Abstract
Predicting how plants respond to drought requires an understanding of how physiological mechanisms and drought response strategies occur, as these strategies underlie rates of gas exchange and productivity. We assessed the response of 11 plant traits to repeated experimental droughts in four co-occurring species of central Australia. The main goals of this study were to: (i) compare the response to drought between species; (ii) evaluate whether plants acclimated to repeated drought; and (iii) examine the degree of recovery in leaf gas exchange after cessation of drought. Our four species of study were two tree species and two shrub species, which field studies have shown to occupy different ecohydrological niches. The two tree species (Eucalyptus camaldulensis Dehnh. and Corymbia opaca (D.J.Carr & S.G.M.Carr) K.D.Hill & L.A.S.Johnson) had large reductions in stomatal conductance (gs) values, declining by 90% in the second drought. By contrast, the shrub species (Acacia aptaneura Maslin & J.E.Reid and Hakea macrocarpa A.Cunn. ex R.Br.) had smaller reductions gs in the second drought of 52 and 65%, respectively. Only A. aptaneura showed a physiological acclimatation to drought due to small declines in gs versus ᴪpd (0.08 slope) during repeated droughts, meaning they maintained higher rates of gs compared with plants that only experienced one final drought (0.19 slope). All species in all treatments rapidly recovered leaf gas exchange and leaf mass per area following drought, displaying physiological plasticity to drought exposure. This research refines our understanding of plant physiological responses to recurrent water stress, which has implications for modelling of vegetation, carbon assimilation and water use in semi-arid environments under drought.
Keywords: Acacia, Australia, carbon flux, photosynthesis, stomatal conductance, water-use efficiency, water-use strategy
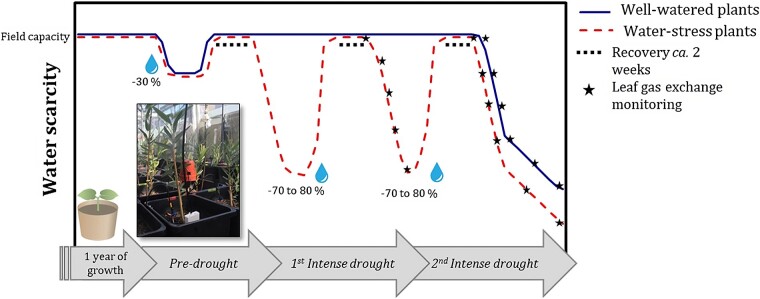
Graphical Abstract
Graphical Abstract.
Introduction
Drought is globally the most widespread climate extreme, with a large influence on the plant carbon cycle (Reichstein et al. 2014, Frank et al. 2015). The Southern Hemisphere experienced a reduction in net primary production (NPP, the difference between photosynthesis and plant respiration), with reduced NPP across 70% of the vegetated land areas due to drought over 2000–2009 (Chapin et al. 2006, Zhao and Running 2010). Globally, a large loss in NPP is projected by the end of the 21st century as a consequence of drought (Cao et al. 2022). However, our understanding of how photosynthesis and respiration respond to drought, notably repeated drought, remains limited. This knowledge gap is especially pronounced for species inhabiting arid and semi-arid ecosystems, which are presumed to be adapted to dry conditions, and therefore drought.
Plants exhibit varied strategies to respond to drought, including differing responses in gas exchange (Peñuelas et al. 2001, Adams et al. 2009, Allen et al. 2010). However, drought studies seldom explore how plants respond to repeated droughts, and whether acclimatation to drought may alter gas exchange during and following release from drought (Vandegeer et al. 2020). There are several ways to assess acclimatation responses by plants to drought: (i) to compare the response from an initial drought to a second drought of the same plants or (ii) comparing plants exposed to a single drought versus species with well-watered conditions (Lemoine et al. 2018). Testing various ways plants respond to droughts is laborious and limited to experimental studies.
In theory, stomata close in response to both declining soil water availability and increasing atmospheric vapour pressure deficits (VPD; Duursma et al. 2014, Sperry and Love 2015). Stomatal closure regulates leaf water potential (ᴪ), preventing rapid declines in ᴪ and subsequent cavitation, which can lead to plant death (McDowell et al. 2022). There is a continuum of water-use strategies with two extreme states: isohydric and anisohydric (Martinez-Vilalta et al. 2014). Isohydric species use rapid and early declines in stomatal conductance (gs) to tightly regulate ᴪ in the early stages of drought. Anisohydric species tolerate a larger decline of ᴪ, thereby allowing the maintenance of gs further into drought (Martinez-Vilalta et al. 2014). In reality, applying the iso/anisohydric paradigm is challenging because most plants fall somewhere between the two behaviours. Furthermore, plant water-use strategies can be dynamic and defined by multiple traits (i.e., hydroscapes; Kannenberg et al. 2022).
An important trait that characterizes plant water-use strategies is water-use efficiency (WUE). WUE describes the trade-off between carbon gain and water loss that occurs when plants photosynthesize. Intrinsic WUE (WUEi) can be defined as the ratio of net photosynthetic assimilation (An) to gs. WUEi responds to (i) stomatal closure and (ii) photosynthesis through changes in photosynthetic parameters such as the maximum rate of carboxylation (Vcmax; maximum rate of the Rubisco activity) and the maximum rate of electron transport (Jmax) (Flexas et al. 2006, Galmes et al. 2007, Sperry and Love 2015). Rates of Vcmax and Jmax provide important insights into plant functioning, in which the Rubisco enzyme and chlorophyll are responsible for An in C3 plants (Cernusak et al. 2011). Typically, values of Vcmax are higher when water is more readily available than under drought (Zhou et al. 2016); by contrast, values are reduced during short-term water stress. Vcmax can maintain the same values during drought if species can acclimate to drought by modifying the Rubisco activity, thus leading to a higher protein content allocated to the Rubisco (Zhou et al. 2016).
Reductions in An due to drought affect the whole leaf carbon balance, changing the respiratory loss of CO2 by plants that account for up to 30–80% of the daily carbon uptake, realized as dark respiration (Rd) (Gimeno et al. 2010, Gauthier et al. 2014). Reductions in Rd usually occur to a lesser extent than reductions in An. In addition to physiological responses to drought, plants can also exhibit morphological responses to drought. Leaf mass per area (LMA) is one such trait. High LMA relates to leaf toughness, less air space, high density and packed cells, hence leaf and plant survival (Poorter et al. 2009), and nitrogen content is generally higher in leaves with high LMA (Dong et al. 2022). Therefore, LMA can be an adaptive response to prolonged water stress in plants.
The aim of this study was to compare the responses of key leaf physiological and morphological traits during repeated droughts in diverse co-occurring semi-arid species. We chose four species that represent the main tree genera within central Australia: Eucalyptus, Corymbia, Acacia and Hakea. These genera exhibit evergreen sclerophyllous foliage and a set of different hydraulic traits (O'Grady et al. 2009, Santini et al. 2015, Nolan et al. 2017a). Eucalyptus and Corymbia are members of the Myrtaceae family which are tall, deep-rooted trees and are known to access deep soil water content (SWC) and groundwater in drylands (O'Grady et al. 2006, Rumman et al. 2018). This means they may rarely experience extremes of low soil moisture content. In contrast, Acacia spp., especially those from the Mulga complex, which dominate ~20 to 25% of the semi-arid Australian continent, are highly tolerant to very low soil moisture content (Page et al. 2011, Eamus et al. 2013, Cleverly et al. 2016). Acacia spp. and Hakea spp. are shrubs that have shallow root systems, possessing specific hydraulic traits, including narrow xylem vessels and small diameter roots, to withstand low water availability (Lamont 1993, Groom et al. 1994, Page et al. 2011, Nolan et al. 2017b). In particular, Hakea have been widely observed to generate root clusters. Root clusters play a beneficial role as they effectively increase the surface area available for water uptake (Lamont 2003).
We hypothesized the following: (i) species with a faster growth rate (i.e., Myrtaceae) will have larger declines in An and gs during the development of drought, than species with lower growth rates (i.e., Acacia and Hakea spp.). (ii) Within species, plants that have experienced drought previously will be less sensitive to subsequent droughts. The sensitivity or acclimatation to drought will be observed as smaller reductions of An and gs as leaf water potential declines during repeated droughts. (iii) The degree of recovery in leaf-scale gas exchange variables (especially An and gs) after repeated droughts will be larger in the Mulga and Hakea species than in Eucalyptus and Corymbia species because of the range of drought-resistant traits exhibited in the former species but not the latter two species.
Materials and methods
Plant taxa and plant growth conditions
Seedlings of Acacia aptaneura Maslin & J.E.Reid (also known as Mulga), Corymbia opaca (D.J.Carr & S.G.M.Carr) K.D.Hill & L.A.S.Johnson, Eucalyptus camaldulensis Dehnh. var. obtusa and Hakea macrocarpa A.Cunn. ex R.Br. were germinated from seeds in the winter of 2015 in a glasshouse at the University of Technology Sydney, Sydney, Australia. Seeds were obtained from Nindethana Seed Service (Albany, Western Australia), which had been collected from wild populations. These species were selected due to their dominance in semi-arid central Australia (Maslin and Reid 2012, Eamus et al. 2013, Cleverly et al. 2016), due to their representativeness within two important biomes, the Mulga woodland and Corymbia savanna (Tarin et al. 2019, 2020), and because of their contrasting functional traits (Table 1). A pre-treatment with boiling water was applied to A. aptaneura seeds to break seed dormancy. Around 20 seeds per species were placed in Petri dishes with 4–8% agar and located in a glasshouse at ~25 °C until germination occurred (ca 3–30 days). Petri dishes were placed within a glass cabinet with a sunlit polythene-covered lid that transmitted 70% of sunlight. Seedlings were transplanted from the agar plates to trays (20 × 30 cm) with the same reduced sunlight until they were >5 cm tall. These trays were filled with sterilized soil to prevent contamination from microbes/fungi. Once seedlings were >10 cm tall, they were planted in 18 L pots (one plant per pot) with a soil mix consisting of 50% native mix with low-phosphorus (Greenlife Native Mix) and 50% river sand. We selected pots of this size to maximum the potential area available for root exploration. Nevertheless, we acknowledge that potted plant studies may limit the potential size of roots. High SWC was maintained until initiation of the drought treatments when seedlings were >12 months old. Pots were irrigated with an automated drip water system every 2 days. Environmental conditions were natural, where daily air temperature ranged from 13 to 38 °C, and daytime VPD (D) was on average ca 1.9 kPa (Nolan et al. 2017c).
Table 1.
Plant species description from the literature. Note A. aptaneura was previously named A. aneura (Maslin and Reid 2012).
|
Family/
species |
Plant functional type | Life form | Leaf morphology | Xylem attributes | Rooting attributes | References |
|---|---|---|---|---|---|---|
| Myrtaceae/ Eucalyptus camaldulensis | Riparian evergreen, sclerophyll angiosperm tree | Tree >15 m | Broad leaves | Low wood density | Deep-rooted |
Santini et al. (2015)
|
| Myrtaceae/ Corymbia opaca | Savanna, evergreen angiosperm tree | Tree >15 m | Broad leaves | Large hydraulic conductivity, relatively low wood density | Deep-rooted |
Santini et al. (2015)
O’Grady et al. (2006, 2009) |
| Proteaceae/ Hakea macrocarpa | Evergreen, angiosperm tree | Shrub 1–3 m | Broad and terete leaves | Low wood density | Strongly dimorphic | Groom et al. (1994) |
| Fabaceae/ Acacia aptaneura | Evergreen, angiosperm tree | As shrub <2 m As tree from 2 to 15 m |
Evergreen phyllodes with high leaf density and thickness | Complex xylem vessel network with small vessels size Large wood density with low hydraulic conductivity |
Shallow root system with taproot and feeder roots N2-fixing species |
Page et al. (2011)
Santini et al. (2015) O’Grady et al. (2009) |
Experimental design
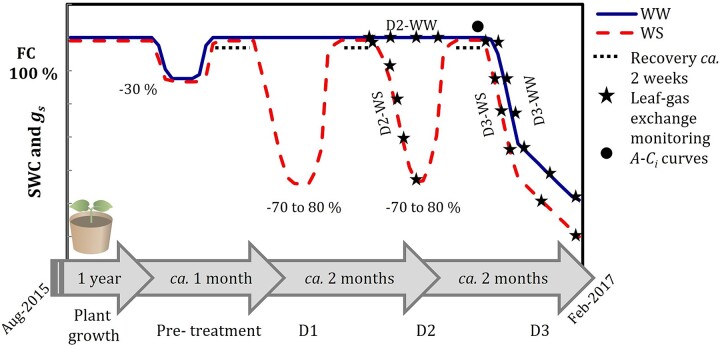
This study consisted of three repeated droughts to test the effect of pre-exposure to drought (Nolan et al. 2017c). The drought-imposed experiment had eight replicates per treatment (well-watered: WW and water-stressed: WS) per species. Plants were assigned randomly to either WS or WW treatments to avoid differences in plant height across treatments. Prior to the imposition of the three experimental droughts, a pre-treatment mild water stress was imposed to all species and all individuals during July–August 2016 (Figure 1), as described in Nolan et al. (2017c). Application of mild water stress is frequently applied to seedlings in nurseries to ‘drought harden’ species, i.e., make them more resistant to drought, and subsequently decrease seedling mortality rates (Landis 1999). This mild water stress was imposed by completely ceasing irrigation until gs dropped by ~30% of maximal gs and there were concomitant declines in SWC with respect to field capacity (FC).
Figure 1.
Drought experiment design for well-watered (WW) and water-stressed (WS) treatments. Stomatal conductance (gs) and SWC were repeatedly monitored during the experiment after the first year of plant growth. Percentages indicate the decline in gs of the maximum. Experimental droughts are indicated by D1, D2 and D3.
Following the drought pre-treatment, three sequential experimental droughts (D1, D2 and D3) were applied to all individuals (including WW individuals but only for D3) between September 2016 and February 2017 (the Austral spring/summer season, Figure 1). SWC, measured gravimetrically, and gs were monitored for all plants. Droughts D1 and D2 were stopped when gs was ~20 to 30% of gs control values (Figure 1). The length of each drought varied among species, due to differences in the rate of decline in gs. The shortest drought events were imposed for E. camaldulensis (21–22 days), and the longest drought was imposed for C. opaca (37–38 days). Drought length for A. aptaneura and H. macrocarpa was 26–31 days. During the entire ~5 month experiment, pots were repeatedly moved every ~3 weeks to reduce the impact of any variation in micro-climate across benches within the glasshouse.
Leaf gas exchange
Leaf gas exchange measurements were made with an infrared gas analyser (IRGA) system (Li-6400XT, Li-Cor Inc., Lincoln, NE, USA) coupled to a 2 × 3 cm broadleaf chamber (6400-02B LED Light Source; Li-Cor Inc.). Two leaves per individual were measured on five replicate plants per treatment per species during the second and third experimental droughts every 2 days. Leaf dark respiration (Rd) was measured during drought D3 on a different leaf each time; this was one leaf per plant (n = 4) per treatment. Leaves for Rd were wrapped in aluminium foil prior to sunrise. Gas exchange measurements, including Rd, were made between 09:00 and 11:00 h; each measurement took between 5 and 10 min, until stomatal conductance approached steady-state conditions. Temperature was maintained on average at 33 ± 2.5 °C during measurements. CO2 concentration was set at 400 p.p.m. inside of the leaf chamber, and photosynthetic photon flux density (PPFD) was 1500 μmol m−2 s−1 and 0 μmol m−2 s−1 when measuring Rd. Projected leaf area was measured using an image analysis system (WinDIAS 3, Delta-T Devices, Cambridge, UK), and leaf gas exchange values were area-corrected.
Photosynthetic responses to sub-stomatal CO2 concentration (A–Ci curves) measurements were made before the start of drought D3, once plants had recovered from the previous drought, usually within 1 day (D2) after FC was applied to the soil. This was additionally confirmed when gs was measured and compared with previous gs measurements at FC (Figure 1). CO2 concentrations in the leaf cuvette were set at 400, 200, 100, 50, 40, 400, 400, 500, 600, 800, 1000, 1200, 1400 and 1700 p.p.m. (n = 4 per species, per treatment) using a 3-min time step between measurements. The plantecophys R package (Duursma 2015) was used in R 3.2.1 (R Development and Core Team, 2016) to estimate Jmax and Vcmax from the fitted A–Ci curves using the Farquhar et al. (1980) model. Leaf temperature was corrected to 25 °C, and D was held as constant as possible during the measurements at 2.3 ± 0.8 kPa.
Plant water status
Pre-dawn leaf water potential (ᴪpd; in negative values) was measured on the same days as leaf gas exchange measurements. Leaves/phyllodes were selected randomly before sunrise between 05:00 and 06:00 h (n = 4 per species per treatment). Excised leaves were immediately placed in Ziploc bags and sealed and transported to the laboratory in an insulated cooler, and their water potential was measured within the following hour in a Scholander-type pressure chamber (PMS Instruments, Albany, OR, USA).
Gas exchange sensitivity to water stress
To estimate the sensitivity of An and gs rates to drought, the value of SWC at 50% loss of leaf gas exchange was calculated following the method of Domec and Gartner (2001). An and gs were normalized to 100% using maximum values. To fit the leaf gas exchange curves as SWC declined, the following Weibull function was used:
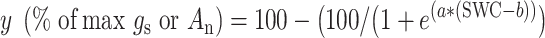 |
(1) |
where 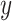 is either An or gs (normalized values),
is either An or gs (normalized values), 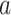 describes the slope of the curve and
describes the slope of the curve and 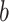 is the SWC at 50% of An or gs (An50 and gs50, respectively).
is the SWC at 50% of An or gs (An50 and gs50, respectively).
LMA and plant growth
Leaf mass per area (LMA: g m−2) was calculated by assessing four individuals (~10 leaves each) per species of the same individuals for all previous measurements (leaf gas exchange and leaf water potentials). Leaf areas of fresh leaves were measured in a leaf area scanner (WinDIAS 3, Delta-T Devices, Cambridge, UK). The same leaves were then oven-dried at 65 °C and dry weights were obtained after 72 h. Additionally, plant growth was monitored at the start of each experimental drought (from D1 to D3) by measuring the number of leaves, stem diameter and plant height.
Statistical analyses
All statistical analyses were undertaken using the R 3.1.1 Project software (R Development Core Team 2016). We used simple averages for leaf gas exchange measurements that were taken in two leaves per individual. To test plant recovery and differences between species in leaf gas exchange variables and plant growth, the interaction term species  treatment was tested using two-way analysis of variance (ANOVA). Tukey’s HSD post-hoc tests were applied to test for significant differences between species. Linear regressions were applied per species to determine the relationship of: ᴪpd between An and gs. Differences in slopes were tested with two approaches separately: (i) for every treatment between species and (ii) within species between treatments. The first approach allowed us to differentiate rates in declining leaf gas exchange variables as drought progressed between species within the same treatment, either WW or WS across droughts. The second approach allowed us to differentiate changes in rates with repeated droughts, for example, to compare plants from D2 versus plants from D3 within species. Slope differences were tested using a standardized major axis method in the SMATR package in R and multiple comparison pair-wise test comparisons among species (Warton et al. 2012). A nonlinear least squares ‘nls’ function in R was used to evaluate the response of gs and An to SWC.
treatment was tested using two-way analysis of variance (ANOVA). Tukey’s HSD post-hoc tests were applied to test for significant differences between species. Linear regressions were applied per species to determine the relationship of: ᴪpd between An and gs. Differences in slopes were tested with two approaches separately: (i) for every treatment between species and (ii) within species between treatments. The first approach allowed us to differentiate rates in declining leaf gas exchange variables as drought progressed between species within the same treatment, either WW or WS across droughts. The second approach allowed us to differentiate changes in rates with repeated droughts, for example, to compare plants from D2 versus plants from D3 within species. Slope differences were tested using a standardized major axis method in the SMATR package in R and multiple comparison pair-wise test comparisons among species (Warton et al. 2012). A nonlinear least squares ‘nls’ function in R was used to evaluate the response of gs and An to SWC.
Results
Plant growth
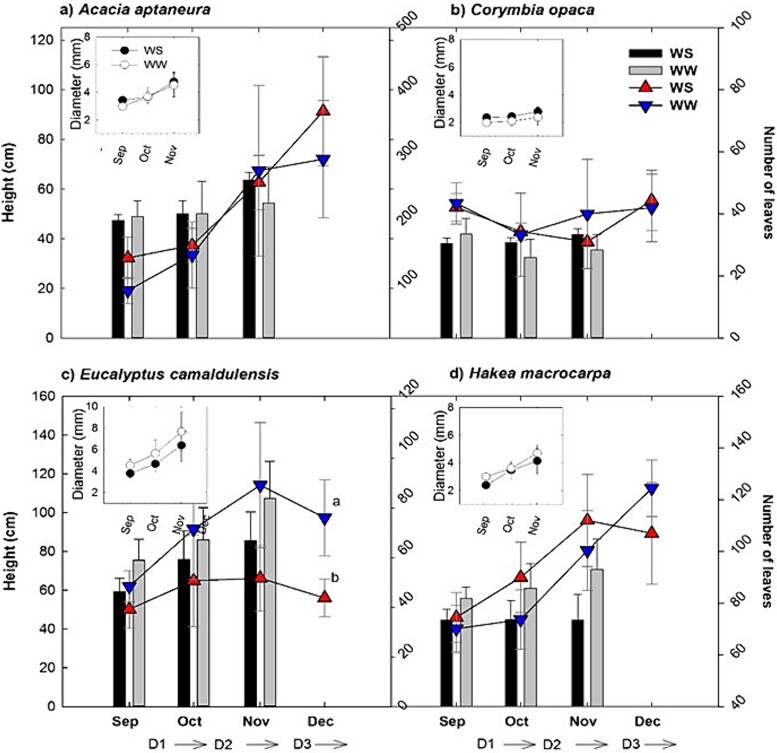
Plant height ranged between 30 and >100 cm (Figure 2). Throughout the experiment, E. camaldulensis was the tallest species, followed by A. aptaneura (Figure 2a; P < 0.05; see Table S1 available as Supplementary data at Tree Physiology Online), while C. opaca was the smallest (30–60 cm) species after 1 year of growth. Within species, there were significant differences between treatments in height and diameter values (P < 0.05, see Table S1 available as Supplementary data at Tree Physiology Online).
Figure 2.
Plant growth during drought. Plant height (bars), number of leaves (triangles) and stem diameter (circles) changes for well-watered (WW) and water-stressed (WS) treatments during September–December 2016 after 1 year of growth. X-axes also indicate estimated starts of each experimental drought (D1, D2, D3; dates vary for each species). (a and b) A. aptaneura, (c and d) Corymbia opaca, (e and f) Eucalyptus camaldulensis and (g and h) Hakea macrocarpa. Error bars represent ±1 SE (n = 4).
There was a considerable reduction in the numbers of leaves for the WS and WW in E. camaldulensis during D3 (P < 0.05). Stem diameter was considerably large in E. camaldulensis during the entire experiment (4–8 mm) but was similar within the remaining three species (2–5 mm) for both treatments WS and WW.
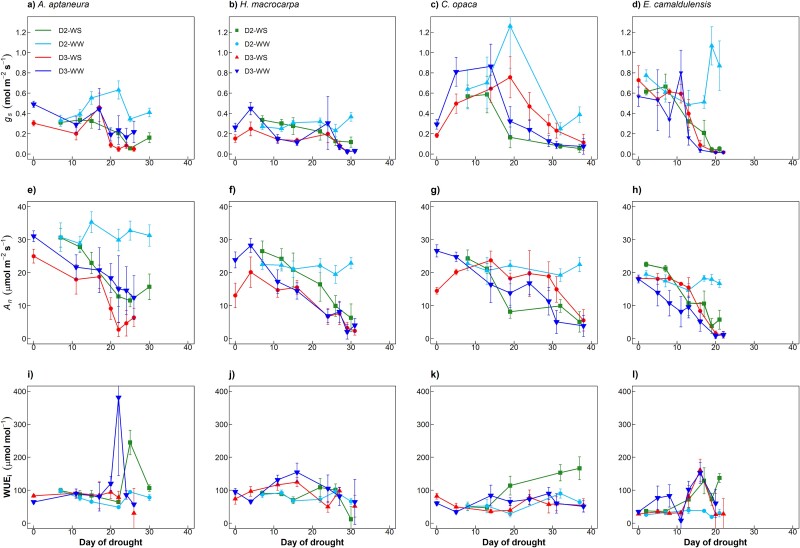
Temporal trends in leaf gas exchange
In all species, gs and An decreased as drought progressed (Figure 3). Among species there was a large range of values of gs (0.02–1.3 mol m−2 s−1; Figure 3a–d), An (0.84–35.32 μmol m−2 s−1; Figure 3e–h) and WUEi (8–380 μmol mol−1) during the drought experiments of D2-WS, D3-WS and D3-WW. During drought, the largest rates of gs were observed in E. camaldulensis with 1.07 and 0.80 mol m−2 s−1 and C. opaca with 1.26 and 0.86 mol m−2 s−1 in droughts D2 and D3, respectively. In contrast, the largest An, hence the largest maximum assimilation rate was observed in A. aptaneura (32.32 μmol m−2 s−1) with minimal differences across the remaining three species (An = 28.2, 26.6 and 22.5 μmol m−2 s−1 for H. macrocarpa, C. opaca and E. camaldulensis, respectively).
Figure 3.
Effect of drought on leaf gas exchange: Stomatal conductance (gs; a–d), net assimilation (An; e–h) and intrinsic water-use-efficiency (WUEi; i–l). Treatments are well-watered (WW) and water-stress (WS) for 2nd and 3rd droughts (D2 and D3; see Figure 1). Error bars represent ±1 SE. Data points are the means of measurements within each treatment (n = 5).
Generally, across all four species, WUEi showed varied responses as drought progressed (Figure 3i and l). WUEi increased in some, but not all, treatments, and this was most pronounced in A. aptaneura and E. camaldulensis (Figure 3i and l). In C. opaca and E. camaldulensis, moderate reductions in SWC, from 0.2 to 0.1 m3 m−3, resulted in increased WUEi but further reductions in SWC decreased WUEi (Figure S1k and l available as Supplementary data at Tree Physiology Online).
SWC values associated with a 50% loss in gas exchange
With the normalized values of gs and An (normalized to 100% using maximum values of gs and An at high SWC), SWC values associated with a 50% loss in gs and An were calculated (Figures S1 and S2 available as Supplementary data at Tree Physiology Online). During D2, values of SWC associated with a 50% of loss in gs (gs50) and An (An50) were larger in H. macrocarpa (0.15 m3 m−3) and C. opaca (0.13 m3 m−3) than E. camaldulensis (0.08 m3 m−3) and A. aptaneura (0.07 m3 m−3). In H. macrocarpa gs50 and An50 were twice as large for the D2-WS (0.15 m3 m−3) as for the D3-WS (0.07 m3 m−3). For E. camaldulensis plants subject to repeated water stress, there was a 10% decline in the value of SWC associated with a 50% decline in gs50 and An50 between the second and third droughts (from 0.08 to 0.07 m3 m−3). In contrast, for C. opaca plants subject to repeated water stress, there was an increase in the value of SWC associated with a 50% decline in gs50 and An50, from 0.13 to 0.18 m3 m−3. Acacia aptaneura did not show changes in either gs50 or An50 (ca 0.07 m3 m−3).
Across all species in the final drought, the value of SWC associated with a 50% decline in gas exchange values was either similar or lower for the plants subject to repeated water stress (Figures S1 and S2 available as Supplementary data at Tree Physiology Online). The largest difference of gs50 between D3-WW (0.12 m3 m−3) and D3-WS (0.07 m3 m−3) was observed in E. camaldulensis, followed for H. macrocarpa and A. aptaneura, with no changes in C. opaca (0.18 m3 m−3).
Gas exchange following release from drought
To compare leaf gas exchange among species following recovery and under moist conditions (during the last recovery period after D2; see Figure 1), averages of gs, An and WUEi were calculated separately for the four species per treatment (Table 2). Contrasting gas exchange values were observed among species, yet the interaction species  treatment was not significantly different (P > 0.05) for gs, An, Rd/An and WUEi (Table S1 available as Supplementary data at Tree Physiology Online).
treatment was not significantly different (P > 0.05) for gs, An, Rd/An and WUEi (Table S1 available as Supplementary data at Tree Physiology Online).
Table 2.
Summary of plant species comparisons of mean values of leaf mass per area (LMA; g m−2), net assimilation (An; μmol m−2 s−1), stomatal conductance (gs; mol m−2 s−1), intrinsic water-use efficiency (WUEi; μmol mol−1), the ratio of night-time respiration to daytime net assimilation (Rd/An), velocities of carboxylase (Vcmax; maximum rate of the rubisco activity; μmol m−2 s−1) and the maximum rate of electron transport (Jmax; μmol m−2 s−1) for each treatment: Well-watered (WW) and water-stressed (WS). Parameters such as An, gs, WUEi, LMA, Vcmax and Jmax correspond to the last recovery period of the experiment. Rd/An ratio values were averaged from different point measurements during the third drought per treatment (WS and WS). Errors represent ±1 SE (n = 5 and 4 for Vcmax and Jmax). Within columns, different letters indicate that means were significantly different at P < 0.05 among species with two-way ANOVAs and the iteration species × treatment (Table S1 available as Supplementary data at Tree Physiology Online); the Tukey’s HSD post-hoc tests for significant differences was applied for differences between species.
| Species | Treatment | LMA | A n | R d /A n | g s | WUE i | V cmax | J max |
|---|---|---|---|---|---|---|---|---|
| A. aptaneura | WS | 543 ± 38.6a | 25.0 ± 2.1a | 0.10 ± 0.04b | 0.30 ± 0.03b | 82.9 ± 4.6b | 85 ± 14.7b | 287 ± 53.4c |
| WW | 385 ± 93.3a | 31.0 ± 1.2a | 0.10 ± 0.3b | 0.49 ± 0.03b | 64.1 ± 4.6b | 95 ± 7.8b | 283.4 ± 31.7c | |
| C. opaca | WS | 286 ± 34.1bc | 14.5 ± 1.0b | 0.18 ± 0.07b | 0.18 ± 0.01b | 80.7 ± 7.8b | 110 ± 3.4b | – |
| WW | 220 ± 53.2bc | 16.5 ± 2.4b | 0.21 ± 0.07b | 0.28 ± 0.06b | 63.5 ± 6.3b | 91 ± 6.5b | 294 ± 14bc | |
| E. camaldulensis | WS | 135 ± 16.7b | 18.3 ± 0.4b | 0.07 ± 0.01b | 0.73 ± 0.06a | 27.9 ± 3.9a | 68 ± 2.4a | 137 ± 13.7bc |
| WW | 130 ± 10.6b | 18.1 ± 1.2b | 0.12 ± 0.03b | 0.57 ± 0.10a | 34.7 ± 5.0a | 60 ± 7.5a | 119 ± 8.5b | |
| H. macrocarpa | WS | 498 ± 54.2ac | 16.7 ± 4.3b | 0.83 ± 0.22a | 0.18 ± 0.03b | 88.0 ± 8.5b | 76 ± 5.7ab | 180 ± 29.5bc |
| WW | 285 ± 52.5ac | 19.8 ± 2.8b | 0.52 ± 0.14a | 0.23 ± 0.03b | 88.9 ± 9.0b | 86 ± 5.7ab | 254 ± 23.2bc |
The most water-use efficient plants were H. macrocarpa (88.8 ± 9.0 μmol mol−1), A. aptaneura (82.9 ± 4.6 μmol mol−1) and C. opaca (80.7 ± 7.8 μmol mol−1), all in the WS treatment. However, A. aptaneura had low WUEi in the WW treatment (64.1 ± 4.6 μmol mol−1) and was not significantly different from the WUEi of C. opaca WW (63.5 ± 6.3 μmol mol−1). The lowest WUEi was for E. camaldulensis (27.9 ± 3.9 μmol mol−1 and 34.7 ± 5.0 μmol mol−1) in both WS and WW, respectively.
After the second drought, Rd/An was largest in H. macrocarpa (WS = 0.83 ± 0.22 and WW = 0.52 ± 0.14) and significantly different from the remaining three species (Table 2). The smallest ratio was observed in A. aptaneura (WS = 0.10 ± 0.04 and WW = 0.10 ± 0.3) and E. camaldulensis (WS = 0.07 ± 0.01 and WW = 0.12 ± 0.03). For WW treatment, H. macrocarpa also had the largest Rd/An value and was statically different from E. camaldulensis, C. opaca and A. aptaneura.
Plant sensitivity to leaf pre-dawn water potentials
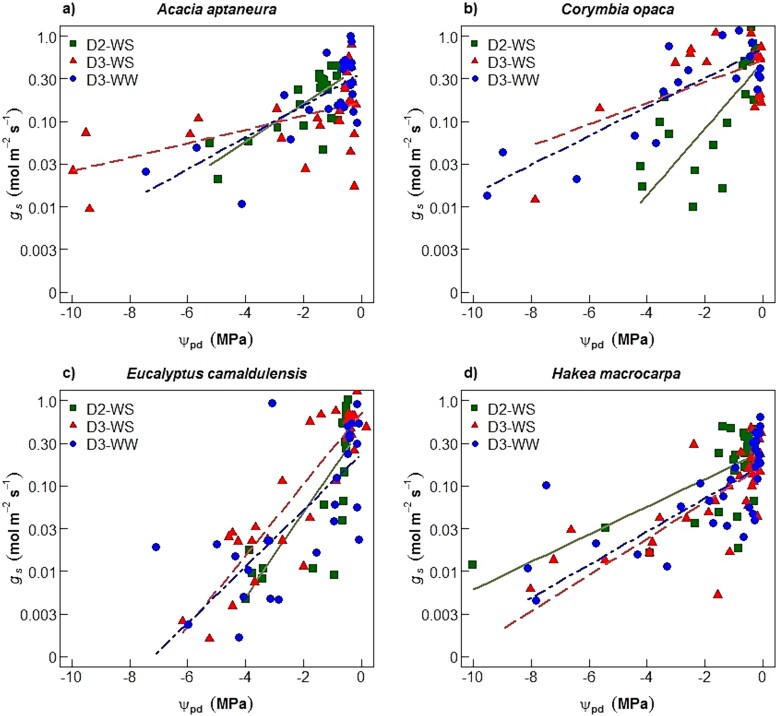
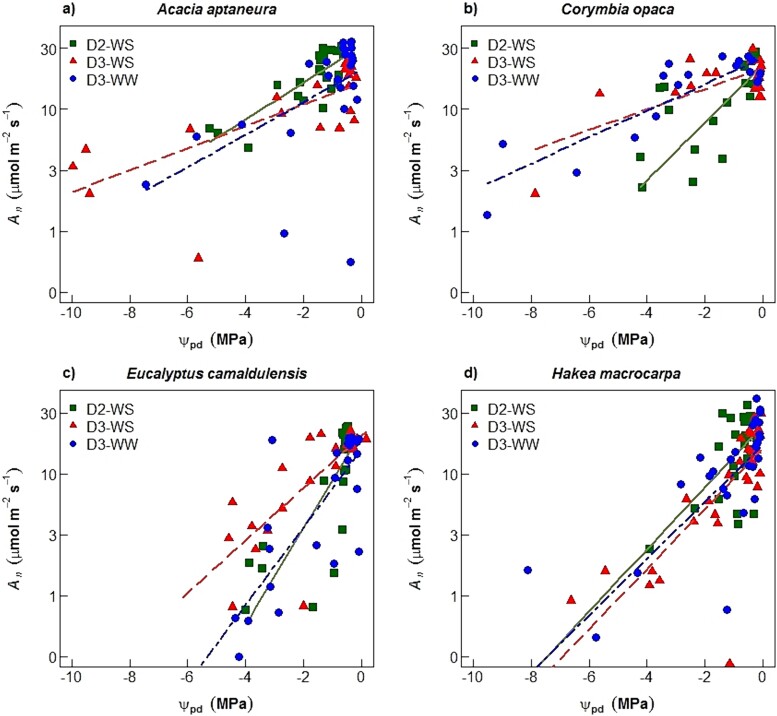
Variations in gs and An (log10 transformed) were linearly correlated with ᴪpd in all four species (Figures 4 and 5 for gs and An, respectively). In D2 imposed on the WS treatment plants (D2-WS), E. camaldulensis showed the largest slope (m) in gs (m = 0.50, Figure 4c and Table 3) and An (m = 0.40, Figure 5c), followed by C. opaca for gs (m = 0.39, Figure 4b) and H. macrocarpa for An (m = 0.26, Figure 5b). Hakea macrocarpa had the smallest slopes for gs (m = 0.16; Figure 4d) in D2-WS and A. aptaneura for An (m = 0.15; Figure 5a) in D2-WS.
Figure 4.
Stomatal conductance (log10(gs) scale; Mol m−2 s−1) versus leaf pre-dawn water potentials (ᴪpd) for all four species (a–d). Symbols indicate treatments as: squares for 2nd drought (D2-WS; water-stressed treatment), triangles for 3rd drought (D3-WS) and circles for D3-WW (well-watered treatment). Regression line colour corresponds to treatment symbol colour. Regression coefficients are shown in Table 3.
Figure 5.
Net assimilation (log10(An) scale; μmol m−2 s−1), versus leaf pre-dawn water potentials (ᴪpd). Symbols indicate treatments as: squares for 2nd drought (D2-WS; water-stressed treatment), triangles for 3rd drought (D3-WS) and circles for D3-WW (well-watered treatment). Regression line colour corresponds to treatment symbol colour. Regression coefficients are shown in Table 3.
Table 3.
Coefficients (±1 SE) of the linear regressions between pre-dawn water potentials (ᴪpd; MPa) and stomatal conductance (Figure 4, log10(gs) scale; Mol m−2 s−1) and ᴪp versus net assimilation (Figure 5, log10(An) scale; μmol m−2 s−1), for each species and treatment. Within columns, asterisk indicate that slopes were significantly different (P-value < 0.05) between treatments within same species and lower-case letters among species and the same treatment (either WS or WW) as tested separately using a standardized major axis method.
| g s versus Ψpd; Figure 4 | A n versus Ψpd; Figure 5 | |||||||
|---|---|---|---|---|---|---|---|---|
| Species | Treatment | Slope | r 2 | P-value | Slope | r 2 | P-value | |
| A. aptaneura | D2-WS | 0.22 ± 0.04 a | 0.55 | <0.001 | 0.15 ± 0.02 a | 0.63 | <0.001 | |
| D3-WS | 0.08 ± 0.02 a* | 0.27 | <0.001 | 0.09 ± 0.02 a* | 0.51 | <0.001 | ||
| D3-WW | 0.19 ± 0.04 a | 0.51 | <0.001 | 0.14 ± 0.02 a | 0.68 | <0.001 | ||
| C. opaca | D2-WS | 0.39 ± 0.09 b | 0.45 | <0.001 | 0.24 ± 0.07 b | 0.35 | 0.003 | |
| D3-WS | 0.12 ± 0.04 ac | 0.31 | 0.01 | 0.08 ± 0.02 a | 0.49 | <0.001 | ||
| D3-WW | 0.17 ± 0.02 ac | 0.67 | <0.001 | 0.11 ± 0.01 b | 0.76 | <0.001 | ||
| E. camaldulensis | D2-WS | 0.50 ± 0.08 b | 0.63 | <0.001 | 0.40 ± 0.06 b | 0.65 | <0.001 | |
| D3-WS | 0.41 ± 0.03 b | 0.81 | <0.001 | 0.22 ± 0.03 b | 0.61 | <0.001 | ||
| D3-WW | 0.33 ± 0.06 b | 0.51 | <0.001 | 0.32 ± 0.04 c | 0.68 | <0.001 | ||
| H. macrocarpa | D2-WS | 0.16 ± 0.03 a | 0.43 | <0.001 | 0.26 ± 0.07 b | 0.32 | <0.001 | |
| D3-WS | 0.21 ± 0.03 c | 0.62 | <0.001 | 0.25 ± 0.03 b | 0.7 | <0.001 | ||
| D3-WW | 0.19 ± 0.03 c | 0.53 | <0.001 | 0.23 ± 0.05 a | 0.41 | <0.001 | ||
First, we tested plant response to drought by comparing D2-WS (second drought to same plants; see Figure 1) with D3-WS (third drought to same plants). Results showed a decrease in the slopes of the response of gs to ᴪpd of 18, 64 and 68% for E. camaldulensis, A. aptaneura and C. opaca, respectively, from D2-WS to D3-WS (Table 3). In contrast, an increase was observed in the slope (of gs to ᴪpd) of 31% in H. macrocarpa (Figure 4d). The slope of the regression of An versus ᴪpd plotted on a semi-log plot significantly decreased between D2-WS and D3-WS treatment for all species except H. macrocarpa (m = 0.26 and m = 0.25, D2-WS and D3-WS, respectively; Table 3).
Second, we tested plant response to drought by comparing D3-WS (three droughts, same plants; see Figure 1) with D3-WW (plant that only experienced one drought at the end of the experiment). Differences between the treatments (D3-WS and D3-WW) during the final drought were observed in A. aptaneura and E. camaldulensis. There were significant differences in slopes only in A. aptaneura for the plants that experienced three droughts (D3-WS) and plants that experienced only the final drought (D3-WW; Table 3). In A. aptaneura, the slope of D3-WS (m = 0.08) was 58% smaller than that of D3-WW (m = 0.19). The slope (An versus ᴪpd) in A. aptaneura of D3-WS (m = 0.09; repeated drought) was 36% smaller than D3-WW (m = 0.14), whereas in E. camaldulensis the difference was 31% smaller in D3-WS (m = 0.22) compared with the D3-WW (0.32) treatment (Table 3). Corymbia opaca did not show significant differences between slopes (D3-WS and D3-WW).
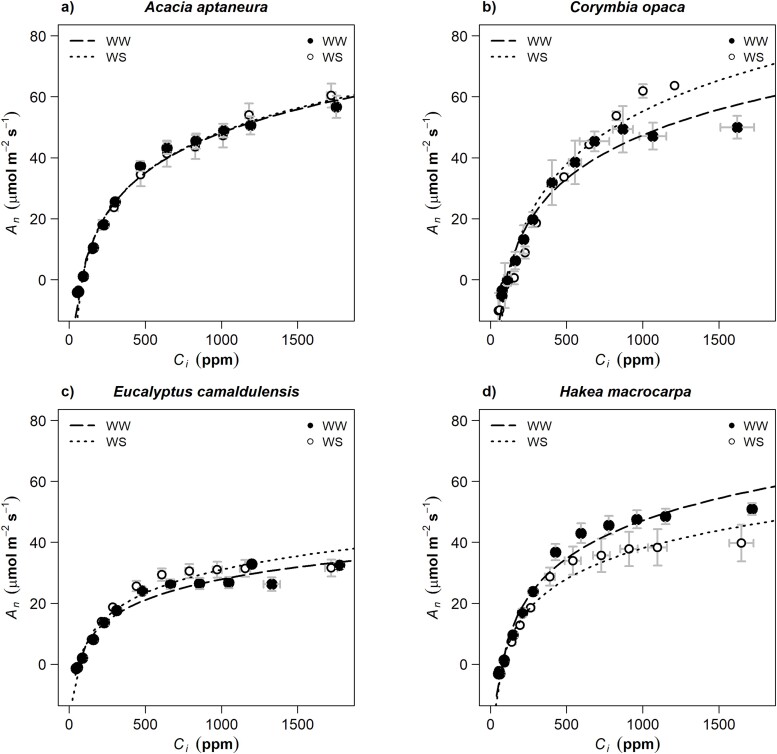
Responses of Vcmax, Jmax and LMA to drought
Mean values for rates of carboxylation (Vcmax; μmol m−2 s−1) and the maximum rate of electron transport (Jmax; μmol m−2 s−1) estimated from A–Ci curves are presented in Table 2 (see also for A–Ci curves Figure 6). The interaction species  treatments were not significantly different for Vcmax (P > 0.05), but they were for Jmax (Table S1 available as Supplementary data at Tree Physiology Online).
treatments were not significantly different for Vcmax (P > 0.05), but they were for Jmax (Table S1 available as Supplementary data at Tree Physiology Online).
Figure 6.
The response of net assimilation (An) to intercellular CO2 (Ci). Open white circles correspond to WW treatment and closed black symbols to WS treatment. Error bars represent ±1SE (n = 4).
Acacia aptaneura and E. camaldulensis had the smallest differences in Jmax between treatments. The largest difference between treatments was in H. macrocarpa (180 ± 29.5 and 254 ± 23.2 μmol m−2 s−1; WS and WW, respectively; Table 2). Corymbia opaca had the highest Vcmax values (110 ± 3.4 μmol m−2 s−1) in WS treatment, while A. aptaneura had the highest Vcmax value (95 ± 7.8 μmol m−2 s−1) in WW treatment (Table 2). Minimum Vcmax values for both WS and WW were observed in E. camaldulensis, but these were not statistically different from H. macrocarpa in both treatments, WW for C. opaca and both treatments for A. aptaneura. Additionally, C. opaca and E. camaldulensis had the smallest LMA compared with the remaining two species. Acacia aptaneura and H. macrocarpa had the largest differences (in LMA), but differences were not statistically significant between treatments (Table 2, Table S1 available as Supplementary data at Tree Physiology Online).
Discussion
In this study, we examined the effects of repeated droughts on four co-occurring semi-arid species that exhibited contrasting physiological strategies observed in their natural habitat (O'Grady et al. 2009, Santini et al. 2015, Nolan et al. 2017a, Rumman et al. 2018). We examined leaf gas exchange, photosynthetic capacity, LMA and plant growth to track the effects of repeated droughts and recovery after releases of drought. Consistent with our hypotheses, we observed differing responses to repeated drought among our four study species; these results are discussed in detail in the following sections.
Coordination in leaf gas exchange and leaf water potential
In semi-arid field conditions, E. camaldulensis and C. opaca are tall, deep-rooted trees, that access groundwater (O'Grady et al. 2009, Cleverly et al. 2016); hence, we hypothesized that these species with relatively fast growth rates would have larger declines in leaf gas exchange than A. aptaneura and H. macrocarpa. In agreement with our hypothesis, results showed that E. camaldulensis and C. opaca had the largest slope values in the regression of gs and An versus ᴪpd, for plants that experienced the second drought (during D2-WS; Table 2). Indeed, E. camaldulensis and C. opaca had greater declines in gs versus ᴪpd (more than 100%) compared with H. macrocarpa, and 77% greater for C. opaca and 130% for E. camaldulensis compared with A. aptaneura in drought D2-WS (Table 3). Likewise, large declines were observed in the regression An versus ᴪpd, yet to a lesser degree compared with gs. Declines of gs for a given decline in ᴪpd can provide an insight into plant water-use strategies, indicating a more isohydric or anisohydric behaviour (Tardieu and Thierry 1998, Klein 2014). We found that E. camaldulensis and C. opaca had large declines in gs and An as drought progressed, meaning they had a tight regulation of leaf gas exchange. This stomatal control decreases plant water use and thus prevents ᴪpd from declining as soil water availability declines, which makes these plants more isohydric. Although we did not measure midday ᴪ, a related glasshouse study on E. camaldulensis and A. aptaneura (Nolan et al. 2017c) found that pre-dawn and midday ᴪ were tightly correlated in both drought and control plants (r2 > 0.93). Furthermore, Nolan et al. (2017c) found that E. camaldulensis had a higher turgor loss point (expressed in negative values as for leaf water potentials), and a shallower slope in the relationship between pre-dawn and midday ᴪ compared with A. aptaneura, and concluded that E. camaludelensis was relatively more isohydric than A. aptaneura. The existence of isohydric behaviour is still debatable (Martínez-Vilalta and Garcia-Forner 2017, Feng et al. 2019), although most isohydric species use rapid and early declines in gs to tightly regulate leaf water potentials in the early stages of drought, resulting in decreasing An (Meinzer et al. 2009, Limousin et al. 2013, Nolan et al. 2017b).
Consistent with our results, Santini et al. (2015, 2017) found that the Myrtaceae species (C. opaca and E. camaldulensis) exhibit larger xylem vessel diameters, lower wood density and larger hydraulic conductivity than Acacia species (A. aneura and A. aptaneura). Wide vessel size makes the xylem more vulnerable to embolism; however, stomatal closure can prevent hydraulic failure when water availability declines and therefore delay drought-induced mortality (Eamus et al. 2000, McDowell et al. 2008). Plant water use is also a function of the dynamics of the below-ground environment, which is crucial to the above-ground process, such as leaf gas exchange Kannenberg et al. 2022). In terms of plant growth, we observed that E. camaldulensis had the widest stem diameter (and hence sapwood area) and was the tallest of all four species examined (Figure 2c). Given all species were germinated from seed at the same time and grown for the same period prior to this study (12 months), these data reflects the faster growth rate of E. camaldulensis compared with the other species. A larger sapwood area and plant size are indicative of larger rates of water flow and overall plant water-use, all of which are related and coordinate to some extent the leaf gas exchange in plants (Katul et al. 2003, Sperry and Love 2015). Corymbia opaca did not grow as tall as E. camaldulensis in this study (Figure 2b), which may be explained by the difference in a number of traits between these species. Corymbia opaca has higher wood density, lower root hydraulic conductance and smaller vessel diameter than those observed in E. camaldulensis (Santini et al. 2015, 2017). Investing in high wood density often occurs at the expense of plant height and diameter growth (Enquist et al. 1999, O'Grady et al. 2009). Investing carbohydrates in plant maintenance and hydraulic architecture, such as deep roots and high venation density (to enhance water transport), is often made at the expense of leaf traits (Brodribb and Jordan 2011, Poorter et al. 2012, Yin et al. 2018). We observed in the final drought that E. camaldulensis reduced its numbers of leaves in the water-stressed (WS) treatment, but this was not observed in C. opaca (Figure 2c). One possible reason is the higher LMA in C. opaca compared with E. camaldulensis, with higher LMA associated with longer leaf life-spans (Wang et al. 2022). This suggests that allocation of assimilated carbohydrates was preferably given to anchorage and a tall structure for E. camaldulensis, rather than maintaining leaves while droughts were imposed (data was not collected for below-ground traits). Although, we did not study the below-ground plant behaviour, above-ground growth can provide insights into plant water-use strategies in variation to root-deep, rood hydraulics and root structure, which ultimately determine water flow, transport and loss in plants.
Our results support our first hypothesis: A. aptaneura showed the smallest slope-values in the regression of gs and An versus ᴪpd during the last drought (DS-WS; Table 3). Hakea macrocarpa only exhibited moderate declines (slopes) for a wider range of water potential values (Figures 4 and 5). Smaller declines in the relationship of gs versus ᴪpd compared with C. opaca and E. camaldulensis implies that A. aptaneura had more risky stomatal regulation for a wider range of leaf water stress (and more negative water potential values). Previous studies have identified A. aptaneura as an anisohydric species (Page et al. 2016, Nolan et al. 2017c). Anisohydric species tolerate a larger decline of leaf water potentials, thereby allowing the maintenance of gs and An further into drought (Martinez-Vilalta et al. 2014). Acacia aptaneura continued to photosynthesize at much lower ᴪpd (Figure 5a). Both A. aptaneura and H. macrocarpa are known for being highly tolerant to drought and high temperatures (Table 1). For example, higher wood density was observed within the two species (A. aptaneura and H. macrocarpa) than species from the Myrtaceae family (Santini et al. 2015). High wood density protects against xylem cavitation, leading to a superior resistance to cell wall collapse, thereby allowing species with high wood density to tolerate low water potentials (Santiago et al. 2004) and to maintain to some extent leaf gas exchange. The response to progressive water stress observed in the four studied species was in agreement with studies that have sought to understand the response of leaf gas exchange to drought in C3 plants (Flexas et al. 2004, Galmes et al. 2007, Cano et al. 2014).
Changes in WUE during drought
In our study, WUEi was maintained at more or less constant low values in the early stages of droughts for all four species (Figure 3i–l). However, as drought progressed, WUEi increased for A. aptaneura and E. camaldulensis but only for a short period, followed by declines in WUEi towards the end of the droughts. Although not all species had a significant increase in WUEi, all species showed a significant decline in WUEi at the end of the droughts, excepting drought D2-WS. This biphasic pattern, an increase in WUEi as soil water stress increases, but consistent decline in WUEi under extreme water stress has been observed across multiple arid and semi-arid plant species (Manzoni et al. 2011, Limousin et al. 2013, 2015). There are different plausible explanations for declining WUEi under extreme drought. One explanation is that stomatal closure results in assimilation rates that are zero or close to them; however, residual conductance via cuticular transpiration and/or leaky stomata results in continued water loss (Petrík et al. 2023). Another explanation is that stomata remain open to provide evaporative cooling, but assimilation does not occur. However, this decoupling of gs from An is likely to only occur under heat wave conditions (Marchin et al. 2023), which were not present in our study. Species that show high WUEi, such as A. aptaneura demonstrate a competitive advantage over the other three species to extreme soil water deficits or droughts.
The increase in WUEi was driven mainly by decreasing gs rather than An as drought progressed, except for H. macrocarpa. For example, reductions of gs were up to 31%, 38% and 46% (for A. aptaneura, C. opaca and E. camaldulensis, respectively) more than the reductions for An in all drought and periods as the soils dried down (Table 3 and Figure S2 available as Supplementary data at Tree Physiology Online). For H. macrocarpa WUEi was pretty much constant during all droughts and only decreased at the end of the droughts due to a decrease in An. Hakea macrocarpa showed greater reductions in An than in gs (for a given reduction in ᴪpd) but only up to 20%, specifically in drought D3-WS. The decoupling of gs from An showed in H. macrocarpa suggests transpiration cooling during soil drying as a strategy to resists drought, or perhaps is related to leaky stomata (Duursma et al. 2019). Plants that experienced drought and have reductions in An tend to consume non-structural carbohydrates to maintain cellular survival, respiratory mechanism and osmotic adjustment (McDowell et al. 2008).
Remarkably, it was observed that H. macrocarpa significantly increased Rd, which led to an increase in the ratio of Rd/An that experienced drought (Table 2). Large values of Rd have been previously observed in field conditions in other Australian species such as Acacia lasiocarpa (Atkin et al. 2015, Falster et al. 2021). Both stomatal closure and the 20% decrease in An as drought progressed were important drivers for the increase in Rd for H. macrocarpa and hence the ratio of Rd/An. This change in the plant carbon balance is a well-understood mechanism in plant mortality (Vandegeer et al. 2020, McDowell et al. 2022). Assessing the response and adaptation of plants to drought is crucial, and understanding the mechanisms inducing a decrease or increase in WUEi will ultimately provide information on which species would be more resistant to drought.
The effect of repeated droughts and plant recovery
We observed an acclimatation response to drought in A. aptaneura (Table 3), i.e., less declines in gas exchange with repeated drought. Acacia aptaneura significantly reduced the rate at which gs and An were declining for a given decline in ᴪpd in plants from D3-WS by 57% for gs and 35% for An compared with individuals of D3-WW. Changes in exogenous hormones (i.e., ABA) may explain this behaviour, since the accumulation of ABA that is expected to occur during droughts enhances stomatal sensitivity to a low water potential levels in repeated droughts (Eamus and Narayan 1989, Thomas et al. 2000, Nolan et al. 2017c). Our previous study (Nolan et al. 2017c) showed a ‘peaking-type’ response, where ABA increased in the early-mid stages of the drought, followed by an abrupt decline once a threshold leaf water potential of −3.9 MPa was reached. Another explanation is the possible accumulation of osmotically active solutes as the soil dries, which allows species to adjust the turgor loss point at which leaf cells lose turgor and close stomatal (Bartlett et al. 2014, Nolan et al. 2017c). A lower turgor loss point (i.e., more negative values) was observed in A. aptaneura following repeated drought in our previous study (Nolan et al. 2017c). These physiological mechanisms allow A. aptaneura to maintain relatively higher rates of gas exchange during repeated droughts. Consequentially, A. aptaneura showed the largest values of WUEi (Figure 3i) by declining gs more than An, which is supported by observations done in field conditions, where Acacia species tend to maintain large rates of primary productivity even outside of the rainy season, while maintaining high WUE values as soil water declines (Tarin et al. 2019, 2020). All this may explain the high dominance of Mulga in the Australian continent, with these species occupying 20–25% of the continent, particularly A. aptaneura and A. aneura (Eamus et al. 2013). Mulga dominates in central Australia, where there is high rainfall variability (Cleverly et al. 2016).
Interestingly, we also observed that the rate of declines in leaf gas exchange for C. opaca were in the same range as A. aptaneura in the third drought and both treatments (D3-WS and D3-WW, Table 2). This suggests that C. opaca has the plasticity to exhibit different plant water-use strategies when exposed to repeated droughts and highlights the importance of examining physiological responses to soil dryness under repeated exposure to drought. Plasticity has been observed in plant water-use strategies, showing other species like Larrea tridentata to have a partial isohydric behaviour during drought conditions but was extremely anisohydric in wet conditions (Guo et al. 2020). Another interesting result was observed in H. macrocarpa, which did not show significant differences between treatments during the final drought (Table 3); however, the point at which both gs and An were reduced by >50% differed from ~0.15 m3 m−3 (during the second drought, Figure S3) to ~ 0.07 m3 m−3 (Figure S2d available as Supplementary data at Tree Physiology Online; during the final drought). It may be possible that root clusters, which are common across Hakea spp. (Lamont 2003), benefited plant–water relations. Root clusters (which in fact were observed at the end of the experiment) can increase the soil volume explored by a factor of up to 300, and release deeply sourced water at night for subsequent uptake the following day through the process of hydraulic lift (Lamont 2003). This may explain why there were no changes in rate declines of gs and An when regressed with ᴪpd between treatments. Similarly, for E. camaldulensis, plants from the final drought did not show differences between treatments, with plants from both treatments exhibiting large photosynthetic rates and stomatal conductance values (Figure 3). In field conditions across different rainfall gradients, eucalypts have shown little or no variation in leaf water potentials among wet and dry seasons due to their ability to access groundwater (Eamus et al. 2000, Mitchell et al. 2014, Nolan et al. 2017a). Thus, hydraulic traits discussed above explain why E. camaldulensis was not sensitive to repeated droughts compared to the Acacia species examined in the present study. In conclusion, although we observed differences between treatments with these three species (C. opaca, E. camaldulensis and H. macrocarpa), there was a lack of memory for repeated droughts, and none of them showed an acclimatation for individuals that experienced three consecutive droughts.
Plant responses to drought have been extensively examined in relation to limitations in leaf gas exchange, the response of mesophyll conductance and biogeochemical limitations occurring at different time-scales during periods of drought (Flexas et al. 2004, 2006, Limousin et al. 2013, Zhou et al. 2016). Thus, plant recovery from droughts depends on species ability to diminish these limitations when water subsequently becomes available (Galmes et al. 2007). We showed full recovery in An and gs during the last re-watering cycle (between the second and final droughts) within the first 3 days after SWC was increased to FC for all species (Table 2). There were no significant differences in leaf gas exchange between WW and WS treatments after cessation of drought, indicating a complete recovery in all four species. Full plant recovery following alleviation of drought has been observed in other C3 plants, in which the period of recovery varied from 1 to 3 days (Flexas et al. 2006, Resco et al. 2009, Cano et al. 2014). As observed in Flexas et al. (2006), usually photosynthesis recovers in 1 day at mild water stress, but photosynthesis can have a slower recovery if the biochemical or hydraulic pathway is damaged (Resco et al. 2009).
The photosynthetic response to drought that occurs independent of stomatal responses can be assessed by evaluating changes in the capacities of carboxylation (Vcmax) and electron transport (Jmax) (Vogan and Maherali 2014). We observed small increases in Vcmax and Jmax in A. aptaneura and H. macrocarpa, but they were not statistically significant. Maintenance of Vcmax and Jmax may indicate that plant species have large resistance to drought in support to our third hypothesis (Table 2). Our results showed that A. aptaneura (which is a N2-fixing species) had the largest LMA values for both treatments (Table 2; Cook and Dawes-Gromadzki 2005, Page et al. 2011). N2-fixing species usually have large foliar nitrogen contents, and foliar nitrogen is positively correlated with LMA (Poorter et al. 2009). Interestingly, A. aptaneura showed an increase of 41% and H. macrocarpa of 75% in LMA from the WW to the WS treatment (Table 2). This reflects the high plasticity of LMA of these two species when exposed to drought. High LMA values >400 g m−2 have been previously reported in other species, such as Hakea acuminata, A. aptaneura, Eucalyptus cyanophylla and E. haemastoma, according to the AusTrait database (Lamont et al. 2002; Dong et al. 2022; Falster et al. 2021). LMA increases when rainfall or water availability is limited to reduce cell expansion rates under drought, and high LMA is also a result of expensive leaf construction and low nutrient content, as semi-arid Australia is well known for having low nitrogen content in its soils.
Conclusions
Our work evaluated the response to repeated experimental droughts using eleven physiological and morphological traits related to leaf gas exchange and plant growth and evaluated plant recovery of four co-occurring species of central Australia. Results showed contrasting physiological behaviours among the four species, but also within species depending on whether plants had been previously exposed to drought. Our results highlight the potential for acclimatation and plasticity in drought responses. By understanding functional attributes of dominant species in the face of climate change through refining metrics of plants physiology, this work has the potential to contribute to reducing uncertainties and improve vegetation and ecosystem modelling approaches for the central Australian region and potentially other similar arid regions across the globe.
Supplementary Material
Acknowledgments
We would like to thank Ralph Faux for technical support in establishing and running the experiment. Thanks also to Rizwana Rumman and John Gallego for the support during data collection.
Contributor Information
Tonantzin Tarin, Instituto de Ecología, Universidad Nacional Autónoma de México, Ciudad Universitaria, Mexico City 04510, Mexico.
Derek Eamus, Terrestrial Ecohydrology Research Group, School of Life Sciences, University of Technology Sydney, Sydney, NSW 2007, Australia.
Nadia S Santini, Instituto de Geología, Universidad Nacional Autónoma de México, Ciudad Universitaria, Mexico City, 04510, Mexico.
Rachael H Nolan, Terrestrial Ecohydrology Research Group, School of Life Sciences, University of Technology Sydney, Sydney, NSW 2007, Australia; Hawkesbury Institute for the Environment, Western Sydney University, Science Rd. Penrith, NSW 2751, Australia.
Authors' contributions
T.T. and R.H.N. conceived and designed the experiment. T.T., R.H.N. and N.S.S. performed the experiment and collected the data. T.T. analysed the data and wrote the manuscript with inputs and edits from R.H.N., D.E., N.S.S. and J.C.
Conflict of interest
None declared.
Funding
This work was supported by an ARC grant awarded to D.E. (DP14101150), a UTS Graduate Scholarship to T.T., the Consejo Nacional de Humanidades, Ciencia y Tecnología (CONAHCYT; 232184) and UNAM-PAPIIT (IA204722).
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author.
References
- Adams HD, Guardiola-Claramonte M, Barron-Gafford GA, Villegas JC, Breshears DD, Zou CB, Troch PA, Huxman TE. 2009. Temperature sensitivity of drought-induced tree mortality portends increased regional die-off under global-change-type drought. Proc Natl Acad Sci USA. 106(17):7063–7066. 10.1073/pnas.0901438106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen CD, Macalady AK, Chenchouni H, Bachelet D, McDowell N, Vennetier M. 2010. A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. For Ecol Manage. 259(4):660–684. 10.1016/j.foreco.2009.09.001. [DOI] [Google Scholar]
- Atkin OK, Bloomfield KJ, Reich PB, Tjoelker MG, Asner GP, Bonal D, Bönisch G, Bradford MG, Cernusak LA, Cosio EGet al. 2015. Global variability in leaf respiration in relation to climate, plant functional types and leaf traits. New Phytol. 206:614–636. 10.1111/nph.13253. [DOI] [PubMed] [Google Scholar]
- Bartlett MK, Zhang Y, Kreidler N, Sun S, Ardy R, Cao K, Sack L. 2014. Global analysis of plasticity in turgor loss point, a key drought tolerance trait. Ecol Lett. 17(12):1580–1590. 10.1111/ele.12374. [DOI] [PubMed] [Google Scholar]
- Brodribb TJ, Jordan GJ. 2011. Water supply and demand remain balanced during leaf acclimation of Nothofagus cunninghamii trees. New Phytol. 192(2):437–448. 10.1111/j.1469-8137.2011.03795.x. [DOI] [PubMed] [Google Scholar]
- Cano FJ, Lopez R, Warren CR. 2014. Implications of the mesophyll conductance to CO2 for photosynthesis and water-use efficiency during long-term water stress and recovery in two contrasting eucalyptus species. Plant Cell Environ. 37(11):2470–2490. 10.1111/pce.12325. [DOI] [PubMed] [Google Scholar]
- Cao D, Zhang J, Han J, Zhang T, Yang S, Wang J. 2022. Projected increases in global terrestrial net primary productivity loss caused by drought under climate change. Earths Future. 10:e2022EF002681. 10.1029/2022EF002681. [DOI] [Google Scholar]
- Cernusak LA, Hutley LB, Beringer J, JAM H, Turner BL. 2011. Photosynthetic physiology of eucalypts along a sub-continental rainfall gradient in northern Australia. Agric For Meteorol. 151(11):1462–1470. 10.1016/j.agrformet.2011.01.006. [DOI] [Google Scholar]
- Chapin FS, Woodwell GM, Randerson JT, Rastetter EB, Lovett GM, Baldocchi DD, Clark DA, Harmon ME, Schimel DS, Valentini Ret al. 2006. Reconciling carbon-cycle concepts, terminology, and methods. Ecosystems. 9(7):1041–1050. 10.1007/s10021-005-0105-7. [DOI] [Google Scholar]
- Cleverly J, Eamus D, Van Gorsel E, Chen C, Rumman R, Luo Q, Coupe NR, Li L, Kljun N, Faux Ret al. 2016. Productivity and evapotranspiration of two contrasting semiarid ecosystems following the 2011 global carbon land sink anomaly. Agric For Meteorol. 220:151–159. 10.1016/j.agrformet.2016.01.086. [DOI] [Google Scholar]
- Cook GD, Dawes-Gromadzki TZ. 2005. Stable isotope signatures and landscape functioning in banded vegetation in arid-Central Australia. Landsc Ecol. 20(6):649–660. 10.1007/s10980-005-0069-1. [DOI] [Google Scholar]
- Domec JC, Gartner BL. 2001. Cavitation and water storage capacity in bole xylem segments of mature and young Douglas-fir trees. Trees. 15(4):204–214. 10.1007/s004680100095. [DOI] [Google Scholar]
- Dong N, Prentice IC, Wright IJ, Wang H, Atkin OK, Bloomfield KJ, Domingues TF, Gleason SM, Maire V, Onoda Yet al. 2022. Leaf nitrogen from the perspective of optimal plant function. J Ecol. 110:2585–2602. 10.1111/1365-2745.13967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duursma RA. 2015. Plantecophys--an R package for analysing and modelling leaf gas exchange data. PloS One. 10(11):e0143346. 10.1371/journal.pone.0143346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duursma RA, Barton CVM, Lin YS, Medlyn BE, Eamus D, Tissue DT, Ellsworth DS, McMurtrie RE. 2014. The peaked response of transpiration rate to vapour pressure deficit in field conditions can be explained by the temperature optimum of photosynthesis. Agric For Meteorol. 189-190:2–10. 10.1016/j.agrformet.2013.12.007. [DOI] [Google Scholar]
- Duursma RA, Blackman CJ, Lopéz R, Martin-StPaul NK, Cochard H, Medlyn BE. 2019. On the minimum leaf conductance: its role in models of plant water use, and ecological and environmental controls. New Phytol. 221:693–705. 10.1111/nph.15395. [DOI] [PubMed] [Google Scholar]
- Eamus D, Narayan AD. 1989. The influence of prior water stress and abscisic acid foliar spraying on stomatal responses to CO2, IAA, ABA, and calcium in leaves of Solanum melongena. J Exp Bot. 40(214):573–579. [Google Scholar]
- Eamus D, O'Grady AP, Hutley LB. 2000. Dry season conditions determine wet season water use in the wet-dry tropical savannas of northern Australia. Tree Physiol. 20:1219–1226. [DOI] [PubMed] [Google Scholar]
- Eamus D, Cleverly J, Boulain N, Grant N, Faux R, Villalobos-Vega R. 2013. Carbon and water fluxes in an arid-zone Acacia savanna woodland: an analyses of seasonal patterns and responses to rainfall events. Agric For Meteorol. 182-183:225–238. 10.1016/j.agrformet.2013.04.020. [DOI] [Google Scholar]
- Enquist B, West GB, Charnov EL, Brown JH. 1999. Allometric scaling of production and life-history variation in vascular plants. Nature. 401:907–911. [Google Scholar]
- Falster D, Gallagher R, Wenk EH, Wright IJ, Indiarto D, Andrew SC, Baxter C, Lawson J, Allen S, Fuchs Aet al. 2021. AusTraits, a curated plant trait database for the Australian flora. Sci Data. 8(1):254. 10.1038/s41597-021-01006-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farquhar GD, Caemmerer S, Berry JA. 1980. A biochemical model of photosynthetic CO2 assimilation in leaves of C3 species. Planta. 149:78–90. [DOI] [PubMed] [Google Scholar]
- Feng X, Ackerly DD, Dawson TE, Manzoni S, McLaughlin B, Skelton RP, Vico G, Weitz AP, Thompson SE. 2019. Beyond isohydricity: the role of environmental variability in determining plant drought responses. Plant Cell Environ. 42(4):1104–1111. 10.1111/pce.13486. [DOI] [PubMed] [Google Scholar]
- Flexas J, Bota J, Loreto F, Cornic G, Sharkey TD. 2004. Diffusive and metabolic limitations to photosynthesis under drought and salinity in C3 plants. Plant Biol. 6(3):269–279. 10.1055/s-2004-820867. [DOI] [PubMed] [Google Scholar]
- Flexas J, Ribas-Carbo M, Bota J, Galmes J, Henkle M, Martinez-Canellas S, Medrano H. 2006. Decreased rubisco activity during water stress is not induced by decreased relative water content but related to conditions of low stomatal conductance and chloroplast CO2 concentration. New Phytol. 172(1):73–82. 10.1111/j.1469-8137.2006.01794.x. [DOI] [PubMed] [Google Scholar]
- Frank D, Reichstein M, Bahn M, Thonicke K, Frank D, Mahecha MD, Smith P, van der Velde M, Vicca S, Babst Fet al. 2015. Effects of climate extremes on the terrestrial carbon cycle: concepts, processes and potential future impacts. Glob Chang Biol. 21(8):2861–2880. 10.1111/gcb.12916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galmes J, Medrano H, Flexas J. 2007. Photosynthetic limitations in response to water stress and recovery in Mediterranean plants with different growth forms. New Phytol. 175(1):81–93. 10.1111/j.1469-8137.2007.02087.x. [DOI] [PubMed] [Google Scholar]
- Gauthier PP, Crous KY, Ayub G, Duan H, Weerasinghe LK, Ellsworth DS, Tjoelker MG, Evans JR, Tissue DT, Atkin OK. 2014. Drought increases heat tolerance of leaf respiration in Eucalyptus globulus saplings grown under both ambient and elevated atmospheric [CO2] and temperature. J Exp Bot. 65(22):6471–6485. 10.1093/jxb/eru367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimeno TE, Sommerville KE, Valladares F, Owen KA. 2010. Homeostasis of respiration under drought and its important consequences for foliar carbon balance in a drier climate: insights from two contrasting Acacia species. Funct Plant Biol. 37:323–333. [Google Scholar]
- Groom PK, Lamont BB, Kupsky L. 1994. Contrasting morphology and mcophysiology of co-occurring broad and terete leaves in Hakea trifurcata (Proteaceae). Aust J Bot. 42:307–320. [Google Scholar]
- Guo JS, Hultine KR, Koch GW, Kropp H, Ogle K. 2020. Temporal shifts in iso/anisohydry revealed from daily observations of plant water potential in a dominant desert shrub. New Phytol. 225:713–726. 10.1111/nph.16196. [DOI] [PubMed] [Google Scholar]
- Kannenberg SA, Guo JS, Novick KA, Anderegg WR, Feng X, Kennedy D, Konings AG, Martínez-Vilalta J, Matheny AM. 2022. Opportunities, challenges and pitfalls in characterizing plant water-use strategies. Funct Ecol. 36:24–37. 10.1111/1365-2435.13945. [DOI] [Google Scholar]
- Katul G, Leuning R, Oren R. 2003. Relationship between plant hydraulic and biochemical properties derived from a steady-state coupled water and carbon transport model. Plant Cell Environ. 26:339–350. [Google Scholar]
- Klein T. 2014. The variability of stomatal sensitivity to leaf water potential across tree species indicates a continuum between isohydric and anisohydric behaviours. Funct Ecol. 28:1313–1320. 10.1111/1365-2435.12289. [DOI] [Google Scholar]
- Lamont BB. 1993. Why are hairy root clusters so abundant in the most nutrient-impoverished soils of Australia? Plant Soil. 155(156):269–272. [Google Scholar]
- Lamont BB. 2003. Structure, ecology and physiology of root clusters - a review. Plant Soil. 248:1–19. [Google Scholar]
- Lamont BB, Groom PK, Cowling RM. 2002. High leaf mass per area of related species assemblages may reflect low rainfall and carbon isotope discrimination rather than low phosphorus and nitrogen concentrations. Funct Ecol. 16:403–412. [Google Scholar]
- Landis CW. 1999. Seedling propagation. The container tree nursery manual, Vol. 4. USDA Forest Service, WA, USA. [Google Scholar]
- Lemoine NP, Griffin-Nolan RJ, Lock AD. 2018. Drought timing, not previous drought exposure, determines sensitivity of two shortgrass species to water stress. Oecologia. 188:965–975. 10.1007/s00442-018-4265-5. [DOI] [PubMed] [Google Scholar]
- Limousin JM, Bickford CP, Dickman LT, Pangle RE, Hudson PJ, Boutz AL, Gehres N, Osuna JL, Pockman WT, NG MD. 2013. Regulation and acclimation of leaf gas exchange in a piñon-juniper woodland exposed to three different precipitation regimes. Plant Cell Environ. 36(10):1812–1825. 10.1111/pce.12089. [DOI] [PubMed] [Google Scholar]
- Limousin JM, Yepez EA, McDowell NG, Pockman WT, Tjoelker M. 2015. Convergence in resource use efficiency across trees with differing hydraulic strategies in response to ecosystem precipitation manipulation. Funct Ecol. 29(9):1125–1136. 10.1111/1365-2435.12426. [DOI] [Google Scholar]
- Manzoni S, Vico G, Katul G, Fay PA, Polley W, Palmroth S, Porporato A. 2011. Optimizing stomatal conductance for maximum carbon gain under water stress: a meta-analysis across plant functional types and climates. Funct Ecol. 25(3):456–467. 10.1111/j.1365-2435.2010.01822.x. [DOI] [Google Scholar]
- Marchin RM, Medlyn BE, Tjoelker MG, Ellsworth DS. 2023. Decoupling between stomatal conductance and photosynthesis occurs under extreme heat in broadleaf tree species regardless of water access. Glob Chang Biol. 29:6319–6335. 10.1111/gcb.16929. [DOI] [PubMed] [Google Scholar]
- Martínez-Vilalta J, Garcia-Forner N. 2017. Water potential regulation, stomatal behaviour and hydraulic transport under drought: deconstructing the iso/anisohydric concept. Plant Cell Environ. 40(6):962–976. [DOI] [PubMed] [Google Scholar]
- Martinez-Vilalta J, Poyatos R, Aguade D, Retana J, Mencuccini M. 2014. A new look at water transport regulation in plants. New Phytol. 204(1):105–115. 10.1111/nph.12912. [DOI] [PubMed] [Google Scholar]
- Maslin BR, Reid JE. 2012. A taxonomic revision of Mulga (Acacia aneura and its close relatives: Fabaceae) in Western Australia. West Aust Herb. 22(4):129–267. [Google Scholar]
- McDowell N, Pockman WT, Allen CD, Breshears DD, Cobb N, Kolb T, Plaut J, Sperry J, West A, Williams DGet al. 2008. Mechanisms of plant survival and mortality during drought: why do some plants survive while others succumb to drought? New Phytol. 178(4):719–739. 10.1111/j.1469-8137.2008.02436.x. [DOI] [PubMed] [Google Scholar]
- McDowell NG, Sapes G, Pivovaroff A, Adams HD, Allen CD, Anderegg WRL, Arend M, Breshears DD, Brodribb T, Choat Bet al. 2022. Mechanisms of woody-plant mortality under rising drought, CO2 and vapour pressure deficit. Nat Rev Earth Environ. 3(5):294–308. 10.1038/s43017-022-00272-1. [DOI] [Google Scholar]
- Meinzer FC, Johnson DM, Lachenbruch B, McCulloh KA, Woodruff DR. 2009. Xylem hydraulic safety margins in woody plants: coordination of stomatal control of xylem tension with hydraulic capacitance. Funct Ecol. 23(5):922–930. 10.1111/j.1365-2435.2009.01577.x. [DOI] [Google Scholar]
- Mitchell PJ, O'Grady AP, Tissue DT, Worledge D, Pinkard EA. 2014. Co-ordination of growth, gas exchange and hydraulics define the carbon safety margin in tree species with contrasting drought strategies. Tree Physiol. 34(5):443–458. 10.1093/treephys/tpu014. [DOI] [PubMed] [Google Scholar]
- Nolan RH, Fairweather KA, Tarin T, Santini NS, Cleverly J, Faux R, Eamus D. 2017a. Divergence in plant water-use strategies in semiarid woody species. Funct Plant Biol. 44:1134–1146. 10.1071/fp17079. [DOI] [PubMed] [Google Scholar]
- Nolan RH, Tarin T, Fairweather KA, Cleverly J, Eamus D. 2017b. Variation in photosynthetic traits related to access to water in semiarid Australian woody species. Funct Plant Biol. 44(11):1087–1097. 10.1071/fp17096. [DOI] [PubMed] [Google Scholar]
- Nolan RH, Tarin T, Santini NS, McAdam SAM, Ruman R, Eamus D. 2017c. Differences in osmotic adjustment, foliar abscisic acid dynamics, and stomatal regulation between an isohydric and anisohydric woody angiosperm during drought. Plant Cell Environ. 40:3122–3134. 10.1111/pce.13077. [DOI] [PubMed] [Google Scholar]
- O'Grady AP, Eamus D, Cook PG, Lamontagne S. 2006. Groundwater use by riparian vegetation in the wet–dry tropics of northern Australia. Aust J Botany. 54(2):145–154. 10.1071/bt04164. [DOI] [PubMed] [Google Scholar]
- O'Grady AP, Cook PG, Eamus D, Duguid A, Wischusen JD, Fass T, Worldege D. 2009. Convergence of tree water use within an arid-zone woodland. Oecologia. 160(4):643–655. 10.1007/s00442-009-1332-y. [DOI] [PubMed] [Google Scholar]
- Page GF, Liu J, Grierson PF. 2011. Three-dimensional xylem networks and phyllode properties of co-occurring Acacia. Plant Cell Environ. 34(12):2149–2158. 10.1111/j.1365-3040.2011.02411.x. [DOI] [PubMed] [Google Scholar]
- Page GFM, Merchant A, Grierson PF. 2016. Inter-specific differences in the dynamics of water use and pulse-response of co-dominant canopy species in a dryland woodland. J Arid Environ. 124:332–340. 10.1016/j.jaridenv.2015.09.004. [DOI] [Google Scholar]
- Peñuelas J, Lloret F, Montoya R. 2001. Severe drought effects on Mediterranean woody flora in Spain. Forest Sci. 47(2):214–218. [Google Scholar]
- Petrík P, Petek-Petrik A, Mukarram M, Schuldt B, Lamarque LJ. 2023. Leaf physiological and morphological constraints of water-use efficiency in C3 plants. AoB Plants. 15(4):plad047. 10.1093/aobpla/plad047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poorter H, Niinemets Ü, Poorter L, Wright IJ, Villar R. 2009. Causes and consequences of variation in leaf mass per area (LMA): a meta-analysis. New Phytol. 182(3):565–588. 10.1111/j.1469-8137.2009.02830.x. [DOI] [PubMed] [Google Scholar]
- Poorter H, Niklas KJ, Reich PB, Oleksyn J, Poot P, Mommer L. 2012. Biomass allocation to leaves, stems and roots: meta-analyses of interspecific variation and environmental control. New Phytol. 193(1):30–50. 10.1111/j.1469-8137.2011.03952.x. [DOI] [PubMed] [Google Scholar]
- R Development Core Team . 2016. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria, http://www.Rproject.org/. [Google Scholar]
- Reichstein M, Bahn M, Mahecha MD, Kattge J, Baldocchi DD. 2014. Linking plant and ecosystem functional biogeography. Proc Natl Acad Sci USA. 111(38):13697–13702. 10.1073/pnas.1216065111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resco V, Ewers BE, Sun W, Huxman TE, Weltzin JF, Williams DG. 2009. Drought-induced hydraulic limitations constrain leaf gas exchange recovery after precipitation pulses in the C3 woody legume, Prosopis velutina. New Phytol. 181(3):672–682. 10.1111/j.1469-8137.2008.02687.x. [DOI] [PubMed] [Google Scholar]
- Rumman R, Cleverly J, Nolan RH, Tarin T, Eamus D. 2018. Speculations on the application of foliar 13C discrimination to reveal groundwater dependency of vegetation, provide estimates of root depth and rates of groundwater use. Hydrol Earth Syst Sci Discuss. 1–25:4875–4889. 10.5194/hess-2017-540. [DOI] [Google Scholar]
- Santiago LS, Goldstein G, Meinzer FC, Fisher JB, Machado K, Woodruff D, Jones T. 2004. Leaf photosynthetic traits scale with hydraulic conductivity and wood density in Panamanian forest canopy trees. Oecologia. 140(4):543–550. 10.1007/s00442-004-1624-1. [DOI] [PubMed] [Google Scholar]
- Santini NS, Cleverly J, Faux R, Lestrange C, Rumman R, Eamus D. 2015. Xylem traits and water-use efficiency of woody species co-occurring in the Ti Tree Basin arid zone. Trees. 30(1):295–303. 10.1007/s00468-015-1301-5. [DOI] [Google Scholar]
- Santini NS, Cleverly J, Faux R, McBean K, Nolan R, Eamus D. 2017. Root xylem characteristics and hydraulic strategies of species co-occurring in semi-arid Australia. IAWA J. 39(1):43–62. 10.1163/22941932-20170188. [DOI] [Google Scholar]
- Sperry JS, Love DM. 2015. What plant hydraulics can tell us about responses to climate-change droughts. New Phytol. 207(1):14–27. 10.1111/nph.13354. [DOI] [PubMed] [Google Scholar]
- Tardieu F, Thierry S. 1998. Variability among species of stomatal control under fluctuating soil water status and evaporative demand: modelling isohydric and anisohydric behaviours. J Exp Bot. 49(Issue Special Issue):419–432. 10.1093/jxb/49.Special_Issue.419. [DOI] [Google Scholar]
- Tarin T, Nolan RH, Medlyn BE, Cleverly J, Eamus D. 2019. Water-use efficiency in a semi-arid woodland with high rainfall variability. Glob Chang Biol. 26(2):496–508. 10.1111/gcb.14866. [DOI] [PubMed] [Google Scholar]
- Tarin T, Nolan RH, Eamus D, Cleverly J. 2020. Carbon and water fluxes in two adjacent Australian semi-arid ecosystems. Agric For Meteorol. 281:107853. 10.1016/j.agrformet.2019.107853. [DOI] [Google Scholar]
- Thomas DS, Eamus D, Shanahan S. 2000. Influence of season, drought and xylem ABA on stomatal responses to leaf-to-air vapour pressure difference of trees of the Australian wet–dry tropics. Aust J Bot. 48:143–151. [Google Scholar]
- Vandegeer RK, Tissue DT, Hartley SE, Glauser G, Johnson SN. 2020. Physiological acclimation of a grass species occurs during sustained but not repeated drought events. Environ Exp Bot. 171:103954. 10.1016/j.envexpbot.2019.103954. [DOI] [Google Scholar]
- Vogan PJ, Maherali H. 2014. Increased photosynthetic capacity as a mechanism of drought adaptation in C3 plants. Int J Plant Sci. 175(9):1033–1041. 10.1086/678088. [DOI] [Google Scholar]
- Wang H, Prentice CI, Wright IJ, Warton DI, Qiao S, Xu X, Zhou J, Kikuzawa K, Stenseth NC. 2022. Leaf economics fundamentals explained by optimality principles. Sci Adv. 9:eadd5667. 10.1126/sciadv.add5667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warton DI, Duursma RA, Falster DS, Taskinen S. 2012. smatr 3- an R package for estimation and inference about allometric lines. Methods Ecol Evol. 3(2):257–259. 10.1111/j.2041-210X.2011.00153.x. [DOI] [Google Scholar]
- Yin Q, Wang L, Lei M, Dang H, Quan J, Tian T, Chai Y, Yue M. 2018. The relationships between leaf economics and hydraulic traits of woody plants depend on water availability. Sci Total Environ. 621:245–252. 10.1016/j.scitotenv.2017.11.171. [DOI] [PubMed] [Google Scholar]
- Zhao M, Running S. 2010. Drought-induced reduction in global terrestrial net primary production from 2000 throught 2009. Science. 329:940–943. [DOI] [PubMed] [Google Scholar]
- Zhou SX, Medlyn BE, Prentice IC. 2016. Long-term water stress leads to acclimation of drought sensitivity of photosynthetic capacity in xeric but not riparian Eucalyptus species. Ann Bot. 117(1):133–144. 10.1093/aob/mcv161. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.