Abstract
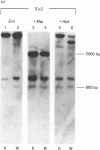
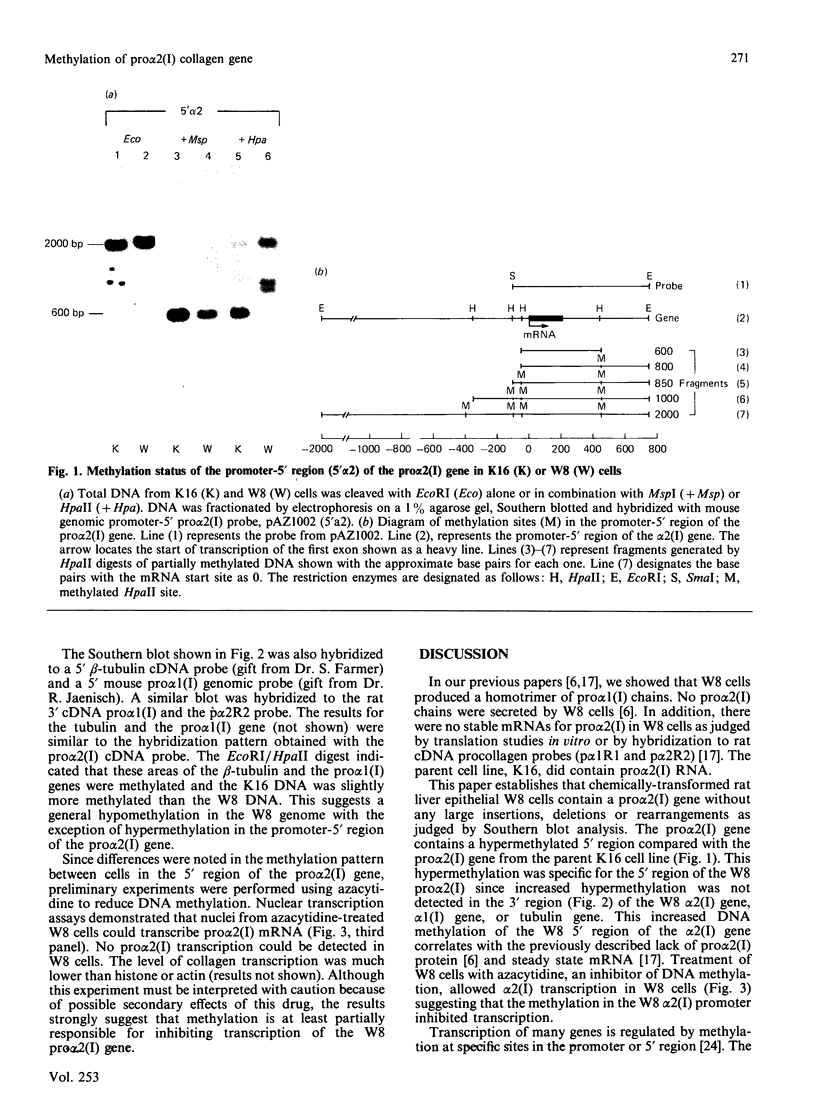
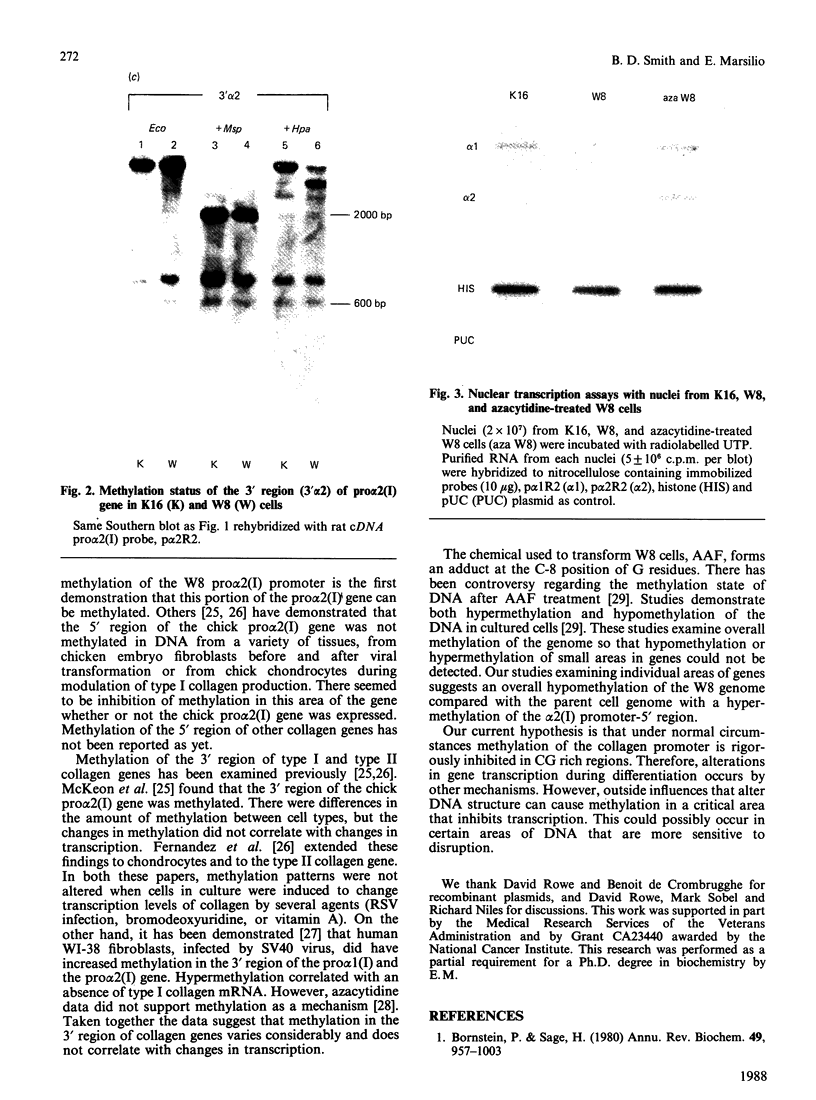
W8 cells, 2-N-(acetoxyacetyl)aminofluorene-transformed rat liver epithelial-like cells, secrete no alpha 2(I) collagen chains. This paper reports the first demonstration of DNA methylation in the promoter-5' region of an alpha 2(I) collagen gene which occurs in W8 cells. Since inhibition of methylation by azacytidine induces transcription of the alpha 2(I) gene, DNA methylation of W8 alpha 2(I) promoter-5' region could contribute to altered collagen production in these cells.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams S. L., Sobel M. E., Howard B. H., Olden K., Yamada K. M., de Crombrugghe B., Pastan I. Levels of translatable mRNAs for cell surface protein, collagen precursors, and two membrane proteins are altered in Rous sarcoma virus-transformed chick embryo fibroblasts. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3399–3403. doi: 10.1073/pnas.74.8.3399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avvedimento E., Yamada Y., Lovelace E., Vogeli G., de Crombrugghe B., Pastan I. Decrease in the levels of nuclear RNA precursors for alpha 2 collagen in Rous sarcoma virus transformed fibroblasts. Nucleic Acids Res. 1981 Mar 11;9(5):1123–1131. doi: 10.1093/nar/9.5.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman J. J., Foidart J. M. Synthesis of collagen by rat liver epithelial cultures. Ann N Y Acad Sci. 1980;349:153–164. doi: 10.1111/j.1749-6632.1980.tb29523.x. [DOI] [PubMed] [Google Scholar]
- Bornstein P., Sage H. Structurally distinct collagen types. Annu Rev Biochem. 1980;49:957–1003. doi: 10.1146/annurev.bi.49.070180.004521. [DOI] [PubMed] [Google Scholar]
- Doerfler W. DNA methylation and gene activity. Annu Rev Biochem. 1983;52:93–124. doi: 10.1146/annurev.bi.52.070183.000521. [DOI] [PubMed] [Google Scholar]
- Fernández M. P., Young M. F., Sobel M. E. Methylation of type II and type I collagen genes in differentiated and dedifferentiated chondrocytes. J Biol Chem. 1985 Feb 25;260(4):2374–2378. [PubMed] [Google Scholar]
- Gay S., Martin G. R., Muller P. K., Timpl R., Kuhn K. Simultaneous synthesis of types I and III collagen by fibroblasts in culture. Proc Natl Acad Sci U S A. 1976 Nov;73(11):4037–4040. doi: 10.1073/pnas.73.11.4037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genovese C., Rowe D., Kream B. Construction of DNA sequences complementary to rat alpha 1 and alpha 2 collagen mRNA and their use in studying the regulation of type I collagen synthesis by 1,25-dihydroxyvitamin D. Biochemistry. 1984 Dec 4;23(25):6210–6216. doi: 10.1021/bi00320a049. [DOI] [PubMed] [Google Scholar]
- Green H., Goldberg B. Synthesis of collagen by mammalian cell lines of fibroblastic and nonfibroblastic origin. Proc Natl Acad Sci U S A. 1965 Jun;53(6):1360–1365. doi: 10.1073/pnas.53.6.1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg M. E., Ziff E. B. Stimulation of 3T3 cells induces transcription of the c-fos proto-oncogene. Nature. 1984 Oct 4;311(5985):433–438. doi: 10.1038/311433a0. [DOI] [PubMed] [Google Scholar]
- Groudine M., Peretz M., Weintraub H. Transcriptional regulation of hemoglobin switching in chicken embryos. Mol Cell Biol. 1981 Mar;1(3):281–288. doi: 10.1128/mcb.1.3.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hata R. I., Peterkofsky B. Specific changes in the collagen phenotype of BALB 3T3 cells as a result of transformation by sarcoma viruses or a chemical carcinogen. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2933–2937. doi: 10.1073/pnas.74.7.2933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hata R., Ninomiya Y., Nagai Y., Tsukada Y. Biosynthesis of interstitial types of collagen by albumin-producing rat liver parenchymal cell (hepatocyte) clones in culture. Biochemistry. 1980 Jan 8;19(1):169–176. doi: 10.1021/bi00542a026. [DOI] [PubMed] [Google Scholar]
- Krawisz B. R., Lieberman M. W. Methylation of deoxycytidine in replicating cells treated with ultraviolet radiation and chemical carcinogens. Carcinogenesis. 1984 Sep;5(9):1141–1144. doi: 10.1093/carcin/5.9.1141. [DOI] [PubMed] [Google Scholar]
- Marsilio E., Sobel M. E., Smith B. D. Absence of procollagen alpha 2(I) mRNA in chemically transformed rat liver epithelial cells. J Biol Chem. 1984 Feb 10;259(3):1401–1404. [PubMed] [Google Scholar]
- Mayne R., Vail M. S., Miller E. J. Analysis of changes in collagen biosynthesis that occur when chick chondrocytes are grown in 5-bromo-2'-deoxyuridine. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4511–4515. doi: 10.1073/pnas.72.11.4511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeon C., Ohkubo H., Pastan I., de Crombrugghe B. Unusual methylation pattern of the alpha 2 (l) collagen gene. Cell. 1982 May;29(1):203–210. doi: 10.1016/0092-8674(82)90104-0. [DOI] [PubMed] [Google Scholar]
- Moro L., Smith B. D. Identification of collagen alpha1(I) trimer and normal type I collagen in a polyoma virus-induced mouse tumor. Arch Biochem Biophys. 1977 Jul;182(1):33–41. doi: 10.1016/0003-9861(77)90280-6. [DOI] [PubMed] [Google Scholar]
- Parker M. I., Gevers W. Demethylation of the type I procollagen genes in transformed fibroblasts treated with 5-azacytidine. Biochem Biophys Res Commun. 1984 Oct 15;124(1):236–243. doi: 10.1016/0006-291x(84)90942-2. [DOI] [PubMed] [Google Scholar]
- Parker M. I., Judge K., Gevers W. Loss of type I procollagen gene expression in SV40-transformed human fibroblasts is accompanied by hypermethylation of these genes. Nucleic Acids Res. 1982 Oct 11;10(19):5879–5891. doi: 10.1093/nar/10.19.5879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe D. W., Moen R. C., Davidson J. M., Byers P. H., Bornstein P., Palmiter R. D. Correlation of procollagen mRNA levels in normal and transformed chick embryo fibroblasts with different rates of procollagen synthesis. Biochemistry. 1978 May 2;17(9):1581–1590. doi: 10.1021/bi00602a001. [DOI] [PubMed] [Google Scholar]
- Sandmeyer S., Gallis B., Bornstein P. Coordinate transcriptional regulation of type I procollagen genes by Rous sarcoma virus. J Biol Chem. 1981 May 25;256(10):5022–5028. [PubMed] [Google Scholar]
- Sandmeyer S., Smith R., Kiehn D., Bornstein P. Correlation of collagen synthesis and procollagen messenger RNA levels with transformation in rat embryo fibroblasts. Cancer Res. 1981 Mar;41(3):830–838. [PubMed] [Google Scholar]
- Schmidt A., Yamada Y., de Crombrugghe B. DNA sequence comparison of the regulatory signals at the 5' end of the mouse and chick alpha 2 type I collagen genes. J Biol Chem. 1984 Jun 25;259(12):7411–7415. [PubMed] [Google Scholar]
- Smith B. D., Biles D., Gonnerman W., Faris B., Levine A., Capparell N., Moolten F., Franzblau C. Collagen synthesis in normal BHK cells and temperature-sensitive chemically transformed BHK cells. In Vitro. 1979 Jun;15(6):455–462. doi: 10.1007/BF02618415. [DOI] [PubMed] [Google Scholar]
- Smith B. D., Niles R. Characterization of collagen synthesized by normal and chemically transformed rat liver epithelial cell lines. Biochemistry. 1980 Apr 29;19(9):1820–1825. doi: 10.1021/bi00550a014. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Weinstein I. B., Orenstein J. M., Gebert R., Kaighn M. E., Stadler U. C. Growth and structural properties of epithelial cell cultures established from normal rat liver and chemically induced hepatomas. Cancer Res. 1975 Jan;35(1):253–263. [PubMed] [Google Scholar]