Abstract
Background
Among people with HIV (PWH), COVID-19 is common and potentially severe. We leveraged REPRIEVE (Randomized Trial to Prevent Vascular Events in HIV) to assess the effects of statin therapy for cardiovascular disease prevention on COVID-19 outcomes (incidence and serious cases) among a global cohort of PWH.
Methods
COVID-19 data collection was implemented April 2020 to capture events from January 2020. COVID-19 was defined by positive test result or clinical diagnosis and serious COVID-19 according to the International Conference on Harmonisation definition. Among participants in follow-up on 1 January 2020, Cox proportional hazards modeling was used to estimate the hazard ratio (HR) of COVID-19 (pitavastatin/placebo), stratified by Global Burden of Disease region. Modification of statin effect following COVID-19 vaccination was evaluated via interaction with time-updated vaccination status.
Results
Among 6905 PWH, 32% were natal female and 41% were Black or African American. The median age was 53 years and the 10-year atherosclerotic cardiovascular disease risk score 4.5%. Statin therapy did not reduce COVID-19 incidence (HR, 1.05; 95% CI, .95–1.15) but appeared to reduce incidence of serious COVID-19 (HR, 0.75; 95% CI, .52–1.09). Among 1701 PWH with COVID-19, the relative risk (pitavastatin/placebo) for serious COVID-19 was 0.73 (95% CI, .52–1.03). The treatment effect size for serious COVID-19 fell within the hypothesized range, but the 95% CI crossed 1 given fewer-than-anticipated cases (117 vs 200). Furthermore, 83% reported COVID-19 vaccination by end of study, with a strong protective effect on serious COVID-19 (HR, 0.27; 95% CI, .14–.53; P < .0001). A protective statin effect was observed prior to vaccination.
Conclusions
Among PWH, statin therapy had no effect on COVID-19 incidence but showed potential to reduce risk of serious COVID-19 prior to COVID-19 vaccination.
Clinical Trials Registration
Keywords: COVID-19, HIV, statin, REPRIEVE, PWH
In a global cohort of people with HIV with low to moderate traditional atherosclerotic cardiovascular disease risk, statin therapy had no effect on COVID-19 incidence but showed potential to reduce the risk of serious COVID-19 prior to COVID-19 vaccination.
Among people with HIV (PWH), SARS-CoV-2 infection is common and potentially serious. A meta-analysis of 32 studies conducted in North America, Europe, Asia, and Africa suggested that SARS-CoV-2 infection was at least as common among individuals with vs without HIV [1]. Meanwhile, several studies have indicated that outcomes of COVID-19 may be more severe among PWH [2]. For example, data from the World Health Organization's global platform for the clinical characterization of COVID-19 outcomes among hospitalized patients showed that PWH (vs people without known HIV) had 15% increased odds of a severe presentation and 38% increased odds of in-hospital death [3]. Adjustment for antiretroviral therapy (ART) use and suppressed viremia only partially mitigated the increased odds of these adverse outcomes [3]. Thus, imperatives exist to better understand effects of medical interventions on COVID-19 incidence and seriousness, specifically among PWH.
Select observational studies conducted among individuals without known HIV have suggested links between clinically prescribed statin therapy (eg, for cardiovascular disease and/or dyslipidemia) and lower risk of severe COVID-19 [4–7]. Mechanisms through which statin therapy may confer protection against COVID-19 severity (but not COVID-19 incidence) have been postulated to include suppression of overly robust virus-induced immune activation, antithrombotic effects, and effects to preserve endothelial function [8]. No prior study has assessed whether statin therapy may protect against serious COVID-19 in a relatively healthy global population of PWH treated with ART.
Due to the timing of its conduct, the Randomized Trial to Prevent Vascular Events in HIV (REPRIEVE) offers a unique opportunity to assess the effects of statin therapy on COVID-19 outcomes among a global cohort. REPRIEVE recruited PWH from 12 countries who were being treated with ART, randomized them to either pitavastatin (4 mg daily) or placebo, and followed them for the development of major adverse cardiovascular events [9, 10]. REPRIEVE participant follow-up ran from March 2015 to August 2023, overlapping with the start and peak of the COVID-19 pandemic. COVID-19–related data collection was implemented in April 2020 to capture all events from 1 January 2020 onward. The current investigation leverages REPRIEVE data to assess COVID-19 incidence in PWH globally and evaluate the effects of pitavastatin therapy for the prevention of COVID-19 (incidence and serious disease).
METHODS
Trial Design and Participants
REPRIEVE (NCT02344290) enrolled 7769 participants from March 2015 to July 2019 in 12 countries across 5 Global Burden of Disease (GBD) regions [11] in a multicenter phase 3 trial based on a prospective, double-blind, placebo-controlled design to evaluate pitavastatin calcium (4 mg daily) vs placebo among PWH undergoing ART [9, 10]. The key inclusion criteria were PWH ≥40 and ≤75 years of age who were undergoing stable ART for ≥6 months with a CD4+ count >100 cells/mm [3] and low to moderate risk for atherosclerotic cardiovascular disease. Primary exclusion criteria included known atherosclerotic cardiovascular disease, diabetes mellitus with low-density lipoprotein cholesterol ≥70 mg/dL, active cancer, renal dysfunction, decompensated cirrhosis, and statin use. REPRIEVE participants were followed until the trial was stopped for demonstrated efficacy in March 2023, with closeout visits completed by August 2023. Analyses were limited to participants who remained in REPRIEVE follow-up at the start of the COVID-19 pandemic, defined as 1 January 2020; follow-up time to the end of REPRIEVE was included.
Patient Consent Statement
The study was approved by the Massachusetts General Brigham Human Research Committee, and each participating clinical research site obtained regulatory entity approvals. Informed consent was obtained from study participants. An independent data and safety monitoring board appointed by the National Institutes of Health reviewed the trial every 6 months.
Outcomes and Characteristics
COVID-19–related data collection, including COVID-19 diagnoses, treatments, and vaccinations, was implemented in April 2020 to capture all events from 1 January 2020 onward. COVID-19 diagnoses were captured as targeted events regardless of grade and included (1) positive results based on SARS-CoV-2 polymerase chain reaction or rapid antigen test (ie, home test) regardless of symptoms and (2) clinical diagnoses. Participant reports of positive testing were accepted, and source documents were not required. Clinical diagnosis by a health care professional without evidence of a positive test result was also accepted. The primary outcome measure was prespecified as serious COVID-19, defined as COVID-19 that resulted in hospitalization or death or was life-threatening, per guideline E2A of the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use [12]. Incidental COVID-19 diagnosis at or during a hospitalization that did not prolong or complicate the hospitalization course was not included as serious. COVID-19 defined as clinical diagnosis or positive test result was a secondary outcome measure. Demographic and clinical characteristics were defined as previously described [13].
Statistical Analysis
Pitavastatin therapy was a priori hypothesized to reduce serious COVID-19 by 35%. We determined that with 200 anticipated serious COVID-19 events (total information), there would be 80% power to detect such effect. Interim efficacy analyses prespecified at 50% and 75% information were foregone in agreement with the data and safety monitoring board, given the direction of the pandemic, increased immunity globally, and availability of vaccines and treatments at that time.
The primary treatment effect was estimated via the relative incidence of serious COVID-19 with prescribed pitavastatin as compared with placebo. In the primary analysis, intercurrent events such as treatment discontinuations and COVID-19 vaccinations were ignored (intention-to-treat policy); deaths without a preceding COVID-19 event of interest were censored as competing events. Event incidence was estimated as the number of events divided by the total person-years (PY) of follow-up at risk, with follow-up time calculated from 1 January 2020 to the first event or last contact, whichever was earlier. The primary treatment effect was estimated by incidence rate ratios from Poisson regression models, adjusted for GBD region to account for regional differences. A supplementary analysis was limited to participants who remained on the study treatment at the start of the COVID-19 pandemic. Given the anti-inflammatory properties of pitavastatin, it was anticipated that while there may not be an effect on COVID-19 infection, serious disease may be mitigated among those infected. In an analysis among participants with COVID-19, a potential mitigation effect was estimated via common relative risk for serious disease with the Mantel-Haenszel method stratified by GBD region.
As a secondary analytic method, stratified Cox proportional hazards regression modeling was conducted with separate cause-specific baseline hazards by GBD region. The relative cause-specific hazard of pitavastatin vs placebo (ie, hazard ratio [HR]) for COVID-19 outcomes was estimated, and the Wald test is presented. This approach was used in the supporting analysis to estimate the pitavastatin effect during treatment (randomized treatment discontinuation was considered a competing risk/censoring event). Modification of the statin effect following COVID-19 vaccination was evaluated via interaction with time-updated vaccination status.
Statistical comparisons were performed with 2-sided significance tests, with a 5% type I error and 2-sided 95% CIs presented. The analysis was conducted with SAS software (version 9.4 for Linux; SAS Institute Inc).
RESULTS
Baseline Characteristics
As of 1 January 2020, 6905 REPRIEVE participants remained in follow-up and were included in this analysis (Supplementary Figure 1). Of those, 49% were in North America and Europe (United States [except Puerto Rico], Canada, Spain), 20% in Latin America and the Caribbean (Brazil, Peru, Haiti, Puerto Rico), 16% in sub-Saharan Africa (Botswana, South Africa, Uganda, Zimbabwe), 8% in Southeast/East Asia (Thailand), and 7% in South Asia (India). Treatment groups were even in size and well balanced with respect to all baseline characteristics (Table 1). There were no substantive differences between these participants and the full cohort of 7769 participants enrolled in REPRIEVE [10]. Regional differences (Supplementary Table 1) are as previously described for the full cohort.
Table 1.
Characteristics of the Participants at Baseline
| Median (Q1-Q3) or No. (%) | |||
|---|---|---|---|
| Total (n = 6905) | Pitavastatin (n = 3451) | Placebo (n = 3454) | |
| Age,* y | 53 (48–57) | 53 (48–57) | 52 (48–57) |
| 40–49 | 2302 (33) | 1139 (33) | 1163 (34) |
| 50–59 | 3536 (51) | 1774 (51) | 1762 (51) |
| ≥60 | 1067 (15) | 538 (16) | 529 (15) |
| Sex at birth | |||
| Male | 4698 (68) | 2346 (68) | 2352 (68) |
| Female | 2207 (32) | 1105 (32) | 1102 (32) |
| Gender identity | |||
| Cisgender | 6769 (98) | 3391 (98) | 3378 (98) |
| Transgender spectrum | 113 (2) | 53 (2) | 60 (2) |
| Not reported | 23 (<0.5) | 7 (<0.5) | 16 (<0.5) |
| Racea | |||
| Black or African American | 2835 (41) | 1386 (40) | 1449 (42) |
| White | 2319 (34) | 1166 (34) | 1153 (33) |
| Asian | 1097 (16) | 551 (16) | 546 (16) |
| Other | 654 (9) | 348 (10) | 306 (9) |
| Ethnicityb | |||
| Hispanic or Latino | 596 (18) | 310 (19) | 286 (18) |
| Not Hispanic or Latino | 2601 (81) | 1290 (80) | 1311 (81) |
| Unknown | 28 (1) | 12 (1) | 16 (1) |
| GBD region | |||
| High income | 3394 (49) | 1696 (49) | 1698 (49) |
| Latin America and Caribbean | 1357 (20) | 670 (19) | 687 (20) |
| Southeast/East Asia | 582 (8) | 298 (9) | 284 (8) |
| South Asia | 478 (7) | 234 (7) | 244 (7) |
| Sub-Saharan Africa | 1094 (16) | 553 (16) | 541 (16) |
| Smoking statusc | |||
| Current | 1643 (24) | 770 (22) | 873 (25) |
| Former | 1684 (24) | 868 (25) | 816 (24) |
| Never | 3570 (52) | 1809 (52) | 1761 (51) |
| Substance used | |||
| Current | 126 (2) | 59 (2) | 67 (2) |
| Former | 1886 (27) | 941 (27) | 945 (27) |
| Never | 4885 (71) | 2448 (71) | 2437 (71) |
| Cholesterol, mg/dLe | |||
| Total | 186 (162–209) | 186 (163–210) | 185 (162–209) |
| LDL, calculated | 108 (87–128) | 109 (87–129) | 108 (87–128) |
| HDL | 48 (39–59) | 48 (39–59) | 48 (39–59) |
| Nonstatin lipid-lowering therapy* | 186 (3) | 101 (3) | 85 (2) |
| Hypertensionf,* | 3117 (45) | 1528 (44) | 1589 (46) |
| Antihypertensive medication* | 1859 (27) | 898 (26) | 961 (28) |
| Diabetes* | 190 (3) | 107 (3) | 83 (2) |
| ASCVD risk score, % | 4.5 (2.1–7.0) | 4.4 (2.1–7.0) | 4.5 (2.2–7.1) |
| BMI,* kg/m2 | |||
| <30 | 5286 (77) | 2610 (76) | 2676 (78) |
| ≥30 | 1613 (23) | 838 (24) | 775 (22) |
| Time since HIV diagnosis,* y | 15 (10–22) | 15 (10–22) | 15 (10–22) |
| Nadir CD4, cells/mm3 | |||
| <200 | 3446 (50) | 1706 (49) | 1740 (50) |
| 200–349 | 1802 (26) | 906 (26) | 896 (26) |
| ≥350 | 1456 (21) | 731 (21) | 725 (21) |
| Unknown | 201 (3) | 108 (3) | 93 (3) |
| Total ART use,* y | 12.1 (7.9–17.3) | 12.0 (7.9–17.4) | 12.1 (7.9–17.3) |
| ART regimen class* | |||
| NRTI + INSTI | 2966 (43) | 1475 (43) | 1491 (43) |
| NRTI + NNRTI | 2540 (37) | 1285 (37) | 1255 (36) |
| NRTI + PI | 815 (12) | 401 (12) | 414 (12) |
| NRTI sparing | 225 (3) | 112 (3) | 113 (3) |
| Other NRTI containing | 359 (5) | 178 (5) | 181 (5) |
| CD4 count,* cells/mm3 | |||
| <500 | 2056 (30) | 1019 (30) | 1037 (30) |
| ≥500 | 4849 (70) | 2432 (70) | 2417 (70) |
| HIV-1 RNA,g,* copies/mL | |||
| <LLQ | 5549 (87) | 2754 (86) | 2795 (87) |
| LLQ to <400 | 645 (10) | 341 (11) | 304 (9) |
| ≥400 | 210 (3) | 103 (3) | 107 (3) |
All statistics are calculated out of participants with data collected. Missing data: smoking status (n = 8), substance use (n = 8), BMI (n = 6), time since HIV diagnosis (n = 4), total ART use (n = 2), and HIV-1 RNA (n = 501).
Abbreviations: ART, antiretroviral therapy; ASCVD, atherosclerotic cardiovascular disease; BMI, body mass index; GBD, Global Burden of Disease; HDL, high-density lipoprotein; INSTI, integrase strand transfer inhibitor; LDL, low-density lipoprotein; LLQ, lower limit of quantitation; NNRTI, nonnucleoside reverse transcriptase inhibitor; NRTI, nucleoside reverse transcriptase inhibitor; PI, protease inhibitor; REPRIEVE, Randomized Trial to Prevent Vascular Events in HIV.
*Data as of 1 January 2020; otherwise, data are shown as of REPRIEVE study entry.
aOther race includes participants self-identifying as native or indigenous to the enrollment region, more than 1 race (with no single race noted as predominant), or unknown.
bEthnicity presented per National Institutes of Health definition for participants in United States (including Puerto Rico) and Canada only; not applicable in other geographic regions.
cSmoking is defined as cigarette smoking.
dSubstance use includes use of cocaine, methamphetamine, and injection drugs.
eScreening lipid panel from clinical care used for eligibility is presented. Fasting was not required if random laboratory values to determine eligibility were within the range specified by the protocol.
fHypertension was defined as any of the following: hypertension diagnosis, use of antihypertensive treatment for elevated blood pressure, systolic blood pressure ≥140 mm Hg, or diastolic blood pressure ≥90 mm Hg.
gHIV-1 RNA was captured if available through standard of care. The assays used for testing varied, including assays with LLQs between 20 and 400 copies/mL.
Study Follow-up
Of the 6905 participants, 90% of the pitavastatin group and 89% of the placebo group remained on the study treatment on 1 January 2020 (Supplementary Figure 1). The last REPRIEVE participant enrolled had been receiving treatment for 5.1 months, and 95% of the participants had been undergoing treatment for over a year. Another 3% in each treatment group discontinued the study treatment after 1 January 2020 but remained in the study follow-up. In total 89% of each treatment group completed the study. The overall median follow-up from 1 January 2020 was 3.3 years. By the end of follow-up, 84% in the pitavastatin group and 83% in the placebo group reported COVID-19 vaccination (Supplementary Figure 2). There were differences in uptake of the COVID-19 vaccine by GBD region, as previously described [14].
COVID-19 Incidence
Of the 6905 participants, 1701 reported COVID-19. The overall incidence of COVID-19 was 8.57 (95% CI, 8.17–8.98) per 100 PY. The incidence rate varied across GBD regions, with the highest incidence in Southeast/East Asia and the lowest in South Asia and sub-Saharan Africa (Supplementary Table 2). A total of 117 participants reported serious COVID-19: the incidence was 0.53 (95% CI, .44–.63) per 100 PY, with the highest incidence in South Asia. Across regions, nearly all serious COVID-19 events occurred within the first 2 years of the pandemic (Supplementary Figure 3).
Statin Effects on COVID-19 Outcomes
The incidence of COVID-19 was 8.79 per 100 PY in the pitavastatin group and 8.35 per 100 PY in the placebo group (incidence rate ratio, 1.05; 95% CI, .96–1.16; Table 2, Supplementary Figure 4). Of the 1701 COVID-19 diagnoses, 1684 were symptomatic, including 15 that resulted in death (8 in the pitavastatin group and 7 in the placebo group). The incidence of serious COVID-19 was 0.45 per 100 PY in the pitavastatin group and 0.60 per 100 PY in the placebo group, representing a 25% lower incidence of serious COVID-19 events with pitavastatin (incidence rate ratio, 0.75; 95% CI, .52–1.08). Among the 1701 participants with COVID-19, the risk of serious COVID-19 was 5.75% in the pitavastatin group as compared with 8.05% in the placebo group (relative risk, 0.73; 95% CI, .52–1.03), reflecting a 27% reduction in serious COVID-19 with pitavastatin. In both treatment groups, COVID-19 treatments were reported in about 24% of those with COVID-19 (Supplementary Table 3).
Table 2.
COVID-19 Incidence by Treatment Group
| At Risk | |||||
|---|---|---|---|---|---|
| Outcome: Treatment Group | No. | Total PY | No. With Event | Incidence Rate per 100 PYa (95% CI) | Incidence Rate Ratiob (95% CI) |
| COVID-19 | |||||
| Pitavastatin | 3451 | 9890 | 869 | 8.79 (8.22–9.39) | 1.05 (.96–1.16) |
| Placebo | 3454 | 9967 | 832 | 8.35 (7.80–8.93) | … |
| Serious COVID-19 | |||||
| Pitavastatin | 3451 | 11 099 | 50 | 0.45 (.34–.59) | 0.75 (.52–1.08) |
| Placebo | 3454 | 11 080 | 67 | 0.60 (.48–.77) | … |
| Serious COVID-19 on treatmentc | |||||
| Pitavastatin | 3123 | 10 138 | 42 | 0.41 (.31–.56) | 0.73 (.49–1.08) |
| Placebo | 3058 | 9936 | 57 | 0.57 (.44–.74) | … |
No. refers to number of participants.
Abbreviation: CI, confidence interval; PY, person-years.
aEvent incidence was estimated as the number of participants with events divided by the total person-years of follow-up at risk, with follow-up time calculated from 1 January 2020 to the first event or last contact, whichever was earlier.
bIncidence rate ratios (reference: placebo) from Poisson models, adjusted for Global Burden of Disease region.
cSerious COVID-19 among those on treatment as of 1 January 2020.
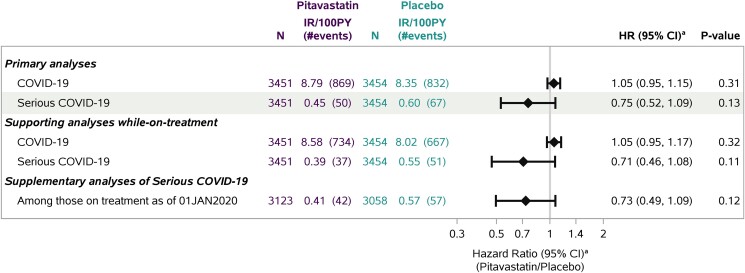
Based on the secondary analytic approach, the estimated HR was 1.05 (95% CI, .95–1.15) for the pitavastatin effect on COVID-19 and 0.75 (95% CI, .52–1.09) for that on serious COVID-19 (Figure 1), consistent with the incidence rate ratio estimates. Results of the supporting analyses while on study treatment were similar, with a slightly larger estimated effect on serious COVID-19. HR estimates within GBD region subgroups were generally consistent with overall estimates (for interaction with treatment, P > .70; Supplementary Figure 5). The estimated pitavastatin effect on serious COVID-19 appeared larger among female participants and among participants without select risk factors for serious COVD-19 (ie, those aged <60 years, body mass index <30 kg/m2, no hypertension), but with the limited number of events, the confidence intervals were nonconclusive (P values >.40; Supplementary Figure 5B).
Figure 1.
Estimated pitavastatin effect on COVID-19 outcomes. aCause-specific hazard ratio (HR) estimates with 95% CIs from Cox proportional hazards models for time to first event, stratified by Global Burden of Disease region. Deaths without COVID-19 were treated as competing events and censored. In the supporting analyses while on treatment, treatment discontinuations were also treated as competing events and censored. For visual purposes, the HRs with 95% CIs are shown in log scale on the x-axis. 100PY, 100 person-years; IR, incidence rate.
In total 83% reported COVID-19 vaccination by end of study, with a 73% reduction in serious COVID-19 events following vaccination (before vs after vaccination: HR, 0.27; 95% CI, .14–.53; P < .0001). Of the 117 serious COVID-19 events, 100 (42 in the pitavastatin group, 58 in the placebo group) occurred before COVID-19 vaccination and only 17 following it. In the analysis examining potential statin effect modification following COVID-19 vaccination, there was a 26% reduction in serious COVID-19 events with pitavastatin prior to vaccination (HR, 0.74; 95% CI, .50–1.10) as compared with a 13% reduction following vaccination (0.87; 95% CI, .33–2.25; for interaction, P = .77; Supplementary Figure 6).
DISCUSSION
Among a global cohort of 6905 PWH randomized to pitavastatin or placebo, the observed overall COVID-19 incidence was 8.57 per 100 PY, varying from 2.80 to 13.74 per 100 PY across regions. The incidence of serious COVID-19 was 0.53 per 100 PY. While statin therapy did not reduce COVID-19 incidence, the incidence of serious COVID-19 was 25% lower. The estimated treatment effect size for serious COVID-19 fell within the hypothesized range, but the fewer-than-anticipated cases (117 vs 200) did not provide sufficient precision to conclude a statin effect. In total 83% reported COVID-19 vaccination by end of study, with a strong protective effect on serious COVID-19. REPRIEVE provides the first randomized comparison assessing statin effects on COVID-19 outcomes among a global cohort of ART-treated HIV, including data during the initial and later years of the COVID-19 pandemic, with findings suggestive of an effect of statins to reduce risk of serious COVID-19. The study also highlights the convincing manner in which vaccination protects against serious COVID-19 among PWH treated with ART globally.
Prior observational studies conducted in the general population assessing statin effects on COVID-19 outcomes have yielded mixed results. Select studies have highlighted a lower risk of severe COVID-19 among individuals with vs without prior statin use [4–7], while other comparative studies have revealed null findings [15, 16]. Overall, however, pooled analysis of available observational studies favors a protective effect of statins commensurate with what we observed in our randomized trial of PWH. Specifically, a meta-analysis of 48 110 patients enrolled in 9 observational studies showed that prior statin use was associated with a 27% lower risk for severe COVID-19 (pooled adjusted relative risk, 0.83; 95% CI, .57–.94) [17]. Patients enrolled in these observational studies were not known to have HIV but did have clinical indications for statin therapy (including dyslipidemia and established heart disease) [17], in contrast to the population of PWH without clinical indications for statin therapy enrolled in REPRIEVE. The randomized allocation of statin treatment in REPRIEVE (vs clinically driven allocation in prior observational studies) reinforces the validity of our findings.
Experimental studies lend biologic plausibility to the finding that statins may protect against serious COVID-19 [18, 19]. Serious COVID-19 is often associated with an overly robust immune response, triggered when SARS-CoV-2 prompts activation of the NLRP3 inflammasome pathway toward hyperproduction of interleukins 1β and 6. Statins suppress activation of this pathway [18, 19]. Serious COVID-19 is associated with endothelial dysfunction and a prothrombotic state. Statins support salutary endothelial cell function by enhancing endothelial nitric oxide synthase expression, with an attendant increase in nitric oxide production [18, 19]. Statins also help prevent against thrombosis, in part by decreasing expression of tissue factor and other procoagulant proteins [18, 19]. Of note, statins have been shown to protect against serious presentations of other viral illnesses, such as influenza [20, 21]. As to why statins did not appear to reduce COVID-19 incidence in our analysis, this finding, too, has a plausible mechanistic explanation [18, 19]. On one hand, statins increase expression of ACE2 (angiotensin-converting enzyme 2) [18, 19], which serves as a membrane receptor facilitating SARS-CoV-2 entry. On the other, statin-mediated inhibition of HMGCoA (hydroxymethylglutaryl–coenzyme A) reduces intracellular cholesterol synthesis [18, 19], in turn disrupting the composition of membrane lipid rafts essential to SARS-CoV-2–mediated cellular entry via ACE2 receptors. The net effects of statins on SARS-CoV-2 infection and ensuing COVID-19 incidence may thus be null due to these countervailing influences.
Our study confirmed that among PWH, vaccination had a strong protective effect on serious COVID-19. By the end of the study, 83% reported COVID-19 vaccination, with differences in uptake by GBD region, as previously published [14]. Across regions, nearly all serious COVID-19 events occurred within the first 2 years of the pandemic. That a limited number of serious events occurred later on was related to the uptake of COVID-19 vaccinations and/or COVID-19 treatments, as well as immunity from previous infection. A protective statin effect against serious COVID-19 was observed prior to vaccination. With few serious cases observed following vaccination, statin effect modification by vaccination status could not be evaluated. The aforementioned findings remain relevant 4 years postpandemic due to recent reports of low COVID-19 booster uptake [22, 23]. Furthermore, although effective treatments are now available to treat potentially serious COVID-19, more serious cases are thought to increase the risk of long COVID or post–COVID-19 conditions (PCCs). Indeed, a meta-analysis including all published studies investigating risk factors and/or predictors of PCCs revealed that patients hospitalized or admitted to the intensive care unit during COVID-19 had a nearly 2.4-fold increased odds of developing PCCs [24]. Meanwhile, COVID-19 vaccination was associated with lower odds of developing PCCs [24]. Thus, prevention of serious COVID-19 through vaccination has ongoing salience.
Overall, in a global cohort of PWH, statin therapy did not affect COVID-19 incidence but showed potential to reduce the risk of serious COVID-19 in the absence of COVID-19 vaccination. Vaccination was protective for serious COVID-19. Potential limitations of our study include the lack of power for meaningful subgroup analyses, as well as ascertainment bias in characterizing COVID-19 incidence due to regional differences in COVID-19 testing and pandemic mitigation strategies. Furthermore, there may have been differences in the availability and uptake of treatment strategies to prevent the progression of COVID-19 to serious COVID-19, as well as in criteria for hospitalization. Given the randomized assignment to statin therapy vs placebo, one would expect access to effective COVID-19 treatments to be balanced between groups. Indeed, our study is unique in illustrating potentially protective effects of randomization to statin therapy vs placebo prior to COVID-19 diagnosis among PWH globally. By contrast, other general-population studies have identified nonsignificant trends toward improved clinical outcomes among individuals randomized to statin therapy vs placebo after COVID-19 diagnosis [25, 26], answering a different question. Additional research is needed to understand potential mechanisms of protective statin effects on serious COVID-19, including those among PWH. Mechanistic insights could inform the development of immune-modulatory strategies that are protective against severe systemic sequelae of future novel viral infections. Such research should occur in tandem with systematic public health efforts to maximize access to and uptake of COVID-19 vaccinations and boosters for PWH globally.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Contributor Information
Markella V Zanni, Metabolism Unit, Massachusetts General Hospital and Harvard Medical School, Boston, Massachusetts, USA.
Triin Umbleja, Center for Biostatistics in AIDS Research, Harvard T.H. Chan School of Public Heath, Boston, Massachusetts, USA.
Carl J Fichtenbaum, Division of Infectious Diseases, College of Medicine, University of Cincinnati, Cincinnati, Ohio, USA.
Kathleen V Fitch, Metabolism Unit, Massachusetts General Hospital and Harvard Medical School, Boston, Massachusetts, USA.
Sara McCallum, Metabolism Unit, Massachusetts General Hospital and Harvard Medical School, Boston, Massachusetts, USA.
Judith A Aberg, Division of Infectious Diseases, Icahn School of Medicine at Mount Sinai, New York, New York, USA.
Edgar Turner Overton, Division of Infectious Diseases, University of Alabama at Birmingham, Birmingham, Alabama, USA.
Carlos D Malvestutto, Division of Infectious Diseases, Ohio State University Medical Center, Columbus, Ohio, USA.
Gerald S Bloomfield, Department of Medicine, Duke Global Health Institute, and Duke Clinical Research Institute, School of Medicine, Duke University, Durham, North Carolina, USA.
Judith S Currier, Division of Infectious Diseases, David Geffen School of Medicine, University of California Los Angeles, Los Angeles, California, USA.
Samuel R Schnittman, Metabolism Unit, Massachusetts General Hospital and Harvard Medical School, Boston, Massachusetts, USA; Division of Infectious Diseases, Massachusetts General Hospital and Harvard Medical School, Boston, Massachusetts, USA.
Kristine M Erlandson, Division of Infectious Diseases, Department of Medicine, University of Colorado–Anschutz Medical Campus, Aurora, Colorado, USA.
Marissa R Diggs, Metabolism Unit, Massachusetts General Hospital and Harvard Medical School, Boston, Massachusetts, USA.
Borek Foldyna, Cardiovascular Imaging Research Center, Department of Radiology, Massachusetts General Hospital and Harvard Medical School, Boston, Massachusetts, USA.
Esteban Martinez, Infectious Diseases Service, Hospital Clinic and University of Barcelona, Barcelona, Spain.
Charurut Somboonwit, Division of Infectious Diseases, Morsani College of Medicine, University of South Florida, Tampa, Florida, USA.
Gary P Wang, Division of Infectious Diseases, School of Medicine, University of Florida, Gainesville, Florida, USA.
David Mushatt, Division of Infectious Diseases, School of Medicine, Tulane University, New Orleans, Louisiana, USA.
Elizabeth Connick, Division of Infectious Diseases, College of Medicine, University of Arizona, Tucson, Arizona, USA.
Michael T Lu, Cardiovascular Imaging Research Center, Department of Radiology, Massachusetts General Hospital and Harvard Medical School, Boston, Massachusetts, USA.
Pamela S Douglas, Duke Clinical Research Institute, School of Medicine, Duke University, Durham, North Carolina, USA.
Heather J Ribaudo, Center for Biostatistics in AIDS Research, Harvard T.H. Chan School of Public Heath, Boston, Massachusetts, USA.
Steven K Grinspoon, Metabolism Unit, Massachusetts General Hospital and Harvard Medical School, Boston, Massachusetts, USA.
Notes
Acknowledgments. We thank the study participants, site staff, and study-associated personnel for their participation in the trial. In addition, we thank the following: the AIDS Clinical Trials Group (ACTG) for clinical site support; ACTG clinical trials specialists (Laura Moran, MPH, and Jhoanna Roa, MD) for protocol development and implementation support; the data management center, Frontier Science Foundation, for data support; the Center for Biostatistics in AIDS Research for statistical support; and the Community Advisory Board for input from the community.
Disclaimer. The views expressed in this article are those of the authors and do not necessarily represent the views of the National Heart, Lung, and Blood Institute or the National Institute of Allergy and Infectious Diseases; the National Institutes of Health; or the US Department of Health and Human Services.
Institutional review board/ethics statement. Each clinical research site obtained institutional review board/ethics committee approval and any other applicable regulatory entity approvals. Participants were provided with study information, including discussion of risks and benefits, and signed the approved declaration of informed consent.
Previous presentation. Conference on Retroviruses and Opportunistic Infections, Denver, CO, USA, 3–6 March 2024.
Financial support. This work was supported by the National Institutes of Health (U01HL123336 and 1UG3HL164285 to the Clinical Coordinating Center; U01HL123339 and 1U24HL164284 to the Data Coordinating Center); Kowa Pharmaceuticals America, Inc; Gilead Sciences; ViiV Healthcare; the National Institute of Allergy and Infectious Diseases (UM1 AI068636, which supports the ACTG Leadership and Operations Center; UM1 AI106701, which supports the ACTG Laboratory Center); and the Nutrition Obesity Research Center at Harvard (P30DK040561 to S. K. G.).
All other authors report no potential conflicts.
References
- 1. Wang Y, Xie Y, Hu S, et al. Systematic review and meta-analyses of the interaction between HIV infection and COVID-19: two years’ evidence summary. Front Immunol 2022; 13:864838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Thornhill J, Orkin C, Cevik M. Estimating the global impact of coronavirus disease 2019 on people living with HIV. Curr Opin Infect Dis 2023; 36:20–5. [DOI] [PubMed] [Google Scholar]
- 3. Bertagnolio S, Thwin SS, Silva R, et al. Clinical features of, and risk factors for, severe or fatal COVID-19 among people living with HIV admitted to hospital: analysis of data from the WHO global clinical platform of COVID-19. Lancet HIV 2022; 9:e486–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Daniels LB, Sitapati AM, Zhang J, et al. Relation of statin use prior to admission to severity and recovery among COVID-19 inpatients. Am J Cardiol 2020; 136:149–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lohia P, Kapur S, Benjaram S, Mir T. Association between antecedent statin use and severe disease outcomes in COVID-19: a retrospective study with propensity score matching. J Clin Lipidol 2021; 15:451–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Santosa A, Franzen S, Natman J, Wettermark B, Parmryd I, Nyberg F. Protective effects of statins on COVID-19 risk, severity and fatal outcome: a nationwide Swedish cohort study. Sci Rep 2022; 12:12047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bouillon K, Baricault B, Semenzato L, et al. Association of statins for primary prevention of cardiovascular diseases with hospitalization for COVID-19: a nationwide matched population-based cohort study. J Am Heart Assoc 2022; 11:e023357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Torres-Pena JD, Katsiki N, Perez-Martinez P. Could statin therapy be useful in patients with coronavirus disease 2019 (COVID-19)? Front Cardiovasc Med 2021; 8:775749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Grinspoon SK, Fitch KV, Overton ET, et al. Rationale and design of the Randomized Trial to Prevent Vascular Events in HIV (REPRIEVE). Am Heart J 2019; 212:23–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Grinspoon SK, Fitch KV, Zanni MV, et al. Pitavastatin to prevent cardiovascular disease in HIV infection. N Engl J Med 2023; 389:687–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. GBD 2019 Diseases and Injuries Collaborators . Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020; 396:1204–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use . ICH harmonised tripartite guideline. Clinical safety data management: definitions and standards for expedited reporting E2A. 1994. https://database.ich.org/sites/default/files/E2A_Guideline.pdf. Accessed 16 September 2024.
- 13. Douglas PS, Umbleja T, Bloomfield GS, et al. Cardiovascular risk and health among people with human immunodeficiency virus (HIV) eligible for primary prevention: insights from the REPRIEVE trial. Clin Infect Dis 2021; 73:2009–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fulda ES, Fitch KV, Overton ET, et al. COVID-19 vaccination rates in a global HIV cohort. J Infect Dis 2022; 225:603–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Butt JH, Gerds TA, Schou M, et al. Association between statin use and outcomes in patients with coronavirus disease 2019 (COVID-19): a nationwide cohort study. BMJ Open 2020; 10:e044421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mitacchione G, Schiavone M, Curnis A, et al. Impact of prior statin use on clinical outcomes in COVID-19 patients: data from tertiary referral hospitals during COVID-19 pandemic in Italy. J Clin Lipidol 2021; 15:68–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yetmar ZA, Chesdachai S, Kashour T, et al. Prior statin use and risk of mortality and severe disease from coronavirus disease 2019: a systematic review and meta-analysis. Open Forum Infect Dis 2021; 8:ofab284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Talasaz AH, Sadeghipour P, Aghakouchakzadeh M, et al. Investigating lipid-modulating agents for prevention or treatment of COVID-19: JACC state-of-the-art review. J Am Coll Cardiol 2021; 78:1635–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Liu C, Yan W, Shi J, et al. Biological actions, implications, and cautions of statins therapy in COVID-19. Front Nutr 2022; 9:927092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Vandermeer ML, Thomas AR, Kamimoto L, et al. Association between use of statins and mortality among patients hospitalized with laboratory-confirmed influenza virus infections: a multistate study. J Infect Dis 2012; 205:13–9. [DOI] [PubMed] [Google Scholar]
- 21. Frost FJ, Petersen H, Tollestrup K, Skipper B. Influenza and COPD mortality protection as pleiotropic, dose-dependent effects of statins. Chest 2007; 131:1006–12. [DOI] [PubMed] [Google Scholar]
- 22. Jacobs ET, Cordova-Marks FM, Farland LV, et al. Understanding low COVID-19 booster uptake among US adults. Vaccine 2023; 41:6221–6. [DOI] [PubMed] [Google Scholar]
- 23. Shah A, Coiado OC. COVID-19 vaccine and booster hesitation around the world: a literature review. Front Med (Lausanne) 2022; 9:1054557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tsampasian V, Elghazaly H, Chattopadhyay R, et al. Risk factors associated with post-COVID-19 condition: a systematic review and meta-analysis. JAMA Intern Med 2023; 183:566–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Talasaz AH, Sadeghipour P, Bakhshandeh H, et al. Atorvastatin versus placebo in ICU patients with COVID-19: ninety-day results of the INSPIRATION-S trial. Thromb Haemost 2023; 123:723–33. [DOI] [PubMed] [Google Scholar]
- 26. REMAP-CAP Investigators . Simvastatin in critically ill patients with COVID-19. N Engl J Med 2023; 389:2341–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.