Abstract
Introduction
Larval source management, particularly larviciding, is mainly implemented in urban settings to control malaria and other mosquito-borne diseases. In Tanzania, the government has recently expanded larviciding to rural settings across the country, but implementation faces multiple challenges, notably inadequate resources and limited know-how by technical staff. This study evaluated the potential of training community members to identify, characterize and target larval habitats of Anopheles funestus mosquitoes, the dominant vector of malaria transmission in south-eastern Tanzania.
Methods
A mixed-methods study was used. First, interviewer-administered questionnaires were employed to assess knowledge, awareness, and perceptions of community members towards larviciding (N = 300). Secondly community-based volunteers were trained to identify and characterize aquatic habitats of dominant malaria vector species, after which they treated the most productive habitats with a locally-manufactured formulation of the biolarvicide, Bacillus thuringiensis var. israelensis. Longitudinal surveys of mosquito adults and larvae were used to assess impacts of the community-led larviciding programme in two villages in rural south-eastern Tanzania.
Results
At the beginning of the program, the majority of village residents were unaware of larviciding as a potential malaria prevention method, and about 20 % thought that larvicides could be harmful to the environment and other insects. The trained community volunteers identified and characterized 360 aquatic habitats, of which 45.6 % had Anopheles funestus, the dominant malaria vector in the area. The preferred larval habitats for An. funestus were deep and had either slow- or fast-moving waters. Application of biolarvicides reduced the abundance of adult An. funestus and Culex spp. species inside human houses in the same villages, by 46.3 % and 35.4 % respectively. Abundance of late-stage instar larvae of the same taxa was also reduced by 74 % and 42 %, respectively.
Conclusion
This study demonstrates that training community members to identify, characterize, and target larval habitats of the dominant malaria vectors can be effective for larval source management in rural Tanzania. Community-led larviciding reduced the densities of adult and late-stage instar larvae of An. funestus and Culex spp. inside houses, suggesting that this approach may have potential for malaria control in rural settings. However, efforts are still needed to increase awareness of larviciding in the relevant communities.
Keywords: Malaria control, Larviciding, Larval source management, Biolarvicides, Community engagement, Tanzania, Ifakara health institute
1. Introduction
Since 2000, malaria incidence and mortality have significantly decreased due primarily to expanded vector control with insecticide-treated nets (ITNs) and indoor residual spraying (IRS), along with improved diagnosis and treatment (Bhatt et al., 2015). However, these gains began plateauing around 2015, and many high-burden countries now report increases in cases (WHO, 2023). The latest estimates from the World Health Organization (WHO) indicate that the disease still causes around 249 million cases and 608,000 deaths, with a majority of this burden being in Africa (WHO, 2023).
In addition to poor socio-economic circumstances and the generally weak health systems in endemic communities (The malERA Consultative Group on Health Systems, 2011; Okumu et al., 2022), the continued burden of malaria is also associated with major biological threats such as the widespread resistance of malaria vectors to insecticides (Hancock et al., 2020; Moyes et al., 2020) and behavioral resistance to indoor interventions (Russell et al., 2011; Moiroux et al., 2012; Sougoufara et al., 2014), as well as growing tolerance of the malaria parasite (Plasmodium spp.) to anti-malarial drugs. Human behaviors, such as staying outdoors unprotected during times of increased malaria transmission, also contribute to ongoing malaria transmission (Finda et al., 2019; Matowo et al., 2013; Monroe et al., 2015). Another important but often less discussed challenge is that sustained malaria prevention is untenable without sufficient engagement of local communities and other stakeholders (Adhikari et al., 2020; Asale et al., 2019).
Research has shown that involving rural communities in vector control programs is beneficial for malaria prevention and control (Asale et al., 2019; Von Seidlein et al., 2019). The success of such interventions is dependent on the level of education and awareness of malaria prevention tools among community members (Mutero et al., 2020). Studies conducted in Tanzania (Maheu-Giroux and Castro, 2013; Chaki et al., 2012), Rwanda (Hakizimana et al., 2022), Burkina Faso (Dambach et al., 2019), Bioko Island (García et al., 2022) and Malawi (Van den Berg et al., 2018; Phiri et al., 2021) have demonstrated the effectiveness of involving community members in routine surveillance, larviciding, and house improvements for vector control. Based on these studies, community engagement has the potential to play a significant role in successful implementation of vector control programs in other African countries.
In Tanzania, the focus of malaria vector control has been on ITNs and IRS (Kramer et al., 2017; Renggli et al., 2013; Mashauri et al., 2013; Kakilla et al., 2020). The 2014–2020 National Malaria Strategic Plan (Ministry of Health and Social Welfare, 2024) also recommended deploying larviciding in urban areas in line with the WHO guidelines (World Health Organization, 2019; World Health Organization, 2013), but this has recently been extended to rural areas (Mapua et al., 2021; MAELEZO TV, 2017; The United Republic of Tanzania Ministry of Health, C. D. G. E. and C, 2020). However, a recent study found limited community knowledge about larviciding and other challenges, including insufficient funding and technical expertise (Mapua et al., 2021), which may hinder the sustainability of the program in rural settings where malaria burden is higher. As in the urban settings, larviciding program in rural Tanzania involves the application of biolarvicides to all water bodies, which is a massive undertaking that severely constrains the program due to limited resources. The expansion of larviciding programs to rural communities in particular requires a deeper understanding of the ecology of the dominant malaria vector species, since the habitats may be more expansive and more numerous than as envisioned in the current WHO guidelines (World Health Organization, 2019; World Health Organization, 2013).
Previous entomological studies in rural south-eastern Tanzania, where An. arabiensis and An. funestus are the predominant malaria vectors, have shown that the latter species accounts for over 90 % of the ongoing malaria transmission (Kaindoa et al., 2017; Lwetoijera et al., 2014). An. funestus is also highly resistant to common public health pesticides targeting adult mosquitoes (Kaindoa et al., 2017; Pinda et al., 2020) thus requiring alternative control options (e.g. the use of the biocontrol agents such as, Bacillus thuringiensis israelensis (Bti) or Lysinibacillus sphaericus (Ls)). While preliminary results indicated that the aquatic habitats of immature An. funestus can be indeed few (i.e., only a small fraction of habitats are occupied by An. funestus), fixed (i.e., they tend to be permanent or semi-permanent) and findable (i.e., have unique characteristics allowing for ease of identification) (Nambunga et al., 2020). Expanded investigations (Msugupakulya et al., Unpublished data) suggest a more expansive range of habitats in different seasons, meaning that any effective control would require extensive engagement with locals to effectively search, characterize and target the habitats.
The aim of this study is to evaluate methods for involving community members in identifying, characterizing, and targeting the primary aquatic habitats of the dominant malaria vector species using biolarvicide, and to assess the impact of such an intervention on the density of the most relevant vector species in rural areas of Tanzania. This study focused on villages in the Kilombero valley, southeastern Tanzania, a meso-endemic community where An. funestus accounts for over 90 % of malaria transmission events.
2. Methods
2.1. Study area
The study was done in two villages, Igumbiro (8.35021°S, 36.67299°E) and Sululu (8.00324°S, 36.83118°E), both located on the Kilombero valley flood plain, south-eastern Tanzania (Fig. 1). Igumbiro is at a slightly higher altitude (340 m) above sea level than Sululu (315 m above sea level). Annual rainfall ranges from 1200 mm to 1400 mm, and the temperature ranges from 20 °C to 32 °C in these villages (Gebrekidan et al., 2020; Msofe et al., 2019). The majority of residents are subsistence rice farmers, but other crops such as sweet potatoes, beans, and maize are also produced (Gebrekidan et al., 2020).
Fig. 1.
Map showing two villages in the Kilombero Valley, south-eastern Tanzania where the study was conducted.
2.2. Longitudinal entomological surveillance of adult mosquitoes
Mosquitoes were routinely collected in the two villages during the same period from March 2020 to September 2021. Three different households were sampled nightly in each village for four nights in a week, with one additional household sampled once per week as a sentinel station. The collections were done using CDC light traps from 6 pm to 6 am for host-seeking mosquitoes (Mboera et al., 1998) and Prokopack aspirators from 6 am to 7 am for resting mosquitoes (Maia et al., 2011). Collected female mosquitoes were sorted by taxa and physiological state (Gillies and De Meillon, 1968). Recent studies in these settings had determined that the An. gambiae s.l. and An. funestus group consisted of An. arabiensis (100 %) and An. funestus s.s. (>93 %) respectively (Kaindoa et al., 2017; Ngowo et al., 2017; Ngowo et al., 2021), but also that majority of the transmission (>90 %) is driven by An. funestus s.s. (Kaindoa et al., 2017; Mapua et al., 2022a).
2.3. Recruitment and training of community volunteers
A total of 300 adult community members, 150 from each village, were randomly selected using residents' lists provided by village authorities. An interviewer-administered questionnaire, conducted in Swahili using KoboToolbox software (Harvard Humanitarian Initiative, 2024), assessed the community members' prior knowledge, awareness, and perception of disease-transmitting mosquitoes and larviciding. The questionnaires were administered following written informed consent. From these, ten community members from each village, meeting specific criteria, were selected for entomological training with an emphasis on equal gender distribution. The criteria were: i) ability to read and write properly, ii) involvement of the participant's household in the entomological surveillance, iii) residency in the village for at least two years, and iv) age between 18 and 50 years.
The training program for community volunteers was comprehensive, covering essential topics related to mosquito larvae identification, GPS usage, and the application of biolarvicides following WHO recommendations (World Health Organization, 2019; World Health Organization, 2013). It consisted of both theoretical and practical components. The theoretical aspect involved presentations and demonstrations (Fig. 2) on mosquito life stages, the biology of malaria vectors, and malaria transmission. The practical aspect included field visits, where volunteers engaged in hands-on training to practice identifying and characterizing mosquito larvae habitats, using GPS for mapping, and applying biolarvicides. Additionally, the practical sessions covered sampling techniques, estimating larval density, processing and storing immature mosquitoes, and recording data. (See Fig. 3.)
Fig. 2.
Theoretical and practical training of the community members for identification and characterization of aquatic habitats and application of larviciding.
Fig. 3.
Example of the common An. funestus larvae habitats found in Sululu and Igumbiro villages, south-eastern Tanzania.
The training spanned three full days, with each day divided into theoretical sessions in the morning and practical sessions in the afternoon. Over the course of three days, three simplified modules were covered: i) an introduction to the control and biology of malaria vectors, ii) the identification and characterization of aquatic habitats of malaria vectors in Ulanga and Kilombero districts, and iii) larval source management. Facilitators from the Ifakara Health Institute and the government-owned biolarvicide plant (Tanzania Biotech LTD) led the training, ensuring high-quality instruction and relevance to local contexts. District malaria focal persons participated as liaisons, enhancing the connection between the community, government, and the Ifakara Health Institute. Although a formal post-assessment of the training program to evaluate the knowledge and skills acquired by community members was not conducted due to budget and timeline constraints, participants' skills were continuously assessed through hands-on activities during the practical sessions.
2.4. Identification and characterization of the aquatic habitats of the dominant malaria vectors, Anopheles funestus
The trained community members were deployed in their respective villages, with 2 km transects allocated to each participation for the identification and characterization of water bodies. Based on our previous survey (Nambunga et al., 2020), the focus was on types of habitats shown to be favored by An. funestus mosquitoes. For each aquatic habitat, a 350 ml larval dipper was used to check for the absence or presence of An. funestus larvae based on WHO larval survey guidelines (World Health Organization, 2013). Larvae counts and mosquito species identification were recorded per dipper sample in a square meter habitat. Each dipper was treated as an individual observation. Habitat types were defined, and environmental features were recorded. Physicochemical parameters (temperature (°C), total dissolved solid (ppm), acidity (pH), electrical conductivity (μS/cm) and dissolved oxygen (ppm)) were also measured, and GPS coordinates were recorded for each habitat.
2.5. Application and monitoring of the efficacy of Bacillus thuringiensis israelensis (Bti)
Following the habitat characterization and larval surveys, the trained community members applied Bti (i.e., 10 ml in liquid formulation for every square meter) to all aquatic habitats within the 2 km transect that has been identified as including An. funestus larvae. Our study employed Bti serotype H-14, a product registered with both the Central Pesticide Registration of Cuba and the Tropical Research Institute of Tanzania. This serotype is manufactured under license by the LABIOFAM Enterprise Group and sourced from Tanzania Biotech LTD, a local manufacturer. It is commercially available under the name BACTIVEC® (ITU 1200/mg). Application of the larvicide in both villages was done using the IK Vector Control Super Pressurized sprayers. This was followed by larval monitoring surveys after 24 h, 7, 23 and 30 days. Data from the larval surveys conducted during aquatic habitat characterization and after application were considered to enable pre and post intervention assessment.
2.6. Data analysis
The open-source software, R programming language version 3.6.0 (R Development Core Team, 2019) was used for the statistical analyses. Linear mixed effect models were used to assess the association between number of female adult mosquitoes collected per night per house, and status of the larviciding intervention (i.e., pre-larviciding and post-larviciding) in each village. In addition, households were used as a random effect. The linear mixed effect model was fitted using lmer function found within lme4 package, whilst the variance estimator was set to Restricted Maximum Likelihood (REML) (Bates et al., 2014). Similar modelling was performed to determine association between number of larvae per habitat per village and status of larviciding, with the community member names as the random effect.
The questionnaire survey data was retrieved from KoboToolbox, cleaned, and coded for easier statistical analyses in R programming language software. The data was used to determine percentage of the community members that had knowledge and awareness of larviciding intervention, but also their perceptions towards larviciding. The Generalized Linear Model (GLM) was also used to investigate the association between community members perception towards larviciding intervention and their socio-demographic characteristics such as gender, age and literacy status.
GLMMs were used to determine the relationship between presence or absence of mosquito larvae and environmental characteristics. Highly correlated independent variables were removed, and the full model was fitted using a glmer function with binomial distribution and logit link function. Model selection was performed by removing insignificant terms. Final models were validated by assessing average residuals against expected values using binned residual plots. The same approach was applied to reveal the association between physicochemical attributes and occurrence of An. funestus larvae and other mosquitoes.
3. Results
3.1. Knowledge, awareness and perception of community members towards larviciding
A total of 300 community members (46 % women and 54 % men) participated in the knowledge assessment on larviciding. Of these, only 39 % were aware of larviciding as a malaria control strategy, a majority of these being men (Table 3). While age of participants did not influence their knowledge, those with secondary education were more than five times more knowledgeable than those without formal education (p < 0.001). Most of the participants had become aware of larviciding from family members or friends (Table 1). A large proportion (86 %) of the participants reported that they had never observed larviciding being implemented in their respective villages. While 8–20 % of the participants believed that larvicides were potentially harmful to other organisms (insects, large animals, and fish), more than 80 % agreed that larviciding could be necessary against disease-transmitting mosquitoes. Indeed, 71 % were willing to participate in the implementation of larviciding programs in their communities (Table 2).
Table 3.
Association between socio-demographic characteristics and community perception towards larviciding.
| Category | Variable | Odds ratio (95 % CI) | P-value |
|---|---|---|---|
| Sex | Female | 1 | – |
| Male | 1.84 (1.13–2.99) | 0.01 | |
| Age category (in years) | 18–25 | 1 | – |
| 26–35 | 0.87 (0.46–1.68) | 0.69 | |
| 36–45 | 0.66 (0.32–1.37) | 0.26 | |
| 46–50 | 0.50 (0.15–1.59) | 0.24 | |
| Above 50 | 0.63 (0.32–1.21) | 0.17 | |
| Education level | No formal education | 1 | – |
| Primary | 1.51 (0.85–2.68) | 0.16 | |
| Secondary | 5.40 (2.20–13.24) | <0.001 |
Table 1.
Knowledge and awareness of larviciding in the communities (N = 300).
| Variable | Response | Percentage (n) |
|---|---|---|
| Awareness of larviciding (N = 300) | Yes | 39 (117) |
| No | 61 (183) | |
| Source of information (N = 117) | Family/Friends | 65 (76) |
| Radio/Television | 14.5 (17) | |
| Village meeting | 5.1 (6) | |
| Village health officer | 6 (7) | |
| Village agricultural officer | 0 (0) | |
| Malaria focal person | 6 (7) | |
| Other sources | 13.7 (16) | |
| Do not remember | 1 (1) | |
| Have you ever witnessed larviciding implemented in the community (N = 300) | Yes | 8 (24) |
| No | 86 (258) | |
| Do not remember | 6 (18) | |
| Where are these larvicides manufactured (N = 300) | Domestic | 6.7 (20) |
| Abroad | 6.7 (20) | |
| Both | 1.3 (4) | |
| Do not know | 85.3 (256) | |
| Which is the first stage during larvicides application (N = 300) | Identification of aquatic habitats | 29 (87) |
| Community sensitization | 28.3 (85) | |
| Estimation of larvicide quantity | 1.7 (5) | |
| Spraying larvicides | 3 (9) | |
| Other | 3.3 (10) | |
| Do not know | 34.7 (104) |
Table 2.
Perception of community members towards larviciding for the malaria prevention (N = 300).
| Variable | Response | Percentage (n) |
|---|---|---|
| Have you ever participated in larviciding (N = 300) | Yes | 1.3 (4) |
| No | 98.7 (296) | |
| Do you think larvicides are harmful to insect (N = 300) | Yes | 20 (60) |
| No | 31.3 (94) | |
| Do not know | 48.7 (146) | |
| Do you think larvicides are harmful to fish (N = 300) | Yes | 8 (24) |
| No | 39.3 (118) | |
| Do not know | 52.7 (158) | |
| Do you think larvicides are harmful to animal (N = 300) | Yes | 12.3 (37) |
| No | 40.7 (122) | |
| Do not know | 47 (141) | |
| Willingness to participate in larviciding (N = 300) | Yes | 71 (213) |
| No | 29 (87) | |
| Acceptance of larviciding (N = 300) | Agree | 82.3 (247) |
| Do not agree | 4.7 (14) | |
| Neutral | 13 (39) |
3.2. Habitat characteristics and mosquito species in different aquatic habitats
A total of 360 aquatic habitats were surveyed, including 167 (46.4 %) in Sululu and 193 (53.6 %) in Igumbiro village (Table 4). Larvae of the dominant malaria vector, An. funestus, exhibited a preference for naturally occurring or man-made wells that receive spring-fed water, river streams, and puddles, rather than swamps (Table 5). Culex spp. and An. arabiensis larvae displayed comparable preferences, except for the latter, which did not favor man-made wells. Furthermore, An. funestus larvae were more commonly found in water bodies with slow (p < 0.001) flow rate compared to other mosquito species (Table 5).
Table 4.
Characteristics of aquatic habitats of Anopheles funestus and other mosquito species.
| Parameter | No. habitats observed (%) | Habitats without larvae | Habitats with An. funestus larvae (%) | Habitats with other Anopheles larvae n(%) | Habitats with Culicine sp. larvae n(%) | |
|---|---|---|---|---|---|---|
| Water movement | Stagnant | 370 (49.7) | 7 (1.9) | 167 (45.1) | 110 (29.7) | 123 (33.2) |
| Slow | 354 (47.6) | 11 (3.1) | 164 (46.3) | 90 (25.4) | 147 (41.5) | |
| Fast | 20 (2.7) | 1 (5) | 11 (55) | 1 (5) | 7 (35) | |
| Water type | Permanent | 525 (70) | 10 (1.9) | 279 (53.1) | 150 (28.6) | 230 (43.8) |
| Temporary | 219 (29.4) | 9 (4.1) | 63 (28.8) | 51 (23.3) | 47 (21.5) | |
| Water colour | Clear | 520 (69.9) | 16 (3.1) | 224 (43.1) | 136 (26.2) | 196 (37.7) |
| Coloured | 161 (21.6) | 3 (1.8) | 89 (55.3) | 46 (28.6) | 57 (35.4) | |
| Polluted | 63 (8.5) | 0 | 29 (46) | 19 (30.2) | 24 (38.1) | |
| Habitat type | Swamp | 168 (24.6) | 2 (1.2) | 97 (57.7) | 41 (24.4) | 76 (45.2) |
| Stream | 390 (56.2) | 12 (3.1) | 185 (47.4) | 91 (23.3) | 157 (40.3) | |
| Rice field | 10 (1.6) | 0 | 4 (40) | 4 (40) | 7 (70) | |
| Natural well | 43 (3.7) | 5 (11.6) | 16 (37.2) | 10 (23.3) | 17 (39.5) | |
| Man-made well | 32 (2.3) | 0 | 7 (21.8) | 7 (21.8) | 3 (9.4) | |
| Puddle | 101 (11.6) | 0 | 33 (32.7) | 48 (47.5) | 17 (16.8) | |
| Shade over habitat | None | 425 (56.2) | 10 (2.4) | 196 (46.1) | 127 (29.9) | 145 (34.1) |
| Partial | 236 (31.9) | 9 (3.8) | 102 (43.2) | 55 (23.3) | 95 (40.3) | |
| Heavy | 83 (11.8) | 0 | 44 (53) | 19 (22.9) | 37 (44.6) | |
| Vegetation quantity | None | 257 (34.5) | 12 (4.7) | 114 (44.4) | 57 (22.2) | 96 (37.4) |
| Scarce | 68 (9.1) | 0 | 34 (50) | 15 (22) | 27 (39.7) | |
| Moderate | 217 (29.2) | 6 (2.7) | 103 (47.5) | 57 (26.3) | 78 (35.9) | |
| Abundant | 202 (27.2) | 1 (0.5) | 91 (45.1) | 72 (35.6) | 76 (37.6) | |
| Vegetation type | None | 121 (16.3) | 0 | 74 (61.2) | 33 (27.3) | 69 (57) |
| Submerged | 46 (6.2) | 0 | 23 (50) | 25 (54.3) | 8 (17.4) | |
| Floating | 99 (13.3) | 0 | 70 (70.7) | 34 (34.3) | 53 (53.5) | |
| Emergent | 478 (64.2) | 19 (4) | 175 (36.6) | 109 (22.8) | 147 (30.8) | |
| Algae quantity | None | 516 (69.4) | 17 (3.3) | 197 (26.5) | 126 (16.9) | 171 (23) |
| Scarce | 48 (6.5) | 0 | 34 (70.8) | 20 (41.7) | 17 (35.4) | |
| Moderate | 129 (17.3) | 0 | 85 (65.9) | 40 (31) | 66 (51.2) | |
| Abundant | 51 (6.9) | 2 (3.9) | 26 (51) | 15 (29.4) | 23 (45.1) | |
| Habitat depth | Less than 10 cm | 361 (48.5) | 15 (4.2) | 196 (54.3) | 72 (19.9) | 178 (49.3) |
| Between 10 and 50 cm | 355 (47.7) | 4 (1.1) | 138 (38.9) | 117 (33) | 96 (27) | |
| Greater than 50 cm | 28 (3.8) | 0 | 8 (28.6) | 12 (42.9) | 3 (10.7) | |
No. habitats observed represents total number of observations per parameter.
Rice field represents habitats specifically found within non-irrigational rice fields.
Table 5.
Results of multivariate regression analysis of habitat characteristics and mosquito larvae.
| Parameter |
Anopheles arabiensis |
Anopheles funestus |
Culex spp. |
||||
|---|---|---|---|---|---|---|---|
| OR (95 % CIs) | p-value | OR (95 % CIs) | p-value | OR (95 % CIs) | p-value | ||
| Water movement | Stagnant | 1 | – | 1 | – | 1 | – |
| Slow | 2.21 (0.60–8.17) | 0.23 | 37.83 (6.99–204.81) | <0.001 | 0.59 (0.07–5.31) | 0.64 | |
| Fast | 0.77 (0.12–4.99) | 0.79 | 21.25 (2.82–160.02) | <0.05 | 0.61 (0.05–6.89) | 0.69 | |
| Water type | Temporary | 1 | – | 1 | – | 1 | – |
| Permanent | 0.14 (0.01–1.87) | 0.14 | 1.04 (0.14–7.91) | 0.97 | 0.21 (0.01–3.73) | 0.29 | |
| Water colour | Clear | 1 | – | 1 | – | 1 | – |
| Coloured | 1.44 (0.72–2.91) | 0.30 | 1.97 (0.97–4.01) | 0.06 | 0.88 (0.44–1.76) | 0.72 | |
| Polluted | 0.71 (0.29–1.76) | 0.46 | 1.78 (0.73–4.32) | 0.20 | 1.20 (0.51–2.84) | 0.68 | |
| Habitat type | Swamp | 1 | – | 1 | – | 1 | – |
| Stream | 0.35 (0.14–0.83) | 0.02 | 6.89 (3.33–14.26) | <0.001 | 0.10 (0.04–0.27) | <0.001 | |
| Rice field | 1.17 (0.22–6.32) | 0.85 | 9.49 (1.71–52.52) | <0.05 | 0.08 (0.01–0.76) | 0.03 | |
| Natural well | 0.10 (0.03–0.36) | <0.001 | 4.26 (1.47–12.38) | <0.05 | 0.10 (0.03–0.38) | <0.001 | |
| Man-made well | 0.40 (0.11–1.44) | 0.16 | 9.66 (3–31.04) | <0.001 | 0.09 (0.02–0.37) | <0.001 | |
| Puddle | 0.32 (0.11–0.91) | 0.03 | 10.26 (4.02–26.16) | <0.001 | 0.07 (0.02–0.23) | <0.001 | |
| Shade over habitat | None | 1 | – | 1 | – | 1 | – |
| Partial | 0.62 (0.19–2.02) | 0.43 | 1.72 (0.54–5.44) | 0.36 | 1.01 (0.37–2.74) | 0.98 | |
| Heavy | 0.48 (0.13–1.75) | 0.27 | 1.78 (0.52–6.16) | 0.36 | 0.57 (0.19–1.73) | 0.32 | |
| Vegetation type | None | 1 | – | 1 | – | 1 | – |
| Floating | 2.65 (1.31–5.38) | <0.05 | 0.52 (0.24–1.11) | 0.09 | 0.44 (0.21–0.89) | 0.02 | |
| Submerged | 2.09 (0.95–4.61) | 0.07 | 1.19 (0.53–2.69) | 0.68 | 0.28 (0.11–0.75) | 0.01 | |
| Emergent | 0.07 (0–1.09) | 0.06 | 0.17 (0.02–1.50) | 0.11 | 0.03 (0–0.68) | 0.03 | |
| Vegetation quantity | None | 1 | – | 1 | – | 1 | – |
| Scarce | 0.27 (0.02–3.70) | 0.33 | 0.35 (0.04–3) | 0.34 | 0.08 (0–1.49) | 0.09 | |
| Moderate | 0.17 (0.01–2.14) | 0.17 | 0.49 (0.61–3.96) | 0.50 | 0.11 (0–1.92) | 0.13 | |
| Abundant | 0.14 (0.01–1.84) | 0.14 | 0.31 (0.04–2.47) | 0.27 | 0.07 (0–1.16) | 0.06 | |
| Algae quantity | None | 1 | – | 1 | – | 1 | – |
| Scarce | 0.29 (0.06–1.32) | 0.11 | 1.85 (0.56–6.10) | 0.32 | 0.15 (0.04–0.60) | <0.05 | |
| Moderate | 0.76 (0.21–2.71) | 0.67 | 2.32 (0.79–6.83) | 0.13 | 0.18 (0.05–0.63) | <0.05 | |
| Abundant | 0.82 (0.21–3.23) | 0.78 | 21.81 (0.56–5.89) | 0.32 | 0.39 (0.10–1.497) | 0.17 | |
| Habitat depth | Less than 10 cm | 1 | – | 1 | – | 1 | – |
| Between 10 and 50 cm | 2.87 (0.69–11.92) | 0.15 | 116.34 (18.22–743.05) | <0.001 | 0.24 (0.03–2.24) | 0.21 | |
| Greater than 50 cm | 1.02 (0.16–6.57) | 0.98 | 138.11 (18.48–1031.95) | <0.001 | 0.12 (0.01–1.45) | 0.10 | |
The presence of An. funestus larvae was found to be independent of vegetation type or quantity. However, An. arabiensis showed a preference for habitats with floating vegetation (Table 5). Furthermore, habitats with vegetation were less likely to host Culex spp. larvae. Moreover, according to the modelling results, there was a positive correlation between the depth of aquatic habitats and the likelihood of encountering An. funestus larvae. Culex spp. larvae were negatively associated with the habitats having scarce or moderate algae quantity, but the same was not observed for other mosquito species (Table 5). The occurrence of mosquitoes was unaffected by the shading over the habitat, water clarity (i.e., clear, coloured, or polluted), or habitat permanency (Table 5).
Acidity (pH), temperature (°C), electrical conductivity (μS/cm), total dissolved solids (ppm) and dissolved oxygen (ppm) were measured in a total of 178 aquatic habitats in Igumbiro village. Temperature, pH, total dissolved solids, and electrical conductivity were found to have an impact on the occurrence of Culex spp. larvae. In contrast, only electrical conductivity affected the presence of An. arabiensis, while pH specifically influenced the presence of An. funestus (Table 6).
Table 6.
Results of multivariate regression analysis of different physicochemical characteristics and their association with occurrence of mosquito larvae.
| Physicochemical characteristic |
Anopheles arabiensis |
Anopheles funestus |
Culex spp. |
|||
|---|---|---|---|---|---|---|
| OR (95 % CIs) | p-value | OR (95 % CIs) | p-value | OR (95 % CIs) | p-value | |
| pH | 0.89 (0.32–2.43) | 0.25 | 10.01 (1.11–90.32) | 0.04 | 0.22 (0.06–0.83) | <0.001 |
| Temperature (°C) | 0.80 (0.62–1.02) | 0.08 | 0.74 (0.35–1.54) | 0.42 | 3.08 (1.82–5.23) | 0.03 |
| Electrical conductivity (μS/cm) | 1.02 (1–1.04) | 0.02 | 0.98 (0.94–1.02) | 0.24 | 1.13 (1.04–1.23) | <0.001 |
| TDS (ppm) | 1 (0.98–1.02) | 0.94 | 1.01 (0.96–1.06) | 0.67 | 0.80 (0.66–0.97) | <0.05 |
| DO (ppm) | 0.96 (0.86–1.08) | 0.53 | 0.83 (0.41–1.68) | 0.60 | 1 (0.75–1.34) | 1 |
3.3. Effects of larviciding on the abundance of adult female mosquitoes and larvae
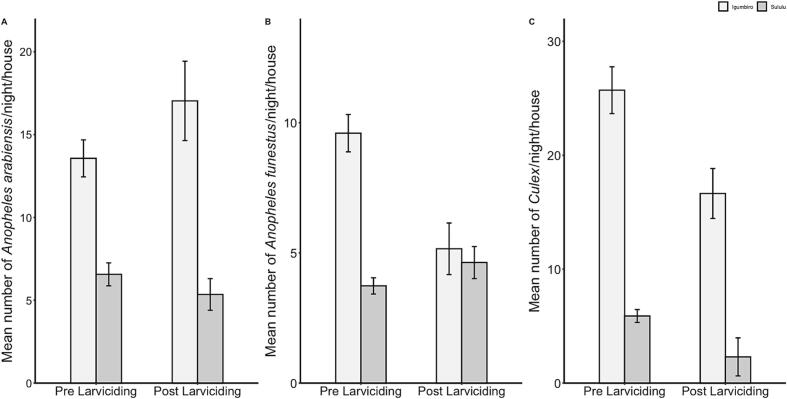
In Igumbiro, there was a significant reduction of indoor densities for An. funestus (p < 0.001) and Culex spp. (p < 0.001) following larviciding. However, the densities of An. arabiensis significantly increased after larviciding (p = 0.005). In Sululu, no significant change was observed in the densities of An. arabiensis (p = 0.268) or An. funestus (p = 0.119), but there was a significant decline in densities of Culex spp. (p = 0.035) (Fig. 4).
Fig. 4.
Effect of a single application of biolarvicides on abundance of adult An. funestus, An. arabiensis and Culex spp. inside houses in Sululu and Igumbiro villages, south-eastern Tanzania.
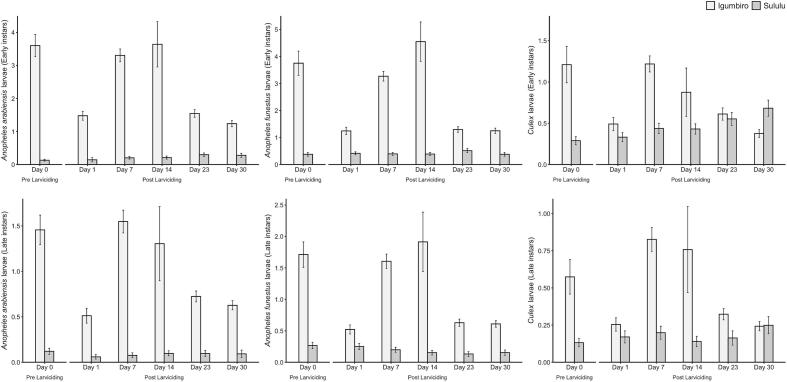
For early instars larvae densities (Fig. 5), there was a significant reduction in the Igumbiro village for An. arabiensis (p < 0.001), An. funestus (p < 0.001) and Culex spp. (p < 0.001) but the densities remained statistically the same for An. arabiensis (p = 0.404) and An. funestus larvae (p = 0.651) in the Sululu village. The densities of Culex spp. at early instars were significantly increased after larviciding intervention (p = 0.001) in Sululu village (Fig. 5). At late instars, the densities of An. arabiensis (p < 0.001), An. funestus (p < 0.001) and Culex spp. (p < 0.001) were significantly reduced in Igumbiro village but not in Sululu, where only the An. funestus densities were marginally reduced (p = 0.045) after larviciding (Fig. 5).
Fig. 5.
Densities of early- and late-instar larvae of An. funestus, An. arabiensis and Culex spp. before and after a single application of biolarvicides in Sululu and Igumbiro villages, south-eastern Tanzania. A previous study (Matowo et al., 2019) identified that Culex spp. mainly consisted of Cx. pipiens pipiens and Cx. quinquefasciatus.
4. Discussion
Effective engagement of community members can ensure sustainability of public health programmes (Holder et al., 2000; Das et al., 2014), in part by creating a sense of ownership and responsibility (Ingabire et al., 2017; Gubler and Clark, 1996). A recent study (Mapua et al., 2021) emphasized the importance of such engagement to improve the larviciding programs in rural Tanzania, following recent expansion of the practice beyond urban settings (The United Republic of Tanzania Ministry of Health, C. D. G. E. and C, 2020). This can be achieved through a combination of community sensitization meetings and targeted training of community-based persons, as demonstrated in urban Dar es Salaam, where community-owned resource persons previously enabled scale-up of larval source management (Maheu-Giroux and Castro, 2013; Geissbühler et al., 2009).
In rural Africa, where mosquito habitats can be more diverse and numerous, and where the existing infrastructure may be inadequate for traditional LSM approaches, a detailed understanding of the ecology of dominant malaria vectors is also necessary to optimize resource use. For example, in the Kilombero valley, Tanzania, where just one of the many vector species, An. funestus, now accounts for nine in every ten malaria infections (Kaindoa et al., 2017; Mapua et al., 2022b), it may be most appropriate to preferentially target habitats for this vector species. Previous studies in the area have provided a baseline for such investigations by mapping the primary characteristics of the vector species habitats (Nambunga et al., 2020) and also by highlighting the importance of such knowledge for practical LSM practices (Mapua et al., 2021). This study tested the potential of training community members to identify, characterize and target larval habitats of dominant malaria vectors in rural south-eastern Tanzania. Biolarvicides were applied to aquatic habitats of An. funestus mosquitoes in the two villages, and the immediate impact assessed by estimating the larval densities and also adult densities of the mosquitoes in people's dwellings.
The current larviciding program in mainland Tanzania is implemented by community health workers (i.e., secondary school graduates with a year of community health training) under the supervision of ward health officers. Limited biolarvicide supply and inadequate funding put severe constraints on implementation of the program, however. The present study has demonstrated the effectiveness of involving lay members of the community in identifying and characterizing aquatic habitats of the most competent malaria vector species and applying the same biolarvicides as provided by the government. Involving community members in larviciding is not uncommon in Tanzania (Maheu-Giroux and Castro, 2013; Geissbühler et al., 2009), but this study demonstrates the role they can play in a species-focused larviciding approach. The training of community members was effective, and they identified habitats and environmental characteristics associated with the occurrence of An. funestus larvae. The aquatic habitats identified by the trained community members had similar characteristics as those previously identified by expert vector biologists (Nambunga et al., 2020). The number of An. funestus mosquitoes inside the houses in the villages was significantly reduced after application of biolarvicides to the aquatic habitats identified by community members. The study suggests that relying on the community to sustainably implement the government-led larviciding program is possible, but recurring training would be better to maximize the program's impact. Additionally, Table 1, Table 2 present the initial awareness and perception of community members, which served as a basis for developing the training modules. Although the subset of community members that underwent training exhibited improved awareness and perception during and after the intervention, we did not seek to quantify this improvement due to the project's tight timeline. The ability of trained community members to carry out habitat identification and larviciding activities indicates that capacity for larval control can be enhanced through a set of simplified yet engaging training sessions.
However, it is important to note that the findings from this study may not be directly generalizable to other rural settings or different ecological zones. The study was conducted in two specific villages within the Kilombero Valley, which has unique environmental and ecological characteristics. Factors such as mosquito species composition, habitat types, and local community practices can vary significantly in different regions. Therefore, while the training program and larviciding approach demonstrated effectiveness in this context, further studies are necessary to evaluate the applicability and efficacy of similar interventions in other rural areas with different ecological conditions.
In the two villages investigated, more than 300 aquatic habitats were surveyed, and it was found that An. funestus larvae had a preference for habitats with slow or fast-moving waters, such as streams. While this has been previously been linked to the higher levels of dissolved oxygen and aeration in such waters (Nambunga et al., 2020; Tchigossou et al., 2017), this current study did not find any significant associations between dissolved oxygen levels and the presence of An. funestus. Similarly, while previous studies have reported the preference of An. funestus for vegetated habitats (Gillies and De Meillon, 1968; Gimnig et al., 2001; Cohuet et al., 2003), no such association was observed here. Except for pH, the physicochemical characteristics of aquatic habitats did not appear to have a significant association with the occurrence of An. funestus larvae, which was also observed in other mosquito species, particularly Culex spp. larvae. These differences may, in part, be due to the limited geographical extent of the current study, which covered only two villages. It was also observed that the main aquatic habitats of An. funestus, including streams and wells, were in close proximity to agricultural activities, suggesting they may be constantly exposed to agricultural pesticide wastes as previously observed in the area (Matowo et al., 2020), potentially exacerbating the challenge of insecticide resistance. Fortunately, the current larviciding programs in Tanzania deploy biolarvicides (i.e., Bacillus thuringiensis israelensis and Lysinibacillus sphaericus), which remain effective against pyrethroid-resistant malaria vectors.
While the approach tested here was clearly effective, we did not investigate the optimal timing of the larviciding. Instead, the single application was done in the dry season, when the habitats were least numerous and least expansive. Also, a recent mathematical simulation (Runge et al., 2021) suggested that the most effective timing for larviciding is during or at the beginning of the rainy season but that was on assumption that the main vector species would be An. gambiae s.s., which breed in temporary pools and whose populations peak during the wet season. It however remains unclear what the optimal timing for larviciding would be in areas dominated by An. funestus, which tends to occupy perennial habitats and therefore remains important throughout the year. The results of this study may be useful for future modelling exercises to assess such scenarios. Moreover, a distinct trend of aquatic habitat recolonization was observed within one to two weeks after treatment, followed by a notable reduction. This pattern suggests the residual effectiveness of Bti. Furthermore, it's important to keep in mind that our results are based on a single larvicide application, which served primarily to showcase the capability of trained community members in targeting disease-transmitting mosquitoes. This also highlights the success of a simpler yet effective training approach.
Tanzania has had great examples of cross-sectoral engagement for malaria prevention. In addition to the supply of locally manufactured biolarvicides from the Tanzania biotechnology industry, there have also been major investments in community sensitization and engagement. For example, the Dar es Salaam Urban Malaria Control Programme (UMCP) organized a highly effective community-based program of environmental management to reduce densities of malaria vectors, which even included a degree of fundraising by the communities (De Castro et al., 2004). More recently, the use of trained community owned resource persons to deploy the larvicides and also monitor adult densities resulted in significant declines of malaria in the city (Maheu-Giroux and Castro, 2013; Chaki et al., 2012; Geissbühler et al., 2009). This current study demonstrate that such community-based strategies can be expanded to rural settings such as the Kilombero Valley. This could significantly reduce implementation costs, especially as community members are generally willing to participate. We did not explicitly investigate the community's willingness to participate without compensation in the current study. Nevertheless, previous studies have indicated that such willingness is possible when there is a higher perceived level of safety and acceptance of the product (Hakizimana et al., 2022; Dambach et al., 2018). Already, the district-level malaria officials have been conducting community sensitization programs to support larval source management (Mapua et al., 2021). However, it is evident that more efforts are needed given that significant proportions of the rural community members (∼60 % in this study), remain unaware of the importance of larviciding for malaria control.
Another important observation was that while An. funestus clearly prefer certain habitat types, they do often cohabit with other mosquito species. In this study the application of the biolarvicides in the habitats of An. funestus also reduced indoor densities of adult Culex spp. but not An. arabiensis. Also, though An. arabiensis preferred irrigational rice fields, their larva densities were also reduced in habitats that they shared with An. funestus. It can be assumed therefore that the current strategy of applying larvicides to all aquatic habitats could be effective as well, especially if there is adequate resources and manpower. However, the findings also indicate that it might be more cost-effective to preferentially target the most competent malaria vector species.
This study also had some limitations. Firstly, the use of a 2 km transects underestimated the potential effectiveness of larviciding because mosquitoes can fly much longer, sometime up to or beyond 4 km (Gillies, 1961). This means that mosquitoes could have emerged from habitats beyond the transect, potentially reducing the larviciding efficacy. Additionally, the study did not gather post-assessment feedback from community members who participated in the program, primarily due to constraints related to the project timeline and available resources. Their views and insights could have provided valuable information on the sustainability of the approach and identified areas for improvement. Furthermore, the study focused solely on assessing the impact of larviciding on the abundance of the major malaria vector, without evaluating its effect on malaria prevalence in the villages. A recent simulation study (Runge et al., 2021) suggests a reduction in malaria prevalence one month after larvicide application. However, due to time limitations, the larvicides were only applied once in this study. While this may have been sufficient for demonstrating the role of community members, it is itself inadequate for assessing efficacy of the intervention on its own. Lastly, the application of biolarvicides was limited to larval habitats that tested positive for An. funestus during the survey, potentially reducing coverage by not treating habitats that could have harbored An. funestus larvae but tested negative at the time of the survey.
In addition to these limitations, potential biases must be considered. Selection bias may have been introduced during the recruitment of volunteers for the entomological training. The criteria for selecting participants—such as the ability to read and write properly, involvement in household entomological surveillance, a minimum residency of two years, and an age range of 18 to 50 years may have excluded certain community members who could have contributed valuable perspectives. This selection process might have favored more educated or engaged individuals, potentially skewing the results. Additionally, response bias in the questionnaires cannot be ruled out. Since the questionnaires were administered by interviewers, there is a possibility that respondents provided socially desirable answers rather than their true perceptions and knowledge levels about disease-transmitting mosquitoes and larviciding. This could lead to an overestimation of the community's baseline awareness and perception. To mitigate these biases in future studies, a more inclusive selection process and the use of anonymous self-administered questionnaires could be considered.
5. Conclusion
Previous studies have shown the effectiveness of larviciding in urban areas of Tanzania, following WHO guidelines. However, the National Malaria Strategic Plan of Tanzania has extended larviciding to rural areas, despite not strictly adhering to these guidelines. This study highlights the potential of species-focused community-led larviciding as a sustainable intervention for malaria control in rural settings. The observed reduction in mosquito densities demonstrates that, with proper training and community engagement, local communities can successfully implement and maintain vector control strategies. These findings suggest that this approach can be effectively adapted to other resource-limited rural settings. Furthermore, by reducing reliance on centralized programs, this model promotes self-sufficiency and community ownership, which are critical for the long-term sustainability of malaria control efforts. The results have important implications for policy makers and public health officials, as community-led interventions could complement existing vector control strategies, thereby enhancing the overall impact of malaria control programs.
The following are the supplementary data related to this article.
Ethics approval and consent to participate
Ethical approvals for this project were obtained from Ifakara Health Institute's Institutional Review Board (Protocol ID: IHI/IRB/No: 29–2019) and the Medical Research Coordinating Committee (MRCC) at the National Institute for Medical Research, in Tanzania (Protocol ID: NIMR/HQ/R.8a/Vol.IX/3517). Written consents were sought from all participants of this study, after they had understood the purpose and procedure of the discussions.
Consent for publication
Permission to publish this study was obtained from National Institute for Medical Research, in Tanzania (No: NIMR/HQ/P. 12 VOL.XXXVI 37).
Funding
This study was supported by the Wellcome Trust International Masters Fellowship in Tropical Medicine and Hygiene (Grant No. 212633/Z/18/Z) awarded to SAM, Bill and Melinda Gates Foundation (Grant Number: OPP1177156) awarded to FOO. All grants were held at Ifakara Health Institute.
Author contributions
SAM, JL, FT and FOO were involved in study design. SAM, AJL, IHN and KK were involved in data collection. SAM conducted data analysis. DK contributed in designing and validating the model selection part of the data analysis. SAM and AJL wrote the manuscript. KU, SM and GJ facilitated training of the community members and vector surveillance officers, and collaboration with district's malaria focal persons. FOO, JL, FT, DK, KU, GJ, WM, IHN and SM provided thorough review of the manuscript. All authors read and approved the final manuscript.
CRediT authorship contribution statement
Salum A. Mapua: Writing – review & editing, Writing – original draft, Project administration, Methodology, Investigation, Funding acquisition, Formal analysis, Data curation, Conceptualization. Alex J. Limwagu: Writing – original draft, Project administration, Methodology, Investigation, Data curation. Dmitry Kishkinev: Validation, Formal analysis. Khamis Kifungo: Project administration, Investigation. Ismail H. Nambunga: Methodology, Investigation. Samuel Mziray: Project administration, Methodology. Gwakisa John: Project administration, Methodology. Wahida Mtiro: Project administration, Methodology. Kusirye Ukio: Project administration, Methodology. Javier Lezaun: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Methodology, Investigation, Funding acquisition, Formal analysis, Data curation, Conceptualization. Frederic Tripet: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Methodology, Investigation, Funding acquisition, Formal analysis, Data curation, Conceptualization. Fredros O. Okumu: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Resources, Methodology, Investigation, Funding acquisition, Formal analysis, Data curation, Conceptualization.
Declaration of competing interest
The authors declare no competing interests.
Acknowledgments
We would like to thank the vector surveillance officers and community members of Ulanga and Kilombero districts for their participation in this study. We would also like to extend our sincere gratitude to Ms. Anna Nyoni, Ms. Elihaika Minja, Dr. Marceline Finda, Ms. Noelia Pama, Ms. Alice Ombella, Mr. Prosper Kobero, Mr. Gerald Tamayamali, Mr. Betwel Msugupakulya and Mr. Japhet Kihonda for their assistance on the execution of the project. We are also grateful to Mr. Augustino Mwambaluka and Ms. Rukia Njalambaha for their transport and administrative supports respectively. Our sincere gratitude goes to Mr. Nicholaus Banzi and Eng. Alejandro Gonzalez both from Tanzania Biotech Products Limited for facilitating training of the community members and vector surveillance officers. Also, we sincerely appreciate the initial inputs on the draft manuscript by Ms. Prisca Kweyamba, Drs. Holly Matthews and Florian Noulin.
Contributor Information
Salum A. Mapua, Email: smapua@ihi.or.tz.
Fredros O. Okumu, Email: fredros@ihi.or.tz.
Data availability
The data will be made available by the corresponding author upon reasonable request.
References
- Adhikari B., Pell C., Cheah P.Y. Community engagement and ethical global health research. Glob. Bioeth. 2020;31(1):1–12. doi: 10.1080/11287462.2020.1751880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asale A., Kussa D., Girma M., Mbogo C., Mutero C.M. Community based integrated vector management for malaria control: lessons from three years’ experience (2016–2018) in Botor-Tolay district, southwestern Ethiopia. BMC Public Health. 2019;19:1–14. doi: 10.1186/s12889-019-6674-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates D., Mächler M., Bolker B., Walker S. arXiv preprint; 2014. Fitting Linear Mixed-Effects Models Using lme4. arXiv:1406.5823. [Google Scholar]
- Bhatt S., Weiss D.J., Cameron E., Bisanzio D., Mappin B., Dalrymple U., et al. The effect of malaria control on Plasmodium falciparum in Africa between 2000 and 2015. Nature. 2015;526:207–211. doi: 10.1038/nature15535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaki P.P., Mlacha Y., Msellemu D., Muhili A., Malishee A.D., Mtema Z.J., Kiware S.S., et al. An affordable, quality-assured community-based system for high-resolution entomological surveillance of vector mosquitoes that reflects human malaria infection risk patterns. Malar. J. 2012;11:1–18. doi: 10.1186/1475-2875-11-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohuet A., Simard F., Toto J.C., Kengne P., Coetzee M., Fontenille D. Species identification within the Anopheles funestus group of malaria vectors in Cameroon and evidence for a new species. Am. J. Trop. Med. Hyg. 2003;69:200–205. doi: 10.4269/ajtmh.2003.69.200. [DOI] [PubMed] [Google Scholar]
- Dambach P., Mendes Jorge M., Traoré I., Phalkey R., Sawadogo H., Zabré P., et al. A qualitative study of community perception and acceptance of biological larviciding for malaria mosquito control in rural Burkina Faso. BMC Public Health. 2018;18:1–11. doi: 10.1186/s12889-018-5841-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dambach P., Baernighausen T., Traoré I., Ouedraogo S., Sié A., Sauerborn R., Becker N., Louis V.R. Reduction of malaria vector mosquitoes in a large-scale intervention trial in rural Burkina Faso using Bti based larval source management. Malar. J. 2019;18:1–9. doi: 10.1186/s12936-019-2882-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das J.K., Salam R.A., Arshad A., Maredia H., Bhutta Z.A. Community based interventions for the prevention and control of non-helminthic NTD. Infect. Dis. Poverty. 2014;3:1–12. doi: 10.1186/2049-9957-3-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Castro M.C., Yamagata Y., Mtasiwa D., Tanner M., Utzinger J., Keiser J., et al. The Intolerable Burden of Malaria II: What’s New, What’s Needed: Supplement to Volume 71 (2) of the American Journal of Tropical Medicine and Hygiene. American Society of Tropical Medicine and Hygiene; 2004. Integrated urban malaria control: a case study in Dar es Salaam, Tanzania. [DOI] [PubMed] [Google Scholar]
- Finda M.F., Moshi I.R., Monroe A., Limwagu A.J., Nyoni A.P., Swai J.K., et al. Linking human behaviours and malaria vector biting risk in South-Eastern Tanzania. PLoS One. 2019;14(6) doi: 10.1371/journal.pone.0217414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García G.A., Fuseini G., Mba Nlang J.A., Olo Nsue Maye V., Rivas Bela N., Wofford R.N., Weppelmann T.A., et al. Evaluation of a multi-season, community-based larval source management program on Bioko Island, Equatorial Guinea. Front. Trop. Dis. 2022;3 doi: 10.3389/fitd.2022.846955. [DOI] [Google Scholar]
- Gebrekidan B.H., Heckelei T., Rasch S. Characterizing farmers and farming system in Kilombero Valley floodplain, Tanzania. Sustainability. 2020;12(17):7114. doi: 10.3390/su12177114. [DOI] [Google Scholar]
- Geissbühler Y., Kannady K., Chaki P.P., Emidi B., Govella N.J., Mayagaya V., et al. Microbial larvicide application by a large-scale, community-based program reduces malaria infection prevalence in urban Dar es Salaam, Tanzania. PLoS One. 2009;4 doi: 10.1371/journal.pone.0005107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillies M.T. Studies on the dispersion and survival of Anopheles gambiae Giles in East Africa, by means of marking and release experiments. Bull. Entomol. Res. 1961;52:99–127. doi: 10.1017/S0007485300024797. [DOI] [Google Scholar]
- Gillies M.T., De Meillon B. 1968. The Anophelinae of Africa South of the Sahara (Ethiopian Zoogeographical Region) p. 343. [Google Scholar]
- Gimnig J.E., Ombok M., Kamau L., Hawley W.A. Characteristics of larval anopheline (Diptera: Culicidae) habitats in Western Kenya. J. Med. Entomol. 2001;38:282–288. doi: 10.1603/0022-2585-38.2.282. [DOI] [PubMed] [Google Scholar]
- Gubler D.J., Clark G.G. Community involvement in the control of Aedes aegypti. Acta Trop. 1996;61:169–179. doi: 10.1016/0001-706X(95)00124-F. [DOI] [PubMed] [Google Scholar]
- Hakizimana E., Ingabire C.M., Rulisa A., Kateera F., van den Borne B., Muvunyi C.M., van Vugt M., et al. Community-based control of malaria vectors using Bacillus thuringiensis var. Israelensis (Bti) in Rwanda. Int. J. Environ. Res. Public Health. 2022;19(11):6699. doi: 10.3390/ijerph19116699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock P.A., Hendriks C.J.M., Tangena J.A., Gibson H., Hemingway J., Coleman M., et al. Mapping trends in insecticide resistance phenotypes in African malaria vectors. PLoS Biol. 2020;18 doi: 10.1371/journal.pbio.3000633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvard Humanitarian Initiative KoBoToolbox. 2024. https://www.kobotoolbox.org Available from:
- Holder H.D., Gruenewald P.J., Ponicki W.R., Treno A.J., Grube J.W., Saltz R.F., Voas R.B., et al. Effect of community-based interventions on high-risk drinking and alcohol-related injuries. JAMA. 2000;284(18):2341–2347. doi: 10.1001/jama.284.18.2341. [DOI] [PubMed] [Google Scholar]
- Ingabire C.M., Hakizimana E., Rulisa A., Kateera F., Van Den Borne B., Muvunyi C.M., et al. Community-based biological control of malaria mosquitoes using Bacillus thuringiensis var. israelensis (Bti) in Rwanda: community awareness, acceptance and participation. Malar. J. 2017;16:1–13. doi: 10.1186/s12936-017-2046-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaindoa E.W., Matowo N.S., Ngowo H.S., Mkandawile G., Mmbando A., Finda M., Okumu F.O. Interventions that effectively target Anopheles funestus mosquitoes could significantly improve control of persistent malaria transmission in South–Eastern Tanzania. PLoS One. 2017;12(5) doi: 10.1371/journal.pone.0177807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakilla C., Manjurano A., Nelwin K., Martin J., Mashauri F., Kinung’hi S.M., Lyimo E., et al. Malaria vector species composition and entomological indices following indoor residual spraying in regions bordering Lake Victoria, Tanzania. Malar. J. 2020;19:1–14. doi: 10.1186/s12936-020-03547-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer K., Mandike R., Nathan R., Mohamed A., Lynch M., Brown N., Mnzava A., Rimisho W., Lengeler C. Effectiveness and equity of the Tanzania National Voucher Scheme for mosquito nets over 10 years of implementation. Malar. J. 2017;16:1–13. doi: 10.1186/s12936-017-2046-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lwetoijera D.W., Harris C., Kiware S.S., Dongus S., Devine G.J., McCall P.J., Majambere S. Increasing role of Anopheles funestus and Anopheles arabiensis in malaria transmission in the Kilombero Valley, Tanzania. Malar. J. 2014;13:1–10. doi: 10.1186/1475-2875-13-331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAELEZO TV Tanzania President Visit Biolarvicide Plant at Kibaha District. 2017. https://www.youtube.com/watch?v=4CzJcsxmptw Available from:
- Maheu-Giroux M., Castro M.C. Impact of community-based larviciding on the prevalence of malaria infection in Dar es Salaam, Tanzania. PLoS One. 2013;8(8) doi: 10.1371/journal.pone.0071638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maia M.F., Robinson A., John A., Mgando J., Simfukwe E., Moore S.J. Comparison of the CDC backpack aspirator and the Prokopack aspirator for sampling indoor-and outdoor-resting mosquitoes in southern Tanzania. Parasit. Vectors. 2011;4:1–10. doi: 10.1186/1756-3305-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mapua S.A., Finda M.F., Nambunga I.H., Msugupakulya B.J., Ukio K., Chaki P.P., et al. Addressing key gaps in implementation of mosquito larviciding to accelerate malaria vector control in southern Tanzania: results of a stakeholder engagement process in local district councils. Malar. J. 2021;20:1–14. doi: 10.1186/s12936-021-03769-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mapua S.A., Hape E.E., Kihonda J., Bwanary H., Kifungo K., Kilalangongono M., Kaindoa E.W., Ngowo H.S., Okumu F.O. Persistently high proportions of Plasmodium-infected Anopheles funestus mosquitoes in two villages in the Kilombero valley, South-Eastern Tanzania. Parasite Epidemiol. Control. 2022;18 doi: 10.1016/j.parepi.2022.e00264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mapua S.A., Hape E.E., Kihonda J., Bwanary H., Kifungo K., Kilalangongono M., et al. Persistently high proportions of Plasmodium-infected Anopheles funestus mosquitoes in two villages in the Kilombero valley, South-Eastern Tanzania. Parasite Epidemiol. Control. 2022;18 doi: 10.1016/j.parepi.2022.e00264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mashauri F.M., Kinung’hi S.M., Kaatano G.M., Magesa S.M., Kishamawe C., Mwanga J.R., Nnko S.E., Malima R.C., Mero C.N., Mboera L.E.G. Impact of indoor residual spraying of lambda-cyhalothrin on malaria prevalence and anemia in an epidemic-prone district of Muleba, North-Western Tanzania. Am. J. Trop. Med. Hyg. 2013;88:841. doi: 10.4269/ajtmh.12-0667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matowo N.S., Moore J., Mapua S., Madumla E.P., Moshi I.R., Kaindoa E.W., et al. Using a new odour-baited device to explore options for luring and killing outdoor-biting malaria vectors: a report on design and field evaluation of the mosquito landing box. Parasit. Vectors. 2013;6:82. doi: 10.1186/1756-3305-6-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matowo N.S., Abbasi S., Munhenga G., Tanner M., Mapua S.A., Oullo D., Koekemoer L.L., et al. Fine-scale spatial and temporal variations in insecticide resistance in Culex pipiens complex mosquitoes in rural South-Eastern Tanzania. Parasit. Vectors. 2019;12:1–13. doi: 10.1186/s13071-019-3676-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matowo N.S., Tanner M., Munhenga G., Mapua S.A., Finda M., Utzinger J., et al. Patterns of pesticide usage in agriculture in rural Tanzania call for integrating agricultural and public health practices in managing insecticide-resistance in malaria vectors. Malar. J. 2020;19:1–16. doi: 10.1186/s12936-020-03623-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mboera L.E., Kihonda J., Al Braks M., Knols B.G. Influence of centers for disease control light trap position, relative to a human-baited bed net, on catches of Anopheles gambiae and Culex quinquefasciatus in Tanzania. Am. J. Trop. Med. Hyg. 1998;59(4):595–596. doi: 10.4269/ajtmh.1998.59.595. [DOI] [PubMed] [Google Scholar]
- Ministry of Health and Social Welfare Tanzania National Malaria Strategic Plan 2014–2020. 2024. https://www.out.ac.tz/wp-content/uploads/2019/10/Malaria-Strategic-Plan-2015-2020-1.pdf Available from:
- Moiroux N., Gomez M.B., Pennetier C., Elanga E., Djènontin A., Chandre F., et al. Changes in Anopheles funestus biting behavior following universal coverage of long-lasting insecticidal nets in Benin. J. Infect. Dis. 2012;206:1622–1629. doi: 10.1093/infdis/jis565. [DOI] [PubMed] [Google Scholar]
- Monroe A., Asamoah O., Lam Y., Koenker H., Psychas P., Lynch M., et al. Outdoor-sleeping and other night-time activities in northern Ghana: implications for residual transmission and malaria prevention. Malar. J. 2015;14:343. doi: 10.1186/s12936-015-0543-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyes C.L., Athinya D.K., Seethaler T., Battle K.E., Sinka M.S., Hadi M.P., et al. Evaluating insecticide resistance across African districts to aid malaria control decisions. Proc. Natl. Acad. Sci. USA. 2020;117:22042–22050. doi: 10.1073/pnas.2002604117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Msofe N.K., Sheng L., Lyimo J. Land use change trends and their driving forces in the Kilombero Valley floodplain, Southeastern Tanzania. Sustainability. 2019;11(2):505. doi: 10.3390/su11020505. [DOI] [Google Scholar]
- Mutero C.M., Okoyo C., Girma M., Mwangangi J., Kibe L., Ng’ang’a P., Kussa D., Diiro G., Affognon H., Mbogo C.M. Evaluating the impact of larviciding with Bti and community education and mobilization as supplementary integrated vector management interventions for malaria control in Kenya and Ethiopia. Malar. J. 2020;19:1–17. doi: 10.1186/s12936-020-03429-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nambunga I.H., Ngowo H.S., Mapua S.A., Hape E.E., Msugupakulya B.J., Msaky D.S., Mhumbira N.T., et al. Aquatic habitats of the malaria vector Anopheles funestus in rural South-Eastern Tanzania. Malar. J. 2020;19:1–11. doi: 10.1186/s12936-020-03704-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngowo H.S., Kaindoa E.W., Matthiopoulos J., Ferguson H.M., Okumu F.O. Variations in household microclimate affect outdoor-biting behaviour of malaria vectors. Wellcome Open Res. 2017;2 doi: 10.12688/wellcomeopenres.12928.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngowo H.S., Hape E.E., Matthiopoulos J., Ferguson H.M., Okumu F.O. Fitness characteristics of the malaria vector Anopheles funestus during an attempted laboratory colonization. Malar. J. 2021;20:1–13. doi: 10.1186/s12936-021-03677-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okumu F., Gyapong M., Casamitjana N., Castro M.C., Itoe M.A., Okonofua F., et al. What Africa can do to accelerate and sustain progress against malaria. PLOS Glob. Public Health. 2022;2 doi: 10.1371/journal.pgph.0000262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phiri M.D., McCann R.S., Kabaghe A.N., van den Berg H., Malenga T., Gowelo S., Tizifa T., et al. Cost of community-led larval source management and house improvement for malaria control: a cost analysis within a cluster-randomized trial in a rural district in Malawi. Malar. J. 2021;20:1–17. doi: 10.1186/s12936-021-04041-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinda P.G., Eichenberger C., Ngowo H.S., Msaky D.S., Abbasi S., Kihonda J., Bwanaly H., Okumu F.O. Comparative assessment of insecticide resistance phenotypes in two major malaria vectors, Anopheles funestus and Anopheles arabiensis in South-Eastern Tanzania. Malar. J. 2020;19:1–11. doi: 10.1186/s12936-020-03705-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Development Core Team . Vol. 1. R Foundation for Statistical Computing; 2019. R: A Language and Environment for Statistical Computing; p. 409. Preprint at: [DOI] [Google Scholar]
- Renggli S., Mandike R., Kramer K., Patrick F., Brown N.J., McElroy P.D., Rimisho W., et al. Design, implementation and evaluation of a national campaign to deliver 18 million free long-lasting insecticidal nets to uncovered sleeping spaces in Tanzania. Malar. J. 2013;12:1–16. doi: 10.1186/1475-2875-12-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Runge M., Mapua S.A., Nambunga I., Smith T.A., Chitnis N., Okumu F.O., et al. Evaluation of different deployment strategies for larviciding to control malaria: a simulation study. Malar. J. 2021;20:1–14. doi: 10.1186/s12936-021-03916-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell T.L., Govella N.J., Azizi S., Drakeley C.J., Kachur S.P., Killeen G.F. Increased proportions of outdoor feeding among residual malaria vector populations following increased use of insecticide-treated nets in rural Tanzania. Malar. J. 2011;10:1–10. doi: 10.1186/1475-2875-10-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sougoufara S., Mocote Diédhiou S., Doucouré S., Diagne N., Sembène P.M., Harry M., et al. Biting by Anopheles funestus in broad daylight after use of long-lasting insecticidal nets: a new challenge to malaria elimination. Malar. J. 2014;13:125. doi: 10.1186/1475-2875-13-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tchigossou G., Akoton R., Yessoufou A., Djegbe I., Zeukeng F., Atoyebi S.M., et al. Water source most suitable for rearing a sensitive malaria vector, Anopheles funestus in the laboratory. Wellcome Open Res. 2017;2 doi: 10.12688/wellcomeopenres.12942.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The malERA Consultative Group on Health Systems A research agenda for malaria eradication: health systems and operational research. PLoS Med. 2011;8 doi: 10.1371/journal.pmed.1000397. Preprint at. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The United Republic of Tanzania Ministry of Health, C. D. G. E. and C . National Malaria Control Program; November 2020. National Malaria Strategic Plan 2021-2025: Transitioning to Malaria Elimination in Phases.http://api-hidl.afya.go.tz/uploads/library-documents/1641210939-jH9mKCtz.pdf Available from: [Google Scholar]
- Van den Berg H., van Vugt M., Kabaghe A.N., Nkalapa M., Kaotcha R., Truwah Z., Malenga T., et al. Community-based malaria control in southern Malawi: a description of experimental interventions of community workshops, house improvement and larval source management. Malar. J. 2018;17:1–12. doi: 10.1186/s12936-018-2585-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Seidlein L., Peto T.J., Landier J., Nguyen T.-N., Tripura R., Phommasone K., Pongvongsa T., et al. The impact of targeted malaria elimination with mass drug administrations on falciparum malaria in Southeast Asia: a cluster randomised trial. PLoS Med. 2019;16(2) doi: 10.1371/journal.pmed.1002745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO World Malaria Report 2023 [Internet] 2023. https://www.who.int/publications/i/item/9789240086173 Available from:
- World Health Organization Larval Source Management: A Supplementary Malaria Vector Control Measure. 2013. https://apps.who.int/iris/handle/10665/85379 Available from:
- World Health Organization . Guidelines for Malaria Vector Control. 2019. Guidelines for malaria vector control.https://www.who.int/publications/i/item/9789241550499 Available from: [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data will be made available by the corresponding author upon reasonable request.