Abstract
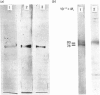
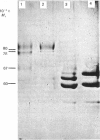
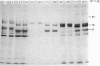
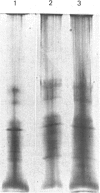
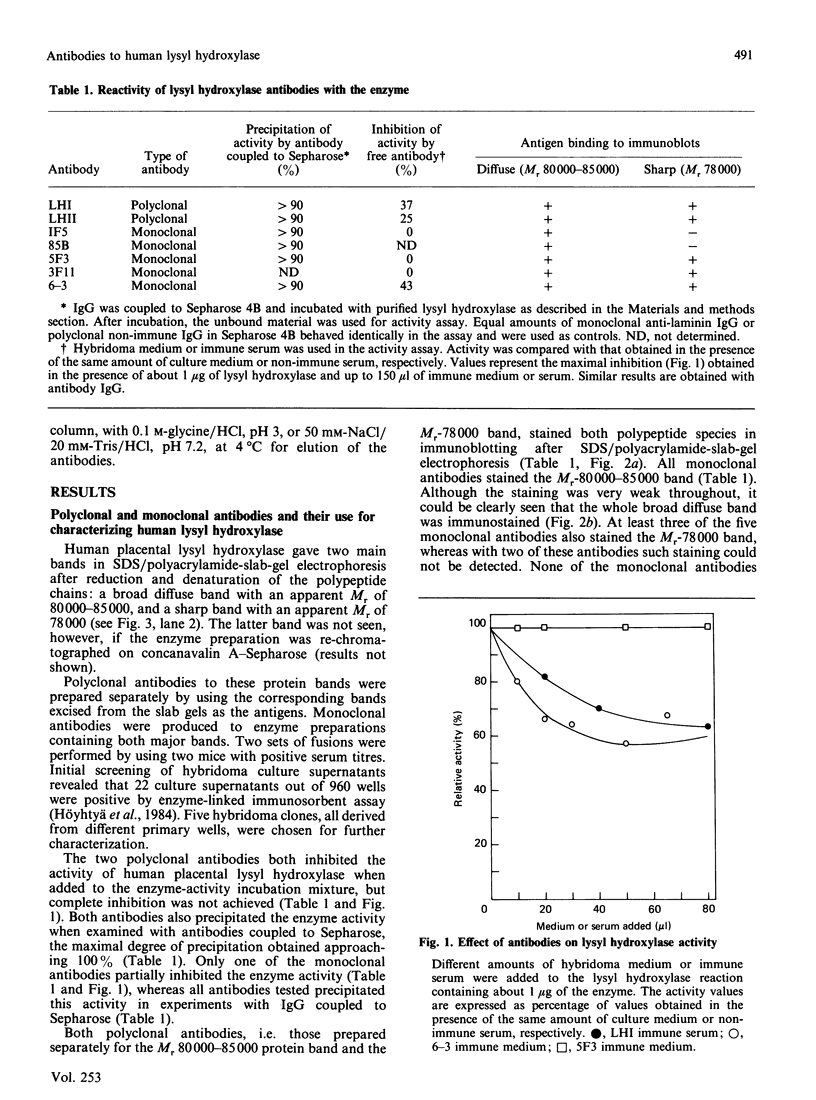
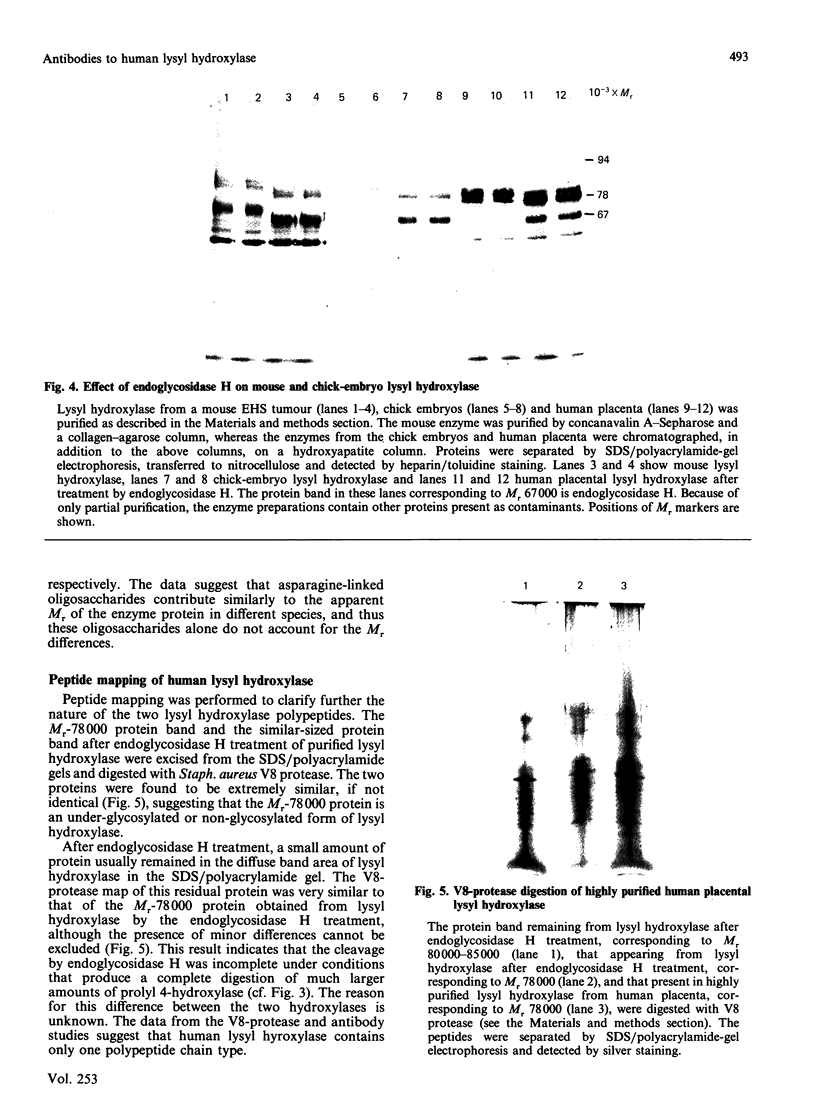
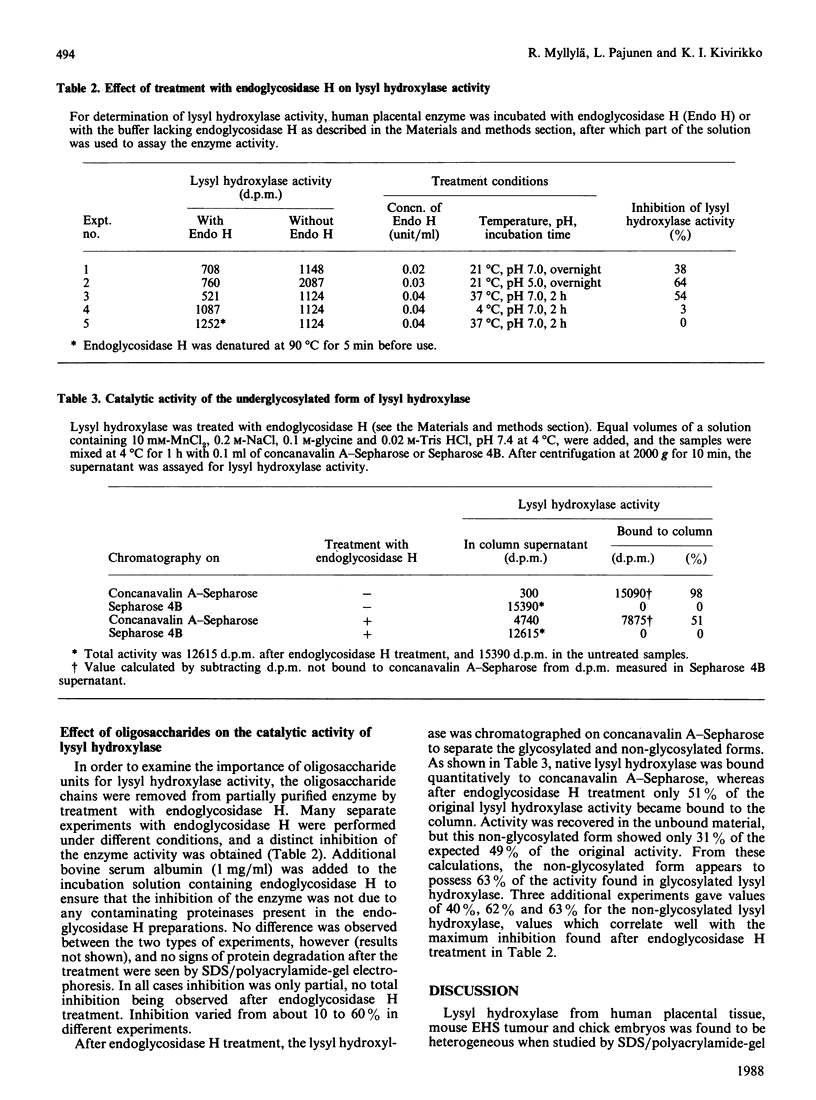
Human placental lysyl hydroxylase gave two bands in SDS/polyacrylamide-slab-gel electrophoresis: a broad, diffuse, major band corresponding to an apparent Mr of 80,000-85,000, and a sharp minor band with Mr 78,000. Mouse and chick-embryo lysyl hydroxylases gave only the broad, diffuse band, whereas the sharp band could not be detected. Polyclonal antibodies were prepared to the two bands of the human enzyme separately, and monoclonal antibodies were prepared to the whole purified enzyme preparation. Both types of polyclonal antibody inhibited and precipitated the enzyme activity, and both stained the two polypeptide bands in immunoblotting after SDS/polyacrylamide-gel electrophoresis. Only one out of five monoclonal antibodies inhibited the enzyme activity, whereas they all precipitated the activity when studied with antibody coupled to Sepharose. All five monoclonal antibodies stained the whole broad band in immunoblotting, and at least three of them also stained the sharp band. Peptide maps produced from the two polypeptide species by digestion with Staphylococcus aureus V8 protease were highly similar. Experiments with endoglycosidase H demonstrated that the Mr-80,000-85,000 polypeptide contains asparagine-linked carbohydrate units, which are required for maximal lysyl hydroxylase activity. The data suggest that the lysyl hydroxylase dimer consists of only one type of monomer, the heterogeneity of which is due to differences in glycosylation.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bruck C., Portetelle D., Glineur C., Bollen A. One-step purification of mouse monoclonal antibodies from ascitic fluid by DEAE Affi-Gel blue chromatography. J Immunol Methods. 1982 Sep 30;53(3):313–319. doi: 10.1016/0022-1759(82)90178-8. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Eckhardt A. E., Hayes C. E., Goldstein I. J. A sensitive fluorescent method for the detection of glycoproteins in polyacrylamide gels. Anal Biochem. 1976 May 21;73(1):192–197. doi: 10.1016/0003-2697(76)90154-8. [DOI] [PubMed] [Google Scholar]
- Eyre D. R. Collagen: molecular diversity in the body's protein scaffold. Science. 1980 Mar 21;207(4437):1315–1322. doi: 10.1126/science.7355290. [DOI] [PubMed] [Google Scholar]
- Galfrè G., Milstein C. Preparation of monoclonal antibodies: strategies and procedures. Methods Enzymol. 1981;73(Pt B):3–46. doi: 10.1016/0076-6879(81)73054-4. [DOI] [PubMed] [Google Scholar]
- Günzler V., Hanauske-Abel H. M., Myllylä R., Kaska D. D., Hanauske A., Kivirikko K. I. Syncatalytic inactivation of prolyl 4-hydroxylase by anthracyclines. Biochem J. 1988 Apr 15;251(2):365–372. doi: 10.1042/bj2510365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Günzler V., Hanauske-Abel H. M., Myllylä R., Mohr J., Kivirikko K. I. Time-dependent inactivation of chick-embryo prolyl 4-hydroxylase by coumalic acid. Evidence for a syncatalytic mechanism. Biochem J. 1987 Feb 15;242(1):163–169. doi: 10.1042/bj2420163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höyhtyä M., Myllylä R., Piuva J., Kivirikko K. I., Tryggvason K. Monoclonal antibodies to human prolyl 4-hydroxylase. Eur J Biochem. 1984 Jun 15;141(3):472–482. doi: 10.1111/j.1432-1033.1984.tb08217.x. [DOI] [PubMed] [Google Scholar]
- Kivirikko K. I., Myllylä R. Post-translational processing of procollagens. Ann N Y Acad Sci. 1985;460:187–201. doi: 10.1111/j.1749-6632.1985.tb51167.x. [DOI] [PubMed] [Google Scholar]
- Kivirikko K. I., Myllylä R. Posttranslational enzymes in the biosynthesis of collagen: intracellular enzymes. Methods Enzymol. 1982;82(Pt A):245–304. doi: 10.1016/0076-6879(82)82067-3. [DOI] [PubMed] [Google Scholar]
- Kivirikko K. I., Myllylä R. Recent developments in posttranslational modification: intracellular processing. Methods Enzymol. 1987;144:96–114. doi: 10.1016/0076-6879(87)44175-x. [DOI] [PubMed] [Google Scholar]
- MacSween J. M., Eastwood S. L. Recovery of antigen from staphylococcal protein A--antibody adsorbents. Methods Enzymol. 1981;73(Pt B):459–471. [PubMed] [Google Scholar]
- Prockop D. J., Kivirikko K. I. Heritable diseases of collagen. N Engl J Med. 1984 Aug 9;311(6):376–386. doi: 10.1056/NEJM198408093110606. [DOI] [PubMed] [Google Scholar]
- Puistola U., Turpeenniemi-Hujanen T. M., Myllylä R., Kivirikko K. I. Studies on the lysyl hydroxylase reaction. I. Initial velocity kinetics and related aspects. Biochim Biophys Acta. 1980 Jan 11;611(1):40–50. doi: 10.1016/0005-2744(80)90040-6. [DOI] [PubMed] [Google Scholar]
- Puistola U., Turpeenniemi-Hujanen T. M., Myllylä R., Kivirikko K. I. Studies on the lysyl hydroxylase reaction. II. Inhibition kinetics and the reaction mechanism. Biochim Biophys Acta. 1980 Jan 11;611(1):51–60. doi: 10.1016/0005-2744(80)90041-8. [DOI] [PubMed] [Google Scholar]
- Tarentino A. L., Maley F. Purification and properties of an endo-beta-N-acetylglucosaminidase from Streptomyces griseus. J Biol Chem. 1974 Feb 10;249(3):811–817. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turpeenniemi-Hujanen T. M., Puistola U., Kivirikko K. I. Human lysyl hydroxylase: purification to homogeneity, partial characterization and comparison of catalytic properties with those of a mutant enzyme from Ehlers-Danlos syndrome type VI fibroblasts. Coll Relat Res. 1981 Jul;1(4):355–366. doi: 10.1016/s0174-173x(81)80012-x. [DOI] [PubMed] [Google Scholar]
- Turpeenniemi-Hujanen T. M., Puistola U., Kivirikko K. I. Isolation of lysyl hydroxylase, an enzyme of collagen synthesis, from chick embryos as a homogeneous protein. Biochem J. 1980 Aug 1;189(2):247–253. doi: 10.1042/bj1890247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turpeenniemi T. M., Puistola U., Anttinen H., Kivirikko K. I. Affinity chromatography of lysyl hydroxylase on concanavalin A-agarose. Biochim Biophys Acta. 1977 Jul 8;483(1):215–219. doi: 10.1016/0005-2744(77)90024-9. [DOI] [PubMed] [Google Scholar]
- Vartio T., Vaheri A. A gelatin-binding 70,000-dalton glycoprotein synthesized distinctly from fibronectin by normal and malignant adherent cells. J Biol Chem. 1981 Dec 25;256(24):13085–13090. [PubMed] [Google Scholar]
- Wray W., Boulikas T., Wray V. P., Hancock R. Silver staining of proteins in polyacrylamide gels. Anal Biochem. 1981 Nov 15;118(1):197–203. doi: 10.1016/0003-2697(81)90179-2. [DOI] [PubMed] [Google Scholar]
- de Waal A., Hartog A. F., de Jong L. Photoaffinity labelling of the 2-oxoglutarate binding site of prolyl 4-hydroxylase with 5-azidopyridine-2-carboxylic acid. Biochim Biophys Acta. 1987 Mar 18;912(1):151–155. doi: 10.1016/0167-4838(87)90260-3. [DOI] [PubMed] [Google Scholar]
- de Waal A., de Jong L., Hartog A. F., Kemp A. Photoaffinity labeling of peptide binding sites of prolyl 4-hydroxylase with N-(4-azido-2-nitrophenyl)glycyl-(Pro-Pro-Gly)5. Biochemistry. 1985 Nov 5;24(23):6493–6499. doi: 10.1021/bi00344a028. [DOI] [PubMed] [Google Scholar]