Abstract
Adenovirus vectors have been studied as vehicles for gene transfer to skeletal muscle, an attractive target for gene therapies for inherited and acquired diseases. In this setting, immune responses to viral proteins and/or transgene products cause inflammation and lead to loss of transgene expression. A few studies in murine models have suggested that the destructive cell-mediated immune response to virally encoded proteins of E1-deleted adenovirus may not contribute to the elimination of transgene-expressing cells. However, the impact of immune responses following intramuscular administration of adenovirus vectors on transgene stability has not been elucidated in larger animal models such as nonhuman primates. Here we demonstrate that intramuscular administration of E1-deleted adenovirus vector expressing rhesus monkey erythropoietin or growth hormone to rhesus monkeys results in generation of a Th1-dependent cytotoxic T-cell response to adenovirus proteins. Transgene expression dropped significantly over time but was still detectable in some animals after 6 months. Systemic levels of adenovirus-specific neutralizing antibodies were generated, which blocked vector readministration. These studies indicate that the cellular and humoral immune response generated to adenovirus proteins, in the context of transgenes encoding self-proteins, hinders long-term transgene expression and readministration with first-generation vectors.
Somatic gene transfer to muscle is being investigated for treatment of muscle-specific disorders, such as muscular dystrophies. In addition, the ability of various viral and nonviral vector systems to transduce muscle fibers with genes encoding secreted products, e.g., coagulation factor IX, α1-antitrypsin, erythropoietin, and growth hormone, may have systemic benefits (2, 24). Skeletal muscles have also proved useful for the delivery of DNA-based vaccines (5). Recombinant adenoviruses transduce fibers of skeletal muscle with great efficiency (3, 16, 21, 28, 29, 35). Furthermore, the low probability of insertional mutagenesis and large capacity for genes such as truncated dystrophin are major advantages of this vector system (8). However, in vivo administration of adenovirus vectors, such as intramuscular, is characterized by inflammation, infiltration of CD4+ and CD8+ T lymphocytes, myonecrosis, and extinction of recombinant gene expression (6, 22, 25, 32, 34, 38).
The mechanism of transient transgene expression has been attributed at least in part to activation of major histocompatibility complex (MHC) class I-restricted cytotoxic T lymphocytes (CTL) directed against the recombinant and viral proteins generated by de novo synthesis in the transduced tissue. Extended transgene expression observed with E1-deleted adenovirus vectors injected into skeletal muscle in rodents with genetic defects in immunity or immune suppressed with pharmacological agents supports the hypothesis that cellular immunity contributes to transgene elimination (4, 14, 15, 20, 30). In addition, administration of adenovirus vectors in vivo leads to generation of CD4+ T-cell-dependent humoral immune responses, characterized by neutralizing antibodies (NAB) against the adenovirus vector capsid proteins. The presence of NAB limits readministration of these vectors into rodent muscle.
Recent observations indicate that the antigenicity of the transgene product encoded within the adenovirus vector can impact the stability of transgene expression (7, 9, 12, 33, 39). Recombinant proteins expressed from adenovirus vectors, recognized as “self,” have been reported to be stably expressed in rodent muscle, suggesting that the destructive cellular response to proteins of viral genes is not significant. Svennson et al. tested this hypothesis in a nonhuman primate model for 84 days. That study was limited not only in the length of observation but also in reporting only a limited number of serum erythropoietin values and therefore not addressing the stability of transduction, and did not evaluate host cellular immune responses to the vector (31).
In this study, rhesus monkey erythropoietin and growth hormone genes were isolated and incorporated into first-generation adenovirus vectors to evaluate the stability of transgene expression in rhesus monkey muscle in the absence of transgene-specific responses. Since both proteins are secreted into the systemic circulation, the protein levels in serum served as surrogate markers for gene transfer and transgene expression. The dynamics of transgene expression and host cellular and humoral immune responses to the viral vector and transgene-encoded proteins were monitored. The extent of transduction of skeletal muscle following a second intramuscular administration of vector was also examined.
MATERIALS AND METHODS
Animals and specimen collection.
Wild-caught juvenile rhesus monkeys were purchased from the Southwest Foundation for Biomedical Research (San Antonio, Tex.) and kept in full quarantine. The monkeys weighed approximately 3 to 4 kg and were serologically negative for simian immunodeficiency virus, simian T-cell lymphotropic virus, other simian retroviruses, and human adenovirus. The protocol was approved by the Infection Control Committee of the Hospital of the University of Pennsylvania, the Environmental Health and Safety Office, the Institutional Biosafety Committee, and the Institutional Animal Care and Use Committee of the University of Pennsylvania.
Cloning of genes.
The cDNA for rhesus erythropoietin was isolated as described (42). The rhesus pituitary growth hormone cDNA was a kind gift from T. G. Golos, Wisconsin Regional Primate Research Center (13).
Vectors.
E1-deleted adenoviruses expressing rhesus monkey growth hormone (H5.010CMVrhGH) or rhesus monkey erythropoietin (H5.010CMVrhEPO), henceforth called Ad-rhGH and Ad-rhEPO, respectively, were made by transfection-infection protocols described earlier (11). Virus was produced according to Good Manufacturing Practices and obtained from the Human Applications Laboratory of the Institute for Human Gene Therapy. The virus was characterized at a particle-to-PFU ratio of 100.
Serum growth hormone and erythropoietin.
Levels of growth hormone and erythropoietin were determined from appropriately diluted serum samples using the human growth hormone enzyme-linked immunosorbent assay (ELISA) kit (Roche Molecular Biochemicals, Mannheim, Germany) and the Quantikine IVD erythropoietin ELISA kit (R&D Systems, Inc., Minneapolis, Minn.) respectively, following the manufacturers' instructions. Repeated analysis of the same sample revealed consistent values that differed by less than 10%. Octreotide acetate (Sandostatin; Sandoz Pharmaceuticals, East Hanover, N.J.) was administered as an intravenous bolus at a dose of 1.4 μg/kg per rhesus monkey for suppression of endogenous secretion of growth hormone.
Lymphoproliferative responses.
Peripheral blood mononuclear cells (PBMC) from the various time points of the study were separated by Ficoll-Hypaque density gradient centrifugation. Triplicate cultures of 100 μl of PBMC (106 cells/ml) were cultured at a multiplicity of infection (MOI) of 10 with inactivated Ad-LacZ, 100 ng of Staphylococcus enterotoxin B (SEB; Toxin Technologies, Sarasota, Fla.) per ml, or medium alone. SEB- and antigen-stimulated cultures were harvested on day 6. Proliferation was measured by a 16-h [3H]thymidine (1 μCi/well) pulse. Results are presented as a stimulation index, which represents the ratio of cpm in adenovirus-stimulated cultures to that in cultures with medium alone.
Cytokine release assays.
PBMC were cultured with or without antigen (inactivated Ad-LacZ at a cell-to-particle ratio of 10) for 48 h in a 24-well plate. Cell supernatants were collected and analyzed for the presence of interleukin-2 (IL-2), IL-4, gamma interferon (IFN-γ), and IL-10 by commercial ELISA kits (BioSource International, Camarrillo, Calif.) using the manufacturers' protocols.
Adenovirus-specific immunoglobulins.
Serum (diluted 1:200) samples from animals were analyzed for adenovirus-specific isotype-specific immunoglobulins (IgM, IgG1, IgG2, and IgG4) by ELISA. For the ELISA, 96-well flat-bottom, high-binding Immulon-IV plates were coated with 100 μl of adenovirus antigen (5 × 109 particles/ml) in phosphate-buffered saline (PBS) overnight at 4°C, washed four times in PBS containing 0.05% Tween, and blocked in PBS supplemented with 1% bovine serum albumin for 1 h at 37°C. Appropriately diluted samples were added to antigen-coated plates and incubated for 4 h at 37°C. Plates were washed four times in PBS–0.05% Tween and incubated with peroxidase-conjugated goat anti-human IgM, IgG1, IgG2, or IgG4 (1:2,000 dilution; Sigma Chemical Co., St. Louis, Mo.) for 2 h at 37°C. Plates were washed as described above, and 2,2′-azinobis(3-ethylbenzthiazolinesulfonic acid) (ABTS) substrate (Kirkegaard and Perry, Gaithersburg, Md.) was added. Optical densities were read at 405 nm on a microplate reader (Dynatech Laboratories, Chantilly, Va.).
Antiadenovirus NAB.
NAB titers were analyzed by determining the ability of serum to inhibit transduction of reporter virus, adenovirus expressing green fluorescent protein (Ad-GFP), into HeLa cells. Various dilutions of antibodies preincubated with the reporter virus for 1 h at 37°C were added to 90% confluent HeLa cell cultures. Cells were incubated for 16 h, and expression of GFP was analyzed with a FluoroImager (Molecular Dynamics, Sunnyvale, Calif.). The neutralizing titer of antibody was calculated as the highest dilution at which 50% of the cells turned green.
CTL. (i) Target cells.
Autologous transformed B-lymphoblastoid cell lines (B-LCL) from the study animal were transformed by incubating PBMC at 37°C with herpesvirus papio-derived supernatant of S594 cells (provided by Norman Letvin, Beth Israel Hospital, Boston, Mass.) and cyclosporin (1 μg/ml) in RPMI 1640 with 20% fetal bovine serum, 10 mM HEPES, 2 mM l-glutamine, 50 IU of penicillin/ml, and 50 μg of streptomycin/ml (complete RPMI). A transformed cell line could be generated in one of the four animals in this study (RQ1828).
(ii) Effector and stimulator cells.
PBMC isolated from heparinized blood were suspended at 2 × 106 cells/ml in complete RPMI medium. For antigen-specific stimulation, B-LCL were infected with recombinant vaccinia viruses (adenovirus types hexon, penton, and fiber [18]) overnight, washed, and inactivated with long-wave UV irradiation (Fisher model IV, 350 to 400 nm wavelength at a distance of 3.5 cm for 10 min) in the presence of 10 μg of psoralen (HRI Associated)/ml. Cells were washed and used as stimulators at a stimulator-responder ratio of 1:10. On day 4 of culture, 20 U of recombinant IL-2 (Boehringer, Mannheim, Germany) was added to the culture. CTL assays were performed 14 days after stimulation.
(iii) Chromium release assays.
Target B-LCL cells were infected with vaccinia viruses (hexon, penton, or fiber) overnight and labeled with 100 μCi of 51chromium per 106 cells. Target cells (103 cells) were dispensed in triplicate for each effector-target cell ratio in 96-well V-bottom plates (Falcon) for 5 h. Plates were spun at 500 rpm for 10 min, after which 30 μl of supernatant was harvested from each well into wells of a LumaPlate-96 (Packard) and allowed to dry overnight. Emitted radioactivity was measured on a MicroBeta liquid scintillation counter (Wallac, Turku, Finland). Spontaneous release was measured from wells containing target cells alone. Maximum release was measured from wells containing target cells and 0.1% Triton X-100 (Sigma). The percent specific cytotoxicity was calculated as follows: [(test release − spontaneous release)/(maximum release − spontaneous release)] × 100.
RESULTS
Skeletal muscle transduction with first-generation adenovirus vectors in nonhuman primates.
Reporter genes encoding the secreted hormones erythropoietin and growth hormone were used in these studies. Both were derived from cDNA clones of rhesus monkey, so the encoded proteins should be viewed as self.
It was necessary to suppress endogenous growth hormone in order to specifically detect recombinant-derived growth hormone. Endogenous growth hormone is secreted from the pituitary in random bursts (17, 26). The hypothalamic peptides growth hormone-releasing hormone and somatostatin critically regulate its secretion. The somatostatin analogue octreotide acetate, administered intravenously at a dose of 1.4 mg/kg, transiently suppresses the endogenous secretion of growth hormone (1) without affecting secretion of recombinant hormone from skeletal muscle. Because of the very short serum half-life of growth hormone (20 min in humans) (36), the transient suppression of secretion by octreotide acetate resulted in a quick decay of the serum levels of endogenous growth hormone, providing a window of several hours in which to measure the transgene product. The effect of octreotide acetate administration on serum growth hormone levels was measured in eight rhesus monkeys (data not shown). At 3 h postadministration of octreotide acetate, serum growth hormone levels reliably reached their lowest point, less than 200 pg/ml. Therefore, serum growth hormone levels in excess of 200 pg/ml at 3 h post-octreotide administration were attributed to the constitutively expressed transgene.
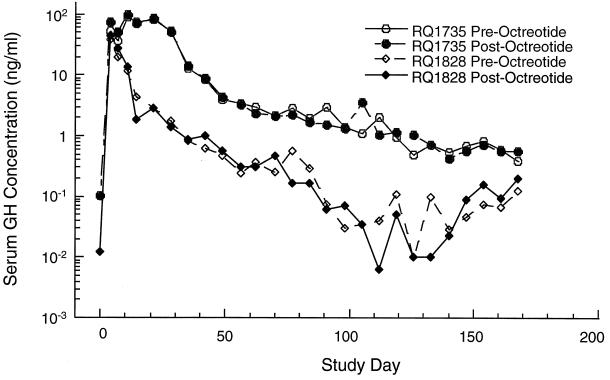
Following a single intramuscular administration of Ad-rhGH, serum growth hormone concentrations were measured prior to and 3 h postadministration of octreotide acetate. Serum growth hormone levels under octreotide acetate suppression in animal RQ1735 were substantially elevated for the first month, peaking at 200-fold above background (Fig. 1); the levels declined rapidly during the next month and then gradually decayed over the remaining 6 months of the experiment. Transgene expression was lost after the second month following vector administration in RQ1828. In this animal, the initial peak of growth hormone was lower than that of RQ1735, and it decreased more rapidly.
FIG. 1.
Serum levels of growth hormone (GH). Rhesus monkeys were administered Ad-rhGH intramuscularly, and serum was obtained at various time points and analyzed for vector-induced GH levels as described in the text. Serum levels following vector administration are presented both before and after octreotide administration. Postoctreotide levels indicate vector-induced growth hormone.
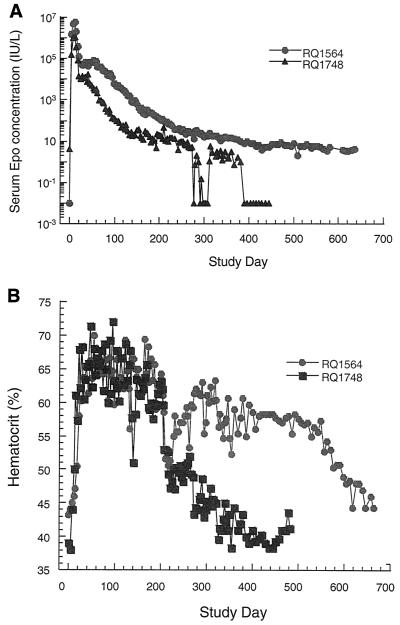
Animals also received vector expressing rhesus monkey erythropoietin from a constitutive promoter. Endogenous erythropoietin levels are low in a nonanemic animal and completely suppressed when excess recombinant erythropoietin drives red blood cell production into polycythemic ranges. Animals that received adenovirus expressing erythropoietin demonstrated an early peak of expression within 2 weeks of vector administration to levels that were 106-fold above baseline (Fig. 2A). A rapid descent of 10- to 100-fold was followed by a short plateau and then a gradual decline in serum levels over the course of the experiment. Erythropoietin levels in RQ1564 were more sustained than in RQ1748. In spite of the differences in the absolute erythropoietin concentrations between the monkeys, the hematocrit values were comparably elevated over the initial 250 days of the experiment for both monkeys (Fig. 2B), requiring weekly therapeutic phlebotomies to maintain levels below 65%.
FIG. 2.
Serum erythropoietin (Epo) levels (A) and hematocrits (B). Rhesus monkeys were administered Ad-rhEPO intramuscularly, and blood obtained at various time points was analyzed for serum Epo levels or hematocrits.
Cellular immune responses to adenovirus vectors.
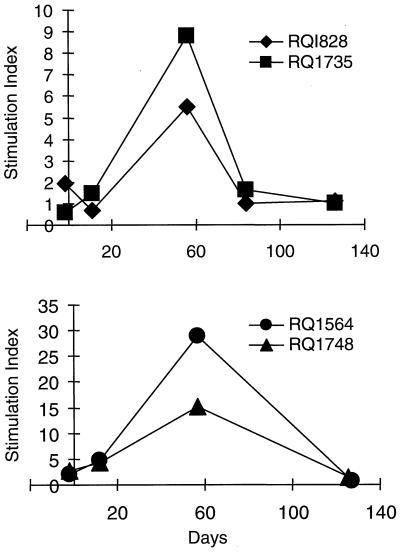
CD4+ T-cell responses to adenovirus vectors were studied from lymphocytes periodically isolated from peripheral blood in the four animals. Lymphoproliferation in response to inactivated adenovirus vector peaked at approximately 2 months post-intramuscular vector administration (Fig. 3). The return to baseline or pre-vector administration response level occurred within 4 months.
FIG. 3.
Lymphoproliferative responses following adenovirus vector administration in muscle. Rhesus monkeys were administered either Ad-rhGH (RQ1828 and RQ1735) or Ad-rhEPO (animals RQ154 and RQ1748) intramuscularly as described in the text. Heparinized blood was drawn prior to and on various days following vector administration. PBMC were isolated by Ficoll-Hypaque density gradient centrifugation and cultured in medium alone or in the presence of adenovirus antigens for 6 days. Responses were measured by [3H]thymidine incorporation and expressed as the stimulation index (see text). The top panel shows responses in animals administered Ad-rhGH, and the lower panel shows responses in animals administered Ad-rhEPO.
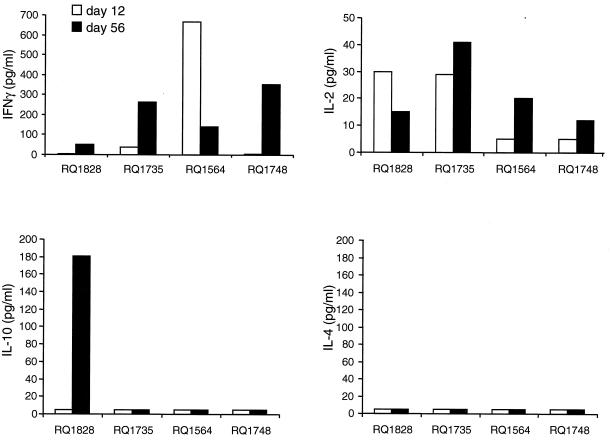
The cytokine secretion profile of activated CD4+ T lymphocytes is shown in Fig. 4. The vector-antigen-specific release of IFN-γ and IL-2 is consistent with a Th1 response. IL-10 secretion was observed in RQ1828 only; however, this cytokine may not differentiate a Th1 from a Th2 response in primates (27). IL-4, a cytokine associated with a Th2 response, was not detected.
FIG. 4.
Cytokine secretion profiles following intramuscular administration of adenovirus vectors in rhesus monkeys. Rhesus monkeys were administered either Ad-rhGH (animals RQ1828 and RQ1735) or Ad-rhEPO (animals RQ1564 and RQ1748) intramuscularly as described in the text on day 1. For induction of cytokines, PBMC obtained on various days were cultured with adenovirus antigens for 48 h. Culture supernatants were collected and measured in duplicate for the presence of IFN-γ, IL-2, IL-4, and IL-10 using commercial ELISA kits (BioSource International) as described by the manufacturer. No significant IL-4 levels could be measured in the culture supernatants, although the extent of cross-reactivity of this human-based assay with rhesus IL-4 is unknown.
At several time points, cellular responses to growth hormone and erythropoietin were studied. Neither reporter molecule stimulated a lymphoproliferative response or induced secretion of IFN-γ, IL-2, IL-4, or IL-10 (data not shown).
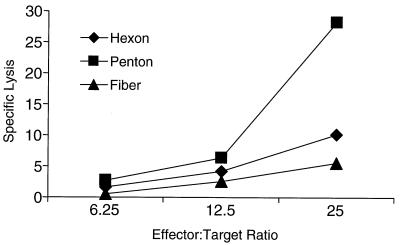
CTL responses were measured in animal RQ1828; other animals could not be tested due to difficulties in generating B-cell lines from those rhesus monkeys. Specific antigens were expressed in target cells by infection with a panel of recombinant vaccinia viruses. Figure 5 shows the presence of CTL responses to penton and minor responses to hexon and fiber on a sample drawn on day 60. No CTL responses to cells infected with vaccinia virus expressing LacZ were observed (data not shown).
FIG. 5.
CTL responses to adenovirus proteins in rhesus monkeys. CTL responses to hexon, penton, and fiber were measured using autologous herpesvirus papio-transformed B lymphocytes from rhesus monkey RQ1828, which received Ad-rhGH. Effector T cells were generated by culturing PBMC for 14 days in the presence of Ad-GFP and IL-2. Autologous target cells were infected with vaccinia virus expressing hexon, penton, or fiber. Specific lysis was measured as described in the text.
Humoral immune responses to adenovirus.
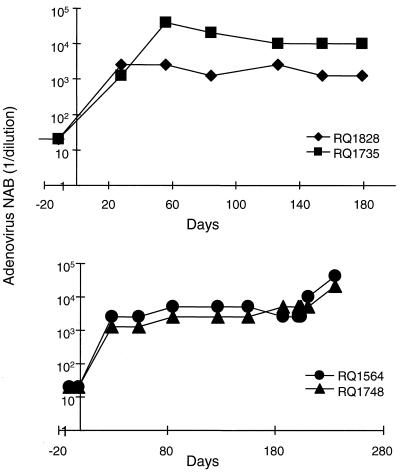
Sera obtained from the monkeys were analyzed for the presence of NAB, which developed within 3 weeks of vector administration in all animals (Fig. 6). The titers were similar in all animals except RQ1828, in which the titers were approximately 10-fold higher on day 56 and remained elevated compared to the samples from the other monkeys.
FIG. 6.
NAB in serum following intramuscular administration of adenovirus vectors in rhesus monkeys. Sera were obtained from animals administered either Ad-rhGH (RQ1828 and RQ1735) or Ad-rhEPO (RQ1564 and RQ1748) on various days. Animals RQ1564 and RQ1748 were readministered Ad-rhGH on day 210. NAB were measured by the ability of sera to interfere with the infection of HeLa cells with adenovirus expressing GFP. Various dilutions of serum preincubated with the reporter virus for 1 h at 37°C were added to 90% confluent HeLa cell cultures. Cells were incubated for 24 h, and expression of GFP was measured by fluoroimaging. The neutralizing titer of the serum was calculated as the highest dilution at which 50% of the cells turned green.
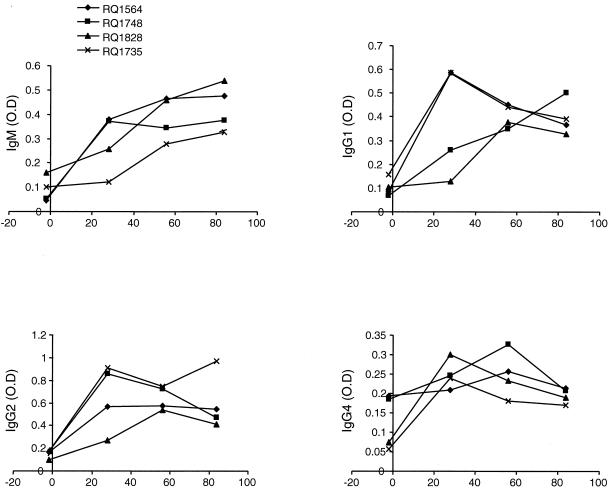
Isotype characterization of the antiadenovirus immunoglobulin was performed with an ELISA. All animals showed an IgM, IgG1, IgG2, and IgG4 response (Fig. 7). Monkeys who received the vector encoding growth hormone had a stronger IgG4 and a weaker IgM response than monkeys who received the erythropoietin vector.
FIG. 7.
Adenovirus vector-specific immunoglobulin isotypes in serum following intramuscular administration of adenovirus vectors in rhesus monkeys. Sera obtained from animals administered Ad-rhGH or Ad-rhEPO were analyzed for the presence of adenovirus-specific immunoglobulin isotypes IgM, IgG1, IgG2, and IgG4 by ELISA as described in the text. Results are expressed as optical density (O.D.).
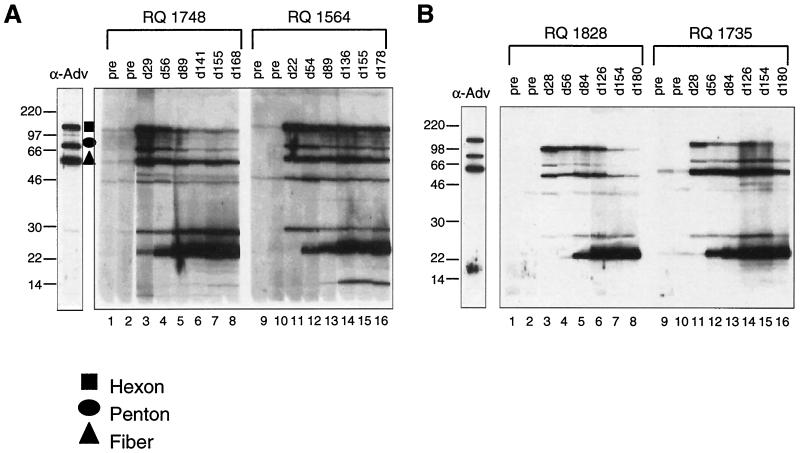
To further define the molecular basis for the humoral response to the adenovirus vector, sera from the animals were evaluated by Western blot analysis for the presence of antibodies to the viral structural components (Fig. 8A and B). Antibodies to epitopes on the major surface proteins (hexon, penton, and fiber; high-molecular-weight bands in Fig. 8) were detected within 1 month of vector administration. A delay in the development of antibodies to some of the minor structural components was observed.
FIG. 8.
Analyses of antibodies to viral capsid proteins. Sera obtained from animals administered either Ad-rhGH or Ad-rhEPO intramuscularly were analyzed for the presence of antibodies to various components of adenovirus capsid proteins by Western blot analyses. Adenovirus antigens were electrophoresed on a 10% polyacrylamide gel and probed with serum obtained preadministration on various days (d) after. Reactivities were visualized by treatment with peroxidase-conjugated goat anti-human IgG followed by chemiluminescence. The positions of hexon, penton, and fiber proteins were determined by using antibodies directed against individual components and are indicated on the figure (data not shown). The lower-molecular-weight proteins comprise late gene proteins associated with the vector particle. Sizes are shown in kilodaltons.
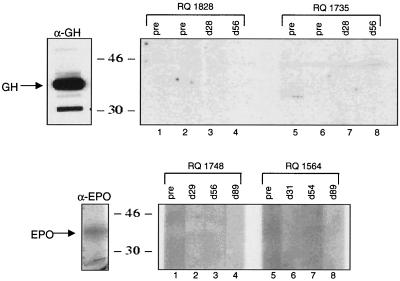
To examine for the presence of antibodies to transgene-encoded proteins, sera were tested for reactivity to growth hormone and erythropoietin on immunoblots. No antibody response to growth hormone or erythropoietin was observed in any of the experimental animals (Fig. 9).
FIG. 9.
Lack of antibodies to growth hormone (GH) and erythropoietin (EPO) in sera from animals administered adenovirus vectors. Sera obtained from animals administered either Ad-rhGH (animals RQ1828 and RQ1735) or Ad-rhEPO (animals RQ1564 and RQ1748) intramuscularly were analyzed for the presence of antibodies to growth hormone (α-GH) or erythropoietin (α-EPO) by Western blot analyses as described in the text. The positions of the proteins were determined using polyclonal antibodies obtained from mice injected with Ad-rhGH or Ad-rhEPO. Sizes are shown in kilodaltons.
Readministration of recombinant adenovirus vectors.
To test the hypothesis that NAB preclude gene transfer in nonhuman primates, RQ1564 and RQ1748, previously treated with adenovirus expressing erythropoietin, were injected with adenovirus expressing growth hormone. Prior to vector administration, a series of octreotide acetate suppression kinetic tests were performed. This required a significant number of blood draws, which decreased the hematocrit despite persistently elevated erythropoietin (Fig. 2B). As in the initial suppression studies, an octreotide acetate dose of 1.4 μg/kg transiently suppressed endogenous growth hormone secretion. A 3-h post-drug administration serum growth hormone level reached a nadir of less than 200 pg/ml (data not shown). Octreotide acetate did not interfere with the assay for erythropoietin, nor was there an impact on the erythropoietin levels, suggesting no effect of the drug on expression from the transgene. All measurements for erythropoietin were performed on samples of blood obtained prior to administration of octreotide acetate.
Adenovirus expressing growth hormone was intramuscularly administered on study day 210. No significant elevation in serum growth hormone was evident during the octreotide acetate suppression except in animal RQ1564, in which there was a transient elevation of growth hormone in the range of 1,000 to 2,000 pg/ml for several weeks. The data do not support significant and sustained transduction upon a second intramuscular administration of adenovirus vectors in nonhuman primates.
DISCUSSION
An objective of this study was to test the hypothesis that transduction of skeletal muscle following intramuscular administration of an adenovirus vector would lead to prolonged transgene expression when the recombinant protein was recognized as self. In fact, transgene expression was transient in rhesus monkeys following intramuscular injection of vector. A correlation between the extent and/or duration of the peak level of serum reporter molecules and decay of expression was observed in both experimental groups; i.e., the more successful the initial transduction, the longer and more sustained was transgene expression. The initial high levels of growth hormone and erythropoietin appeared to decay in two phases. There was a pronounced fall during the first 6 to 8 weeks, followed by a more gradual rate of decline over 3 to 6 months. Levels of the recombinant proteins at the latter time points were a small fraction of the peak levels.
Previous studies have indicated that adenovirus vectors, in spite of the E1A and E1B gene deletions, express early and late viral proteins in transduced cells which serve as potential targets for MHC class I-restricted CTL responses (6, 10, 22, 25, 32, 34, 40). In addition, manipulations that block the development of CTL prolong adenovirus-mediated gene transfer and transgene expression in mice (4, 14, 15, 19, 20, 23, 30). Cellular immune responses to the vector-encoded viral proteins were detected in the rhesus monkeys in this experiment. CD4+ T-cell responses returned to baseline, as assayed from peripheral blood lymphocytes. Secretion of IFN-γ and IL-2 cytokines from vector-stimulated peripheral blood lymphocytes was consistent with a CD4+ Th1 phenotype. In addition, CTL responses directed towards hexon, penton, and fiber were demonstrated in the one animal studied.
The relative contributions of transgene product and viral gene expression to the activation of destructive CTL remain controversial. Our studies eliminated the transgene product as an immunologic target by selecting an open reading frame identical to an endogenous gene (i.e., rhesus monkey-derived erythropoietin or growth hormone). The fact that expression rapidly diminished 10- to 100-fold soon after the immediate peak argues that factors inherent to the E1-deleted vector can lead to instability. Whether this is indeed caused by CTL to viral antigens has not been directly addressed, although the concurrent activation of CD4+ T cells of the Th1 phenotype and CD8+ CTL to viral capsid protein with loss of transgene expression is suggestive.
Another interesting finding in the erythropoietin studies is that transgene expression was still detectable after 6 months, although it diminished 10,000- to 100,000-fold. Apparently a very small but detectable fraction of vector-transduced cells escape the transgene extinction processes. Detection of this small fraction of cells is clearly a function of the sensitivity of the assay, which in the case of erythropoietin is extremely high. In fact, if one simply measured hemotocrit as a biological read-out one would miss the fact that the erythropoietin level dropped 1,000,000-fold over 6 months, during which hemotocrit was persistently elevated. This also illustrates the problems of using hemotocrit as a sole measure of transgene expression. Persistence of some transgene expression for over 6 months also indicates that one cannot assume that immune-mediated clearance of vector-transduced cells will occur in therapeutic applications where transient expression is desired and persistent expression may lead to toxicity (e.g., growth factors).
Antibodies to the adenovirus structural components (major capsid proteins) developed rapidly in these rhesus monkeys. Over time, there was a gradual decrease in the signal generated against the hexon, penton, and fiber proteins, consistent with a decrease in titer to those antigens. A delay in antibodies to lower-molecular-weight structural proteins was observed. This delay may reflect the de novo synthesis of viral proteins, which would be consistent with previous evidence that indicates that vector-encoded viral genes are transcriptionally active in adenovirus vectors (37, 41).
To test the ability to transduce nonhuman primate skeletal muscle in the setting of the presence of NAB, animals who initially received Ad-rhEPO (RQ1564 and RQ1748) received a second intramuscular injection of adenovirus vector (Ad-rhGH). Limited transgene expression was observed in only one animal (RQ1564). In the setting of gene therapy, the presence of NAB to the same vector serotype will limit, if not abrogate, skeletal muscle transduction. This experiment also demonstrates that the NAB titer was maintained for at least 7 months to preclude effective gene transfer. It is possible that low-level antigen generated from residual transduced tissue was a continuous stimulus for the B-cell response.
In summary, experiments with nonhuman primate muscle indicate that factors other than immunity to the transgene product contribute to instability of transgene expression. The development of T-cell responses to viral capsid antigens supports immuno-mediated clearance. Nevertheless, residual transgene expression is detected for up to 6 months, suggesting that partial avoidance of these clearance mechanisms raises potential safety issues with intramuscular injection of first-generation adenoviruses if persistent transgene expression is unwanted.
ACKNOWLEDGMENTS
P. W. Zoltick and N. Chirmule contributed equally to this work.
We thank the personnel of the Vector, Immunology, and Toxicology cores of the Institute for Human Gene Therapy.
The work was funded by NIH (P30 DK47757-08; NHLBI R01 HL49040-10; P01 AR43648-05), the Muscular Dystrophy Association, and Genovo, Inc., a company that J. M. Wilson founded and holds equity in.
REFERENCES
- 1.Battershill P E, Clissold S P. Octreotide: a review of its pharmacodynamic and pharmacokinetic properties, and therapeutic potential in conditions associated with excessive peptide secretion. Drugs. 1989;38:658–702. doi: 10.2165/00003495-198938050-00002. [DOI] [PubMed] [Google Scholar]
- 2.Blau H M, Springer M L. Muscle-mediated gene therapy. N Engl J Med. 1995;23:1554–1556. doi: 10.1056/NEJM199512073332308. [DOI] [PubMed] [Google Scholar]
- 3.Brody S L, Crystal R G. Adenovirus-mediated in vivo gene transfer. Ann NY Acad Sci. 1994;716:90–103. doi: 10.1111/j.1749-6632.1994.tb21705.x. [DOI] [PubMed] [Google Scholar]
- 4.Bromberg J S, Debruyne L A, Qin L. Interactions between the immune system and gene therapy vectors: bidirectional regulation of response and expression. Adv Immunol. 1998;69:353–409. [PubMed] [Google Scholar]
- 5.Chattergoon M, Boyer J, Weiner D B. Genetic immunization: a new era in vaccines and immune therapeutics. FASEB J. 1997;11:753–763. doi: 10.1096/fasebj.11.10.9271360. [DOI] [PubMed] [Google Scholar]
- 6.Chen H H, Mack L M, Kelly R, Ontell M, Kochanek S, Clemens P R. Persistence in muscle of an adenoviral vector that lacks all viral genes. Proc Natl Acad Sci USA. 1997;94:1645–1650. doi: 10.1073/pnas.94.5.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chirmule N, Hughes J V, Gao G-P, Raper S E, Wilson J M. Role of E4 in eliciting CD4 T-cell and B-cell responses to adenovirus vectors delivered to murine and nonhuman primate lungs. J Virol. 1998;72:6138–6145. doi: 10.1128/jvi.72.7.6138-6145.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crystal R. Transfer of genes to humans: early lessons and obstacles to success. Science. 1995;270:404–410. doi: 10.1126/science.270.5235.404. [DOI] [PubMed] [Google Scholar]
- 9.Dai Y, Schwartz E, Gu D, Zhang W, Sarvetnick N, Verma I. Cellular and humoral immune responses to adenoviral vectors containing factor IX gene: tolerization of factor IX and vector antigens allows for long-term expression. Proc Natl Acad Sci USA. 1995;92:1401–1405. doi: 10.1073/pnas.92.5.1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Engelhardt J, Ye X, Doranz B, Wilson J. Ablation of E2A in recombinant adenoviruses improves transgene persistence and decreases inflammatory response in mouse liver. Proc Natl Acad Sci USA. 1994;91:6196–6200. doi: 10.1073/pnas.91.13.6196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Engelhardt J F, Simon R H, Yang Y, Zepeda M, Pendleton S W, Doranz B, Grossman M, Wilson J M. Adenovirus-mediated transfer of the CFTR gene to lung of nonhuman primates: biological efficacy study. Hum Gene Ther. 1993;4:759–769. doi: 10.1089/hum.1993.4.6-759. [DOI] [PubMed] [Google Scholar]
- 12.Gao G P, Yang Y, Wilson J M. Biology of adenovirus vectors with E1 and E4 deletions for liver-directed gene therapy. J Virol. 1996;70:8934–8943. doi: 10.1128/jvi.70.12.8934-8943.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Golos T G, Durning M, Fisher J M, Fowler P D. Cloning of four growth hormone/chorionic somatomammotropin-related complementary deoxyribonucleic acids differentially expressed during pregnancy in the rhesus monkey placenta. Endocrinology. 1993;133:1744–1752. doi: 10.1210/endo.133.4.8404617. [DOI] [PubMed] [Google Scholar]
- 14.Ilan Y, Attavar P, Takahashi M, Davidson A, Horwitz M S, Guida J, Chowdhury N R, Chowdhury J R. Induction of central tolerance by intrathymic inoculation of adenoviral antigens into the host thymus permits long-term gene therapy in Gunn rats. J Clin Investig. 1996;98:2640–2647. doi: 10.1172/JCI119085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ilan Y, Jona V K, Sengupta K, Davidson A, Horwitz M S, Roy-Chowdhury N, Roy-Chowdhury J. Transient immunosuppression with FK506 permits long-term expression of therapeutic genes introduced into the liver using recombinant adenoviruses in the rat. Hepatology. 1997;26:949–956. doi: 10.1002/hep.510260422. [DOI] [PubMed] [Google Scholar]
- 16.Inui K, Okada S, Dickson G. Gene therapy in Duchenne muscular dystrophy. Brain Dev. 1996;18:357–361. doi: 10.1016/0387-7604(96)00043-5. [DOI] [PubMed] [Google Scholar]
- 17.Jacoby J H, Sassin J F, Greenstein M, Weitzman E D. Patterns of spontaneous cortisol and growth hormone secretion in rhesus monkeys during the sleep-waking cycle. Neuroendocrinology. 1974;14:165–173. doi: 10.1159/000122256. [DOI] [PubMed] [Google Scholar]
- 18.Jooss K, Ertl H C, Wilson J M. Cytotoxic T-lymphocyte target proteins and their major histocompatibility complex class I restriction in response to adenovirus vectors delivered to mouse liver. J Virol. 1998;72:2945–2954. doi: 10.1128/jvi.72.4.2945-2954.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jooss K, Turka L A, Wilson J M. Blunting of immune responses to adenoviral vectors in mouse liver and lung with CTLA4Ig. Gene Ther. 1998;5:309–319. doi: 10.1038/sj.gt.3300595. [DOI] [PubMed] [Google Scholar]
- 20.Kaplan J M, Smith A E. Transient immunosuppression with deoxyspergualin improves longevity of transgene expression and ability to readminister adenoviral vector to the mouse lung. Hum Gene Ther. 1997;8:1095–1104. doi: 10.1089/hum.1997.8.9-1095. [DOI] [PubMed] [Google Scholar]
- 21.Karpati G, Acsadi G. The potential for gene therapy in Duchenne muscular dystrophy and other genetic muscle diseases. Muscle Nerve. 1993;16:1141–1153. doi: 10.1002/mus.880161102. [DOI] [PubMed] [Google Scholar]
- 22.Kass-Eisler A, Falck-Pedersen E, Elfenbein D H, Alvira M, Buttrick P M, Leinwand L A. The impact of developmental stage, route of administration and the immune system on adenovirus-mediated gene transfer. Gene Ther. 1994;1:395–402. [PubMed] [Google Scholar]
- 23.Lee W C, Wan Y H, Li W, Fu F, Sime P J, Gauldie J, Thomson A W, Fung J J, Lu L, Qian S. Enhancement of dendritic cell tolerogenicity by genetic modification using adenoviral vectors encoding cDNA for TGF beta 1. Transplant Proc. 1999;31:1195. doi: 10.1016/s0041-1345(98)01960-5. [DOI] [PubMed] [Google Scholar]
- 24.Marshall D J, Leiden J M. Recent advances in skeletal-muscle-based gene therapy. Curr Opin Genet Dev. 1998;8:360–365. doi: 10.1016/s0959-437x(98)80094-4. [DOI] [PubMed] [Google Scholar]
- 25.Morsy M A, Gu M, Motzel S, Zhao J, Lin J, Su Q, Allen H, Franlin L, Parks R J, Graham F L, Kochanek S, Bett A J, Caskey C T. An adenoviral vector deleted for all viral coding sequences results in enhanced safety and extended expression of a leptin transgene. Proc Natl Acad Sci USA. 1998;95:7866–7871. doi: 10.1073/pnas.95.14.7866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Natelson B H, Holaday J, Meyerhoff J, Stokes P E. Temporal changes in growth hormone, cortisol, and glucose: relation to light onset and behavior. Am J Physiol. 1975;229:409–415. doi: 10.1152/ajplegacy.1975.229.2.409. [DOI] [PubMed] [Google Scholar]
- 27.Pretolani M. Interleukin-10: an anti-inflammatory cytokine with therapeutic potential. Clin Exp Allergy. 1999;29:1164–1171. doi: 10.1046/j.1365-2222.1999.00456.x. [DOI] [PubMed] [Google Scholar]
- 28.Quantin B, Perricaudet L D, Tajbakhsh S, Mandel J-L. Adenovirus as an expression vector in muscle cell in vivo. Proc Natl Acad Sci USA. 1992;89:338–340. doi: 10.1073/pnas.89.7.2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ragot T, Vincent N, Chafey P, Vigne E, Gilgenkrantz H, Couton D, Cartaud J, Briand P, Kaplan J C, Perricaudet M, Kahn A. Efficient adenovirus-mediated transfer of a human minidystrophin gene to skeletal muscle of mdx mice. Nature. 1993;361:647–650. doi: 10.1038/361647a0. [DOI] [PubMed] [Google Scholar]
- 30.Smith T A, White B D, Gardner J M, Kaleko M, McClelland A. Transient immunosuppression permits successful repetitive intravenous administration of an adenovirus vector. Gene Ther. 1996;3:496–502. [PubMed] [Google Scholar]
- 31.Svensson E C, Black H B, Dugger D L, Tripathy S K, Goldwasser E, Hao Z, Chu L, Leiden J M. Long-term erythropoietin expression in rodents and non-human primates following intramuscular injection of a replication-defective adenoviral vector. Hum Gene Ther. 1997;8:1797–1806. doi: 10.1089/hum.1997.8.15-1797. [DOI] [PubMed] [Google Scholar]
- 32.Thomas C E, Schiedner G, Kochanek S, Castro M G, Lowenstein P R. Peripheral infection with adenovirus causes unexpected long-term brain inflammation in animals injected intracranially with first-generation, but not with high-capacity, adenovirus vectors: toward realistic long-term neurological gene therapy for chronic diseases. Proc Natl Acad Sci USA. 2000;97:7482–7487. doi: 10.1073/pnas.120474397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tripathy S K, Goldwasser B H, Leiden J M. Immune responses to transgene-encoded proteins limit the stability of gene expression after injection of replication-defective adenovirus vectors. Nat Med. 1996;2:545–550. doi: 10.1038/nm0596-545. [DOI] [PubMed] [Google Scholar]
- 34.van Ginkel F W, McGhee J R, Liu C, Simecka J W, Yamamoto M, Frizzell R A, Sorscher E J, Kiyono H, Pascual D W. Adenoviral gene delivery elicits distinct pulmonary-associated T helper cell responses to the vector and to its transgene. J Immunol. 1997;159:685–693. [PubMed] [Google Scholar]
- 35.Vincent N, Ragot T, Gilgenkrantz H, Couton D, Chafey P, Gregoire A, Briand P, Kaplan J C, Petticaudet M, Kahn A. Long term correction of mouse dystrophic degenration by adenovirus transfer of a mini-dystrophin gene. Nat Genet. 1993;5:130–134. doi: 10.1038/ng1093-130. [DOI] [PubMed] [Google Scholar]
- 36.Wilson J, Foster D, Kronenberg H, Larsen P. Williams textbook of endocrinology. 9th ed. Philadelphia, Pa: W. B. Saunders; 1998. [Google Scholar]
- 37.Yang Y, Ertl H C J, Wilson J M. MHC class I restricted cytotoxic T lymphocytes to viral antigens destroy hepatocytes in mice infected with E1-deleted recombinant adenoviruses. Immunity. 1994;1:433–442. doi: 10.1016/1074-7613(94)90074-4. [DOI] [PubMed] [Google Scholar]
- 38.Yang Y, Haecker S, Su Q, Wilson J. Immunology of gene therapy with adenoviral vectors in mouse skeletal muscle. Hum Mol Genet. 1996;5:1703–1712. doi: 10.1093/hmg/5.11.1703. [DOI] [PubMed] [Google Scholar]
- 39.Yang Y, Jooss K, Su Q, Ertl H C J, Wilson J M. Immune responses to viral antigens versus transgene product in the elimination of recombinant adenovirus-infected hepatocytes in vivo. Gene Ther. 1995;3:137–144. [PubMed] [Google Scholar]
- 40.Yang Y, Nunes F A, Berencsi K, Gonczol E, Engelhardt J F, Wilson J M. Inactivation of E2a in recombinant adenoviruses improves the prospect for gene therapy in cystic fibrosis. Nat Genet. 1994;7:362–369. doi: 10.1038/ng0794-362. [DOI] [PubMed] [Google Scholar]
- 41.Yang Y, Xiang Z, Ertl H C, Wilson J M. Upregulation of class I major histocompatibility complex antigens by interferon gamma is necessary for T-cell-mediated elimination of recombinant adenovirus-infected hepatocytes in vivo. Proc Natl Acad Sci USA. 1995;92:7257–7261. doi: 10.1073/pnas.92.16.7257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ye X, Rivera V M, Zoltick P, Cerasoli F, Schnell M A, Gao G P, Hughes J V, Gilman M, Wilson J M. Regulated delivery of therapeutic proteins after in vivo somatic cell gene transfer. Science. 1999;283:88–91. doi: 10.1126/science.283.5398.88. [DOI] [PubMed] [Google Scholar]