Abstract
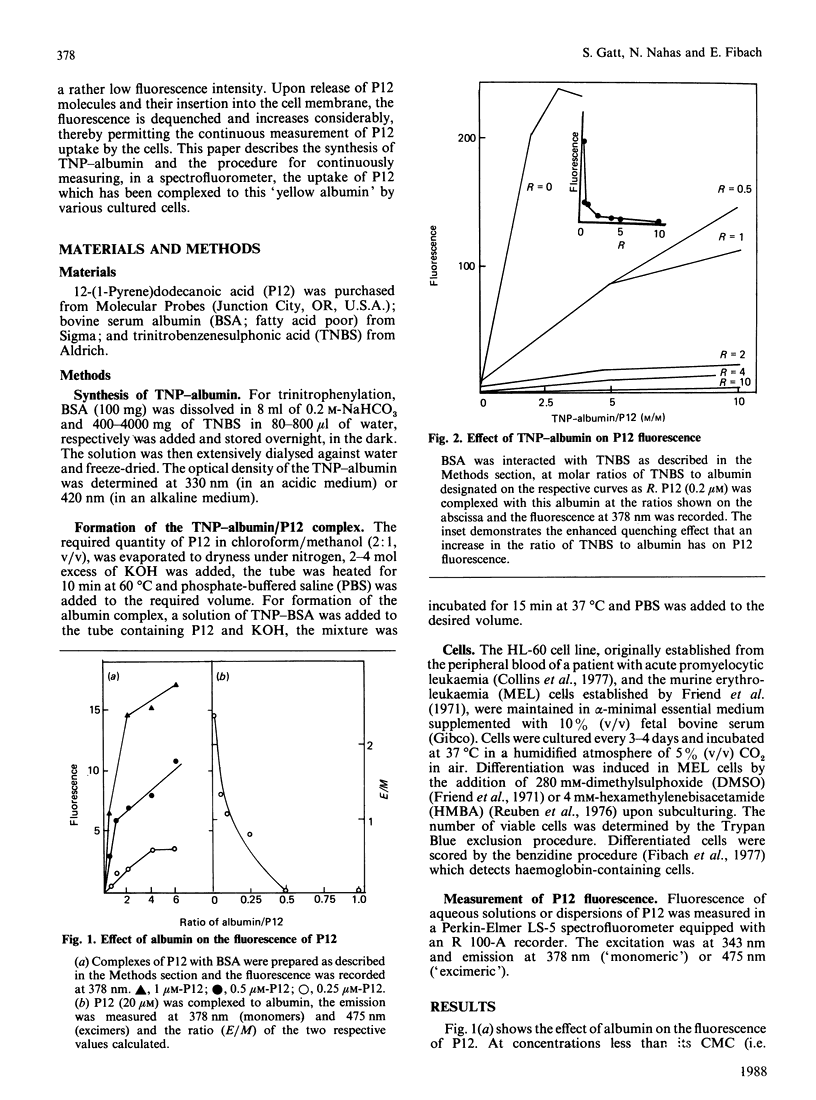
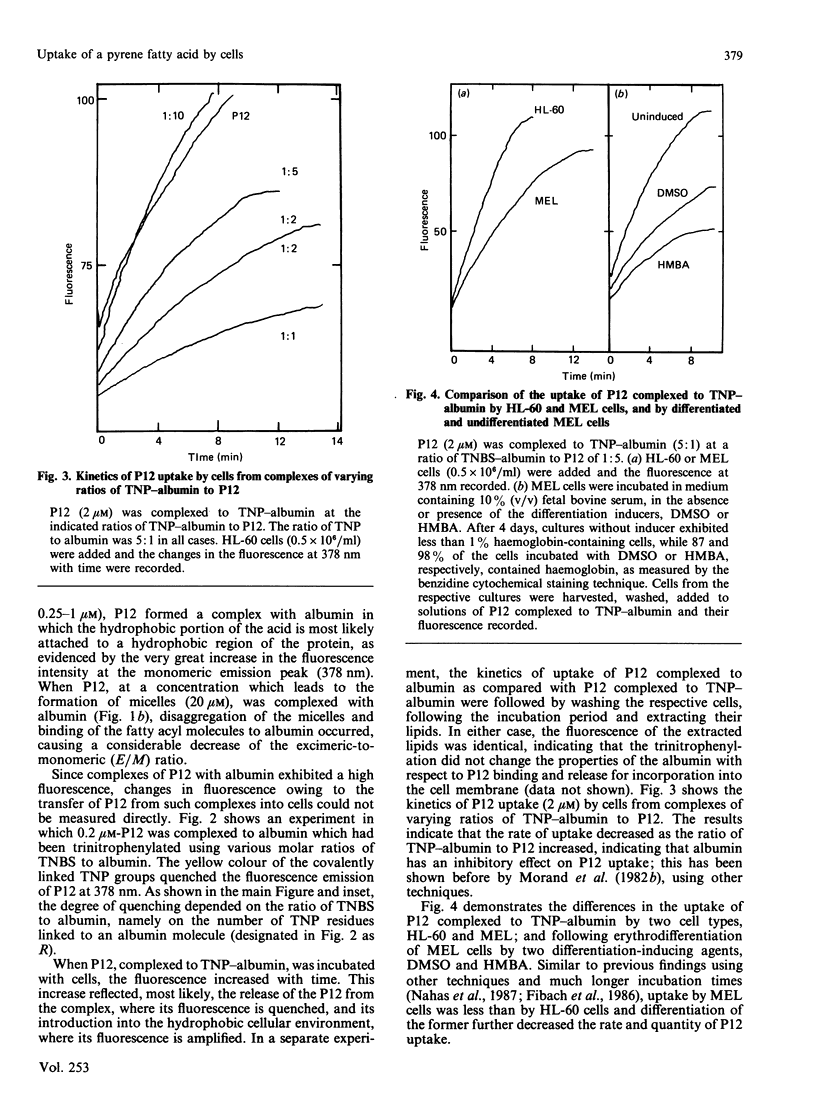
Aqueous dispersions of 12-(1-pyrene)-dodecanoic acid (P12), a medium-chain fatty acid to which the fluorescent probe pyrene has been covalently linked, shows a considerable increase in fluorescence when the probe is introduced into a hydrophobic environment. This enables the uptake of P12 by liposomes and cells to be followed directly in a spectrofluorometer, without separating the cells from the P12-containing medium. In the present study, we show that complexing P12 to albumin produced a very high fluorescence emission intensity. This made direct measurements of the uptake by cells of albumin-bound P12 impossible. Such direct measurements could, however, be made using albumin which had been interacted with trinitrobenzenesulphonic acid (TNBS). The yellow trinitrophenyl (TNP) residues, which were thereby covalently linked to the albumin, quenched the fluorescence of pyrene in the TNP-albumin/P12 complex. Upon release of the P12 molecules from this complex and their subsequent uptake by cells, fluorescence increased. This technique was utilized for the continuous monitoring of the uptake of P12 by different cell types and cells at various stages of maturation.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Charlton S. C., Olson J. S., Hong K. Y., Pownall H. J., Louie D. D., Smith L. C. Stopped flow kinetics of pyrene transfer between human high density lipoproteins. J Biol Chem. 1976 Dec 25;251(24):7952–7955. [PubMed] [Google Scholar]
- Collins S. J., Gallo R. C., Gallagher R. E. Continuous growth and differentiation of human myeloid leukaemic cells in suspension culture. Nature. 1977 Nov 24;270(5635):347–349. doi: 10.1038/270347a0. [DOI] [PubMed] [Google Scholar]
- Fibach E., Nahas N., Giloh H., Gatt S. Uptake of fluorescent fatty acids by erythroleukemia cells. Effect of differentiation. Exp Cell Res. 1986 Sep;166(1):220–228. doi: 10.1016/0014-4827(86)90521-5. [DOI] [PubMed] [Google Scholar]
- Fibach E., Reuben R. C., Rifkind R. A., Marks P. A. Effect of hexamethylene bisacetamide on the commitment to differentiation of murine erythroleukemia cells. Cancer Res. 1977 Feb;37(2):440–444. [PubMed] [Google Scholar]
- Friend C., Scher W., Holland J. G., Sato T. Hemoglobin synthesis in murine virus-induced leukemic cells in vitro: stimulation of erythroid differentiation by dimethyl sulfoxide. Proc Natl Acad Sci U S A. 1971 Feb;68(2):378–382. doi: 10.1073/pnas.68.2.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoekstra D., Klappe K., de Boer T., Wilschut J. Characterization of the fusogenic properties of Sendai virus: kinetics of fusion with erythrocyte membranes. Biochemistry. 1985 Aug 27;24(18):4739–4745. doi: 10.1021/bi00339a005. [DOI] [PubMed] [Google Scholar]
- Morand O., Fibach E., Dagan A., Gatt S. Transport of fluorescent derivatives of fatty acids into cultured human leukemic myeloid cells and their subsequent metabolic utilization. Biochim Biophys Acta. 1982 Jun 11;711(3):539–550. doi: 10.1016/0005-2760(82)90070-4. [DOI] [PubMed] [Google Scholar]
- Morand O., Fibach E., Gatt S. Effect of albumin, low temperature and metabolic inhibitors on transport of fatty acids into cultured human leukemic myeloid cells. Biochim Biophys Acta. 1982 Dec 8;693(1):143–150. doi: 10.1016/0005-2736(82)90480-1. [DOI] [PubMed] [Google Scholar]
- Morand O., Fibach E., Livni N., Gatt S. Induction of lipid storage in cultured leukemic myeloid cells by pyrene-dodecanoic acid. Biochim Biophys Acta. 1984 Mar 27;793(1):95–104. doi: 10.1016/0005-2760(84)90057-2. [DOI] [PubMed] [Google Scholar]
- Nahas N., Fibach E., Giloh H., Gatt S. Use of the fluorescence activated cell sorter for studying uptake of fluorescent fatty acids into cultured cells. Biochim Biophys Acta. 1987 Jan 13;917(1):86–91. doi: 10.1016/0005-2760(87)90287-6. [DOI] [PubMed] [Google Scholar]
- Owen C. S. A membrane-bound fluorescent probe to detect phospholipid vesicle-cell fusion. J Membr Biol. 1980;54(1):13–20. doi: 10.1007/BF01875372. [DOI] [PubMed] [Google Scholar]
- Pownall H. J., Hickson D., Gotto A. M., Jr, Massey J. B. Kinetics of spontaneous and plasma-stimulated sphingomyelin transfer. Biochim Biophys Acta. 1982 Jul 20;712(1):169–176. doi: 10.1016/0005-2760(82)90099-6. [DOI] [PubMed] [Google Scholar]
- Reuben R. C., Wife R. L., Breslow R., Rifkind R. A., Marks P. A. A new group of potent inducers of differentiation in murine erythroleukemia cells. Proc Natl Acad Sci U S A. 1976 Mar;73(3):862–866. doi: 10.1073/pnas.73.3.862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roseman M. A., Thompson T. E. Mechanism of the spontaneous transfer of phospholipids between bilayers. Biochemistry. 1980 Feb 5;19(3):439–444. doi: 10.1021/bi00544a006. [DOI] [PubMed] [Google Scholar]
- Sengupta P., Sackmann E., Kühnle W., Scholz H. P. An optical study of the exchange kinetics of membrane bound molecules. Biochim Biophys Acta. 1976 Jul 15;436(4):869–878. doi: 10.1016/0005-2736(76)90414-4. [DOI] [PubMed] [Google Scholar]
- Spector A. A. Fatty acid binding to plasma albumin. J Lipid Res. 1975 May;16(3):165–179. [PubMed] [Google Scholar]
- Spector A. A. Lipids, hormones, and atherogenesis. The transport and utilization of free fatty acid. Ann N Y Acad Sci. 1968 Nov 21;149(2):768–783. doi: 10.1111/j.1749-6632.1968.tb53834.x. [DOI] [PubMed] [Google Scholar]
- Struck D. K., Hoekstra D., Pagano R. E. Use of resonance energy transfer to monitor membrane fusion. Biochemistry. 1981 Jul 7;20(14):4093–4099. doi: 10.1021/bi00517a023. [DOI] [PubMed] [Google Scholar]


