Abstract
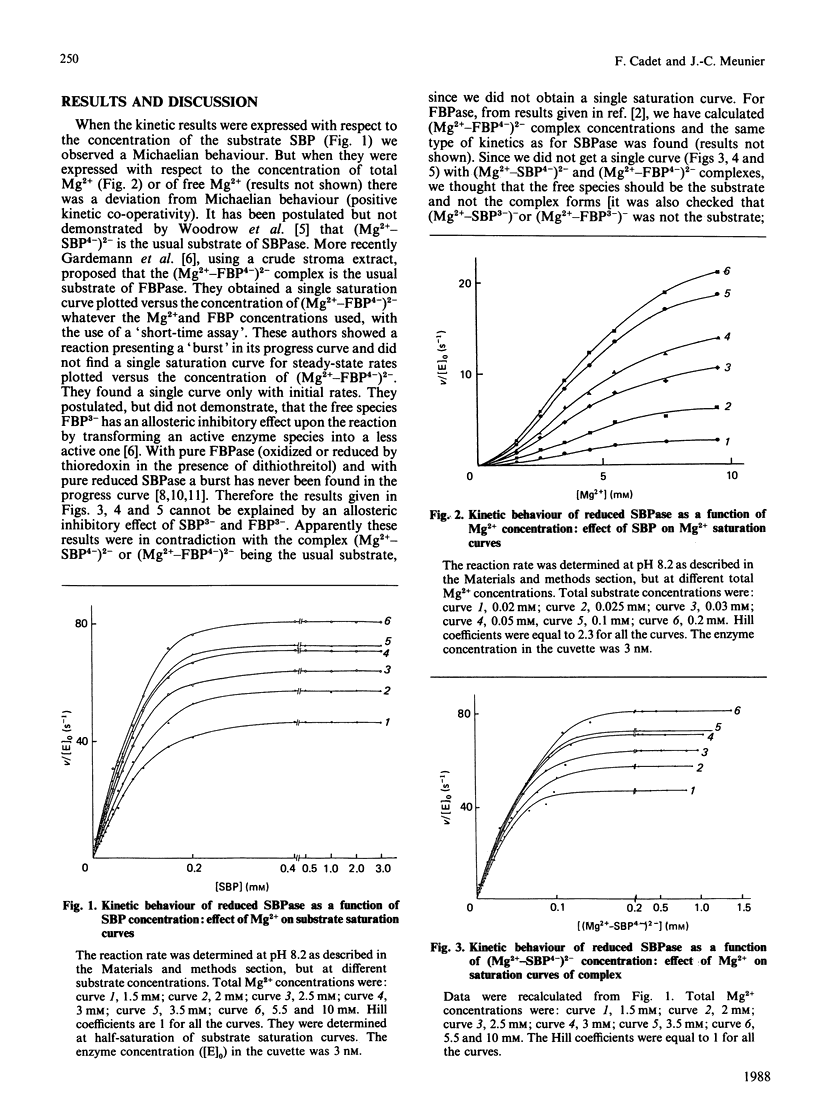
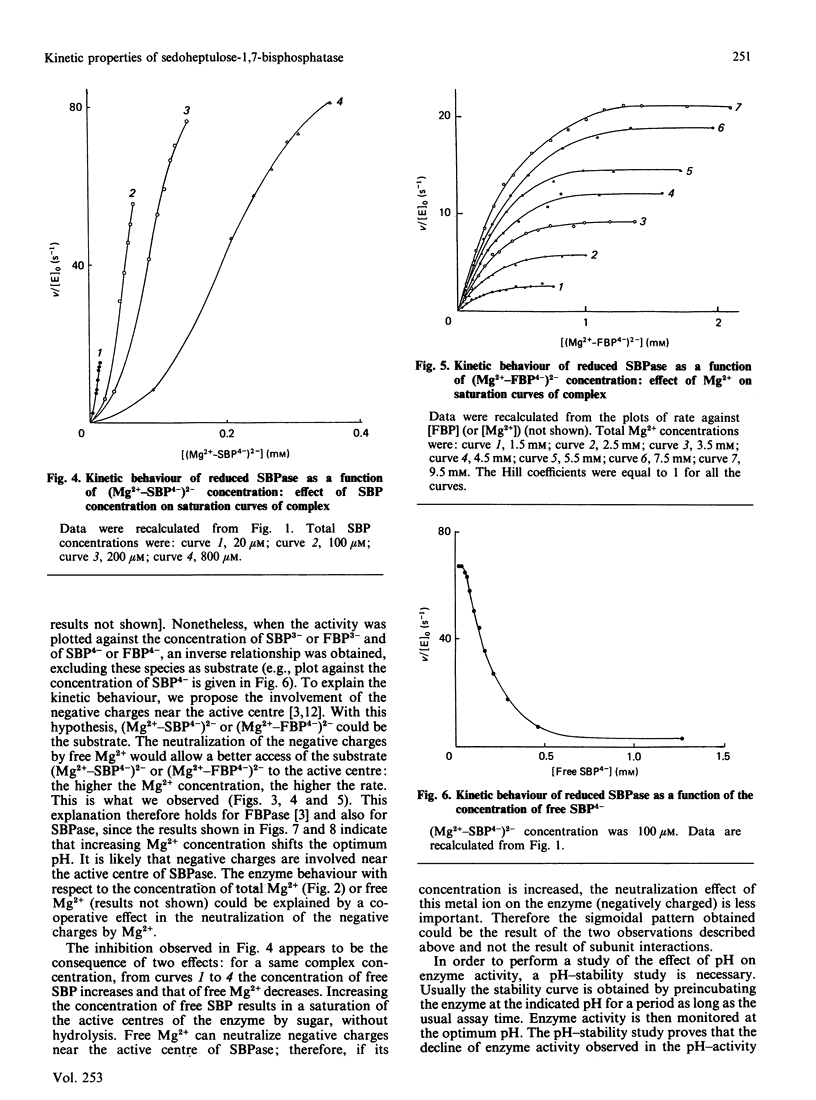
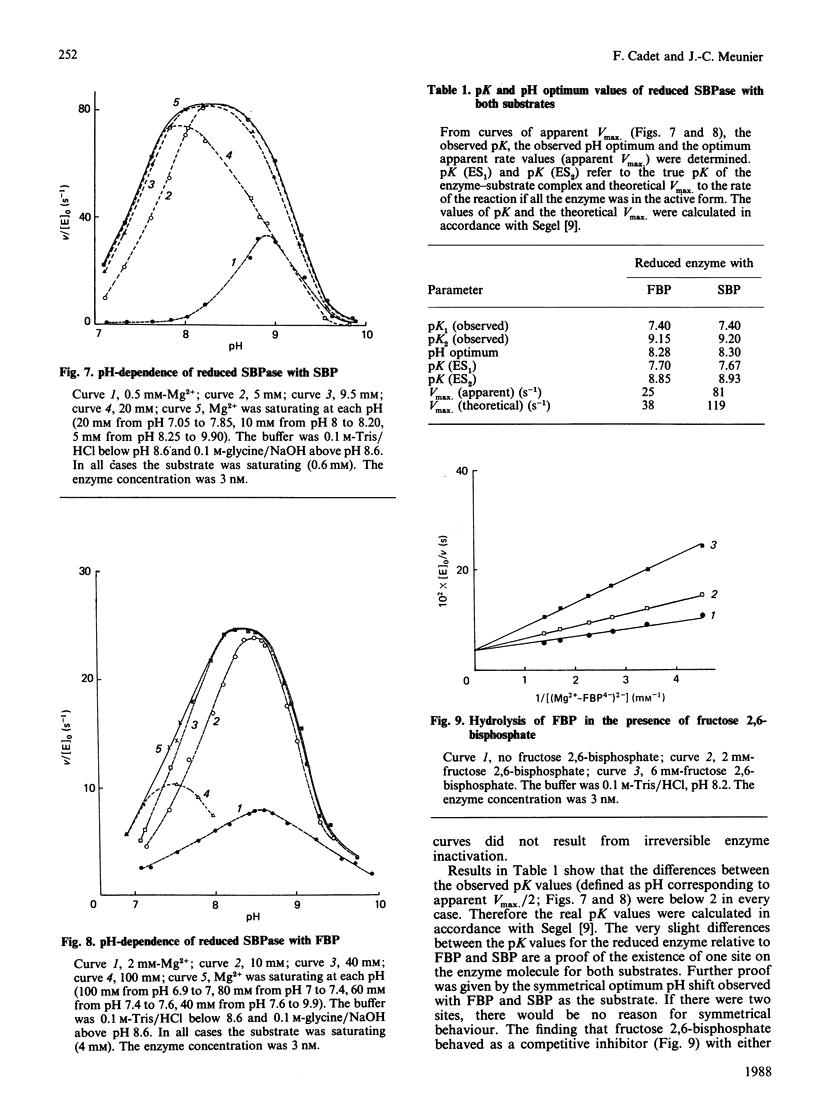
The aim of this paper is to study some steady-state kinetic properties of sedoheptulose-1,7-bisphosphatase, its pH-dependence and the effect of a substrate analogue, fructose 2,6-bisphosphate. Studies were carried out with sedoheptulose 1,7-bisphosphate and with fructose 1,6-bisphosphate, an alternative substrate. The pK values are identical for both substrates, and fructose 2,6-bisphosphate behaves like a competitive inhibitor. These results suggest that there exists a unique active site for either sedoheptulose 1,7-bisphosphate or fructose 1,6-bisphosphate on the enzyme molecule. Increasing Mg2+ concentrations shifted the optimum pH. As for fructose-1,6-bisphosphatase, we believe that this shift is due to the neutralization of negative charges near the active centre [Cadet, Meunier & Ferté (1987) Eur. J. Biochem. 162, 393-398]. The free species of sedoheptulose 1,7-bisphosphate and fructose 1,6-bisphosphate are not the usual substrates of enzyme, nor is Mg2+. But the kinetics relative to the (Mg2+-substrate4-)2- complex is not consistent with this complex being the substrate. An explanation of this discrepancy is proposed, involving both the negative charges near the active centre and the positive charges of Mg2+. The observed Vmax. of the reduced enzyme is 65% of the theoretical Vmax. for both substrates, but the observed Vmax. relative to sedoheptulose 1,7-bisphosphate is 3 times the one relative to fructose 1,6-bisphosphate. The specificity constant (kcat./Km), 1.62 x 10(6) M-1.s-1 with respect to sedoheptulose 1,7-bisphosphate compared with 5.5 x 10(4) M-1.s-1 with respect to fructose 1,6-bisphosphate, indicates that the enzyme specificity towards sedoheptulose 1,7-bisphosphate is high but not absolute.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cadet F., Meunier J. C., Ferté N. Effects of pH and fructose 2,6-bisphosphate on oxidized and reduced spinach chloroplastic fructose-1,6-bisphosphatase. Eur J Biochem. 1987 Jan 15;162(2):393–398. doi: 10.1111/j.1432-1033.1987.tb10614.x. [DOI] [PubMed] [Google Scholar]
- Cadet F., Meunier J. C. Spinach (Spinacia oleracea) chloroplast sedoheptulose-1,7-bisphosphatase. Activation and deactivation, and immunological relationship to fructose-1,6-bisphosphatase. Biochem J. 1988 Jul 1;253(1):243–248. doi: 10.1042/bj2530243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottschalk M. E., Chatterjee T., Edelstein I., Marcus F. Studies on the mechanism of interaction of fructose 2,6-bisphosphate with fructose-1,6-bisphosphatase. J Biol Chem. 1982 Jul 25;257(14):8016–8020. [PubMed] [Google Scholar]
- Pradel J., Soulié J. M., Buc J., Meunier J. C., Ricard J. On the activation of fructose-1,6-bisphosphatase of spinach chloroplasts and the regulation of the Calvin cycle. Eur J Biochem. 1981 Jan;113(3):507–511. doi: 10.1111/j.1432-1033.1981.tb05092.x. [DOI] [PubMed] [Google Scholar]
- Schürmann P., Wolosiuk R. A. Studies on the regulatory properties of chloroplast fructose-1,6-bisphosphatase. Biochim Biophys Acta. 1978 Jan 12;522(1):130–138. doi: 10.1016/0005-2744(78)90329-7. [DOI] [PubMed] [Google Scholar]
- Woodrow I. E., Murphy D. J., Latzko E. Regulation of stromal sedoheptulose 1,7-bisphosphatase activity by pH and Mg2+ concentration. J Biol Chem. 1984 Mar 25;259(6):3791–3795. [PubMed] [Google Scholar]
- Woodrow I. E., Walker D. A. Activation of wheat chloroplast sedoheptulose bisphosphatase: a continuous spectrophotometric assay. Arch Biochem Biophys. 1982 Jul;216(2):416–422. doi: 10.1016/0003-9861(82)90230-2. [DOI] [PubMed] [Google Scholar]
- Zimmermann G., Kelly G. J., Latzko E. Efficient purification and molecular properties of spinach chloroplast fructose 1,6-bisphosphatase. Eur J Biochem. 1976 Nov 15;70(2):361–367. doi: 10.1111/j.1432-1033.1976.tb11025.x. [DOI] [PubMed] [Google Scholar]


