Abstract
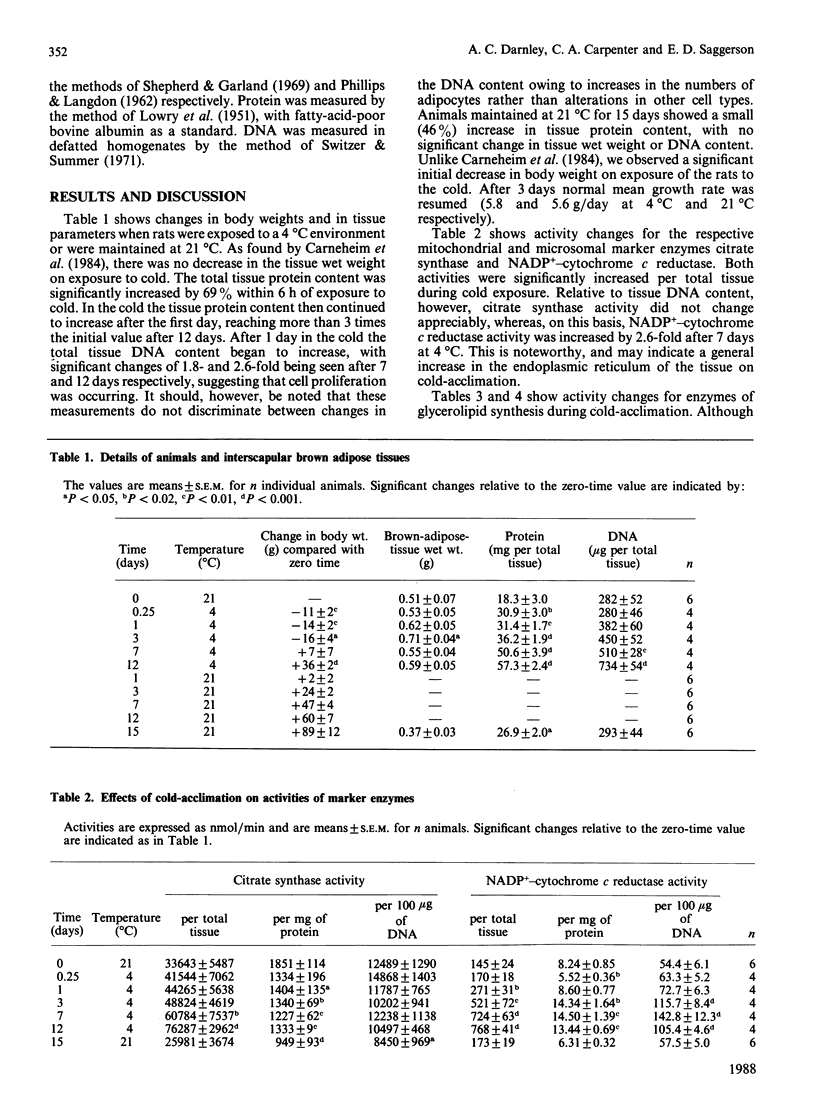
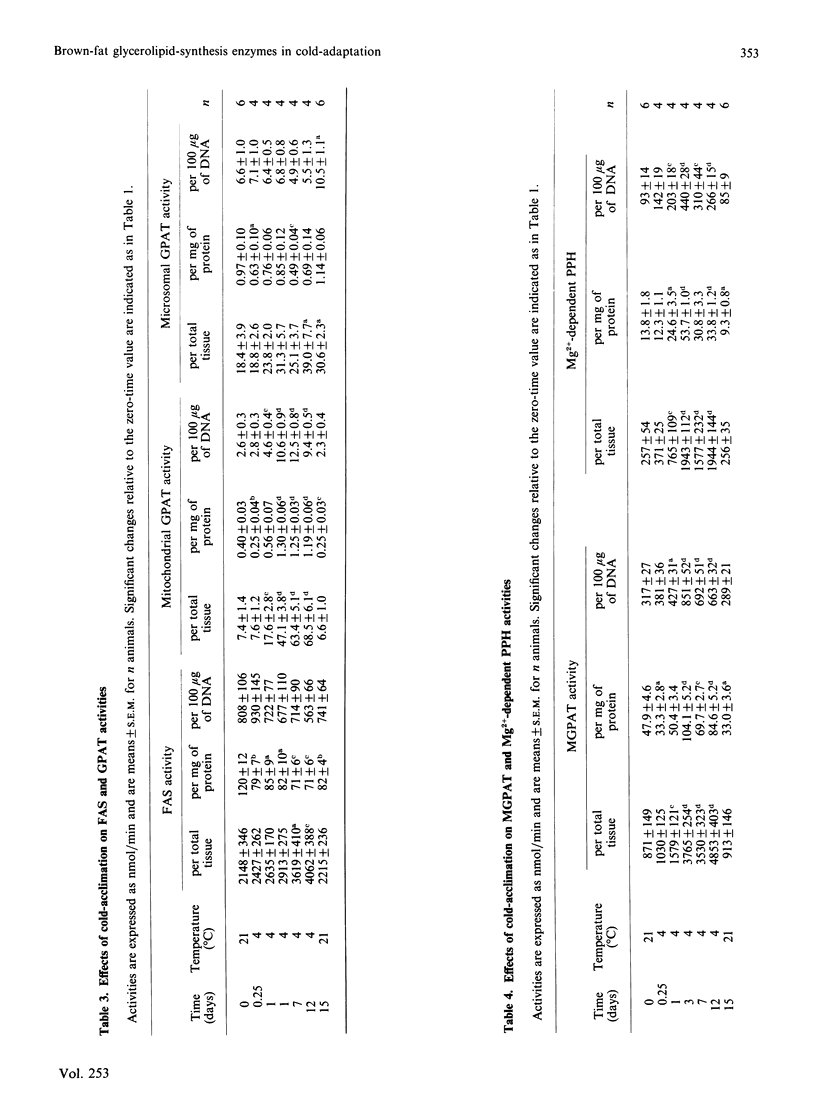
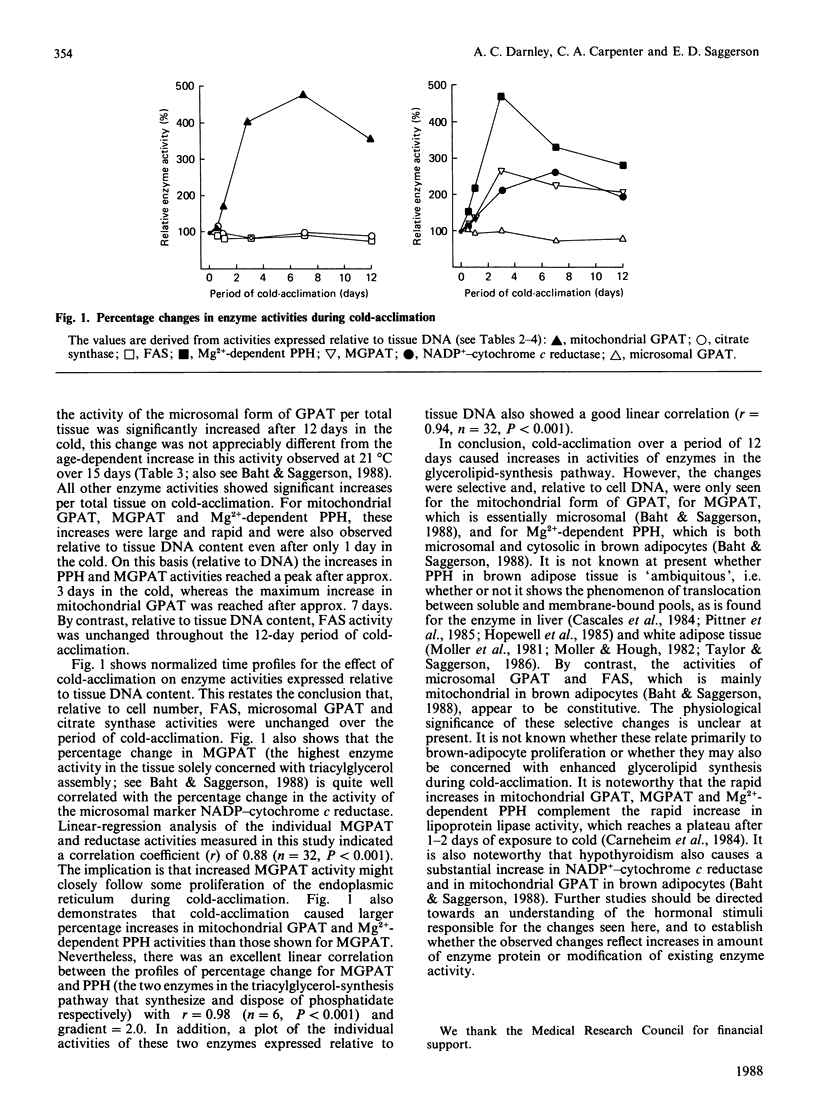
1. Measurements were made of the activities of the following enzymes of glycerolipid synthesis in homogenates of interscapsular brown adipose tissue obtained from rats subjected to a 4 degrees C environment for time periods of 6 h up to 12 days: fatty acyl-CoA synthetase (FAS), mitochondrial and microsomal forms of glycerolphosphate acyltransferase (GPAT), monoacylglycerolphosphate acyltransferase (MGPAT) and Mg2+-dependent phosphatidate phosphohydrolase (PPH). 2. Relative to tissue DNA content, the activities of mitochondrial GPAT, MGPAT and Mg2+-dependent PPH were significantly increased after 1 day of exposure to cold, and continued to increase thereafter. By contrast, FAS and microsomal GPAT activities were unchanged relative to tissue DNA. 3. The time profile of the increase in MGPAT activity correlated well with a concomitant increase in the microsomal marker NADP+-cytochrome c reductase. Changes in mitochondrial GPAT and in Mg2+-dependent PPH activities were larger in amplitude than that of MGPAT. 4. It is proposed that these selective changes in enzyme activity may be associated with the onset of brown-adipose-tissue hyperplasia or possibly with an increase in triacylglycerol synthesis during cold-acclimation.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agius L., Williamson D. H. Lipogenesis in interscapular brown adipose tissue of virgin, pregnant and lactating rats. The effects of intragastric feeding. Biochem J. 1980 Aug 15;190(2):477–480. doi: 10.1042/bj1900477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agius L., Williamson D. H. The utilization of ketone bodies by the interscapular brown adipose tissue of the rat. Biochim Biophys Acta. 1981 Oct 23;666(1):127–132. doi: 10.1016/0005-2760(81)90098-9. [DOI] [PubMed] [Google Scholar]
- Baht H. S., Saggerson E. D. Comparison of triacylglycerol synthesis in rat brown and white adipocytes. Effects of hypothyroidism and streptozotocin-diabetes on enzyme activities and metabolic fluxes. Biochem J. 1988 Mar 1;250(2):325–333. doi: 10.1042/bj2500325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley M. G., Rath E. A. Regulation of fatty acid synthesis and malonyl-CoA content in mouse brown adipose tissue in response to cold-exposure, starvation or re-feeding. Biochem J. 1987 Apr 15;243(2):437–442. doi: 10.1042/bj2430437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAMERON I. L., SMITH R. E. CYTOLOGICAL RESPONSES OF BROWN FAT TISSUE IN COLD-EXPOSED RATS. J Cell Biol. 1964 Oct;23:89–100. doi: 10.1083/jcb.23.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carneheim C., Nedergaard J., Cannon B. Beta-adrenergic stimulation of lipoprotein lipase in rat brown adipose tissue during acclimation to cold. Am J Physiol. 1984 Apr;246(4 Pt 1):E327–E333. doi: 10.1152/ajpendo.1984.246.4.E327. [DOI] [PubMed] [Google Scholar]
- Cascales C., Mangiapane E. H., Brindley D. N. Oleic acid promotes the activation and translocation of phosphatidate phosphohydrolase from the cytosol to particulate fractions of isolated rat hepatocytes. Biochem J. 1984 May 1;219(3):911–916. doi: 10.1042/bj2190911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopewell R., Martin-Sanz P., Martin A., Saxton J., Brindley D. N. Regulation of the translocation of phosphatidate phosphohydrolase between the cytosol and the endoplasmic reticulum of rat liver. Effects of unsaturated fatty acids, spermine, nucleotides, albumin and chlorpromazine. Biochem J. 1985 Dec 1;232(2):485–491. doi: 10.1042/bj2320485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Moller F., Hough M. R. Effect of salts on membrane binding and activity of adipocyte phosphatidate phosphohydrolase. Biochim Biophys Acta. 1982 Jun 11;711(3):521–531. doi: 10.1016/0005-2760(82)90068-6. [DOI] [PubMed] [Google Scholar]
- Moller F., Wong K. H., Green P. Control of fat cell phosphohydrolase by lipolytic agents. Can J Biochem. 1981 Jan;59(1):9–15. doi: 10.1139/o81-002. [DOI] [PubMed] [Google Scholar]
- PHILLIPS A. H., LANGDON R. G. Hepatic triphosphopyridine nucleotide-cytochrome c reductase: isolation, characterization, and kinetic studies. J Biol Chem. 1962 Aug;237:2652–2660. [PubMed] [Google Scholar]
- Pittner R. A., Fears R., Brindley D. N. Interactions of insulin, glucagon and dexamethasone in controlling the activity of glycerol phosphate acyltransferase and the activity and subcellular distribution of phosphatidate phosphohydrolase in cultured rat hepatocytes. Biochem J. 1985 Sep 1;230(2):525–534. doi: 10.1042/bj2300525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radomski M. W., Orme T. Response of lipoprotein lipase in various tissues to cold exposure. Am J Physiol. 1971 Jun;220(6):1852–1856. doi: 10.1152/ajplegacy.1971.220.6.1852. [DOI] [PubMed] [Google Scholar]
- Shepherd D., Garland P. B. The kinetic properties of citrate synthase from rat liver mitochondria. Biochem J. 1969 Sep;114(3):597–610. doi: 10.1042/bj1140597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Switzer B. R., Summer G. K. A modified fluorometric micromethod for DNA. Clin Chim Acta. 1971 Apr;32(2):203–206. doi: 10.1016/0009-8981(71)90333-0. [DOI] [PubMed] [Google Scholar]
- Taylor S. J., Saggerson E. D. Adipose-tissue Mg2+-dependent phosphatidate phosphohydrolase. Control of activity and subcellular distribution in vitro and in vivo. Biochem J. 1986 Oct 15;239(2):275–284. doi: 10.1042/bj2390275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trayhurn P. Fatty acid synthesis in mouse brown adipose tissue. The influence of environmental temperature on the proportion of whole-body fatty acid synthesis in brown adipose tissue and the liver. Biochim Biophys Acta. 1981 Jun 23;664(3):549–560. doi: 10.1016/0005-2760(81)90132-6. [DOI] [PubMed] [Google Scholar]
- Trayhurn P. Fatty acid synthesis in vivo in brown adipose tissue, liver and white adipose tissue of the cold-acclimated rat. FEBS Lett. 1979 Aug 1;104(1):13–16. doi: 10.1016/0014-5793(79)81075-3. [DOI] [PubMed] [Google Scholar]


