Abstract
Background
Vital pulp therapy (VPT) has recently been reported as an effective approach for preventing and treating carious pulp injury in permanent teeth. Compared with root canal treatment (RCT), which involves complete removal of the pulp tissue, VPT effectively maintains pulp vitality and retains the physiological functions of the pulp. In the research pool, large-scale randomized controlled trials evaluating the treatment outcome of VPT using calcium silicate cements and RCT in cariously exposed permanent teeth are lacking. Here, we present a monocentric clinical protocol to compare the effects of VPT using iRoot BP Plus (Innovative Bioceramix, Vancouver, BC, Canada) as a pulp-capping material with RCT.
Methods
The proposed trial is an open-label, single-centre, randomized, controlled, noninferiority trial. In total, 462 patients will be included in this trial according to the following criteria: adult patients (18–50 years old), pulp exposure during the treatment of deep caries in mature permanent teeth, a diagnosis of reversible or partially irreversible pulpitis without apical translucency on X-ray, without periodontitis or systemic disease. Patients with signed informed consent forms will be enrolled and randomly divided into two groups (VPT and RCT) with a balanced treatment allocation (1:1). Clinical evaluations will be conducted at baseline and at 3, 6, 12, and 24 months after treatment, with the potential for extension. The primary outcome measure will be the duration of success. The secondary outcomes will include the success rate at the 1-year follow-up and any adverse reactions. The Kaplan‒Meier method and log-rank test will be used to compare the duration of success of both treatments. For other outcomes, the χ2 test or Fisher’s exact test will be used for categorical variables, and the t test or Mann‒Whitney U test will be used for continuous variables to assess the differences between groups.
Discussion
The results of this trial will provide a clinical reference for selecting treatments for carious pulp injuries in permanent teeth.
Trial registration
ClinicalTrials.gov ChiCTR2100051369. The study has been registered in the Chinese Clinical Trial Registry (ChiCTR) (www.chictr.org.cn). Registered on 21 September 2021
Supplementary Information
The online version contains supplementary material available at 10.1186/s13063-024-08559-y.
Keywords: Vital pulp therapy, Root canal treatment, Carious pulp injury, Mature permanent teeth
Administrative information
Note: the numbers in curly brackets in this protocol refer to SPIRIT checklist item numbers. The order of the items has been modified to group similar items (see http://www.equator-network.org/reporting-guidelines/spirit-2013-statement-defining-standard-protocol-items-for-clinical-trials/).
| Title {1} | Efficacy of vital pulp therapy for carious pulp injury in permanent teeth: a study protocol for an open-label randomized controlled noninferiority trial |
|---|---|
| Trial registration {2a and 2b}. | This trial is registered in the Chinese Clinical Trial Registry ChiCTR2100051369. |
| Protocol version {3} | Trial protocol version 3.0, dated 24 Mar 2021 |
| Funding {4} | This research was supported by grants from the Guangdong Financial Fund for High-Caliber Hospital Construction (174–2018-XMZC-0001–03–0125/D-08). |
| Author details {5a} |
1 Hospital of Stomatology, Sun Yat-Sen University; Guangdong Provincial Key Laboratory of Stomatology, Guangzhou, 510,055, China 2 Guanghua School of Stomatology, Sun Yat-Sen University, Guangzhou, 510,055, China 3 Guangdong Provincial Clinical Research Center of Oral Diseases |
| Name and contact information for the trial sponsor {5b} |
SPIRIT guidance: Name and contact information for the trial sponsor. Hospital of Stomatology, Sun Yat-Sen University |
| Role of sponsor {5c} | This is an investigator-initiated study. The sponsor will play no role in the study design; data collection, management, analysis, or interpretation; writing of the report; or decision to submit the report for publication. |
Introduction
Background and rationale {6a}
The vitality of the pulp is essential for long-term tooth survival. Once pulpitis is diagnosed, root canal therapy (RCT) is conventionally used to eliminate the root canal infection, as the extent of bacterial infection is often unknown. Recent advancements in dental microscopy and endodontic techniques have improved the success rate of RCT, which now ranges between 68 and 93% [1–4]. However, RCT presents certain challenges. The removal of hard tissue during root canal preparation may increase the risk of root fracture [5]. Additionally, the complex anatomy of the root canal system requires high technical proficiency, making RCT difficult for general dentists to perform [6]. Moreover, RCT procedures are time-consuming and labour-intensive, placing a greater burden on patients, clinicians, and social healthcare resources [7].
Vital pulp therapy (VPT) is a treatment that aims to eliminate infection from the dentin‒pulp complex, preserve the pulp viability and maintain the pulp function. VPT includes indirect pulp capping (IPC), direct pulp capping (DPC), partial pulpotomy (PP), and full pulpotomy (FP) [8]. IPC involves capping the material covering the affected dentin over the unexposed pulp, whereas in DPC, the covering agent is placed over the exposed pulp. Pulpotomy involves the removal of a minute amount of the coronal pulp (PP) and complete amputation of the coronal pulp (FP) followed by direct coverage of the remaining pulp tissue [8]. Recently, VPT has attracted increasing attention and has been used in mature permanent teeth with pulp exposure caused by dental caries [9, 10]. In one systematic review, the clinical and radiographic success rates of full pulpotomy for mature permanent teeth were reported to range from 92.2 to 99.4%, whereas the success rates of partial pulpotomy were between 78.2 and 80.6% [11]. Previous studies have demonstrated that the 1-year success rate of VPT for carious mature teeth with symptomatic pulpitis ranges from 88 to 97.4% [12, 13], and the 2-year success rate of VPT in cariously exposed mature teeth without symptoms is as high as 92% [14]. The European Society of Endodontics (ESE) proposed that VPT in the form of pulp capping or partial pulpotomy is indicated after the exposure of reversibly inflamed pulp tissue during caries excavation [8]. In 2021, the American Association of Endodontists (AAE) reported that a diagnosis of irreversible pulpitis is not an indication for pulp removal, as more conservative treatments, such as VPT, could be considered [15]. In addition, several studies have shown that VPT is less costly than RCT and is a cost-effective treatment for younger patients who have occlusal exposure sites or lower Willingness-To-Pay values [6, 16, 17]. Therefore, VPT may be considered an alternative treatment to RCT when appropriate indications and full patient consent are achieved, as this approach will prolong the lifespan of the natural teeth.
Currently, calcium silicate cements, including mineral trioxide aggregate (MTA), Biodentine, and iRoot BP Plus, are recommended for pulp capping in VPT [18, 19]. Among these materials, iRoot BP Plus has excellent sealing properties, biocompatibility, and osteoconductive potential [20–23]. Our previous study revealed that Si and Ca released from iRoot BP Plus increase in the acidic environment of inflammation, which may contribute to its osteogenic/odontogenic potential [24, 25]. Clinical studies have demonstrated that the 1-year success rates of pulpotomy using iRoot BP Plus in immature permanent teeth with carious exposure range from 71.5 to 90.9% [26, 27]. Liu et al. reported that the success rates of DPC by iRoot BP Plus in cariously exposed mature teeth at 1 year, 2 years, and 3 or more years were 98%, 89%, and 81%, respectively [28]. Another prospective study revealed that the overall success rate at the 1–3 year follow-up timepoint after VPT using iRoot BP Plus was 90.5% in 59 permanent teeth with irreversible pulpitis caused by caries [29]. These studies demonstrated the efficacy of iRoot BP Plus as a pulp-capping material in VPT.
Notably, accurate diagnosis of the pulp condition is crucial for determining the appropriate therapy [30]. Conventionally, reversible pulpitis can be resolved with VPT, and RCT is indicated for irreversible pulpitis on the basis of the AAE classification of pulpitis. However, recent studies have reported successful outcomes of VPT for irreversible pulpitis [29, 31, 32]. This may be explained by the fact that clinical signs and symptoms cannot adequately present the degree of histologic damage and the extent of microbe invasion [33]. The AAE classification of pulpitis as reversible or irreversible has been contested for not reflecting the variations in pulpal responses to pathological stimuli. Therefore, Wolters proposed a new classification system based on symptoms and categorized pulpitis into initial, mild, moderate, and severe types [34]. This classification provides an alternative interpretation of disease progression. ESE has further suggested that vital pulps should be considered reversibly inflamed, alternatively partially or completely irreversibly inflamed under deep or extreme caries. Irreversible damage (partial or total) is characterized by episodes of spontaneous, radiating pain that lingers after removal of the stimulus. Reversible pulpitis is either symptomless or involves episodes of less intense, shorter-lasting pain. For reversible pulpitis and partially irreversible pulpitis, VPT can be considered; in contrast, RCT is required to manage completely irreversible pulpitis [8]. This classification can guide decision-making in vital pulp treatment. However, with respect to clinical manifestations, distinguishing between irreversible pulpitis and partial irreversible pulpitis is difficult. Regarding treatment decision-making, integrating the ESE classification and Wolters classification offers a more effective approach. However, better long-term prospective randomized data are needed before this protocol can be established as the preferred treatment.
Although VPT shows promise as an alternative to conventional RCT in mature teeth with carious exposure, there is insufficient evidence to conclusively compare the clinical outcomes of these two treatments. Current studies indicate that full pulpotomy has comparable success rates to RCT in cariously exposed mature teeth, regardless of symptoms [6, 35–38]. However, the sample sizes of these studies, ranging from 54 to 157 teeth, are not large enough to provide robust evidence. Although a multicentre randomized clinical trial included a larger sample size (407 teeth), the recall rate was low (67%, 271/407). Additionally, the procedures were performed by general dentists, which might have affected the RCT outcomes compared with those obtained by specialists. Furthermore, different VPT procedures (DPC, PP, and FP) should be considered on the basis of the ESE classification of pulpitis to comprehensively evaluate the clinical efficacy of VPT and RCT. Therefore, further large-scale prospective clinical studies are necessary.
Objectives {7}
The objective of this study is to compare the clinical outcomes between VPT using iRoot BP Plus as a pulp-capping material and RCT in carious-induced pulp injury in mature permanent teeth to guide future clinical decision-making.
The primary aim is to compare the duration of success between VPT and RCT. The secondary aim is to determine the success rates of VPT and RCT at the 1-year follow-up and identify any adverse reactions associated with VPT.
Trial design {8}
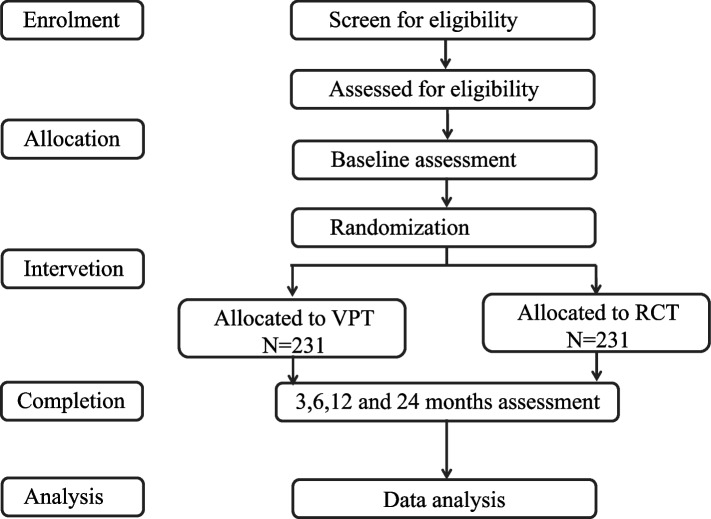
This is an investigator-initiated, mono-centric, open-label, noninferiority, randomized controlled trial. The flow chart is presented in Fig. 1.
Fig. 1.
Study flow chart
Methods: participants, interventions, and outcomes
Study setting {9}
The study is being conducted at the Department of Operative Dentistry and Endodontics, Hospital of Stomatology, Guanghua School of Stomatology, Sun Yat-sen University, Guangzhou, Guangdong Province, China.
Eligibility criteria {10}
Inclusion criteria
Patients who currently meet the following criteria will be included:
Age range of 18–50 years;
Pulp exposure during treatment of deep caries;
Diagnosis with reversible or partially irreversible pulpitis without apical translucency on X-ray (reversible pulpitis is either symptomless or has episodes of less intense, shorter-lasting pain). Partial irreversible pulpitis is a heightened and lengthened reaction to cold, warm and sweet stimuli that can last up to 20 s but then subsides, possibly percussion sensitive, or elicits clear symptoms, with strong, heightened and prolonged reactions to cold, which can last for minutes, possibly percussion sensitive with spontaneous, dull pain that can be suppressed with pain medication.
Absence of periodontitis or systemic disease;
Only one or two proximal surfaces lost with remaining walls > 2 mm; and
Good compliance and signed informed consent forms.
Exclusion criteria
Patients who meet any of the following criteria will be excluded:
Teeth with pulp calcification, root fracture or internal/external absorption;
Teeth with unrestorable large defects or the need for RCT for aesthetic reasons;
Current orthodontic treatment;
Current pregnancy or breast feeding;
Poor compliance or inability to complete the trial.
Who will take informed consent? {26a}
The investigators will provide information about the study and be responsible for obtaining informed consent from individuals who may qualify for participation.
Additional consent provisions for collection and use of participant data and biological specimens {26b}
This information is not available, as there will be no collection of biological specimens as part of this trial.
Interventions
Explanation for the choice of comparators {6b}
RCT is the conventional treatment modality for pulp injury in mature teeth and involves removing the entire pulp. As the minimally invasive concept is currently well accepted, VPT has been used in mature teeth with carious exposure as a promising alternative to conventional RCT. However, there is no sufficient evidence for a comprehensive comparison of the clinical outcomes of these two modalities. The present trial will compare these interventions to guide decision-making for mature teeth with symptomatic or asymptomatic pulp injury.
Intervention description {11a}
The specific intervention plan is shown in Table 1. Patients diagnosed with reversible or partially irreversible pulpitis will be randomly assigned at a 1:1 ratio to the intervention group (VPT) or the control group (RCT). Data on the duration of success and other outcomes will be collected at baseline and at 3, 6, 12, and 24 months after treatment. Moreover, data on adverse events will be collected during the study.
Table 1.
SPIRIT (Standard Protocol Items: Recommendations for Interventional Trials)
| Study period | ||||||
|---|---|---|---|---|---|---|
| Timepoint | Pretreatment | Posttreatment | 3-month follow-up |
6-month follow-up |
12-month follow-up |
24-month follow-up |
| Enrolment | ||||||
| Eligibility screen | X | |||||
| Informed consent | X | |||||
| Baseline data collection | X | |||||
| Randomized subjects | X | |||||
| Allocation | X | |||||
| Intervention | ||||||
| VPT | X | X | ||||
| RCT | X | X | ||||
| Assessments | ||||||
| Primary outcome | X | X | X | |||
| Secondary outcome | X | X | X | X | ||
The intervention group
The intervention group will receive VPT using iRoot BP plus as the pulp-capping material. The VPT will be performed in the following steps: (1) After surface disinfection and local anaesthesia, decayed areas near the pulp will be removed under a rubber dam and dental operating microscope. (2) One per cent sodium hypochlorite will be used for haemostasis after pulp exposure for 3 min. Direct pulp capping (DPC), partial pulpotomy (PP) and full pulpotomy (FP) using a 2-mm-thick layer of iRoot BP plus as the pulp-capping material will be performed on the basis of the pulp tissue conditions and haemostasis. If pulp exposure is less than 1 mm with healthy dentin and pulp tissue, while bleeding can be controlled within 3 min, DPC should be performed; if haemostasis cannot be achieved within 3 min, the tissue will be excised to the superficial 1 ~ 2 mm of the coronal pulp (PP) until the coronal pulp tissue is amputated to the level of the root canal orifices (FP). (3) The composite resin permanent restoration will be completed immediately following pulp capping.
The control group
The control group will be treated with RCT. First, after surface disinfection and local anaesthesia, the decayed tissue will be removed, and the access cavity will be prepared with high-speed burs. A rubber dam will then be applied for isolation, followed by root canal preparation via rotary nickel‒titanium files and root canal filling with warm gutta‒percha under a dental operating microscope. The composite resin permanent restoration will be completed at the subsequent visit if there are no abnormalities.
Criteria for discontinuing or modifying allocated interventions {11b}
Patients will be followed up until treatment completion at 24 months. The interventions will be continued as far as possible unless the following situations occur:
The participants do not return on time or are lost to follow-up, with poor compliance; and
The participants actively quit the trial (the investigators will diligently attempt to ascertain and record the reasons for withdrawal). The participants are free to withdraw at any time and for any reason, without consequence.
Strategies to improve adherence to interventions {11c}
The research team will provide standard treatment strategies to improve adherence to interventions, such as counselling on the necessity of treatment. Counselling will be performed to support adherence at all visits.
Relevant concomitant care permitted or prohibited during the trial {11d}
This topic is not applicable, as no relevant concomitant care is needed during the trial.
Provisions for post-trial care {30}
This topic is not applicable, as no post-trial care is needed in this study.
Outcomes {12}
Clinical and radiographic evaluations will be performed by two independent evaluators at baseline and 3, 6, 12, and 24 months after treatment. Prior to initiating this study, we established standardized operative procedures (SOPs) for all of the procedures, including the evaluations. The evaluators receive training on the research process and standard operative procedures for evaluation. Each patient will be independently evaluated by two senior doctors. In cases where there is a disagreement between the evaluators, a third evaluator will be consulted to reach a consensus. The primary outcome will be the duration of success. The secondary outcomes will include the success rate at the 1-year follow-up and adverse reactions. Clinical success is defined as normal symptoms and signs without pain, abscess, swelling, or sinus tract formation. In addition, pulp vitality tests should reveal normal results in patients managed with DPC or PP. Radiographic success is defined as no periapical radiolucency, no root resorption and no root fracture. The data will be recorded by dentists at each follow-up.
Participant timeline {13}
Table 1 outlines the schedule of enrolment, interventions, and assessments.
Sample size {14}
This trial is designed to test the noninferiority of the duration of success in VPT compared with RCT. According to previous studies, the 18-month success rate is 85% for the patients receiving RCT and 87.5% for patients receiving VPT, indicating a true hazard ratio (HR) of 0.82. In those studies, the noninferiority margin was set as − 5% for the 18-month success rate, which indicates a noninferiority margin with an HR of 1.37. To achieve 80% power and a one-sided type I error of 2.5%, we need at least 462 patients to account for a 15% dropout rate.
Recruitment {15}
The trial will be conducted at the Hospital of Stomatology, Sun Yat-Sen University. The incentives for participant enrolment are not provided. A training session for the research team has been conducted for them to understand the study enrolment criteria and learn the skills needed for effective enrolment. The department staff have been informed of this trial and will refer appropriate patients directly to the study team. All trial information sheets and consent forms are available electronically and in paper form.
Assignment of interventions: allocation
Sequence generation {16a}
Patients meeting the inclusion criteria will be randomly assigned to the intervention group or the control group at a 1:1 ratio via stratified randomization, with this stratification based on the reversibility of pulpitis (reversible/partially irreversible). The randomization sequence will be generated by a statistician using computer software.
Concealment mechanism {16b}
The randomization sequence will be sealed in envelopes by the statistician, who will not enrol the participants or assign the interventions. The randomization list will be inaccessible to the clinical investigators. Allocation concealment will be ensured, as the sealed envelopes will not be provided until the patient has been recruited into the trial, which takes place after all baseline assessments are completed.
Implementation {16c}
For eligible patients who provide signed informed consent, the researchers will open the envelopes sequentially according to the stratification (reversible/partially irreversible) to determine the treatment allocation and proceed with the intervention accordingly.
Assignment of interventions: blinding
Who will be blinded {17a}
No one in this trial will be blinded.
Procedure for unblinding if needed {17b}
The trial team and participants will not be blinded to the treatment allocation because both the treatment procedure and radiography can be easily identified. Therefore, this study is an open-label trial with no blinding of the researchers and participants.
Data collection and management
Plans for assessment and collection of outcomes {18a}
For this trial, X-rays will be used for the observation of apical translucency, root resorption and root fracture, and pulp vitality tests will be performed for the confirmation of dental pulp vitality by senior doctors. The X-ray data will be independently evaluated by two senior doctors who have been trained in the research process and standard operative procedures. Pulp vitality tests will be performed in duplicate by a senior dentist. Case report forms (CRFs) in paper form, which are considered the main source of documentation, will be completed by research team members in a timely manner. These CRFs will be used to collect demographic, baseline and clinical data. The data will be uploaded to the electronic database REDCap. Two study team members will be responsible for checking the consistency of the data from the CRFs and the database.
Plans to promote participant retention and complete follow-up {18b}
The research team will make telephonic contact with the participants before their appointment dates to remind them of the visits. The participants will have access to trial phone numbers that may be called if they have any questions.
Data management {19}
All clinical data will be electronically uploaded to REDCap, which allows data quality checks to ensure data quality and reliability. Two study team members will administratively control the form and data uploading and downloading.
Confidentiality {27}
All participant data will be anonymized, and the participants will be assigned a unique ID number. REDCap, a secure web application for building and managing databases, can ensure the confidentiality and security of electronic information. Access to the REDCap database is password protected, with each study member having their own username and password. No information concerning the participants will be released to an unauthorized third party, without written approval of the participant except as necessary for trial monitoring or regulatory review.
Plans for collection, laboratory evaluation and storage of biological specimens for genetic or molecular analysis in this trial/future use {33}
This step is not applicable, as no biological specimens will be collected in this trial.
Statistical methods
Statistical methods for primary and secondary outcomes {20a}
Efficacy analyses will be conducted in both the intention-to-treat population, which includes all randomly assigned patients, and the per-protocol population, which includes all patients who received the assigned therapy. For the safety analysis, all randomly assigned participants will be included, except for those who did not receive either RCT or VPT. The duration of success will be calculated via the Kaplan‒Meier method, and the different curves will be compared by using the log-rank test. Missing time-to-event data, such as those of patients who are lost to follow-up or without an observed event at the last follow-up date, will be treated as censored. Hazard ratios (HRs) and 95% confidence intervals (CIs) will be calculated via Cox proportional hazards models, with the assumption of proportional hazards confirmed by the Schoenfeld residuals. Multivariate analyses using the Cox proportional hazards model will be performed, considering the randomized factor and other significant factors identified in univariate analyses. For other outcomes, we will use the χ2 test or Fisher’s exact test for categorical variables and the T test or Mann‒Whitney U test for continuous variables to assess differences between groups.
For the primary outcome, the noninferiority margin of the HR is 1.37. If the upper limit of the 95% CI of the HR is less than the margin, noninferiority will be considered to have been achieved.
All analyses will be performed with Stata/MP 14.0. All tests will be two-sided, and P < 0.05 will be considered to indicate statistical significance.
Interim analyses {21b}
The committee members will meet every 6 months to review the trial process and data concerns. No interim analysis will be performed for the effect inference of the study.
Methods for additional analyses (e.g. subgroup analyses) {20b}
Stratified analyses will be conducted on the basis of randomization strata and baseline characteristics.
Methods in analysis to handle protocol non-adherence and any statistical methods to handle missing data {20c}
Patients who complete the protocol will be included in the per-protocol (PP) analysis; patients who participate in the randomization but do not complete the protocol will still be included in the intention-to-treat (ITT) analysis. We will strive to ensure data completeness, and for unavoidable missing data, multiple imputation methods will be employed during the data analysis.
Plans to give access to the full protocol, participant-level data and statistical code {31c}
Requests for access to the full protocol, participant-level data and statistical code will be considered by the corresponding author on a case-by-case basis upon reasonable request and conditions.
Oversight and monitoring
Composition of the coordinating centre and trial steering committee {5d}
The trial has a coordinating committee composed of the principal investigators (PIs), lead investigator, study coordinator, data manager and statistics expert. This team will ensure that the day-to-day operations of the trial run smoothly. The team will meet monthly to discuss the implementation and quality improvement of the interventions. No data monitoring committee is being considered, as this is a low-risk intervention.
The trial steering committee, including the PIs, lead investigators, senior clinicians and trial assistants, will be responsible for providing technical review and inputs to the design of the study protocol; reviewing semiannual progress reports; overseeing the data safety monitoring processes; sharing relevant information; observing the study implementation; and providing feedback and recommendations to the study team.
Composition of the data monitoring committee, its role and reporting structure {21a}
This trial has no independent data monitoring committee. The oversight of the trial is provided by the trial steering committee. This committee will be responsible for monitoring the progress of the trial, overseeing the data safety monitoring processes, and addressing any issues that may arise during the trial. The committee members will meet every 6 months to review the trial process and data concerns.
Adverse event reporting and harms {22}
All enrolled participants will be provided with the contact information of the research project manager on their copy of the informed consent form. They will also be asked to inform their dentists of any perceived adverse events. These adverse events or harms will be recorded and reported to the trial steering committee.
Frequency and plans for auditing trial conduct {23}
The trial steering group will play a central role in the daily coordination of the study. The implementation and quality improvement of the interventions will be discussed in monthly group meetings. The trial steering committee members will meet every 6 months to review the trial process and data concerns, whereas the ethics committee will meet yearly to review the conduct.
Plans for communicating important protocol amendments to relevant parties (e.g. trial participants, ethical committees) {25}
Any protocol amendment will be first communicated to the funder (Hospital of Stomatology, Sun Yat-sen University), and then a copy of the revised protocol will be sent to the PI to add to the Investigator Site File. Any deviations from the protocol will be fully documented via a breach report form. The amended protocol will also be submitted to the research ethics committee for approval before being implemented. The modification of the protocol will also be updated in the Chinese Clinical Trial Registry.
Patient public involvement
Neither the patients nor the public is involved in the design of this protocol.
Dissemination plans {31a}
The trial results will be published in a medical journal and presented at academic conferences. There will be no publication restrictions.
Discussion
VPT has garnered increasing attention in recent years because of its advantages in preserving vital pulp, maintaining the physiological function of dental pulp, and enhancing the long-term survival rate of teeth. It is considered a viable alternative to RCT when appropriate indications are met. However, the current classification of pulpitis is ambiguous, leading to unclear indications for VPT. According to the ESE statement, pulpitis can be classified as reversible, partially irreversible, or completely irreversible in the presence of deep or extensive caries. This classification aids in decision-making for VPT or RCT. Nevertheless, there is limited evidence on the efficacy of VPT and RCTs on the basis of the ESE classification of pulpitis. Additionally, new bioactive materials have been available for a short period and lack long-term efficacy evaluation.
To address this issue, we will conduct a prospective randomized controlled clinical trial to compare the efficacy of VPT using iRoot BP Plus as a capping material with that of RCT based on the ESE classification of pulpitis. This study will evaluate the treatment outcomes and related risk factors for VPT in cariously exposed permanent teeth, providing an evidence-based option for the precise and minimally invasive treatment of pulpitis in permanent teeth. Additionally, to our knowledge, this will be the first prospective clinical study assessing the efficacy of vital pulp therapy using a combination of the ESE and Wolter classifications for pulpitis classification. This study will validate a new standard for pulpitis diagnosis as a reliable predictor of treatment outcomes.
Trial status
The current protocol is version 3.0, dated 24 March 2021. Ethical approval for the trial was obtained from the Medical Ethics Committee of the Hospital of Stomatology, Sun Yat-sen University, on 7 October 2020 (KQEC-2020–43-01) and amended on 5 July 2021 (KQEC-2020–43-02). Recruitment began on 11 August 2021. The last patient follow-up is scheduled for 26 December 2024. We neglected the requirement to submit this manuscript before the first patient's enrolment, mistakenly believing that it should be submitted before the completion of the experiment, which led to our failure to submit this manuscript earlier.
Supplementary Information
Acknowledgements
Not applicable.
Abbreviations
- VPT
Vital pulp therapy
- RCT
Root canal treatment
- IPC
Indirect pulp capping
- DPC
Direct pulp capping
- PP
Partial pulpotomy
- FP
Full pulpotomy
- ESE
European Society of Endodontics
- AAE
American Association of Endodontists
- MTA
Mineral trioxide aggregate
Authors’ contributions {31b}
LHY and WX conceived this study and have overall responsibility for the trial design. LHY, WX, and ZQ contributed to the treatment design. ZQ and LHY drafted the trial protocol and are managing the staff training. CMC and ZSY are responsible for data management and statistical analysis. All authors provided critical reviews of the trial protocol and approved the final manuscript.
Funding {4}
This research is supported by grants from the Guangdong Financial Fund for High-Caliber Hospital Construction (174–2018-XMZC-0001–03–0125/D-08).
Data availability {29}
The datasets generated from this trial will be available from the corresponding author upon reasonable request.
Declarations
Ethics approval and consent to participate {24}
The study design, protocol, and informed consent procedures were approved by the Medical Ethics Committee of the Hospital of Stomatology, Sun Yat-sen University. Written informed consent will be obtained from all of the participants by delegated research members before any trial-related procedures, and they will be informed that they are free to withdraw from the study at any time.
Consent for publication {32}
Such consent is not applicable, as no identifying images or other personal or clinical details of the participants are presented here. All enrolled participants will be provided with a copy of the informed consent form. The participant information materials and informed consent forms will be available from the corresponding author upon request.
Competing interests {28}
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Xi Wei, Email: weixi@mail.sysu.edu.cn.
Hongyan Liu, Email: liuhyan@mail.sysu.edu.cn.
References
- 1.Friedman S, Mor C. The success of endodontic therapy–healing and functionality. J Calif Dent Assoc. 2004;32(6):493–503. [PubMed] [Google Scholar]
- 2.Moazami F, Sahebi S, Sobhnamayan F, Alipour A. Success rate of nonsurgical endodontic treatment of nonvital teeth with variable periradicular lesions. Iran Endod J. 2011;6(3):119–24. [PMC free article] [PubMed] [Google Scholar]
- 3.Asgary S, Shadman B, Ghalamkarpour Z, Shahravan A, Ghoddusi J, Bagherpour A, et al. Periapical status and quality of root canal fillings and coronal restorations in iranian population. Iran Endod J. 2010;5(2):74–82. [PMC free article] [PubMed] [Google Scholar]
- 4.Ng YL, Mann V, Gulabivala K. Tooth survival following non-surgical root canal treatment: a systematic review of the literature. Int Endod J. 2010;43(3):171–89. [DOI] [PubMed] [Google Scholar]
- 5.Silva AA, Belladonna FG, Rover G, Lopes RT, Moreira EJL, De-Deus G, et al. Does ultraconservative access affect the efficacy of root canal treatment and the fracture resistance of two-rooted maxillary premolars? Int Endod J. 2020;53(2):265–75. [DOI] [PubMed] [Google Scholar]
- 6.Taha NA, Abuzaid AM, Khader YS. A randomized controlled clinical trial of pulpotomy versus root canal therapy in mature teeth with irreversible pulpitis: outcome, quality of life, and patients’ satisfaction. J Endod. 2023;49(6):624-631.e2. [DOI] [PubMed] [Google Scholar]
- 7.Passarelli PC, Pagnoni S, Piccirillo GB, Desantis V, Benegiamo M, Liguori A, et al. Reasons for tooth extractions and related risk factors in adult patients: a cohort study. Int J Environ Res Public Health. 2020;17(7):2575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.European Society of Endodontology (ESE) developed by:, Duncan HF, Galler KM, Tomson PL, Simon S, El-Karim I, et al. European Society of Endodontology position statement: Management of deep caries and the exposed pulp. Int Endod J. 2019;52(7):923–34 [DOI] [PubMed]
- 9.Duncan HF. Present status and future directions-Vital pulp treatment and pulp preservation strategies. Int Endod J. 2022;55 Suppl 3(Suppl 3):497–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Iaculli F, Rodríguez-Lozano FJ, Briseño-Marroquín B, Wolf TG, Spagnuolo G, Rengo S. Vital pulp therapy of permanent teeth with reversible or irreversible pulpitis: an overview of the literature. J Clin Med. 2022;11(14):4016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lin GSS, Hisham ARB, Cher CY, Cheah KK, Ghani NRNA, Noorani TY. Success rates of coronal and partial pulpotomies in mature permanent molars: a systematic review and single-arm meta-analysis. Quintessence Int Berl Ger 1985. 2021;52(3):196–208. [DOI] [PubMed] [Google Scholar]
- 12.Sharma R, Kumar V, Logani A, Chawla A, Mir RA, Sharma S, et al. Association between concentration of active MMP-9 in pulpal blood and pulpotomy outcome in permanent mature teeth with irreversible pulpitis - a preliminary study. Int Endod J. 2021;54(4):479–89. [DOI] [PubMed] [Google Scholar]
- 13.Cushley S, Duncan HF, Lappin MJ, Tomson PL, Lundy FT, Cooper P, et al. Pulpotomy for mature carious teeth with symptoms of irreversible pulpitis: a systematic review. J Dent. 2019;88:103158. [DOI] [PubMed] [Google Scholar]
- 14.Alqaderi H, Lee CT, Borzangy S, Pagonis TC. Coronal pulpotomy for cariously exposed permanent posterior teeth with closed apices: a systematic review and meta-analysis. J Dent. 2016;44:1–7. [DOI] [PubMed] [Google Scholar]
- 15.American Association of Endodontists. AAE position statement on vital pulp therapy. J Endod. 2021;47(9):1340–4. [DOI] [PubMed] [Google Scholar]
- 16.Schwendicke F, Stolpe M. Direct pulp capping after a carious exposure versus root canal treatment: a cost-effectiveness analysis. J Endod. 2014;40(11):1764–70. [DOI] [PubMed] [Google Scholar]
- 17.Naved N, Umer F, Khowaja AR. Irreversible pulpitis in mature permanent teeth: a cost-effectiveness analysis of pulpotomy versus root canal treatment. BMC Oral Health. 2024;24(1):285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rao Q, Kuang J, Mao C, Dai J, Hu L, Lei Z, et al. Comparison of iRoot BP plus and calcium hydroxide as pulpotomy materials in permanent incisors with complicated crown fractures: a retrospective study. J Endod. 2020;46(3):352–7. [DOI] [PubMed] [Google Scholar]
- 19.Awawdeh L, Al-Qudah A, Hamouri H, Chakra RJ. Outcomes of vital pulp therapy using mineral trioxide aggregate or biodentine: a prospective randomized clinical trial. J Endod. 2018;44(11):1603–9. [DOI] [PubMed] [Google Scholar]
- 20.Zhu L, Yang J, Zhang J, Lei D, Xiao L, Cheng X, et al. In vitro and in vivo evaluation of a nanoparticulate bioceramic paste for dental pulp repair. Acta Biomater. 2014;10(12):5156–68. [DOI] [PubMed] [Google Scholar]
- 21.De-Deus G, Canabarro A, Alves GG, Marins JR, Linhares ABR, Granjeiro JM. Cytocompatibility of the ready-to-use bioceramic putty repair cement iRoot BP Plus with primary human osteoblasts. Int Endod J. 2012;45(6):508–13. [DOI] [PubMed] [Google Scholar]
- 22.Rifaey HS, Villa M, Zhu Q, Wang YH, Safavi K, Chen IP. Comparison of the osteogenic potential of mineral trioxide aggregate and endosequence root repair material in a 3-dimensional culture system. J Endod. 2016;42(5):760–5. [DOI] [PubMed] [Google Scholar]
- 23.Liu S, Wang S, Dong Y. Evaluation of a bioceramic as a pulp capping agent in vitro and in vivo. J Endod. 2015;41(5):652–7. [DOI] [PubMed] [Google Scholar]
- 24.Tian J, Zhang Y, Lai Z, Li M, Huang Y, Jiang H, et al. Ion release, microstructural, and biological properties of iRoot BP Plus and ProRoot MTA exposed to an acidic environment. J Endod. 2017;43(1):163–8. [DOI] [PubMed] [Google Scholar]
- 25.Giraud T, Jeanneau C, Bergmann M, Laurent P, About I. Tricalcium silicate capping materials modulate pulp healing and inflammatory activity in vitro. J Endod. 2018;44(11):1686–91. [DOI] [PubMed] [Google Scholar]
- 26.Han S, Zhang Q. Vital pulp therapy following pulpotomy in immature permanent teeth with carious exposure. J Clin Pediatr Dent. 2023;47(5):65–72. [DOI] [PubMed] [Google Scholar]
- 27.Sheng M, Zhang D, Yan J, Li W. Relationship between time to hemostasis and outcomes of pulpotomy using iRoot BP Plus in symptomatic young permanent teeth: a prospective study. J Clin Pediatr Dent. 2023;47(6):142–9. [DOI] [PubMed] [Google Scholar]
- 28.Liu SY, Gong WY, Liu MQ, Long YZ, Dong YM. Clinical efficacy observation of direct pulp capping using iRoot BP Plus therapy in mature permanent teeth with carious pulp exposure. Zhonghua Kou Qiang Yi Xue Za Zhi Zhonghua Kouqiang Yixue Zazhi Chin J Stomatol. 2020;55(12):945–51. [DOI] [PubMed] [Google Scholar]
- 29.Guan X, Zhou Y, Yang Q, Zhu T, Chen X, Deng S, et al. Vital pulp therapy in permanent teeth with irreversible pulpitis caused by caries: a prospective cohort study. J Pers Med. 2021;11(11):1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Patro S, Meto A, Mohanty A, Chopra V, Miglani S, Das A, et al. Diagnostic accuracy of pulp vitality tests and pulp sensibility tests for assessing pulpal health in permanent teeth: a systematic review and meta-analysis. Int J Environ Res Public Health. 2022;19(15):9599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ramani A, Sangwan P, Tewari S, Duhan J, Mittal S, Kumar V. Comparative evaluation of complete and partial pulpotomy in mature permanent teeth with symptomatic irreversible pulpitis: a randomized clinical trial. Int Endod J. 2022;55(5):430–40. [DOI] [PubMed] [Google Scholar]
- 32.Jassal A, Nawal RR, Yadav S, Talwar S, Yadav S, Duncan HF. Outcome of partial and full pulpotomy in cariously exposed mature molars with symptoms indicative of irreversible pulpitis: a randomized controlled trial. Int Endod J. 2023;56(3):331–44. [DOI] [PubMed] [Google Scholar]
- 33.Ricucci D, Loghin S, Siqueira JF. Correlation between clinical and histologic pulp diagnoses. J Endod. 2014;40(12):1932–9. [DOI] [PubMed] [Google Scholar]
- 34.Wolters WJ, Duncan HF, Tomson PL, Karim IE, McKenna G, Dorri M, et al. Minimally invasive endodontics: a new diagnostic system for assessing pulpitis and subsequent treatment needs. Int Endod J. 2017;50(9):825–9. [DOI] [PubMed] [Google Scholar]
- 35.Asgary S, Eghbal MJ, Shahravan A, Saberi E, Baghban AA, Parhizkar A. Outcomes of root canal therapy or full pulpotomy using two endodontic biomaterials in mature permanent teeth: a randomized controlled trial. Clin Oral Investig. 2022;26(3):3287–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Koli B, Chawla A, Logani A, Kumar V, Sharma S. Combination of nonsurgical endodontic and vital pulp therapy for management of mature permanent mandibular molar teeth with symptomatic irreversible pulpitis and apical periodontitis. J Endod. 2021;47(3):374–81. [DOI] [PubMed] [Google Scholar]
- 37.Galani M, Tewari S, Sangwan P, Mittal S, Kumar V, Duhan J. Comparative evaluation of postoperative pain and success rate after pulpotomy and root canal treatment in cariously exposed mature permanent molars: a randomized controlled trial. J Endod. 2017;43(12):1953–62. [DOI] [PubMed] [Google Scholar]
- 38.Asgary S, Eghbal MJ, Fazlyab M, Baghban AA, Ghoddusi J. Five-year results of vital pulp therapy in permanent molars with irreversible pulpitis: a non-inferiority multicenter randomized clinical trial. Clin Oral Investig. 2015;19(2):335–41. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated from this trial will be available from the corresponding author upon reasonable request.