Abstract
Background:
Cutaneous leishmaniasis (CL) infection is caused by the Leishmania major (L. major) parasite and affects 1.5 to 2 million people worldwide each year. Although research into vaccines and antiparasitic drugs has been somewhat successful, their adverse effects include high toxicity, prolonged regeneration, and scarring. This has highlighted the importance of research to replace natural products with antibacterial and antioxidant properties, such as vegetable extracts and oils. Since, the anti-leishmaniasis effect of each of the components of Shirvan herbal ointment (aloe vera, Brazembel, Nigella sativa, propolis, lavender, and olive oil) has been separately studied and confirmed, it seems that the combination of these components can have an increasing anti-leishmanial effect to treat CL. Therefore, this study investigated the therapeutic impact of Shirvan herbal ointment on Iranian patients with leishmaniasis in comparison with glucantime (meglumine).
Materials and Methods:
Sixty patients with leishmaniasis were divided into the control and test groups. The control and test groups received intralesional glucantime and Shirvan herbal cream (two times daily), respectively.
Results:
The size mean of the lesion was 51.5 ± 32.5 before and 11.11 ± 16.28 after treatment in the control group and 50.8 ± 31.2 before and 0.0 ± 0.0 after treatment in the test group. In addition, the period mean of treatment was 43.9 ± 14.4 days and 30.5 ± 7.4 days in the control and test groups, respectively. There was a significant difference in lesion size between the two groups after treatment.
Conclusion:
Data suggested that Shirvan herbal ointment can be an alternative drug in the treatment of human CL.
Keywords: Aloe vera, cutaneous leishmaniasis, lavender, Leishmania major, meglumine, Nigella sativa, olive oil, propolis
INTRODUCTION
A protozoan parasite (Leishmania) causes leishmaniasis infection that is transferred through the sting of a female sand fly of Phlebotomus species and includes cutaneous leishmaniasis (CL), visceral (kala-azar), and mucocutaneous types.[1] There are about 21 species of human pathogenic Leishmania that affect between 700000 and 1.2 million new cases worldwide annually.[2] In Iran, an incidence rate of 15.8 cases per 100000 population was reported in 2019.[3] CL as the most common form of leishmaniasis is caused by the Leishmania tropica (L. tropica) (dry type) and Leishmania major (L. major) (wet type). In vector mosquitoes, parasites live as promastigotes in the gastrointestinal tract and enter the wound during bites, and within the phagolysosome of the host, macrophages become amastigotes, due to the parasite hiding inside the cell. Host immunity will generally depend on cellular immunity rather than humoral immunity. At the site of the bite, a pimple-like bulge is formed due to the aggregation of lymphocytes, macrophages, and plasma, and a wound develops with inflammation that will remain for several months to several years after the healing period.[4] Although research into the discovery of vaccines and antiparasitic drugs, such as 5-valent antimony, has been somewhat successful, their limitations and side effects, including the risk of recurrence, drug resistance, drug toxicity, high cost, long recovery period, scarring, and mental and physical problems caused by them, especially in women and children, have resulted in the introduction of novel and effective therapies of natural origin and without side effects, such as vegetable oils (less toxic).[5] There are about 300 essential oils known to have antiviral, fungal, bacterial, and parasitic properties that have commercial value for agriculture, pharmaceuticals, food, etc.[6] For example, many previous studies have emphasized the anti-leishmaniasis activity of aloe vera, Brazembel, propolis, olive oil, Nigella sativa, and lavender.[6,7,8,9,10,11,12] Also, the systematic review and meta-analysis studies of Iranian medicinal plants with anti-leishmaniasis effect showed that Brazembel, propolis, and Nigella sativa extracts have drastic anti-leishmaniasis activity.[12] Furthermore, in other studies conducted in Iran and other countries, lavender, aloe vera, and olive oil were active against Leishmania in vitro and in vivo.[7,11,12] Also, the study of Saberi et al.[13] proved the good potential of Shirvan herbal ointment formulation (containing aloe vera, Nigella sativa, Brazembel, lavender, olive oil, and propolis) in the treatment of CL in vivo. So that, the ointment showed anti-leishmaniasis effects on the lesion size in the infected BALB/c mice (without any adverse effects on the lesions). Moreover, a significant reduction in parasite burden was reported in infected tissues. Therefore, according to the positive results of Shirvan herbal ointment in vitro and also in vivo, in this study, the therapeutic effect of Shirvan herbal ointment was investigated on human leishmaniasis in comparison with glucantime and the good effects of the ointment showed that Shirvan herbal ointment can be used as an alternative drug to repair leishmaniasis lesions and even other wounds.
MATERIALS AND METHODS
This study was conducted in 2021–2022 in Isfahan Province, Central Iran (30–34 degrees north and 49–55 degrees east longitude), with dry and moderate weather. Isfahan people have Iranian or Caucasian ethnicity. The outbreak of CL caused by L. major in Isfahan is higher than in other cities.[14]
Preparing ointment formula
The formulation of this ointment consists of Perovskia abrotanoides leaves and flowers, fresh aloe vera leaves, lavender Nigella sativa, olive oil, propolis, and beeswax. Dried flowers and leaves of Perovskia abrotanoides (400 g) and lavender (70 g), Nigella sativa (50 g), and fresh aloe vera gel (80 g) were powdered to prepare the ointment, mixed with olive oil (200 ml), and then stored at room temperature for 15 days. The propolis (50 g) and beeswax (150 g) were heated to 100°C using a water bath and added to the plant mixture. Next, the mixture was cooled and solidified at room temperature and then stored at 4–8°C for use.[13]
Study description
The present case–control study included sixty patients with confirmed CL who were referred to healthcare centers affiliated with the Isfahan University of Medical Sciences. The following formula was used to calculate the sample size:
N = (Z1-α/2 + Z1-β)2 [P1 (1-P1) + (P2 (1-P2)/d2, where α = 0.05, β = 0.2, and d = 0.3.
Patients were selected randomly from a list prepared in advance by a computer program. Before treatment, the patients were divided randomly into two groups of 30 patients, including the test and control groups. The size of the lesions was measured using a Digimatic caliper (VWR Brand Digital Calipers, Bridgeport, NJ).
Inclusion criteria
The inclusion criteria were confirmed cases of leishmaniasis based on clinical presentation (direct smear or culture in the NNN environment from the sample taken from the wound margin), age group of 1 to 60 years, willingness to take part in the study, and signature of informed consent by the patient (or parents of patients under 18 years).[15]
Exclusion criteria
The exclusion criteria were pregnant or lactating women, lesions on joints and cartilage, number of lesions of more than five cases, disease period of more than 8 weeks, use of any treatment for CL in a recent month, and company in any kind of research project in the last 2 months.[15]
Test group
Thirty patients were administered the Shirvan herbal ointment for each ulcerative lesion topically (twice a day at a dose of 0.5 ml/mm2 for 56 consecutive days).
Control group
Thirty patients received intralesional glucantime (the approved drug for CL) at a dose of 20 mg Sb5+/kg over 56 days.
Follow-up
The period of treatment was considered to be 2 months. During treatment, patients were interviewed weekly in terms of skin side effects (such as erythema, edema, and pain). Furthermore, the length and width of each lesion (mm2) were measured weekly using digital calipers. After completing the period of treatment, patients from the control and test groups were followed for 6 months. Evaluation of clinical improvement was performed based on the complete epithelialization of the lesions as the main criterion (no recurrence at the 6-month follow-up) for the effectiveness of treatment. The treatment and failure of the lesion were determined by a doctor blinded to the treatment group of the patients. The endpoint of the protocol was the treatment of the patient with the removal of all lesions. Finally, the therapeutic effect of Shirvan herbal ointment was investigated by comparing the size of the skin lesions in the control and test groups.
Ethical standards
The present study was confirmed by the Institutional Review Board (IRB) of Isfahan University of Medical Sciences (No. IR.ARI.MUI.REC.1401.339). The researcher-made questionnaire of participants was filled out, and written informed consent was signed by patients.
Statistical analysis
Data were reported as mean ± standard error of the mean (SEM). A statistical analysis of the difference in the size of the lesion was performed using repeated measure and t-test in different treatment groups. Statistical Package for the Social Sciences (SPSS) for Windows version 16 was used for the statistical analysis, and statistical significance was defined as a P value of 0.05 or less.
RESULTS
Patient characteristics
In the present study, 30 patients (8 females and 22 males, F:M = 1: 2.7) with a mean age of 26.9 (±13.3) years were randomly assigned to the test group and 30 cases (11 females and 19 males, F:M = 1: 1.7) with a mean age of 33.16 (±12.80) years were randomly assigned to the control group. The mean number of lesions was 2 per patient, as 56.7% presented two to five lesions and 43.3% of the patients had a unique lesion. 65.1% of patients had lesions on their upper limbs, mainly on the face and hands (53.4%), and lesions manifested on the legs and thoracic–abdominal regions were 35% and 11.7%, respectively. The lesions included ulcerative and non-ulcerative lesion types (nodular and plaques). The patients in the control group received at least one complete period of glucantime (20 mgSb5+/kg/day over 20 days), and only 13.6% and 4,9% of patients had two or three complete rounds, respectively. Arthralgia (19.7%), myalgia (13.5%), fever (11.3%), headache (26.2%), diarrhea (5.2%), nausea (7.6%), and other symptoms, such as facial swelling (17.6%), were the most common side effects of glucantime. In contrast, the treatment with the Shirvan herbal ointment was without adverse effects and was well tolerated; however, a transient burning sensation in some of the patients was reported. Moreover, the mean of treatment duration with the Shirvan herbal ointment was 43.9 (±14.4) days in the control group versus 30.5 (±7.4) days in the test group (P < 0.0), and also, the cure rate in the group receiving the Shirvan herbal ointment was higher than in the group treated with glucantime. As opposed to the control group, no scars were left after treating the patients in the test group with Shirvan herbal ointment. There was also a significant association between the treatment duration and the number of lesions, as longer treatment was needed to reduce the area or complete the disappearance of small lesions. The rate of recovery in both groups was related to age and lesion region, as younger patients and lesions on the face had a faster improvement rate. The basic data for all subjects (test and control) are detailed in Table 1.
Table 1.
Baseline data of test and control groups
| Test (n=30) | Control (n=30) | P | |
|---|---|---|---|
| Age (years) | 26.9±13.3 | 33.16±12.80 | 0.07 |
| Mean number of lesions (±SD) | 1.8±1.1 | 2.1±1.1 | 0.265 |
| Mean duration of treatment (day) | 30.5±7.4 | 43.9±14.4 | 0.00 |
| Mean lesion location (upper limbs) | 0.9±0.92 | 0.8±0.84 | 0.66 |
The measurement of lesion size
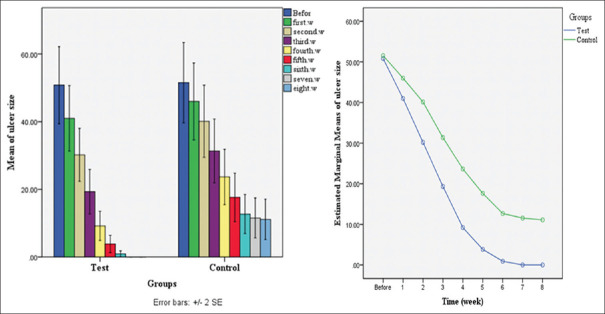
Lesion size was measured at the beginning and weekly for 8 weeks. The data indicated a significant reduction in the size of the lesions in the control group at different weeks, while this reduction was more frequent and continuous in the test group. Therefore, the size of the lesion decreased up to 4 weeks in the test group and reached zero in the last 3 weeks, but it did not change in the control group in 7 and 8 weeks. This difference can be due to the use of Shirvan herbal ointment. The therapeutic effects of the Shirvan herbal ointment and glucantime on the lesion size (mm2) of the control and test groups are detailed weekly in Table 2 and Figure 1.
Table 2.
Frequency distribution±SD of the average size of lesions in different groups (mm2)
| Week | Test (n=30) | Control (n=30) | P |
|---|---|---|---|
| Before treatment | 50.8±31.2 | 51.5±32.5 | 0.93 |
| First week | 40.9±26.4 | 45.9±31.1 | 0.5 |
| Second week | 30.1±21.4 | 40.1±29.2 | 0.139 |
| Third week | 19.3±18.0 | 31.3±25.8 | 0.04 |
| Fourth week | 9.2±11.7 | 23.6±22.4 | 0.003 |
| Fifth week | 3.8±6.9 | 17.6±19.6 | 0.001 |
| Sixth week | 0.92±2.3 | 12.6±15.7 | 0.00 |
| Seventh week | 0.00±0.00 | 11.5±16.1 | 0.00 |
| Eighth week | 0.00±0.00 | 11.1±16.2 | 0.00 |
Figure 1.
Mean of lesion size (mm2) in the control and test groups weekly
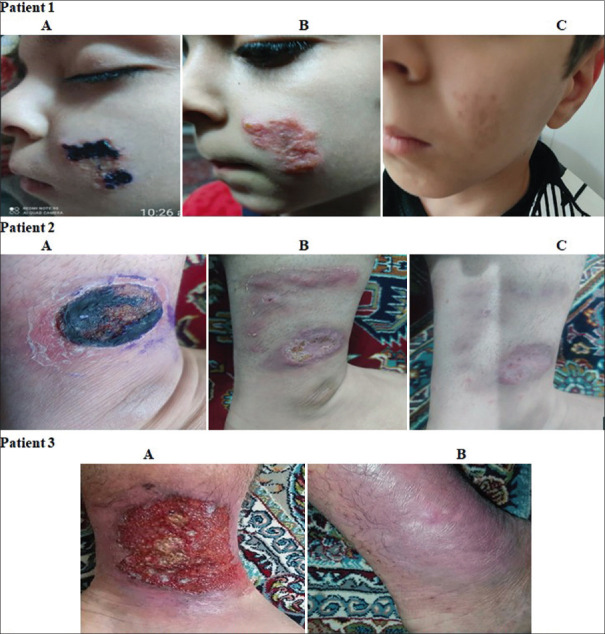
Moreover, our study showed that local consumption of Shirvan herbal ointment significantly reduced the intensity of the created lesion. Furthermore, healing was remarkable and all lesions flattened significantly, and most lesions left only surface scarring or mild post-inflammatory hyperpigmentation. During the 6 months after treatment, patients did not show any symptoms of relapsing lesions. The changes are shown in Figure 2.
Figure 2.
Process of healing wounds in different organs of three patients treated with Shirvan herbal ointment, A: Before treatment. B: 15 days after treatment. C: 30 days after treatment.
DISCUSSION
CL is caused by L. major, and as anti-leishmanial chemotherapy is associated with limitations and several side effects, the evaluation of natural compounds with a history of antimicrobial properties that are economically feasible is of great importance.[6] In this regard, plant extracts with high antiparasitic effects on Leishmania parasites and low cytotoxicity to human cells are desirable and candidates for CL treatment.[16,17] In the present study, we investigated the anti-leishmanial effect of six plants (aloe vera, propolis, Brazembel, lavender, Nigella sativa, and olive oil) in the mixed form of Shirvan ointment in leishmaniasis patients.
Several in vitro and in vivo studies have shown the inhibitory effects of each of these plants and natural products individually, and satisfactory results have been obtained.[6,7,8,9,10,11,12] Also, our results from the previous study indicated significant anti-leishmanial effects of mixed natural ointment formulation on the reduction in lesion size in infected BALB/c mice without adverse effects on lesions compared with the control group. Moreover, a significant reduction in the burden of parasites in the infected tissue and organs was shown.[13] In the present study, the effect of Shirvan herbal ointment on lesion treatment of human CL was investigated and findings showed remarkable improvement in the leishmaniasis. As the morphometrical analysis revealed that the wound sizes treated with Shirvan herbal ointment decreased significantly (from 50.8 ± 31.2 mm2 to 0 mm2) compared with lesions treated with glucantime, complete reepithelialization was observed in lesions 1 week after the end of treatment. Also, improvement in the signs, such as pain, burning, erythema, and edema, was seen in all patients treated with Shirvan herbal ointment. According to our results, Shamsi et al.[7] reported that aloe vera leaf exudates significantly reduced the ulcer size compared with the control. Moreover, in Iranian traditional medicine, the dry root of Perovskia abrotanoides is used to treat CL.[8] According to the research conducted by Fattahi Bafghi et al.[9] Nigella sativa can be useful in the treatment of leishmaniosis by inhibiting the growth and survival of the parasite. In addition, Nilforoushzadeh et al.[10] found positive biological effects on propolis hydroalcoholic extracts that were more efficient in decreasing ulcer size as compared to meglumine. In Shokri et al.’s study[11] anti-leishmanial activity of Lavandula angustifolia on L. major was assessed and lavender essential oil remarkably decreased the number of amastigotes in macrophages compared with the control. The results of a previous study by our research team[6] also showed that ozonated olive oil has potent therapeutic effects on human CL, and this effect is maintained even after 6 months of follow-up. Therefore, the obtained results are consistent with the results of previous studies, indicating that medicinal plants, such as brazembel, aloe vera, lavender, propolis, olive oil, and Nigella sativa, have the potential to produce novel drugs to be used as alternative or in combination with existing drugs and more studies are needed to evaluate the importance of this finding.
CONCLUSION
The potential effects of Shirvan herbal ointment in the present study suggest that this ointment can be used as monotherapy for healing leishmaniasis lesions and even other ulcers. Therefore, further studies should be conducted in larger multivariable cohorts and controlled trials to express more accurate evidence in the future.
Declaration of patient consent
The authors declare that they have obtained consent from patients. Patients have given their consent for their images and other clinical information to be reported in the journal. Patients understand that their names will not be published and due efforts will be made to conceal their identity but anonymity cannot be guaranteed.
Author contribution
M.A contributed to the conception of the work, conducted the study, drafted and revised the draft, and approved the final version of the manuscript. Sh.A contributed to the drafting and revising of the draft and analyzed the data. A.G.Z contributed to the approval of the final version of the manuscript. N.A contributed to the drafting and revising of the draft. M.F contributed to the drafting and revising of the draft. SM.H analyzed the data. Z.SH contributed to the approval of the final version of the manuscript. SH.H contributed to the conception of the work, conducted the study, drafted and revised the draft, and approved the final version of the manuscript.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
The authors wish to thank the Vice-Chancellor of Skin Diseases and Leishmaniasis Research Centre, Isfahan University of Medical Sciences, for their approval of this study.
REFERENCES
- 1.Barrett MP, Croft SL. Management of trypanosomiasis and leishmaniasis. Br Med Bull. 2012;104:175–96. doi: 10.1093/bmb/lds031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Inceboz T. Epidemiology and ecology of leishmaniasis. Current topics in neglected tropical diseases. 2019:1–15. [Google Scholar]
- 3.Razavi MR, Shirzadi MR, Mohebali M, Yaghoobi-Ershadi MR, Vatandoost H, Shirzadi M, et al. Human cutaneous leishmaniosis in Iran, up to date-2019. J Arthropod Borne Dis. 2021;15:143–51. doi: 10.18502/jad.v15i2.7483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pulvertaft R, Hoyle G. Stages in the life-cycle of Leishmania donovani. Trans R Soc Trop Med Hyg. 1960;54:191–6. doi: 10.1016/0035-9203(60)90057-2. [DOI] [PubMed] [Google Scholar]
- 5.Singh N, Kumar M, Singh RK. Leishmaniasis: Current status of available drugs and new potential drug targets. Asian Pac J Trop Med. 2012;5:485–97. doi: 10.1016/S1995-7645(12)60084-4. [DOI] [PubMed] [Google Scholar]
- 6.Aghaei M, Aghaei S, Sokhanvari F, Ansari N, Hosseini SM, Mohaghegh M-A, et al. The therapeutic effect of ozonated olive oil plus glucantime on human cutaneous leishmaniasis. Iran J Basic Med Sci. 2019;22:25. doi: 10.22038/ijbms.2018.29232.7064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shamsi H, Shaddel M, Yakhchali M, Akbarzadeh M, Raoufi N, Tavakoli P, et al. The antileishmanial activity of Aloe vera leaf exudates: In vitro and in vivo. Iran J Dermatol. 2019;22:18–24. [Google Scholar]
- 8.Sairafianpour M, Christensen J, Stærk D, Budnik BA, Kharazmi A, Bagherzadeh K, et al. Leishmanicidal, antiplasmodial, and cytotoxic activity of novel diterpenoid 1, 2-quinones from Perovskia abrotanoides: New source of tanshinones. J Nat Prod. 2001;64:1398–403. doi: 10.1021/np010032f. [DOI] [PubMed] [Google Scholar]
- 9.Bafghi AF, Mirzaei F. Antileishmanial activity of Nigella sativa extract against Leishmania major: An in vitro study. J Chem Pharm Res. 2015;7:1239–44. [Google Scholar]
- 10.Nilforoushzadeh M, Shirani-Bidabadi L, Zolfaghari-Baghbaderani A, Saberi S, Siadat A, Mahmoudi M. Comparison of thymus vulgaris (thyme), achillea millefolium (yarrow) and propolis hydroalcoholic extracts versus systemic glucantime in the treatment of cutaneous leishmaniasis in balb/c mice. J Vector Borne Dis. 2008;45:301–6. [PubMed] [Google Scholar]
- 11.Shokri A, Saeedi M, Fakhar M, Morteza-Semnani K, Keighobadi M, Teshnizi SH, et al. Antileishmanial activity of lavandula angustifolia and rosmarinus officinalis essential oils and nano-emulsions on leishmania major (MRHO/IR/75/ER) Iran J Parasitol. 2017;12:622–31. [PMC free article] [PubMed] [Google Scholar]
- 12.Kheirandish F, Mahmoudvand H, Khamesipour A, Ebrahimzadeh F, Behrahi F, Rezaei S, et al. The therapeutic effects of olive leaf extract on leishmania major infection in BALB/c mice. Marmara Pharm J. 2017;21:837–42. [Google Scholar]
- 13.Saberi R, Ghelich Zadeh A, Afshar MJA, Fakhar M, Keighobadi M, Mohtasebi S, et al. In vivo anti-leishmanial activity of concocted herbal topical preparation against leishmania major (MRHO/IR/75/ER) Ann Parasitol. 2021;67:483–8. doi: 10.17420/ap6703.361. [DOI] [PubMed] [Google Scholar]
- 14.Karami M, Doudi M, Setorki M. Assessing epidemiology of cutaneous leishmaniasis in Isfahan, Iran. J Vector Borne Dis. 2013;50:30. [PubMed] [Google Scholar]
- 15.Firooz A, Khatami A, Khamesipour A, Nassiri-Kashani M, Behnia F, Nilforoushzadeh M, Pazoki-Toroudi H, Dowlati Y. Intralesional injection of 2% zinc sulfate solution in the treatment of acute old world cutaneous leishmaniasis: A randomized, double-blind, controlled clinical trial. J Drugs Dermatol. 2005;4:73–9. [PubMed] [Google Scholar]
- 16.Al Nasr I. In vitro anti-leishmanial assessment of some medicinal plants collected from Al Qassim, Saudi Arabia. Acta Parasitologica. 2020;65:696–703. doi: 10.2478/s11686-020-00205-2. [DOI] [PubMed] [Google Scholar]
- 17.Nilforoushzadeh MA, Heidari-Kharaji M, Zare M, Torkamaniha E, Rafati S. Novel strategies and pharmaceutical agents for the treatment of leishmaniasis: A review. Anti-Infective Agents. 2020;18:89–100. [Google Scholar]