Abstract
Hyoscyamus niger is an important medicinal plant used in medicine and contains tropane alkaloid compounds such as hyoscyamine and scopolamine. In this study, after the selection of the solvent for extracting hyoscyamine and scopolamine, the central composite design of the response surface methodology was used to study the effect of solvent concentrations (0, 25, 50, 75, and 100 %), temperatures (25, 30, 35, 40, and 45 °C) and ultrasonication times (10, 20, 30, 40, and 50 min). The hyoscyamine and scopolamine content were obtained by HPLC-DAD. The results indicated that the predicted optimal condition for hyoscyamine and scopolamine extraction from H. niger root was as follows. Hyoscyamine: 100 % methanol, temperature 45 °C and ultrasonication time 10 min, obtained 172.06 μg/g dry weight; and scopolamine: 98.50 % methanol, temperature 25 °C and ultrasonication time 10 min, provided 229.48 μg/g dry weight. To confirm the predicted extraction conditions, a separate experiment was conducted, and the results showed that the hyoscyamine and scopolamine contents were 164.72 and 209.23 μg/g dry weight, respectively.
Keywords: Henbane, High-performance liquid chromatography, Response surface methodology, Secondary metabolites, Ultrasound-assisted extraction
1. Introduction
Studies show that human health and nutrition are related, and the bioactive compounds in raw food have a significant impact on health. Therefore, plant materials have been considered due to having abundant amounts of bioactive compounds. Bioactive compounds are usually located inside the cell and need to be released into the solvent during the extraction process. In recent years, extraction methods based on ultrasound have been widely considered. Ultrasonic easily breaks the cell wall of the plant by producing cavitation bubbles, and extreme shear force and the intracellular substances are released in the solvent. Ultrasound-assisted extraction is one of the green extraction methods and compared to other methods requires little time and temperature and has high efficiency. Extraction with ultrasound is simple and affordable compared to extraction with microwaves and enzyme extraction [1,2]. In ultrasound-assisted extraction, various factors such as type of solvent, temperature, and duration of ultrasound have a significant impact on the extraction efficiency, and optimization of these factors can increase the amount of extracted bioactive compounds [3]. Therefore, this method has been widely used to optimize of extraction of bioactive compounds such as azadirachtin, mevalonic acid and squalene from the Azadirachta indica [4], glabridin and isoliquiritigenin from Glycyrrhiza glabra [5] and taxanes from Taxus cuspidate [6].
In designing the extraction of bioactive compounds from plants, the response surface methodology (RSM) is used, a statistical method to optimize extraction factors through the analysis of regression equations [7]. The RSM method is a cost-effective method by reducing the number of required tests and obtaining much information. Also, it allows investigating the simultaneous effect of several factors and predicts the optimal conditions [8]. In choosing the response surface design, the most important properties should be considered: 1) result in good fit of the model to the data, 2) provide good model parameter estimates, 3) provide a good distribution of prediction variance of the response throughout the region of interest, 4) provide an estimate of “pure” experimental error, 5) give sufficient information to allow for lack of fit test, 6) Provide a check on the homogeneous variance assumption, 7) be insensitive to the presence of outliers in the data, 8) be robust to errors in the control of design levels, 9) allow models of increasing order to be constructed sequentially, 10) allow for experiments to be done in blocks, and 11) be cost-effective [9]. Therefore, Box-Behnken (BBD) and Central Composite (CCD) designs are mainly used, which show the relationships between independent and dependent variables. In addition, RSM has 3D and 2D graphs that provide response levels [10,11].
Hyoscyamus niger belongs to the Solanaceae family and is one of the medicinal plants used to treat many diseases. This plant has tropane alkaloid compounds such as hyoscyamine and scopolamine, which have many medicinal properties, but their high doses are very toxic for humans. Despite its toxicity, hyoscyamine is used in the treatment of Parkinson's disease, bradyarrhythmias, and genitourinary symptoms, and scopolamine is also used to treat respiratory and gastrointestinal diseases such as acute respiratory distress syndrome and gastrointestinal motility disorders [12,13]. So far, no study has been conducted to extract hyoscyamine and scopolamine from the roots of the H. niger by the RSM method. In this study, for the first time, the optimal conditions for extracting these compounds have been obtained. In this study, the aim was to select the appropriate solvent and predict and determine the optimal conditions in terms of solvent concentration, temperature, and duration of ultrasonication to increase the extraction efficiency of hyoscyamine and scopolamine.
2. Materials and methods
2.1. Plant material
The H. niger seeds were collected from Imam Khomeini International University, and after washing with tap water, they were immersed in 200 mg/L gibberellic acid and kept at 4 °C for 48 h to break dormancy. Then, seeds were surface sterilized with 70 % ethanol for 45 s and 2.5 % hypochlorite sodium containing one drop of Tween 80 for 20 min and cultured on MS basal medium [14] for germination and seedling growth. The cultures were maintained in the growth chamber at 25 ± 2 °C with a 16/8 h photoperiod. After three weeks, germinated seeds were transferred to 200 mL culture jars and incubated in the growth chamber. After another three weeks, the roots of the seedlings were separated to extract hyoscyamine and scopolamine.
2.2. Root harvesting
The roots of the seedlings were separated using a scalpel, and after washing with tap water, they were kept in an oven at 40 °C for 72 h to dry. Then, the dried roots were powdered with a porcelain mortar and stored at −20 °C until extraction.
2.3. Selection of solvent
To select the solvent, 100 mg of dried and powdered root tissue was separated, and 1 mL of solvent was added. The solvents used included methanol, ethanol, dichloromethane, acetonitrile, and water. Then, the resulting mixture was sonicated (Elmasonic E30H, 37 kHz, 320 W, Germany) for 25 min at room temperature, centrifuged at 12,000 rpm for 15 min, and the supernatant was collected. The supernatant was kept at −20 °C until analysis by HPLC-DAD.
2.4. Treatment for hyoscyamine and scopolamine extraction
After selecting of solvent, 100 mg of dried and powdered root tissue was extracted with 1 mL of solvent and sonicated for 10, 20, 30, 40, and 50 min at 25, 30, 35, 40, and 45 °C. The mixtures were centrifuged at 12,000 rpm for 15 min, and the supernatant was collected and kept at −20 °C until analysis by HPLC-DAD.
2.5. HPLC-DAD analysis
The hyoscyamine and scopolamine contents of extracts were obtained using the Knauer HPLC-DAD system (DAD detector, Azura, Germany) according to Vakili, Karimi, Sharifi and Behmanesh [15] with a modification. The stationary phase was the C18 column (TSKgel-ODS C-18, 5 μm, 4.6 × 250 mm, Japan), and the mobile phase was 83 % water, 15 % methanol, 1.5 % glacial acetic acid, and 0.5 triethylamine. The injection volume was 20 μL and the column temperature was room temperature. The flow rate was 0.5 mL/min, and the absorbance of hyoscyamine and scopolamine was monitored at 258 nm. The calibration curve was obtained by hyoscyamine and scopolamine sulfate standards (Sigma, U.S.A), and their content was estimated from the standard curve.
2.6. Statistical analysis and central composite design
In order to select the suitable solvent, the experiment was carried out in a completely randomized design with five different types of solvents in three repetitions. Variance analysis was performed using IBM SPSS statistics 27.0 (Armonk, NY, USA) software. The mean comparison of different solvents was also done using Duncan's multi-range test at the probability level of 5 %. For the prediction and optimization of hyoscyamine and scopolamine extraction, the central composite design (CCD) of RSM with three variables at five levels (−2, −1, 0, +1, and +2) was used. The number of central and axial points were 6 and 14, respectively. Independent variables included solvent concentrations, temperatures, and ultrasonication times, and dependent variables were the amount of extracted hyoscyamine and scopolamine. Overall, 20 experiments were conducted to test the five levels of solvent concentrations, temperatures, and ultrasonication times. The predicted responses were calculated, and the quadratic model was shown by Farjaminezhad and Garoosi [16]. The RSM analysis was performed using Design Expert 13.0 software. To confirm the predicted extraction conditions, a separate experiment was conducted in three repetitions.
3. Results
3.1. Selection of solvent
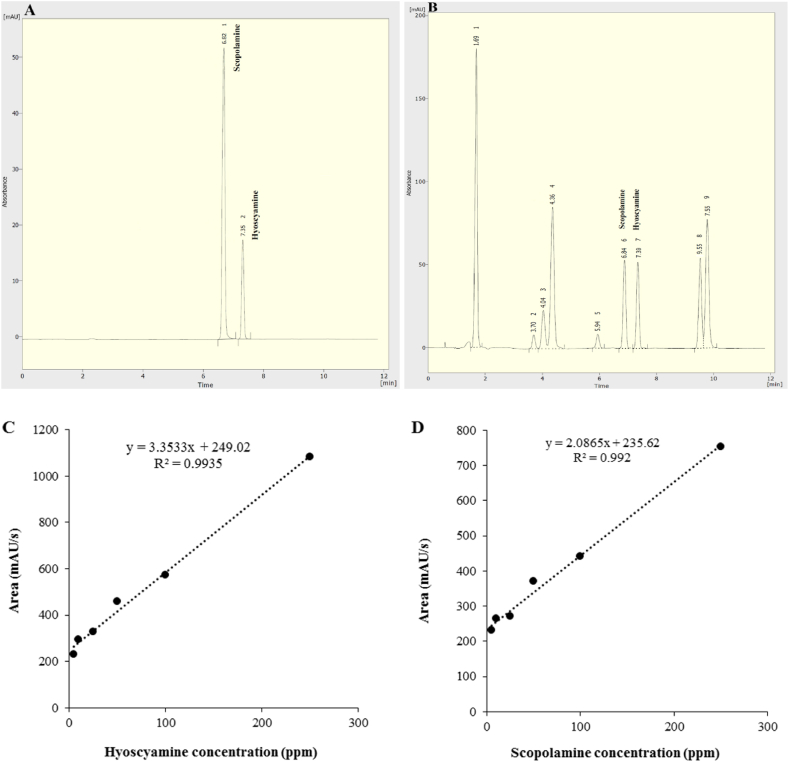
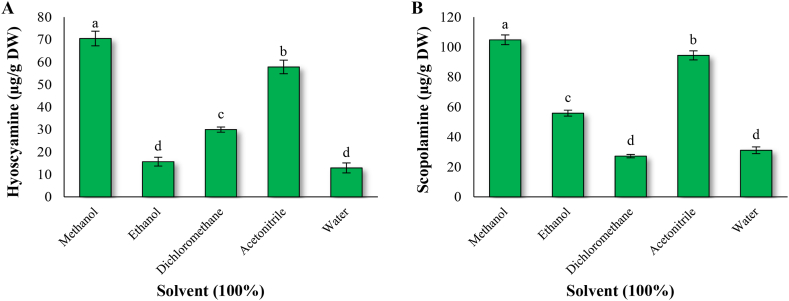
HPLC-DAD chromatograms of standard, extracted samples, standard calibration curves of hyoscyamine and scopolamine are shown in Fig. 1. In this study, the results of analysis of variance showed that depending on the solvent used, there are significant differences in the hyoscyamine and scopolamine content (Table 1). Among the five types of solvents used in this study, the highest hyoscyamine (70.45 μg/g dry weight) and scopolamine (104.84 μg/g dry weight) content was observed in methanol, and compared to ethanol, dichloromethane, acetonitrile, and water, the hyoscyamine content was 4.49-, 2.35-, 1.22-, and 5.46- fold higher, and scopolamine content was 1.88-, 3.85-, 1.11-, and 3.37-fold higher, respectively (Fig. 2). Therefore, methanol was selected as a suitable solvent for further study.
Fig. 1.
HPLC-DAD standard and extracted sample chromatograms (A and B) and standard calibration curves of hyoscyamine and scopolamine (C and D) and (B). The retention time for hyoscyamine and scopolamine standards was 7.35 and 6.82 min at 258 nm.
Table 1.
ANOVA results of the effect of different solvents on hyoscyamine and scopolamine extraction in H. niger root.
| Source of variation | df | Mean of square |
|
|---|---|---|---|
| Hyoscyamine | Scopolamine | ||
| Solvent | 4 | 1977.639a | 3813.737a |
| Error | 10 | 9.939 | 18.009 |
| CV (%) | 8.44 | 6.77 | |
Significant at 1 % probability.
Fig. 2.
Effect of solvents on hyoscyamine (A) and scopolamine (B) extraction in H. niger root.
3.2. Optimization of hyoscyamine extraction
3.2.1. Prediction of single factors and their interactions affect
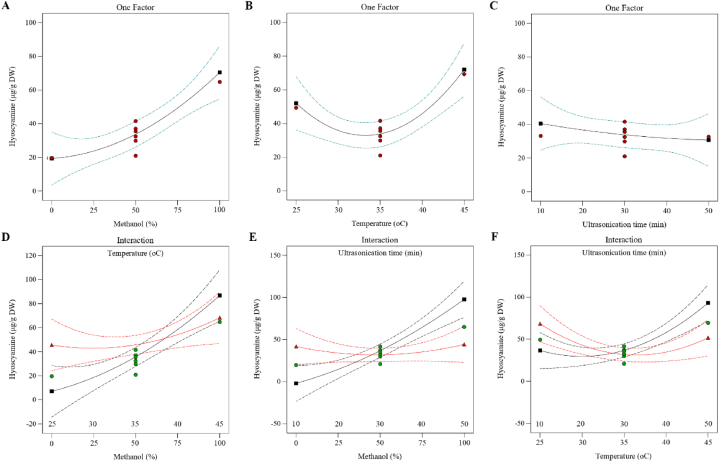
Single factors and their interaction experiments of hyoscyamine extraction were carried out with three parameters, including methanol concentration, extraction temperature, and ultrasonication time. According to the results, hyoscyamine content increased with the methanol concentration from 0 % to 100 %, and it was predicted that hyoscyamine content in 0 and 100 % methanol could be 19.42 and 70.56 μg/g dry weight, respectively. (Fig. 3A). The hyoscyamine content decreased with increasing temperature from 25 °C to 35 °C but increased again with increasing temperature from 35 °C to 45 °C. However, it can be predicted that the hyoscyamine content at 25 °C and 45 °C without considering the concentration of methanol and duration of ultrasonication can be 52.04 and 71.99 μg/g dry weight, respectively (Fig. 3B). Also, by studying the effect of different times of ultrasonication, it was found that the hyoscyamine content decreases with the increase in the duration of ultrasonication, and it was predicted that regardless of methanol concentration and temperature, hyoscyamine content of 10 and 50 min of sonication can be 40.52 and 30.07 μg/g dry weight, respectively (Fig. 3C). By examining the interaction effect of methanol concentration and temperature, it can be concluded that the maximum and minimum content of hyoscyamine in methanol 0 % and 25 °C can 45.74 and 7.19 μg/g dry weight, respectively, and its maximum and minimum content in methanol 100 % and 45 °C can 86.91 and 68.30 μg/g dry weight, respectively (Fig. 3D). Also, by studying the interaction effect of methanol concentration and duration of ultrasonication, it was predicted that in 0 % methanol and 10 min of ultrasonication, the minimum and maximum hyoscyamine content can be 0 and 41.72 μg/g dry weight, and in 100 % methanol and 50 min of ultrasonication it can be 44.24 and 97.77 μg/g dry weight, respectively (Fig. 3E). By investigating the interaction effect of temperature and ultrasonication time, it can be expected that the minimum and maximum hyoscyamine content at 25 °C and 10 min of ultrasonication are 36.64 and 68.33 μg/g dry weight and at 45 °C and 50 min of ultrasonication are 51.68 and 92.19 μg/g dry weight, respectively (Fig. 3F).
Fig. 3.
Effect of single factors and their interactions on hyoscyamine extraction in H. niger root.
3.2.2. Model fitting and analysis of variance
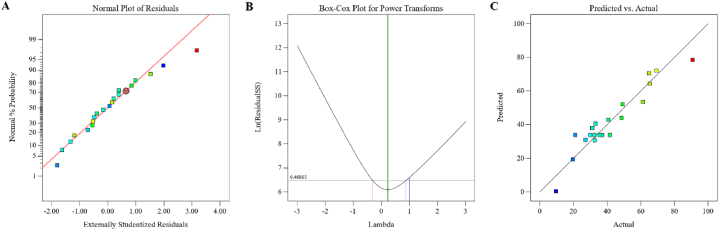
The first step in the response surface methodology is validation, shown in Table 2 and Fig. 4. Normality plots for hyoscyamine content are presented in Fig. 4A. Linear fitting of data in the normality plots, and the slight difference between predicted vs. actual values affirms the fitness of quadratic models for the responses. The Box-Cox plot of power transforms represents the Lambda value + 0.23 with a lower confidence interval of −0.31 and a higher confidence interval of +0.87 (Fig. 4B). Also, there is an excellent correlation between the values obtained from the experiment and the model (Fig. 4C). The hyoscyamine content was in the range of 9.56–90.67 μg/g dry weight. In experiment #15, the powdered root was extracted by 75 % methanol at 40 °C and sonicated for 40 min, producing 90.67 μg/g dry weight hyoscyamine. However, the lowest hyoscyamine content was obtained in experiment #10 using 25 % methanol, 30 °C, and 20 min sonication (Table 2). ANOVA analysis showed that the studied model was significant for hyoscyamine content, and the coefficient of determination (R2) was 0.9009. This shows that the model is suitable for predicting hyoscyamine content in the studied ranges [11,17]. In this model, linear coefficients A and B were significant, and linear coefficient C was insignificant. The interaction terms of AB, AC, and BC were also significant. The quadratic term B2 was significant and A2 and C2 were non-significant (Table 3). In general, the final equation for predicting the extracted hyoscyamine content was as follows:
Table 2.
Central composite design (CCD) and response values for hyoscyamine and scopolamine extraction.
| Run | A: Methanol (%) |
B: Temperature (°C) |
C: Ultrasonication time (min) |
Hyoscyamine (μg/g DW) |
Scopolamine (μg/g DW) |
||
|---|---|---|---|---|---|---|---|
| Actual Value | Predicted Value | Actual Value | Predicted Value | ||||
| 1 | 75 (+1) | 40 (+1) | 40 (+1) | 27.13 | 30.98 | 104.21 | 113.91 |
| 2 | 50 (0) | 45 (+2) | 30 (0) | 69.34 | 71.99 | 86.51 | 76.98 |
| 3 | 25 (−1) | 40 (+1) | 40 (+1) | 48.56 | 44.01 | 55.58 | 66.92 |
| 4 | 75 (+1) | 30 (−1) | 40 (+1) | 61.17 | 53.59 | 87.56 | 89.21 |
| 5 | 50 (0) | 25 (−2) | 30 (0) | 49.32 | 52.04 | 126.1 | 140.51 |
| 6 | 0 (−2) | 35 (0) | 30 (0) | 19.71 | 19.42 | 13.44 | 18.16 |
| 7 | 50 (0) | 35 (0) | 30 (0) | 41.58 | 33.83 | 102.77 | 95.54 |
| 8 | 50 (0) | 35 (0) | 30 (0) | 35.55 | 33.83 | 107.82 | 95.54 |
| 9 | 75 (+1) | 30 (−1) | 20 (−1) | 65.34 | 64.52 | 165.98 | 149.75 |
| 10 | 25 (−1) | 30 (−1) | 20 (−1) | 9.56 | 0.3442 | 111.41 | 96.83 |
| 11 | 50 (0) | 35 (0) | 50 (+2) | 32.65 | 30.7 | 108.84 | 97.32 |
| 12 | 100 (+2) | 35 (0) | 30 (0) | 64.89 | 70.56 | 117.89 | 118.06 |
| 13 | 50 (0) | 35 (0) | 10 (−2) | 33.20 | 40.52 | 83.14 | 99.54 |
| 14 | 50 (0) | 35 (0) | 30 (0) | 21.00 | 33.83 | 96.86 | 95.54 |
| 15 | 75 (+1) | 40 (+1) | 20 (−1) | 90.67 | 78.5 | 118.2 | 117.86 |
| 16 | 50 (0) | 35 (0) | 30 (0) | 32.52 | 33.83 | 100.65 | 95.54 |
| 17 | 25 (−1) | 30 (−1) | 40 (+1) | 31.25 | 38.05 | 103.11 | 98.56 |
| 18 | 50 (0) | 35 (0) | 30 (0) | 37.10 | 33.83 | 79.54 | 95.54 |
| 19 | 50 (0) | 35 (0) | 30 (0) | 29.87 | 33.83 | 80.74 | 95.54 |
| 20 | 25 (−1) | 40 (+1) | 20 (−1) | 40.71 | 42.91 | 15.14 | 8.61 |
The bold value indicates the maximum value.
Fig. 4.
Normal plot (A), Box-Cox plot (B) predicated vs. Actual plot (C) for hyoscyamine content in H. niger root.
Table 3.
ANOVA results for the response surface quadratic model for optimization of extraction parameters.
| Source | df | Hyoscyamine |
Scopolamine |
||||
|---|---|---|---|---|---|---|---|
| Mean Square | F-value | p-value | Mean Square | F-value | p-value | ||
| Model | 9 | 745.24a | 10.11 | 0.0006 | 2331.12a | 10.63 | 0.0005 |
| A-Methanol | 1 | 2615.58a | 35.47 | 0.0001 | 9980.11a | 45.50 | <0.0001 |
| B-Temperature | 1 | 397.87a | 5.40 | 0.0426 | 4035.68a | 18.40 | 0.0016 |
| C-Ultrasonication time | 1 | 96.44 ns | 1.31 | 0.2794 | 4.93 ns | 0.0225 | 0.8838 |
| AB | 1 | 408.37a | 5.54 | 0.0404 | 1587.04a | 7.24 | 0.0227 |
| AC | 1 | 1182.22a | 16.03 | 0.0025 | 1938.84a | 8.84 | 0.0140 |
| BC | 1 | 669.80a | 9.08 | 0.0130 | 1601.04a | 7.30 | 0.0222 |
| A2 | 1 | 195.64 ns | 2.65 | 0.1344 | 1183.05a | 5.39 | 0.0426 |
| B2 | 1 | 1248.45a | 16.93 | 0.0021 | 273.99 ns | 1.25 | 0.2898 |
| C2 | 1 | 4.98 ns | 0.0675 | 0.8003 | 13.09 ns | 0.0597 | 0.8120 |
| Residual | 10 | 73.74 | 219.32 | ||||
| Lack of Fit | 5 | 97.31 ns | 1.94 | 0.2424 | 298.24 ns | 2.12 | 0.2140 |
| Pure Error | 5 | 50.17 | 140.41 | ||||
|
Cor Total |
19 |
||||||
| R2 | 0.9009 | 0.9054 | |||||
| CV (%) | 20.42 | 15.88 | |||||
indicates significant and ns indicates non-significant.
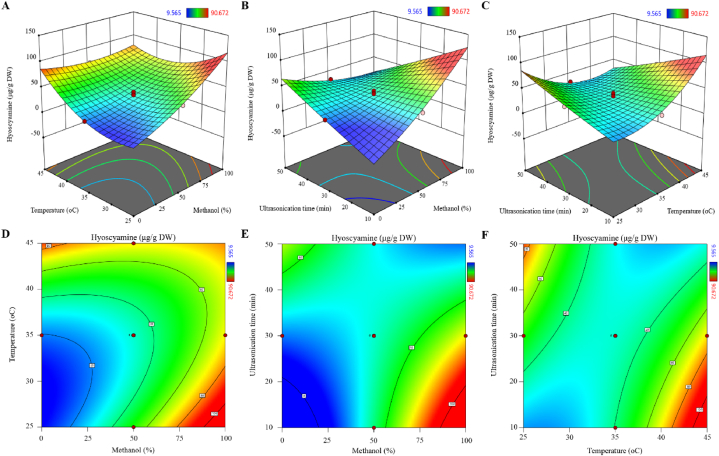
3.2.3. Response surface analysis
The 3D response surface and 2D contour plots were obtained based on the above equation (Fig. 5). In the figure of the 3D response surface plot, the higher slope indicated greater interaction. In the 2D contour plot, the elliptical and circular contours indicate more and less interaction, respectively. According to Fig. 5A and D, hyoscyamine content decreases with the simultaneous increase of methanol percentage and solvent temperature. While in Fig. 5B and E, hyoscyamine content increases with the increase of methanol percentage and ultrasonication time. In Fig. 5C and F, hyoscyamine content increases slightly with increasing temperature and duration of ultrasonication. The optimum condition for maximum hyoscyamine content (172.06 μg/g dry weight) was predicted at extraction in 100 % methanol at 45 °C and ultrasonication for 10 min. In another experiment, the predicted methanol concentration, temperature, and ultrasonication time were applied and the hyoscyamine content was obtained 164.72 μg/g dry weight.
Fig. 5.
3D response surface plots (A, B, and C) and contour plots (D, E, and F) indicated the effects of the studied factors on the hyoscyamine content in H. niger root.
3.3. Optimization of scopolamine extraction
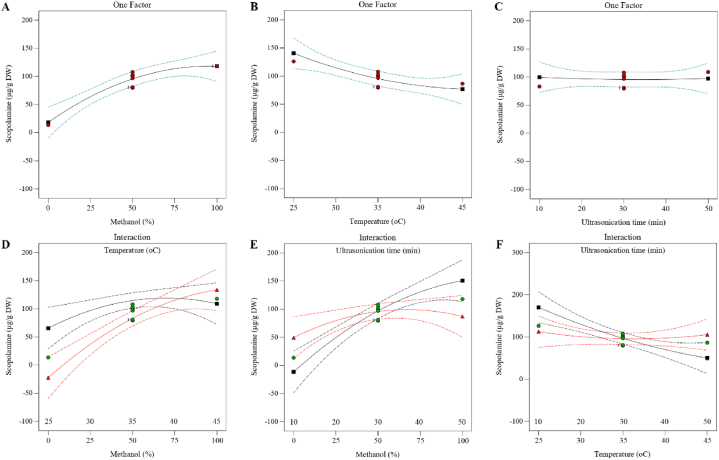
3.3.1. Prediction of single factors and their interactions affect
Single factors and their interactions experiments of scopolamine extraction were carried out with three parameters, including methanol concentration, extraction temperature, and ultrasonication time. According to the results, scopolamine content increased with the methanol concentration from 0 % to 100 %, and it was predicted that scopolamine content in 0 and 100 % methanol could be 18.15 and 118.06 μg/g dry weight, respectively. (Fig. 6A). The scopolamine content decreased with increasing temperature from 25 °C to 45 °C, however, it can be predicted that the scopolamine content at 25 °C and 45 °C without considering the concentration of methanol and duration of ultrasonication can be 140.51 and 76.98 μg/g dry weight, respectively (Fig. 6B). Also, by studying the effect of different times of ultrasonication, it was found that the scopolamine content is no significant change, and it was predicted that regardless of methanol concentration and temperature, scopolamine content of 10 and 50 min of sonication can be 99.54 and 97.32 μg/g dry weight, respectively (Fig. 6C). By examining the interaction effect of methanol concentration and temperature, it can be concluded that the maximum and minimum content of scopolamine in methanol 0 % and 25 °C can 65.51 and 0 μg/g dry weight, respectively, and its maximum and minimum content in methanol 100 % and 45 °C can 137.64 and 109.07 μg/g dry weight, respectively (Fig. 6D). Also, by studying the interaction effect of methanol concentration and duration of ultrasonication, it was predicted that in 0 % methanol and 10 min of ultrasonication, the minimum and maximum scopolamine content can be 0 and 49.16 μg/g dry weight, and in 100 % methanol and 50 min of ultrasonication it can be 87.08 and 150.47 μg/g dry weight, respectively (Fig. 6E). By investigating the interaction effect of temperature and ultrasonication time, it can be expected that the minimum and maximum scopolamine content at 25 °C and 10 min of ultrasonication are 112.38 and 170.08 μg/g dry weight and at 45 °C and 50 min of ultrasonication are 49.97 and 107.44 μg/g dry weight, respectively (Fig. 6F).
Fig. 6.
Effect of single factors and their interactions on scopolamine extraction in H. niger root.
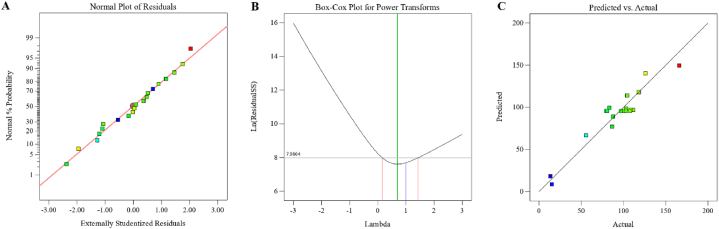
3.3.2. Model fitting and analysis of variance
Normality plots for scopolamine content are presented in Fig. 7A. Linear fitting of data in the normality plots, and the slight difference between predicted vs. actual values affirms the fitness of quadratic models for the responses. The Box-Cox plot of power transforms represents the Lambda value + 0.70 with a lower confidence interval of +0.16 and a higher confidence interval of +1.43 (Fig. 7B). Also, it is observed a very good correlation between the values obtained from the experiment and the model (Fig. 7C). The scopolamine content was in the range from 13.44 to 165.98 μg/g dry weight. In experiment #9, the powdered root was extracted by 75 % methanol at 30 °C and sonicated for 30 min, produced 165.98 μg/g dry weight scopolamine. However, the lowest scopolamine content was obtained in experiment #6 using 0 % methanol, 35 °C, and 30 min sonication (Table 2). ANOVA analysis showed that the studied model was significant for scopolamine content, and the coefficient of determination (R2) was 0.9054. This shows that the model is suitable for predicting scopolamine content in the studied ranges [18]. In this model, linear coefficients A and B were significant, and linear coefficient C was non-significant. The interaction terms of AB, AC, and BC were also significant. The quadratic term A2 was significant, and B2 and C2 were non-significant (Table 3). In general, the final equation for predicting the extracted scopolamine content was as follows:
Fig. 7.
Normal plot (A), Box-Cox plot (B) predicated vs. actual plot (C) for scopolamine content in H. niger root.
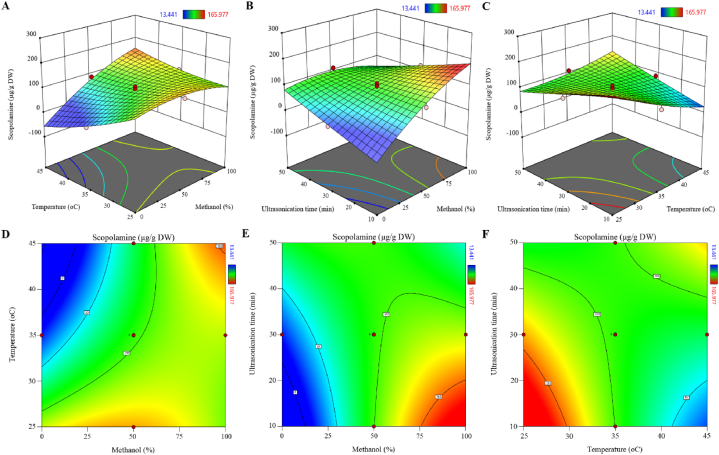
3.3.3. Response surface analysis
The 3D response surface and 2D contour plots were obtained based on the above equation (Fig. 8). In the figure of the 3D response surface plot, the higher slope indicated more significant interaction. In the 2D contour plot, the elliptical and circular contours indicate more and less interaction, respectively. According to Fig. 8A and D, scopolamine content decreases with increased methanol percentage and solvent temperature. In Fig. 8B and E, scopolamine content increases with the increase of methanol percentage and ultrasonication time. In Fig. 8C and F, scopolamine content decreases slightly with increasing temperature and duration of ultrasonication. The optimum condition for maximum scopolamine content (229.48 μg/g dry weight) was predicted at extraction in 98.50 % methanol at 25 °C and ultrasonication for 10 min. In another experiment, the predicted methanol concentration, temperature, and ultrasonication time were applied and the scopolamine content was obtained 209.23 μg/g dry weight.
Fig. 8.
3D response surface plots (A, B and C) and contour plots (D, E, and F) indicated the effects of the studied factors on scopolamine content in H. niger root.
4. Discussion
The use of plants to treat diseases is very common and isolated compounds and their extracts have been used to treat various diseases including cancer. In recent years, due to the high use of chemotherapy agents, cancer cells have become resistant to these compounds, but due to the presence of special compounds in plants, they can be used to re-sensitize resistant cancer cells to chemotherapy agents [19]. H. niger is one of the plants that have been studied in recent years for the treatment of various diseases, including ovarian cancer and COVID-19 [20,21]. However, in these studies, general methods are usually used to extract compounds, and as a result, the amount of compounds effective on the disease in these extracts may be low. Therefore, obtaining optimal extraction conditions for different compounds can be useful in the treatment of different diseases. Studies show that the extraction of medicinal compounds from different plants depends on various factors such as temperature and time of extraction as well as the type of solvent. Therefore, it is very important to find the optimal conditions for the extraction of these compounds. To investigate the effect of various factors on the extraction of medicinal compounds, all factors must be examined simultaneously, which leads to an increase in the number of experiments, costs, and loss of time. The response surface methodology reduces costs and saves time due to the reduction in the number of experiments to assess the impact of various factors in extraction [4]. For this reason, in this study, we first selected a suitable solvent in an experiment, and then we investigated the effect of different solvent concentrations, temperatures, and durations of ultrasonication using the response surface curve method.
The solvent used to extract of bioactive compounds is critical and should be selected depending on the target compound. Therefore, in the extraction process, the first step should be to focus on the effect of the solvent [22]. For example, Saha, Walia, Kundu, Sharma and Paul [23] used different solvents to extract β-carotene from carrots and observed that the use of a mixture of acetone and hexane resulted in the lowest carotenoid content, while the mixture of ethanol and hexane had the highest amount of lutein and β-carotene. In this study, methanol was selected as a suitable solvent for further study. Studies show that the amount of phenols, flavonoids, alkaloids, and terpenoids in methanolic extract is higher than other solvents, which is due to their high solubility in methanol [24]. Solvents are different in terms of polarity, and have different effects on extracting phytochemicals [25]. Zachová, Tříska, Vrchotová, Balík, Sajfrtová and Sovová [26] used methanol, acetone, and ethanol to extract stilbene from grapes and observed that ethanol was more selective than methanol. Liu, Guo, Chang, Jiang and Wang [27] extracted two alkaloids, evodiamine, and rutaecarpine, from Evodia rutaecarpa using different solvents and reported that methanol increases the yield of these two alkaloids. Rezaei and Ghasemi Pirbalouti [28] indicated that methanol is an effective solvent for polyphenols and flavonoid extraction from Zaravschanica membranacea and Ferulago angulata.
Studies show that the solubility of some bioactive compounds increases with a long extraction time, but it should be noted that some compounds may decompose in a long time and high temperature [29]. Temperature is an important factor affecting the extraction, which can differ depending on the composition [30]. For example, in foxtail millet, increasing the temperature improves the yield of total phenol [31]. In other studies, the extraction of bioactive compounds has been predicted under different conditions. For example, Farjaminezhad and Garoosi [4], by studying the effect of solvent concentration, temperature, and duration of ultrasonication on the extraction of azadirachtin from neem cells, predicted that the highest azadirachtin content obtained with the use of water, a temperature of 35 °C and ultrasonication for 20 min. In the study of Mudaser, Mumtaz, Akhtar, Mukhtar, Raza, Shami and Touqeer [32], the maximum yield of α-glucosidase extraction was observed when the plant sample was extracted with 60 % ethanol and sonication for 75 min at 35 °C.
The efficiency of extraction with the ultrasound depends on the cavitation created in the solvent, which leads to the formation of tiny pores in the cell wall and the penetration of the solvent into the cell, but the long extraction time is also important and has a direct effect on the extraction of compounds. Long extraction time can lead to higher yields, but too much time has a negative effect and results in the decomposition of the compound [1]. As seen, increasing the extraction temperature has decreased the efficiency of scopolamine extraction. This was unexpected, as increasing temperature increases kinetic energy and diffusion speed, but this trend has also been reported by other researchers [33]. One of the reasons for the decrease in extraction efficiency is the increase in vapor pressure, which affects the intensity of the cavitation of ultrasound. At low temperatures, ultrasound creates few bubbles, but these bubbles explode with greater force, which is due to the more pressure difference on the surface of the bubbles, which reduces the vapor pressure. However, at high temperatures, despite the fact that more bubbles are formed, the pressure difference between the inside and outside of the bubble is slight, and they explode without the need for energy [34].
In neem cells, it was also predicted that the highest mevalonic acid and squalene content is obtained by applying 50 % ethanol at 45 °C and ultrasonication for 30 min and 50 % ethanol at 55 °C and ultrasonication for 10 min, respectively [4]. In the study of bioactive components and antioxidant activity of Poria cocos, the optimal conditions were predicted as 55.53 % ethanol for 48.64 min sonication in 60 mL solvent for triterpenoid acids and 40.49 % ethanol for 30.25 min sonication in 20 mL solvent for antioxidant activity and total polysaccharide and phenolic contents [11].
5. Conclusion
In this study, the CCD design of RSM was used to determine the optimal conditions for hyoscyamine and scopolamine extraction from H. niger root. In the first step, we experimented to select the appropriate solvent for hyoscyamine and scopolamine extraction. In this experiment, we used five solvents, including methanol, ethanol, dichloromethane, acetonitrile, and water, and we chose methanol as the suitable solvent for extracting hyoscyamine and scopolamine. In the next step, we used different concentrations of methanol (0, 25, 50, 75, and 100 %), different temperatures (25, 30, 35, 40, and 45 °C), and different ultrasonication times (10, 20, 30, 40, and 50 min). We obtained the best conditions for extracting hyoscyamine and scopolamine. The results showed that the optimal conditions for extracting of hyoscyamine and scopolamine are 100 % methanol, 45 °C, and 10 min ultrasonication, and 98.50 % methanol, 25 °C, and 10 min ultrasonication, respectively.
Data availability statement
Data will be made available on request.
Additional information
No additional information is available for this paper.
CRediT authorship contribution statement
Roghayeh Ahmadpour: Writing – review & editing, Writing – original draft, Methodology, Investigation. Bahram Maleki Zanjani: Project administration. Ghasem-ali Garoosi: Project administration. Reza Farjaminezhad: Software, Formal analysis, Data curation. Raheem Haddad: Supervision.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors are grateful to the University of Zanjan and Plant tissue culture laboratory of Imam Khomeini International University for financial and technical support.
Contributor Information
Roghayeh Ahmadpour, Email: r.ahmadpour65@gmail.com.
Bahram Maleki Zanjani, Email: bmalekiz@znu.ac.ir.
Ghasem-ali Garoosi, Email: garoosi@eng.ikiu.ac.ir.
Reza Farjaminezhad, Email: farjaminezhad@org.ikiu.ac.ir.
Raheem Haddad, Email: r.haddad@eng.ikiu.ac.ir.
References
- 1.Sirichan T., Kijpatanasilp I., Asadatorn N., Assatarakul K. Optimization of ultrasound extraction of functional compound from makiang seed by response surface methodology and antimicrobial activity of optimized extract with its application in orange juice. Ultrason. Sonochem. 2022;83 doi: 10.1016/j.ultsonch.2022.105916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yin D., Sun X., Li N., Guo Y., Tian Y., Wang L. Structural properties and antioxidant activity of polysaccharides extracted from Laminaria japonica using various methods. Process Biochem. 2021;111:201–209. doi: 10.1016/j.procbio.2021.10.019. [DOI] [Google Scholar]
- 3.Sang J., Sang J., Ma Q., Hou X.-f., Li C.-q. Extraction optimization and identification of anthocyanins from Nitraria tangutorun Bobr. seed meal and establishment of a green analytical method of anthocyanins. Food Chem. 2017;218:386–395. doi: 10.1016/j.foodchem.2016.09.093. [DOI] [PubMed] [Google Scholar]
- 4.Farjaminezhad R., Garoosi G.-a. Establishment of green analytical method for ultrasound-assisted extraction of azadirachtin, mevalonic acid and squalene from cell suspension culture of Azadirachta indica using response surface methodology. Ind. Crops Prod. 2020;144 doi: 10.1016/j.indcrop.2019.111946. [DOI] [Google Scholar]
- 5.Xing C., Cui W.-Q., Zhang Y., Zou X.-S., Hao J.-Y., Zheng S.-D., Wang T.-T., Wang X.-Z., Wu T., Liu Y.-Y., Chen X.-Y., Yuan S.-G., Zhang Z.-Y., Li Y.-H. Ultrasound-assisted deep eutectic solvents extraction of glabridin and isoliquiritigenin from Glycyrrhiza glabra: optimization, extraction mechanism and in vitro bioactivities. Ultrason. Sonochem. 2022;83 doi: 10.1016/j.ultsonch.2022.105946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang Y., Zhao Z., Meng H., Li W., Wang S. Ultrasonic extraction and separation of taxanes from Taxus cuspidata optimized by response surface methodology. Separations. 2022;9:193. doi: 10.3390/separations9080193. [DOI] [Google Scholar]
- 7.Li J., Chen Z., Shi H., Yu J., Huang G., Huang H. Ultrasound-assisted extraction and properties of polysaccharide from Ginkgo biloba leaves. Ultrason. Sonochem. 2023;93 doi: 10.1016/j.ultsonch.2023.106295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kusuma H.S., Amenaghawon A.N., Darmokoesoemo H., Neolaka Y.A.B., Widyaningrum B.A., Onowise S.U., Anyalewechi C.L. A comparative evaluation of statistical empirical and neural intelligence modeling of Manihot esculenta-derived leaves extract for optimized bio-coagulation-flocculation of turbid water. Ind. Crops Prod. 2022;186 doi: 10.1016/j.indcrop.2022.115194. [DOI] [Google Scholar]
- 9.R.H. Myers, D.C. Montgomery, C.M. Anderson-Cook, Response Surface Methodology: Process and Product Optimization Using Designed Experiments, John Wiley & Sons2016.
- 10.Almeida D.G., Soares da Silva R.d.C.F., Luna J.M., Rufino R.D., Santos V.A., Sarubbo L.A. Response surface methodology for optimizing the production of biosurfactant by Candida tropicalis on industrial waste substrates. Front. Microbiol. 2017;8 doi: 10.3389/fmicb.2017.00157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen J., Lan X., Jia R., Hu L., Wang Y. Response surface methodology (RSM) mediated optimization of medium components for mycelial growth and metabolites production of Streptomyces alfalfae XN-04. Microorganisms. 2022;10:1854. doi: 10.3390/microorganisms10091854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ahmadpour R., Maleki Zanjani B., Garoosi G.-a., Haddad R., Farjaminezhad R. Prediction of the concentration of plant growth regulators for somatic embryogenesis and regeneration of Hyoscyamus niger using Box–Behnken design of response surface methodology. Plant Cell Tissue Organ Cult. 2023;154:55–71. doi: 10.1007/s11240-023-02510-w. [DOI] [Google Scholar]
- 13.Shi Z., Zou W., Zhu Z., Xiong Z., Li S., Dong P., Zhu Z. Tropane alkaloids (hyoscyamine, scopolamine and atropine) from genus Datura: extractions, contents, syntheses and effects. Ind. Crops Prod. 2022;186 doi: 10.1016/j.indcrop.2022.115283. [DOI] [Google Scholar]
- 14.Murashige T., Skoog F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant. 1962;15:473–497. doi: 10.1111/j.1399-3054.1962.tb08052.x. [DOI] [Google Scholar]
- 15.Vakili B., Karimi F., Sharifi M., Behmanesh M. Chromium-induced tropane alkaloid production and H6H gene expression in Atropa belladonna L. (Solanaceae) in vitro-propagated plantlets. Plant Physiol. Biochem. 2012;52:98–103. doi: 10.1016/j.plaphy.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 16.Farjaminezhad R., Garoosi G. Prediction of the effect of chitosan on cell suspension culture of Azadirachta indica by response surface methodology. Plant Cell Tissue Organ Cult. 2021;146:323–337. doi: 10.1007/s11240-021-02072-9. [DOI] [Google Scholar]
- 17.Thu Dao T.A., Webb H.K., Malherbe F. Optimization of pectin extraction from fruit peels by response surface method: conventional versus microwave-assisted heating. Food Hydrocoll. 2021;113 doi: 10.1016/j.foodhyd.2020.106475. [DOI] [Google Scholar]
- 18.Taofiq O., Silva A.R., Costa C., Ferreira I., Nunes J., Prieto M.A., Simal-Gandara J., Barros L., Ferreira I.C.F.R. Optimization of ergosterol extraction from Pleurotus mushrooms using response surface methodology. Food Funct. 2020;11:5887–5897. doi: 10.1039/D0FO00301H. [DOI] [PubMed] [Google Scholar]
- 19.Dehelean C.A., Marcovici I., Soica C., Mioc M., Coricovac D., Iurciuc S., Cretu O.M., Pinzaru I. Plant-derived anticancer compounds as new perspectives in drug discovery and alternative therapy. Molecules. 2021;26:1109. doi: 10.3390/molecules26041109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kosari M., Khorvash F., Sayyah M.K., Ansari Chaharsoughi M., Najafi A., Momen-Heravi M., Karimian M., Akbari H., Noureddini M., Salami M., Ghaderi A., Amini Mahabadi J., Khamechi S.P., Yeganeh S., Banafshe H.R. The influence of propolis plus Hyoscyamus niger L. against COVID-19: a phase II, multicenter, placebo-controlled, randomized trial. Phytother Res. 2024;38:400–410. doi: 10.1002/ptr.8047. [DOI] [PubMed] [Google Scholar]
- 21.Lekmine S., Benslama O., Kadi K., Ignacio Martín-García A., Shamsul Ola M., Abdullah Yilmaz M., Ali A. Therapeutic potential of Hyoscyamus niger-derived compounds: targeting ovarian cancer through antioxidant activity and EGFR tyrosine kinase inhibition. J. King Saud Univ. Sci. 2024;36 doi: 10.1016/j.jksus.2024.103103. [DOI] [Google Scholar]
- 22.Lefebvre T., Destandau E., Lesellier E. Selective extraction of bioactive compounds from plants using recent extraction techniques: a review. J. Chromatogr. A. 2021;1635 doi: 10.1016/j.chroma.2020.461770. [DOI] [PubMed] [Google Scholar]
- 23.Saha S., Walia S., Kundu A., Sharma K., Paul R.K. Optimal extraction and fingerprinting of carotenoids by accelerated solvent extraction and liquid chromatography with tandem mass spectrometry. Food Chem. 2015;177:369–375. doi: 10.1016/j.foodchem.2015.01.039. [DOI] [PubMed] [Google Scholar]
- 24.Do Q.D., Angkawijaya A.E., Tran-Nguyen P.L., Huynh L.H., Soetaredjo F.E., Ismadji S., Ju Y.-H. Effect of extraction solvent on total phenol content, total flavonoid content, and antioxidant activity of Limnophila aromatica. JFDA. 2014;22:296–302. doi: 10.1016/j.jfda.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alara O.R., Abdurahman N.H., Ukaegbu C.I. Extraction of phenolic compounds: a review. Curr. Res. Food Sci. 2021;4:200–214. doi: 10.1016/j.crfs.2021.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zachová Z., Tříska J., Vrchotová N., Balík J., Sajfrtová M., Sovová H. Combining high-pressure methods for extraction of stilbenes from grape cane. J. Supercrit. Fluids. 2018;142:38–44. doi: 10.1016/j.supflu.2018.05.021. [DOI] [Google Scholar]
- 27.Liu B., Guo F., Chang Y., Jiang H., Wang Q. Optimization of extraction of evodiamine and rutaecarpine from fruit of Evodia rutaecarpa using modified supercritical CO2. J. Chromatogr. A. 2010;1217:7833–7839. doi: 10.1016/j.chroma.2010.10.079. [DOI] [PubMed] [Google Scholar]
- 28.Rezaei M., Ghasemi Pirbalouti A. Phytochemical, antioxidant and antibacterial properties of extracts from two spice herbs under different extraction solvents. J. Food Meas. Char. 2019;13:2470–2480. doi: 10.1007/s11694-019-00167-8. [DOI] [Google Scholar]
- 29.Xue H., Tan J., Li Q., Tang J., Cai X. Optimization ultrasound-assisted deep eutectic solvent extraction of anthocyanins from raspberry using response surface methodology coupled with genetic algorithm. Foods. 2020;9:1409. doi: 10.3390/separations9080193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zeng J., Dou Y., Yan N., Li N., Zhang H., Tan J.-N. Optimizing ultrasound-assisted deep eutectic solvent extraction of bioactive compounds from Chinese wild rice. Molecules. 2019;24:2718. doi: 10.3390/molecules24152718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zheng B., Yuan Y., Xiang J., Jin W., Johnson J.B., Li Z., Wang C., Luo D. Green extraction of phenolic compounds from foxtail millet bran by ultrasonic-assisted deep eutectic solvent extraction: optimization, comparison and bioactivities. LWT. 2022;154 doi: 10.1016/j.lwt.2021.112740. [DOI] [Google Scholar]
- 32.Mudaser B., Mumtaz M.W., Akhtar M.T., Mukhtar H., Raza S.A., Shami A.A., Touqeer T. Response surface methodology based extraction optimization to improve pharmacological properties and 1H NMR based metabolite profiling of Azadirachta indica. Phytomed. Plus. 2021;1 doi: 10.1016/j.phyplu.2020.100015. [DOI] [Google Scholar]
- 33.Mohammadpour H., Sadrameli S.M., Eslami F., Asoodeh A. Optimization of ultrasound-assisted extraction of Moringa peregrina oil with response surface methodology and comparison with Soxhlet method. Ind. Crops Prod. 2019;131:106–116. doi: 10.1016/j.indcrop.2019.01.030. [DOI] [Google Scholar]
- 34.Zhang Z.-S., Wang L.-J., Li D., Jiao S.-S., Chen X.D., Mao Z.-H. Ultrasound-assisted extraction of oil from flaxseed. Sep. Purif. Technol. 2008;62:192–198. doi: 10.1016/j.seppur.2008.01.014. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.