Abstract
Background
Multiheme cytochromes c (MHC) provide prokaryotes with a broad metabolic versatility that contributes to their role in the biogeochemical cycling of the elements and in energy production in bioelectrochemical systems. However, MHC have only been isolated and studied in detail from a limited number of species. Among these, Desulfuromonadia spp. are particularly MHC-rich. To obtain a broad view of the diversity of MHC, we employed bioinformatic tools to study the cytochromome encoded in the genomes of the Desulfuromonadia class.
Results
We found that the distribution of the MHC families follows a different pattern between the two orders of the Desulfuromonadia class and that there is great diversity in the number of heme-binding motifs in MHC. However, the vast majority of MHC have up to 12 heme-binding motifs. MHC predicted to be extracellular are the least conserved and show high diversity, whereas inner membrane MHC are well conserved and show lower diversity. Although the most prevalent MHC have homologues already characterized, nearly half of the MHC families in the Desulforomonadia class have no known characterized homologues. AlphaFold2 was employed to predict their 3D structures. This provides an atlas of novel MHC, including examples with high beta-sheet content and nanowire MHC with unprecedented high numbers of putative heme cofactors per polypeptide.
Conclusions
This work illuminates for the first time the universe of experimentally uncharacterized cytochromes that are likely to contribute to the metabolic versatility and to the fitness of Desulfuromonadia in diverse environmental conditions and to drive biotechnological applications of these organisms.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12864-024-10872-4.
Keywords: Multiheme cytochromes, Desulfuromonadia, Pangenome, Nanowire, Protein structure prediction, Heme-binding motifs, Protein diversity
Background
Multiheme c-type cytochromes (MHC) are metalloproteins found widespread across the Bacteria and Archaea domains that play central roles in diverse anabolic and catabolic pathways. These pathways contribute significantly to the biogeochemical cycling of key elements such as metals (e.g. iron), nitrogen, and sulfur [1, 2]. The functional versatility of these proteins reflects their tunable coordination, redox, spin, and acid–base properties. MHC contain two or more heme cofactors covalently bound to the protein polypeptide chain. This typically occurs through two thioether bonds between two nearby cysteines of the apoprotein and the two vinyl groups of the heme cofactors [3]. Although the most common heme-binding motif is CX2CH, where ‘X’ represents any possible amino acid, other less common heme-binding motifs are described in the literature, namely CXCK [4], CX2CK [5], CX3CH [6], CX4CH [7], CX11CH, CX15CH [8] and CX17CH [9]. In all these cases, the iron is coordinated by the nitrogen atoms of the protoporphyrin plane and is axially coordinated by residues of the protein, forming a square pyramidal (penta-coordinated) or octahedral coordination (hexa–coordinated) geometries. The proximal axial ligand comes from the histidine or lysine that follows the second bound cysteine in the polypeptide chain [2].
The known oligomerization states of MHC range from monomers to the current maximum of an octamer of trimers (24-mer) for the Hydrazine Dehydrogenase complex (PDB ID: 6HIF), containing a total of 192 hemes that are closely packed to facilitate fast electron transfer [10]. Another oligomerization mode is found in the so-called extracellular cytochrome nanowires from electroactive bacteria [11–13] and archaea [14]. These proteins are composed of repetitive MHC subunits that extend outwards from the cell surface reaching micrometer-long lengths. Concerning the number of heme cofactors per polypeptide chain, molecular structures were experimentally obtained for MHC spanning from dihemes [15] to the hexadecaheme HmcA [16]. However, genome mining has revealed putative MHC with significantly higher numbers of heme cofactors per polypeptide chain. The current record holder is a sequence encoding a putative MHC containing 113 heme-binding motifs [17]. The extensive variety of existing MHC was observed to be the result of a complex process of fusion and fission processes involving heme and protein modules across time in a widespread group of MHCs [9, 18–20].
The first bioinformatics investigation of the rich diversity of MHC as a whole analyzed 594 representative prokaryotic genomes [21]. From these genomes, 258 were found to contain sequences coding for MHC, resulting in the identification of a total of 1659 MHC sequences. This analysis revealed a significant gap in our understanding of the ‘cytochromome’ [22]. Out of the 124 clusters generated from sequence alignments, only 12 had representative structures, accounting for approximately 10% of the total clusters. Since then, many MHC were characterized [2, 10] and among prokaryotes, the Desulfuromonadia class (former Desulforomonadales class [23]) comprises members with the highest known number of predicted MHC per genome. For example, the genome of Geotalea uraniireducens Rf4 (former Geobacter uranireducens [23]) was reported to contain a total of 75 MHC, constituting approximately 1.7% of all the protein-coding genes in the genome (CP000698.1). This organism stands out as having the highest number of MHC per genome identified thus far [21]. In addition, Desulfuromonadia genomes also contain protein-coding sequences that exhibit some of the highest numbers of heme-binding motifs per MHC sequence ever found. Specifically, protein-coding sequences with 69 and 53 heme-binding motifs were identified in this group [24, 25]. Members of this class of organisms participate in the biogeochemical cycling of elements, particularly of metals, and perform extracellular electron transfer to exchange electrons with metallic minerals in their environment [26] or to electrodes where they generate some of the highest current densities ever recorded in bioelectrochemical systems [27]. These systems use microorganisms as living catalysts to exchange electrons with electrodes to develop sustainable industrial applications, such as electricity production coupled with wastewater treatment, water desalination, biosensing and production of added-value compounds [28]. The best-studied microorganism of the Desulfuromonadia class is Geobacter sulfurreducens PCA, which together with the Gammaproteobacterium Shewanella oneidensis MR-1 are model electroactive organisms that contain extracellular electron transfer (EET) pathways fully composed of MHC. To a lesser extent, other Desulfuromonadia members have also been studied in terms of current production in bioelectrochemical systems and/or fundamental components of EET. These include other Geobacter spp [29–31], Desulfuromonas acetoxidans [32–34] and Geoalkalibacter spp [35, 36].
In this study, we focused on the ‘cytochromome’ of the Desulfuromonadia class and obtained for the first time a comprehensive overview of the diversity of MHC in these organisms and conservation patterns of their number of heme-binding motifs. This analysis highlighted MHC variants with predicted topology and heme content that have not been previously characterized through genetic, biochemical, or structural biology methods. These findings identify significant MHC candidates for future research, particularly in the development of sustainable bioelectrochemical technologies and for the understanding of the molecular foundations of metabolic pathways sustaining biogeochemical cycles of key elements such as sulfur, iron and nitrogen.
Results
Distribution of the number of heme-binding motifs per MHC reflects conflicting evolutionary pressures
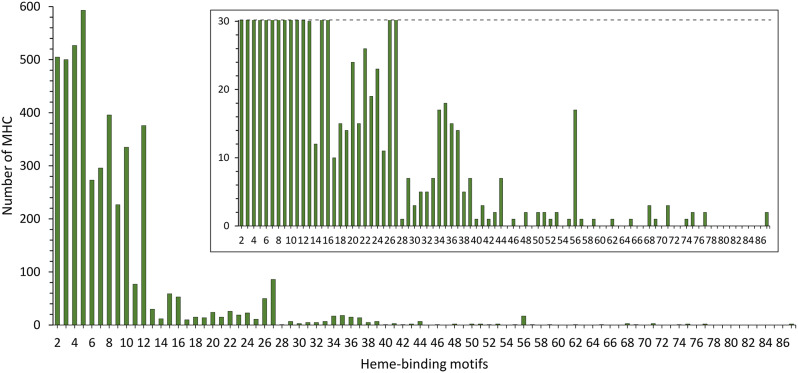
We gathered a total of 84 genomes from members of the Desulfuromonadia class (Additional file 1; Table S1). These genomes are rich in MHC and yielded 4716 protein sequences identified as MHC within this dataset (Additional file 2: Data S1) using InterProScan. This contrasts with just 1659 MHC sequences previously identified in a much larger sample of 594 prokaryotic genomes from diverse phylogenetic backgrounds [21]. The current result represents an almost 3-fold increase in the number of MHC studied, as a consequence of many more genomes being currently available and the larger coverage of the InterPro database vs. the Pfam [21]. More importantly, it represents a nearly 20-fold increase in the average number of MHC per genome with 56.1 MHC in the present dataset vs. 2.8 MHC in the previous dataset of diverse prokaryotes, reflecting the specific content of the bioenergetic electron transfer chains of Desulfuromonadia members. Heme-binding motifs of the 4716 MHC protein sequences were identified showing an overall trend in the shape of the distribution of frequency of heme binding motifs per polypeptide similar to that previously found (Fig. 1). Overall, the distribution of heme-binding motifs per polypeptide is not monotonic and predominantly favors small numbers of heme-binding motifs with high frequency counts at the beginning. The data show a moderate increase in prevalence from two to five heme binding sites per polypeptide followed by a steep decay until 12 heme binding sites per polypeptide, with 87% of all putative MHC found in this range. This is then followed by a long tail with very few examples which extend to 87 heme-binding sites per polypeptide. It is nonetheless interesting to observe a spike in prevalence with a maximum at 27 heme binding sites, a broad maximum centered at 35 heme binding sites and a lone spike at 56 heme binding sites (Fig. 1). Sequence alignments (Additional file 3: Data S2) showed that all of these cases (27, 35 and 56) are composed of mixed species populations and therefore include different MHC families. The spikes at 27 and 56 are composed of sequences predicted to be either periplasmic or extracellular MHC. The peak at 35 is composed solely of sequences predicted to be extracellular MHC.
Fig. 1.
Distribution of the number of heme-binding motifs across the MHC of all the Desulfuromonadia genomes analyzed. Inset represents a zoomed-in view of the graph for better visualization of the small prevalences at the high numbers of putative heme-binding motifs per polypeptide
The fact that the prevalence of MHC does not follow a monotonic function of the number of hemes likely indicates that conflicting fitness pressures operate in the development of MHC. Factors promoting a low number of hemes per MHC polypeptide chain, may include: (1) the pressure to maintain function while conserving iron and heme resources for metabolic efficiency; (2) limitations related to protein size imposed by poor mobility of large MHC in the crowded periplasmic space from their biogenesis in the inner-membrane of Gram-negative bacteria to their final location; (3) the fact that as the number of hemes in a MHC increases, the number of options for connecting the distal ligand of the hemes increases with a power of two, making the folding of MHC more prone to errors; (4) the fact that there is no obvious pressure from the side of biological catalysis to go beyond eight hemes in a MHC, given that eight electrons is the maximum necessary for reactions of biological relevance such as the conversions of sulfate to sulfide, nitrate to ammonia or CO2 to methane, and; (5) the option for protein polymerization as observed in nanowires of electroactive bacteria [11–13] and archaea [14] over producing a single, long polypeptide chain of high molecular weight and fixed length. By contrast, the long tails may reflect specific requirements, of which two can be envisaged on the bases of current knowledge: (1) storage of electrons [37], and; (2) long-distance electron transfer [13]. The combination of these pressures may have resulted in the most common number of heme-binding motifs per sequence in the Desulfuromonadia MHC being five, followed by four, two, three and eight. This result is slightly different from that reported earlier [21] where it was found that four was the most common number of heme-binding motifs, followed by two, five, ten and eight. These differences can be rationalized on two accounts: (1) [21] analyzed prokaryotes in general, while we focused on the Desulfuromonadia, which along with being more MHC-rich than the average of known prokaryotes, also seem to have differences in the distribution of heme-binding motifs; (2) [21] only considered the canonical CXXCH heme-binding motif, while we also take in consideration the other non-canonical heme-biding motifs. For example, the pentaheme NrfA, has four hemes with the canonical heme-binding motifs and frequently one catalytic heme with the CXXCK binding site [5]. As NrfA is highly ubiquitous in prokaryotes [9], this likely contributed to the underestimation of heme-binding motif counts, skewing the count of the most common number of heme-binding motifs from five to four in [21]. The example of NrfA hints at the balance of the conflicting pressures likely to be responsible for limiting the number of heme-binding motifs in MHC. Indeed, the five hemes of NrfA cannot hold all the necessary electrons to perform the six-electron reduction of nitrite to ammonia [5] However, natural selection found it more efficient to make the protein operate as a dimer than adding the additional necessary heme. Indeed, oligomerization may be a strong driver of the maximum of 5 heme-binding motifs per MHC since pentaheme cytochrome sequences are also present in homologues of the families OmcS and OmcE (next section, Fig. 2) that are highly oligomerized as cytochrome nanowires and also abundant in our data set [13].
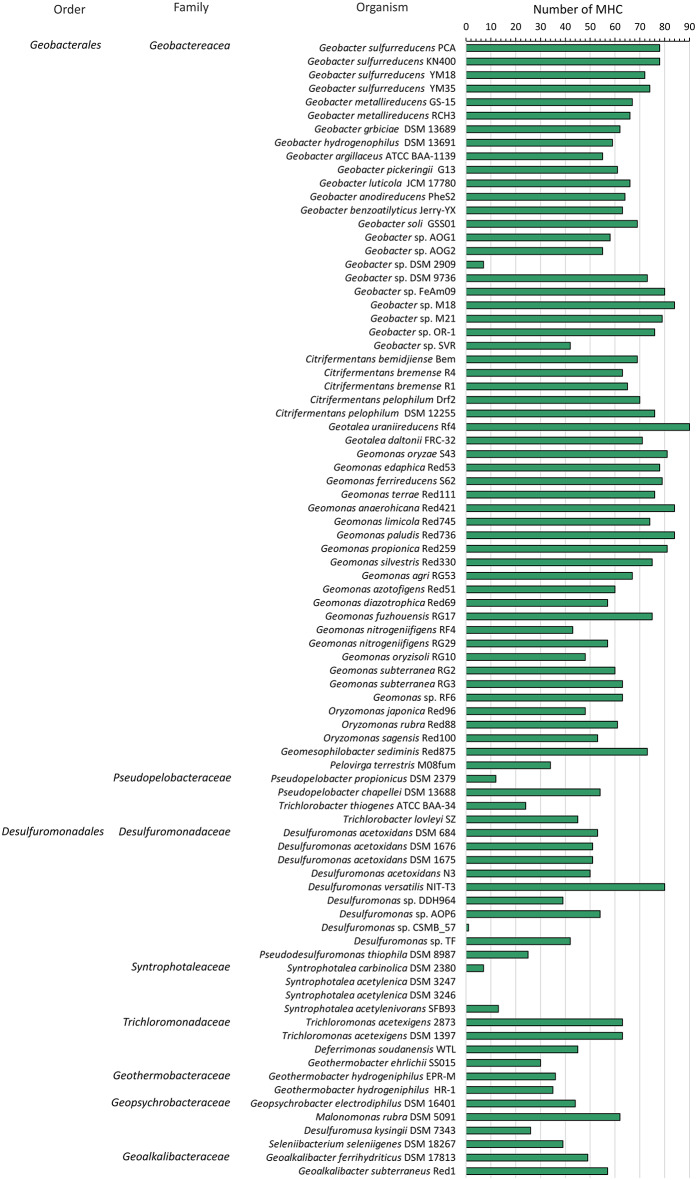
Fig. 2.
Number of putative MHC coding sequences per genome. MHC counts for each Desulfuromonadia genome are shown. Taxonomical classification at the level of family and order is presented on the left-hand side
The MHC repertoire shows differences between Geobacterales and Desulfuromonadales
Our data show that the Desulfuromonadia class is more MHC-rich than the average found in a broad sample of prokaryotes [21]. They also show that members of the Geobacterales order typically had a higher number of MHC per genome than members of the Desulfuromonadales order with an average of 64 and 39, respectively (Fig. 3). In line with this observation, the elevated iron content of G. sulfurreducens, that constitutes 0.2% of the cell dry weight, was proposed to result from the presence of a high number of MHC. Geotalea uranireducens displayed the highest number of MHC (90 MHC) corresponding to 2% of all the genes encoded in its genome. By contrast, Geobacter sp. DSM 2909 had only 6 MHC, an unexpectedly low number of MHC for a strain affiliated with the Geobacter genus. This could be a result of a different evolutionary metabolic path towards a more fermentative metabolism that does not rely on MHC as is thought to have happened in the Desulfuromonadaceae family [38] or an incorrect classification of this strain that has not been subject to a rigorous phylogenetic characterization in order to place it in a particular Geobacter species. For the Desulfuromonadales order, Desulfuromonas versatilis had the highest number of MHC, with a total of 80. This observation is in line with the fact that Desulfuromonas versatilis exhibits enhanced anaerobic respiratory versatility compared to other species of the Desulfuromonadales order [25]. On the other hand, members of the Syntrophotalea genus are usually described as fermentative [39], which agrees well with the fact that for species of this genus, we could find few or no MHC. This contrasts with our discovery of 62 putative MHC in the genome of Malonomonas rubra DSM 5091 which was described as strictly fermentative. However, the capacity for extracellular electron transfer of this organism was not tested [40].
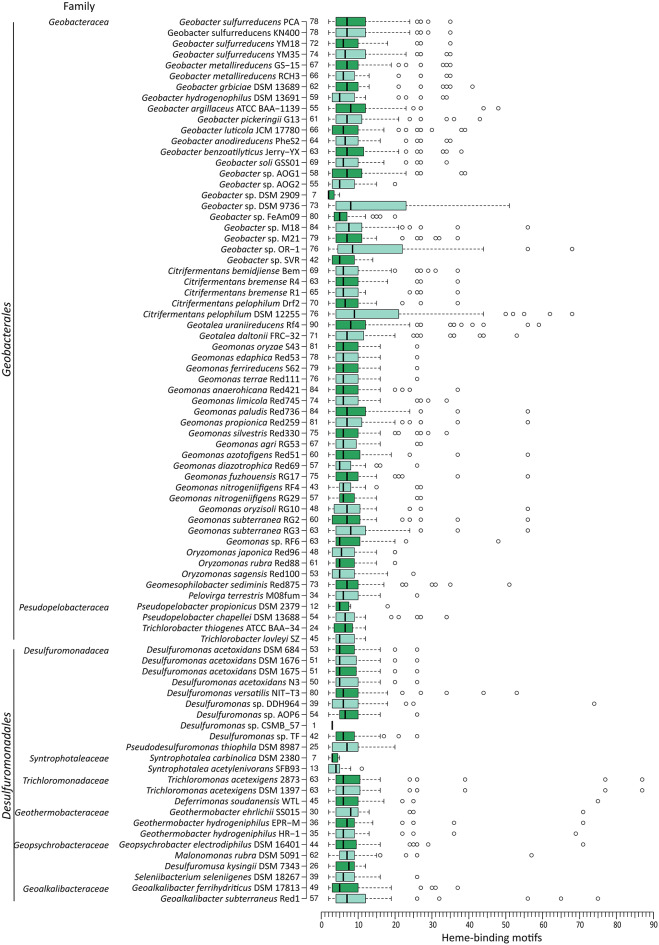
Fig. 3.
Distribution of the number of heme-binding motifs for each genome. Family and order are indicated at the left of each organism, while the number of MHC is indicated at the right. The whiskers of the boxplots extend to data points that are less than 1.5 times the interquartile range away from 1st /3rd quartile (Tukey whiskers). Circles represent outlier values using the above criteria
MHC with a large number of hemes are typically outliers within the genome
Heme-binding motif distributions in individual species follow the global trend, with the median number of heme-binding motifs in the range of 2 to 9 (Fig. 4). Some species do not exhibit a distribution characterized by a long tail of MHC with high numbers of heme-binding motifs. Examples of such species include strict fermenters, such as Syntrophotalea acetylenivorans [39] and S. carbinolica [41]. In addition, some species that respire soluble and insoluble electron acceptors display distributions of heme-binding motifs that lack the long tail, such as Trichlorobacter lovleyi [42] and T. thiogenes [43]. Some species, like Geobacter sp. DSM 9736 and Geobacter sp. OR-1 [44] and Citrifermentans pelophilum DSM 11255 [45] display a large interquartile range. These species have a high frequency of MHC with a large number of heme-binding motifs.
Fig. 4.
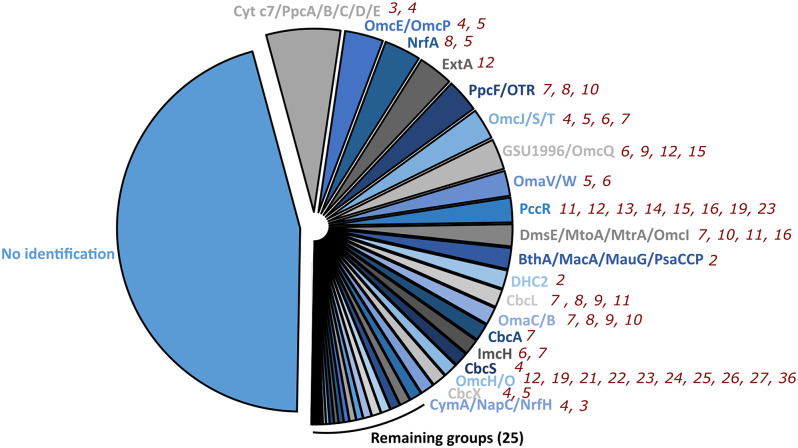
Pie-chart distribution of the number of sequences of the homologous groups identified within the MHC dataset from the Desulfuromonadia. No identification represents groups that do not display significant homology to representative sequences. Out of the 45 homologous groups identified, the top 20 largest ones are labelled. The number of heme-binding motifs found in each homologous group in members of Desulfuromonadia is presented near each group. Detailed information can be accessed in the Additional file 5: Table S2
Nearly half of the MHC in Desulfuromonadia have no known homologues
To characterize the large pool of MHC from the Desulfuromonadia, we constructed a curated database to provide templates of MHC described in the literature that could be used to define clusters based on sequence homology (see Materials and Methods; Additional file 4: Data S3). This database included MHC that were characterized genetically, biochemically, or structurally. Despite using template MHC that in some cases were only characterized through gene knockout studies, a significant portion of the clusters (45.5% of the dataset) remained to be identified. We were able to identify homologues for 54.4% of the Geobacterales MHC and 54.6% of the Desulfuromonadales MHC. These homologues have diverse numbers of heme binding sites as a consequence of the modular evolution of MHCs involving grafting and pruning of protein modules with heme binding sites [9]. The clusters with the most abundant sequences are homologous to MHC already characterized (Fig. 2 and Additional file 5: Table S2). For a significant number of clusters, the characterization did not include structural information and were only predicted using Alphafold2 (Additional file 6: Fig. S1).
We found homologues of putative inner membrane quinone/quinol oxidoreductases such as ActA [46], CbcA [47], CbcL [48], CbcS [49], CbcX [49], CymA/NapC/NrfH [50], and ImcH [51]. Homologues of the periplasmic MHC were also found, including of BthA/MacA/MauG/PsaCCP [52], cytochrome c3 [53], cytochrome c7/PpcA/B/C/D/E [54], DHC2 [55], DsrJ [56], FccA [57], GSU0105 [58], GSU0616 [59], GSU1786 [49], GSU1996/OmcQ [37], HAO/HDH [60], IhOCC [61], MccA [8], NrfA [5], PccG [49], PccR [49], PpcF/OTR [62], QHNDH [63], sb-DHC [64], Split Soret cytochrome c [65], and STC [66]. As for homologues of outer membrane MHC we found DmsE/MtoA/MtrA/OmcI [19], ExtA [67], ExtK [67], ExtG [67], andOmaC/B [68]. Extracellular MHC were also found, including OmaV/W(ExtC) [67], ExtD [67], GSU1334/OmcZ [11], GSU2884(OmcA) [49], MtrC/F [19], OmcB/C [69], OmcE/OmcP [12], OmcG [70], OmcH/O [70], OmcJ/S/T [13], OmcM [49] and PgcA [71]. NCBI accession numbers for all these proteins are provided in Additional file 4: Data S3.
The identification process showed that the most prevalent homologous group (12.0% of the identified groups) consisted of homologs of the well-known periplasmic triheme cytochromes: cytochrome c7 and PpcA-E. These are amongst the most abundant MHC found in Desulfuromonadia members [33, 72, 73]. The second most prevalent group (6.2% of the identified groups) was the OmcE/P group. Additionally, a considerable portion of the sequences was categorized into groups comprising nanowire MHC, such as the ones that included the well-characterized OmcS (5.0%), GSU1996 (4.9%) and OmcZ (1%). This totalized 17.1% of the identified groups, which demonstrates the importance of ‘nanowire-like’ MHC for Desulfuromonadia. Homologous sequences for the catalytic MHC NrfA/ONR and OTR were also abundant and together constitute 11.3% of the identified sequences. Inner membrane-associated MHC seem to be less abundant overall, where CbcL homologues’ sequences were found to be dominant, representing 2.9% of the sequences that could be identified. Surprisingly, a substantial number of sequences homologous to the DmsE/MtoA/MtrA/OmcI group was also found, which have not previously been reported in Geobacter or related species thus far. This group includes well-characterized proteins involved in the reduction and oxidation of soluble and insoluble compounds, organized in what is referred to as porin-cytochrome complexes that permeate the outer-membrane of several Gram-negative bacteria [74]. A similar topological organization is also expected in members of the Desulfuromonadia class which possess DmsE/MtoA/MtrA/OmcI in the same operon as the MtrB/PioB-like porin gene. Even more surprising, some members, from the Geobacterales order, possess operons containing genes annotated as “ABC transporter ATP-binding protein” and “ABC transporter substrate-binding protein”, in addition to the DmsE/MtoA/MtrA/OmcI and MtrB/PioB genes. This suggests that in these organisms, DmsE/MtoA/MtrA/OmcI MHC could have a role in the transport of substances, such as the uptake of metals [75].
The distribution of MHC within species shows differences between Desulfuromonadales and Geobacterales
To relate this information with the corresponding species we represented the distribution of MHC identified by homology within each species (Fig. 5; Additional file 7: Data S4). Within the inner membrane, ActA is the least represented in Desulfuromonadia, appearing in only 26% of the species. CymA/Nap/NrfH is poorly represented in the Geobacterales, while CbcS, CbcX and ImcH are poorly represented in the Desulfuromonadales (≤ 50% of the species). By contrast, CbcA and CbcL are both well distributed among the Desulfuromonadia (present in ≥ 84% of the species). This difference between the two orders of organisms may hint at differences in the redox potential range of the preferential terminal electron acceptors, by analogy to what is observed in Geobacter sulfureducens [81]. Typically, each genome contains only one copy of these inner membrane MHC sequences. However, there are a few exceptions to this, such as Desulfuromonas acetoxidans strains that have 3 or 4 CymA/Nap/NrfH genes per genome. Periplasmic MHC genes are globally dominated by the cytochrome c7/PpcA-E family. With a few exceptions, this family is widely distributed, featuring multiple paralogues per genome. In addition, BthA/MacA/MauG/PsaCCP, DHC2, NrfA, PccR and PpcF/OTR groups are also well distributed within the Desulfuromonadia class (≥ 54% of the species). The GSU0105 and GSU1996/OmcQ groups are widely distributed, but only within the Geobacterales order. MccA, QHNGH and sb-DHC were only found in one species, while cyt c3, MtrC/F and DsrJ were only found in two species. The low conservation suggests that these genes likely originated from horizontal gene transfer from other families where they are more prevalent, including Shewanellacea for MtrC/F [76] or Desulfovibrionaceae for cytochrome c3 [2]. From the sequences related to MHC predicted to be located at the outer membrane, DmsE/MtoA/MtrA/OmcI, ExtA, OmaC/B are well distributed in the Desulfuromonadia class (≥ 58% of the species). The remaining MHC are not well distributed within the Desulfuromonadia class. ExtK is only well distributed in the Geobacterales, while ExtG is poorly distributed overall. For the sequences related to extracellular MHC the scenario is quite different since there is a pattern of global low conservation among species. The only MHC well distributed among Desulfuromonadia class is OmcE/P (≥ 54% of the species), while OmcH/O and OmaV/W (ExtC) are well distributed among Geobacterales and OmcJ/S/T group in the Desulfuronadales order. This suggests that exoelectrogenic EET in this class of organisms is less restrained, having more room for diversification. This result aligns with earlier bioinformatics studies conducted on the Geobacterales order [77] and Shewanellaceae [76], as well as experimental evolutionary studies on Geobacter sulfurreducens, focusing on adaptation to microbial fuel cells [78]. Almost half of the sequences could not be classified and it remains uncertain whether this pattern will persist in these cases, or if MHC families yet to be discovered are so distinct from those currently known that even the conservation pattern based on their location is significantly different.
Fig. 5.
Heat map distribution of the different MHC across the species of the Desulfuromonadia class. The number of sequences found per homologous group is shown. High values are shown with darker red colors as the background. Species names are abbreviated and are shown in the same order as in Figs. 1 and 2. Raw data is provided in Additional file 7: Data S4. Only DmsE/MtoA/MtrA/OmcI, ExtA, ExtK, ExtG, OmaC/B, OmaV/W (ExtC), ExtD, GSU2884(OmcA), MtrC/F, OmcB/C MHC are in operons coding for cytochrome-porin complexes. *Outer membrane
Globally, Desulfuromonadia organisms exhibit diverse MHC sequences, featuring varying degrees of redundancy. Within these organisms, multiple parallel pathways seem to exist, at least as options at the level of the genome. This matches the observation in G. sulfurreducens and S. oneidensis of multiple pathways that offer alternative routes for reduction or oxidation of insoluble acceptors and donors, respectively [67, 79], which is often linked to their ability in fine-tuning access to various redox potential ranges and in conserving energy [80, 81]. Ultimately, this underscores the importance of having diverse MHC as a beneficial option to adapt to multiple conditions, as previously proposed [82]. However, due to the lack of detailed information about the native environment of the organisms studied in this work and the properties of the individual MHC, it is challenging to establish a correlation between the presence of specific MHC with specific metabolic diversity or habitat. Further studies are needed to explore the relationship between MHC presence and environmental conditions, and the contribution that the properties of particular MHC would provide to the fitness of organisms in the operation of sustainable bioelectrochemical technologies.
MHC diversity correlates with subcellular location
From the 4716 MHC sequences of the dataset, 51% could be assigned to a specific cellular sublocation. From these 11.1% were assigned to the inner membrane, 62.8% to the periplasm, 0.1% to the outer membrane and 25.9% to the extracellular space. Figure 6 shows that inner membrane MHC are more conserved with respect to size and number of hemes, while extracellular MHC are the least conserved. The inner membrane MHC group only contains three sequences that have more than 10 heme-binding motifs. Conversely, the extracellular group of sequences contains elements ranging from 2 to 75 heme-binding motifs, which are well distributed at much higher numbers of heme-binding motifs. In this group, 50% of the sequences have 10 or more hemes. MHC sequences assigned to the periplasm are also well-distributed towards relatively high numbers of heme-binding motifs. Only a few sequences were assigned to the outer membrane region. This can be a consequence of the fact that the distinction between outer membrane and periplasmic location can be difficult to establish. For example, some outer membrane MHC such as MtrA are also proposed to function in the periplasm [83, 84]. The pattern of diversification, in terms of size and number of heme binding sites versus cell location, shows that there is more room for diversification at the cell surface than on the inner membrane. In addition, the sequence identity against reference MHC (Additional file 6, Fig. S2; Additional file 7, Data S4) also reflects the same pattern of decrease conservation of MHC from the inner membrane to the extracellular space. This likely reflects the fact that extracellular MHC confer an adaptative advantage to these organisms in diverse ecological contexts. By contrast, MHC of the inner-membrane are functionally more homogeneous and involved in metabolic functions that are conserved across the organisms of the class, namely exchanging electrons between the quinone/quinol pool and the periplasmic redox partners, and translocation of protons across the inner-membrane for energy conservation [47, 51]. In this sense, evolution appears to have more leeway to modify extracellular MHC, accommodating multiple paralogues and changing the sequence and structure of these MHC, leading to a high degree of diversity.
Fig. 6.
Plot of size vs. number of hemes in the MHC sequences for which a subcellular location could be assigned
The unidentified MHC show that structure is more conserved than sequence
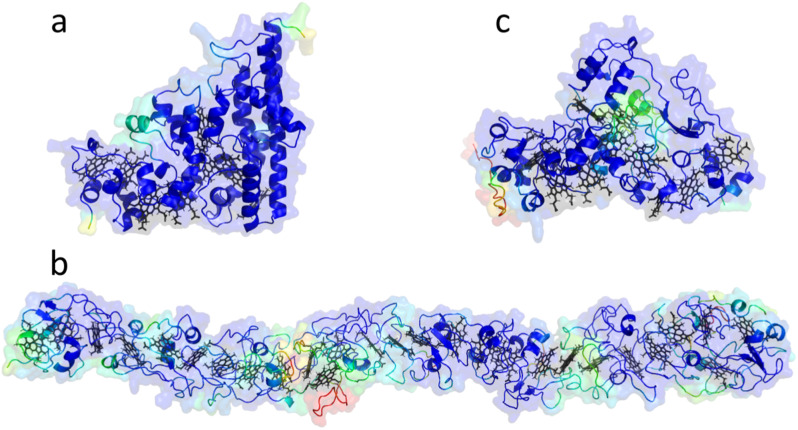
Since almost half of the MHC sequences lacked characterized homologues, we explored the structural characteristics of the most representative groups. We assembled clusters based on sequence alignments and predicted the structure of representatives from clusters containing more than 15 sequences (41 clusters in total) using Alphafold2 [85]. These conserved sequences are more likely to represent important proteins within Desulfuromonadia class, bridging the gaps in our current knowledge of their function and structural properties, and suggesting targets to be prioritized in future research. The most populated cluster, cluster 1, consists of 116 homologous sequences and the corresponding MHC are predicted to be located in the periplasmic space. The predicted structure reported in (Fig. 7A) is from a sequence from Citrifermentans bemidjiense Bem with eight heme-binding motifs. It distinctly displays the three long C-terminal alpha-helices and topological organization of the hemes typical of the group of octaheme cytochromes that catalyze key reactions in the nitrogen and sulfur cycles [9]. Regarding the homologous octaheme MHC, the relative position of the distal ligands within this structure is highly similar to that of ONR, HAO/HDH and IhOCC [9]. The fact that sequence identies range between 17.5 and 23% emphasizes that structure is more conserved than sequence (Additional file 6, Fig. S3). The second largest cluster displays a predicted structure that forms a long wire with closely spaced hemes similar to the already characterized nanowires [11–14, 37] but forming a longer wire per monomer/polypeptide chain. This predicted structure has the closest neighboring hemes with predicted iron-to-iron distances lower than 15 Ångstroms, which is compatible with fast electron transfer along the length of the protein [86]. As a representative, we display the predicted structure of a putative MHC from Geobacter metallireducens RCH3, featuring 27 heme-binding motifs and a molecular weight of 130.7 kDa (Fig. 6B). The third largest cluster is predicted to be periplasmic, has 11 heme-binding motifs and the representative depicted in Fig. 7C is from Desulfuromusa kysingii with a predicted molecular weight of 51.3 kDa. Cluster 2 (Fig. 7B) is predicted to be extracellular and is not part of an operon coding for a porin-cytochrome complex. Proteins from this cluster have a predicted length that is more than the average width of the periplasm (350 Ångstroms vs. 170 Ångstroms) [87]. Maturation and translocation to the outer membrane of proteins of this size may pose potential challenges in the crowded periplasmic space. However, judging by the high prevalence of regions without secondary structural elements across the length of the protein we can expect a high degree of flexibility in regions of low confidence of the Alphafold2 model (Fig. 7B), potentially easing its processing into the mature form and delivery to the target cell location. Indeed, an architecture of modules connected by linkers that provide some flexibility may have been an evolved strategy to solve two problems. On the one hand, it limits the search space for distal ligands of the hemes to residues in each module, decreasing the risk of misfolding. On the other hand, the flexibility allows for easier navigation in the crowded periplasmic space when the mature protein needs to reach its final destination outside of the cell surface. Experimental characterization for a MHC with these characteristics was only achieved for GSU1996 that shows a wire-like structure, proposed to arise from the duplication and concatenation of cytochrome c7 modules [37, 88]. The molecular wire clusters identified here might have originated through similar processes, or GSU1996 could be the result of the pruning of larger ancestral cytochromes as found for other MHC [9]. We provide an atlas of the remaining predicted structures from the representatives of the top 41 clusters in Additional file 6: Fig. S4 (corresponding protein structure files in Additional file 8: Data S5 and confidence estimation in Additional file 6: Fig. S5), showing that there are likely still novel MHC folds that have not been characterized to date, including long tubular MHC, and MHC with novel beta-sheet domains (Additional file 9: Table S3). The NrfA heme arrangement seems to be highly common across different clusters (cluster 1,3 and 8 – periplasm; clusters 4, 11 and 13 –unknown localization; Additional file 6: Fig. S4). Some of these have 5 hemes and contain a C-terminal domain of beta sheets (cluster 4, 11 and 13 –unknown localization; Additional file 6: Fig. S4) instead of alpha helixes as in NrfA [5]. Additional file 6: Fig. S4 also shows the presence of an extensive pool of periplasmic wire MHCs that begs the question of what is their physiological function, particularly considering that they have an average length similar to that generally associated with the width of the periplasm of Gram-negative bacteria [89]. Either the periplasm of the corresponding Desulfuromonadia members is wider than average, or respiratory pathways in organisms that contain these putative MHC may possess features in the periplasm that are currently unknown. These MHC might directly link the two membranes, offering an alternative to small soluble cytochromes that depend on diffusion for encountering partner proteins to engage in electron transfer from the quinone pool to the cell surface. The top three MHC clusters with a representative predicted to have an extracellar location showed the presence of one or more hematite-binding motifs [90] that were surface exposed and near a heme. From these, cluster 1 was predicted to form a long nanowire with 27 hemes and displays this feature unlike what is observed for the structurarly characterized extracellular nanowires OmcS, OmcE and OmcZ of Geobacter sulfurreducens [11–13]. Among the MHC predicted to have an extracellular location and that were characterized only on the basis of genetics, GSU2884 (OmcA), OmcH, OmcG, OmcM also showed the presence of one or multiple hematite-binding motifs. From these, GSU2884 (OmcA), OmcH, OmcG are also predicted to form long naowires with high number of hemes per polypeptide chain (Additional file 6: Fig. S1). Figure 5 shows that species of the Desulfuromonadia class contain MHC that are typically associated with Shewanella spp. and other related Gamma-proteobacteria, namely FccA, STC, DmsE/MtoA/MtrA/OmcI MtrC/F. From the pool of unidentified sequences based on sequence alignments, we also found structures resembling MtrC/F/OmcA like MHC (Additional file 6: Fig. S4; the second largest cluster of extracellular MHC composed of 57 sequences), which are not associated with cytochrome-porin complexes according to the operon configuration from the reference sequence. Indeed, in Shewanella oneidensis MR-1, OmcA and UndA that also belong to this family are also not part of the same operon [91, 92]. Further investigation is however required to understand if these sequences derive from vertical or horizontal gene transfer. Several studies point out that MHC have a high likelihood of horizontal gene transfer, including some of those extensively characterized in Shewanella spp [93]. Alternatively, these could be distantly related MHC derived from a common ancestor of the two phyla to which the Desulfuromonadia and Shewanella belong.
Fig. 7.
Predicted structures from the top three representative clusters for which no experimentally determined structure is available. Structures are oriented with the N-terminal at the left-hand side. Model structures are colored (red to blue) according to the pLDTT confidence values (see Additional file 6, Fig. S5 for detailed information). a Structure representative of the largest cluster whose sequence is from Citrifermentans bemidjiense and is predicted to be located in the periplasm. b Structure representative of the second largest cluster whose sequence is from Geobacter metallireducens and is predicted to be located at the extracellular space. c Structure representative of the third largest cluster whose sequence is from Desulfuromusa kysingii and is predicted to be located in the periplasm
Discussion
MHC are key metalloproteins that play a role in the biogeochemical cycles of the elements. Even though MHCs have been characterized in great detail for almost half a century, experimental characterization has only focused on a few representatives from the vast universe of MHC. Given that Desulfuromonadia class have an average prevalence of putative MHC in their genomes that is larger than what is typical for other prokaryotes, it was targeted to expand our understanding of this important class of proteins. This study revealed for the first time the diversity of MHC of the Desulfuromonadia class that contains metabolically diverse organisms with fermentative and/or respiratory metabolisms. Those that have a respiratory energetic metabolism have a relatively high number of MHC per genome, with the highest numbers found in some members of the Geobacterales order. The number of heme-binding motifs per polypeptide chain is highly variable being several fold higher in number than the existing maximum of the MHC currently characterized structurally. We presently have limited knowledge about the underlying reasons for the distinct option between oligomerization and repetition of polypeptide chain modules in modular evolution. Nevertheless, the frequency of heme-binding motifs is concentrated towards relatively low numbers. This shows biological constraints that only allow the number of heme-binding motifs to reach a relatively high number on very specific occasions. Experimental work is still far from giving answers on what factors come to bear on the solutions found by nature on these specific occasions. Nevertheless, this study reinforces the need to focus on this matter in order to be able to rationally manipulate MHC for biotechnological applications without being limited to using MHC with a relatively low number of hemes. This will open the opportunity to benefit from the interesting electrical properties of MHC [94]. MHC from the different cellular locations show different patterns of conservation. Inner membrane MHC, although having low relative abundance in total, are highly conserved compared to extracellular MHC. By contrast, extracellular MHC are the least conserved in general. This difference in variability may be associated with the particular environment of the two cellular locations. Whereas innermembrane MHC for the most part, interact with a homogeneous set of conditions involving electron exchange with the quinone pool, outer-membrane and extracellular MHC may have evolved to match the diversity of environmental conditions of the ecosystem of the organism to which they belong. However, even for inner-membrane MHC, we still lack detailed characterization of some of these for the model organisms that perform extracellular electron transfer. We expect that in the future, with additional experimentally characterized MHC and integrated studies of their structure and function, it will be possible to confidently assign novel MHC to known MHC families. Indeed, this work reveals that we lack knowledge of the diversity of characterized MHC in general, because it was only possible to identify half of the MHC sequences from the Desulfuromonadia. This observation reinforces the potential of the so-called protein structuromics in leveraging structural prediction tools to explore the role of proteins that have not yet been characterized biochemically, and identify interesting targets for this characterization [95]. On the one hand, some uncharacterized examples represent novel nanowire-like MHC that have higher molecular weight and heme content than the currently characterized nanowire MHC. On the other hand, some of the sequences that did not fall into recognized MHC families turned out to have predicted structures that match known families. This shows that just as there is redundancy in the genetic code when translated to protein sequence, there is redundancy in the sequence code when translated to structure, as already discussed in the literature [96–98]. This redundancy also translates into functional redundancy as has been shown for some MHC families such as those containing NrfA, where some members have diverse potential catalytic activities [9]. This provides a mechanism for the transition between different functions as natural selection operates on existing proteins to provide additional fitness to the host organisms in a changing environment.
Conclusions
Overall, this study provides an atlas of novel MHC that have not been investigated experimentally, but that were identified in the genomes of Desulfuromonadia owing to the presence of several heme-binding motifs in genes coding for proteins with a signal peptide. Their predicted structures are also compatible with the binding of several heme c cofactors as bona fide MHC. This study reveals the diversity of MHC of Desulfuromonadia with respect to their structure, function, and evolutionary plasticity generated presumably to match diverse environmental conditions. It shows that evolution has found diverse solutions for extracellular exposed MHC, in agreement with the need to match diverse environmental conditions that at the same time use the minimum amount of energy to sustain metabolism [82]. This information broadens our capability to understand the functional role of individual MHC and to improve their properties to be suitable for artificial environments such as those found in microbial electrochemical technologies.
Materials and methods
Data acquisition and distribution of MHC and heme-binding motifs across the Desulfuromonadia class
Genomes of Desulfuromonadia were obtained from the RefSeq database [99] using the NCBI genome server on 5th of April, 2022. All genomes assigned to ‘Desulfuromonadales’ were downloaded given that NCBI still had the previous classification that is now further substituted by the class Desulfuromonadia. When several genomes from the same strain existed, the one which had a higher level of genome assembly was chosen. If the same level of assembly was present, the most recent genome submission was chosen. A list of the genomes and their global characteristics is shown in Additional file 1: Table S1. Corresponding protein-coding sequences were obtained from each genome and text manipulation tools of Ubuntu 18.04 (Bash version 4.4.19(1)) were used to tag each protein sequence to the corresponding genome reference number. MHC were identified using InterProScan 5.55-88.0 [100]. NCBI eliminates redundancy from dataset copies where paralogues with 100% identity are assigned to only one reference code and proteome datasets only contain one entry in this case. To circumvent this and take into account MHC identical copies in the genome, each genome was searched for copies using Ubuntu 18.04 (Bash version 4.4.19(1)). When there were copies of MHC these were further added to the MHC protein coding sequences dataset. MHC sequences were converted to table format and transferred to Microsoft Excel, where heme-binding motifs were counted using the wildcard pattern recognition function. The canonical CXXCH and alternative heme-binding motifs were searched [4, 9]. Sequences showing less than two putative heme-binding motifs were removed. Graphs for the number of MHC per genome and the global distribution of the number of heme-binding motifs across all genomes were generated using Microsoft Excel. Box-plots corresponding to the genomes’ individual distributions of the number of heme-binding motifs were generated using BoxPlotR [101]. MHC localization was predicted using PSORTb 3.0.3 [102]. Molecular weight was predicted using Expasy PI/MW tool [103] and adding 616.18 Da for each heme.
Identification, clustering and structure prediction of MHC
A curated database of characterized MHC was constructed using the table reported in the literature [2] complemented with MHC characterized in the meantime. The source databases from which these sequences were collected were the NCBI-RefSeq [99] and PDB. Each sequence was aligned against each sequence of this database using BLASTp suite-2sequences [104]) with a cutoff of 1− 5e-value. Alignment data were retrieved and query and subject length and coverage were calculated using Microsoft Excel. Alignments were then further filtered using a minimum of 70% query and subject coverage, 30% length difference between query and subject and a minimum of 25% identity. Sequences from the constructed database were clustered using MMseqs 2 [105] with 25% identity, 70% coverage with coverage mode 0 (which applies this threshold to both query to subject and subject to query) in order to group them as homologous groups. These criteria provide confidence for homologous sequence collection and avoid entering the so-called twilight zone of protein identity [106, 107]. Each sequence that matched the alignment criteria was labeled with the subject protein sequence from the constructed database. In addition, each sequence was also labeled with the homologous group where that subject sequence belonged. Unidentified MHC were further clustered using MMseqs 2 [105] with 20% identity, 70% coverage with coverage mode 0 (Additional file 10: Data S6). Predicted structures were generated using AlphaFold2 (2.3.2) [85] within the COSMIC2 platform [108], using as parameters the full database sequence for the multiple sequence alignments (MSA), five predictions per model, monomer mode for the Predicted Template Modeling Score (pTM) and Amber relaxation for the best model generated. For the characterized homologous groups from which no representative structure is available in the PDB, the predicted structure was gathered from the AlphaFold Protein Structure database [109]. For structure prediction the signal peptide was cut using SignalIP 5.0 [110]. To incorporate hemes into the structural models, a PyMOL script was devised. In brief, it operates by identifying CXXCH and CXXXXCH binding motifs and then iterates the PyMOL align function to incorporate heme 3 from PDB entry 6HR0 at those binding motifs. This script has now been made available at https://github.com/BenjaminNash5/C_heme_insert_script_repository. The structures with the hemes were subsequently water refined using the protocol implemented in the interface of the HADDOCK 2.4 webserver [111, 112]. Image processing was performed using PyMOL Molecular Graphics System (version 2.0 Schrödinger, LLC). Pairwise structural alignments were performed using FATCAT flexible pairwise comparison [113]. Fold comparison was performed using Foldseek with the TM-align mode [114] against the PDB100, CATH50 and AlphaFold/Uniprot50 databases.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors are grateful to Tom Clarke and Irene Diaz-Moreno for helpful discussions and suggestions.
Author contributions
All authors participated in the conceptualization of the study and in the manuscript review and editing process. RS, BMF, BWN, ROL were involved in the methodology. RS, BMF, ROL were involved in the investigation of the work. BWN designed the PyMoL script for heme c incorporation in the predicted MHC structures. RS produced the first draft, including all the figures and tables. CMP, ROL supervised all stages of the work.
Funding
Financial support was provided by the following institutes and funding agencies with the respective projects specified: MOSTMICRO-ITQB base funding with references UIDB/04612/2020 and UIDP/04612/2020, LS4FUTURE Associated Laboratory (LA/P/0087/2020), Portuguese Foundation for Science and Technology (FCT) grant PTDC/BIA-BQM/4143/2021.
Data availability
All relevant data are available in the supplementary materials. The PyMoL script for heme c incorporation within protein structure files is available together with the respective readme file through this repository: https://github.com/BenjaminNash5/C_heme_insert_script_repository. Predicted AlphaFold structures were deposited in https://modelarchive.org/ and the accession codes are as follows:
ma-uj7c5 Extracellular cluster 1
ma-ojjr4 Extracellular cluster 2
ma-ql9kg Extracellular cluster 3
ma-b0tlf Extracellular cluster 4
ma-7j10t Extracellular cluster 5
ma-4fv90 Inner membrane cluster 1
ma-r3esc Inner membrane cluster 2
ma-y8d7g Inner membrane cluster 3
ma-wg0ws Periplasm cluster 1
ma-ymq5q Periplasm cluster 2
ma-p0jfx Periplasm cluster 3
ma-sg0b3 Periplasm cluster 4
ma-exqi1 Periplasm cluster 5
ma-0082i Periplasm cluster 6
ma-po1tf Periplasm cluster 7
ma-hkfk1 Periplasm cluster 8
ma-u1ym6 Unknown location cluster 1
ma-949p6 Unknown location cluster 2
ma-mo2je Unknown location cluster 3
ma-x25fy Unknown location cluster 4
ma-q9tly Unknown location cluster 5
ma-z8dxy Unknown location cluster 6
ma-3ymrw Unknown location cluster 7
ma-pgrps Unknown location cluster 8
ma-ooza4 Unknown location cluster 9
ma-opfjj Unknown location cluster 10
ma-rkl6m Unknown location cluster 11
ma-bgk7z Unknown location cluster 12
ma-rtshj Unknown location cluster 13
ma-cp75i Unknown location cluster 14
ma-gxx5s Unknown location cluster 15
ma-4fwjx Unknown location cluster 16
ma-s76an Unknown location cluster 17
ma-87fdd Unknown location cluster 18
ma-nn4b3 Unknown location cluster 19
ma-3xtcv Unknown location cluster 20
ma-x5zmm Unknown location cluster 21
ma-mwjws Unknown location cluster 22
ma-k6guf Unknown location cluster 23
ma-5vbm3 Unknown location cluster 24
ma-n6eqr Unknown location cluster 25
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
The original online version of this article was revised: the ‘Data availability’ declaration was incomplete
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
11/21/2024
A Correction to this paper has been published: 10.1186/s12864-024-11050-2
References
- 1.Bird LJ, Bonnefoy V, Newman DK. Bioenergetic challenges of microbial iron metabolisms. Trends Microbiol. 2011;19:330–40. [DOI] [PubMed] [Google Scholar]
- 2.Paquete CM, Rusconi G, Silva AV, Soares R, Louro RO. A brief survey of the cytochromome. Adv Microb Physiol. 2019;75:69–135. [DOI] [PubMed] [Google Scholar]
- 3.Daltrop O, Allen JWA, Willis AC, Ferguson SJ. In vitro formation of a c-type cytochrome. Proc Natl Acad Sci U S A. 2002;99:7872–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ferousi C, Lindhoud S, Baymann F, Hester ER, Reimann J, Kartal B. Discovery of a functional, contracted heme-binding motif within a multiheme cytochrome. J Biol Chem. 2019;294:16953–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Einsle O, Messerschmidt A, Stach P, Bourenkov GP, Bartunik HD, Huber R, et al. Structure of cytochrome c nitrite reductase. Nature. 1999;400:476–80. [DOI] [PubMed] [Google Scholar]
- 6.Aragão D, Frazão C, Sieker L, Sheldrick GM, LeGall J, Carrondo MA. Structure of dimeric cytochrome c3 from Desulfovibrio gigas at 1.2 a resolution. Acta Crystallogr D Biol Crystallogr. 2003;59:644–53. [DOI] [PubMed] [Google Scholar]
- 7.Czjzek M, Guerlesquin F, Bruschi M, Haser R. Crystal structure of a dimeric octaheme cytochrome c3 (Mr 26000) from Desulfovibrio desulfuricans Norway. Structure. 1996;4:395–404. [DOI] [PubMed] [Google Scholar]
- 8.Hermann B, Kern M, La Pietra L, Simon J, Einsle O. The octahaem MccA is a haem c-copper sulfite reductase. Nature. 2015;520:706–9. [DOI] [PubMed] [Google Scholar]
- 9.Soares R, Costa NL, Paquete CM, Andreini C, Louro RO. A new paradigm of Multiheme Cytochrome evolution by Grafting and pruning protein modules. Mol Biol Evol. 2022;39:msac139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Akram M, Dietl A, Mersdorf U, Prinz S, Maalcke W, Keltjens J, et al. A 192-heme electron transfer network in the hydrazine dehydrogenase complex. Sci Adv. 2019;5:eaav4310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gu Y, Guberman-Pfeffer MJ, Srikanth V, Shen C, Giska F, Gupta K, et al. Structure of Geobacter cytochrome OmcZ identifies mechanism of nanowire assembly and conductivity. Nat Microbiol. 2023;8:284–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang F, Mustafa K, Suciu V, Joshi K, Chan CH, Choi S, et al. Cryo-EM structure of an extracellular Geobacter OmcE cytochrome filament reveals tetrahaem packing. Nat Microbiol. 2022;7:1291–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang F, Gu Y, O’Brien JP, Yi SM, Yalcin SE, Srikanth V, et al. Structure of Microbial Nanowires reveals stacked hemes that Transport Electrons over Micrometers. Cell. 2019;177:361–e36910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baquero DP, Cvirkaite-Krupovic V, Hu SS, Fields JL, Liu X, Rensing C, et al. Extracellular cytochrome nanowires appear to be ubiquitous in prokaryotes. Cell. 2023;186:2853–e28648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carpenter JM, Zhong F, Ragusa MJ, Louro RO, Hogan DA, Pletneva EV. Structure and redox properties of the diheme electron carrier cytochrome c4 from Pseudomonas aeruginosa. J Inorg Biochem. 2020;203:110889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matias PM, Coelho AV, Valente FMA, Plácido D, LeGall J, Xavier AV, et al. Sulfate respiration in Desulfovibrio vulgaris Hildenborough. Structure of the 16-heme cytochrome c HmcA AT 2.5-A resolution and a view of its role in transmembrane electron transfer. J Biol Chem. 2002;277:47907–16. [DOI] [PubMed] [Google Scholar]
- 17.Leu AO, Cai C, McIlroy SJ, Southam G, Orphan VJ, Yuan Z, et al. Anaerobic methane oxidation coupled to manganese reduction by members of the Methanoperedenaceae. ISME J. 2020;14:1030–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Klotz MG, Schmid MC, Strous M, op den Camp HJM, Jetten MSM, Hooper AB. Evolution of an octahaem cytochrome c protein family that is key to aerobic and anaerobic ammonia oxidation by bacteria. Environ Microbiol. 2008;10:3150–63. [DOI] [PubMed] [Google Scholar]
- 19.Edwards MJ, White GF, Butt JN, Richardson DJ, Clarke TA. The Crystal structure of a Biological Insulated Transmembrane Molecular Wire. Cell. 2020;181:665–e67310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Costa NL, Hermann B, Fourmond V, Faustino MM, Teixeira M, Einsle O et al. How thermophilic gram-positive organisms perform Extracellular Electron transfer: characterization of the cell surface terminal reductase OcwA. mBio. 2019;10. [DOI] [PMC free article] [PubMed]
- 21.Sharma S, Cavallaro G, Rosato A. A systematic investigation of multiheme c-type cytochromes in prokaryotes. J Biol Inorg Chem. 2010;15:559–71. [DOI] [PubMed] [Google Scholar]
- 22.Londer YY, Giuliani SE, Peppler T, Collart FR. Addressing Shewanella oneidensis cytochromome: the first step towards high-throughput expression of cytochromes c. Protein Expr Purif. 2008;62:128–37. [DOI] [PubMed] [Google Scholar]
- 23.Waite DW, Chuvochina M, Pelikan C, Parks DH, Yilmaz P, Wagner M, et al. Proposal to reclassify the proteobacterial classes Deltaproteobacteria and Oligoflexia, and the phylum Thermodesulfobacteria into four phyla reflecting major functional capabilities. Int J Syst Evol Microbiol. 2020;70:5972–6016. [DOI] [PubMed] [Google Scholar]
- 24.Badalamenti JP, Summers ZM, Chan CH, Gralnick JA, Bond DR. Isolation and genomic characterization of ‘Desulfuromonas Soudanensis WTL’, a metal- and electrode-respiring bacterium from Anoxic Deep Subsurface Brine. Front Microbiol. 2016;7:913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xie L, Yoshida N, Ishii S, Meng L. Isolation and polyphasic characterization of Desulfuromonas Versatilis sp. Nov., an Electrogenic Bacteria Capable of versatile metabolism isolated from a Graphene Oxide-reducing Enrichment Culture. Microorganisms. 2021;9:1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roden EE, Lovley DR. Dissimilatory Fe(III) reduction by the Marine Microorganism Desulfuromonas acetoxidans. Appl Environ Microbiol. 1993;59:734–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rossi R, Logan BE. Using an anion exchange membrane for effective hydroxide ion transport enables high power densities in microbial fuel cells. Chem Eng J. 2021;422:130150. [Google Scholar]
- 28.Fonseca B, Soares R, Paquete CM, Louro RO. Chapter 8: bacterial power: an Alternative Energy source. In: Moura JJG, Moura I, Maia LB, editors. Enzymes for solving Humankind’s problems. Springer International Publishing; 2020. pp. 1–32.
- 29.Huang L, Tang J, Chen M, Liu X, Zhou S. Two modes of riboflavin-mediated Extracellular Electron transfer in Geobacter uraniireducens. Front Microbiol. 2018;9:2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Portela PC, Fernandes TM, Dantas JM, Ferreira MR, Salgueiro CA. Biochemical and functional insights on the triheme cytochrome PpcA from Geobacter metallireducens. Arch Biochem Biophys. 2018;644:8–16. [DOI] [PubMed] [Google Scholar]
- 31.Rotaru A-E, Woodard TL, Nevin KP, Lovley DR. Link between capacity for current production and syntrophic growth in Geobacter species. Front Microbiol. 2015;6:744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Czjzek M, Haser R, Shepard W. Structure of cytochrome c7 from Desulfuromonas acetoxidans at 1.9 Å resolution. Acta Crystallogr Sect D. 2001;57:670–8. [DOI] [PubMed] [Google Scholar]
- 33.Correia IJ, Paquete CM, Louro RO, Catarino T, Turner DL, Xavier AV. Thermodynamic and kinetic characterization of trihaem cytochrome c3 from Desulfuromonas acetoxidans: characterization of a trihaem cytochrome c3. Eur J Biochem. 2002;269:5722–30. [DOI] [PubMed] [Google Scholar]
- 34.Alves A, Ly HK, Hildebrandt P, Louro RO, Millo D. Nature of the surface-exposed cytochrome-electrode interactions in Electroactive biofilms of Desulfuromonas acetoxidans. J Phys Chem B. 2015;119:7968–74. [DOI] [PubMed] [Google Scholar]
- 35.Carmona-Martínez AA, Pierra M, Trably E, Bernet N. High current density via direct electron transfer by the halophilic anode respiring bacterium Geoalkalibacter subterraneus. Phys Chem Chem Phys. 2013;15:19699–707. [DOI] [PubMed] [Google Scholar]
- 36.Yadav S, Singh R, Sundharam SS, Chaudhary S, Krishnamurthi S, Patil SA. Geoalkalibacter halelectricus SAP-1 sp. nov. possessing extracellular electron transfer and mineral-reducing capabilities from a haloalkaline environment. Environ Microbiol. 2022;24:5066–81. [DOI] [PubMed] [Google Scholar]
- 37.Pokkuluri PR, Londer YY, Duke NEC, Pessanha M, Yang X, Orshonsky V, et al. Structure of a novel dodecaheme cytochrome c from Geobacter sulfurreducens reveals an extended 12 nm protein with interacting hemes. J Struct Biol. 2011;174:223–33. [DOI] [PubMed] [Google Scholar]
- 38.Butler JE, Young ND, Lovley DR. Evolution from a respiratory ancestor to fill syntrophic and fermentative niches: comparative genomics of six Geobacteraceae species. BMC Genomics. 2009;10:103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Baesman SM, Sutton JM, Fierst JL, Akob DM, Oremland RS. Syntrophotalea acetylenivorans sp. nov., a diazotrophic, acetylenotrophic anaerobe isolated from intertidal sediments. Int J Syst Evol Microbiol. 2021;71:004698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dehning I, Schink B. Malonomonas rubra gen. nov. sp. nov., a microaerotolerant anaerobic bacterium growing by decarboxylation of malonate. Arch Microbiol. 1989;151:427–33. [Google Scholar]
- 41.Schink B. Fermentation of 2,3-butanediol by Pelobacter carbinolicus sp. nov. and Pelobacter propionicus sp. nov., and evidence for propionate formation from C2 compounds. Arch Microbiol. 1984;137:33–41.
- 42.Sung Y, Fletcher KE, Ritalahti KM, Apkarian RP, Ramos-Hernández N, Sanford RA, et al. Geobacter lovleyi sp. nov. Strain SZ, a Novel reducingducintetrachloroetheneedechlorinatingnbacteriumterium. Appl Environ Microbiol. 2006;72:2775–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.De Wever H, Cole JR, Fettig MR, Hogan DA, Tiedje JM. Reductive dehalogenation of trichloroacetic acid by Trichlorobacter thiogenes gen. nov., sp. nov. Appl Environ Microbiol. 2000;66:2297–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ohtsuka T, Yamaguchi N, Makino T, Sakurai K, Kimura K, Kudo K, et al. Arsenic Dissolution from Japanese Paddy Soil by a dissimilatory arsenate-reducing bacterium Geobacter sp. OR-1. Environ Sci Technol. 2013;47:6263–71. [DOI] [PubMed] [Google Scholar]
- 45.Straub KL, Buchholz-Cleven BE. Geobacter bremensis sp. nov. and Geobacter pelophilus sp. nov., two dissimilatory ferric-iron-reducing bacteria. Int J Syst Evol Microbiol. 2001;51:1805–8. [DOI] [PubMed] [Google Scholar]
- 46.Sousa JS, Calisto F, Langer JD, Mills DJ, Refojo PN, Teixeira M, et al. Structural basis for energy transduction by respiratory alternative complex III. Nat Commun. 2018;9:1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Joshi K, Chan CH, Bond DR. Geobacter sulfurreducens inner membrane cytochrome CbcBA controls electron transfer and growth yield near the energetic limit of respiration. Mol Microbiol. 2021;116:1124–39. [DOI] [PubMed] [Google Scholar]
- 48.Antunes JMA, Silva MA, Salgueiro CA, Morgado L. Electron Flow from the inner membrane towards the Cell Exterior in Geobacter sulfurreducens: biochemical characterization of cytochrome CbcL. Front Microbiol. 2022;13. [DOI] [PMC free article] [PubMed]
- 49.Aklujkar M, Coppi MV, Leang C, Kim BC, Chavan MA, Perpetua LA, et al. Proteins involved in electron transfer to Fe(III) and mn(IV) oxides by Geobacter sulfurreducens and Geobacter uraniireducens. Microbiol (Reading). 2013;159:515–35. [DOI] [PubMed] [Google Scholar]
- 50.Rodrigues ML, Scott KA, Sansom MSP, Pereira IAC, Archer M. Quinol Oxidation by c-Type cytochromes: structural characterization of the Menaquinol binding site of NrfHA. J Mol Biol. 2008;381:341–50. [DOI] [PubMed] [Google Scholar]
- 51.Pimenta AI, Paquete CM, Morgado L, Edwards MJ, Clarke TA, Salgueiro CA, et al. Characterization of the inner membrane cytochrome ImcH from Geobacter reveals its importance for extracellular electron transfer and energy conservation. Protein Sci. 2023;32:e4796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rizzolo K, Cohen SE, Weitz AC, López Muñoz MM, Hendrich MP, Drennan CL, et al. A widely distributed diheme enzyme from Burkholderia that displays an atypically stable bis-Fe(IV) state. Nat Commun. 2019;10:1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Messias AC, Kastrau DH, Costa HS, LeGall J, Turner DL, Santos H, et al. Solution structure of Desulfovibrio vulgaris (Hildenborough) ferrocytochrome c3: structural basis for functional cooperativity. J Mol Biol. 1998;281:719–39. [DOI] [PubMed] [Google Scholar]
- 54.Assfalg M, Banci L, Bertini I, Bruschi M, Turano P. 800 MHz 1H NMR solution structure refinement of oxidized cytochrome c7 from Desulfuromonas acetoxidans. Eur J Biochem. 1998;256:261–70. [DOI] [PubMed] [Google Scholar]
- 55.Heitmann D, Einsle O. Structural and biochemical characterization of DHC2, a novel diheme cytochrome c from Geobacter sulfurreducens. Biochemistry. 2005;44:12411–9. [DOI] [PubMed] [Google Scholar]
- 56.Grein F, Venceslau SS, Schneider L, Hildebrandt P, Todorovic S, Pereira IAC, et al. DsrJ, an essential part of the DsrMKJOP Transmembrane Complex in the Purple Sulfur Bacterium Allochromatium vinosum, is an unusual triheme cytochrome c. Biochemistry. 2010;49:8290–9. [DOI] [PubMed] [Google Scholar]
- 57.Taylor P, Pealing SL, Reid GA, Chapman SK, Walkinshaw MD. Structural and mechanistic mapping of a unique fumarate reductase. Nat Struct Biol. 1999;6:1108–12. [DOI] [PubMed] [Google Scholar]
- 58.Fernandes TM, Folgosa F, Teixeira M, Salgueiro CA, Morgado L. Structural and functional insights of GSU0105, a unique multiheme cytochrome from G. sulfurreducens. Biophys J. 2021;120:5395–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shelobolina ES, Coppi MV, Korenevsky AA, DiDonato LN, Sullivan SA, Konishi H, et al. Importance of c-Type cytochromes for U(VI) reduction by Geobacter sulfurreducens. BMC Microbiol. 2007;7:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Maalcke WJ, Dietl A, Marritt SJ, Butt JN, Jetten MSM, Keltjens JT, et al. Structural basis of biological NO generation by octaheme oxidoreductases. J Biol Chem. 2014;289:1228–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Parey K, Fielding AJ, Sörgel M, Rachel R, Huber H, Ziegler C, et al. In meso crystal structure of a novel membrane-associated octaheme cytochrome c from the Crenarchaeon Ignicoccus hospitalis. FEBS J. 2016;283:3807–20. [DOI] [PubMed] [Google Scholar]
- 62.Mowat CG, Rothery E, Miles CS, McIver L, Doherty MK, Drewette K, et al. Octaheme tetrathionate reductase is a respiratory enzyme with novel heme ligation. Nat Struct Mol Biol. 2004;11:1023–4. [DOI] [PubMed] [Google Scholar]
- 63.Datta S, Ikeda T, Kano K, Mathews FS. Structure of the phenylhydrazine adduct of the quinohemoprotein amine dehydrogenase from Paracoccus denitrificans at 1.7 a resolution. Acta Crystallogr D Biol Crystallogr. 2003;59:1551–6. [DOI] [PubMed] [Google Scholar]
- 64.De March M, Di Rocco G, Hickey N, Geremia S. High-resolution crystal structure of the recombinant diheme cytochrome c from Shewanella baltica (OS155). J Biomol Struct Dyn. 2015;33:395–403. [DOI] [PubMed] [Google Scholar]
- 65.Abreu IA, Lourenço AI, Xavier AV, LeGall J, Coelho AV, Matias PM, et al. A novel iron centre in the split-soret cytochrome c from Desulfovibrio desulfuricans ATCC 27774. J Biol Inorg Chem. 2003;8:360–70. [DOI] [PubMed] [Google Scholar]
- 66.Fonseca BM, Silva L, Trindade IB, Moe E, Matias PM, Louro RO et al. Optimizing Electroactive organisms: the Effect of Orthologous proteins. Front Energy Res. 2019;7.
- 67.Jiménez Otero F, Chan CH, Bond DR. Identification of different putative outer membrane Electron conduits necessary for Fe(III) citrate, Fe(III) Oxide, Mn(IV) Oxide, or Electrode Reduction by Geobacter sulfurreducens. J Bacteriol. 2018;200:e00347–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Liu Y, Fredrickson JK, Zachara JM, Shi L. Direct involvement of ombB, omaB, and omcB genes in extracellular reduction of Fe(III) by Geobacter sulfurreducens PCA. Front Microbiol. 2015;6:1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Leang C, Coppi MV, Lovley DR. OmcB, a c-Type polyheme cytochrome, involved in Fe(III) reduction in Geobacter sulfurreducens. J Bacteriol. 2003;185:2096–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kim B-C, Qian X, Leang C, Coppi MV, Lovley DR. Two putative c-type multiheme cytochromes required for the expression of OmcB, an outer membrane protein essential for optimal Fe(III) reduction in Geobacter sulfurreducens. J Bacteriol. 2006;188:3138–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fernandes TM, Silva MA, Morgado L, Salgueiro CA. Hemes on a string: insights on the functional mechanisms of PgcA from Geobacter sulfurreducens. J Biol Chem. 2023;299. [DOI] [PMC free article] [PubMed]
- 72.Ding Y-HR, Hixson KK, Aklujkar MA, Lipton MS, Smith RD, Lovley DR, et al. Proteome of Geobacter sulfurreducens grown with Fe(III) oxide or fe(III) citrate as the electron acceptor. Biochim et Biophys Acta (BBA) - Proteins Proteom. 2008;1784:1935–41. [DOI] [PubMed] [Google Scholar]
- 73.Morgado L, Bruix M, Pessanha M, Londer YY, Salgueiro CA. Thermodynamic characterization of a Triheme Cytochrome Family from Geobacter sulfurreducens reveals mechanistic and functional diversity. Biophys J. 2010;99:293–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Richardson DJ, Butt JN, Fredrickson JK, Zachara JM, Shi L, Edwards MJ, et al. The porin-cytochrome model for microbe-to-mineral electron transfer. Mol Microbiol. 2012;85:201–12. [DOI] [PubMed] [Google Scholar]
- 75.Locher KP. Mechanistic diversity in ATP-binding cassette (ABC) transporters. Nat Struct Mol Biol. 2016;23:487–93. [DOI] [PubMed] [Google Scholar]
- 76.Zhong C, Han M, Yu S, Yang P, Li H, Ning K. Pan-genome analyses of 24 Shewanella strains re-emphasize the diversification of their functions yet evolutionary dynamics of metal-reducing pathway. Biotechnol Biofuels. 2018;11:193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Butler JE, Young ND, Lovley DR. Evolution of electron transfer out of the cell: comparative genomics of six Geobacter genomes. BMC Genomics. 2010;11:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Butler JE, Young ND, Aklujkar M, Lovley DR. Comparative genomic analysis of Geobacter sulfurreducens KN400, a strain with enhanced capacity for extracellular electron transfer and electricity production. BMC Genomics. 2012;13:471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fonseca BM, Paquete CM, Neto SE, Pacheco I, Soares CM, Louro RO. Mind the gap: cytochrome interactions reveal electron pathways across the periplasm of Shewanella oneidensis MR-1. Biochem J. 2013;449:101–8. [DOI] [PubMed] [Google Scholar]
- 80.Howley E, Krajmalnik-Brown R, Torres CI. Cytochrome gene expression shifts in Geobacter sulfurreducens to maximize energy conservation in response to changes in redox conditions. Biosens Bioelectron. 2023;237:115524. [DOI] [PubMed] [Google Scholar]
- 81.Levar CE, Hoffman CL, Dunshee AJ, Toner BM, Bond DR. Redox potential as a master variable controlling pathways of metal reduction by Geobacter sulfurreducens. ISME J. 2017;11:741–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Deng X, Dohmae N, Nealson KH, Hashimoto K, Okamoto A. Multi-heme cytochromes provide a pathway for survival in energy-limited environments. Sci Adv. 2018;4. [DOI] [PMC free article] [PubMed]
- 83.Firer-Sherwood MA, Bewley KD, Mock J-Y, Elliott SJ. Tools for resolving complexity in the electron transfer networks of multiheme cytochromes c. Metallomics. 2011;3:344–8. [DOI] [PubMed] [Google Scholar]
- 84.Schuetz B, Schicklberger M, Kuermann J, Spormann AM, Gescher J. Periplasmic electron transfer via the c-type cytochromes MtrA and FccA of Shewanella oneidensis MR-1. Appl Environ Microbiol. 2009;75:7789–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jumper J, Evans R, Pritzel A, Green T, Figurnov M, Ronneberger O, et al. Highly accurate protein structure prediction with AlphaFold. Nature. 2021;596:583–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Page CC, Moser CC, Dutton PL. Mechanism for electron transfer within and between proteins. Curr Opin Chem Biol. 2003;7:551–6. [DOI] [PubMed] [Google Scholar]
- 87.Seltmann G, Holst O. Periplasmic Space and rigid layer. In: Seltmann G, Holst O, editors. The bacterial cell wall. Berlin, Heidelberg: Springer; 2002. pp. 103–32. [Google Scholar]
- 88.Morgado L, Fernandes AP, Londer YY, Pokkuluri PR, Schiffer M, Salgueiro CA. Thermodynamic characterization of the redox centres in a representative domain of a novel c-type multihaem cytochrome. Biochem J. 2009;420:485–92. [DOI] [PubMed] [Google Scholar]
- 89.Dohnalkova AC, Marshall MJ, Arey BW, Williams KH, Buck EC, Fredrickson JK. Imaging Hydrated Microbial Extracellular polymers: comparative analysis by Electron Microscopy. Appl Environ Microbiol. 2011;77:1254–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lower BH, Lins RD, Oestreicher Z, Straatsma TP, Hochella MF, Shi L, et al. In vitro evolution of a peptide with a hematite binding motif that may constitute a natural metal-oxide binding archetype. Environ Sci Technol. 2008;42:3821–7. [DOI] [PubMed] [Google Scholar]
- 91.Edwards MJ, Baiden NA, Johs A, Tomanicek SJ, Liang L, Shi L, et al. The X-ray crystal structure of Shewanella oneidensis OmcA reveals new insight at the microbe–mineral interface. FEBS Lett. 2014;588:1886–90. [DOI] [PubMed] [Google Scholar]
- 92.Myers JM, Myers CR. Isolation and sequence of omcA, a gene encoding a decaheme outer membrane cytochrome c of Shewanella putrefaciens MR-1, and detection of omcA homologs in other strains of S. putrefaciens. Biochim Biophys Acta. 1998;1373:237–51. [DOI] [PubMed] [Google Scholar]
- 93.Baker IR, Conley BE, Gralnick JA, Girguis PR. Evidence for Horizontal and Vertical transmission of mtr-mediated Extracellular Electron transfer among the Bacteria. mBio. 2022;:e0290421. [DOI] [PMC free article] [PubMed]
- 94.Nakahara Y, Kimura K, Inokuchi H, Yagi T. Electrical conductivity of an anhydrous cytochrome c3 film as a function of temperature and ambient pressure. Chem Phys Lett. 1980;73:31–4. [Google Scholar]
- 95.Wu L, Liu H, Xu Y, Nie Y. Entering an era of protein structuromics. Biochemistry. 2023;62:3167–9. [DOI] [PubMed] [Google Scholar]
- 96.Illergård K, Ardell DH, Elofsson A. Structure is three to ten times more conserved than sequence–a study of structural response in protein cores. Proteins. 2009;77:499–508. [DOI] [PubMed] [Google Scholar]
- 97.Ingles-Prieto A, Ibarra-Molero B, Delgado-Delgado A, Perez-Jimenez R, Fernandez JM, Gaucher EA, et al. Conservation of protein structure over four billion years. Structure. 2013;21:1690–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chothia C, Lesk AM. The relation between the divergence of sequence and structure in proteins. EMBO J. 1986;5:823–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.O’Leary NA, Wright MW, Brister JR, Ciufo S, Haddad D, McVeigh R, et al. Reference sequence (RefSeq) database at NCBI: current status, taxonomic expansion, and functional annotation. Nucleic Acids Res. 2016;44:D733–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Jones P, Binns D, Chang H-Y, Fraser M, Li W, McAnulla C, et al. InterProScan 5: genome-scale protein function classification. Bioinformatics. 2014;30:1236–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Spitzer M, Wildenhain J, Rappsilber J, Tyers M. BoxPlotR: a web tool for generation of box plots. Nat Methods. 2014;11:121–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Yu NY, Wagner JR, Laird MR, Melli G, Rey S, Lo R, et al. PSORTb 3.0: improved protein subcellular localization prediction with refined localization subcategories and predictive capabilities for all prokaryotes. Bioinformatics. 2010;26:1608–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Walker JM, editor. The Proteomics protocols Handbook. Totowa, NJ: Humana; 2005. [Google Scholar]
- 104.Tatusova TA, Madden TL. BLAST 2 sequences, a new tool for comparing protein and nucleotide sequences. FEMS Microbiol Lett. 1999;174:247–50. [DOI] [PubMed] [Google Scholar]
- 105.Steinegger M, Söding J. MMseqs2 enables sensitive protein sequence searching for the analysis of massive data sets. Nat Biotechnol. 2017;35:1026–8. [DOI] [PubMed] [Google Scholar]
- 106.Chung SY, Subbiah S. A structural explanation for the twilight zone of protein sequence homology. Structure. 1996;4:1123–7. [DOI] [PubMed] [Google Scholar]
- 107.Punetha A, Sarkar P, Nimkar S, Sharma H, KNR Y, Nagaraj S. Structural Bioinformatics: Life through the 3D glasses. In: Shanker A, editor. Bioinformatics: sequences, structures, phylogeny. Singapore: Springer; 2018. pp. 191–253. [Google Scholar]
- 108.Cianfrocco MA, Wong-Barnum M, Youn C, Wagner R, Leschziner A. COSMIC2: A Science Gateway for Cryo-Electron Microscopy Structure Determination. In: Proceedings of the Practice and Experience in Advanced Research Computing 2017 on Sustainability, Success and Impact. New York, NY, USA: Association for Computing Machinery; 2017. pp. 1–5.
- 109.Varadi M, Anyango S, Deshpande M, Nair S, Natassia C, Yordanova G, et al. AlphaFold protein structure database: massively expanding the structural coverage of protein-sequence space with high-accuracy models. Nucleic Acids Res. 2022;50:D439–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Almagro Armenteros JJ, Tsirigos KD, Sønderby CK, Petersen TN, Winther O, Brunak S, et al. SignalP 5.0 improves signal peptide predictions using deep neural networks. Nat Biotechnol. 2019;37:420–3. [DOI] [PubMed] [Google Scholar]
- 111.de Vries SJ, van Dijk M, Bonvin AMJJ. The HADDOCK web server for data-driven biomolecular docking. Nat Protoc. 2010;5:883–97. [DOI] [PubMed] [Google Scholar]
- 112.van Zundert GCP, Rodrigues JPGLM, Trellet M, Schmitz C, Kastritis PL, Karaca E, et al. The HADDOCK2.2 web server: user-friendly integrative modeling of Biomolecular complexes. J Mol Biol. 2016;428:720–5. [DOI] [PubMed] [Google Scholar]
- 113.Li Z, Jaroszewski L, Iyer M, Sedova M, Godzik A. FATCAT 2.0: towards a better understanding of the structural diversity of proteins. Nucleic Acids Res. 2020;48:W60–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.van Kempen M, Kim SS, Tumescheit C, Mirdita M, Lee J, Gilchrist CLM, et al. Fast and accurate protein structure search with Foldseek. Nat Biotechnol. 2024;42:243–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All relevant data are available in the supplementary materials. The PyMoL script for heme c incorporation within protein structure files is available together with the respective readme file through this repository: https://github.com/BenjaminNash5/C_heme_insert_script_repository. Predicted AlphaFold structures were deposited in https://modelarchive.org/ and the accession codes are as follows:
ma-uj7c5 Extracellular cluster 1
ma-ojjr4 Extracellular cluster 2
ma-ql9kg Extracellular cluster 3
ma-b0tlf Extracellular cluster 4
ma-7j10t Extracellular cluster 5
ma-4fv90 Inner membrane cluster 1
ma-r3esc Inner membrane cluster 2
ma-y8d7g Inner membrane cluster 3
ma-wg0ws Periplasm cluster 1
ma-ymq5q Periplasm cluster 2
ma-p0jfx Periplasm cluster 3
ma-sg0b3 Periplasm cluster 4
ma-exqi1 Periplasm cluster 5
ma-0082i Periplasm cluster 6
ma-po1tf Periplasm cluster 7
ma-hkfk1 Periplasm cluster 8
ma-u1ym6 Unknown location cluster 1
ma-949p6 Unknown location cluster 2
ma-mo2je Unknown location cluster 3
ma-x25fy Unknown location cluster 4
ma-q9tly Unknown location cluster 5
ma-z8dxy Unknown location cluster 6
ma-3ymrw Unknown location cluster 7
ma-pgrps Unknown location cluster 8
ma-ooza4 Unknown location cluster 9
ma-opfjj Unknown location cluster 10
ma-rkl6m Unknown location cluster 11
ma-bgk7z Unknown location cluster 12
ma-rtshj Unknown location cluster 13
ma-cp75i Unknown location cluster 14
ma-gxx5s Unknown location cluster 15
ma-4fwjx Unknown location cluster 16
ma-s76an Unknown location cluster 17
ma-87fdd Unknown location cluster 18
ma-nn4b3 Unknown location cluster 19
ma-3xtcv Unknown location cluster 20
ma-x5zmm Unknown location cluster 21
ma-mwjws Unknown location cluster 22
ma-k6guf Unknown location cluster 23
ma-5vbm3 Unknown location cluster 24
ma-n6eqr Unknown location cluster 25