Abstract
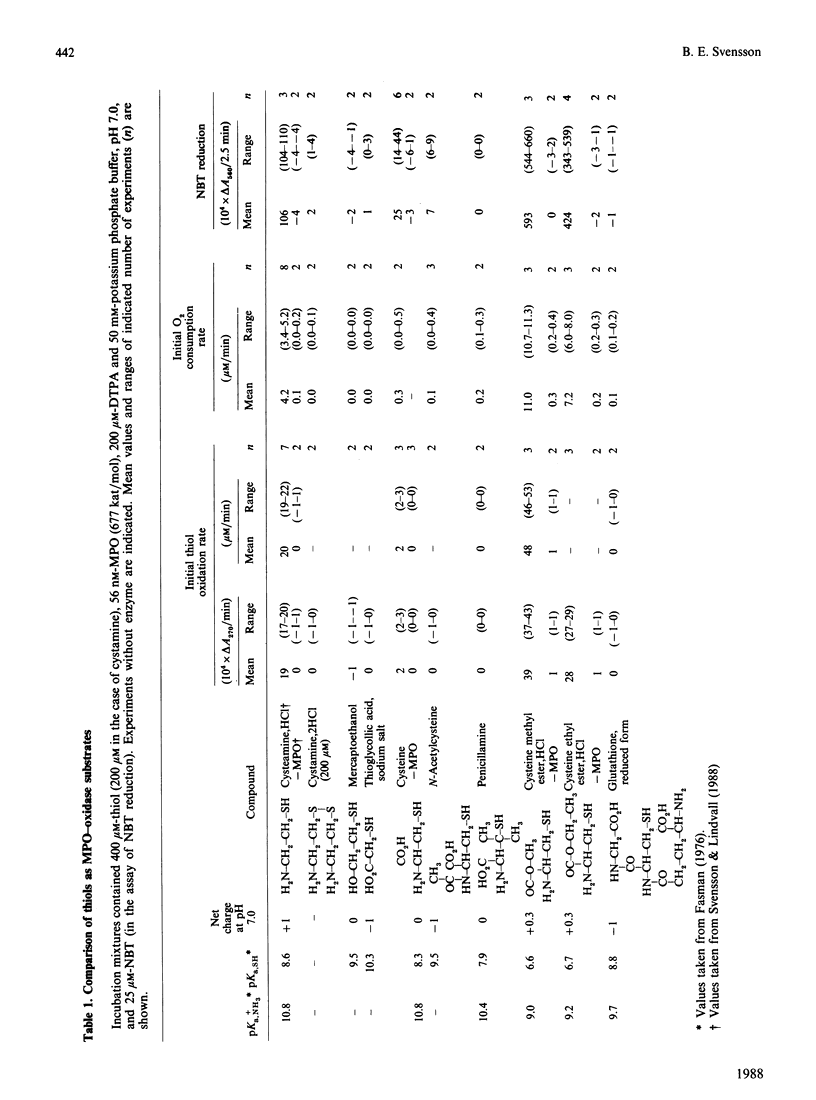
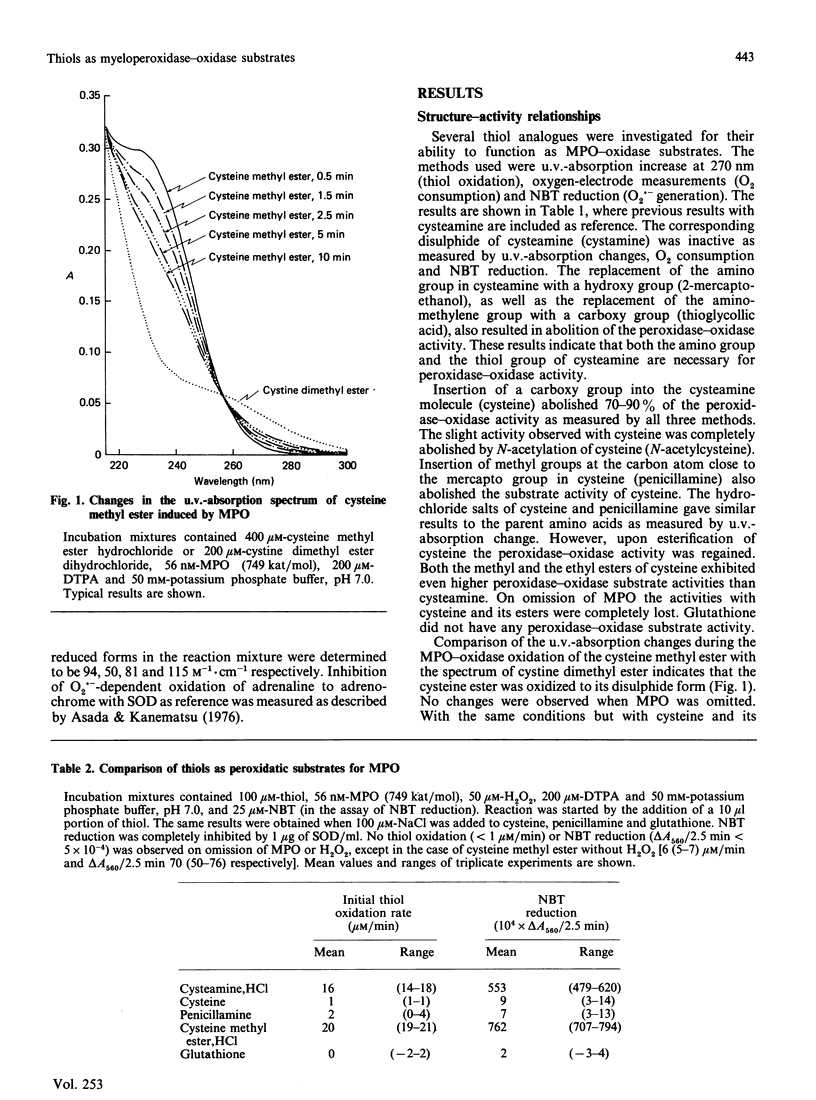
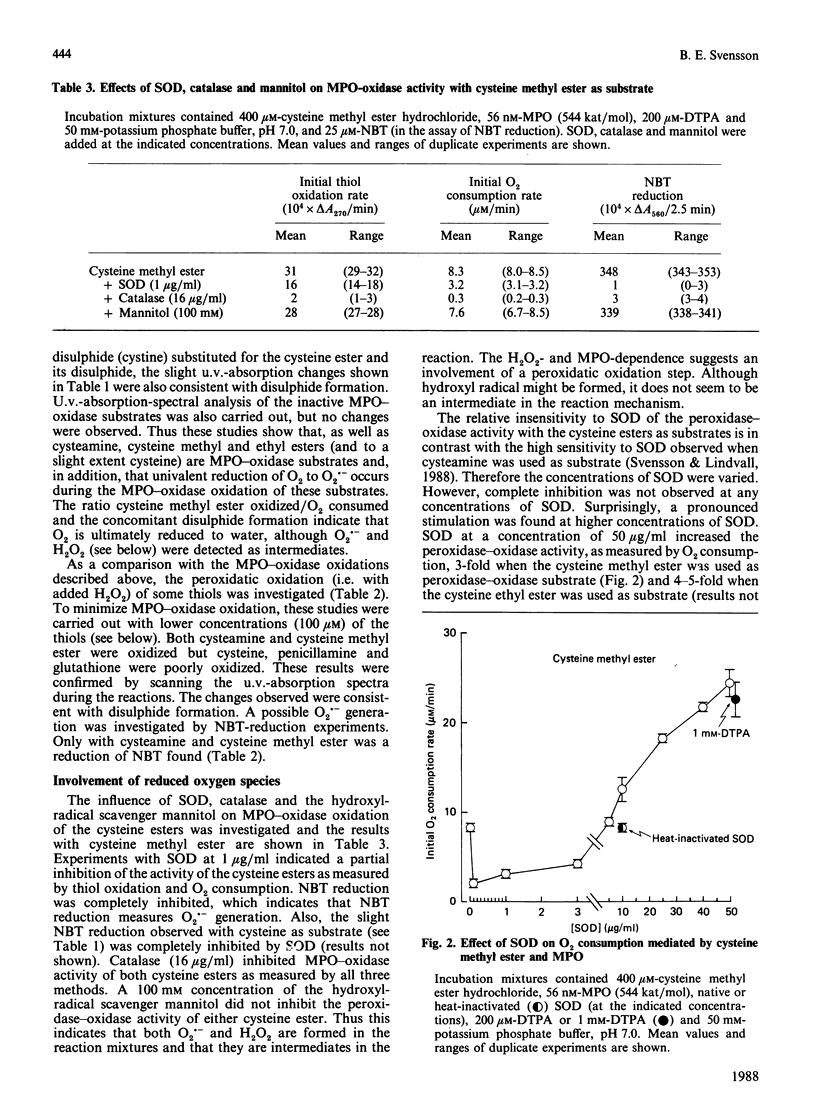
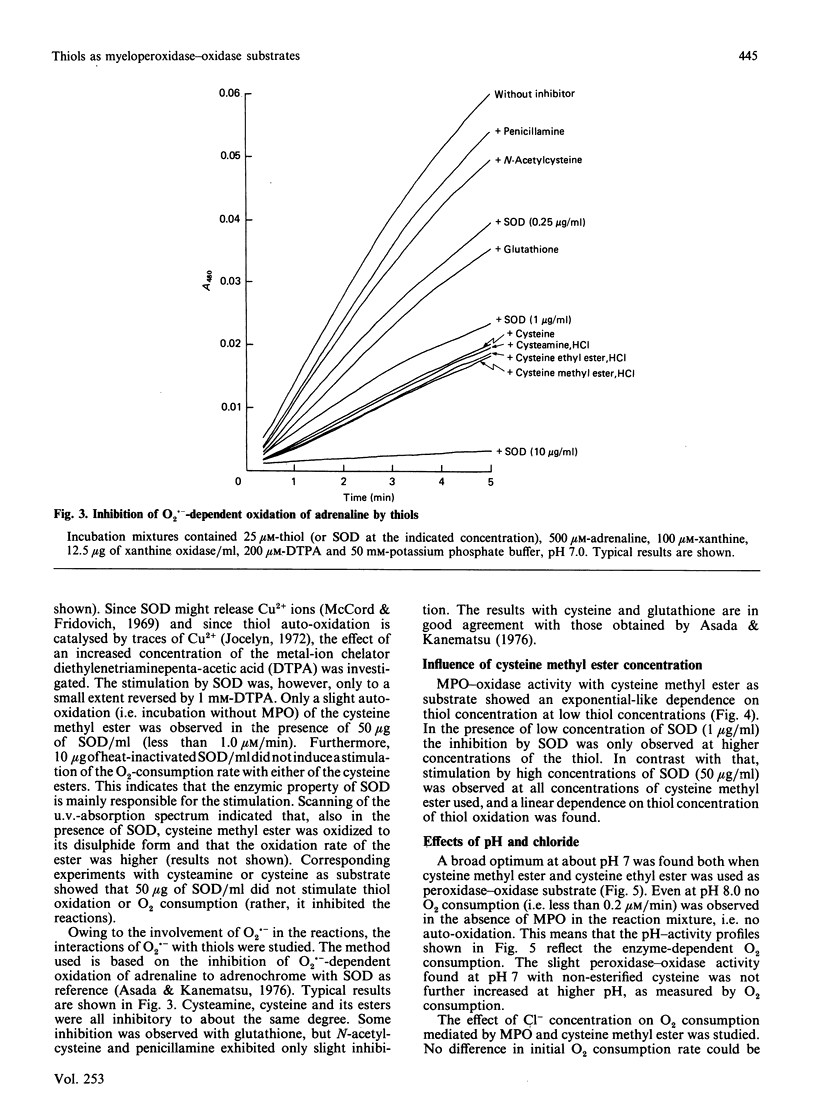
Nine low-Mr thiols were compared with regard to their ability to function as myeloperoxidase-oxidase substrates under conditions where no auto-oxidation of the thiols could be observed. The methyl and ethyl esters of cysteine were found to be about twice as active as cysteamine at pH 7.0, in terms of increased O2 consumption. Cysteine itself was poorly active, whereas glutathione, N-acetylcysteine and penicillamine were completely inactive as myeloperoxidase-oxidase substrates under these conditions. The structure-activity relationships indicated that both a free thiol and free amino group were required for peroxidase-oxidase activity, and also that a free carboxy group abolished activity. In analogy with cysteamine, the activities of both cysteine esters were inhibited by superoxide dismutase (less than 5 micrograms/ml) and by catalase and not by the hydroxyl-radical scavenger mannitol. In contrast with cysteamine, the activities of both cysteine esters were stimulated more than 2-fold by high concentrations (greater than 5 micrograms/ml) of superoxide dismutase. The activities of both cysteine esters exhibited broad pH optima at pH 7. A mechanism for the myeloperoxidase-oxidase oxidation of the cysteine esters is proposed, which is partly different from that previously proposed for cysteamine.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babior B. M. Oxygen-dependent microbial killing by phagocytes (first of two parts). N Engl J Med. 1978 Mar 23;298(12):659–668. doi: 10.1056/NEJM197803232981205. [DOI] [PubMed] [Google Scholar]
- Harman L. S., Mottley C., Mason R. P. Free radical metabolites of L-cysteine oxidation. J Biol Chem. 1984 May 10;259(9):5606–5611. [PubMed] [Google Scholar]
- McCord J. M., Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem. 1969 Nov 25;244(22):6049–6055. [PubMed] [Google Scholar]
- Meister A. Selective modification of glutathione metabolism. Science. 1983 Apr 29;220(4596):472–477. doi: 10.1126/science.6836290. [DOI] [PubMed] [Google Scholar]
- Odajima T., Yamazaki I. Myeloneperoxidase of the leukocyte of normal blood. 3. The reaction of ferric myeloperoxidase with superoxide anion. Biochim Biophys Acta. 1972 Oct 12;284(2):355–359. doi: 10.1016/0005-2744(72)90130-1. [DOI] [PubMed] [Google Scholar]
- Odajima T., Yamazaki I. Myeloperoxidase of the leukocyte of normal blood. I. Reaction of myeloperoxidase with hydrogen peroxide. Biochim Biophys Acta. 1970 Apr 22;206(1):71–77. doi: 10.1016/0005-2744(70)90083-5. [DOI] [PubMed] [Google Scholar]
- Olsen J., Davis L. The oxidation of dithiothreitol by peroxidases and oxygen. Biochim Biophys Acta. 1976 Sep 14;445(2):324–329. doi: 10.1016/0005-2744(76)90086-3. [DOI] [PubMed] [Google Scholar]
- Pryor W. A. Oxy-radicals and related species: their formation, lifetimes, and reactions. Annu Rev Physiol. 1986;48:657–667. doi: 10.1146/annurev.ph.48.030186.003301. [DOI] [PubMed] [Google Scholar]
- Quintiliani M., Badiello R., Tamba M., Esfandi A., Gorin G. Radiolysis of glutathione in oxygen-containing solutions of pH7. Int J Radiat Biol Relat Stud Phys Chem Med. 1977 Aug;32(2):195–202. doi: 10.1080/09553007714550891. [DOI] [PubMed] [Google Scholar]
- Sevilla M. D., Becker D., Swarts S., Herrington J. Sulfinyl radical formation from the reaction of cysteine and glutathione thiyl radicals with molecular oxygen. Biochem Biophys Res Commun. 1987 Apr 29;144(2):1037–1042. doi: 10.1016/s0006-291x(87)80068-2. [DOI] [PubMed] [Google Scholar]
- Svensson B. E., Domeij K., Lindvall S., Rydell G. Peroxidase and peroxidase-oxidase activities of isolated human myeloperoxidases. Biochem J. 1987 Mar 15;242(3):673–680. doi: 10.1042/bj2420673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svensson B. E., Lindvall S. Myeloperoxidase-oxidase oxidation of cysteamine. Biochem J. 1988 Jan 15;249(2):521–530. doi: 10.1042/bj2490521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Steveninck J., Boegheim J. P., Dubbelman T. M., Van der Zee J. The mechanism of potentiation of horseradish peroxidase-catalysed oxidation of NADPH by porphyrins. Biochem J. 1987 Mar 1;242(2):611–613. doi: 10.1042/bj2420611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wefers H., Riechmann E., Sies H. Excited species generation in horseradish peroxidase-mediated oxidation of glutathione. J Free Radic Biol Med. 1985;1(4):311–318. doi: 10.1016/0748-5514(85)90137-0. [DOI] [PubMed] [Google Scholar]
- Yamazaki I., Yokota K. Oxidation states of peroxidase. Mol Cell Biochem. 1973 Nov 15;2(1):39–52. doi: 10.1007/BF01738677. [DOI] [PubMed] [Google Scholar]


