatory scores are associated with increased risk of chronic kidney disease in Iranian adults. Nutr J. 2023;22(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li Z, Gao Y, Byrd DA, Gibbs DC, Prizment AE, Lazovich D, et al. Novel dietary and lifestyle inflammation scores directly associated with all-cause, all-cancer, and all-cardiovascular disease mortality risks among women. J Nutr. 2021;151(4):930–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, et al. Multi-ethnic study of atherosclerosis: objectives and design. Am J Epidemiol. 2002;156(9):871–81. [DOI] [PubMed] [Google Scholar]
- 21.Ainsworth BE, Irwin ML, Addy CL, Whitt MC, Stolarczyk LM. Moderate physical activity patterns of minority women: the cross-cultural activity participation study. J Womens Health Gend Based Med. 1999;8(6):805–13. [DOI] [PubMed] [Google Scholar]
- 22.Detrano RC, Anderson M, Nelson J, Wong ND, Carr JJ, McNitt-Gray M, et al. Coronary calcium measurements: effect of CT scanner type and calcium measure on rescan reproducibility—MESA study. Radiology. 2005;236(2):477–84. [DOI] [PubMed] [Google Scholar]
- 23.Paixao AR, Chakravorty R, Khera A, Leonard D, DeFina LF, Barlow CE, et al. Disagreement between different definitions of coronary artery calcium progression. JACC: Cardiovasc Imaging. 2015;8(6):743–4. [DOI] [PubMed] [Google Scholar]
- 24.Nettleton JA, Rock CL, Wang Y, Jenny NS, Jacobs DR. Associations between dietary macronutrient intake and plasma lipids demonstrate criterion performance of the multi-ethnic study of atherosclerosis (MESA) food-frequency questionnaire. Br J Nutr. 2009;102(8):1220–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gao Y, Byrd DA, Prizment A, Lazovich D, Bostick RM. Associations of novel lifestyle-and whole foods-based inflammation scores with incident colorectal cancer among women. Nutr Cancer. 2022;74(4):1356–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hu T, Jacobs DR, Bazzano LA, Bertoni AG, Steffen LM. Low-carbohydrate diets and prevalence, incidence and progression of coronary artery calcium in the multi-ethnic study of atherosclerosis (MESA). Br J Nutr. 2019;121(4):461–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gao J-W, Hao Q-Y, Zhang H-F, Li X-Z, Yuan Z-M, Guo Y et al. Low-carbohydrate diet score and coronary artery calcium progression: results from the CARDIA study. Arteriosclerosis, thrombosis, and vascular biology. 2021;41(1):491–500. [DOI] [PMC free article] [PubMed]
- 28.Gorgulho B, Alves MA, Teixeira JA, Santos RO, de Matos SA, Bittencourt MS, et al. Dietary patterns associated with subclinical atherosclerosis: a cross-sectional analysis of the Brazilian Longitudinal Study of Adult Health (ELSA-Brasil) study. Public Health Nutr. 2021;24(15):5006–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Richardson LA, Basu A, Chien L-C, Alman AC, Snell-Bergeon JK. Associations of the Mediterranean-style dietary pattern score with coronary artery calcification and pericardial adiposity in a sample of US adults. Nutrients. 2022;14(16):3385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moreno-Franco B, Rodríguez-Ayala M, Donat-Vargas C, Sandoval-Insausti H, Rey-García J. Association of Cooking Patterns with inflammatory and cardio-metabolic. Risk Biomarkers. 2021;13(2). [DOI] [PMC free article] [PubMed]
- 31.Poljsak B, Kovač V, Antioxidants. Food Process Health. 2021;10(3). [DOI] [PMC free article] [PubMed]
- 32.Bailey RL. Overview of dietary assessment methods for measuring intakes of foods, beverages, and dietary supplements in research studies. Curr Opin Biotechnol. 2021;70:91–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen D, Liang M, Jin C, Sun Y, Xu D, Lin Y. Expression of inflammatory factors and oxidative stress markers in serum of patients with coronary heart disease and correlation with coronary artery calcium score. Experimental Therapeutic Med. 2020;20(3):2127–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jenny NS, Brown ER, Detrano R, Folsom AR, Saad MF, Shea S, et al. Associations of inflammatory markers with coronary artery calcification: results from the multi-ethnic study of atherosclerosis. Atherosclerosis. 2010;209(1):226–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhou H-T, Zhao D-L, Wang G-K, Wang T-Z, Liang H-W, Zhang J-L. Assessment of high sensitivity C-reactive protein and coronary plaque characteristics by computed tomography in patients with and without diabetes mellitus. BMC Cardiovasc Disord. 2020;20:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Severance LM, Contijoch FJ, Carter H, Fan CC, Seibert TM, Dale AM, et al. Using a genetic risk score to calculate the optimal age for an individual to undergo coronary artery calcium screening. J Cardiovasc Comput Tomogr. 2019;13(4):203–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang M, Hou Z-H, Xu H, Liu Y, Budoff MJ, Szpiro AA, et al. Association of estimated long-term exposure to air pollution and traffic proximity with a marker for coronary atherosclerosis in a nationwide study in China. JAMA Netw open. 2019;2(6):e196553–e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Elks CM, Francis J. Central adiposity, systemic inflammation, and the metabolic syndrome. Curr Hypertens Rep. 2010;12:99–104. [DOI] [PubMed] [Google Scholar]
- 39.Wang H, Chen H, Fu Y, Liu M, Zhang J, Han S et al. Effects of Smoking on inflammatory-related cytokine levels in human serum. Molecules. 2022;27(12). [DOI] [PMC free article] [PubMed]
- 40.Khanna D, Khanna S, Khanna P, Kahar P, Patel BM. Obesity: a chronic low-Grade inflammation and its markers. Cureus. 2022;14(2):e22711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.van de Loo AJ, Mackus M, Kwon O, Krishnakumar IM, Garssen J, Kraneveld AD, et al. The inflammatory response to alcohol consumption and its role in the pathology of alcohol hangover. J Clin Med. 2020;9(7):2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pescatore LA, Gamarra LF, Liberman M. Multifaceted mechanisms of vascular calcification in aging. Arteriosclerosis, thrombosis, and vascular biology. 2019;39(7):1307–16. [DOI] [PubMed]
- 43.Roy SK, Zeb I, Kadakia J, Li D, Budoff MJ. Body surface area is a predictor of coronary artery calcium, whereas body mass index is not. Coron Artery Dis. 2012;23(2):113–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cardoso R, Generoso G, Staniak HL, Foppa M, Duncan BB, Pereira AC, et al. Predictors of coronary artery calcium incidence and progression: the Brazilian Longitudinal Study of Adult Health (ELSA-Brasil). Atherosclerosis. 2020;309:8–15. [DOI] [PubMed] [Google Scholar]
- 45.Tsai J-P, Jan Y-T, Yun C-H, Sung K-T, Liu C-C, Kuo J-Y, et al. Associations of cigarette smoking and burden of thoracic aortic calcification in asymptomatic individuals: a dose-response relationship. PLoS ONE. 2020;15(1):e0227680. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data used for this study were obtained from The Biologic Specimen and Data Repository Information Coordinating Center (BioLINCC) is accessed at https://biolincc.nhlbi.nih.gov/.