Abstract
Background
Understanding the interaction between environmental conditions, crop yields, and soil health is crucial for sustainable agriculture in a changing climate. Management practices to limit disease are a balancing act. For example, in potato production, dry conditions favour common scab (Streptomyces spp.) and wet conditions favour blackleg disease (Pectobacterium spp.). The exact mechanisms involved and how these link to changes in the soil microbiome are unclear. Our objectives were to test how irrigation management and bacterial pathogen load in potato seed stocks impact: (i) crop yields; (ii) disease development (blackleg or common scab); and (iii) soil microbial community dynamics.
Methods
We used stocks of seed potatoes with varying natural levels of Pectobacterium (Jelly [high load], Jelly [low load] and Estima [Zero – no Pectobacterium]). Stocks were grown under four irrigation regimes that differed in the timing and level of watering. The soil microbial communities were profiled using amplicon sequencing at 50% plant emergence and at harvest. Generalised linear latent variable models and an annotation-free mathematical framework approach (ensemble quotient analysis) were then used to show the interacting microbes with irrigation regime and Pectobacterium pathogen levels.
Results
Irrigation increased blackleg symptoms in the plots planted with stocks with low and high levels of Pectobacterium (22–34%) but not in the zero stock (2–6%). However, withholding irrigation increased common scab symptoms (2–5%) and reduced crop yields. Irrigation did not impact the composition of the soil microbiome, but planting stock with a high Pectobacterium burden resulted in an increased abundance of Planctomycetota, Anaerolinea and Acidobacteria species within the microbiome. Ensemble quotient analysis highlighted the Anaerolinea taxa were highly associated with high levels of Pectobacterium in the seed stock and blackleg symptoms in the field.
Conclusions
We conclude that planting seed stocks with a high Pectobacterium burden alters the abundance of specific microbial species within the soil microbiome and suggest that managing pathogen load in seed stocks could substantially affect soil communities, affecting crop health and productivity.
Video Abstract
Supplementary Information
The online version contains supplementary material available at 10.1186/s40168-024-01918-6.
Keywords: Potato blackleg, Common scab disease, Soil microbial communities, Potato crop health, Pectobacterium, Bioinformatics
Background
To meet the growing global demand for food, it has been estimated that agricultural crop production needs to more than double by 2050 [1]. Production rates are predicted to decrease due to the impact of climate change and requirements for environmentally sustainable production [2]. Potatoes (Solanum tuberosum) are one of the most important food crops, with over 359 million metric tons produced globally [3]. However, production yields have stagnated over the past decade and land use for potatoes has decreased [4, 5]. Potatoes are highly susceptible to infection from a plethora of pests and pathogens. These include for example; oomycetes (late blight; Phytophthora infestans [6, 7]), bacteria (blackleg; Pectobacterium spp. [8]), (common scab; Streptomyces spp. [9]), potato cyst nematodes (Globodera spp. [10]) and viruses [11]. These diseases reduce plant growth, the quality of tubers and overall potato production yields. Changing environmental conditions are anticipated to exacerbate disease prevalence and future potato crop production [12–15]. For example, even within the UK, there is extensive variation in sustainable management solutions, which need to be customised to the local environmental conditions. Growers must also make trade-offs between crop value (based on consumer choice and supply chains) and pathogen management. While cultivar selection is largely driven by crop value, there are known to be both climate-induced and cultivar-induced differences in disease susceptibility [16–18]. However, this effect is highly variable and not clearly understood. Thus, a deeper understanding of the impact of management practices, potato stock selection and pathogen load on plant susceptibility and soil diversity would be invaluable.
Soil moisture content is a key parameter in the development of many potato crop diseases. In dry conditions, crops are typically more prone to common scab, Streptomyces species [19, 20]. In contrast, wet conditions promote the development of blackleg disease, Pectobacterium spp. [21], while a combination of wet, warm and dry conditions favours late blight, Phytophthora infestans [21]. In this work, we will focus on two of these diseases: common scab and blackleg disease.
Common scab disease results in scab-like lesions on potato tubers. Timing is crucial for disease development, with potatoes being the most vulnerable up to 6 weeks post-tuber initiation (i.e. tuber development; [19]). Irrigation applied during the period when plants are most susceptible to common scab infection can reduce disease and increase crop yields [22–25], but some authors have noted that this is not a reliable means of control [26]. Indeed, the mechanisms of control via irrigation or other means (soil pH amendment) are not clearly understood. Moreover, disease control is challenging as many Streptomyces spp. are naturally present in the soil environment [27].
Blackleg disease of potato stems and soft rot disease of tubers are typically caused by Pectobacterium atrosepticum in the UK but may be caused by other Pectobacterium and Dickeya species elsewhere [28]. A high soil moisture content induces blackleg infection in two ways. Firstly, anaerobic conditions from water logging cause the suppression of plant oxidative stress response-mediated defence mechanisms and increase plant cell permeability [29–31]. Secondly, P. atrosepticum is a facultative anaerobe and thus anoxic conditions enable it to proliferate and take advantage of the previously mentioned weaknesses in the plant host [30]. Pectobacterium species are thought to have limited survival in soil, with disease primarily spread through contaminated seed tubers. There are no chemical control options for blackleg and control of the disease has primarily been through stringent seed lot certification. For example, in the UK there is zero tolerance in the first-generations of progeny seed [pre-basic seed grade] [32]. In terms of management practice, tubers are typically only grown for 5–6 years due to the build-up of P. atrosepticum [33, 34]. However, other factors such as irrigation, aerosols and contaminated seed handling equipment may also play a role [35, 36]. Additional management practices during the growing season are not well defined and are less evidenced in the literature, but some practices include rogueing fields to remove crops showing characteristic above-ground wilting associated with blackleg disease [37]. Blackleg disease development is heavily influenced by climate; thus, management must be modified to local conditions [31]. For example, Scotland produces most UK potato seed stock, which are mainly grown without irrigation to avoid blackleg disease, whilst in England irrigation is used to manage common scab, to avoid plant desiccation and increase yield in what are mainly ware crops. This example nicely highlights the balancing act placed on potato growers, where they must trade off management practices with the environmental niches of multiple pathogens.
Management practices to control crop disease could also affect soil fertility and health, which in turn could impact crop productivity. For example, fertilisation can lead to soil degradation, while mechanical tillage can lead to soil erosion [38]. Soil microbial communities are also influenced by agricultural practices such as crop rotations [39], soil compaction [40], tillage and crop choice [41, 42]. This is important, as the microorganisms in soil are part of a wider interactive web involving animals and plants whose activities contribute to soil fertility, diversity and plant health. These factors are essential for global food production [43], but the exact mechanisms of these interactions and how these intricate systems respond to management practices and changing environmental conditions remain unclear [44]. Nevertheless, regulating, predicting and manipulating these responses is crucial for healthy soil and global food security in a changing global environment.
We are beginning to recognise that the soil microbiome is intrinsic to ecosystem health, services and plant, animal and human health [45]. Agricultural management practices can disrupt or alter the native soil microbiomes [46, 47]. However, the ramifications of such impacts are not clearly understood. Soil and plant microbiome networks are complex and shaped by interactions with surrounding fauna and responses to the abiotic and biotic environment. Despite this complexity, it has been observed that some plants show a bespoke local microbiome that is distinct from the surrounding soil microbiome [48]. In some cases, this microbiome can be modulated using the plant’s defence system for protection against pathogens [49]. The soil microbiome can also play a role in defence against diseases, e.g. in disease-suppressive soils [50, 51]. Which soil microorganisms are involved in the interactions essential for plant health and what variables drive these key microbial taxa remains to be unravelled. A perspective by Toju et al. [52] discussed this concept and highlighted the potential for multiple states of healthy soil microbiome (core microbiomes) akin to human gut-associated microbiomes (enterotypes) which, if understood, could allow us to unlock the potential of sustainable agroecosystems.
In this study, our overall aim was to determine whether there are interactions between agricultural management practices, potato seed stocks and crop health and how these link to soil microbial communities. Using agricultural field trials at NIAB Cambridge, UK we tested whether irrigation practices and/or starting pathogen burden (Pectobacterium sp.) in different stocks of seed potatoes affected crop yields, disease symptoms (common scab and blackleg) and overall diversity (taxonomic and functional) of the core soil microbial communities. To more specifically quantify differences in ‘key’ microbial taxa or the contributions of rare taxa to the microbiome, we then used differential heat-tree analysis and generalised linear latent variable modelling (GLLVM) to specifically compare microbial community composition in relation to the experimental treatments and used ensemble quotient analysis (EQO) to test whether there was a correlation between disease symptoms (blackleg and common scab) with particular microbial genera.
Materials and methods
Seed stock selection
To select appropriate seed stocks with varying Pectobacterium levels, we used blackleg-susceptible commercial potato stocks which were tested for pathogen prevalence by SASA (Science and Advice for Scottish Agriculture). Two of the stocks were of the Jelly variety: JellyHigh (Pre-basic Class Generation 3 stock [PB3] with peel containing a mean of 1.47 × 103 colony forming units (cfu)/g of P. atrosepticum and 5.74 × 104 cfu/g of Pectobacterium carotovorum) and JellyLow (Pre-basic Class Generation 2 stock [PB2] containing a mean of 400 cfu/g of P. atrosepticum and 1.88 × 103 cfu/g of P. carotovorum). No commercial Jelly stocks available to us were free of Pectobacterium and Jelly mini-tubers were unavailable. Therefore, for the stock without Pectobacterium we used Estima mini-tubers (EstimaZero) stock [Pre-basic PB1], which were found contain zero Pectobacterium species. Note: Pectobacterium atrosepticum is the causative agent of blackleg disease, while Pectobacterium carotovorum is not typically associated with the disease in the field, although authors have shown potential for disease outcome using inoculation experiments [53].
Irrigation regimes
We compared four irrigation treatments: (1) rainfed only (Unirrigated); (2) irrigated when the soil moisture deficit (SMD) reached 40 mm (Irrigation 1); (3) irrigated maintaining SMD < 15 mm during the common scab control period (the 4 weeks following the onset of tuber initiation) and unirrigated for the rest of the season (Irrigation 2); and (4) irrigated maintaining SMD < 15 mm during the common scab control period and then < 25 mm throughout the rest of the season (Irrigation 3). Irrigation was applied using 20–25 mm applications for all irrigation regimes. The total amount of water applied during the season was 150 mm for Irrigation 1 and Irrigation 2, and 251 mm for Irrigation 3. The total rainfall recorded from plant emergence was 202 mm. No plots were protected from rainfall.
Field trials
The field-trial experiments were carried out at NIAB, Cambridge (52.2417°N, 0.0987°E). The soil profile is included in the Supplementary Information. The field was randomised into a factorial block design using combinations of the three seed stocks (JellyHigh, JellyLow and EstimaZero) and four irrigation regimes (Unirrigated, Irrigation 1, Irrigation 2, and Irrigation 3), which were replicated in triplicate plots. Details of the schematic of the experimental design (Supplementary Figure S1) and layout of the plots (Supplementary datasheet 1) are provided in Supplementary Information. The experiment was planted on the 23rd of April 2020 using 30–40 mm Jelly seed (1206 tuber count/50 kg) and 25–30 mm Estima mini-tubers (2078 tuber count/50 kg) at a within-row spacing of 30 cm in 75 cm rows. The seed was dibbed 12 cm deep into pre-formed ridges, which were raked after planting to re-form the original ridge. Plots were 3.6 m long and four rows wide, with a 2-m pathway between strips of plots. An extra guard row was planted on either side of each plot to act as an additional buffer against overland and aerial movement of water from the irrigator. Plots were tie-bunded at either end to prevent over-land water flow between plots to minimise the risk of between-plot transfer of blackleg. Post-planting (30/04/2020), ammonium nitrate was applied at a rate of 200 kg N/ha and herbicides and fungicides were applied as required to keep the experiment free from weeds and blight. Irrigation was scheduled using the Cambridge University Farm (CUF) Potato Irrigation Scheduling Model based on meteorological data obtained from a Delta-T Devices weather station c. 450 m from the experimental plot. The irrigation was carried out using a diesel engine-driven Briggs VR4 90/400 hosereel and R50 boom equipped with Senninger LDN UP3 Single Pad nozzle dropper pipes to allow discrete irrigation between plots.
Crop monitoring and crop yield
Plant emergence was recorded by counting the number of plants that had emerged within the two central harvest rows. Emergence was first recorded on the 19th of May 2020 and recorded every 3–4 days thereafter until the 15th of June 2020. The number of plants with initiated tubers in a two-plant sample harvested in every plot was counted every 2 days, commencing on the 8th of June. At harvest (23–25th September 2020), tubers were graded, counted and weighed in 10 mm increments to provide total tuber yield and volume per hectare.
Blackleg incidence and common scab prevalence
Plants were scored for blackleg symptoms on 18th June, 26th June, 6th July, 31st July and 20th of August 2020. Blackleg symptoms were considered as either black decaying lesions on stems or wilted leaves on a single stem. At harvest tubers showing rot symptoms were bagged separately, counted and the weight recorded. A total of 50 tubers from the final harvest were selected for common scab assessment, which was determined as the total surface area infected with common scab which was further sorted into the following threshold levels: 0, 1, 5, 10, 15, 20, 25, 30, 40, 50, 60, 70, 80 and 90% surface area infected [54].
Complete metadata for the field-trial was collated and included plot number, plot area (based on location in the field—Supplementary data files 1–2), seed stock, irrigation regime, rainfall, temperature, irrigation volumes, starting levels of P. atrosepticum and of P. carotovorum in the starting seed stock), blackleg incidence, blackleg percentage prevalence, the weight of rotted tubers and common scab percentage.
DNA extraction and amplicon sequencing of the 16S rRNA gene
Soil samples were taken for microbiome analysis at 50% plant emergence (22nd May 2020) and at final harvest (15th September 2020). Samples were taken across treatments (Unirrigated, Irrigation 1, Irrigation 2, and Irrigation 3) and potato stock/pathogen levels (JellyHigh, JellyLow and EstimaZero). Comparisons in microbial communities across time were made using soil (20 samples per plot) sampled from the ridge (near the top of the furrow) at 50% plant emergence (T_E Ridge) and harvest (T_H Ridge). In addition, to determine if there were differences in microbial communities sampled closer to the plant roots, additional samples (20 per plot) were also taken at the bottom of the furrow at harvest only (T_H Root), to avoid damaging roots in the emerging plants (Supplementary Figure S1). Samples were taken using a soil corer. Corer diameter was 29 mm and the depth of insertion was 150 mm, giving a core volume of 99 cm3. Twenty cores were taken per plot (c. 1 kg soil), the soil was bulked together and mixed thoroughly. Samples were stored in cold storage at NIAB before shipping to the University of Glasgow for further homogenisation of sample material by mixing in large sampling bags and taking aliquots for storage at − 80 ºC. DNA was extracted with the Fast DNA™ SPIN Kit for Soil (MP Biomedicals, CA, USA) using 0.5 g of soil material. Negative controls (nuclease-free water) were additionally passed through the extraction procedure for each set of extractions. DNA quality was assessed by agarose gel electrophoresis and quantified using a QuBit 3 Fluorometer (Thermo-Fisher Scientific, Renfrew, Scotland). Amplicon sequencing was then carried out using the universal bacterial and archaeal primer set (515f and 806r; [55]) that contained the Illumina adapter sequence ‘TCGTCGGCAGCGTCAGATGTGTATAAGAGACAG’ on the forward primer and the adapter sequence ‘GTCTCGTGGGCTCGGAGATGTGTATAAGAGACAG’ on the reverse primer. Samples were indexed using the Nextera XT DNA Library Preparation Kit (Illumina Inc., Hayward, CA, USA Illumina). Amplicon libraries were pooled and quality checked using a Bioanalyser (Agilent, Santa Clara, CA, USA) and RT-qPCR (quantitative reverse transcription polymerase chain reaction) using the Kapa library Quantification kit on an Applied Biosystems StepOne plus system (Applied Biosystems, MA, USA). Sequencing was carried out using the Illumina technology at the Glasgow Polyomics sequencing facility using a standard flow cell and 600 cycle v3 reagent cartridge for 2 × 300 bp (base pair) reads on an Illumina MiSeq instrument (Illumina Inc., Hayward, CA, USA).
Bioinformatic analysis
The paired-end reads were demultiplexed and converted to FastQ format sequence files for further analysis. These raw sequences were submitted to the sequence read archive (SRA) database under Bioproject Submission PRJNA992106. A total number of 13,575,540 reads were obtained from 222 samples. Briefly, we used Qiime2 software for the sequencing analysis [56]. Within the Qiime2 framework, the DEBLUR algorithm [57] was used for the generation of amplicon sequencing variants (ASVs), after quality trimming the reads with a Phred quality score of 20. The final ASVs (p = 33,464 ASVs for n = 222 samples) were then classified against the SILVA v138.1 SSU Ref NR database [58]. The abundance table was then combined with the taxonomy to produce a biom file, on which downstream statistics were applied. In addition, predictive functional analysis was carried out using PICRUSt2 software within the Qiime2 framework, which produced sample-level KEGG orthologs and MetaCyc pathways as biom and functional.tsv files [59]. As a pre-processing step, we removed typical eukaryotic contaminants such as Mitochondria, Chloroplasts and any ASVs unassigned at all levels, as per recommendations given at https://docs.qiime2.org/2022.8/tutorials/filtering/. Moreover, singletons and samples with reads < 5000 were excluded from analysis. This resulted in 31,114 ASVs from 208 samples. Prior to the downstream statistical analysis, we used the ‘decontam’ package in R to remove contaminants by comparing the prevalence of ASVs in the negative controls to true samples [60].
Abundant and core microbial genera
The top 25 most abundant microbial families as a proportion of total relative abundance per sample were visualised using ggplot2 [61]. We considered the common ‘core’ microbiome to constitute the genera present in all field-trial samples (with > 1% compositional abundance in at least 85% of samples). The common core microbiome has been summarised in a review Shetty et al. [62]. Analysis was conducted in R (R version 4.1.3: [63]) using ‘phyloseq’ [64]. The core microbiome was calculated using R’s microbiome package [65] based on the preliminary work of [66].
Statistical analysis
Statistical analysis methods are provided in the Supplementary Information.
Results
Crop yields
The choice of potato stock (with associated pathogen levels) and irrigation regime both impacted crop yield. Not irrigating reduced the tuber yield for all potato stocks, particularly for the EstimaZero mini-tubers (Table 1). Irrigation regime 1 (irrigated when SMD reached 40 mm) resulted in the highest yield for both Jelly stocks (JellyHigh 69 tons/HA; JellyLow 67.4 tons/HA). In contrast, irrigation regime 3 (irrigated maintaining SMD < 15 mm during the common scab control period and then < 25 mm throughout the rest of the season) resulted in the highest yield for the EstimaZero stock, which was the highest tuber yield observed for all stocks and treatments (75 tons/HA).
Table 1.
Potato crop yields from the experimental field trials at harvest
| Stock | Irrigation | Total no. of tubers (000/ha) | Total tuber yield (t/ha) |
|---|---|---|---|
| JellyHigh | Unirrigated | 267 | 45.2 |
| Irrigation 1 | 349 | 69.0 | |
| Irrigation 2 | 277 | 50.0 | |
| Irrigation 3 | 299 | 63.8 | |
| JellyLow | Unirrigated | 339 | 49.9 |
| Irrigation 1 | 369 | 67.4 | |
| Irrigation 2 | 341 | 54.5 | |
| Irrigation 3 | 308 | 64.9 | |
| EstimaZero | Unirrigated | 264 | 29.8 |
| Irrigation 1 | 324 | 60.5 | |
| Irrigation 2 | 394 | 59.0 | |
| Irrigation 3 | 401 | 75.0 | |
| Fprob Stock | 0.020 | 0.501 | |
| Fprob Irrigation | 0.032 | < 0.001 | |
| Fprob Stock*Irrigation | 0.011 | 0.006 |
Table 1 shows tuber yield information for each potato stock (JellyHigh, JellyLow and EstimaZero) and each irrigation regime (Unirrigated, Irrigation 1, Irrigation 2 and Irrigation 3). Tuber yield was calculated by the total number of tubers per hectare (HA) and the tuber yield in tonnes per hectare. Results of the statistical analysis (P values) testing the impact of ‘Stock’, ‘Irrigation’ and ‘Stock and Irrigation’ are shown at the bottom of the table. Values are significant when P < 0.05. We have highlighted in bold which values are significant.
Disease prevalence
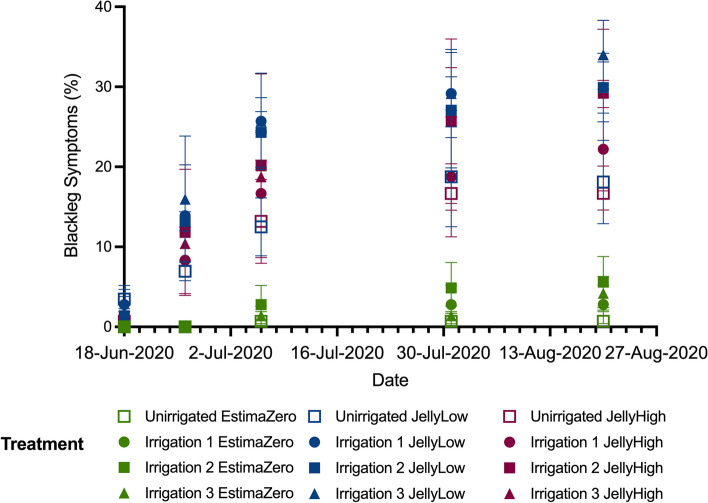
The Jelly stocks with both high and low initial Pectobacterium loads both showed a high percentage of blackleg symptoms at the end of the field trial (Table 2). The percentage of blackleg symptoms was strongly impacted by the irrigation regime, with the unirrigated treatment showing the lowest symptom prevalence (16.7% JellyHigh and 18.1% JellyLow) and irrigation 3 showing the highest symptom prevalence (34% for both Jelly stocks), although there was some variation among plots (Supplementary Table S1). Examining the blackleg symptoms over time highlights that disease incidence developed at a similar rate in the stock with low starting levels of Pectobacterium (JellyLow) as compared to the stock with high starting levels (JellyHigh) (Fig. 1). This trend was exacerbated in the irrigation treatments (Irrigation 1, Irrigation 2 and Irrigation 3). In contrast, the EstimaZero with no initial Pectobacterium detected showed very low levels of blackleg symptoms across the entire experiment (reaching only 0.7% in the unirrigated treatment and ranging from 2.8 to 5.6% in the irrigated treatments at harvest; Table 2). Despite substantial blackleg symptoms recorded in the field for the Jelly stocks, overall, the degree of tuber rotting at harvest was low, with < 1.5% of rotted tubers (Supplementary Table S2). Moreover, no significant difference was observed with respect to irrigation or potato stock on the observed proportion of rotting tubers.
Table 2.
Blackleg and common scab symptoms at the end of the experimental trial
| Stock | Irrigation | Blackleg symptoms (%) | Common scab severity (%) |
|---|---|---|---|
| JellyHigh | Unirrigated | 16.7 | 2.74 |
| Irrigation 1 | 22.2 | 2.13 | |
| Irrigation 2 | 29.2 | 1.72 | |
| Irrigation 3 | 34 | 1.51 | |
| JellyLow | Unirrigated | 18.1 | 3.29 |
| Irrigation 1 | 29.9 | 1.83 | |
| Irrigation 2 | 29.9 | 1.56 | |
| Irrigation 3 | 34 | 1.73 | |
| EstimaZero | Unirrigated | 0.7 | 5.43 |
| Irrigation 1 | 2.8 | 2.46 | |
| Irrigation 2 | 5.6 | 2.27 | |
| Irrigation 3 | 4.2 | 1.94 |
Fig. 1.
Blackleg symptoms (%) plotted against time from plant emergence (18/06/2020) to plant harvest (27/08/2020). Symptoms are based on the average of blackleg symptoms per plot based on the incidences of recorded symptoms in the field. Values are shown by stock (EstimaZero, JellyLow and JellyHigh) and irrigation regimes (Unirrigated, Irrigation 1, Irrigation 2 and Irrigation 3). Standard deviation bounds are shown on the horizontal lines on the box-plot whiskers per condition
While irrigation increased blackleg disease symptoms, a lack of irrigation (rainfed only) strongly impacted common scab severity in the tubers upon harvest (Table 2). The unirrigated treatments showed the highest common scab levels, and the Estima tubers were the most affected (5.43% EstimaZero, 3.29% JellyLow and 2.74% JellyHigh). In general, tubers from irrigation regime 3 (irrigated maintaining SMD < 15 mm during the common scab control period and then < 25 mm throughout the rest of the season) showed the lowest common scab severity (1.94% EstimaZero, 1.73% JellyLow and 1.51% JellyHigh—Fig. 1; Table 2).
Table 2 shows the percentage of the crop showing blackleg symptoms on 20th August 2020 and the percentage of common scab severity from tubers from the final harvest (23–25th September 2020) for the three potato stocks (JellyHigh, JellyLow and EstimaZero) and the four irrigation regimes (Unirrigated, Irrigation 1, Irrigation 2 and Irrigation 3).
Dominant microbial community composition and core microbiome
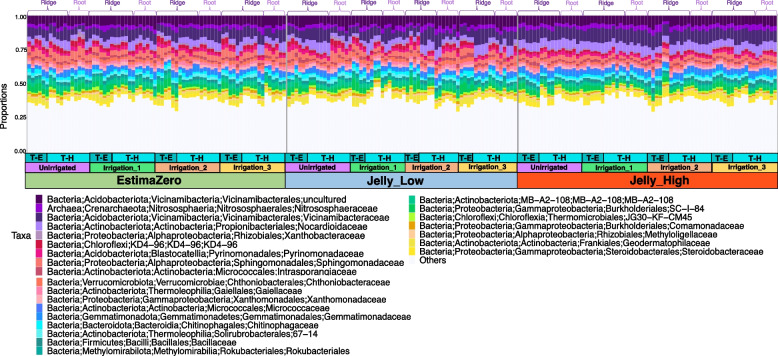
The 25 most abundant microbial families in the soil communities were remarkably stable (Fig. 2) in relation to initial Pectobacterium levels (Zero, Low and High), potato variety (Jelly seed or Estima mini-tubers) and irrigation regime (Unirrigated, Irrigation_1, Irrigation_2 and Irrigation_3). Some of the most abundant taxa were species within the Vicinamibacterales (~ 20%), Nitrososphaeraceae (~ 12%), Nocardiodaceae (~ 8%) and Sphingomonodaceae (~ 8%) families (Fig. 2). Although we did observe some shifts in relative abundance across time in the ridge samples (T-E—50% plant emergence and T-H—plant harvest) and in the relative abundances of the top 25 families between the ridge and root samples at harvest, the pattern of changes were similar across stocks and irrigation treatments (Fig. 2). Based on a threshold of presence in at least 85% of samples, we observed that 65 genera were part of the ‘core microbiome’ that did not change in relation to treatments (Supplementary Figure S3; Supplementary Table S4). Of these, Vicinibacterales, Nocardioides, Pyrinomonadaceae (RB41) and Chloroflexi (KD4-96) species showed the highest prevalence (75–100%) and the highest number of amplicon sequencing variants (ASVs) detected (298–1362 ASVs).
Fig. 2.
Stacked vertical bar chart showing the soil microbiome composition, comparing the relative abundance of the top 25 microbial families found in soil across treatments (irrigation regime), stocks/ pathogen levels (JellyHigh, JellyLow, Estima), time (50% plant emergence T_E, or harvest, T-H) and whether samples were taken from the ridge (background soil community at both timepoints) or root (rhizosphere at harvest). Colours represent the relative abundance of each taxonomic family. See Supplementary Table S3 for the taxonomic description). The white portion of the bars indicate the contribution of microbial families that were not represented in the top 25
Alpha and beta diversity
Alpha diversity represents the number and evenness of different ASVs within a single sample, while beta diversity is a measure of similarity or dissimilarity between ASVs from two communities [67]. In addition to considering ASVs (taxonomic diversity) we also considered functional diversity, which was based on the number and evenness of KEGG orthologs (KOs) within a single sample (alpha diversity) and the dissimilarity of KOs between two communities (beta diversity).
Irrigation
At 50% plant emergence, the irrigation management practice did not significantly impact taxonomic or functional microbial diversity either within or between treatments (Supplementary Figure S4–S5). At harvest, there were no significant differences in taxonomic diversity within treatments (Supplementary Figure S6A). However, there were differences in taxonomic diversity between treatments, with samples showing some clustering with respect to Irrigation 2 and whether samples were from the root or ridge (Supplementary Figure S6B; Dim 1 6.85%, Dim 2 2.9%). At harvest, functional diversity within treatments was altered, with more unique KOs detected in the Irrigation 1 ridge samples, which was significantly higher (0.01 or more) than the ridge samples from Unirrigated, Irrigation 2 and Irrigation 3 regimes (Supplementary Figure S7A). We also observed differences with respect to functional diversity between treatments, with samples clustering according to irrigation regime (particularly Unirrigated and Irrigation 2) and whether samples were from root or ridge (Supplementary Figure S7B; Dim 1–77.89%, Dim 2–5.16%).
Potato stock
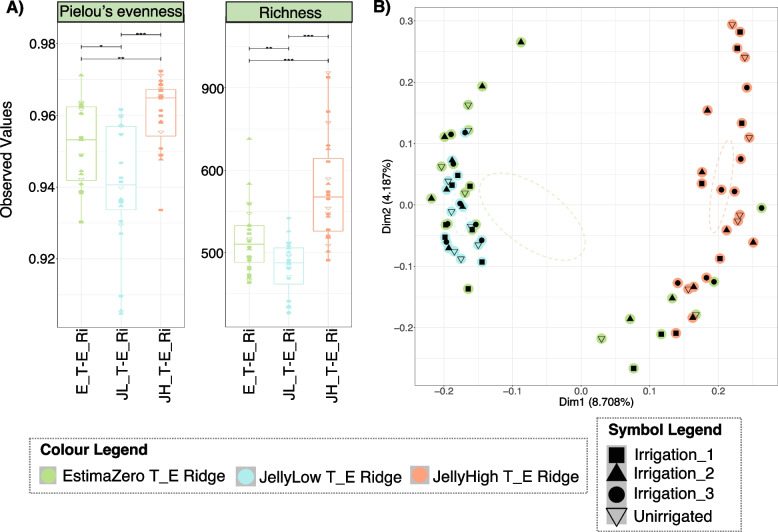
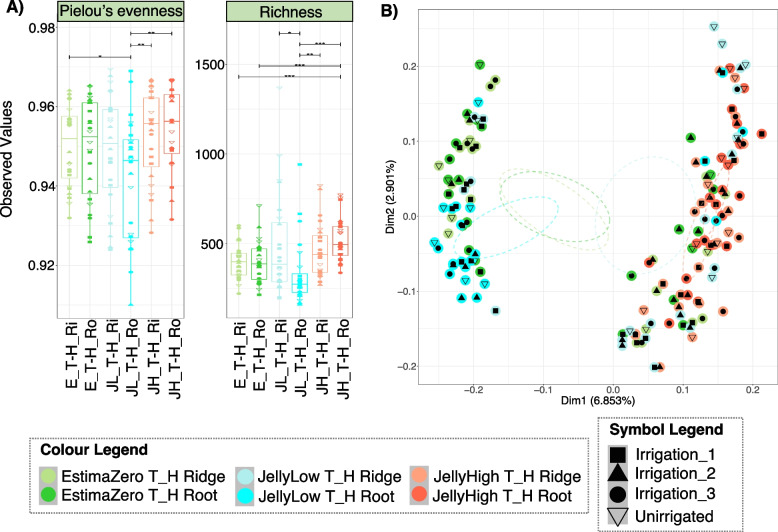
In the above analysis, the taxonomic and functional diversity of the microbial communities between treatments indicated that samples from the same potato stock were highly similar irrespective of irrigation regime. We, therefore, repeated the analysis using potato stock as the grouping variable. At 50% plant emergence, taxonomic diversity was significantly different within treatments. For example, JellyHigh treatments showed both an increased number of ASVs (richness values of ~ 480–920) and a more balanced distribution in the abundance of these ASVs (evenness values of ~ 0.935–0.975), while JellyLow showed reduced values, with richness values of 400–550 and evenness values of 0.90–0.96, Fig. 3A). It must be noted that the scale of these changes was relatively low, with evenness values ranging from 0.90 to 1.0 and richness values ranging from 400–900 (Fig. 3A). There were also significant differences in taxonomic diversity between treatments. JellyHigh samples with a high pathogen burden were highly similar and clustered together, whereas in general, the low (JellyLow) and zero (EstimaZero) stocks were more similar to each other and dissimilar to the JellyHigh (Fig. 3B; Dim1 8.7%, Dim2 4.19%). Some Estima samples (from plots 3–4, 3–8, 3–9, 3–11) clustered within this JellyHigh cluster. At harvest, there were fewer differences in taxonomic diversity within treatments. However, values for JellyLow root samples were lower than the other treatments, with evenness values of 0.95 and richness values of 450 (Fig. 4A). At harvest, we again observed significant differences in taxonomic diversity between treatments, with EstimaZero and JellyLow samples showing greater similarity, while JellyHigh samples were more dissimilar and formed a distinct cluster (Fig. 4B; Dim1 6.85%, Dim2 2.9%). Interestingly, while some JellyLow/EstimaZero samples clustered with the JellyHigh samples, no JellyHigh was observed outside the JellyHigh signature cluster (Fig. 4B). When we considered the taxonomic diversity within treatments over time (ridge samples only), evenness values decreased over time in the EstimaZero and JellyHigh but were increased in the JellyLow (Supplementary Figure S8A), while richness values increased over time in all but the JellyHigh samples (Supplementary Figure 8A). No distinct temporal pattern was observed in taxonomic diversity between treatments (Supplementary Figure S8B).
Fig. 3.
Taxonomic diversity of the soil microbiomes at 50% plant emergence (T_E). Diversity is influenced by potato stock Pectobacterium levels and potentially potato variety. Colours represent potato stock (EstimaZero—E, JellyLow—JL and JellyHigh—JH), time-point (50% plant emergence–T_E and soil sample type (Ridge–Ri). Symbols represent the irrigation regimes (Unirrigated, Irrigation_1, Irrigation_2 and Irrigation 3). A The relative distribution of taxa (Pielou’s evenness) and diversity (Richness) within the communities. Pair-wise ANOVA P-values are displayed P < 0.05*, P < 0.01**, and P < 0.001***. B Principal coordinate analysis (PCoA) based on Bray–Curtis distance of beta diversity dissimilarity between the communities
Fig. 4.
Taxonomic diversity of the soil microbiomes at plant harvest (T_H). Diversity is influenced by potato stock Pectobacterium levels and potentially potato variety. Colours represent potato stock (EstimaZero—E, JellyLow—JL and JellyHigh—JH), time-point (plant harvest—T_H) and soil sample type (Ridge—Ri, Root—Ro). Symbols represent the irrigation regimes (Unirrigated, Irrigation_1, Irrigation_2, and Irrigation 3). A The relative distribution of taxa (Pielou’s evenness) and diversity (Richness) within the communities. Pair-wise ANOVA P-values are displayed P < 0.05*, P < 0.01**, and P < 0.001***. B Principal coordinate analysis (PCoA) based on Bray–Curtis distance of beta diversity dissimilarity between the communities
In terms of functional diversity within treatments, at 50% plant emergence and at plant harvest, the distribution in the abundance of KEGG Orthologs (KOs) was lower in the JellyHigh samples (evenness), and the number of detected functions was slightly higher in the JellyHigh samples (Supplementary Figure S9A, Supplementary Figure S10A). Again, the scale of these changes was very low (differences of 0.01 for evenness and 100 for richness). At 50% plant emergence, functional diversity between treatments showed some clustering with respect to potato stock (Supplementary Figure S9B; Dim 1 74.48, Dim2 7.29%). At harvest, this was more pronounced with sample clustering according to potato stock and sample type (Ridge or Root) (Supplementary Figure S10B; Dim 1 77.29, Dim2 5.17%). However, these were not as clearly differentiated as observed in the taxonomic diversity plots (Fig. 4B; Dim1 6.85%, Dim2 2.9%). When we considered the functional diversity within treatments over time (ridge samples only), evenness values increased in the EstimaZero and JellyLow, but reduced in the JellyLow (Supplementary Figure S11A), while richness values increased over time in all but the JellyHigh samples, which remained stable (Supplementary Figure S8A). Functional diversity between treatments showed that samples loosely clustered according to time-point and potato stock (Supplementary Figure S11B).
Statistical analysis of taxonomic diversity between treatments
For the taxonomic diversity between treatments (beta diversity), we also implemented permutational multivariate analysis of variance (PERMANOVA) to determine what categorical variables could explain dissimilarity between such treatments. We determined that potato stock and plot number were highly significant variables (P = 0.001), explaining 8.3% and 43% of the variance between treatments, respectively. The irrigation regime was also a significant variable (P = 0.043; R2 4.5%). At harvest, potato stock, plot area and plot number were again highly significant (P = 0.001), and sample type (Root or Ridge; P = 0.007) and explained 3.4%, 6%, 17.85% and 0.9% of the variance, respectively. Irrigation was also significant (P = 0.007; R2 2.4%). Notably, the remaining residual variation was 69%, suggesting that the experimental variables explained a relatively low amount of variation. PERMANOVA analysis of the ridge samples over time showed that time was also a highly significant variable (P = 0.001; R2 1.9%). Redundancy analysis (RDA) with forward selection and subsequent PERMANOVA analysis revealed that blackleg and common scab symptoms per plot were highly significant between the ridge samples from EstimaZero, JellyLow and JellyHigh stocks (P < 0.001), explaining 42% and 17% of the variance, respectively. Irrigation volume was determined not to be significant (P = 0.138). The same pattern was observed in the root samples, although here irrigation volume was slightly significant (P = 0.049).
Contribution of rare taxa to betadiversity
We further assessed how ‘Rare’ microbial taxa (< 1% relative abundance) contribute to taxonomic diversity between potato stocks. We found that all taxa at the ASV-level fell into the ‘Rare’ category and at this level, no ASVs were categorised as ‘Abundant’ (> 1% relative abundance) or ‘Conditionally Rare’ (ASVs with max:min > 100 across groups) [data not shown]. The majority of ASVs were ‘Persistently Rare’ (ASVs with a maximum relative abundance < 5 times their minimum value [max:min < = 5]) with some ‘Other Rare’ [ASVs whose abundances were outwith the thresholds for ‘Conditionally Rare’ and ‘Persistently Rare’] ASVs (data not shown). ‘Persistently Rare’ ASVs contributed 42–95% to taxonomic diversity between potato stocks, and ‘Other Rare’ ASVs contributed 10–55% (Supplementary Figure S9). The contribution of ‘Persistently Rare’ taxa to taxonomic diversity increased over time in the EstimaZero and JellyHigh samples but decreased in the JellyLow, while the opposite was observed for the contribution of ‘Other Rare’ ASVs (Supplementary Figure S12).
Differential microbial taxa associated with irrigation management practice
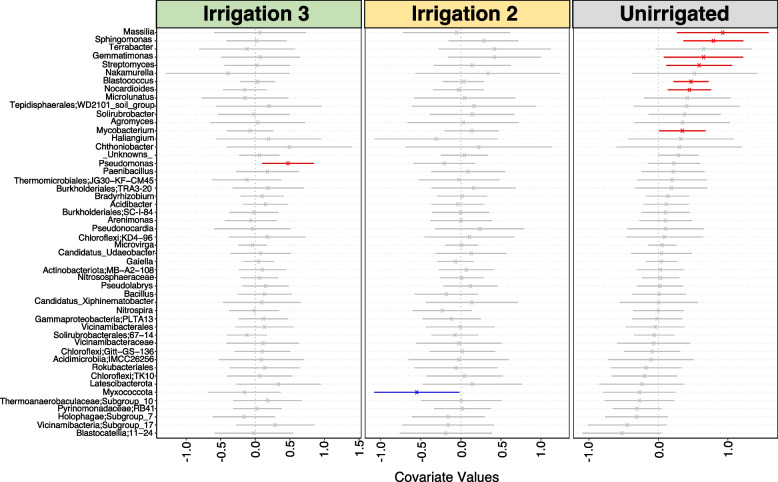
To assess the impact of irrigation regimes on microbial communities, we first implemented differential heat tree analysis, which determines microbial taxa with a Log2 fold difference in abundance in pair-wise comparisons between irrigation regimes. This analysis revealed that no taxa showed a Log2 fold difference between irrigation regimes at 50% plant emergence or at harvest (Supplementary Figures 13–16). We then applied a generalised linear latent variable model (GLLVM) to the dataset to find individual microbial genera either positively or negatively associated with each irrigation regime. At 50% plant emergence, we found no significant associations between microbial taxa and irrigation regime (data not shown). However, at harvest, we found that seven microbial genera (out of the top 50 genera) were positively associated with a lack of irrigation in the root samples. These included Massilia, Sphingomonas, Gemmatimonas, Streptomyces, Microlunatus, Nocardiodes and Mycobacterium genera (Fig. 5), while in the ridge sample, 16 microbial genera (out of the top 50 genera) were negatively associated with Irrigation 3. These included Acidimicrobiia, Chthoniobacter, Streptomyces, Sphingomonas, Nocardiodes and Vicinimibacteraceae (Supplementary Figure S17).
Fig. 5.
Plot highlighting the impact of ‘Irrigation Regime’ on the abundance of microbial taxa in the potato harvest (T_H) root soil samples based on the generalised linear latent variable model (GLVMM) analysis. The microbial genera names are indicated on the y-axis and the environmental covariate values are indicated on the x-axis, which in this case is (e.g. Unirrigated, Irrigation_1 and Irrigation_3 as compared to the Irrigation 1 treatment). The values range from positive, neutral to negative associations with the specific taxa on the y-axis. The red lines show taxa that are positively associated with the indicated environmental covariate, and the blue lines show taxa that are negatively associated; the lines in grey are not significantly different. Note: Irrigation 1 treatment is not displayed as the analysis uses this treatment as the reference. The results showing the analysis excluding the Unirrigated treatment instead are shown in Supplementary Figure S18
Differential microbial taxa associated with potato stock and time
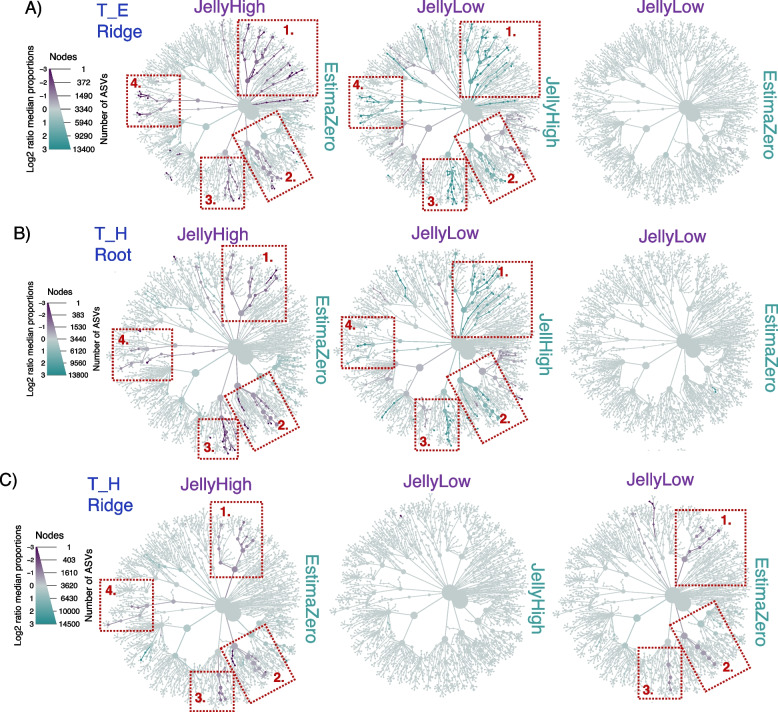
We repeated the above analysis with potato stock as the grouping variable. The differential heat tree analysis revealed substantial differentiation in the abundance of particular taxonomic groups between potato stocks at both time points (T_E and T_H) and in root and ridge samples. At 50% plant emergence, the soil planted with potato stock with a high Pectobacterium load (JellyHigh) showed Log2 fold increases in Planctomycetota phylum (Phycisphaeae [WD2101 soil group], Pirellulales [Pirellula] and Gemmatales [Gemmatela]), Chloroflexi phylum (Anaerolineae [Caldilineales, SBR1031, A4B]) and Acidobacteria phylum (Vincinamibacteraceae members) as compared to both the JellyLow and Estima stocks (Fig. 6A). In contrast, there were almost no differences between the low (JellyLow) and zero (EstimaZero) stocks. This trend was also observed in the harvest root samples (Fig. 6B). However, in the ridge samples at potato harvest, the JellyLow showed significantly increased Planctomycetota, Vicinibacteria and Anaerolinea as compared to the EstimaZero stock and showed no difference in taxa with the JellyHigh (Fig. 6C).
Fig. 6.
Heat tree analysis to visualise the differential taxa between potato stock soil microbiomes. A high initial pathogen burden in the potato seed stocks impacts the ridge and root-soil microbial communities. The full legend for the tree branches is shown in Supplementary Figure S13. In each plot four red boxes are highlighted, which correspond to: 1. Planctomycetota; 2. Vicinibacteria; 3. Anaerolinea; and 4. Verrucomicrobiae. The size of the branch nodes shows the number of ASVs, while the differential Log2 ratio median proportion is shown by the colours. The figures show a comparison between JellyHigh (purple) compared to EstimaZero (blue), JellyLow (purple) compared to JellyHigh (blue) and JellyLow (purple) compared to EstimaZero (blue) A at the 50% plant emergence (T_E) sampling period of the ridge microbiome; B at the harvest (T_H) sampling period of the ridge microbiome; and C at the harvest (T_H) sampling period of the root microbiome
Using the GLLVM analysis we further observed that at 50% plant emergence 16 (out of the top 50) microbial genera were positively associated with the stock with a high pathogen burden [JellyHigh] (Supplementary Figure S19). The most positively associated were Chthoniobacter, Anaerolineae, Planctomycetota, Tepidisphaerales, Pirellula and Vicinamibacteria species (Supplementary Figure S19). Eight of these were also positively associated with the JellyLow samples. Interestingly, at harvest, we observed a different pattern in the JellyLow root samples, which showed more negative or neutral associations with microbial genera that were typically positively associated with the JellyHigh samples (e.g. Chthoniobacter and Vicinamibacteria; Supplementary Figure S20). Typically, genera that were positively associated with JellyHigh or JellyLow (e.g. Planctomycetota, Anaerolineae, Tepidisphaerales, Latescibacterota and Vicinamibacteria) were negatively associated with the EstimaZero group (Supplementary Figure 21).
The GLLVM analysis on temporal samples (ridge samples from 50% plant emergence and harvest) further revealed that Planctomycetota (OM190), Anaerolinea (SBR1031, A4b and RBG-13), Haliangium, Pseudocardia, Latescibacterota, Pirellula and Pseudomonas were all positively associated with the potato harvest time point (Supplementary Figure S21). In contrast, the following taxa were negatively associated with the potato harvest time point; Massilia, Sphingomonas, Flavobacterium, Devosia, Arenomonas and Acidobacteria (Supplementary Figure S22).
Microbial taxa associated with blackleg and common scab disease symptoms
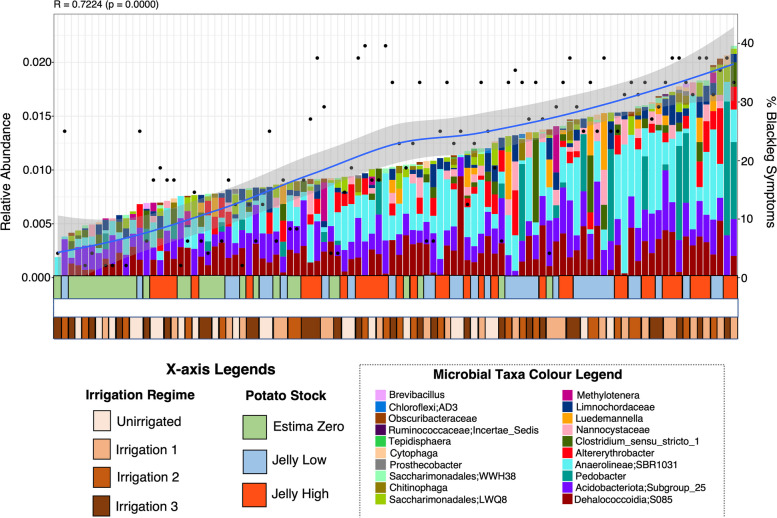
Finally, we wanted to determine which microbial genera were correlated to blackleg disease symptoms or common scab disease symptoms at potato harvest. This was achieved using Ensemble Quotient Optimisation (EQO), which returns the subset of microbial genera (ensemble) associated with a continuous variable. In the ridge samples, approximately eight genera comprised the minimum subset correlated with blackleg disease symptoms. Within these, the Anaerolineae (SBR1031) consistently showed increased relative abundance with increasing blackleg symptoms (Fig. 7). A clear trend was seen between the increasing percentage blackleg symptoms and the increasing abundance of the minimum subset of taxa identified by EQO. This contrasts with the patterns observed in the harvest root samples, where there was no trend indicating a key microbe to blackleg disease prevalence, with many genera in the subset (12–20) present across low to high blackleg symptoms (Supplementary Figure S23). For common scab symptoms, there was also no trend correlating key taxa to common scab symptoms, with most genera of the subset found across low to high common scab symptoms (Supplementary Figure S24). A similar result was observed in the root samples, though the relative abundance of Terrabacter and Saccharamonidales appeared to increase with increasing common scab symptoms (Supplementary Figure S25).
Fig. 7.
Minimum subset of amplicon sequencing variants (ASVs) associated with percentage blackleg symptoms during the field trial, based on the Ensemble Quotient Optimisation (EQO) analysis, considering the at most 20 microbial taxa for the ridge samples at harvest. The left y-axis shows the relative abundance of the microbial taxa, and the right y-axis shows the percentage blackleg symptoms observed. The x-axis shows the sample identity, with colour legends for potato stock (Green = EstimaZero, Blue = JellyLow and Red = JellyHigh) and the irrigation regimes (Unirrigated, Irrigation 1, Irrigation 2 and Irrigation 3)
Discussion
The factors underlying disease development in food crops like potatoes are a global issue. Management practices and climate conditions can mitigate or exacerbate disease symptoms, influence crop yields and perturb the supporting microbial communities [38–40]. In this study, we assessed the influence of Pectobacterium load, seed stock and irrigation on potato yields, common scab and blackleg disease symptoms and the impact on the soil microbial communities. While we observed that the irrigation regimes impacted both crop yield and disease prevalence, we found that neither this nor pathogen burden translated to disruption in the dominant soil microbial communities since the most abundant members of the microbial communities were stable irrespective of irrigation regime or initial pathogen burden of the seed potato stocks. However, by also including analyses that allowed consideration of changes in less abundant taxa, we observed that the initially high Pectobacterium pathogen burden on the JellyHigh seed stock was correlated with an increased abundance of particular microbial groups, even though the final disease burdens were similar between the low and high Jelly stocks. In contrast, we did not observe substantial changes in rarer taxa in comparisons between the JellyLow or EstimaZero stocks or in relation to irrigation regimes. This suggests that the conditions under which seed stocks are maintained and which lead to a high pathogen burden are more of an important driver of microbial community composition than the irrigation regime. Our results also emphasise the value of basing the interpretation of microbiome community dynamics not only on the effects of treatments on the “core microbiome” but also on more fine-scale analyses of rarer taxa, as suggested in a meta-analysis of agricultural studies in China [68].
Crop yields
Crop yields were highly influenced by the irrigation regime. Not irrigating reduced yields for all planted stocks, and this was most pronounced for the EstimaZero mini-tubers, indicating that the rainfall for the region (Cambridge, UK) was insufficient to fulfil the growth requirements of the crop, as is often the case, given the need for irrigation management as reported by UK growers. However, irrigating when the soil moisture deficit (SMD) reached 40 mm increased the yields for all stocks. Potatoes are vulnerable to drought stress, as the rooting system is relatively shallow [69] but there is variation in water requirements for different potato varieties [22, 70]. In addition, the method of irrigation supply can impact crop yields [71, 72]. Like other studies [73, 74], we noted an increase in crop yields with irrigation. However, we only analysed the conventional furrow irrigation methodology that would be the typical system of growers in England. Rainfed growth (unirrigated) is a practice that is used in Scotland for seed crops, which experiences 2–3 times more rainfall than in England. This emphasises regional variations and the need for adaptable management practices, particularly in the face of future climactic changes [14]. Potato production models simulating climate change (temperature, solar radiation, precipitation and CO2 levels) on potato growth development and yield have shown that by 2055 yields will increase in some areas (e.g. Western Europe) and decrease in others (e.g. North America and Eastern Europe) but that overall, production will have declined globally by 2085 [75]. Given that such models do not include impacts of biotic interactions (pests, pathogens and beneficial microbes) or management practices, the impacts of climate change could be even more dramatic. Our results emphasise the importance of matching management practices to local climatic conditions and carefully monitoring impacts on the most serious threats to production.
Disease prevalence
Although we observed only very low levels of rotting in our study, we observed that blackleg symptoms increased to a similar level in seed stocks with an initial Pectobacterium species burden regardless of whether the pathogen level was low or high. Importantly, even low levels of Pectobacterium in the seed stock translated into symptoms in the field that were on par with stock carrying a much higher initial burden. Our results contrast with those of authors who correlated the development of disease with Pectobacterium inoculum levels in seed tubers in both field trials with native levels and with artificial infections following in vitro inoculation of blackleg-causing species [76, 77]. However, while our results were based on multiple repetitions, they may have differed from those above due to only single stocks being used in our study and the levels of P. atrosepticum in both our high and low stocks are low as compared to both studies mentioned. Authors observed in field trials in Switzerland that the levels of Dickeya spp., Pectobacterium wasabiae and P. carotovorum subsp. brasiliense (now classified as P. brasiliense) could be correlated to initial inoculum levels but did not see the same trend with P. atrosepticum [35]. In our study, blackleg symptoms were worsened with applied irrigation regimes and were highest in the regime that maintained a soil moisture deficit of less than 15 mm during the common scab control period and continued irrigation through the rest of the growing season. This confirms the link between blackleg disease and soil moisture content [30]. In contrast, blackleg symptoms were very low in the stock without initial Pectobacterium contamination (EstimaZero). These results, therefore, reinforce the requirement for stringent seed certification for blackleg as even low levels of initial bacterial contamination greatly increased disease incidence.
Although common scab disease prevalence was under 5% in our study, we observed increased disease symptoms in the unirrigated (rainfed only) treatments compared to all irrigated treatments; this is in agreement with previous field-trial studies [23, 25]. The irrigation regime specifically designed to reduce common scab (Irrigation 2) was effective for all the stocks, and the regimes that continued watering post-tuber-initiation (Irrigations 1 and 3) also reduced common scab as well as increasing the total yield of tubers. This emphasises the potential trade-off in disease management practices since these irrigation treatments also resulted in higher blackleg incidence. Once again, local decisions about irrigation practices are predicted to have even more importance as climate change will increase the unpredictability of rainfall [75].
Impact of irrigation on the soil microbial communities
It is encouraging that our results show that differences in irrigation regimes did not perturb the dominant species or overall diversity of microbial communities associated with potatoes. Many studies have assessed the impact of irrigation on potato crop production [21, 22, 70] but so far there have been limited specific considerations about interactions between crop irrigation and soil microbial communities [78]. Of those studies that have, they explore the impacts of irrigation on soil microbial communities and do so with the aim of understanding recycled wastewater rather than in an agricultural setting [79–82]. The GLLVM approach by Niku et al. allows computationally efficient analysis of the correlations between environmental variables and microbial taxa [83]. Using this method, we observed that certain microbial groups were positively associated with the unirrigated treatments. These include Streptomyces, Terrabacter, Gemmatimonas, Sphingomonas and Massilia genera. Streptomyces, Sphingomonas and Gemmatimonas, are common taxa that have been observed previously in soil from potato field trials [84, 85]. Although amplicon sequencing alone did not allow resolution to species, Streptomyces scabies and other Streptomyces spp. are the causative agents of common scab. However, species of Streptomyces and Terrabacter have also been linked to disease-suppressive soils for many bacterial and fungal plant diseases [86, 87]. Massilia spp. play a role in the hydrolysis of inorganic phosphates and are thought to be more abundant in soils with limited phosphate [88]. Similarly, Gemmatimonas spp. are phosphate-solubilizing species that can make phosphate bioavailable to plants [89, 90]. These results suggest more complex interactions between the microbial communities and other environmental parameters (such as nutrient content).
Impact of pathogen burden on the soil microbial communities
Interestingly, we observed that the initial seed stock pathogen burden impacted beta diversity (between treatments) of soil microbial communities and the differential abundance of key taxa. Using Bray–Curtis dissimilarities, the communities from the EstimaZero and JellyLow stocks were more similar than the JellyHigh stock. The differential heat tree and GLLVM analysis confirmed that a high Pectobacterium burden in the starting seed stock resulted in increased Anaerolineae, Chthoniobacter, Planctomycetota, Tepidisphaerales, Pirellula and Vicinamibacteria species, at both 50% plant emergence and at harvest, compared to the other stocks. The JellyLow stocks also became more similar to the JellyHigh stocks at harvest, showing increased Planctomycetota, Vicinibactiera and Anaerolinea compared to the EstimaZero stocks. Buckley et al. [91] found that Planctomycetota taxa could be correlated with soil management history and these taxa showed increased diversity with increased nitrate concentrations. Vicinamibacteria are characteristic species in loamy soil, confirming the soil type of our field conditions [92]. Anaerolineae are strictly anaerobic bacteria that are fermentative species capable of growth on a diverse range of substrates [93–95] and have been associated previously with waterlogged agricultural fields [96]. Our research provides evidence of a distinct soil response to the potato stock with a high pathogen burden that is stronger than the effect of irrigation.
The challenge in microbiome studies is moving beyond statistical correlations to a mechanistic understanding. Therefore, we used the ensemble quotient (EQO) analysis, which identified the minimal subset of taxa linked to blackleg or common scab disease prevalence values based on patterns of statistical variation. This method further revealed that in the ridge samples, the Anaerolinea (SBR1031) showed a high relative proportion in the subset of taxa that were linked to increasing blackleg prevalence. The underlying cause for the link between the taxa and the planting of seed stock with a high Pectobacterium pathogen burden (as revealed by the differential and EQO analysis) is unclear. These taxa could merely be responding to environmental conditions that coincidentally favour blackleg disease but could also be involved in synergistic activities with Pectobacterium. An alternate hypothesis is the ‘cry-for-help’ model, where plants modulate their phytohormones to alter the composition of the rhizosphere microbiome to promote pathogen-suppressive microbes to help protect them from attack [97–99]. Thus, taxa from the Anaerolinea may get recruited during infection. Evidence for this hypothesis could be the development of a similar signature in the JellyLow at harvest. The passing of altered microbial signatures to the next ‘seed’ generation has been shown previously [100]. This theory may be possible given that the JellyHigh was a generation 3 stock and blackleg levels accumulate over time [31]. Why we observe a strong signature in the ridge microbiome as compared to the root microbiome remains to be ascertained but may relate to the larger build-up of Pectobacterium at the ridge site. Moreover, our study only considers bacterial populations and, given the symbiotic association of potatoes with fungi (mycorrhiza), we are almost certainly not capturing the complexity of these interactions. Therefore, future research efforts should focus on untangling the interactions between potato pathogen burden, plant defence responses and the soil microbiome (for multiple trophic groups including fungi), and more specifically on investigating further the roles of genera such as Streptomyces and Anaerolinea, e.g. in highly controlled pot experiments. Understanding the associations between genera and management practices such as irrigation (e.g. Streptomyces) or a high pathogen burden (e.g. Anaerolinea) could help to develop strategies to modulate the response of agricultural potato soil microbiomes.
Conclusions
Our work underscores the importance of employing advanced bioinformatic tools to unravel the complex associations between management practices, soil microbiomes and crop outcomes by identifying specific bacterial genera, such as Streptomyces and Anaerolinea, as influenced by management practices or high pathogen burden. Thus, providing insights for developing strategies to modulate the response of agricultural potato soil microbiomes. Overall, this research enhances our understanding of the interplay between management practices, soil microbiomes, disease prevalence and crop yields. The findings contribute to the potential improvement of sustainable agriculture by enabling the development of targeted strategies to optimise crop productivity and mitigate disease risks in potato cultivation.
Supplementary Information
Supplementary Material 1: Supplementary data file 1. Layout of the plots
Supplementary Material 2: Supplementary data file 2. Complete metadata for the field-trial
Supplementary Material 3: Supplementary Figure S1. Schematic diagram showing the experimental design of this study. This includes the choice of potato seed stock with high, low (both Jelly varieties) and zero (Estima mini-tubers) starting levels of Pectobacterium species and irrigation regimes (Unirrigated [rainfed only], Irrigation 1 [SMD 40 mm], Irrigation 2 [SMD < 15 mm during the common scab control period then unirrigated], and Irrigation 3 [SMD < 15 mm during the common scab control period then unirrigated]. Comparisons in microbial communities across time were made using soil sampled from the ridge (near the top of the furrow) at 50% plant emergence (T_E Ridge) and harvest (T_H Ridge). In addition, to determine if there were differences in microbial communities sampled closer to the plant roots, samples were also taken at the bottom of the furrow at harvest only (T_H Root), to avoid damaging roots in the emerging plants. Details about the plot layout can be found in Supplementary Data File 2. Supplementary Figure S2. Rarefaction curves showing the number of reads from the 16S rRNA gene in DNA from the soil samples on the x-axis and the number of OTUs within a 97% percent sequence similarity threshold on the y-axis. Treatment groups are indicated by colour-coded lines. Supplementary Table S1. Percentage of blackleg disease symptoms in potato plants per plot over the course of the experimental field trial in 2020. Supplementary Table S2. Proportion of tubers with rotting (%) and weight of rotted tubers (tubers/hectare) at potato harvest. Table also shows the results of the statistical analysis of individual stocks and irrigation regimes. The respective degrees of freedom (D.F.) is given within the standard error (S.E.). Supplementary Table S3. Genus identifier list for Supplementary Fig. 2 and the average percentage prevalence in all samples. Supplementary Figure S3.The ‘core microbiome of the soil microbial communities from the field trial. The ‘core microbiome’ was set at 85% minimum prevalence of samples. Numbers referring to the individual genera are shown on the x-axis (defined in Supplementary Table 3), with colours highlighting the taxonomic phyla. The y-axis shows the detection threshold of absolute genera abundances and heatmap colours showing the average prevalence of each genus within all samples, ranging from 0–1. Supplementary Table S4. Genus identifier list for Supplementary Fig. 2 and the average percentage prevalence in all samples. Supplementary Figure S4. Taxonomic diversity of the soil microbiomes (ridge samples) at the 50% plant emergence (T_E) timepoint. Colours represent the irrigation regime (Unirrigated, Irrigation 1, Irrigation 2, and Irrigation 3). Symbols represent the potato seed stock (EstimaZero, JellyLow, and JellyHigh). A) Shows alpha diversity based on the relative distribution of taxa (Pielou’s evenness) and the number of different ASVs (Richness) within the communities; there were no significant differences between the watering regimes and no obvious clustering based on potato stock. B) Principal coordinate analysis (PCoA) based on Bray–Curtis distance of beta diversity dissimilarity between the communities; note that there was no distinct clustering in relation to irrigation regime, but there was clustering with respect to potato stock. Supplementary Figure S5. Functional diversity of the soil microbiomes (ridge samples) at the 50% plant emergence (T_E) timepoint. Colours represent the irrigation regime (Unirrigated, Irrigation 1, Irrigation 2, and Irrigation 3). Symbols represent the potato seed stock (EstimaZero, JellyLow, and JellyHigh). A) Shows diversity based on the relative distribution of KEGG Orthologs (KOs; Pielou’s evenness) and number of KEGG Orthologs (KOs; Richness) within the communities; there were no significant differences in relation to irrigation treatment and no obvious clustering by potato stock. B) Principal coordinate analysis (PCoA) based on Bray–Curtis distance of functional beta diversity dissimilarity between the communities as assessed through PiCrust2 and hierarchical metastorms analysis; note that there was no distinct clustering in relation to any of the variables. Supplementary Figure S6. Taxonomic diversity of the soil microbiomes at the harvest (T_H) timepoint. Colours represent the irrigation regime (Unirrigated, Irrigation 1, Irrigation 2, and Irrigation 3) and whether the sample was from the ‘Ridge’ or ‘Root’. Symbols represent the potato seed stock (EstimaZero, JellyLow, and JellyHigh). A) Shows diversity based on the relative distribution of taxa (Pielou’s evenness) and the number of different ASVs (Richness) within the communities; which were similar across irrigation regimes. B) Principal coordinate analysis (PCoA) based on Bray–Curtis distance of beta diversity dissimilarity between the communities; note that samples clustered according to potato stock, sample type (ridge or root) and Irrigation 2 samples showed some clustering. Supplementary Figure S7. Functional diversity of the soil microbiomes at the harvest (T_H) timepoint. Colours represent the irrigation regime (Unirrigated, Irrigation 1, Irrigation 2, and Irrigation 3) and the sample was from the ‘Ridge’ or ‘Root’. Symbols represent the potato seed stock (EstimaZero, JellyLow, and JellyHigh). A) Shows diversity based on the relative distribution of KEGG Orthologs (KOs; Pielou’s evenness) and number of KEGG Orthologs (KOs; Richness) within the communities; there were no significant differences in relation to irrigation treatment and no obvious clustering by potato stock. B) Principal coordinate analysis (PCoA) based on Bray–Curtis distance of functional beta diversity dissimilarity between the communities as assessed through PiCrust2 and hierarchical metastorms analysis; note that samples loosely cluster according to irrigation regime, sample type (root or ridge) and primarily cluster according to potato stock. Supplementary Figure S8. Taxonomic diversity of the soil microbiomes at the 50% plant emergence (T_E) and plant harvest (T_H) time points from ‘Ridge’ samples only. Colours represent the irrigation regime (Unirrigated, Irrigation 1, Irrigation 2, and Irrigation 3). Symbols represent the potato seed stock (EstimaZero, JellyLow, and JellyHigh). A) Shows diversity based on the relative distribution of taxa (Pielou’s evenness) and the number of different ASVs (Richness) within the communities; which were similar across irrigation regimes. B) Principal coordinate analysis (PCoA) based on Bray–Curtis distance of beta diversity dissimilarity between the communities; note that samples clustered according to potato stock. Supplementary Figure S9. Functional diversity of the soil microbiomes at 50% plant emergence (T_E). Colours represent potato stock (EstimaZero – E, JellyLow – JL, and JellyHigh – JH). Symbols represent the irrigation regimes (Unirrigated, Irrigation_1, Irrigation_2, and Irrigation 3). A) Shows diversity based on the relative distribution of KEGG Orthologs (KOs; Pielou’s evenness) and number of KEGG Orthologs (KOs; Richness) within the communities; there were no significant differences in relation to irrigation treatment and no obvious clustering by potato stock. B) Principal coordinate analysis (PCoA) based on Bray–Curtis distance of functional beta diversity dissimilarity between the communities as assessed through PiCrust2 and hierarchical metastorms analysis; note that samples very loosely cluster according to potato stock. Supplementary Figure S10. Functional diversity of the soil microbiomes at plant harvest (T_H). Colours represent potato stock (EstimaZero – E, JellyLow – JL, and JellyHigh – JH), time-point (50% plant emergence – T_H), and soil sample type (Ridge – Ri, Root – Ro). Symbols represent the irrigation regimes (Unirrigated, Irrigation_1, Irrigation_2, and Irrigation 3). A) Shows diversity based on the relative distribution of KEGG Orthologs (KOs; Pielou’s evenness) and number of KEGG Orthologs (KOs; Richness) within the communities; there were no significant differences in relation to irrigation treatment and no obvious clustering by potato stock. B) Principal coordinate analysis (PCoA) based on Bray–Curtis distance of functional beta diversity dissimilarity between the communities as assessed through PiCrust2 and hierarchical metastorms analysis; note that samples loosely cluster according to potato stock and sample type. Supplementary Figure S11. Functional diversity of the soil microbiomes at the 50% plant emergence (T_E) and plant harvest (T_H) time points from ‘Ridge’ samples only. Colours represent the irrigation regime (Unirrigated, Irrigation 1, Irrigation 2, and Irrigation 3). Symbols represent the potato seed stock (EstimaZero, JellyLow, and JellyHigh). A) Shows diversity based on the relative distribution of KEGG Orthologs (KOs; Pielou’s evenness) and number of KEGG Orthologs (KOs; Richness) within the communities; there were no significant differences in relation to irrigation treatment and no obvious clustering by potato stock. B) Principal coordinate analysis (PCoA) based on Bray–Curtis distance of functional beta diversity dissimilarity between the communities as assessed through PiCrust2 and hierarchical metastorms analysis; note that samples loosely cluster according to potato stock and time point. Supplementary Figure S12. Analysis of rare taxa from the Estima, JellyLow and JellyHigh samples at T_E (50% plant emergence) and T_H (plant harvest) divided into ridge and root samples (Ri and Ro). These figures show the percentage contribution (%) to taxonomic beta-diversity dissimilarity of: A) ‘Persistently Rare’ taxa B) ‘Abundant’ taxa; C) ‘Conditionally Rare’ taxa; and D) ‘Other Rare’ taxa. Supplementary Figure S13. Taxonomic map for differential heat tree analysis. The branches show the taxonomic identification of all ASVs. Note that the same tree can be used for each differential heat tree analysis instance. Supplementary Figure S14. Heat tree analysis to visualise the differential taxa between irrigation regimes at 50% plant emergence (T_E). The full legend for the tree branches is shown in Supplementary Fig. 10. Each tree shows a pair-wise comparison between irrigation regimes (denoted by either purple or blue). The size of the branch nodes shows the number of ASVs, while the differential Log2 ratio median proportion is shown by the colours. Note that there are no Log2 fold differences between taxa across the irrigation regimes. Supplementary Figure S15. Heat tree analysis to visualise the differential taxa between irrigation regimes at potato harvest (T_H) in soil ridge samples. The full legend for the tree branches is shown in Supplementary Fig. 10. Each tree shows a pair-wise comparison between irrigation regimes (denoted by either purple or blue). The size of the branch nodes shows the number of ASVs, while the differential Log2 ratio median proportion is shown by the colours. Note that there are no Log2 fold differences between taxa across the irrigation regimes. Supplementary Figure S16. Heat tree analysis to visualise the differential taxa between irrigation regimes at potato harvest (T_H) in soil root samples. The full legend for the tree branches is shown in Supplementary Fig. 10. Each tree shows a pair-wise comparison between irrigation regimes (denoted by either purple or blue). The size of the branch nodes shows the number of ASVs, while the differential Log2 ratio median proportion is shown by the colours. Note that there are no Log2 fold differences between taxa across the irrigation regimes. Supplementary Figure S17. Plot highlighting the impact of ‘Irrigation Regime’ on the abundance of microbial taxa in the potato harvest (T_H) ridge soil samples, based on the GLVMM analysis. The microbial genera names are indicated on the y-axis. The environment covariates values are indicated on the x-axis which are the coefficient values against the environmental covariates (e.g. Unirrigated, Irrigation_2, and Irrigation_3 as compared to the Irrigation 1 treatment). The values range from positive, neutral to negative associations with the specific taxa on the y-axis. The red lines show significantly positive taxa associated with the indicated environmental covariate and the blue lines show significantly negative taxa; grey lines are not statistically significant. Note: Irrigation 1 treatment is not displayed as the analysis uses this treatment as the reference. Supplementary Figure S18. Plot highlighting the impact of ‘Irrigation Regime’ on the abundance of microbial taxa in the potato harvest (T_H) root soil samples, based on the GLVMM analysis. The microbial genera names are indicated on the y-axis. The environment covariates values are indicated on the x-axis which are the coefficient values against the environmental covariates (e.g. Irrigation_1, Irrigation_2, and Irrigation_3, as compared to the Unirrigated treatment). The values range from positive, neutral to negative associations with the specific taxa on the y-axis. The red lines show significantly positive taxa associated with the indicated environmental covariate and the blue lines show significantly negative taxa; grey lines are not statistically significant. Note: Unirrigated treatment is not displayed as the analysis uses this as the reference. Supplementary Figure S19. Plot highlighting the impact of ‘Potato Stock’ on the abundance of microbial taxa at 50% plant emergence (T_E), based on the GLVMM analysis. The microbial genera names are indicated on the y-axis. The environmental covariates values are indicated on the x-axis which are the coefficient values against the environmental covariates (e.g. ‘Potato Stock’ either JellyHigh, JellyLow as compared to the EstimaZero. The values range from positive, and neutral to negative associations with the specific taxa on the y-axis. The red lines show significantly positive taxa associated with the indicated environmental covariate and the blue lines show significantly negative taxa; grey lines are not statistically significant. Note: The EstimaZero group is not displayed as the analysis uses this as the reference. Supplementary Figure S20. Plot highlighting the impact of ‘Potato Stock’ on the abundance of microbial taxa in the root samples at potato harvest (T_H), based on the GLVMM analysis. The microbial genera names are indicated on the y-axis. The environment covariates values are indicated on the x-axis which are the coefficient values against the environmental covariates (e.g. ‘Potato Stock’ either JellyHigh, JellyLow as compared to the EstimaZero. The values range from positive, neutral to negative associations with the specific taxa on the y-axis. The red lines show significantly positive taxa associated with the indicated environmental covariate and the blue lines show significantly negative taxa; grey lines are not statistically significant. Note: The EstimaZero group is not displayed as the analysis uses this as the reference. Supplementary Figure S21. Plot highlighting the impact of ‘Potato Stock’ on the abundance of microbial taxa in the ridge samples at potato harvest (T_H), based on the GLVMM analysis. The microbial genera names are indicated on the y-axis. The environment covariates values are indicated on the x-axis which are the coefficient values against the environmental covariates (e.g. ‘Potato Stock’ either JellyHigh, EstimaZero as compared to the JellyLow. The values range from positive, neutral to negative associations with the specific taxa on the y-axis. The red lines show significantly positive taxa associated with the indicated environmental covariate and the blue lines show significantly negative taxa; grey lines are not statistically significant. Note: The JellyLow group is not displayed as the analysis uses this as the reference. Supplementary Figure S22. Plot highlighting the impact of time on the abundance of microbial taxa in the ridge soil samples, based on the GLVMM analysis. The microbial genera names are indicated on the y-axis. The environment covariates values are indicated on the x-axis which are the coefficient values against the environmental covariate (e.g. Harvest [T_H] time point, as compared to the 50% plant emergence [T_E] time point. The values range from positive, neutral to negative associations with the specific taxa on the y-axis. The red lines show significantly positive taxa associated with the indicated environmental covariate and the blue lines show significantly negative taxa; g grey lines are not statistically significant. Note: The 50% emergence time point (T_E) is not displayed, as the analysis uses this as the reference. Supplementary Figure S23. Minimum subset of amplicon sequencing variants (ASVs) associated with percentage blackleg symptoms during the field trial. Analysis using the Ensemble Quotient Optimisation (EQO) technique considering at most 20 microbial taxa and the root samples at harvest. The left y-axis shows the relative abundance of the microbial taxa, and the right y-axis shows the percentage blackleg symptoms observed. The x-axis shows the sample identity with a colour legend for potato stock (Green EstimaZero, Blue JellyLow, and Red JellyHigh) and the irrigation regimes are shown in text (Unirrigated, Irrigation 1, Irrigation 2, and Irrigation 3). Supplementary Figure S24. Continuous ensemble quotient analysis (EQO) of the harvest ridge samples. Plot shows a stacked bar chart showing the minimum subset of amplicon sequencing variants (ASVs) associated with common scab symptoms. The x-axis shows the sample details including the potato stock (EstimaZero – Green, JellyLow – Blue, and JellyHigh – Red) and the irrigation regime is indicated in text. The right y-axis shows the relative abundance of the ASVs and the right y-axis shows the percentage (%) of common scab symptoms. Supplementary Figure S25. Continuous ensemble quotient analysis (EQO) of the harvest root samples. Plot shows a stacked bar chart showing the minimum subset of amplicon sequencing variants (ASVs) associated with common scab symptoms. The x-axis shows the sample details including the potato stock (EstimaZero – Green, JellyLow – Blue, and JellyHigh – Red) and the irrigation regime is indicated in text. The right y-axis shows the relative abundance of the ASVs and the right y-axis shows the percentage (%) of common scab symptoms.
Acknowledgements
We would like to thank the field and sampling staff at NIAB, Cambridge, UK, for their contributions to the fieldwork. We thank Simon Smart for the discussions on the fieldwork data. We additionally thank Simon Alexander and Eric Anderson for useful feedback on potato production and agronomy within the UK. In addition, we thank Julie Galbraith, David McGuinness and others at the Glasgow Polyomics sequencing facility for processing the sequencing libraries and providing data. Finally, we thank the Bacterial Plant Diseases Coordination Team and Sarah McLusky for communication and support of the project.
Authors’ contributions
C.K. (Data curation: Equal; Methodology: Equal; Formal analysis: Equal; Investigation: Equal; Visualisation; Lead; Writing – original draft: Lead; Writing – review & editing: Lead). E.K. (Investigation: Equal; Methodology: Equal; Data curation: Equal; Writing – review & editing: Supporting). M.A.S. (Conceptualisation: Supporting; Formal analysis: Supporting; Methodology: supporting; Writing – review & editing: supporting). C.F.N. (Resources: Supporting; Writing – review & editing: Supporting). J.M. (Resources: Supporting; Conceptualisation: Supporting; Investigation; Supporting: Writing – reviewing and editing; Supporting). S.H. (Conceptualisation: Supporting; Visualization: Supporting; Writing – review and editing: Supporting). I.T. (Conceptualization: Supporting; Visualization: Supporting; Writing – review and editing: Supporting). B.K.M. (Conceptualisation: Equal; Resources: Equal; Investigation: Equal; Formal analysis: Supporting; Writing – review & editing: Equal). U.Z.I. (Conceptualisation: Equal; Resources: Equal; Software: Lead; Investigation: Equal; Formal analysis: Equal; Writing – review & editing: Equal). All authors reviewed the manuscript.
Funding
This work was funded by BBSRC, NERC, Defra and the Scottish Government through the Bacterial Plant Diseases Program. Specifically, the James Hutton team (I.T. and S.H.) were funded by BB/T010657/1; the Glasgow team (B.M., U.Z.I. and J.M.) were funded by UKRI BB/T010649/1; and the NIAB team (M.S. and C.N.) were funded by UKRI BB/T011025/1.
Data availability
Sequence data is available from the sequence read archive (SRA) database under Bioproject Submission PRJNA992106.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Ciara Keating, Email: ciara.keating@durham.ac.uk.
Umer Zeeshan Ijaz, Email: umer.ijaz@glasgow.ac.uk.
References
- 1.Ray DK, Ramankutty N, Mueller ND, West PC, Foley JA. Recent patterns of crop yield growth and stagnation. Nat Commun. 2012;3:1293. [DOI] [PubMed] [Google Scholar]
- 2.Ray DK, Gerber JS, MacDonald GK, West PC. Climate variation explains a third of global crop yield variability. Nat Commun. 2015;6:5989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Statista. Potato production worldwide. 2020. Available from: https://www.statista.com/statistics/382174/global-potato-production/. Cited 2022 Oct 19.
- 4.Potato production worldwide, 2020. Statista. Available from: https://www.statista.com/statistics/382174/global-potato-production/. Cited 2022 Oct 19.
- 5.Griffin D, Bourke L, Mullins E, Hennessy M, Phelan S, Kildea S, et al. Potatoes in Ireland: sixty years of potato research and development, market evolution and perspectives on future challenges. Ir J Agric Food Res. 2022. Available from: https://scienceopen.com/hosted-document?doi=10.15212/ijafr-2020-0144. Cited 2022 Nov 28.
- 6.Fry WE. Phytophthora infestans: the itinerant invader; “late blight”: the persistent disease. Phytoparasitica. 2020. 10.1007/s12600-019-00778-3. Cited 2023 Mar 24.
- 7.Yuen J. Pathogens which threaten food security: Phytophthora infestans, the potato late blight pathogen. Food Sec. 2021;13:247–53. 10.1007/s12571-021-01141-3. Cited 2023 Mar 24. [Google Scholar]
- 8.Van Gijsegem F, Toth IK, van der Wolf JM. Soft rot pectobacteriaceae: a brief overview. In: Van Gijsegem F, van der Wolf JM, Toth IK, editors. Plant diseases caused by Dickeya and Pectobacterium species. Cham: Springer International Publishing; 2021. p. 1–11. Available from: http://link.springer.com/10.1007/978-3-030-61459-1_1. Cited 2023 Mar 24. [Google Scholar]
- 9.Braun S, Gevens A, Charkowski A, Allen C, Jansky S. Potato common scab: a review of the causal pathogens, management practices, varietal resistance screening methods, and host resistance. Am J Potato Res. 2017;94:283–96. Available from: http://link.springer.com/10.1007/s12230-017-9575-3. Cited 2022 Sep 9. [Google Scholar]
- 10.Price JA, Coyne D, Blok VC, Jones JT. Potato cyst nematodes Globodera rostochiensis and G. pallida. Mol Plant Pathol. 2021;22:495–507. Available from: https://onlinelibrary.wiley.com/doi/abs/10.1111/mpp.13047. Cited 2023 Mar 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gray SM, Power AG. Anthropogenic influences on emergence of vector-borne plant viruses: the persistent problem of Potato virus Y. Curr Opin Virol. 2018;33:177–83. Available from: https://www.sciencedirect.com/science/article/pii/S1879625718300865. Cited 2023 Jul 2. [DOI] [PubMed] [Google Scholar]
- 12.Dahal K, Li X-Q, Tai H, Creelman A, Bizimungu B. Improving potato stress tolerance and tuber yield under a climate change scenario – a current overview. Front Plant Sci. 2019;10. Available from: https://www.frontiersin.org/articles/10.3389/fpls.2019.00563. Cited 2022 Nov 22. [DOI] [PMC free article] [PubMed]
- 13.Divya KL, Mhatre PH, Venkatasalam EP, Sudha R. Crop simulation models as decision-supporting tools for sustainable potato production: a review. Potato Res. 2021;64:387–419. 10.1007/s11540-020-09483-9. Cited 2022 Oct 21. [Google Scholar]
- 14.Haverkort AJ, Verhagen A. Climate change and its repercussions for the potato supply chain. Potato Res. 2008;51:223. 10.1007/s11540-008-9107-0. Cited 2022 Nov 22. [Google Scholar]
- 15.Luck J, Spackman M, Freeman A, Tre˛ bicki P, Griffiths W, Finlay K, et al. Climate change and diseases of food crops. Plant Pathol. 2011;60:113–21. Available from: https://onlinelibrary.wiley.com/doi/abs/10.1111/j.1365-3059.2010.02414.x. Cited 2022 Nov 22. [Google Scholar]
- 16.Ardanov P, Lyastchenko S, Karppinen K, Häggman H, Kozyrovska N, Pirttilä AM. Effects of Methylobacterium sp. on emergence, yield, and disease prevalence in three cultivars of potato (Solanum tuberosum L.) were associated with the shift in endophytic microbial community. Plant Soil. 2016;405:299–310. 10.1007/s11104-015-2500-y. Cited 2023 Jan 24. [Google Scholar]
- 17.Garrett KA, Zúñiga LN, Roncal E, Forbes GA, Mundt CC, Su Z, et al. Intraspecific functional diversity in hosts and its effect on disease risk across a climatic gradient. Ecol Appl. 2009;19:1868–83. Available from: https://onlinelibrary.wiley.com/doi/abs/10.1890/08-0942.1. Cited 2023 Jan 24. [DOI] [PubMed] [Google Scholar]
- 18.Velásquez AC, Castroverde CDM, He SY. Plant-pathogen warfare under changing climate conditions. Curr Biol. 2018;28:R619–34. Available from: https://www.sciencedirect.com/science/article/pii/S0960982218304123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lapwood DH, Lewis BG. Observations on the timing of irrigation and the incidence of potato common scab (Streptomyces scabies). Plant Pathol. 1967;16:131–5. Available from: https://onlinelibrary.wiley.com/doi/abs/10.1111/j.1365-3059.1967.tb00388.x. Cited 2022 Sep 9. [Google Scholar]
- 20.Wale S, Sutton M. Non-water control measures for potato common scab. British Potato Council. Res Rev Suppl. 2005. Ref: R248.
- 21.Shock CC, Pereira AB, Eldredge EP. Irrigation best management practices for potato. Am J Potato Res. 2007;84:29–37. Available from: https://link.springer.com/10.1007/BF02986296. Cited 2022 Sep 9. [Google Scholar]
- 22.Djaman K, Irmak S, Koudahe K, Allen S. Irrigation management in potato (Solanum tuberosum L.) production: a review. Sustainability. 2021;13:1504. Available from: https://www.mdpi.com/2071-1050/13/3/1504. Cited 2022 Sep 9. [Google Scholar]
- 23.Lapwood DH, Wellings LW, Hawkins JH. Irrigation as a practical means to control potato common scab (Streptomyces scabies): final experiment and conclusions. Plant Pathol. 1973;22:35–41. Available from: https://onlinelibrary.wiley.com/doi/abs/10.1111/j.1365-3059.1973.tb01766.x. Cited 2022 Nov 23. [Google Scholar]
- 24.Lapwood DH, Wellings LW, Rosser WR. The control of common scab of potatoes by irrigation. Ann Appl Biol. 1970;66:397–405. Available from: https://onlinelibrary.wiley.com/doi/abs/10.1111/j.1744-7348.1970.tb04619.x. Cited 2022 Sep 9. [Google Scholar]
- 25.Wilson CR, Pemberton BM, Ransom LM. The effect of irrigation strategies during tuber initiation on marketable yield and development of common scab disease of potato in Russet Burbank in Tasmania. Potato Res. 2001;44:243–51. 10.1007/BF02357902. Cited 2022 Nov 23. [Google Scholar]
- 26.Dees MW, Wanner LA. In search of better management of potato common scab. Potato Res. 2012;55:249–68. Available from: http://link.springer.com/10.1007/s11540-012-9206-9. Cited 2022 Sep 9. [Google Scholar]
- 27.Lapwood DH. The relative importance of weather, soil- and seed-borne inoculum in determining the incidence of common scab (Streptomyces scabies) in potato crops. Plant Pathol. 1972;21:105–8. Available from: https://bsppjournals.onlinelibrary.wiley.com/10.1111/j.1365-3059.1972.tb01736.x. Cited 2022 Nov 25. [Google Scholar]
- 28.Van Gijsegem F, van der Wolf JM, Toth IK, editors. Plant diseases caused by Dickeya and Pectobacterium species. Cham: Springer International Publishing; 2021. Available from: http://link.springer.com/10.1007/978-3-030-61459-1. Cited 2022 Nov 23. [Google Scholar]
- 29.Burton WG, Wigginton MJ. The effect of a film of water upon the oxygen status of a potato tuber. Potato Res. 1970;13:180–6. Available from: http://link.springer.com/10.1007/BF02355973. Cited 2022 Nov 23. [Google Scholar]
- 30.Pérombelon MCM. Potato diseases caused by soft rot erwinias: an overview of pathogenesis. Plant Pathol. 2002;51:1–12. Available from: https://onlinelibrary.wiley.com/doi/abs/10.1046/j.0032-0862.2001.Shorttitle.doc.x. Cited 2022 Nov 23. [Google Scholar]
- 31.Pérombelon MCM. Potato blackleg: epidemiology, host-pathogen interaction and control. Neth J Plant Pathol. 1992;98:135–46. Available from: http://link.springer.com/10.1007/BF01974480. Cited 2022 Nov 23. [Google Scholar]
- 32.Explanatory guide to the Seed Potato Classification Scheme and Approved Stock Scheme 2023 to 2024. UK: Animal and Plant Health Agency; 2023. https://assets.publishing.service.gov.uk/media/66ab5d58ce1fd0da7b59315e/SPCS_and_AS_explanatory_guide_2024_25.pdf.
- 33.Pérombelon MCM, Hyman LJ. Control of potato blackleg: production of healthy seed. Asp Appl Biol. 1992:77–84. Available from: https://www.cabdirect.org/cabdirect/abstract/19932334426. Cited 2023 Feb 11.
- 34.Toth IK, Sullivan L, Brierley JL, Avrova AO, Hyman LJ, Holeva M, et al. Relationship between potato seed tuber contamination by Erwinia carotovora ssp. atroseptica, blackleg disease development and progeny tuber contamination. Plant Pathol. 2003;52:119–26. Available from: https://onlinelibrary.wiley.com/doi/abs/10.1046/j.1365-3059.2003.00821.x. Cited 2022 Nov 28. [Google Scholar]
- 35.de Werra P, Kopp C, Häberli M, Stöcker I, Keil A, Debonneville C, et al. Monitoring potato seed lots to control blackleg in fields in Switzerland and southern Germany. Plant Pathol. 2020;69:1331–46. Available from: https://onlinelibrary.wiley.com/doi/abs/10.1111/ppa.13226. Cited 2022 Sep 9. [Google Scholar]
- 36.van der Wolf JM, De Boer SH, Czajkowski R, Cahill G, Van Gijsegem F, Davey T, et al. management of diseases caused by Pectobacterium and Dickeya species. In: Van Gijsegem F, van der Wolf JM, Toth IK, editors., et al., Plant diseases caused by Dickeya and Pectobacterium species. Cham: Springer International Publishing; 2021. p. 175–214. 10.1007/978-3-030-61459-1_6. Cited 2023 Mar 24. [Google Scholar]
- 37.Czajkowski R, Pérombelon MCM, van Veen JA, van der Wolf JM. Control of blackleg and tuber soft rot of potato caused by Pectobacterium and Dickeya species: a review. Plant Pathol. 2011;60:999–1013. Available from: https://onlinelibrary.wiley.com/doi/abs/10.1111/j.1365-3059.2011.02470.x. Cited 2022 Nov 25. [Google Scholar]
- 38.Carretta L, Tarolli P, Cardinali A, Nasta P, Romano N, Masin R. Evaluation of runoff and soil erosion under conventional tillage and no-till management: a case study in northeast Italy. Catena. 2021;197:104972. Available from: https://www.sciencedirect.com/science/article/pii/S0341816220305221. Cited 2022 Nov 27. [Google Scholar]
- 39.Chamberlain LA, Bolton ML, Cox MS, Suen G, Conley SP, Ané J-M. Crop rotation, but not cover crops, influenced soil bacterial community composition in a corn-soybean system in southern Wisconsin. Appl Soil Ecol. 2020;154:103603. [Google Scholar]
- 40.Longepierre M, Widmer F, Keller T, Weisskopf P, Colombi T, Six J, et al. Limited resilience of the soil microbiome to mechanical compaction within four growing seasons of agricultural management. ISME Commun. 2021;1:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li T, Li Y, Shi Z, Wang S, Wang Z, Liu Y, et al. Crop development has more influence on shaping rhizobacteria of wheat than tillage practice and crop rotation pattern in an arid agroecosystem. Appl Soil Ecol. 2021;165:104016. [Google Scholar]
- 42.Hills K, Collins H, Yorgey G, McGuire A, Kruger C. Improving soil health in pacific northwest potato production: a review. Am J Potato Res. 2020;97:1–22. [Google Scholar]
- 43.Schratzberger M, Holterman M, van Oevelen D, Helder J. A worm’s world: ecological flexibility pays off for free-living nematodes in sediments and soils. Bioscience. 2019;69:867–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dubey A, Malla MA, Khan F, Chowdhary K, Yadav S, Kumar A, et al. Soil microbiome: a key player for conservation of soil health under changing climate. Biodivers Conserv. 2019;28:2405–29. [Google Scholar]
- 45.Banerjee S, van der Heijden MGA. Soil microbiomes and one health. Nat Rev Microbiol. 2023;21:6–20. Available from: http://www.nature.com/articles/s41579-022-00779-w. Cited 2023 Jan 3. [DOI] [PubMed] [Google Scholar]
- 46.Bünemann EK, Schwenke GD, Zwieten LV, Bünemann EK, Schwenke GD, Zwieten LV. Impact of agricultural inputs on soil organisms—a review. Soil Res. 2006;44:379–406. Available from: https://www.publish.csiro.au/sr/SR05125. Cited 2023 Feb 27. [Google Scholar]
- 47.Tsiafouli MA, Thébault E, Sgardelis SP, de Ruiter PC, van der Putten WH, Birkhofer K, et al. Intensive agriculture reduces soil biodiversity across Europe. Glob Change Biol. 2015;21:973–85. Available from: https://onlinelibrary.wiley.com/doi/abs/10.1111/gcb.12752. Cited 2023 Feb 27. [DOI] [PubMed] [Google Scholar]
- 48.DiLegge MJ, Manter DK, Vivanco JM. Soil microbiome disruption reveals specific and general plant-bacterial relationships in three agroecosystem soils. PLoS One. 2022;17:e0277529. Available from: https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0277529. Cited 2023 Feb 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Berendsen RL, Vismans G, Yu K, Song Y, de Jonge R, Burgman WP, et al. Disease-induced assemblage of a plant-beneficial bacterial consortium. ISME J. 2018;12:1496–507. Available from: https://www.nature.com/articles/s41396-018-0093-1. Cited 2023 Feb 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mareckova M, Omelka M, Kopecky J. The golden goal of soil management – disease-suppressive soils. Phytopathology. 2022. Available from: https://apsjournals.apsnet.org/doi/10.1094/PHYTO-09-22-0324-KD. Cited 2023 Feb 27. [DOI] [PubMed]
- 51.Schlatter D, Kinkel L, Thomashow L, Weller D, Paulitz T. Disease suppressive soils: new insights from the soil microbiome. Phytopathology. 2017;107:1284–97. Available from: https://apsjournals.apsnet.org/doi/full/10.1094/PHYTO-03-17-0111-RVW. Cited 2023 Feb 27. [DOI] [PubMed] [Google Scholar]
- 52.Toju H, Peay KG, Yamamichi M, Narisawa K, Hiruma K, Naito K, et al. Core microbiomes for sustainable agroecosystems. Nat Plants. 2018;4:247–57. Available from: https://www.nature.com/articles/s41477-018-0139-4. Cited 2023 Feb 27. [DOI] [PubMed] [Google Scholar]
- 53.de Haan EG, Dekker-Nooren TCEM, van den Bovenkamp GW, Speksnijder AGCL, van der Zouwen PS, van der Wolf JM. Pectobacterium carotovorum subsp. carotovorum can cause potato blackleg in temperate climates. Eur J Plant Pathol. 2008;122:561–9. 10.1007/s10658-008-9325-y. Cited 2023 Jul 2. [Google Scholar]
- 54.AHDB. Common scab control: reducing the irrigation water requirements and understanding the effects of beneficial soil microorganisms. Ref 448. Mark Stalham, Marc Allison, David Firman, Jeff Peters, Richard Thwaites, and Melanie Sapp; 2015. https://potatoes.ahdb.org.uk/common-scab-control.
- 55.Caporaso JG, Lauber CL, Walters WA, Berg-Lyons D, Huntley J, Fierer N, et al. Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. ISME J. 2012;6:1621–4. Available from: http://www.nature.com/articles/ismej20128. Cited 2020 May 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, et al. QIIME allows analysis of high-throughput community sequencing data. Nat Methods. 2010;7:335–6. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3156573/. Cited 2020 May 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Amir A, McDonald D, Navas-Molina JA, Kopylova E, Morton JT, Zech Xu Z, et al. Deblur rapidly resolves single-nucleotide community sequence patterns. mSystems. 2017;2:e00191–16. Available from: https://journals.asm.org/doi/full/10.1128/mSystems.00191-16. Cited 2022 Nov 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Quast C, Pruesse E, Yilmaz P, Gerken J, Schweer T, Yarza P, et al. The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res. 2012;41:D590–6. Available from: http://academic.oup.com/nar/article/41/D1/D590/1069277/The-SILVA-ribosomal-RNA-gene-database-project. Cited 2020 May 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Douglas GM, Maffei VJ, Zaneveld JR, Yurgel SN, Brown JR, Taylor CM, et al. PICRUSt2 for prediction of metagenome functions. Nat Biotechnol. 2020;38:685–8. Available from: http://www.nature.com/articles/s41587-020-0548-6. Cited 2021 Apr 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Davis NM, Proctor DM, Holmes SP, Relman DA, Callahan BJ. Simple statistical identification and removal of contaminant sequences in marker-gene and metagenomics data. Microbiome. 2018;6:226. 10.1186/s40168-018-0605-2. Cited 2023 Feb 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wickham H. ggplot2: Elegant Graphics for Data Analysis. Springer-Verlag New York. ISBN; 2016. 978-3-319-24277-4. https://ggplot2.tidyverse.org.
- 62.Shetty SA, Hugenholtz F, Lahti L, Smidt H, de Vos WM. Intestinal microbiome landscaping: insight in community assemblage and implications for microbial modulation strategies. FEMS Microbiol Rev. 2017;41:182–99. Available from: https://academic.oup.com/femsre/article/41/2/182/2979411. Cited 2020 Jun 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.R Core Team . R: A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; 2021. https://www.R-project.org/.
- 64.McMurdie PJ, Holmes S. phyloseq: an R package for reproducible interactive analysis and graphics of microbiome census data. PLoS One. 2013;8:e61217. Available from: http://link.gale.com/apps/doc/A478172249/EAIM?u=glasuni&sid=zotero&xid=7741c358. Cited 2020 May 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Introduction to the microbiome R package. Available from: https://microbiome.github.io/tutorials/. Cited 2022 Nov 28.
- 66.Jalanka-Tuovinen J, Salonen A, Nikkilä J, Immonen O, Kekkonen R, Lahti L, et al. Intestinal microbiota in healthy adults: temporal analysis reveals individual and common core and relation to intestinal symptoms. PLoS One. 2011;6. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3145776/. Cited 2020 May 15. [DOI] [PMC free article] [PubMed]
- 67.Finotello F, Mastrorilli E, Di Camillo B. Measuring the diversity of the human microbiota with targeted next-generation sequencing. Brief Bioinform. 2018;19:679–92. 10.1093/bib/bbw119. Cited 2023 Mar 24. [DOI] [PubMed] [Google Scholar]
- 68.Jiao S, Wang J, Wei G, Chen W, Lu Y. Dominant role of abundant rather than rare bacterial taxa in maintaining agro-soil microbiomes under environmental disturbances. Chemosphere. 2019;235:248–59. Available from: https://www.sciencedirect.com/science/article/pii/S0045653519314158. Cited 2023 Feb 17. [DOI] [PubMed] [Google Scholar]
- 69.Yamaguchi J, Tanaka A. Quantitative observation on the root system of various crops growing in the field. Soil Sci Plant Nutr. 1990;36:483–93. 10.1080/00380768.1990.10416917. Cited 2022 Nov 28. [Google Scholar]
- 70.Xing Y, Zhang T, Jiang W, Li P, Shi P, Xu G, et al. Effects of irrigation and fertilization on different potato varieties growth, yield and resources use efficiency in the Northwest China. Agric Water Manag. 2022;261:107351. Available from: https://www.sciencedirect.com/science/article/pii/S0378377421006284. Cited 2022 Nov 28. [Google Scholar]
- 71.Onder S, Caliskan ME, Onder D, Caliskan S. Different irrigation methods and water stress effects on potato yield and yield components. Agric Water Manag. 2005;73:73–86. Available from: https://www.sciencedirect.com/science/article/pii/S0378377404002720. Cited 2022 Nov 28. [Google Scholar]
- 72.Sarker S, Lamb JJ, Hjelme DR, Lien KM. A review of the role of critical parameters in the design and operation of biogas production plants. Appl Sci. 2019;9:1915. Available from: https://www.mdpi.com/2076-3417/9/9/1915. Cited 2022 Jan 10. [Google Scholar]
- 73.Larkin RP, Honeycutt CW, Griffin TS, Olanya OM, Halloran JM, He Z. Effects of different potato cropping system approaches and water management on soilborne diseases and soil microbial communities. Phytopathology. 2011;101:58–67. Available from: https://apsjournals.apsnet.org/doi/10.1094/PHYTO-04-10-0100. Cited 2023 Feb 15. [DOI] [PubMed] [Google Scholar]
- 74.Sarker KK, Hossain A, Timsina J, Biswas SK, Kundu BC, Barman A, et al. Yield and quality of potato tuber and its water productivity are influenced by alternate furrow irrigation in a raised bed system. Agric Water Manag. 2019;224:105750. Available from: https://www.sciencedirect.com/science/article/pii/S0378377419308029. Cited 2022 Nov 28. [Google Scholar]
- 75.Raymundo R, Asseng S, Robertson R, Petsakos A, Hoogenboom G, Quiroz R, et al. Climate change impact on global potato production. Eur J Agron. 2018;100:87–98. Available from: https://www.sciencedirect.com/science/article/pii/S1161030117301818. Cited 2022 Nov 28. [Google Scholar]
- 76.Bain RA, Pérombelon MCM, Tsror L, Nachmias A. Blackleg development and tuber yield in relation to numbers of Erwinia carotovora subsp. atroseptica on seed potatoes. Plant Pathol. 1990;39:125–33. Available from: https://onlinelibrary.wiley.com/doi/abs/10.1111/j.1365-3059.1990.tb02483.x. Cited 2022 Nov 28. [Google Scholar]
- 77.Toth IK, Bell KS, Holeva MC, Birch PRJ. Soft rot erwiniae: from genes to genomes: soft rot erwiniae. Mol Plant Pathol. 2003;4:17–30. Available from: 10.1046/j.1364-3703.2003.00149.x. Cited 2022 Sep 9. [DOI] [PubMed] [Google Scholar]
- 78.Mavrodi DV, Mavrodi OV, Elbourne LDH, Tetu S, Bonsall RF, Parejko J, et al. Long-term irrigation affects the dynamics and activity of the wheat rhizosphere microbiome. Front Plant Sci. 2018;9:345. Available from: https://www.frontiersin.org/articles/10.3389/fpls.2018.00345. Cited 2022 Nov 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dang Q, Tan W, Zhao X, Li D, Li Y, Yang T, et al. Linking the response of soil microbial community structure in soils to long-term wastewater irrigation and soil depth. Sci Total Environ. 2019;688:26–36. Available from: https://www.sciencedirect.com/science/article/pii/S0048969719327093. Cited 2022 Nov 28. [DOI] [PubMed] [Google Scholar]
- 80.Entry JA, Mills D, Mathee K, Jayachandran K, Sojka RE, Narasimhan G. Influence of irrigated agriculture on soil microbial diversity. Appl Soil Ecol. 2008;40:146–54. Available from: https://www.sciencedirect.com/science/article/pii/S0929139308000607. Cited 2023 Feb 17. [Google Scholar]
- 81.Ibekwe AM, Gonzalez-Rubio A, Suarez DL. Impact of treated wastewater for irrigation on soil microbial communities. Sci Total Environ. 2018;622–623:1603–10. Available from: https://www.sciencedirect.com/science/article/pii/S0048969717327432. Cited 2023 Feb 17. [DOI] [PubMed] [Google Scholar]
- 82.Zolti A, Green SJ, Ben Mordechay E, Hadar Y, Minz D. Root microbiome response to treated wastewater irrigation. Sci Total Environ. 2019;655:899–907. Available from: https://www.sciencedirect.com/science/article/pii/S0048969718346084. Cited 2022 Nov 28. [DOI] [PubMed] [Google Scholar]
- 83.Niku J, Brooks W, Herliansyah R, Hui FKC, Taskinen S, Warton DI. Efficient estimation of generalized linear latent variable models. PLoS One. 2019;14:e0216129. Available from: https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0216129. Cited 2022 Nov 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Marković S, Popović T, Berić T, Dimkić I, Jelušić A, Iličić R, et al. Metabarcoding approach for evaluation of bacterial diversity in soft rotting potato tubers and corresponding geocaulospheres. Potato Res. 2022. 10.1007/s11540-022-09601-9. Cited 2022 Nov 28.
- 85.Wright PJ, Frampton RA, Anderson C, Hedderley D. Factors associated with soils suppressive to black scurf of potato caused by Rhizoctonia solani. N Z Plant Prot. 2022;75:31–49. Available from: https://journal.nzpps.org/index.php/nzpp/article/view/11761. Cited 2022 Nov 28. [Google Scholar]
- 86.Heinsch SC, Hsu S-Y, Otto-Hanson L, Kinkel L, Smanski MJ. Complete genome sequences of Streptomyces spp. isolated from disease-suppressive soils. BMC Genomics. 2019;20:994. 10.1186/s12864-019-6279-8. Cited 2023 Feb 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wei Z, Gu Y, Friman V-P, Kowalchuk GA, Xu Y, Shen Q, et al. Initial soil microbiome composition and functioning predetermine future plant health. Sci Adv. 2019;5:eaaw0759. Available from: https://www.science.org/doi/10.1126/sciadv.aaw0759. Cited 2022 Nov 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Samaddar S, Chatterjee P, Truu J, Anandham R, Kim S, Sa T. Long-term phosphorus limitation changes the bacterial community structure and functioning in paddy soils. Appl Soil Ecol. 2019;134:111–5. Available from: https://www.sciencedirect.com/science/article/pii/S0929139318308400. Cited 2022 Nov 28. [Google Scholar]
- 89.Lu H, Wu Y, Liang P, Song Q, Zhang H, Wu J, et al. Alkaline amendments improve the health of soils degraded by metal contamination and acidification: crop performance and soil bacterial community responses. Chemosphere. 2020;257:127309. Available from: https://www.sciencedirect.com/science/article/pii/S0045653520315022. Cited 2023 Feb 27. [DOI] [PubMed] [Google Scholar]
- 90.Zhang C, Zhou T, Zhu L, Juhasz A, Du Z, Li B, et al. Response of soil microbes after direct contact with pyraclostrobin in fluvo-aquic soil. Environ Pollut. 2019;255:113164. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0269749119337674. Cited 2023 Feb 27. [DOI] [PubMed] [Google Scholar]
- 91.Buckley DH, Huangyutitham V, Nelson TA, Rumberger A, Thies JE. Diversity of planctomycetes in soil in relation to soil history and environmental heterogeneity. Appl Environ Microbiol. 2006;72:4522–31. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1489350/. Cited 2022 Apr 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kandasamy S, Weerasuriya N, White JF, Patterson G, Lazarovits G. Chapter 23 - Soil’s physical and nutritional balance is essential for establishing a healthy microbiome. In: White J, Kumar A, Droby S, editors. Microbiome stimulants for crops. Woodhead Publishing; 2021. p. 381–404. Available from: https://www.sciencedirect.com/science/article/pii/B9780128221228000042. Cited 2022 Nov 28. [Google Scholar]
- 93.Sekiguchi Y. Anaerolinea thermophila gen. nov., sp. nov. and Caldilinea aerophila gen. nov., sp. nov., novel filamentous thermophiles that represent a previously uncultured lineage of the domain Bacteria at the subphylum level. Int J Syst Evol Microbiol. 2003;53:1843–51. Available from: http://ijs.microbiologyresearch.org/content/journal/ijsem/10.1099/ijs.0.02699-0. Cited 2019 Feb 23. [DOI] [PubMed] [Google Scholar]
- 94.Song Z, Massart S, Yan D, Cheng H, Eck M, Berhal C, et al. Composted chicken manure for anaerobic soil disinfestation increased the strawberry yield and shifted the soil microbial communities. Sustainability. 2020;12:6313. Available from: https://www.mdpi.com/2071-1050/12/16/6313. Cited 2022 Nov 28. [Google Scholar]
- 95.Xia Y, Wang Y, Wang Y, Chin FYL, Zhang T. Cellular adhesiveness and cellulolytic capacity in Anaerolineae revealed by omics-based genome interpretation. Biotechnol Biofuels. 2016;9:111. 10.1186/s13068-016-0524-z. Cited 2022 Nov 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gschwend F, Aregger K, Gramlich A, Walter T, Widmer F. Periodic waterlogging consistently shapes agricultural soil microbiomes by promoting specific taxa. Appl Soil Ecol. 2020;155:103623. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0929139319316105. Cited 2022 Apr 25. [Google Scholar]
- 97.Doornbos RF, van Loon LC, Bakker PAHM. Impact of root exudates and plant defense signaling on bacterial communities in the rhizosphere. A review. Agron Sustain Dev. 2012;32:227–43. 10.1007/s13593-011-0028-y. Cited 2023 Feb 17. [Google Scholar]
- 98.Halim VA, Vess A, Scheel D, Rosahl S. The role of salicylic acid and jasmonic acid in pathogen defence. Plant Biol. 2006;8:307–13. Available from: https://onlinelibrary.wiley.com/doi/abs/10.1055/s-2006-924025. Cited 2023 Feb 17. [DOI] [PubMed] [Google Scholar]
- 99.Rolfe SA, Griffiths J, Ton J. Crying out for help with root exudates: adaptive mechanisms by which stressed plants assemble health-promoting soil microbiomes. Curr Opin Microbiol. 2019;49:73–82. Available from: https://www.sciencedirect.com/science/article/pii/S1369527419300578. Cited 2023 Feb 17. [DOI] [PubMed] [Google Scholar]
- 100.Kong HG, Song GC, Ryu C-M. Inheritance of seed and rhizosphere microbial communities through plant–soil feedback and soil memory. Environ Microbiol Rep. 2019;11:479–86. Available from: https://onlinelibrary.wiley.com/doi/abs/10.1111/1758-2229.12760. Cited 2023 Feb 17. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material 1: Supplementary data file 1. Layout of the plots
Supplementary Material 2: Supplementary data file 2. Complete metadata for the field-trial
Supplementary Material 3: Supplementary Figure S1. Schematic diagram showing the experimental design of this study. This includes the choice of potato seed stock with high, low (both Jelly varieties) and zero (Estima mini-tubers) starting levels of Pectobacterium species and irrigation regimes (Unirrigated [rainfed only], Irrigation 1 [SMD 40 mm], Irrigation 2 [SMD < 15 mm during the common scab control period then unirrigated], and Irrigation 3 [SMD < 15 mm during the common scab control period then unirrigated]. Comparisons in microbial communities across time were made using soil sampled from the ridge (near the top of the furrow) at 50% plant emergence (T_E Ridge) and harvest (T_H Ridge). In addition, to determine if there were differences in microbial communities sampled closer to the plant roots, samples were also taken at the bottom of the furrow at harvest only (T_H Root), to avoid damaging roots in the emerging plants. Details about the plot layout can be found in Supplementary Data File 2. Supplementary Figure S2. Rarefaction curves showing the number of reads from the 16S rRNA gene in DNA from the soil samples on the x-axis and the number of OTUs within a 97% percent sequence similarity threshold on the y-axis. Treatment groups are indicated by colour-coded lines. Supplementary Table S1. Percentage of blackleg disease symptoms in potato plants per plot over the course of the experimental field trial in 2020. Supplementary Table S2. Proportion of tubers with rotting (%) and weight of rotted tubers (tubers/hectare) at potato harvest. Table also shows the results of the statistical analysis of individual stocks and irrigation regimes. The respective degrees of freedom (D.F.) is given within the standard error (S.E.). Supplementary Table S3. Genus identifier list for Supplementary Fig. 2 and the average percentage prevalence in all samples. Supplementary Figure S3.The ‘core microbiome of the soil microbial communities from the field trial. The ‘core microbiome’ was set at 85% minimum prevalence of samples. Numbers referring to the individual genera are shown on the x-axis (defined in Supplementary Table 3), with colours highlighting the taxonomic phyla. The y-axis shows the detection threshold of absolute genera abundances and heatmap colours showing the average prevalence of each genus within all samples, ranging from 0–1. Supplementary Table S4. Genus identifier list for Supplementary Fig. 2 and the average percentage prevalence in all samples. Supplementary Figure S4. Taxonomic diversity of the soil microbiomes (ridge samples) at the 50% plant emergence (T_E) timepoint. Colours represent the irrigation regime (Unirrigated, Irrigation 1, Irrigation 2, and Irrigation 3). Symbols represent the potato seed stock (EstimaZero, JellyLow, and JellyHigh). A) Shows alpha diversity based on the relative distribution of taxa (Pielou’s evenness) and the number of different ASVs (Richness) within the communities; there were no significant differences between the watering regimes and no obvious clustering based on potato stock. B) Principal coordinate analysis (PCoA) based on Bray–Curtis distance of beta diversity dissimilarity between the communities; note that there was no distinct clustering in relation to irrigation regime, but there was clustering with respect to potato stock. Supplementary Figure S5. Functional diversity of the soil microbiomes (ridge samples) at the 50% plant emergence (T_E) timepoint. Colours represent the irrigation regime (Unirrigated, Irrigation 1, Irrigation 2, and Irrigation 3). Symbols represent the potato seed stock (EstimaZero, JellyLow, and JellyHigh). A) Shows diversity based on the relative distribution of KEGG Orthologs (KOs; Pielou’s evenness) and number of KEGG Orthologs (KOs; Richness) within the communities; there were no significant differences in relation to irrigation treatment and no obvious clustering by potato stock. B) Principal coordinate analysis (PCoA) based on Bray–Curtis distance of functional beta diversity dissimilarity between the communities as assessed through PiCrust2 and hierarchical metastorms analysis; note that there was no distinct clustering in relation to any of the variables. Supplementary Figure S6. Taxonomic diversity of the soil microbiomes at the harvest (T_H) timepoint. Colours represent the irrigation regime (Unirrigated, Irrigation 1, Irrigation 2, and Irrigation 3) and whether the sample was from the ‘Ridge’ or ‘Root’. Symbols represent the potato seed stock (EstimaZero, JellyLow, and JellyHigh). A) Shows diversity based on the relative distribution of taxa (Pielou’s evenness) and the number of different ASVs (Richness) within the communities; which were similar across irrigation regimes. B) Principal coordinate analysis (PCoA) based on Bray–Curtis distance of beta diversity dissimilarity between the communities; note that samples clustered according to potato stock, sample type (ridge or root) and Irrigation 2 samples showed some clustering. Supplementary Figure S7. Functional diversity of the soil microbiomes at the harvest (T_H) timepoint. Colours represent the irrigation regime (Unirrigated, Irrigation 1, Irrigation 2, and Irrigation 3) and the sample was from the ‘Ridge’ or ‘Root’. Symbols represent the potato seed stock (EstimaZero, JellyLow, and JellyHigh). A) Shows diversity based on the relative distribution of KEGG Orthologs (KOs; Pielou’s evenness) and number of KEGG Orthologs (KOs; Richness) within the communities; there were no significant differences in relation to irrigation treatment and no obvious clustering by potato stock. B) Principal coordinate analysis (PCoA) based on Bray–Curtis distance of functional beta diversity dissimilarity between the communities as assessed through PiCrust2 and hierarchical metastorms analysis; note that samples loosely cluster according to irrigation regime, sample type (root or ridge) and primarily cluster according to potato stock. Supplementary Figure S8. Taxonomic diversity of the soil microbiomes at the 50% plant emergence (T_E) and plant harvest (T_H) time points from ‘Ridge’ samples only. Colours represent the irrigation regime (Unirrigated, Irrigation 1, Irrigation 2, and Irrigation 3). Symbols represent the potato seed stock (EstimaZero, JellyLow, and JellyHigh). A) Shows diversity based on the relative distribution of taxa (Pielou’s evenness) and the number of different ASVs (Richness) within the communities; which were similar across irrigation regimes. B) Principal coordinate analysis (PCoA) based on Bray–Curtis distance of beta diversity dissimilarity between the communities; note that samples clustered according to potato stock. Supplementary Figure S9. Functional diversity of the soil microbiomes at 50% plant emergence (T_E). Colours represent potato stock (EstimaZero – E, JellyLow – JL, and JellyHigh – JH). Symbols represent the irrigation regimes (Unirrigated, Irrigation_1, Irrigation_2, and Irrigation 3). A) Shows diversity based on the relative distribution of KEGG Orthologs (KOs; Pielou’s evenness) and number of KEGG Orthologs (KOs; Richness) within the communities; there were no significant differences in relation to irrigation treatment and no obvious clustering by potato stock. B) Principal coordinate analysis (PCoA) based on Bray–Curtis distance of functional beta diversity dissimilarity between the communities as assessed through PiCrust2 and hierarchical metastorms analysis; note that samples very loosely cluster according to potato stock. Supplementary Figure S10. Functional diversity of the soil microbiomes at plant harvest (T_H). Colours represent potato stock (EstimaZero – E, JellyLow – JL, and JellyHigh – JH), time-point (50% plant emergence – T_H), and soil sample type (Ridge – Ri, Root – Ro). Symbols represent the irrigation regimes (Unirrigated, Irrigation_1, Irrigation_2, and Irrigation 3). A) Shows diversity based on the relative distribution of KEGG Orthologs (KOs; Pielou’s evenness) and number of KEGG Orthologs (KOs; Richness) within the communities; there were no significant differences in relation to irrigation treatment and no obvious clustering by potato stock. B) Principal coordinate analysis (PCoA) based on Bray–Curtis distance of functional beta diversity dissimilarity between the communities as assessed through PiCrust2 and hierarchical metastorms analysis; note that samples loosely cluster according to potato stock and sample type. Supplementary Figure S11. Functional diversity of the soil microbiomes at the 50% plant emergence (T_E) and plant harvest (T_H) time points from ‘Ridge’ samples only. Colours represent the irrigation regime (Unirrigated, Irrigation 1, Irrigation 2, and Irrigation 3). Symbols represent the potato seed stock (EstimaZero, JellyLow, and JellyHigh). A) Shows diversity based on the relative distribution of KEGG Orthologs (KOs; Pielou’s evenness) and number of KEGG Orthologs (KOs; Richness) within the communities; there were no significant differences in relation to irrigation treatment and no obvious clustering by potato stock. B) Principal coordinate analysis (PCoA) based on Bray–Curtis distance of functional beta diversity dissimilarity between the communities as assessed through PiCrust2 and hierarchical metastorms analysis; note that samples loosely cluster according to potato stock and time point. Supplementary Figure S12. Analysis of rare taxa from the Estima, JellyLow and JellyHigh samples at T_E (50% plant emergence) and T_H (plant harvest) divided into ridge and root samples (Ri and Ro). These figures show the percentage contribution (%) to taxonomic beta-diversity dissimilarity of: A) ‘Persistently Rare’ taxa B) ‘Abundant’ taxa; C) ‘Conditionally Rare’ taxa; and D) ‘Other Rare’ taxa. Supplementary Figure S13. Taxonomic map for differential heat tree analysis. The branches show the taxonomic identification of all ASVs. Note that the same tree can be used for each differential heat tree analysis instance. Supplementary Figure S14. Heat tree analysis to visualise the differential taxa between irrigation regimes at 50% plant emergence (T_E). The full legend for the tree branches is shown in Supplementary Fig. 10. Each tree shows a pair-wise comparison between irrigation regimes (denoted by either purple or blue). The size of the branch nodes shows the number of ASVs, while the differential Log2 ratio median proportion is shown by the colours. Note that there are no Log2 fold differences between taxa across the irrigation regimes. Supplementary Figure S15. Heat tree analysis to visualise the differential taxa between irrigation regimes at potato harvest (T_H) in soil ridge samples. The full legend for the tree branches is shown in Supplementary Fig. 10. Each tree shows a pair-wise comparison between irrigation regimes (denoted by either purple or blue). The size of the branch nodes shows the number of ASVs, while the differential Log2 ratio median proportion is shown by the colours. Note that there are no Log2 fold differences between taxa across the irrigation regimes. Supplementary Figure S16. Heat tree analysis to visualise the differential taxa between irrigation regimes at potato harvest (T_H) in soil root samples. The full legend for the tree branches is shown in Supplementary Fig. 10. Each tree shows a pair-wise comparison between irrigation regimes (denoted by either purple or blue). The size of the branch nodes shows the number of ASVs, while the differential Log2 ratio median proportion is shown by the colours. Note that there are no Log2 fold differences between taxa across the irrigation regimes. Supplementary Figure S17. Plot highlighting the impact of ‘Irrigation Regime’ on the abundance of microbial taxa in the potato harvest (T_H) ridge soil samples, based on the GLVMM analysis. The microbial genera names are indicated on the y-axis. The environment covariates values are indicated on the x-axis which are the coefficient values against the environmental covariates (e.g. Unirrigated, Irrigation_2, and Irrigation_3 as compared to the Irrigation 1 treatment). The values range from positive, neutral to negative associations with the specific taxa on the y-axis. The red lines show significantly positive taxa associated with the indicated environmental covariate and the blue lines show significantly negative taxa; grey lines are not statistically significant. Note: Irrigation 1 treatment is not displayed as the analysis uses this treatment as the reference. Supplementary Figure S18. Plot highlighting the impact of ‘Irrigation Regime’ on the abundance of microbial taxa in the potato harvest (T_H) root soil samples, based on the GLVMM analysis. The microbial genera names are indicated on the y-axis. The environment covariates values are indicated on the x-axis which are the coefficient values against the environmental covariates (e.g. Irrigation_1, Irrigation_2, and Irrigation_3, as compared to the Unirrigated treatment). The values range from positive, neutral to negative associations with the specific taxa on the y-axis. The red lines show significantly positive taxa associated with the indicated environmental covariate and the blue lines show significantly negative taxa; grey lines are not statistically significant. Note: Unirrigated treatment is not displayed as the analysis uses this as the reference. Supplementary Figure S19. Plot highlighting the impact of ‘Potato Stock’ on the abundance of microbial taxa at 50% plant emergence (T_E), based on the GLVMM analysis. The microbial genera names are indicated on the y-axis. The environmental covariates values are indicated on the x-axis which are the coefficient values against the environmental covariates (e.g. ‘Potato Stock’ either JellyHigh, JellyLow as compared to the EstimaZero. The values range from positive, and neutral to negative associations with the specific taxa on the y-axis. The red lines show significantly positive taxa associated with the indicated environmental covariate and the blue lines show significantly negative taxa; grey lines are not statistically significant. Note: The EstimaZero group is not displayed as the analysis uses this as the reference. Supplementary Figure S20. Plot highlighting the impact of ‘Potato Stock’ on the abundance of microbial taxa in the root samples at potato harvest (T_H), based on the GLVMM analysis. The microbial genera names are indicated on the y-axis. The environment covariates values are indicated on the x-axis which are the coefficient values against the environmental covariates (e.g. ‘Potato Stock’ either JellyHigh, JellyLow as compared to the EstimaZero. The values range from positive, neutral to negative associations with the specific taxa on the y-axis. The red lines show significantly positive taxa associated with the indicated environmental covariate and the blue lines show significantly negative taxa; grey lines are not statistically significant. Note: The EstimaZero group is not displayed as the analysis uses this as the reference. Supplementary Figure S21. Plot highlighting the impact of ‘Potato Stock’ on the abundance of microbial taxa in the ridge samples at potato harvest (T_H), based on the GLVMM analysis. The microbial genera names are indicated on the y-axis. The environment covariates values are indicated on the x-axis which are the coefficient values against the environmental covariates (e.g. ‘Potato Stock’ either JellyHigh, EstimaZero as compared to the JellyLow. The values range from positive, neutral to negative associations with the specific taxa on the y-axis. The red lines show significantly positive taxa associated with the indicated environmental covariate and the blue lines show significantly negative taxa; grey lines are not statistically significant. Note: The JellyLow group is not displayed as the analysis uses this as the reference. Supplementary Figure S22. Plot highlighting the impact of time on the abundance of microbial taxa in the ridge soil samples, based on the GLVMM analysis. The microbial genera names are indicated on the y-axis. The environment covariates values are indicated on the x-axis which are the coefficient values against the environmental covariate (e.g. Harvest [T_H] time point, as compared to the 50% plant emergence [T_E] time point. The values range from positive, neutral to negative associations with the specific taxa on the y-axis. The red lines show significantly positive taxa associated with the indicated environmental covariate and the blue lines show significantly negative taxa; g grey lines are not statistically significant. Note: The 50% emergence time point (T_E) is not displayed, as the analysis uses this as the reference. Supplementary Figure S23. Minimum subset of amplicon sequencing variants (ASVs) associated with percentage blackleg symptoms during the field trial. Analysis using the Ensemble Quotient Optimisation (EQO) technique considering at most 20 microbial taxa and the root samples at harvest. The left y-axis shows the relative abundance of the microbial taxa, and the right y-axis shows the percentage blackleg symptoms observed. The x-axis shows the sample identity with a colour legend for potato stock (Green EstimaZero, Blue JellyLow, and Red JellyHigh) and the irrigation regimes are shown in text (Unirrigated, Irrigation 1, Irrigation 2, and Irrigation 3). Supplementary Figure S24. Continuous ensemble quotient analysis (EQO) of the harvest ridge samples. Plot shows a stacked bar chart showing the minimum subset of amplicon sequencing variants (ASVs) associated with common scab symptoms. The x-axis shows the sample details including the potato stock (EstimaZero – Green, JellyLow – Blue, and JellyHigh – Red) and the irrigation regime is indicated in text. The right y-axis shows the relative abundance of the ASVs and the right y-axis shows the percentage (%) of common scab symptoms. Supplementary Figure S25. Continuous ensemble quotient analysis (EQO) of the harvest root samples. Plot shows a stacked bar chart showing the minimum subset of amplicon sequencing variants (ASVs) associated with common scab symptoms. The x-axis shows the sample details including the potato stock (EstimaZero – Green, JellyLow – Blue, and JellyHigh – Red) and the irrigation regime is indicated in text. The right y-axis shows the relative abundance of the ASVs and the right y-axis shows the percentage (%) of common scab symptoms.
Data Availability Statement
Sequence data is available from the sequence read archive (SRA) database under Bioproject Submission PRJNA992106.