Abstract
Background
Loss of human leukocyte antigen (HLA) class I expression and loss of heterozygosity (LOH) are common events implicated in the primary resistance of non-small cell lung cancer (NSCLC) to immunotherapy. However, there is no data on perioperative chemoimmunotherapy (ChIO) efficacy or response mechanisms in the context of HLA class I defects.
Methods
Baseline HLA class I tumor status (HLA-deficient (HLA-DEF) or HLA-proficient (HLA-PRO)) was determined by DNA LOH combined with immunohistochemistry for protein levels in tissue of 24 patients with NSCLC treated with perioperative nivolumab plus chemotherapy from NADIM trial (NCT03081689). We integrated HLA tumor status with molecular data (programmed death-ligand 1 (PD-L1), TMB, TCR repertoire, TILs populations, bulk RNA-seq, and spatial transcriptomics (ST)) and clinical outcomes (pathological response and survival data) to study the activity of perioperative ChIO considering HLA class I defects.
Results
HLA-DEF tumors comprised 41.7% of analyzed tumors and showed a desert-like microenvironment at baseline, with lower PD-L1 levels and reduced immune infiltrate. However, perioperative ChIO induced similar complete pathological response (CPR) rates in both HLA-DEF and PRO tumors (50% and 60% respectively, p=0.670), as well as 3-year survival rates: Progression-free survival (PFS) and overall survival (OS) of 70% (95% CI 32.9% to 89.2%) for HLA-DEF, and PFS 71.4% (95% CI 40.6% to 88.2%) and OS 92.9% (95% CI 59.1% to 99.0%) for HLA-PRO (log-rank PFS p=0.909, OS p=0.137). Proof-of-concept ST analysis of a CPR HLA-DEF tumor after ChIO showed a strong immune response with tertiary lymphoid structures (TLS), CD4+T cells with HLA class II colocalization, and activated CD8+T cells.
Conclusions
Our findings highlight the activity of perioperative ChIO, and the potential role of TLS and T-cell immune response, in NSCLC HLA-DEF tumors.
Keywords: non-small cell lung cancer, HLA, neoadjuvant, biomarker, immune checkpoint inhibitor
WHAT IS ALREADY KNOWN ON THIS TOPIC.
WHAT THIS STUDY ADDS
HLA-deficient (HLA-DEF) tumors presented a colder microenvironment at baseline, however ChIO induced similar complete pathological response and survival rates, regardless of HLA class I status. In this sense, neoadjuvant ChIO can induce a strong immune response involving tertiary lymphoid structures.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
Our results support the activity of ChIO in HLA-DEF tumors, providing the first results regarding any HLA-based biomarker in the perioperative ChIO setting of NSCLC.
Introduction
Lung cancer is one of the most common malignancies in the world. Approximately 234,580 new cases will be diagnosed, and 125,070 people will lose their lives in the USA in 2024 for this type of cancer, where most of them (85%) will be non-small cell lung cancer (NSCLC).1 Immunotherapy (IO), particularly through the blockade of the programmed death-1 or programmed death ligand 1 (PD-1/PD-L1) axis, has been a paradigm shift in the treatment of these patients.2 Recently, neoadjuvant chemoimmunotherapy (ChIO) has significantly improved pathological response and survival rates in patients with resectable locally advanced NSCLC, establishing a new standard of treatment.3 4
Despite the improvement, around 30–60% of patients present non-complete pathological responses (non-CPR), and 20–30% relapse at 2 years.4,8 In this sense, there is a scarcity of reliable biomarkers to identify responders, and doubts arise regarding the effectiveness of ChIO in tumors with specific characteristics due to a limited understanding of response mechanisms.
Different studies have demonstrated the implication of different elements responsible for eliciting an effective antitumor immune response after IO regimens, including tumor mutational burden (TMB) and a proficient antigen presentation through human leukocyte antigen (HLA) genes.9,11 In this way, previous studies of our group and others have shown that neoadjuvant ChIO seems to be effective even in NSCLC tumors with low TMB, however, there is no data on neoadjuvant ChIO activity in the context of HLA-mediated antigenic presentation defects.12
The HLA system is a large complex of genes which includes HLA class I, II and III genes located in the short arm of chromosome 6 (6p21.3), playing an important role in the T-cell responses.11 HLA class I complex consists of an alpha-heavy chain interacting with a β-2 microglobulin, encoded in chromosome 15, and the HLA class II complex is composed of a similar-sized alpha and beta chains.13 Classical HLA class I proteins (HLA-A, HLA-B, and HLA-C) are responsible for the presentation of endogenous peptides to CD8+T cells, and classical HLA class II proteins (HLA-DR, HLA-DP, and HLA-DQ) present exogenous peptides to CD4+T cells.14 In addition, antigen-presenting cells (APCs) can also present exogenous peptides through HLA class I in a process called cross-priming, activating adaptive CD8 T-cell responses.15 However, tumor cell recognition by CD8+T cell TCR is not possible in tumors with loss of HLA expression, being necessary innate receptors such as NKG2D in tumor resolution.16
Classically, HLA class I expression has been studied in different tumors, being common the loss of HLA expression as an immune escape mechanism, which is associated with poor prognosis of patients.17,19 Additionally, loss of heterozygosity (LOH) of HLA class I (HLA LOH), affecting the chromosome 6 (HLA) and/or chromosome 15 (β-2 microglobulin), has become increasingly important as a potential immune escape mechanism, decreasing the neoantigen repertoire presented to T-cells.20 HLA LOH may be involved in the response of some tumors to IO, but HLA LOH rates vary across tumor types, and its prognostic value as a single biomarker is still unclear.21,25 Regarding ChIO, studies are scarcer and further investigations are needed.26 27
In the present study, we analyzed the baseline HLA class I LOH and HLA class I expression at diagnosis and its relationship to clinical outcomes in a cohort of 24 patients with NSCLC after perioperative ChIO from NADIM I clinical trial (NCT03081689). Furthermore, we also studied the composition of the tumor microenvironment (TME) to elucidate the underlying mechanisms of response and resistance to ChIO based on the tumor HLA class I status.
Methods
Patients and samples
Patients in this study were participants of NADIM clinical trial (NCT03081689), which included 46 patients with resectable stage IIIA NSCLC who received neoadjuvant ChIO treatment prior to surgery consisting of three cycles of nivolumab (360 mg) combined with paclitaxel (200 mg/m2) and carboplatin (area under the curve 6; 6 mg/mL per min), followed by adjuvant nivolumab monotherapy for 1 year (240 mg every 2 weeks for 4 months, followed by 480 mg every 4 weeks for 8 months). The pathological responses of the tumors were assessed by determination of the percentage of residual viable tumor in the primary tumor and lymph nodes tested and classified into complete pathological response (CPR, 0% of viable tumor cells) or non-CPR. The median follow-up time was 38.0 months (95% CI 36.7 to 40.7).
Baseline (at diagnosis) and post-neoadjuvant treatment (at surgery) tissue samples were collected from these patients. Pretreatment HLA class I status (proficient (PRO) or deficient (DEF) at any level) was determined in 24 pretreatment tissue samples by DNA LOH (ImmunoArray-24 V.2 kit) combined with pan-HLA immunohistochemistry (IHC) (online supplemental figure S1). HLA defects were not studied in the other 22 patients in the NADIM clinical trial due to limited baseline tissue samples. The techniques performed on samples from the 24 patients with known HLA class I status are summarized in online supplemental table S1.
Immunohistological analysis
We could analyze 5 NSCLC cases from the patient cohort present in this study. 4 µm-thick tissues were cut from formalin-fixed paraffin-embedded (FFPE) tissue samples and tissue sections were mounted on pretreated slides. Immunohistological techniques were performed with the biotin-streptavidin system (supersensitive Multilink HRP/DAB kit, BioGenex, The Hague, The Netherlands). Tumor samples were evaluated by two independent pathologists.
The following mouse monoclonal antibodies (mAbs) were used to analyze HLA class I expression: EMR8-5 against HLA-ABC heavy chain (Clone ab70328, Abcam), anti-b2m (Dako, Denmark) HC-10, specific for HLA-B and C free heavy chain (Dako, Denmark) and HCA2 which recognizes a subset of HLA-A locus-encoded gene products (Dako, Denmark). Total loss of HLA class I molecules was defined by negative staining with EMR85 and anti-b2m mAbs according to the criteria established by the HLA and Cancer component of the 1996 International Histocompatibility Workshop.28 The following cells were excluded from scoring: Infiltrating immune cell, normal cells, and non-specific staining of necrotic cells. Immunostained tissue sections were further scanned and analyzed using a “Panoramic Scanner MIDI II” and “Panoramic Viewer” (3DHISTECH, Budapest, Hungary).
LOH in HLA class I region of chromosome 6 and 15 analyzed by single nucleotide polymorphism arrays
Evaluation of the LOH in tumor samples was done based on the method described.29,32 DNA samples were genotyped using the Illumina Infinium HTS Assay on the ImmunoArray-24 V.2 BeadChip (Illumina) according to manufacturer protocol, which detects over 250,000 single-nucleotide polymorphisms (SNPs) selected based on GWAS (Genome-wide association study) of the diseases of the immune system. Data for LOH analysis and copy number (CN) analysis were obtained from the Illumina GenomeStudio Genotyping Module V.2.0 software as “Norm Theta” and “R” values. “Norm Theta” represents the B-allele frequency (BAF) and “R” the joined fluorescence intensity of both channels. While “Norm Theta” can be interpreted directly to detect LOH, “R” needs to be compared with a standard to detect regions of CN loss or gain. We used immunochip data from unrelated samples of European ancestry to obtain a median fluorescence value per probe to create such a standard and to subsequently obtain Log R ratios.31 33 A Log R ratio distribution around zero can be regarded as CN neutral, while chromosomal intervals of mainly positive (or negative) Log R ratios can be interpreted as CN gains (or losses). Chromosomal stretches of BAF with values of mainly zero or one can be interpreted as LOH. Dots with a value of “1” represents SNPs with the “AA” genotype, those with a value of “0” indicate SNPs with the “BB” genotype, and dots at “0.5” represent heterozygous “AB” genotype. When a BAF pattern has all three mentioned above values, the sample is heterozygous (AB) and there is no LOH. Complete loss of all SNPs with the AB genotype indicates regions of LOH. We also used the University of California in Santa Cruz (UCSC, USA) genome browser (http://genome.ucsc.edu/, accessed on January 31, 2021) to map and characterize the range of the missing regions in chromosomes 6 and 15 (GRCh38/hg38 assembly).
TMB, PD-L1 expression and TCR repertoire
TMB and PD-L1 tumor proportion score (TPS) were estimated from 22 pretreatment tissue samples using the Oncomine Tumor Mutational Load assay and IHC (22C3), respectively, as previously described.4 TMB higher than the median (5.89, range 1.68–73.95) was used to classify tumors with high and low TMB.
TCR sequencing was performed from FFPE using the Oncomine TCR Beta-SR Assay (RNA) assay as previously described.10 Frequency classifications of the clones in the TCR repertoire were done as previously described, and the Top 1% clonal space (CS) were defined as the aggregate frequencies of the Top 1% most frequent clones.10 34 A high CS of the Top 1% CS was defined using a 20% threshold.
Multiplex immunofluorescence
Multiplex immunofluorescence (mIF) was performed in 11 pretreatment tissues and 12 post-treatment tissues using Opal 7-Color fIHC Kit (Akoya Biosciences, Marlborough, MA) and the stained slides were scanned by a Vectra multispectral microscope (Akoya Biosciences). The immunofluorescence (IF) markers used were grouped into two 6-antibody panels: Panel 1 consisted of CD3 (red), CD8 (pink), PD-1 (green), PD-L1 (orange), CD68 (yellow) and pancytokeratin (cyan). Panel 2 consisted of CD3 (red), CD8 (pink), FOXP3 (green), granzyme B (orange), CD45RO (yellow) and pancytokeratin (cyan). Different immunophenotypes of T cells (CD3+), cytotoxic T cells (CD3+CD8+), T regulatory cells (CD3+FOXP3+) and macrophages (CD68+) were characterized and quantified using inForm image analysis software. Individual markers for each mIF panel were quantified and expressed as density per mm2.35
RNA expression: bulk RNA-seq and Visium spatial transcriptomics
Tumor bulk-RNA sequencing was achieved using the Oncomine Immune Response Assay.36 RNA was obtained from 12 FFPE tissue samples by truXTRAC FFPE DNA kit (Covaris). Differential-expressed genes (DEGs) and pathway enrichment analysis between HLA class I status categories were assessed using DESeq2 and gene set enrichment analysis (GSEA).
The spatial features of two post-treatment tissue samples were analyzed using Visium Spatial for FFPE Gene Expression Kit for Human Transcriptome (1000336, 10x Genomics). Prior to library construction, the RNA quality of the tissue was assessed by calculating DV200 of RNA extracted from FFPE-collected tissue sections. FFPE tissue sections were cut and placed in Visium spatial gene expression slides (1000344) and stained using H&E. The complementary DNA library was constructed using Dual Index Kit TS Set A (1000251) following Visium Spatial Gene Expression Reagent Kits for FFPE User Guide (CG000407, Rev D), and was sequenced on a NovaSeq X Plus (Illumina). Space Ranger pipeline (V.2.1.1; 10x Genomics) and Loupe Browser V.7.0.1 software (10x Genomics) were used to preprocess, process, and visualize the sequencing data. The areas of interest were previously defined and characterized by a pathologist.
Statistics
IBM SPSS software (V.25.0) and GraphPad Prism (V.8.0.2) were employed in the statistical analysis. Non-parametric Mann-Whitney U test was used to analyze differences between HLA-PRO and HLA-DEF tumors. Progression-free survival (PFS) and overall survival (OS) analysis were carried out using Kaplan-Meier, log-rank tests, and HR. The Ggplot2R package was used to build heatmaps and PCA (Principal Component Analysis) plots, and volcano plots were developed by VolcaNoseR18 based on the data obtained from DESeq2 analysis. A p value of <0.05 was considered statistically significant.
Results
Tumor HLA class I status at diagnosis and clinical outcomes
Baseline LOH or decreased HLA class I protein levels occurred in 10 out of 24 tumors analyzed (41.7%) and were therefore categorized as HLA-DEF status. Tumors without any alterations in HLA (58,3%) were classified as HLA-PRO. Illustrative immunostainings and SNPs arrays for HLA class I status determination are shown in online supplemental figure S1.
Patient clinical characteristics were representative of the complete NADIM cohort and are shown in table 1. There were no differences regarding age (p=1.000), sex (p=1.000), histology (p=0.590), smoking status (p=1.000), and clinical response (p=0.673), between patients with HLA-PRO or HLA-DEF tumors.
Table 1. Demographic and clinical characteristics of patients according to pretreatment HLA class I status. An adjusted p value of <0.05 was considered statistically significant.
| Characteristic | HLA-DEF (n=10) | HLA-PRO (n=14) | P value |
| Age (years)—median (range) | 66.5 (59.0–76.0) | 64.5 (41.0–74.0) | 1.000 |
| Sex—n (%) | 1.000 | ||
| Male | 8 (80.0) | 11 (78.6) | |
| Female | 2 (20.0) | 3 (21.4) | |
| Histology—n (%) | 0.590 | ||
| Adenocarcinoma | 5 (50.0) | 8 (57.1) | |
| Squamous | 5 (50.0) | 5 (35.7) | |
| No other specified | 0 (0.0) | 1 (7.1) | |
| Smoking status—n (%) | 1.000 | ||
| Former | 5 (50.0) | 6 (42.9) | |
| Current | 5 (50.0) | 8 (57.1) | |
| Packs-year—median (range) | 57.0 (24.0–111.0) | 50 (23.0–114.0) | 1.000 |
| Clinical response—n (%) | 0.673 | ||
| Partial | 6 (60.0) | 10 (71.4) | |
| Stable | 4 (40.0) | 4 (28.6) | |
| Surgery | 0.239 | ||
| Yes | 10 (100.0) | 11 (78.6) | |
| No | 0 (0.0) | 3 (21.4) | |
| Pathological response—n (%) | 0.670*, 1.000† | ||
| Complete | 5 (50.0) | 7 (50.0) | |
| Major | 2 (20.0) | 1 (7.1) | |
| Incomplete | 3 (30.0) | 3 (21.4) | |
Complete responses versus other.
Incomplete responses versus other.
HLAhuman leukocyte antigenHLA-DEFHLA-deficientHLA-PROHLA-proficient
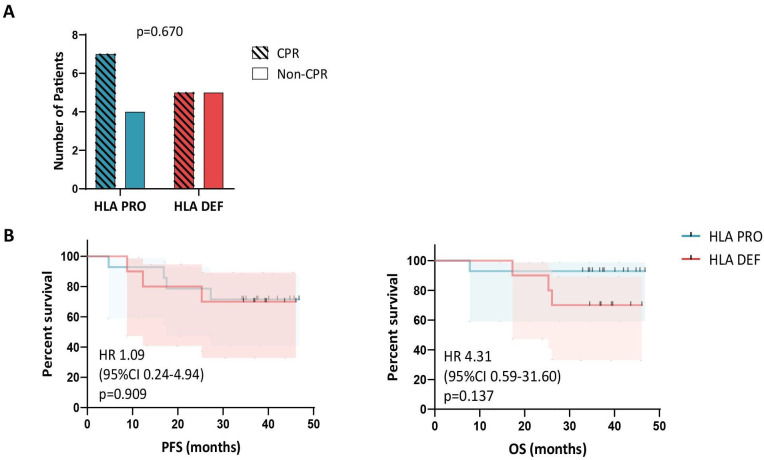
Tumor HLA-PRO status at baseline was not associated with pathological response achieved after neoadjuvant ChIO (p=0.670) (figure 1A). The percentage of CPR rates were similar regardless of HLA class I status. 50% of tumors with HLA-DEF status (5 out of 10 tumors) showed CPR, meanwhile 64% of HLA-PRO tumors showed CPR (7 out of 11). The pathological response of three HLA PRO tumors was not evaluated since the patients did not receive surgery.
Figure 1. HLA class I status is not associated with pathological response and survival after neoadjuvant treatment. (A) Number of patients with HLA-proficient (n=11) or deficient (n=10) tumors, and CPR (n=12) or non-CPR (n=9) (p=0.670). Pathological response of three HLA-PRO tumors was not evaluated since the patients did not receive surgery. (B) Kaplan-Meier survival curves of progression-free survival (PFS, on the left) and overall survival (OS, on the right) for HLA-proficient (n=14) and HLA-deficient (n=10) patients (p=0.909 and p=0.137). An adjusted p value of<0.05 was considered statistically significant. CPR, complete pathological response; HLA, human leukocyte antigen; HLA-DEF, HLA-deficient; HLA-PRO, HLA-proficient.
Similarly, HLA class I status was not associated with long-term survival outcomes (figure 1B). Patients with HLA-PRO and DEF tumors showed similar 3 year PFS and OS rates: PFS 71.4% (95% CI 40.6% to 88.2%) and OS 92.9% (95% CI 59.1% to 99.0%), and PFS 70% (95% CI 32.9% to 89.2%) and OS 70% (95% CI 32.9% to 89.2%), respectively.
Remarkably, the combination of TMB and HLA class I status in a single parameter seems to improve the identification of patients that showed long-term survival compared with each of those biomarkers alone (online supplemental figure S2). Although not statistically significant, probably due to the low number of double positive cases, all patients with TMB-high HLA-PRO tumors (n=5) were free of disease progression and alive at 3 years. On the contrary, for the rest of the patients (n=17) the PFS and OS rates were 58.8% (95% CI 32.5% to 77.8%) and 76.5% (95% CI 48.8% to 90.5%), respectively.
Tumor HLA class I status influence baseline microenvironment
Although the rates of complete responses and 3-year survival following neoadjuvant ChIO were similar between both HLA class I status groups of patients, we decided to investigate potential disparities in the TME composition at baseline. These differences may define two distinct immunological entities with possible unique mechanisms of response and resistance (figure 2).
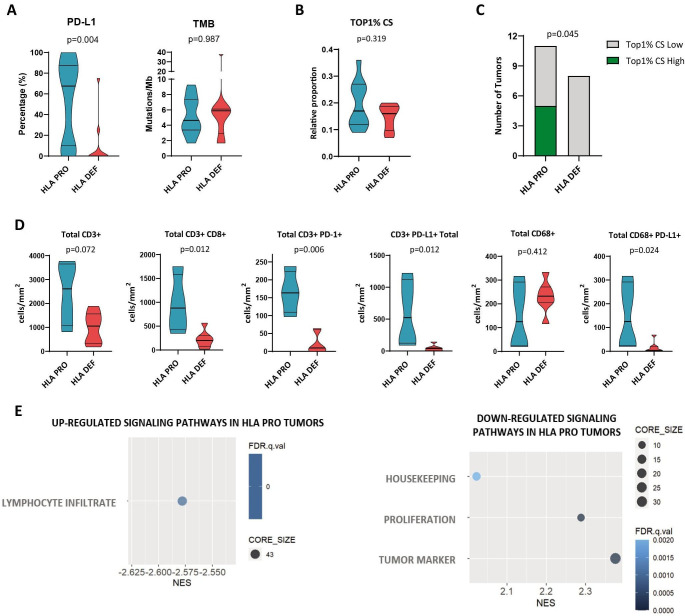
Figure 2. Tumor HLA class I status influence baseline tumor microenvironment. (A) Tumor proportion score (TPS) of PD-L1 (n=22), TMB levels (n=22). (B) Top 1% CS in HLA-DEF and PRO tumors (n=19). T cell clonotypes were ranked according to their frequency as the Top 1% most frequent clonotypes. (C) Patients with HLA-DEF and PRO classified as high or low Top 1% CS. A high CS of the Top 1% was defined using a 20% threshold. (D) Density of total T-cells (CD-3+), cytotoxic T-cells (CD3+CD8+), PD-1+T cells (CD3+PD-1+), PD-L1+T cells (CD3+PD-L1+), macrophages (CD68+) and PD-L1+macrophages (CD68+PD-L1+) (n=11 for all immune cell populations). (E) Gene set enrichment analysis for HLA class I status. Differentially upregulated and downregulated pathways in HLA-proficient (n=7) and HLA-deficient (n=5) pretreatment tumors. Non-parametric Mann-Whitney test was used for comparisons. An adjusted p value of<0.05 was considered statistically significant. FDR, False Discovery Rate; HLA, human leukocyte antigen; HLA-DEF, HLA-deficient; HLA-PRO, HLA-proficient; NES, Normalized Enrichment Score; PD-L1, programmed death ligand 1; TMB, tumor mutational burden; Top 1% CS, Top 1% clonal space.
In terms of the established response biomarkers PD-L1 and TMB, tumors with HLA class I defects had statistically significantly lower PD-L1 TPS levels than tumors without HLA class I alterations (HLA-PRO 67.5%, IQR 10–87.5%; vs HLA-DEF 0%, IQR 0–10%; p=0.004). In contrast, no differences in TMB levels were observed between the two groups (p=0.987) (figure 2A).
Next, we evaluated the TCR repertoire in baseline tumors (figure 2B,C), since its features, specially Top 1% CS (ie, the aggregate frequency of the percentile one most frequent clonotypes), has been associated with strong and antigen-specific immune responses in the neoadjuvant scenario (both IO and ChIO).10 34 No statistically significant differences in Top 1% CS were observed between groups (Mann-Whitney U test, p=0.319) (figure 2B). However, 5 out of 11 (45%) HLA-PRO tumors showed a high CS occupied by the top 1% most frequent clones (higher than 20% of the repertoire), while none of the 8 HLA-DEF tumors showed high Top 1% CS (Fisher’s exact test, p=0.045) (figure 2C). This supports that the HLA-class I function is necessary to generate a clonal T-cell response at baseline.
To determine the different immunophenotypes of the immune cell populations, mIF was performed on pretreatment tissue samples. Concerning lymphocytes populations, HLA-DEF tumors seemed to exhibit a desertic TME, displaying decreased densities of CD3+ (total T-cells; p=0.072), CD3+CD8+ (cytotoxic T-cells; p=0.012), CD3+PD-1+ (antigen-experienced T-cells37 ; p=0.006), and CD3+PD-L1+ (inhibitory T-cells,38 p=0.012) cells (figure 2D). On the other hand, the macrophage component seemed to be conserved in HLA-DEF tumors, with no differences in total macrophages (CD68+cells, p=0.412). However, HLA-DEF tumors showed reduced densities of macrophages PD-L1+ (p=0.024) (figure 2D). No differences were observed when analyzing the other immune populations detected by mIF. Similar differences in cell densities were observed considering both the tumor area and stroma (online supplemental figure S3).
Consistent with these results, GSEA using RNA-bulk tumor expression data showed that HLA-DEF tumors exhibited downregulation of the lymphocyte infiltrate pathway, as well as upregulation of both proliferation and tumor marker pathways (figure 2E). Analysis by DESeq2 identified four DEGs that were upregulated in HLA-DEF tumors, specifically CCL22, CCL17, IL1B, and CXCL8 (online supplemental figure S4), which are related to immunosuppressive TME, tumor growth, metastasis and lack of response to IO.39,41
Treatment-induced changes are HLA class I status-dependent
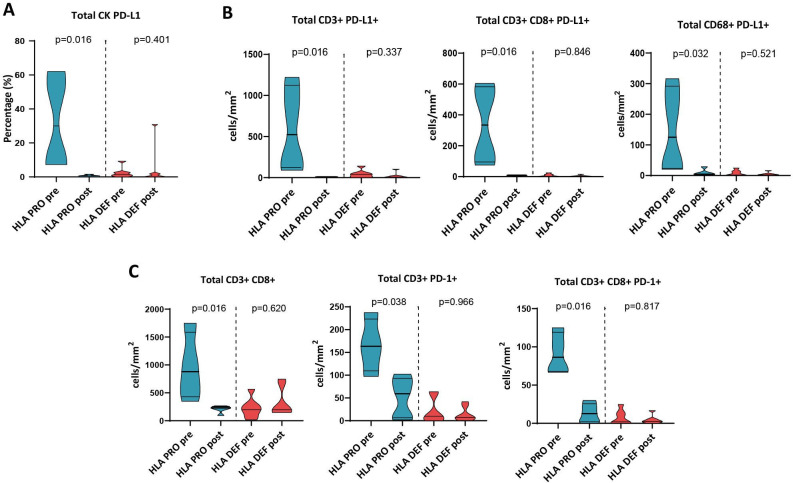
To understand the treatment effects in TMEs according to baseline HLA class I status, we analyzed the differences between the tumor immune component at diagnosis and post-treatment. HLA-PRO tumors decreased their percentage of tumor cells expressing PD-L1 after treatment (CK PD-L1+) (HLA-PRO pretreatment 30%, IQR 7.7–59.3%; vs HLA-PRO post-treatment 0.6%, IQR 0.1–1.2%; p=0.016), while HLA-DEF tumors maintained their levels low (p=0.401) (figure 3A). Similar changes occurred for different PD-L1 positive immune cell populations, including T-cells (CD3+PD-L1+) (HLA-PRO pretreatment 523.5, IQR 122.8–1124; vs HLA-PRO post-treatment 10.79, IQR 6.5–12.4; p=0.016), cytotoxic T-cells (CD3+CD8+ PD-L1+) (HLA-PRO pretreatment 334.2, IQR 94–582.1; vs HLA-PRO post-treatment 7.9, IQR 4.4–11.4; p=0.016) and macrophages (CD68+PD-L1+) (HLA-PRO pretreatment 125.1, IQR 23.5–291.8; vs HLA-PRO post-treatment 4.7, IQR 2.6–18.44; p=0.032) (figure 3B). In the same direction, the densities of cytotoxic T-cells, and PD-1 positive T-cells and cytotoxic T-cells decreased after treatment in HLA-PRO tumors (HLA-PRO pretreatment 879,3, IQR 429,3–1585; vs HLA-PRO post-treatment 229, IQR 154.3–252.2; p=0.016 in T-cells; HLA-PRO pretreatment 163,4, IQR 109.5–223.2; vs HLA-PRO post-treatment 59.2, IQR 6.7–92.9; p=0.038 in PD-1+T cells; and HLA-PRO pretreatment 86.4, IQR 68.12–119; vs HLA-PRO post-treatment 12.7, IQR 2.1–25.7; p=0.016 in PD-1+cytotoxic T-cells), but there were no differences between post-treatment immune infiltration of HLA-DEF and PRO tumors (figure 3C). Similar differences were observed in both tumor area and stroma of the tumors (online supplemental figure S5).
Figure 3. Treatment-induced changes in tumors are HLA class I status-dependent. (A) Percentage of total PD-L1+tumor cells (CK PD-L1+). (B) Density of total PD-L1+T cells (CD3+PD-L1+), PD-L1+cytotoxic T-cells (CD3+CD8+PD-L1+) and PD-L1+macrophages (CD68+PD-L1+). (C) Density of total cytotoxic T-cells (CD3+CD8+), PD-1+T cells (CD3+PD-1+) and PD-1+cytotoxic T-cells (CD3+CD8+PD-1+). n=11 and n=12 for pretreatment and post-treatment multiplex IF data, respectively. Non-parametric Mann-Whitney test was used for comparisons. An adjusted p value of<0.05 was considered statistically significant. HLA, human leukocyte antigen; HLA-DEF post, HLA-deficient post-treatment; HLA-DEF pre, HLA-deficient pretreatment; HLA-PRO post, HLA-proficient post-treatment; HLA-PRO pre, HLA-proficient pretreatment; IF, immunofluorescence; PD-1, programmed cell death protein-1; PD-L1, programmed death ligand 1.
Perioperative ChIO is capable of inducing a strong immune response in an HLA-DEF tumor context involving tertiary lymphoid structures
Despite HLA defects, 50% of HLA-DEF tumors achieved CPR after neoadjuvant ChIO. To investigate the mechanisms underlying these complete responses in a defective antigenic presentation scenario, we studied the TME after induction with ChIO by spatial transcriptomics (ST) in two tumor resection specimens at surgery, corresponding to HLA-DEF tumors with CPR and non-CPR responses.
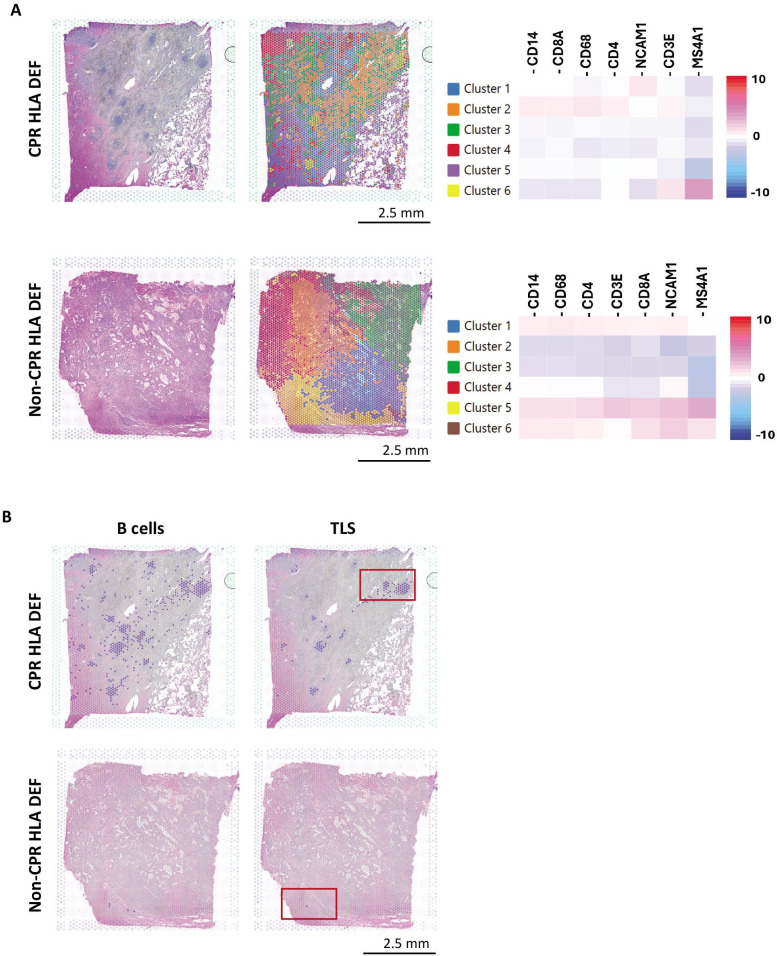
As a first approximation, we grouped the ST data into 6 k-means based clusters, which matched the tissue microanatomy observed by H&E, and tested the expression of different genes related to basic immune populations in each cluster (figure 4A).
Figure 4. Spatial transcriptomic analysis reveals a strong immune response and TLS presence in an HLA-deficient CPR tumor after neoadjuvant chemoimmunotherapy. (A) Clusters defined in both CPR and non-CPR HLA-deficient tumors and their corresponding heatmap of immune cell genes. (B) B cells infiltrate (MS4A1, CD19) and TLS (MS4A1, CD19, CR2, CXCL13) in CPR and non-CPR HLA-deficient tumors. Dots in purple express simultaneously the genes listed in each category. Marked in red are areas of interest with increased immune infiltration for subsequent analysis. CPR, complete pathological response; HLA, human leukocyte antigen; HLA-DEF, HLA-deficient; TLS, tertiary lymphoid structure.
Regarding CPR HLA-DEF tumor, immune infiltration was predominately in clusters 2 and 6. Cluster 6 was notably enriched in MS4A1 expression (CD20, B-cell marker), meanwhile cluster 2 was enriched in CD14, CD68, CD3E, CD4, and CD8A expression, compatible with high macrophages and T-cells infiltration. In the case of the non-CPR tumor, immune infiltration was restrained to clusters 1, 5, and 6.
Notably, in the immune infiltrate of the tumor that achieved complete response, there was a high number of lymphoid aggregates visible by H&E that were not present in the sample with incomplete response, which presented a more dispersed inflammatory component. These lymphoid aggregates exhibit a different expression profile, assigned to cluster 6, and are compatible with tertiary lymphoid structures (TLS) in which B-cells are predominant (figure 4A).
Thus, CPR HLA-DEF TME showed abundant and large aggregates of organized B-cells (based on MS4A1 and CD19 positivity), whereas B-cells were barely detected in non-CPR tumor. To determine whether any of these aggregates were mature TLS, we evaluated a TLS signature including MS4A1, CD19, CR2, and CXCL13 genes, which encode CD20, CD19, CD21 and CXCL13, respectively. Most of the B-cell aggregates were mature TLS, demonstrating strong immune responses after neoadjuvant ChIO in HLA-DEF tumors that achieve CPR, and suggesting a response mechanism in the context of impaired HLA antigen presentation (figure 4B).
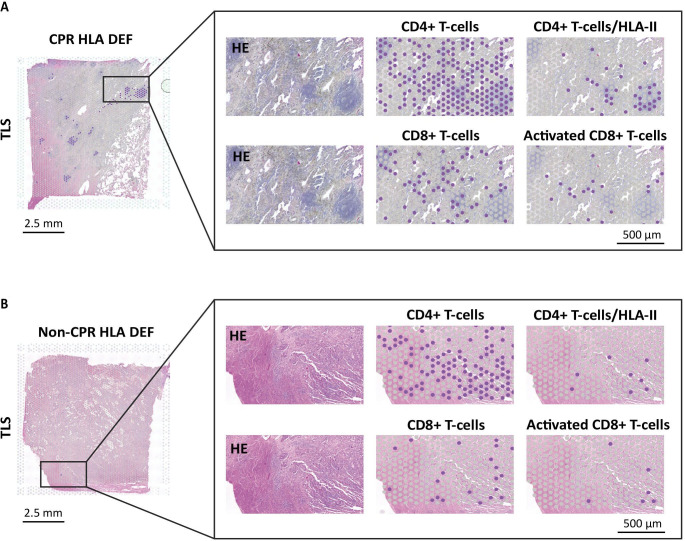
To shed light on this, we studied the role of T-cells in each type of response. To do that, we selected a TLS-rich region of the CPR tumor and the area of increased immune infiltration of non-CPR tumor (figure 5).
Figure 5. CPR HLA-deficient tumor presents a CD4+and CD8+ immune response involving TLS. Infiltrating CD4+T cells (CD4), CD4 T-cells coexisting with HLA class II (CD4, CD80, CIITA), infiltrating CD8+T cells (CD8A) and activated CD8+T cells (CD8, GZMB, KLRK1) in both CPR (A) and non-CPR (B) HLA-deficient tumors. Dots in purple express simultaneously the genes listed in each category. CPR, complete pathological response; HLA, human leukocyte antigen; HLA-DEF, HLA-deficient; TLS, tertiary lymphoid structure.
On the one hand, CD4 T-cells (based on CD3E and CD4 positivity) were detected in both CPR and non-CPR HLA-DEF tumors, but the infiltration was higher in CPR tumor. In addition, we included some genes related to HLA class II antigenic presentation (CIITA, CD80) to study whether these T-cells may coexist in regions of high presentation of antigens through HLA class II. Including these genes expression, the spots marked as CD4+T cell infiltrate mostly disappeared in non-CPR tumor, whereas were well maintained in CPR tissue, notably inside of TLS, suggesting that TLS may potentiate the activation of CD4+T cells recognizing their cognate antigens via HLA class II (figure 5). Furthermore, we observed that CPR tissue had higher expression of HLA-DRA, HLA-DPA1 and HLA-DQA1 compared with non-CPR tumor, further supporting the idea of the importance of class II antigenic presentation in the response of HLA-DEF tumors (online supplemental figure S6).
On the other hand, CD8+T cells (based on CD3E and CD8A positivity) were detected in both tumors, although the infiltrate seemed to be higher in the CPR tumors, where lymphocytes were located around TLS. To determine whether these CD8+T cells were activated, we included the GZMB and KLRK1 genes, which encode granzyme B and NKG2D proteins. A significant fraction of the detected CD8+T cell infiltrate was activated in CPR tumor, but not in non-CPR tumor, highlighting that the cytotoxic action of CD8+T cells is also an important factor in generating complete responses in HLA-DEF tumors (figure 5).
Discussion
In this study we evaluated the relevance of HLA class I status in the NADIM clinical trial patient cohort, studying the association with key clinical outcomes and identifying possible mechanisms of response to neoadjuvant ChIO. To our knowledge these are the first results regarding the predictive value of any HLA-based biomarker in the perioperative IO setting of NSCLC.12
The presentation of tumor antigens through HLA is one of the most important elements in tumor recognition by the immune system.11 Both HLA expression and the repertoire of neoantigens that can be presented may be involved in tumor evolution and treatment responses.17 18 42 43 In the NADIM clinical trial cohort, 41.7% of tested tumors at baseline were HLA-DEF, similarly to other studies.23 33 42 This means that almost half of patients with NSCLC could be categorized according to their tumor HLA class I status, with possible implications in their TME, response to therapy, and long-term survival.
Nevertheless, HLA class I status did not seem to be associated with either pathological response or survival after perioperative ChIO in our study. Some authors have associated defects that contribute to a lower diversity of presented neoantigens (such as HLA LOH or more recently HED, ie, germline HLA class I evolutionary divergence) with a poor response to IO in NSCLC.21 44 However, the predictive value of HLA class I status for ChIO may be less straightforward. The study by Jiang et al on patients with NSCLC with metastatic disease treated with ChIO, showed that HLA LOH alone failed to predict the benefit of ChIO, similar to our results in the perioperative scenario. However, a predictive value for HED was observed.27 In this regard, HED could serve as a more refined biomarker and might better capture the diversity of presented peptides in a more gradual manner than HLA heterozygosity status. Thus, beyond HLA LOH, HED could play a key role, especially in ChIO, whose value in the perioperative context would need to be validated in future studies.
Similarly, the combined HLA class I status with TMB may be informative in the perioperative ChIO context, overcoming observed limitations for HLA class I status alone. Although the differences were not statistically significant, probably due to the limited number of cases, all patients with tumors with HLA-PRO status and high TMB from the NADIM study were alive and free of disease progression at 3 years. TMB predictive value has been limited, specifically for ChIO.45 In this sense, this is not the first time that correction of TMB with HLA class I status is described to improve its prognostic value.25 46
In any case, apart from its potential value as a predictive biomarker for response to IO-based therapies, there appears to be a growing consensus on HLA role in tumor evolution and its interaction with the microenvironment.23 27 31 33 47
Thus, higher PD-L1 TPS has been described in tumors with higher HED.27 In the same line, we found that HLA-PRO status was associated with high PD-L1 TPS and, on the contrary, HLA-DEF status was associated with low PD-L1 TPS.
HLA class I defects, such as low HLA class I expression, have been correlated with a reduction of tumor-infiltrating lymphocytes (TILs), especially CD8+T cells, in a gradual transition involving cancer immunoediting, leading to a poorly immunogenic tumor phenotype.33 48 Baseline HLA-DEF tissue samples analyzed here also showed a decreased infiltrate of different immune cell populations, including cytotoxic T-cells or macrophages, generating a “cold” TME, confirmed by their downregulation of the lymphocyte infiltrate pathway in the GSEA of bulk RNAseq data, as well as a lack of T cell clonal expansion revealed by TCR repertoire analysis.
In contrast, it seems that HLA-PRO tumors showed a “hot” but exhausted immune infiltrate, in which tumor cells were able to elicit an immune response to the extent of recruiting T-cells, that recognized their cognate antigen (and therefore express PD-1), generating T cell clonal expansions, but by definition (there is a macroscopic tumor that is in the escape phase) are probably inhibited by increased expression of PD-L1 in the TME and associated immune infiltrate (higher densities of PD-L1+macrophages and T-cells).
One limitation of the study is the potential oversimplification of the determined HLA status, as proper antigenic presentation involves numerous proteins, such as TAP and others.49 50 However, our results support that, despite the lack of predictive value for response to ChIO, the HLA class I status measured in our study has a clear impact on the biology of the tumor and its microenvironment at baseline. Likewise, the results reinforce the role of HLA in tumor evolution, where the presence of an HLA defect at some point in development conditions the TME at diagnosis, making the overexpression of PD-L1 less relevant as an immune escape mechanism for HLA-DEF tumors.
Therefore, the absence of differences in patient prognosis according to HLA class I status does not exclude its importance in tumor biology, but it could be related to the efficacy of ChIO in patients with HLA defects and low immune infiltrate, compared with IO alone,44 supporting the improved clinical outcomes observed with neoadjuvant ChIO over IO.3
It is difficult to know exactly what occurs during neoadjuvant therapy, due to the impossibility of re-biopsy during treatment, but surgical tumor specimens after neoadjuvant treatment may give evidence of the processes that have taken place in the TME. From a descriptive standpoint, ST showed large infiltrates of B-cells forming TLS in an HLA-DEF tumor showing CPR. In the last few years, TLS have been described as a central element in tumor resolution and associated with strong responses to IO and ChIO in NSCLC, probably because of their role in the development and organization of adaptative antitumor responses.51,53 This implies a clear immune mechanism in the response of some tumors with defects in HLA beyond the activity of chemotherapy alone. Comparing CPR and non-CPR HLA-DEF tumors, CD4+T cell infiltrate was detected in both tumors. However, coexistence with HLA class II APCs occurred only inside TLS of CPR tumor, suggesting that this interaction could be a major contributor to the response of HLA-DEF tumors.54 Similarly to CD4+T cells, CD8+T cells were infiltrating both HLA-DEF tumors. However, activated CD8+T cells were detected mainly surrounding the TLS of the CPR tumor. CD8+T cells generally recognize endogenous antigens through HLA class I, but may recognize exogenous antigens presented by APCs, such as tumor antigens, through a cross-activation mechanism that primes CD8 T-cell cytotoxicity against the tumor and could therefore be activated in HLA-DEF tumors despite defects in the HLA class I of the tumor.15 Following activation, T-cells can carry out an adaptive response through HLA class I, but it has also been described that the expression of receptors such as NKG2D allows these cells to exert an innate cytotoxicity against tumors with defects in antigenic presentation.16 55
Recently, PD-1/PD-L1 axis inhibitors have been reported to increase tumor-specific γδ T-cell tumor infiltration, especially in HLA-defective tumors,56 57 being likely that they also participate in the neoadjuvant ChIO response scenario. This, in turn, reinforces that there are differential mechanisms of response to IO-based treatment based on the basal HLA status of the tumors.
In any case, the presence of TLS following neoadjuvant therapy in an HLA class I DEF context highlights the importance of the immune system in their response. Additionally, it demonstrates the capacity to develop TLS despite a baseline HLA defect and unveils a potential response mechanism to be therapeutically enhanced in non-responsive tumors.
Conclusions
In conclusion, our findings highlight the activity of perioperative ChIO in NSCLC HLA-DEF tumors, and the potential role of TLS, as well as the action of helper and cytotoxic T-cells, in tumor resolution. However, further investigations in larger cohorts will be needed to dilucidate the complex interplay between HLA, immune response, and clinical outcomes in perioperative ChIO scenarios for NSCLC.
supplementary material
Acknowledgements
We would like to thank the patients, their families, all the participating clinical teams, and all the Spanish Lung Cancer Group, BMS, for making this study possible.
Footnotes
Funding: Work in the authors’ laboratories was supported by “Instituto de Salud Carlos III” (ISCIII) PI19/01652 and PI22/01223 grants co-founded by European Regional Development Fund (ERDF), Bristol Myers Squibb (BMS), Ministry of Science and Innovation RTC2017-6502-1 “INmunoSIGHT”, RTC2019-007359-1 “BLI-O”, CPP2022-009545 “STRAGEN-IO”, and European Union’s Horizon 2020 research and innovation programme, CLARIFY 875160 grant, to MP. ThermoFisher provided reagents for TCR sequencing. AC-B is supported by a “Miguel Servet” contract CP23/00044 by ISCIII and received an ISCIII project grant PI23/01054 both co-founded by European Union. CM-T is supported by Comunidad de Madrid PIPF-2022/SAL-GL-25283 contract granted to MP. MM-A is supported by Ayuda Predoctoral Asociación Española Contra el Cáncer (AECC) Madrid 2023 contract granted to MP. FG, NA, and FRC was supported by grants from Andalusian Government and confounding by FEDER funds (B-CTS-410-UGR-20) and (Group CTS-143) and Ministry of Science and Innovation (PID2020-115087GB-100).
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Not applicable.
Ethics approval: Informed consent for the collection of research samples and study protocol were approved by the clinical research ethics committee of Hospital Puerta de Hierro (ref. 20.16) and the Spanish Lung Cancer Group (SLCG) Board (ref. N/A) in accordance with the International Conference on Harmonization Guidelines on Good Clinical Practice and the Declaration of Helsinki. Participants gave informed consent to participate in the study before taking part.
Data availability free text: Data are available on reasonable request. The data sets supporting this study are available from the corresponding author upon justified demand.
Contributor Information
Marta Molina-Alejandre, Email: mmolina@idiphim.org.
Francisco Perea, Email: franciscoj.perea.garcia@juntadeandalucia.es.
Virginia Calvo, Email: vircalvo@hotmail.com.
Cristina Martinez-Toledo, Email: cmartinez@idiphim.org.
Ernest Nadal, Email: esnadal@iconcologia.net.
Belén Sierra-Rodero, Email: bsierra@idiphim.org.
Marta Casarrubios, Email: mcasarrubios@idiphim.org.
Joaquín Casal-Rubio, Email: joaquin.casal.rubio@sergas.es.
Alex Martinez-Martí, Email: alexmartinezmarti.oncologia@gmail.com.
Amelia Insa, Email: ameliainsamolla@gmail.com.
Bartomeu Massuti, Email: bmassutis@seom.org.
Santiago Viteri, Email: sviteri@uomi.es.
Isidoro Barneto Aranda, Email: isidoroc.barneto@gmail.com.
Delvys Rodriguez-Abreu, Email: delvysra@gmail.com.
Javier de Castro, Email: javier.decastro@salud.madrid.org.
Joaquín Mosquera Martínez, Email: joaquin.mosquera.martinez@gmail.com.
Manuel Cobo, Email: manuelcobodols@yahoo.es.
Ignacio I Wistuba, Email: iiwistuba@mdanderson.org.
Edwin R Parra, Email: erparra@mdanderson.org.
Javier Martín-López, Email: jmartinl@salud.madrid.org.
Diego Megías, Email: diego.megias@isciii.es.
Rafael Muñoz-Viana, Email: Rmviana@idiphim.org.
Federico Garrido, Email: federico.garrido.sspa@juntadeandalucia.es.
Natalia Aptsiauri, Email: naptsiauri@ugr.es.
Francisco Ruiz-Cabello, Email: francisco.ruizcabello.sspa@juntadeandalucia.es.
Mariano Provencio, Email: mprovencio@gmail.com.
Alberto Cruz-Bermúdez, Email: alberto.cruz.bermudez@gmail.com.
Data availability statement
Data are available upon reasonable request.
References
- 1.Siegel RL, Giaquinto AN, Jemal A. Cancer statistics, 2024. CA Cancer J Clin. 2024;74:12–49. doi: 10.3322/caac.21820. [DOI] [PubMed] [Google Scholar]
- 2.Chen LN, Wei AZ, Shu CA. Neoadjuvant immunotherapy in resectable non-small-cell lung cancer. Ther Adv Med Oncol. 2023;15:17588359231163798. doi: 10.1177/17588359231163798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Provencio M, Calvo V, Romero A, et al. Treatment Sequencing in Resectable Lung Cancer: The Good and the Bad of Adjuvant Versus Neoadjuvant Therapy. Am Soc Clin Oncol Educ Book. 2022;42:1–18. doi: 10.1200/EDBK_358995. [DOI] [PubMed] [Google Scholar]
- 4.Provencio M, Nadal E, Insa A, et al. Neoadjuvant chemotherapy and nivolumab in resectable non-small-cell lung cancer (NADIM): an open-label, multicentre, single-arm, phase 2 trial. Lancet Oncol. 2020;21:1413–22. doi: 10.1016/S1470-2045(20)30453-8. [DOI] [PubMed] [Google Scholar]
- 5.Provencio M, Nadal E, González-Larriba JL, et al. Perioperative Nivolumab and Chemotherapy in Stage III Non-Small-Cell Lung Cancer. N Engl J Med. 2023;389:504–13. doi: 10.1056/NEJMoa2215530. [DOI] [PubMed] [Google Scholar]
- 6.Heymach JV, Harpole D, Mitsudomi T, et al. Perioperative Durvalumab for Resectable Non-Small-Cell Lung Cancer. N Engl J Med. 2023;389:1672–84. doi: 10.1056/NEJMoa2304875. [DOI] [PubMed] [Google Scholar]
- 7.Wakelee H, Liberman M, Kato T, et al. Perioperative Pembrolizumab for Early-Stage Non-Small-Cell Lung Cancer. N Engl J Med. 2023;389:491–503. doi: 10.1056/NEJMoa2302983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cascone T, Awad MM, Spicer JD, et al. Perioperative Nivolumab in Resectable Lung Cancer. N Engl J Med. 2024;390:1756–69. doi: 10.1056/NEJMoa2311926. [DOI] [PubMed] [Google Scholar]
- 9.Jardim DL, Goodman A, de Melo Gagliato D, et al. The Challenges of Tumor Mutational Burden as an Immunotherapy Biomarker. Cancer Cell. 2021;39:154–73. doi: 10.1016/j.ccell.2020.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Casarrubios M, Cruz-Bermúdez A, Nadal E, et al. Pretreatment Tissue TCR Repertoire Evenness Is Associated with Complete Pathologic Response in Patients with NSCLC Receiving Neoadjuvant Chemoimmunotherapy. Clin Cancer Res. 2021;27:5878–90. doi: 10.1158/1078-0432.CCR-21-1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hazini A, Fisher K, Seymour L. Deregulation of HLA-I in cancer and its central importance for immunotherapy. J Immunother Cancer. 2021;9:e002899. doi: 10.1136/jitc-2021-002899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dimou A. Time to Think about HLA-Based Diagnostics in Lung Cancer? Clin Cancer Res. 2023;29:4706–8. doi: 10.1158/1078-0432.CCR-23-2152. [DOI] [PubMed] [Google Scholar]
- 13.Wu Y, Zhang N, Hashimoto K, et al. Structural Comparison Between MHC Classes I and II; in Evolution, a Class-II-Like Molecule Probably Came First. Front Immunol. 2021;12:621153. doi: 10.3389/fimmu.2021.621153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.An J, Lein K, Kie A, et al. Review Articles Advances in Immunology: The HLA System. N Engl J Med. 2000;343:702–9. [Google Scholar]
- 15.Luri-Rey C, Gomis G, Glez-Vaz J, et al. Cytotoxicity as a form of immunogenic cell death leading to efficient tumor antigen cross-priming. Immunol Rev. 2024;321:143–51. doi: 10.1111/imr.13281. [DOI] [PubMed] [Google Scholar]
- 16.Rudd CE. CD8+ T cell killing of MHC class I–deficient tumors. Nat Cancer . 2023;4:1214–6. doi: 10.1038/s43018-023-00606-y. [DOI] [PubMed] [Google Scholar]
- 17.Garrido F, Cabrera T, Concha A, et al. Natural history of HLA expression during tumour development. Immunol Today. 1993;14:491–9. doi: 10.1016/0167-5699(93)90264-L. [DOI] [PubMed] [Google Scholar]
- 18.Cordon-Cardo C, Fuks Z, Drobnjak M, et al. Expression of HLA-A,B,C antigens on primary and metastatic tumor cell populations of human carcinomas. Cancer Res. 1991;51:6372–80. http://aacrjournals.org/cancerres/article-pdf/51/23_Part_1/6372/2445672/cr05123p16372.pdf Available. [PubMed] [Google Scholar]
- 19.Kikuchi E, Yamazaki K, Torigoe T, et al. HLA class I antigen expression is associated with a favorable prognosis in early stage non-small cell lung cancer. Cancer Sci. 2007;98:1424–30. doi: 10.1111/j.1349-7006.2007.00558.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang Q-L, Wang T-M, Deng C-M, et al. Association of HLA diversity with the risk of 25 cancers in the UK Biobank. EBioMedicine. 2023;92:104588. doi: 10.1016/j.ebiom.2023.104588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chowell D, Krishna C, Pierini F, et al. Evolutionary divergence of HLA class I genotype impacts efficacy of cancer immunotherapy. Nat Med. 2019;25:1715–20. doi: 10.1038/s41591-019-0639-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abed A, Calapre L, Lo J, et al. Prognostic value of HLA-I homozygosity in patients with non-small cell lung cancer treated with single agent immunotherapy. J Immunother Cancer. 2020;8:e001620. doi: 10.1136/jitc-2020-001620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang X, Tang H, Luo H, et al. Integrated investigation of the prognostic role of HLA LOH in advanced lung cancer patients with immunotherapy. Front Genet. 2022;13:1066636. doi: 10.3389/fgene.2022.1066636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao J, Xiao X, Li Y, et al. The prevalence of HLA-I LOH in Chinese pan-cancer patients and genomic features of patients harboring HLA-I LOH. Hum Mutat. 2021;42:1254–64. doi: 10.1002/humu.24255. [DOI] [PubMed] [Google Scholar]
- 25.Montesion M, Murugesan K, Jin DX, et al. Somatic HLA Class I Loss Is a Widespread Mechanism of Immune Evasion Which Refines the Use of Tumor Mutational Burden as a Biomarker of Checkpoint Inhibitor Response. Cancer Discov. 2021;11:282–92. doi: 10.1158/2159-8290.CD-20-0672. [DOI] [PubMed] [Google Scholar]
- 26.Lee D, Park J, Choi H, et al. Association of HLA class I homozygosity with unfavorable clinical outcomes in patients with non-small cell lung cancer treated with chemo-immunotherapy or immunotherapy as first-line therapy. Heliyon. 2021;7:e07916. doi: 10.1016/j.heliyon.2021.e07916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jiang T, Jin Q, Wang J, et al. HLA-I Evolutionary Divergence Confers Response to PD-1 Blockade plus Chemotherapy in Untreated Advanced Non-Small Cell Lung Cancer. Clin Cancer Res. 2023;29:4830–43. doi: 10.1158/1078-0432.CCR-23-0604. [DOI] [PubMed] [Google Scholar]
- 28.Garrido F. 12th International Histocompatibility Conference. Genetic diversity of HLA: functional and medical implications. Hum Immunol. 1996;47:1–184. doi: 10.1016/S0198-8859(96)85603-5. [DOI] [PubMed] [Google Scholar]
- 29.Montes P, Bernal M, Campo LN, et al. Tumor genetic alterations and features of the immune microenvironment drive myelodysplastic syndrome escape and progression. Cancer Immunol Immunother. 2019;68:2015–27. doi: 10.1007/s00262-019-02420-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Betensky M, Babushok D, Roth JJ, et al. Clonal evolution and clinical significance of copy number neutral loss of heterozygosity of chromosome arm 6p in acquired aplastic anemia. Cancer Genet. 2016;209:1–10. doi: 10.1016/j.cancergen.2015.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Perea F, Sánchez-Palencia A, Gómez-Morales M, et al. HLA class I loss and PD-L1 expression in lung cancer: impact on T-cell infiltration and immune escape. Oncotarget. 2018;9:4120–33. doi: 10.18632/oncotarget.23469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jasek M, Gondek LP, Bejanyan N, et al. TP53 mutations in myeloid malignancies are either homozygous or hemizygous due to copy number-neutral loss of heterozygosity or deletion of 17p. Leukemia. 2010;24:216–9. doi: 10.1038/leu.2009.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Perea F, Bernal M, Sánchez-Palencia A, et al. The absence of HLA class I expression in non-small cell lung cancer correlates with the tumor tissue structure and the pattern of T cell infiltration. Int J Cancer. 2017;140:888–99. doi: 10.1002/ijc.30489. [DOI] [PubMed] [Google Scholar]
- 34.Zhang J, Ji Z, Caushi JX, et al. Compartmental Analysis of T-cell Clonal Dynamics as a Function of Pathologic Response to Neoadjuvant PD-1 Blockade in Resectable Non-Small Cell Lung Cancer. Clin Cancer Res. 2020;26:1327–37. doi: 10.1158/1078-0432.CCR-19-2931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Parra ER, Uraoka N, Jiang M, et al. Validation of multiplex immunofluorescence panels using multispectral microscopy for immune-profiling of formalin-fixed and paraffin-embedded human tumor tissues. Sci Rep. 2017;7:13380. doi: 10.1038/s41598-017-13942-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Casarrubios M, Provencio M, Nadal E, et al. Tumor microenvironment gene expression profiles associated to complete pathological response and disease progression in resectable NSCLC patients treated with neoadjuvant chemoimmunotherapy. J Immunother Cancer. 2022;10:e005320. doi: 10.1136/jitc-2022-005320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Simon S, Labarriere N. PD-1 expression on tumor-specific T cells: Friend or foe for immunotherapy? Oncoimmunology. 2018;7:e1364828. doi: 10.1080/2162402X.2017.1364828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Diskin B, Adam S, Cassini MF, et al. PD-L1 engagement on T cells promotes self-tolerance and suppression of neighboring macrophages and effector T cells in cancer. Nat Immunol. 2020;21:442–54. doi: 10.1038/s41590-020-0620-x. [DOI] [PubMed] [Google Scholar]
- 39.Marshall LA, Marubayashi S, Jorapur A, et al. Tumors establish resistance to immunotherapy by regulating Treg recruitment via CCR4. J Immunother Cancer. 2020;8:e000764. doi: 10.1136/jitc-2020-000764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Han ZJ, Li YB, Yang LX, et al. Roles of the CXCL8-CXCR1/2 Axis in the Tumor Microenvironment and Immunotherapy. Molecules. 2022;27:137. doi: 10.3390/molecules27010137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Diwanji R, O’Brien NA, Choi JE, et al. Targeting the IL1β Pathway for Cancer Immunotherapy Remodels the Tumor Microenvironment and Enhances Antitumor Immune Responses. Cancer Immunol Res. 2023;11:777–91. doi: 10.1158/2326-6066.CIR-22-0290. [DOI] [PubMed] [Google Scholar]
- 42.McGranahan N, Rosenthal R, Hiley CT, et al. Allele-Specific HLA Loss and Immune Escape in Lung Cancer Evolution. Cell. 2017;171:1259–71. doi: 10.1016/j.cell.2017.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Aptsiauri N, Garrido F. The Challenges of HLA Class I Loss in Cancer Immunotherapy: Facts and Hopes. Clin Cancer Res. 2022;28:5021–9. doi: 10.1158/1078-0432.CCR-21-3501. [DOI] [PubMed] [Google Scholar]
- 44.Chowell D, Morris LGT, Grigg CM, et al. Patient HLA class I genotype influences cancer response to checkpoint blockade immunotherapy. Science. 2018;359:582–7. doi: 10.1126/science.aao4572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Strickler JH, Hanks BA, Khasraw M. Tumor Mutational Burden as a Predictor of Immunotherapy Response: Is More Always Better? Clin Cancer Res. 2021;27:1236–41. doi: 10.1158/1078-0432.CCR-20-3054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shim JH, Kim HS, Cha H, et al. HLA-corrected tumor mutation burden and homologous recombination deficiency for the prediction of response to PD-(L)1 blockade in advanced non-small-cell lung cancer patients. Ann Oncol. 2020;31:902–11. doi: 10.1016/j.annonc.2020.04.004. [DOI] [PubMed] [Google Scholar]
- 47.Flores-Martín JF, Perea F, Exposito-Ruiz M, et al. A Combination of Positive Tumor HLA-I and Negative PD-L1 Expression Provides an Immune Rejection Mechanism in Bladder Cancer. Ann Surg Oncol. 2019;26:2631–9. doi: 10.1245/s10434-019-07371-2. [DOI] [PubMed] [Google Scholar]
- 48.Aptsiauri N, Ruiz-Cabello F, Garrido F. The transition from HLA-I positive to HLA-I negative primary tumors: the road to escape from T-cell responses. Curr Opin Immunol. 2018;51:123–32. doi: 10.1016/j.coi.2018.03.006. [DOI] [PubMed] [Google Scholar]
- 49.Dersh D, Hollý J, Yewdell JW. A few good peptides: MHC class I-based cancer immunosurveillance and immunoevasion. Nat Rev Immunol . 2021;21:116–28. doi: 10.1038/s41577-020-0390-6. [DOI] [PubMed] [Google Scholar]
- 50.Tu Z, Li K, Ji Q, et al. Pan-cancer analysis: predictive role of TAP1 in cancer prognosis and response to immunotherapy. BMC Cancer. 2023;23:133. doi: 10.1186/s12885-022-10491-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sautès-Fridman C, Petitprez F, Calderaro J, et al. Tertiary lymphoid structures in the era of cancer immunotherapy. Nat Rev Cancer. 2019;19:307–25. doi: 10.1038/s41568-019-0144-6. [DOI] [PubMed] [Google Scholar]
- 52.Cottrell TR, Thompson ED, Forde PM, et al. Pathologic features of response to neoadjuvant anti-PD-1 in resected non-small-cell lung carcinoma: a proposal for quantitative immune-related pathologic response criteria (irPRC) Ann Oncol. 2018;29:1853–60. doi: 10.1093/annonc/mdy218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xie M, Jing Y, Xue X. EP06.02-07 Tertiary Lymphoid Structures Predict Clinical Outcomes of Neoadjuvant Chemoimmunotherapy in Resectable NSCLC. J Thorac Oncol. 2023;18:S481–2. doi: 10.1016/j.jtho.2023.09.892. [DOI] [Google Scholar]
- 54.Johnson DB, Estrada MV, Salgado R, et al. Melanoma-specific MHC-II expression represents a tumour-autonomous phenotype and predicts response to anti-PD-1/PD-L1 therapy. Nat Commun. 2016;7:10582. doi: 10.1038/ncomms10582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lerner EC, Woroniecka KI, D’Anniballe VM, et al. CD8+ T cells maintain killing of MHC-I-negative tumor cells through the NKG2D-NKG2DL axis. Nat Cancer . 2023;4:1258–72. doi: 10.1038/s43018-023-00600-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lien SC, Ly D, Yang SYC, et al. Tumor reactive γδ T cells contribute to a complete response to PD-1 blockade in a Merkel cell carcinoma patient. Nat Commun. 2024;15:1094. doi: 10.1038/s41467-024-45449-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.de Vries NL, van de Haar J, Veninga V, et al. γδ T cells are effectors of immunotherapy in cancers with HLA class I defects. Nature New Biol. 2023;613:743–50. doi: 10.1038/s41586-022-05593-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available upon reasonable request.