Abstract
Purpose
Ischemic colitis appears to be a rare but serious complication of COVID-19. About 33.3% hospitalized COVID-19 patients who underwent endoscopy showed features resembling ischemic colitis. The aim of this prospective study was to describe the symptoms, treatment, and outcomes of these patients, particularly their colonoscopy and histologic findings.
Patients and methods
We conducted a prospective study on ischemic bowel disease associated with COVID-19 across four centers from December 2022 to January 2023. All cases were identified through a comprehensive search of electronic medical records for procedure-related data. The initial diagnosis of ischemic bowel disease was confirmed using colonoscopy and biopsy findings. After the patients were discharged, a 12-month follow-up was conducted through regular phone interviews to assess their clinical outcomes.
Results
Overall, the study included 3 male patients and 9 female patients (age range, 33–76 years). Abdominal pain and hematochezia always occurred within 2 weeks after COVID-19 infection (average 5.5 days). Gastrointestinal manifestations did not parallel the severity of COVID-19 infection. The descending colon was the most susceptible segment, which was involved in 10 patients (83.33%). Colonoscopy revealed diffuse redness, edema, bleeding, erosion, and ulceration of the intestinal mucosa, similar to the findings of ischemic colitis, and biopsy revealed crypt atrophy, reduction, and interstitial bleeding. All patients were self-limited without converting to chronic changes in the next 12 months.
Conclusion
SARS-CoV-2 infection may induce transient acute colon ischemia within 2 weeks, accompanied by severe clinical symptoms such as acute abdominal pain and hematochezia, which are self-limiting and do not lead to chronic symptoms.
Keywords: SARS-CoV-2, lower gastrointestinal bleeding, ischemic colitis, colonoscopy
Introduction
The emergence and spread of COVID-19 have brought significant challenges to global public health. Its clinical manifestations are variable, ranging from a flu-like syndrome with dry cough, sore throat, and conjunctivitis to dysgeusia and anosmia, up to a picture of bilateral interstitial pneumonia, even acute respiratory distress syndrome, and multiorgan failure, with a fatal outcome.1 Fever and cough are usually the earliest and most commonly reported symptoms in patients with COVID-19, but according to a previous study, 15% of patients have gastrointestinal (GI) symptoms, with nausea or vomiting, diarrhea, and loss of appetite being the three most common symptoms, and 10% of patients present with GI symptoms alone without respiratory features,2 as the ACE-2 receptors, by which SARS-CoV-2 invades cells, are highly expressed not only in the respiratory system but also in the GI tract, especially in the gut.3
Research has shown that GI involvement in COVID-19 patients is often linked to delayed diagnosis and an increased risk of developing acute respiratory distress syndrome.2 GI symptoms in COVID-19 patients have been noticed since early 2020,4 and they have been reported to have a higher detection rate of fecal SARS-CoV-2 RNA.5 Endoscopic examinations have revealed abnormalities in both the upper and lower GI tracts of infected patients, including ulceration in the stomach, while bleeding and ischemic changes are commonly observed in the lower GI tract.6 Endoscopic abnormalities have included esophagitis, erosive gastritis, bulbar ulcer, diverticulitis, colon erythema, and ischemia.6 When COVID-19 first surged during China’s loosening of epidemic control measures, we collected 12 cases of ischemic colitis, and a 12-month follow-up was conducted via telephone.
Method
This prospective observational study describes the colonoscopy and histological findings of COVID-19 patients with bowel ischemic changes. This study was approved by the institutional review board (IRB) of the Second Affiliated Hospital of Xi’an Jiaotong University (Approval number: 2023410). The inclusion criterion for this study was the presence of ischemic bowel disease under colonoscopy with SARS-CoV-2 infection. The standard for exclusion was previous abnormal colonoscopy findings. 12 cases from 4 centers in December 2022 who were tested for COVID-19 daily regularly during hospitalization were collected. Informed consent was obtained from all patients. A review of clinical data was performed, including a query of the electronic medical records and the electronic procedure reporting system. The following clinical and demographic data were collected for the study patients: age, sex, BMI, clinical presentation, and endoscopic findings. All pathological reports were issued by the same experienced pathologist. Two of the patients underwent multiple colonoscopies over the next two months. All patients underwent telephone follow-up for symptom assessment after 12 months. Descriptive statistics were used to present the data.
Results
This study included a total of 12 patients, including 9 females, accounting for 75%, with an average age of 58.17± 12.42 years. Only two patients (16.67%) developed pneumonia, and the other ten (83.33%) had no respiratory symptoms. No patient experienced hypoxemia. Six patients (50%) developed GI symptoms one day before testing positive for SARS-CoV-2, and the other six patients had GI symptoms after testing positive for SARS-CoV-2 for 0 to 13 days (average 5.5 days). None of them had preexisting lower GI symptoms such as ulcers or melena or had previously reported any abnormalities on colonoscopy. The patients’ clinical characteristics and a summary of endoscopic findings are presented in Table 1.
Table 1.
Basic Characteristics of the Patients
| Sex | Age (Year) | BMI (kg/m2) | Past Medical History | Symptom Onset time (Days) | Digestive Symptoms | Colonoscopy Time (Days) | Extent | Endoscopic Performance | D-Dimer (ng/mL) | CRP (mg/L) | Hospitalization Days | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Female | 33 | 24.65 | — | −7 | Celialgia, Hematochezia, nausea, weakness | −2 | Descending colon | Mucosal congestion and swelling, multiple superficial ulcers and erosions. | 940 | hs-CRP 7.12 | 11 |
| 2 | Female | 51 | 23.14 | Hypertension | 6 | Celialgia | 12 | Transverse colon to sigmoid colon | Mucosal congestion, edema, erosion | 500 | — | 4 |
| 3 | Female | 51 | 22.34 | — | 3 | Hematochezia | 3 | Transverse colon | Mucosal congestion and swelling, superficial ulcers covered with yellow white moss and dark red scabs | — | 95.9 | 11 |
| 4 | Female | 56 | 22.04 | — | −1 | Celialgia, Hematochezia | 0 | Descending colon | Mucosal congestion, edema and ecchymosis. superficial ulcers, vascular network disappeared | 546 | 19.01 | 8 |
| 5 | Female | 59 | 18.75 | — | −1 | Celialgia, Hematochezia | 11 | Transverse colon | Mucosal congestion and swelling with granular hyperplasia, multiple ulcers covered with white moss, | 630 | <10 | 7 |
| 6 | Female | 66 | 24.09 | — | −1 | Hematochezia | 3 | Transverse colon to descending colon | Mucosal congestion, edema, erosion | 1050 | 48.19 | 6 |
| 7 | Female | 67 | 23.88 | Hypertension | −1 | Celialgia, Hematochezia | 1 | Descending colon | Mucosal congestion and swelling, multiple erosion and blood spots, superficial ulcers. The vascular network was disordered. | 341 | <10 | 12 |
| 8 | Female | 75 | 22.89 | Hypertension | 8 | Hematochezia | 6 | Rectum | Mucosal congestion and swelling, multiple rectal deep ulcers with | 1850 | — | 10 |
| 9 | Female | 76 | 20.31 | Hypertension | 0 | Celialgia, Hematochezia | 2 | Ascending and descending colon | Mucosal congestion and swelling with nodular hyperplasia.Multiple erosion and longitudinal ulcers covered with white moss.The submucosal vascular network was disordered and disappeared. | 239 | 32.31 | 10 |
| 10 | Male | 44 | 26.73 | — | −1 | Celialgia, Hematochezia | 1 | Descending and sigmoid colon | Mucosal congestion and bleeding with multiple ulcers | 560 | 53.09 | 9 |
| 11 | Male | 62 | 20.07 | — | 13 | Hematochezia | 14 | Descending colon and sigmoid colon | Mucosal congestion and erosion, superficial ulcers | 730 | <10 | 6 |
| 12 | Male | 58 | 20.57 | — | −1 | Celialgia, Hematochezia | 6 | Descending colon | Mucosal congestion and erosion | 990 | 33.2 | 7 |
Notes: The onset time of symptoms refer to abdominal pain and hematochezia, based on the time of diagnosis of COVID-19, with positive numbers being after diagnosis and negative numbers being before diagnosis.
Abbreviations: CRP, C-reactive protein; hs-CRP, hypersensitive C-reactive protein.
The ischemic changes manifested as segmental changes, and multiple intestinal segments were involved in some patients. All patients had biopsies taken during the first colonoscopy after disease onset. None of the patients experienced hypoxemia during hospitalization or received anticoagulation or antiviral treatment. All lesions were self-limited without conversion to chronic changes (Supplementary Figures 1–12).
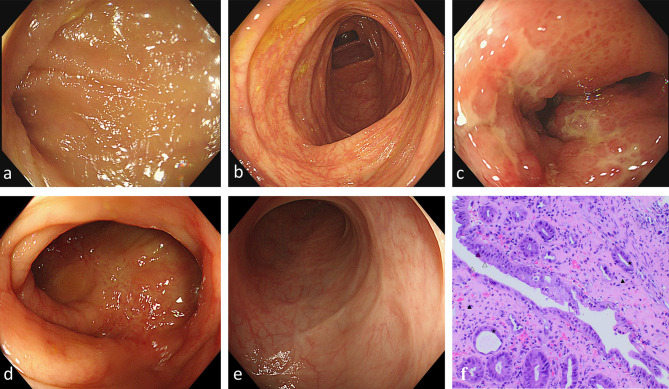
The descending colon was the most susceptible segment, and it was involved in 10 (83.33%) of the patients. Figure 1 shows the colonoscopic changes of a 59-year-old female patient who was admitted with acute abdominal pain and hematochezia as her initial symptoms. On the second day after admission, the first colonoscopy examination was completed. Her terminal ileum (Figure 1a) and ascending colon (Figure 1b) were normal, but ischemic changes could be observed in the descending colon (Figure 1c), similar to ulcerative colitis. Ulcers and white pseudomembranes were scattered on the friable mucosa with distinct hyperemia and edema, and granular hyperplasia protrusions diffused throughout the affected intestinal wall. Histologically, mucosal surface ischemia and epithelial damage were observed (Figure 1f). Six days later, the patient underwent her second colonoscopy (Figure 1d): the boundary of the ulcers in the ascending colon had narrowed, with mucosal aggregation, edema, and hyperemia partially relieved, and the primary lesion in the descending colon had disappeared. On her third colonoscopy, 2 months later, the ulcer erosion, congestion, and edema in the ascending colon disappeared, with a smooth mucosal surface and visible white scars (Figure 1e).
Figure 1.
Endoscopic and histopathological findings in ischemic colitis in patients with COVID-19 across multiple colonoscopies. (a–c) First colonoscopy. (a) Terminal ileum. (b) Ascending colon. (c) Decreasing colon: discontinuous lesion, superficial ulcer, erosion, hyperemia and edema, disappearance of vascular texture. (d) 2nd colonoscopy: ascending colon. (e) 3rd colonoscopy: ascending colon. (f) Histopathology of biopsy specimens from the descending colon from the first colonoscopy showing ischemic colitis. mucus deficiencyΔ, atrophy and reduction of crypts★, intercellular substance hemorrhage and hyalinization▲ (hematoxylin and eosin [H&E]×10). The purple triangle observed in the pathological images is identified as a staining artifact, which does not represent a true tissue feature. This has been clarified to ensure accurate interpretation of the results.
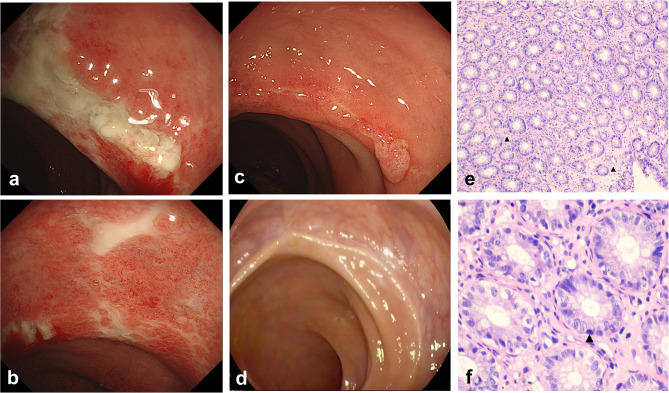
There was one case of rectal involvement in a 75-year-old female (Figure 2). COVID-19 infection with rectal ischemia has rarely been reported before. Colonoscopy revealed typical manifestations of ischemic colitis: multiple circumferential ulcers were present 5 cm from the anus, with the intermittent distribution of lesions covered with a thick white coating and congestion of the surrounding mucosa (Figure 2a and b). Two weeks later, the ulcers became less extensive, with mucosal aggregation and scarring. Moreover, edema and hyperemia were alleviated without special treatment (Figure 2c). The 3rd colonoscopy was performed 2 months later: a white scar was observed at the original ulcer site, and mucosal hyperemia and edema almost disappeared (Figure 2d). Histologically, the mucosa showed lymphocytic infiltration, as the intercellular substance hyaline was degenerative. The crypt epithelial nucleus was slightly larger and deeply stained with intercellular substance hyalinization (Figure 2e and f).
Figure 2.
Endoscopic and histopathological changes in ischemic colitis of the rectum in patients with COVID-19. (a–d) Endoscopic images of the colon. (a and b) 1st colonoscopy: rectum. Multiple deep ulcers, mucosal hyperemia and edema, and vascular texture disappeared. (c) 2nd colonoscopy: ulcers became less extensive. (d) 3rd colonoscopy: A white scar was observed at the original ulcer site. (e and f) Histopathology of biopsy specimens from the rectum from the first colonoscopy showing ischemic colitis. (e) ([H&E], ×10) Lymphocyte infiltration and intercellular substance hyalinization. (f) ([H&E], ×40) Deeply stained nucleus of the crypt▲.
We conducted a telephone follow-up for all patients after 12 months and learned that they did not experience abdominal pain or hematochezia as before after discharge.
Discussion
Ischemic bowel disease is an acute and chronic intestinal wall ischemic damage disease caused by poor blood perfusion, with typical clinical features usually presenting with sudden onset of abdominal pain, diarrhea, and hematochezia, of which colonic ischemia is the most common.7 75% percent of our patients were women, which was consistent with the findings of a previous study: females are more likely to develop gastrointestinal symptoms when infected with SARS-CoV-2.8 Endoscopic features of transient ischemic colitis include petechial hemorrhages, edematous and fragile mucosa, segmental erythema, scattered erosion, longitudinal ulcerations, and sharply defined segments of involvement.9 Pathology often shows infiltration of lymphocytes and neutrophilic granulocytes into edematous mucosa with gland degeneration, atrophy, or necrosis in ischemic colitis.9 Segmental mucosal hyperemia, erythema and edema, and scattered irregular ulcers were common in our patients, and ischemic bowel disease was also confirmed by pathology. Among hospitalized COVID-19 patients who underwent endoscopy, about 33.3% showed features resembling ischemic colitis.6 However, how SARS-CoV-2 causes these ischemic changes remains uncertain.
Anatomic studies have revealed several weak points in the colonic vasculature resulting from incomplete anastomoses of the marginal arteries, where ischemia quickly occurs when blood pressure drops.10 However, the affected section in our patients did not show a distribution pattern, and no hypotension was observed.
SARS-CoV-2 has been shown to invade gastrointestinal epithelial cells via the ACE-2 receptor, initiating a cytokine storm that drives a severe inflammatory response. This inflammatory cascade disrupts the intestinal mucosal barrier and may lead to bacterial translocation.11,12 In such cases, we would expect the lesions to be continuous and diffuse, rather than segmental. However, our findings of segmental lesions suggest that additional mechanisms may contribute to the pathogenesis. Recent study has proposed that a hypercoagulable state induced by SARS-CoV-2 infection can lead to intestinal ischemia.13 It has been reported that almost half of COVID-19 patients with bowel ischemia had macrovascular arterial/venous thrombosis, and overall mortality in COVID-19 patients with GI ischemia and radiologically evident mesenteric ischemia was 38% and 40%, respectively.14 However, abdominal vascular ultrasound and D-dimer levels were always normal in all patients in our cohort, and they improved without anticoagulant treatment within 2 weeks.
We inferred that this may be because all of our patients were admitted to the hospital at the early stage of ischemic bowel disease, and their rapid recovery might benefit from necessary supportive treatment, such as fluid infusion and oral probiotics, which can prevent intestinal hypoperfusion and bacterial translocation. However, the exact mechanism remains unclear. We must admit that the small sample size limited our conclusions, but the phenomenon we describe deserves further research.
Conclusion
We found that SARS-CoV-2 infection may induce transient acute colon ischemia in a minority of patients, usually within 2 weeks, accompanied by severe clinical symptoms such as acute abdominal pain and hematochezia, which are self-limiting and do not lead to chronic symptoms. High-quality colonoscopy images of patients with COVID-19 combined with acute ischemic colitis are provided in the Supplementary Materials. This case series, to some extent, could provide a basis for the management of this type of patient.
Data Sharing Statement
The data that support the findings of this study are available from the corresponding author (Bin Qin) upon reasonable request.
Ethical Approval
The study was approved by the Ethics Committee of the Second Affiliated Hospital of Xi’an Jiaotong University (Approval number: 2023410), and strictly complied with the Declaration of Helsinki.
Consent for Publication
Every patient whose data are included in this manuscript has provided their approval for publication.
Consent for Participate
Written informed consent was obtained from the patients.
Disclosure
The authors declared that there were no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Chams N, Chams S, Badran R, et al. COVID-19: a Multidisciplinary Review. Front Public Health. 2020;8:383. doi: 10.3389/fpubh.2020.00383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mao R, Qiu Y, He JS, et al. Manifestations and prognosis of gastrointestinal and liver involvement in patients with COVID-19: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2020;5(7):667–678. doi: 10.1016/S2468-1253(20)30126-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.ACE2 protein expression summary-The Human Protein Atlas. 2023.
- 4.Lin L, Jiang X, Zhang Z, et al. Gastrointestinal symptoms of 95 cases with SARS-CoV-2 infection. Gut. 2020;69(6):997–1001. doi: 10.1136/gutjnl-2020-321013 [DOI] [PubMed] [Google Scholar]
- 5.Wong MC, Huang J, Lai C, Ng R, Chan FKL, Chan PKS. Detection of SARS-CoV-2 RNA in fecal specimens of patients with confirmed COVID-19: a meta-analysis. J Infect. 2020;81(2):e31–e38. doi: 10.1016/j.jinf.2020.06.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vanella G, Capurso G, Burti C, et al. Gastrointestinal mucosal damage in patients with COVID-19 undergoing endoscopy: an international multicentre study. BMJ Open Gastroenterol. 2021;8(1):e000578. doi: 10.1136/bmjgast-2020-000578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ahmed M. Ischemic bowel disease in 2021. World J Gastroenterol. 2021;27(29):4746–4762. doi: 10.3748/wjg.v27.i29.4746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Han C, Duan C, Zhang S, et al. Digestive Symptoms in COVID-19 Patients With Mild Disease Severity: clinical Presentation, Stool Viral RNA Testing, and Outcomes. Am J Gastroenterol. 2020;115(6):916–923. doi: 10.14309/ajg.0000000000000664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zou X, Cao J, Yao Y, Liu W, Chen L. Endoscopic findings and clinicopathologic characteristics of ischemic colitis: a report of 85 cases. Dig Dis Sci. 2009;54(9):2009–2015. doi: 10.1007/s10620-008-0579-1 [DOI] [PubMed] [Google Scholar]
- 10.Yamazaki T, Shirai Y, Tada T, Sasaki M, Sakai Y, Hatakeyama K. Ischemic colitis arising in watershed areas of the colonic blood supply: a report of two cases. Surg Today. 1997;27(5):460–462. doi: 10.1007/BF02385714 [DOI] [PubMed] [Google Scholar]
- 11.Cardinale V, Capurso G, Ianiro G, Gasbarrini A, Arcidiacono PG, Alvaro D. Intestinal permeability changes with bacterial translocation as key events modulating systemic host immune response to SARS-CoV-2: a working hypothesis. Dig Liver Dis. 2020;52(12):1383–1389. doi: 10.1016/j.dld.2020.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leppkes M, Knopf J, Naschberger E, et al. Vascular occlusion by neutrophil extracellular traps in COVID-19. EBioMedicine. 2020;58:102925. doi: 10.1016/j.ebiom.2020.102925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Poor HD. Pulmonary Thrombosis and Thromboembolism in COVID-19. Chest. 2021;160(4):1471–1480. doi: 10.1016/j.chest.2021.06.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Keshavarz P, Rafiee F, Kavandi H, Goudarzi S, Heidari F, Gholamrezanezhad A. Ischemic gastrointestinal complications of COVID-19: a systematic review on imaging presentation. Clin Imaging. 2021;73:86–95. doi: 10.1016/j.clinimag.2020.11.054 [DOI] [PMC free article] [PubMed] [Google Scholar]