Abstract
Numerous clinical trials for myasthenia gravis (MG) treatment have been conducted recently, with satisfactory cognitive and clinical results. However, due to the limited evidence for direct comparison of the safety and effectiveness of various drugs, there is a need for further exploration of the advantages and disadvantages of different monoclonal antibodies and immunosuppressants. Thus, in the present network meta-analysis (NMA), we aimed to compare the efficacy and safety of immunosuppressants and monoclonal antibodies in treating MG. We systematically searched for randomized controlled trials published in PubMed, Embase, Web of Science, and the Cochrane Library between January 1, 2000 and March 6, 2024. Statistical analyses were performed using R software (version 4.2.3), JAGS, and STATA (version 15.0). The surface under the cumulative ranking curve (SUCRA) value was calculated to assess the potential efficacy of each drug and the likelihood of adverse events (AEs), with higher SUCRA values indicating better efficacy or a lower likelihood of AEs. This NMA included 21 randomized controlled trials involving 13 drugs and 1,657 patients. Based on changes in Quantitative MG and MG Composite scores, batoclimab was most likely to exert the best therapeutic effects, with SUCRA values of 99% and 92%, respectively. Rozanolixzumab performed better than the other drugs in terms of the MG Activities of Daily Living score (85%). Eculizumab exhibited the highest potential in reducing the 15-item revised version of the MG Quality of Life score (96%). Regarding safety, belimumab had the highest SUCRA value (85%), demonstrating the lowest likelihood of AEs. In conclusion, all immunosuppressants and monoclonal antibodies analyzed in this study were more effective than the placebo in treating MG, with rozanolixzumab and batoclimab potentially being the most effective. Regarding safety, rozanolixzumab exhibited a higher likelihood of AEs than did placebo. The conclusions guide the clinical selection of effective drugs and offer insights for future drug experiments.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12967-024-05751-1.
Keywords: Generalized myasthenia gravis, Monoclonal antibody, Immunosuppressants, FcRn inhibitor, Complement inhibitor, Meta-analysis
Introduction
Myasthenia gravis (MG) is a relatively rare autoimmune disease of the nervous system that is mainly mediated by B cells and damages the prominent posterior membrane of the neuromuscular junction [1]. As an autoimmune disease, its primary pathogenic antibodies include antibodies against the nicotinic acetylcholine receptor (AChR; detected in 80% of patients) and muscle-specific kinase (MuSK) (detected in approximately 6% of patients) and anti-lipoprotein receptor-related protein 4 (occurs more rarely) [2–4]. The primary clinical symptoms of MG include fluctuating fatigue and weakness of the extraocular, pharyngeal, laryngeal, trunk, and limb muscles, and the respiratory muscles can be seriously involved. The incidence of GM is approximately 0.15–61.33 per million person-years; however, in recent years, the incidence of MG has increased significantly [5, 6].
Currently, treatment for MG mainly includes cholinesterase inhibitors and conventional immunosuppressants (glucocorticoids and nonsteroidal immunosuppressive agents) [7, 8]. The condition of many patients has been controlled using these drugs; however, poor curative effects are still observed in some patients, and approximately 20% of patients with MG do not respond to conventional immunosuppressive treatment [9]. In addition, hormones and immunosuppressive agents have significant side effects, such as diabetes, osteoporosis, hypertension, obesity, and skin lesions, making some patients unable to adhere to immunosuppressive treatments [10, 11]. Therefore, new drugs with stronger targeting, higher safety, and better efficacy, particularly monoclonal antibody drugs, have been developed recently.
According to their mechanism of action, new immune drugs for MG can be divided into the following three categories: neonatal Fc receptor inhibitors (FcRn), complex inhibitors, and B-cell therapies [12, 13]. After 2017, the US Food and Drug Administration (FDA) successively approved eculizumab, ravulizumab, efgartigimod, and rozanolixizumab as treatments for patients with MG [14–17]. In October 2023, the FDA approved zilucoplan as a treatment for adult generalized MG. However, owing to the limited evidence for the direct comparison of the safety and effectiveness of various drugs, the advantages and disadvantages of different monoclonal antibodies and immunosuppressants need to be further explored. Through a Bayesian network meta-analysis (NMA), the effectiveness and safety of different drugs can be compared, and their effects can be ranked by collecting direct or indirect comparison evidence [18]. Therefore, in the present study, we conducted an NMA of all relevant immunotherapy methods and comprehensively compared and ranked the strategies for MG treatment.
Methods
Search strategy
The study protocol was prospectively registered in the International Prospective Register for Systematic Reviews (CRD42024519160). This NMA complies with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement [19].
Two reviewers independently performed a comprehensive literature search of PubMed, Embase, Cochrane Library, and Web of Science. The search covered all studies published between January 1, 2000 and March 6, 2024. Discrepancies between the two reviewers were resolved through discussion or arbitration by a third reviewer (Shu-Yan Cong).
All studies were double-blind, randomized, placebo-controlled trials on the efficacy of MG treatment and were published in English. To accurately search for the required studies, we searched for a combination of terms in Medical Subject Headings and general terms.
The included studies were those related to immunosuppressants and monoclonal antibodies and reported information about the treatment effects. Details of the search strategy and extraction of specific literature are presented in Supplementary Table S1.
Inclusion criteria
We included all MG-related immunosuppressants and monoclonal antibodies that underwent randomized controlled trials (RCTs) after 2000. Participants in the included studies were adult patients diagnosed with generalized MG who met the Myasthenia Gravis Foundation of America (MGFA) class II–V clinical classification at the time of screening. The patients were treated with immunosuppressants or monoclonal antibodies, and different doses of the same drug were administered to the same intervention group. The control group included in the experiment received a placebo treatment. At least one of the following four scores was the most important outcome in the included studies: MG Activities of Daily Living (MG-ADL), Quantitative MG (QMG), MG Composite (MGC), and 15-item revised version of the MG Quality of Life (MG-QoL 15r) scores. The included studies reported adverse events (AEs) and severe AEs (SAEs) as adverse reactions.
Data extraction and outcome measures
We collected the following data: [1] the last name of the first author and the year of publication; [2] study phase, sample size, patient sex, patient age, intervention measures, disease duration, history of thymectomy, duration and dose of medication, serotype (AB AChR-positive and anti-MuSK antibody-positive and seronegative), and MGFA category; [3] outcome data for efficacy: MG-ADL, QMG, MGC, and MG-QOL 15r scores (mean, standard deviation [SD]); and [4] outcome data for safety: drug-related AEs and SAEs during the follow-up period. Data were extracted by two reviewers (Yue Qiao and Jian Gu) using a standardized data extraction table, and another reviewer (Shu-Yan Cong) checked the data. Any conflicting observations were discussed, and a consensus was reached.
Quality assessment and risk of bias
In this NMA, we strictly adhered to the predefined inclusion and exclusion criteria and only incorporated RCTs. To assess the risk of bias in the included studies, we used the Cochrane Collaboration’s risk of bias assessment tool [20], which covers six key domains: random sequence generation (selection bias), allocation concealment (selection bias), blinding of participants and personnel (performance bias), blinding of outcome assessment (detection bias), incomplete outcome data (attrition bias), and selective reporting (reporting bias) and other biases. The risk of bias in each study was evaluated by two independent reviewers (Yue Qiao and Jian Gu), who rated each domain as having “low risk,” “high risk,” or “unclear risk” according to the guidelines provided by the Cochrane tool. Any disagreements during the assessment were resolved through discussion or consultation with a third reviewer (Shu-Yan Cong).
Statistical analysis
The Bayesian NMA was performed using R statistical software (Version 4.2.3) [21] and STATA software (version 15.0). The primary measures of effect were the odds ratio (OR) and mean difference (MD) for dichotomous and continuous outcomes, respectively, both with corresponding 95% credible intervals (CrIs). If there were no raw data (for example, no SDs, only P-values or ranges were reported), the SDs and 95% CIs indicated in the publication were calculated using established methods for estimation [22]. When significant heterogeneity is detected (I² ≥ 50%), a random-effects model is applied to account for variability across studies. In contrast, when heterogeneity is low (I² < 50%) indicating greater consistency among studies, a fixed-effects model is used.Network comparisons of various interventions are illustrated in network maps, where each node represents an intervention, and the thickness of the connecting lines indicates the number of trials comparing the two interventions. The size of each node indicates the number of cases to which the intervention was applied. Global inconsistency was evaluated by comparing the Deviance Information Criterion (DIC) between the random- and fixed-effects models. A DIC difference of < 11 suggests superior global consistency [23]. Given that all comparisons involved monoclonal antibodies or immunosuppressive agents versus placebo or varying drug dosages, without direct comparisons between different monoclonal antibodies or immunosuppressive agents, and closed loops were absent in the network plots, consistency was not assessed using the node-splitting method [24]. The surface under the cumulative ranking curve (SUCRA) value was calculated to rank the interventions according to their efficacy and safety, with larger SUCRA values indicating superior performance [25]. For the outcomes of each trial, potential publication bias was assessed by visually inspecting the symmetry of the funnel plot.
Results
Study characteristics
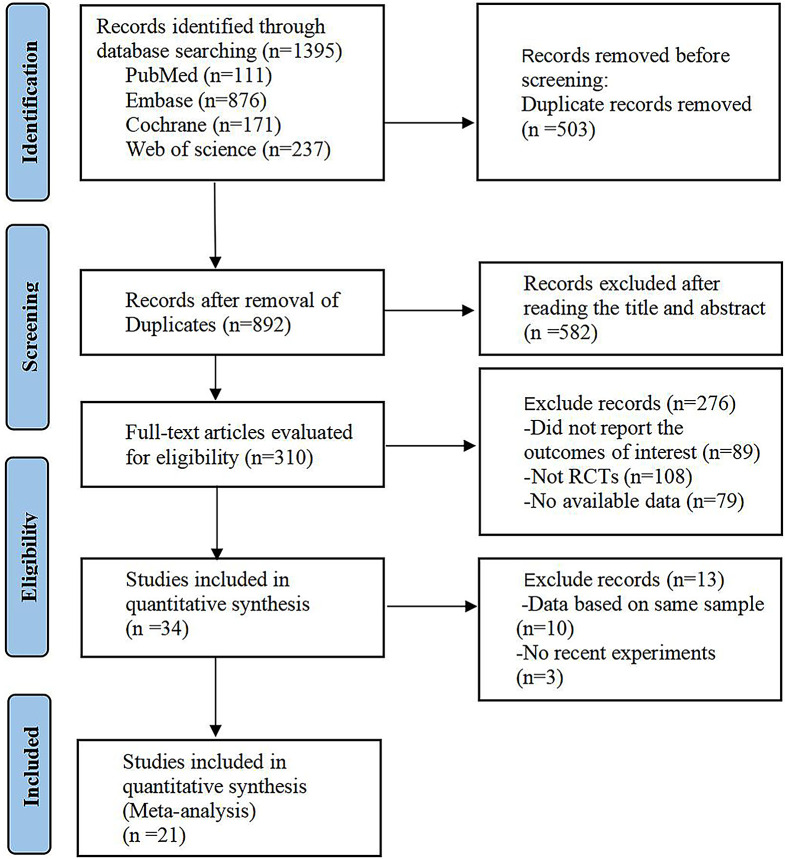
After searching various databases, 1395 studies were identified. Two reviewers read the titles and abstracts and excluded duplicate studies, animal experiments, non-RCTs, and studies with outcome times and designs that did not match those of the required studies. Finally, 20 studies were included in the present NMA [26–46]. Three trials included three intervention groups, whereas the remaining included two. A flowchart of the search process is shown in Fig. 1. In total, 1657 patients diagnosed with generalized MG were enrolled. Thirteen types of immunosuppressants or monoclonal antibodies were summarized: batoclimab, efgartigimod, nipocalimab, rozanolixizumab, ravulizumab, eculizumab, zilucoplan, iscalimab, belimumab, rituximab, tacrolimus, methotrexate, and mycophenolate mofetil. Treatment groups with different doses of the same drug were summarized. Patients’ baseline characteristics are shown in Table 1. The study duration ranged between 29 and 52 weeks. The number of participants ranged from 14 to 200. A total of 1216 patients provided a serum AChR antibody status, among which 1137 provided positive serum samples.
Fig. 1.
PRISMA flow diagram of the present network meta-analysis. PRISMA, Preferred Reporting Items for Systematic review and Meta-analysis
Table 1.
Trial features and baseline characteristics of participants for 21 trials included in the network meta-analysis
| Study | phase | Sample size | Gender(M/F) | Mean age(years) | intervention | Time since onset (y) | Thymectomy | Intervention periods | AChR+ | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|
|
Yan 2022(26) |
2 |
BAT680mg: 11 BAT340mg: 10 PLA:9 |
BAT680mg: 2/9 BAT340mg: 2/8 PLA:2/7 |
BAT680mg: 40.6 ± 16.8 BAT340mg: 36.4 ± 9.8 PLA:40.2 ± 9.3 |
BAT |
BAT680mg: 6.4 ± 5.7 BAT340mg: 9.8 ± 10.8 PLA:6.0 ± 6.8 |
BAT680mg: 3 BAT340mg: 3 PLA:2 |
43 days |
BAT680mg: 11 BAT340mg: 9 PLA:8 |
a.b.c.d.e.f. |
|
Yan 2024(45) |
3 |
BAT:67 PLA:64 |
BAT:27/40 PLA:16/48 |
BAT:43.8 ± 13.9 PLA:43.7 ± 13.5 |
BAT | NA |
BAT:23 PLA:14 |
6 weeks |
BAT:65 PLA:59 |
a.b.c.d.e. |
|
Hewett 2018(27) |
2 |
BEL: 18 PLA: 21 |
BEL: 8/10 PLA: 7/14 |
BEL: 52.7 ± 17.32 PLA: 59 ± 13.88 |
BEL |
BEL: 6.95 ± 9.03 PLA: 8.30 ± 8.06 |
BEL: 6 PLA: 7 |
24 weeks |
BEL: 18 PLA: 20 |
a.b.c.e.f. |
|
Howard 2017(28) |
3 |
ECU:62 PLA:63 |
ECU:21/41 PLA:22/41 |
ECU:47.5 ± 15.7 PLA:46.9 ± 18 |
ECU |
ECU:9.9 ± 8.1 PLA:9.2 ± 8.4 |
ECU:37 PLA:31 |
26 weeks | NA | a.b.c.d.f. |
|
Howard 2019(29) |
2 |
EFG:12 PLA:12 |
EFG:5/7 PLA:4/8 |
EFG:55.3 ± 13.6 PLA:43.5 ± 19.3 |
EFG |
EFG:8.2 ± 9 PLA:13.3 ± 11.2 |
NA | 78 days | NA | a.b.c.d.e.f. |
|
Howard 2021(30) |
3 |
EFG:84 PLA:83 |
EFG:21/63 PLA:28/55 |
EFG:45.9 ± 14.4 PLA:48.2 ± 15 |
EFG |
EFG:10.1 ± 9 PLA:8.8 ± 7.6 |
EFG:59 PLA:36 |
8 weeks |
EFG:65 PLA:64 |
a.b.c.d.e.f. |
|
GomezMancilla 2024(31) |
2 |
ISC:22 PLA:22 |
ISC:10/12 PLA:6/16 |
ISC:44.7 ± 13.5 PLA:43.3 ± 13.9 |
ISC |
ISC:8.2 ± 6.96 PLA:8.4 ± 8.47 |
NA | 25 weeks |
ISC:22 PLA:22 |
a.b.c.d.e.f. |
|
Antozzi 2024(32) |
2 |
NIP:54 PLA:14 |
NIP:25/29 PLA:6/8 |
NIP:57.5 ± 14.8 PLA:60.5 ± 14.5 |
NIP |
NIP:7.3 ± 7.31 PLA:13.2 ± 9.81 |
NA | 57 days |
NIP:51 PLA:3 |
a.b.d.e.f. |
|
Tuan 2022(33) |
3 |
RAV:86 PLA:89 |
RAV:42/44 PLA:44/45 |
RAV:58.0 ± 13.8 PLA:53.3 ± 16.1 |
RAV |
RAV:9.8 ± 9.7 PLA:10.0 ± 8.9 |
NA | 26 weeks |
RAV:86 PLA:89 |
a.b.d.e.f. |
|
Bril 2021(34) |
2 |
ROZ:21 PLA:22 |
ROZ:8/13 PLA:8/14 |
ROZ:50.5 ± 14.7 PLA:53 ± 15.7 |
ROZ | NA |
ROZ:11 PLA:10 |
29 days |
ROZ:19 PLA:21 |
a.b.c.e.f. |
|
Bril 2023(35) |
3 |
ROZ 7 mg:66 ROZ 10 mg:67 PLA:67 |
ROZ 7 mg:27/39 ROZ 10 mg:32/35 PLA:20/47 |
ROZ 7 mg:53.2 ± 14.7 ROZ 10 mg:51.9 ± 16.5 PLA:50.4 ± 17.7 |
ROZ | NA |
ROZ 7 mg:32 ROZ 10 mg:20 PLA:31 |
43 days |
ROZ 7 mg:60 ROZ 10 mg:60 PLA:59 |
a.b.c.e.f. |
|
Howard 2020(36) |
2 |
ZIL0.1 mg:15 ZIL0.3 mg: 14 PLA:15 |
ZIL:7/8 ZIL0.3 mg: 10/4 PLA:4/11 |
ZIL0.1 mg:45.5 ± 15.7 ZIL0.3 mg: 54.6 ± 15.5 PLA:48.4 ± 15.7 |
ZIL |
ZIL0.1 mg:6.5 ± 5.63 ZIL0.3 mg: 5.3 ± 6.38 PLA:6.3 ± 5.2 |
ZIL0.1 mg:8 ZIL0.3 mg: 7 PLA:5 |
12 weeks |
ZIL0.1 mg:15 ZIL0.3 mg: 14 PLA:15 |
a.b.c.d.e.f. |
|
Howard 2023(37) |
3 |
ZIL:86 PLA:88 |
ZIL:34/52 PLA:41/47 |
ZIL:52.6 ± 14.6 PLA:53.3 ± 15.7 |
ZIL |
ZIL:9.3 ± 9.5 PLA:9 ± 10.4 |
ZIL:45 PLA:37 |
12 weeks |
ZIL:86 PLA:88 |
a.b.c.d.e.f. |
|
Piehl 2022(38) |
3 |
RIT:25 PLA:22 |
RIT:18/7 PLA:15/7 |
RIT:67.4 ± 13.4 PLA:58 ± 18.6 |
RIT | NA | NA | 16 weeks |
RIT:23 PLA:22 |
a.b.d.e.f. |
|
Nowak 2022(39) |
2 |
RIT:25 PLA:27 |
RIT:14/11 PLA:15/12 |
RIT:53.2 ± 17.5 PLA:56.8 ± 17 |
RIT |
RIT:6.7 ± 6.5 PLA:4.4 ± 5.3 |
RIT:8 PLA:14 |
52 weeks |
RIT:25 PLA:27 |
a.b.c.d.e.f. |
|
Zhou 2017(41) |
3 |
TAC:44 PLA:38 |
TAC:16/28 PLA:20/18 |
TAC:41 ± 12.8 PLA:44 ± 12.1 |
TAC |
TAC:27.9 ± 37.8 M PLA:63.5 ± 90.2 M |
NA | 24 weeks | NA | a.b.e.f. |
|
Yoshikawa 2011(40) |
3 |
TAC:40 PLA:40 |
TAC:17/23 PLA:13/27 |
TAC:45.9 ± 11.5 PLA:44.4 ± 12.36 |
TAC |
TAC:7.41 ± 9 PLA:7.94 ± 9.54 |
TAC:28 PLA:30 |
28 weeks | NA | a.b.e.f. |
|
Pasnoor 2016(42) |
3 |
MTX:25 PLA:25 |
MTX:19/6 PLA:16/9 |
MTX:66.5 ± 13.85 PLA:68.6 ± 15.15 |
MTX | NA | NA | 52 weeks | NA | a.b.c.d.e. |
| Meriggioli 2003(44) | 2 |
MMF:7 PLA:7 |
MMF:2/5 PLA:2/5 |
MMF:57.7 ± 8.75 PLA:51.3 ± 12.75 |
MMF |
MMF:8.99 PLA:9.91 |
MMF:3 PLA:5 |
21 weeks |
MMF:5 PLA:6 |
b. |
| Sanders 2008a(43) | 3 |
MMF:88 PLA:88 |
MMF:42/46 PLA:40/48 |
MMF:49 ± 18 PLA:49.7 ± 18.4 |
MMF |
MMF:35.1 ± 30.8 PLA:41.1 ± 39.2 M |
MMF:23 PLA:25 |
36 weeks | NA | a.b.e.f. |
| Sanders 2008b(46) | 3 |
MMF:41 PLA:39 |
MMF:24/17 PLA:23/16 |
MMF:57.1 ± 18.8 PLA:55.3 ± 17.7 |
MMF |
MMF:2 ± 4.1 PLA:2 ± 4.4 |
NA | 12 weeks | NA | a.b.e. |
a. Myasthenia Gravis Activities of Daily Living (MG-ADL) score
b. Quantitative Myasthenia Gravis (QMG) score
c. Myasthenia Gravis Composite (MGC) score
d. 15-item revised version of the Myasthenia Gravis Quality of Life (MG-QoL 15r) score
e. adverse effects (AEs)
f. several adverse effects (SAEs)
ROZ, rozanolixzumab; BAT, batoclimab; EFG, efgartigimod; NIP, nipocalimab; ECU, eculizumab; ZIL, zilucoplan; RAV, ravulizumab; BEL, belimumab; RIT, rituximab; ISC, iscalimab; PLA, placebo; MMF, mycophenolate mofetil; TAC, Tacrolimus; MTX, Methotrexate; NA, not applicable; AChR, nicotinic acetylcholine receptor
NMA
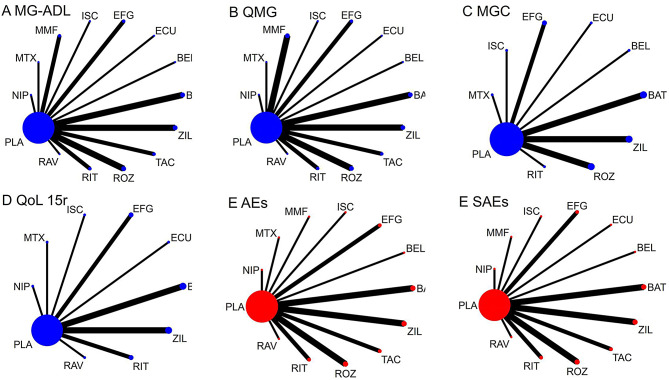
Figure 2 shows a network map of various immunosuppressants and monoclonal antibodies, focusing on their efficacy and safety outcomes. Each node represents a different intervention, with the node size indicating the participant count. The thickness of the connecting lines or edges between nodes indicates the number of trials comparing the two strategies.
Fig. 2.
Risk of bias graph and summary
MG-ADL score network
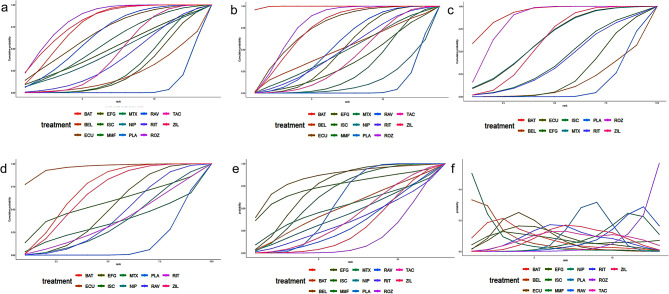
The NMA of MG-ADL included 20 studies involving 13 drugs, in which different dosages of the same drug were not considered as separate treatment methods. Batoclimab (MD: -2, 95% CrI: -2.7 to -1.3), eculizumab (MD: -1.8, 95% CrI: -3.2 to -0.42), methotrexate (MD: -1.5, 95% CrI: -2.9 to -0.029), ravulizumab (MD: -1.7, 95% CrI: -2.7 to -0.66), rozanolixzumab (MD: -2.2, 95% CrI: -3 to -1.4), tacrolimus (MD: -1.1, 95% CrI: -1.8 to -0.41), and zilucoplan (MD: -2.1, 95% CrI − 3.2 to -1.1) demonstrated superiority to the placebo. Notably, rozanolixzumab was superior to belimumab, efgartigimod, and mycophenolate mofetil (MDs ranging between 1.52 and 1.89). Zilucoplan demonstrated superiority to efgartigimod and mycophenolate mofetil (MDs ranging between 1.46 and 1.57). Batoclimab was superior to efgartigimod and mycophenolate mofetil (MDs ranging between − 1.43 and − 1.32). According to the SUCRA values, rozanolixzumab ranked first (85%), followed by zilucoplan (82%) and batoclimab (78%) (Table 2). Belimumab (21%), efgartigimod (25%), and placebo (7%) were the least effective therapies. The detailed results are presented in Supplementary Table S2. Cumulative probability analysis showed that rozanolixizumab was associated with the greatest benefit in terms of MG-ADL, as shown in Fig. 3A.
Table 2.
The SUCRA values for each treatment
| Treatment | AEs | Rank | SAEs | Rank | MG-ADL | Rank | QMG | Rank | MGC | Rank | MG-QoL 15r | Rank |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Batoclimab | 49.86% | 7 | 72.27% | 3 | 78.02% | 3 | 99.66% | 1 | 92.48% | 1 | 72.04% | 2 |
| Efgartigimod | 73.25% | 3 | 61.25% | 5 | 25.49% | 12 | 48.68% | 7 | 24.96% | 8 | 49.45% | 5 |
| Nipocalimab | 42.62% | 8 | 89.08% | 1 | 47.03% | 8 | 13.18% | 13 | - | - | 35.30% | 8 |
| Rozanolixizumab | 19.99% | 13 | 6.52% | 13 | 84.53% | 1 | 79.34% | 2 | 85.40% | 2 | - | - |
| Ravulizumab | 32.32% | 11 | 16.91% | 12 | 66.43% | 5 | 54.43% | 5 | - | - | 41.25% | 6 |
| Eculizumab | - | - | 70.93% | 4 | 68.95% | 4 | 74.84% | 3 | 64.02% | 3 | 96.66% | 1 |
| Zilucoplan | 28.97% | 12 | 42.62% | 9 | 81.61% | 2 | 74.55% | 4 | 61.40% | 5 | 66.98% | 3 |
| Iscalimab | 74.64% | 2 | 54.99% | 6 | 50.87% | 7 | 34.89% | 11 | 41.33% | 6 | 56.20% | 4 |
| Belimumab | 84.85% | 1 | 85.30% | 2 | 21.03% | 13 | 49.84% | 6 | 15.34% | 9 | - | - |
| Rituximab | 35.30% | 10 | 49.32% | 7 | 38.86% | 10 | 48.60% | 8 | 39.57% | 7 | 36.49% | 7 |
| Tacrolimus | 39.49% | 9 | 42.86% | 8 | 44.60% | 9 | 39.53% | 10 | - | - | - | - |
| Placebo | 55.23% | 5 | 37.05% | 10 | 6.65% | 14 | 7.41% | 14 | 11.53% | 10 | 11.78% | 10 |
| Methotrexate | 50.75% | 6 | - | - | 57.11% | 6 | 46.96% | 9 | 63.96% | 4 | 34.86% | 9 |
| Mycophenolate Mofetil | 65.74% | 4 | 20.92% | 11 | 28.82% | 11 | 28.09% | 12 | - | - | - | - |
AEs, adverse events; SAEs, severe adverse events; MG-ADL, Myasthenia Gravis Activities of Daily Living; QMG, Quantitative Myasthenia Gravis; MGC, Myasthenia Gravis
Composite; MG-QoL 15r,15-item revised version of the Myasthenia Gravis Quality of Life
Fig. 3.
Network graphs of randomized controlled trials comparing the efficacy and safety of mAbs and immunosuppressants for treating myasthenia gravis: (A) MG-ADL, (B) QMG, (C) MGC, (D) MG-QoL 15r, (E) AEs, and (F) SAEs. mAbs, monoclonal antibodies; MG-ADL, Myasthenia Gravis Activities of Daily Living; QMG, Quantitative Myasthenia Gravis; MGC, Myasthenia Gravis Composite; MG-QoL 15r, 15-item revised version of the Myasthenia Gravis Quality of Life; AEs, adverse events; SAEs, severe adverse events
QMG score network
The NMA of QMG included 21 studies involving 13 drugs, in which different dosages of the same drug were not considered as separate treatment methods.
Batoclimab (MD: -5.3, 95% CrI: -6.5 to -4.1), eculizumab (MD: -2.9, 95% CrI: -4.6 to -1.2), efgartigimod (MD: -1.8, 95% CrI: -2.9 to -0.69), ravulizumab (MD: -2, 95% CrI: -3.3 to -0.74), rozanolixzumab (MD: -3, 95% CrI: -3.9 to -2.1), tacrolimus (MD: -1.4, 95% CrI: -2.8 to -0.066), and zilucoplan (MD: -2.8, 95% CrI: -4.1 to -1.5) demonstrated superiority to the placebo. Notably, batoclimab demonstrated significant superiority to all the other treatments. Batoclimab had the highest SUCRA value (99%), followed by rozanolixzumab (79%) and eculizumab (75%) (Table 2). Rozanolixzumab demonstrated significant superiority to nipocalimab and mycophenolate mofetil. Zilucoplan demonstrated significant superiority to nipocalimab and mycophenolate mofetil. Eculizumab demonstrated significant superiority to nipocalimab. Nipocalimab was the least effective treatment according to the SUCRA value (13%), except for the placebo. The detailed results are presented in Supplementary Table S3. The cumulative probability analysis showed that batoclimab was associated with the greatest benefit in terms of QMG score, as shown in Fig. 3B.
MGC score network
The NMA of MGC included 13 studies involving nine drugs, in which different dosages of the same drug were not considered as separate treatment methods.
Batoclimab (MD: -5.1, 95% CrI: -6.8 to -3.4), eculizumab (MD: -3.3, 95% CrI: -6.1 to -0.52), methotrexate (MD: -3.3, 95% CrI: -6.2 to -0.41), rozanolixzumab (MD: -4.5, 95% CrI: -5.3 to -3.8), and zilucoplan (MD: -3.1, 95% CrI: -4.9 to -1.3) demonstrated superiority to the placebo. Rozanolixzumab demonstrated significant superiority to belimumab and efgartigimod. Batoclimab had the highest SUCRA value (92%), followed by rozanolixzumab (85%), eculizumab (64%), and methotrexate (64%). Batoclimab demonstrated significant superiority to efgartigimod and belimumab. Belimumab was the least effective treatment according to the SUCRA value (15%), except for the placebo (Table 2). The detailed results are presented in Supplementary Table S4. The cumulative probability analysis showed that batoclimab was associated with the greatest benefit in terms of MGC score, as shown in Fig. 3C.
MG-QoL 15r score network
The NMA of MG-QoL 15r included 13 studies involving nine drugs, in which different dosages of the same drug were not considered as separate treatment methods.
Batoclimab (MD: -3.4, 95% CrI: -4.9 to -1.8), eculizumab (MD: -7.1, 95% CrI: -12 to -2.7), efgartigimod (MD: -2.1, 95% CrI: -3.8 to -0.53), and zilucoplan (MD: -3.1, 95% CrI: -4.9 to -1.3) demonstrated superiority to the placebo. Eculizumab demonstrated significant superiority to efgartigimod, nipocalimab, rituximab, and ravulizumab. Eculizumab had the highest SUCRA value (96%), followed by batoclimab (72%) and zilucoplan (67%). Methotrexate was the least effective treatment according to the SUCRA value (35%), except for the placebo (Table 2). The detailed results are presented in Supplementary Table S5. The cumulative probability analysis showed that eculizumab was associated with the greatest benefit in terms of MG-QoL 15r score, as shown in Fig. 3D.
AE and SAE network
The NMA of AEs included 19 studies involving 12 drugs.
Rozanolixzumab was associated with a higher risk of AEs than did efgartigimod, mycophenolate mofetil, and the placebo. The results of the ranking according to SUCRA values showed that belimumab (85%) exhibited the highest safety, followed by iscalimab (75%), efgartigimod (73%), mycophenolate mofetil (66%), placebo (55%), methotrexate (51%), batoclimab (50%), nipocalimab (43%), tacrolimus (39%), rituximab (35%), ravulizumab (32%), zilucoplan (29%), and rozanolixzumab (17%) (Table 2).
The NMA of SAEs included 18 studies involving 12 drugs. Rozanolixzumab was associated with a higher risk of SAEs than did belimumab, eculizumab, batoclimab, nipocalimab, and the placebo. Additionally, ravulizumab was associated with a higher risk of SAEs than did belimumab, eculizumab, and nipocalimab. Mycophenolate mofetil was associated with a higher risk of SAEs than did belimumab, eculizumab, and nipocalimab. Nipocalimab had the highest SUCRA value (89%), whereas rozanolixzumab had the lowest SUCRA value (7%) (Table 2). The detailed results are presented in Supplementary Tables S6 and S7. The cumulative probability analysis showed that belimumab was associated with AEs, as shown in Fig. 3E, and that nipocalimab was associated with SAEs, as shown in Fig. 3F.
Risk of bias and heterogeneity
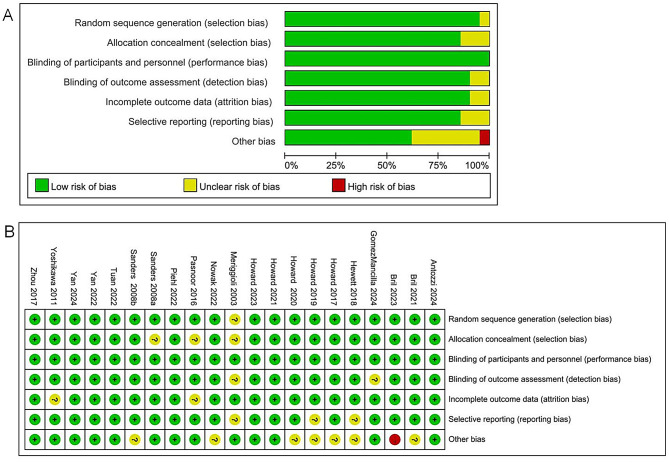
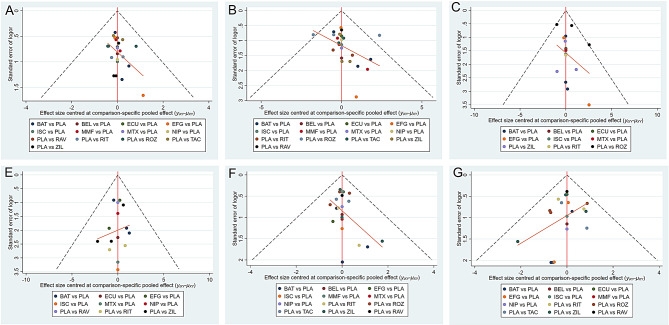
The outcomes of risk of bias are shown in Fig. 4, revealing that most included studies had a low risk of bias. Eight studies had one uncertain risk of bias, three had two, and one had four. These uncertainties in the risk levels were predominantly found in the category of other biases. In addition, one study had a high risk of bias in the category of other biases. Funnel plots were used to assess publication bias for the outcomes, as shown in Fig. 5. The funnel plot appeared visually symmetrical, indicating no potential publication bias, thereby further supporting the robustness of the study results. Global inconsistency was evaluated through the development of consistency and inconsistency models. The negligible discrepancies observed in the DIC and additional parameters between the fixed- and random-effects models suggested minimal inconsistency, underscoring the reliability and stability of our results (Supplementary Table S8). Furthermore, a heterogeneity analysis of multiple outcomes was performed. The findings showed that most comparisons exhibited low heterogeneity, except those in the studies of Bril et al. (Supplementary Figures S1–S6).
Fig. 4.
Ranking of the cumulative probabilities for basic parameters and adverse events: (A) MG-ADL, (B) QMG, (C) MGC, (D) MG-QoL 15r, (E) AEs, and (F) SAEs. MG-ADL, Myasthenia Gravis Activities of Daily Living; QMG, Quantitative Myasthenia Gravis; MGC, Myasthenia Gravis Composite; MG-QoL 15r, 15-item revised version of the Myasthenia Gravis Quality of Life; AEs, adverse events; SAEs, severe adverse events
Fig. 5.
Funnel plots for efficacy and safety outcomes: (A) MG-ADL, (B) QMG, (C) MGC, (D) MG-QoL 15r, (E) AEs, and (F) SAEs. MG-ADL, Myasthenia Gravis Activities of Daily Living; QMG, Quantitative Myasthenia Gravis; MGC, Myasthenia Gravis Composite; MG-QoL 15r, 15-item revised version of the Myasthenia Gravis Quality of Life; AEs, adverse events; SAEs, severe adverse events
Discussion
In total, 1657 participants from 21 studies were included in the present NMA to investigate the efficacy and safety of immunosuppressants and monoclonal antibodies as treatments for MG. The results of the pairwise meta-analyses showed that, compared with other interventions, batoclimab had a better effect on improving the QMG and MGC scores, with SUCRA values of 99% and 92%, respectively. According to the MG-ADL assessment, rozanolixzumab demonstrated the best performance in improving the MG symptoms. Eculizumab had a SUCRA value of 96% and performed better than the other drugs in improving the MG-QoL 15r score. Batoclimab, eculizumab, and zilucoplan were superior to the placebo in terms of all four efficacy scores. The risk of AEs due to immunosuppressants and monoclonal antibodies for treating MG was also investigated. Compared with the placebo, none of the drugs significantly increased the risk of AEs, except for rozanolixzumab.
To assess the efficacy of the drugs, we used the MG-ADL, QMG, MGC, and MG-QoL 15r scores. Notably, the MG-ADL and QMG scores were used in almost all studies, with the MG-ADL score frequently designated as the primary outcome measure. When the MG-ADL score was prioritized as the primary evaluation criterion, rozanolixizumab emerged as the top candidate based on the ranking probability, followed by zilucoplan and batoclimab. When drug efficacy was assessed using the QMG and MGC scores, batoclimab ranked first, with the highest probability ranking, whereas rozanolixizumab ranked second. An NMA published in 2023 on the efficacy and safety of monoclonal antibodies for treating MG suggested that rozanolixzumab had the highest probability ranking, followed by batoclimab and zilucoplan [47]. A recent meta-analysis [48] suggested that rozanolixizumab was more effective than the placebo when efficacy was assessed using the MG-ADL score, whereas batoclimab was more effective than the placebo when efficacy was evaluated using the QMG score. This finding was largely consistent with that of the present study; however, the ranking of batoclimab slightly decreased in the present study when efficacy was assessed using the MG-ADL score, possibly due to the inclusion of the most recent research findings related to batoclimab in our analysis [45]. The most recent Phase 3 study of batoclimab included more patients, thus reducing bias and making the results more credible. Rozanolixizumab is a humanized monoclonal antibody designed to treat MG [34]. Its therapeutic mechanism is based on the targeting and inhibition of FcRn. Rozanolixizumab blocks FcRn to induce the degradation of immunoglobulins (IgGs) [49], including the pathogenic autoantibodies responsible for MG. This degradation can decrease the immune system’s attack on the neuromuscular junction, thereby alleviating MG symptoms. Additionally, rozanolixzumab was the first and only drug approved by the FDA to treat adult patients with anti-AChR and anti-MuSK antibody-positive generalized MG. Recent clinical trials indicate that rozanolixizumab has therapeutic benefits that are not inferior to those of plasma exchange and intravenous immunoglobulin and that its administration through subcutaneous injection facilitates its dissemination. However, its safety and efficacy require further evaluation after its market launch. If the effectiveness of rozanolixizumab is validated in the real world, it may become the preferred medication for the treatment of MG in the future [50]. Previous NMAs have primarily confirmed the efficacy of rozanolixizumab [47], with fewer analyses of its safety, indicating that its AEs are not superior to those of other monoclonal antibodies. Recent meta-analyses have shown that rozanolixizumab has superior efficacy; however, the incidence of AEs was higher in the rozanolixizumab group than in the placebo group [48, 51]. The AEs associated with rozanolixizumab were mostly mild to moderate in severity. The most commonly reported AEs included headache, diarrhea, pyrexia, and nausea [34, 35]. The increased incidence of rozanolixizumab-related AEs was likely due to rozanolixizumab-induced mild to moderate headaches, which required no additional treatment [35]. Regarding safety, rozanolixizumab ranked last regarding the likelihood of AEs and SAEs in the present study. The forest plot showed that the safety of rozanolixizumab was significantly worse than that of the placebo, which is consistent with the findings of previous studies. A recent meta-analysis [48] has suggested that batoclimab is superior to the placebo in terms of QMG, MGC, and MG-QoL 15r scores. The present study indicated that batoclimab ranked first in the likelihood of improving the QMG and MGC scores, which is consistent with the findings of recent meta-analyses. However, an NMA by Chen et al. [47] suggested that batoclimab did not rank first in the likelihood of improving the QMG and MGC scores, which is inconsistent with the findings of the present study. The present NMA, which incorporated the results of the most recent clinical trials of batoclimab, had higher credibility. Based on the ranking for the likelihood of AEs, batoclimab was in the middle range, whereas it was ranked third based on the ranking for the likelihood of SAEs. This suggests that batoclimab has a relatively good safety profile, particularly regarding severe adverse reactions. Batoclimab treatment was associated with hypercholesterolemia. However, after administering batoclimab to patients, a rapid recovery and no related complications are observed [26]. An RCT of batoclimab showed that serum cholesterol levels increased and then decreased after discontinuing the drug [52]. Previous studies did not directly compare the safety of batoclimab with that of other FcRn inhibitors [53]. The present NMA validated the efficacy and safety of batoclimab. Although batoclimab can be recommended as an option to reduce the risk of adverse events associated with rozanolizumab, the final treatment decision should be based on the patient’s individual needs and specific clinical requirements. Eculizumab inhibits the cleavage of complement protein C5 into C5a and C5b, which are key components in the formation of the membrane attack complex that damages cells [54]. In cases of MG, the activation of the complement system contributes to damage at the neuromuscular junction, leading to muscle weakness [55]. By preventing the formation of C5a and C5b, eculizumab reduces complement-mediated damage at the neuromuscular junction, thereby improving muscle strength and reducing MG symptoms. Eculizumab was first approved for the treatment of MG by the United States FDA in 2017 [14], followed by approval in other regions, including the European Union. In the present NMA, eculizumab ranked high in terms of the likelihood of improving the MG-QoL 15r, MGC, and QMG scores, ranking first in terms of the MG-QoL 15r score. Studies on patients with generalized anti-AChR antibody-positive MG treated with eculizumab indicated that eculizumab demonstrated higher efficacy than that of rituximab [56]. However, owing to its specific targeting of the complement system and associated costs, its use may be reserved for specific patient populations or those with severe diseases not adequately controlled by other therapies.
In the present study, three immunosuppressants were included: methotrexate, mycophenolate mofetil, and tacrolimus. Among these, methotrexate ranked higher than the other two drugs in terms of both the MG-ADL and QMG scores. The other two immunosuppressants were not included in the comparison regarding the MGC and MG-QoL 15r scores. Methotrexate has been used to treat MG in some patients, particularly those who are not sensitive enough to other treatments or develop an adverse reaction [57]. The present study indicates that methotrexate has better efficacy than that of other immunosuppressants; however, its effectiveness is moderate compared with that of some monoclonal antibodies. A systematic review of the use of methotrexate for generalized MG showed that it is a potentially safe and effective alternative to azathioprine as a steroid-sparing agent, especially in developing countries where the high cost of azathioprine limits compliance [58]. This finding supports those of the present study.
In the present study, drug safety was assessed using two indicators: AEs and SAEs. When evaluated based on AEs, the top three drugs were belimumab, iscalimab, and efgartigimod, respectively, with rozanolixizumab ranking last. When assessed based on SAEs, the top three drugs in the probability ranking were nipocalimab, belimumab, and batoclimab, respectively. Belimumab targets and inhibits B lymphocyte stimulation, which plays a role in reducing abnormal B-cell activity in autoimmune conditions. A study evaluated the effectiveness of belimumab in treating MG and revealed no significant difference in improvement between belimumab and the placebo based on the primary endpoint, which was the change in the QMG score; however, it did not increase the risk of AEs [27]. This is consistent with the findings in the present study.
The present study is the most recent NMA to comprehensively compare common monoclonal antibodies and immunosuppressants for treating MG. This NMA included a wide range of drugs, incorporated high-quality RCTs, and included the most recent drug trial results [45]. Clinically, individualized treatment plans that balance efficacy and safety are essential for optimizing patient outcomes in the management of myasthenia gravis. However, the present study has some limitations. First, the number of included studies was limited, with few direct comparisons; therefore, indirect estimates were relied on rather than direct estimates. Second, we compared the overall drug outcomes without considering different doses, with significant variations in the follow-up period. Third, we included only published studies and excluded unpublished studies. Finally, some studies lacked data on the percentage of antibody-negative patients, changes in serum IgG levels, and antibody levels compared to the baseline values. Future research should focus on evaluating the long-term safety profiles and real-world effectiveness of these treatments.
Conclusion
The results of this study indicated that the included immunosuppressants and monoclonal antibodies were more effective than the placebo. Rozanolixizumab and batoclimab may be the most effective treatments for generalized MG. However, rozanolixizumab was associated with a higher likelihood of AEs and SAEs. Methotrexate may be superior to other immunosuppressants in terms of efficacy.The conclusions provide a strong reference for the clinical selection of more effective therapeutic drugs and also offer insights for the further development of related drug experiments. Owing to the lack of trials with direct comparisons and statistical uncertainty in SUCRA rankings, specific case analyses of clinical medications are needed to determine treatment plans.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Material 1: Table S1. Detailed search strategy for the four databases. Table S2. League table for MG-ADL. Table S3. League table for QMG. Table S4. League table for MGC. Table S5. League table for MG-QoL 15r. Table S6. League table for AEs. Table S7. League table for SAEs. Table S8. The results of consistency and inconsistency tests. Figure S1. Sensitivity analysis of MG-ADL. Figure S2. Sensitivity analysis of QMG. Figure S3. Sensitivity analysis of MGC. Figure S4. Sensitivity analysis of MG-QoL 15r. Figure S5. Sensitivity analysis of AEs. Figure S6. Sensitivity analysis of SAEs
Author contributions
All authors contributed to the conception and design of this study. JG: subject design and writing—original draft. YQ: investigation. SC: conceptualization, supervision, reviewing, and editing. RH: conceptualization, supervision, reviewing, and editing. All the authors contributed to the manuscript and approved the submitted version.
Funding
None.
Data availability
The original contributions presented in this study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Declarations
Conflict of interest
The authors declare no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Jian Gu, Email: jackgu0801@126.com.
Shuyan Cong, Email: congshuyan@hotmail.com.
References
- 1.Gilhus NE, Tzartos S, Evoli A, Palace J, Burns TM, Verschuuren J. Myasthenia gravis. Nat Rev Dis Primers. 2019;5(1):30. [DOI] [PubMed] [Google Scholar]
- 2.Gilhus NE, Verschuuren JJ. Myasthenia gravis: subgroup classification and therapeutic strategies. Lancet Neurol. 2015;14(10):1023–36. [DOI] [PubMed] [Google Scholar]
- 3.Lazaridis K, Tzartos SJ. Autoantibody specificities in Myasthenia gravis; implications for Improved Diagnostics and therapeutics. Front Immunol. 2020;11:212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Verschuuren JJ, Huijbers MG, Plomp JJ, Niks EH, Molenaar PC, Martinez-Martinez P, et al. Pathophysiology of myasthenia gravis with antibodies to the acetylcholine receptor, muscle-specific kinase and low-density lipoprotein receptor-related protein 4. Autoimmun Rev. 2013;12(9):918–23. [DOI] [PubMed] [Google Scholar]
- 5.Bubuioc AM, Kudebayeva A, Turuspekova S, Lisnic V, Leone MA. The epidemiology of myasthenia gravis. J Med Life. 2021;14(1):7–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Joensen P. Myasthenia gravis incidence in a general North Atlantic isolated population. Acta Neurol Scand. 2014;130(4):222–8. [DOI] [PubMed] [Google Scholar]
- 7.Sanders DB, Wolfe GI, Benatar M, Evoli A, Gilhus NE, Illa I, et al. International consensus guidance for management of myasthenia gravis: executive summary. Neurology. 2016;87(4):419–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Narayanaswami P, Sanders DB, Wolfe G, Benatar M, Cea G, Evoli A, et al. International Consensus Guidance for Management of Myasthenia gravis: 2020 update. Neurology. 2021;96(3):114–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang Q, Ge H, Gui M, Yang M, Bi Z, Ma X, et al. Polymorphisms in drug metabolism genes predict the risk of refractory myasthenia gravis. Ann Transl Med. 2022;10(21):1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Suh J, Goldstein JM, Nowak RJ. Clinical characteristics of refractory myasthenia gravis patients. Yale J Biol Med. 2013;86(2):255–60. [PMC free article] [PubMed] [Google Scholar]
- 11.Silvestri NJ, Wolfe GI. Treatment-refractory myasthenia gravis. J Clin Neuromuscul Dis. 2014;15(4):167–78. [DOI] [PubMed] [Google Scholar]
- 12.Menon D, Bril V. Pharmacotherapy of generalized myasthenia gravis with special emphasis on newer biologicals. Drugs. 2022;82(8):865–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zeng R, Glaubitz S, Schmidt J. Antibody therapies in Autoimmune Inflammatory myopathies: Promising Treatment options. Neurotherapeutics. 2022;19(3):911–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.ALEXION. Fda Approves Soliris® (Eculizumab) for the Treatment of Patients with Generalized Myasthenia Gravis (Gmg) 2017 [ https://myasthenia.org/Portals/0/Press%20Release%20Soliris%20FDA%20Approval.pdf
- 15.AstraZeneca. Ultomiris Approved in the Us for Adults with Generalised Myasthenia Gravis 2022 [ https://www.astrazeneca.com/media-centre/press-releases/2022/ultomiris-approved-in-the-us-for-adults-with-generalisedmyasthenia-gravis.html
- 16.U N, UCB Announces US. FDA Approval of Rystiggo[®] (RozanolixizumabNoli) for the Treatment of Adults with Generalized Myasthenia Gravis 2023 [ https://www.ucb.com/stories-media/Press-Releases/article/UCB-announces-USFDA-approval-of-RYSTIGGOR-rozanolixizumab-noli-for-the-treatment-of-adultswith-generalized-myasthenia-gravis
- 17.Argenx. Argenx Announces US. Food and Drug Administration Approval of Vyvgart Hytrulo (Efgartigimod Alfa and Hyaluronidase-Qvfc) Injection for Subcutaneous Use in Generalized Myasthenia Gravis 2023 [ https://www.globenewswire.com/news-release/2023/06/20/2691658/0/en/argenx-AnnouncesU-S-Food-and-Drug-Administration-Approval-of-VYVGART-Hytrulo-efgartigimodalfa-and-hyaluronidase-qvfc-Injection-for-Subcutaneous-Use-in-GeneralizedMyasthenia-Grav.html
- 18.Rouse B, Chaimani A, Li T. Network meta-analysis: an introduction for clinicians. Intern Emerg Med. 2017;12(1):103–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van Valkenhoef G, Lu G, de Brock B, Hillege H, Ades AE, Welton NJ. Automating network meta-analysis. Res Synth Methods. 2012;3(4):285–99. [DOI] [PubMed] [Google Scholar]
- 22.Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Spiegelhalter DJ, Best NG, Carlin BP, Van Der Linde A. Bayesian measures of Model Complexity and Fit. J Royal Stat Soc Ser B: Stat Methodol. 2002;64(4):583–639. [Google Scholar]
- 24.Chen K, Li H, Yang L, Jiang Y, Wang Q, Zhang J, et al. Comparative efficacy and safety of antidepressant therapy for the agitation of dementia: a systematic review and network meta-analysis. Front Aging Neurosci. 2023;15:1103039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shim S, Yoon BH, Shin IS, Bae JM. Network meta-analysis: application and practice using Stata. Epidemiol Health. 2017;39:e2017047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yan C, Duan RS, Yang H, Li HF, Zou Z, Zhang H, et al. Therapeutic effects of Batoclimab in Chinese patients with generalized Myasthenia gravis: a Double-Blinded, randomized, placebo-controlled phase II study. Neurol Ther. 2022;11(2):815–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hewett K, Sanders DB, Grove RA, Broderick CL, Rudo TJ, Bassiri A, et al. Randomized study of adjunctive belimumab in participants with generalized myasthenia gravis. Neurology. 2018;90(16):e1425–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Howard JF Jr., Utsugisawa K, Benatar M, Murai H, Barohn RJ, Illa I, et al. Safety and efficacy of eculizumab in anti-acetylcholine receptor antibody-positive refractory generalised myasthenia gravis (REGAIN): a phase 3, randomised, double-blind, placebo-controlled, multicentre study. Lancet Neurol. 2017;16(12):976–86. [DOI] [PubMed] [Google Scholar]
- 29.Howard JF Jr., Bril V, Burns TM, Mantegazza R, Bilinska M, Szczudlik A, et al. Randomized phase 2 study of FcRn antagonist efgartigimod in generalized myasthenia gravis. Neurology. 2019;92(23):e2661–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Howard JF Jr., Bril V, Vu T, Karam C, Peric S, Margania T, et al. Safety, efficacy, and tolerability of efgartigimod in patients with generalised myasthenia gravis (ADAPT): a multicentre, randomised, placebo-controlled, phase 3 trial. Lancet Neurol. 2021;20(7):526–36. [DOI] [PubMed] [Google Scholar]
- 31.GomezMancilla B, Meriggioli MN, Genge A, Roubenoff R, Espié P, Dupuy C, et al. Efficacy and safety of iscalimab, a novel anti-CD40 monoclonal antibody, in moderate-to-severe myasthenia gravis: a phase 2 randomized study. J Clin Neurosci. 2024;119:76–84. [DOI] [PubMed] [Google Scholar]
- 32.Antozzi C, Guptill J, Bril V, Gamez J, Meuth SG, Nowak RJ, et al. Safety and Efficacy of Nipocalimab in patients with generalized Myasthenia Gravis. Neurology. 2024;102(2):e207937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vu T, Meisel A, Mantegazza R, Annane D, Katsuno M, Aguzzi R, et al. Terminal complement inhibitor ravulizumab in generalized Myasthenia Gravis. NEJM Evid. 2022;1(5):EVIDoa2100066. [DOI] [PubMed] [Google Scholar]
- 34.Bril V, Benatar M, Andersen H, Vissing J, Brock M, Greve B, et al. Efficacy and safety of Rozanolixizumab in moderate to severe generalized Myasthenia gravis: a phase 2 Randomized Control Trial. Neurology. 2021;96(6):e853–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bril V, Drużdż A, Grosskreutz J, Habib AA, Mantegazza R, Sacconi S, et al. Safety and efficacy of rozanolixizumab in patients with generalised myasthenia gravis (MycarinG): a randomised, double-blind, placebo-controlled, adaptive phase 3 study. Lancet Neurol. 2023;22(5):383–94. [DOI] [PubMed] [Google Scholar]
- 36.Howard JF Jr., Nowak RJ, Wolfe GI, Freimer ML, Vu TH, Hinton JL, et al. Clinical effects of the self-administered subcutaneous complement inhibitor zilucoplan in patients with moderate to severe generalized Myasthenia gravis: results of a phase 2 Randomized, Double-Blind, Placebo-Controlled, Multicenter Clinical Trial. JAMA Neurol. 2020;77(5):582–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Howard JF Jr., Bresch S, Genge A, Hewamadduma C, Hinton J, Hussain Y, et al. Safety and efficacy of zilucoplan in patients with generalised myasthenia gravis (RAISE): a randomised, double-blind, placebo-controlled, phase 3 study. Lancet Neurol. 2023;22(5):395–406. [DOI] [PubMed] [Google Scholar]
- 38.Piehl F, Eriksson-Dufva A, Budzianowska A, Feresiadou A, Hansson W, Hietala MA, et al. Efficacy and safety of Rituximab for New-Onset generalized Myasthenia gravis: the RINOMAX Randomized Clinical Trial. JAMA Neurol. 2022;79(11):1105–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nowak RJ, Coffey CS, Goldstein JM, Dimachkie MM, Benatar M, Kissel JT, et al. Phase 2 trial of Rituximab in Acetylcholine receptor antibody-positive generalized Myasthenia gravis: the BeatMG Study. Neurology. 2022;98(4):e376–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yoshikawa H, Kiuchi T, Saida T, Takamori M. Randomised, double-blind, placebo-controlled study of tacrolimus in myasthenia gravis. J Neurol Neurosurg Psychiatry. 2011;82(9):970–7. [DOI] [PubMed] [Google Scholar]
- 41.Zhou L, Liu W, Li W, Li H, Zhang X, Shang H, et al. Tacrolimus in the treatment of myasthenia gravis in patients with an inadequate response to glucocorticoid therapy: randomized, double-blind, placebo-controlled study conducted in China. Ther Adv Neurol Disord. 2017;10(9):315–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pasnoor M, He J, Herbelin L, Burns TM, Nations S, Bril V, et al. A randomized controlled trial of methotrexate for patients with generalized myasthenia gravis. Neurology. 2016;87(1):57–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sanders DB, Hart IK, Mantegazza R, Shukla SS, Siddiqi ZA, De Baets MH, et al. An international, Phase III, randomized trial of mycophenolate mofetil in myasthenia gravis. Neurology. 2008;71(6):400–6. [DOI] [PubMed] [Google Scholar]
- 44.Meriggioli MN, Rowin J, Richman JG, Leurgans S. Mycophenolate mofetil for myasthenia gravis: a double-blind, placebo-controlled pilot study. Ann N Y Acad Sci. 2003;998:494–9. [DOI] [PubMed] [Google Scholar]
- 45.Yan C, Yue Y, Guan Y, Bu B, Ke Q, Duan R et al. Batoclimab vs Placebo for generalized Myasthenia gravis: a Randomized Clinical Trial. JAMA Neurol. 2024;81(4):336-345. [DOI] [PMC free article] [PubMed]
- 46.A trial of. Mycophenolate mofetil with prednisone as initial immunotherapy in myasthenia gravis. Neurology. 2008;71(6):394–9. [DOI] [PubMed] [Google Scholar]
- 47.Chen H, Qiu Y, Yin Z, Wang Z, Tang Y, Ni H, et al. Efficacy and safety of the innovative monoclonal antibodies in adults with generalized myasthenia gravis: a bayesian network analysis. Front Immunol. 2023;14:1280226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li J, Wu X, Chu T, Tan X, Wang S, Qu R et al. The efficacy and safety of FcRn inhibitors in patients with myasthenia gravis: a systematic review and meta-analysis. J Neurol. 2024;271(5):2298-2308. [DOI] [PubMed]
- 49.Flammer J, Neziraj T, Rüegg S, Pröbstel AK. Immune mechanisms in Epileptogenesis: update on diagnosis and treatment of Autoimmune Epilepsy syndromes. Drugs. 2023;83(2):135–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Matic A, Alfaidi N, Bril V. An evaluation of rozanolixizumab-noli for the treatment of anti-AChR and anti-MuSK antibody-positive generalized myasthenia gravis. Expert Opin Biol Ther. 2023;23(12):1163–71. [DOI] [PubMed] [Google Scholar]
- 51.Rissardo JP, Caprara ALF, Durante Í, Rauber A. Lithium-associated movement disorder: a literature review. Brain Circ. 2022;8(2):76–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kahaly GJ, Dolman PJ, Wolf J, Giers BC, Elflein HM, Jain AP, et al. Proof-of-concept and Randomized, Placebo-controlled trials of an FcRn inhibitor, Batoclimab, for thyroid Eye Disease. J Clin Endocrinol Metab. 2023;108(12):3122–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhu LN, Hou HM, Wang S, Zhang S, Wang GG, Guo ZY, et al. FcRn inhibitors: a novel option for the treatment of myasthenia gravis. Neural Regen Res. 2023;18(8):1637–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Patriquin CJ, Kuo KHM. Eculizumab and Beyond: the past, Present, and future of complement therapeutics. Transfus Med Rev. 2019;33(4):256–65. [DOI] [PubMed] [Google Scholar]
- 55.Conti-Fine BM, Milani M, Kaminski HJ. Myasthenia gravis: past, present, and future. J Clin Invest. 2006;116(11):2843–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nelke C, Schroeter CB, Stascheit F, Pawlitzki M, Regner-Nelke L, Huntemann N, et al. Eculizumab versus Rituximab in generalised myasthenia gravis. J Neurol Neurosurg Psychiatry. 2022;93(5):548–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rodolico C, Bonanno C, Brizzi T, Nicocia G, Trimarchi G, Lupica A, et al. Methotrexate as a steroid-sparing Agent in Myasthenia gravis: a preliminary retrospective study. J Clin Neuromuscul Dis. 2021;23(2):61–5. [DOI] [PubMed] [Google Scholar]
- 58.Prado MB Jr., Adiao KJB. Methotrexate in generalized myasthenia gravis: a systematic review. Acta Neurol Belg. 2023;123(5):1679–91. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material 1: Table S1. Detailed search strategy for the four databases. Table S2. League table for MG-ADL. Table S3. League table for QMG. Table S4. League table for MGC. Table S5. League table for MG-QoL 15r. Table S6. League table for AEs. Table S7. League table for SAEs. Table S8. The results of consistency and inconsistency tests. Figure S1. Sensitivity analysis of MG-ADL. Figure S2. Sensitivity analysis of QMG. Figure S3. Sensitivity analysis of MGC. Figure S4. Sensitivity analysis of MG-QoL 15r. Figure S5. Sensitivity analysis of AEs. Figure S6. Sensitivity analysis of SAEs
Data Availability Statement
The original contributions presented in this study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.