Abstract
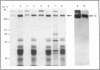
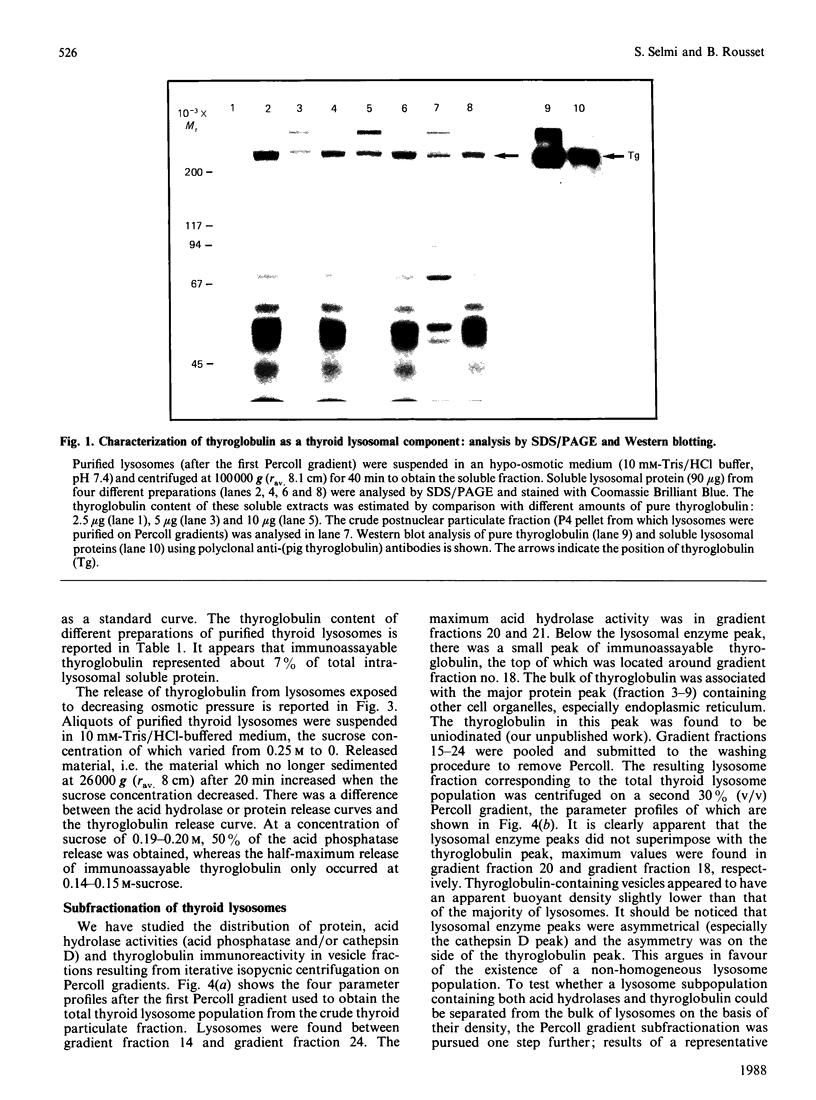
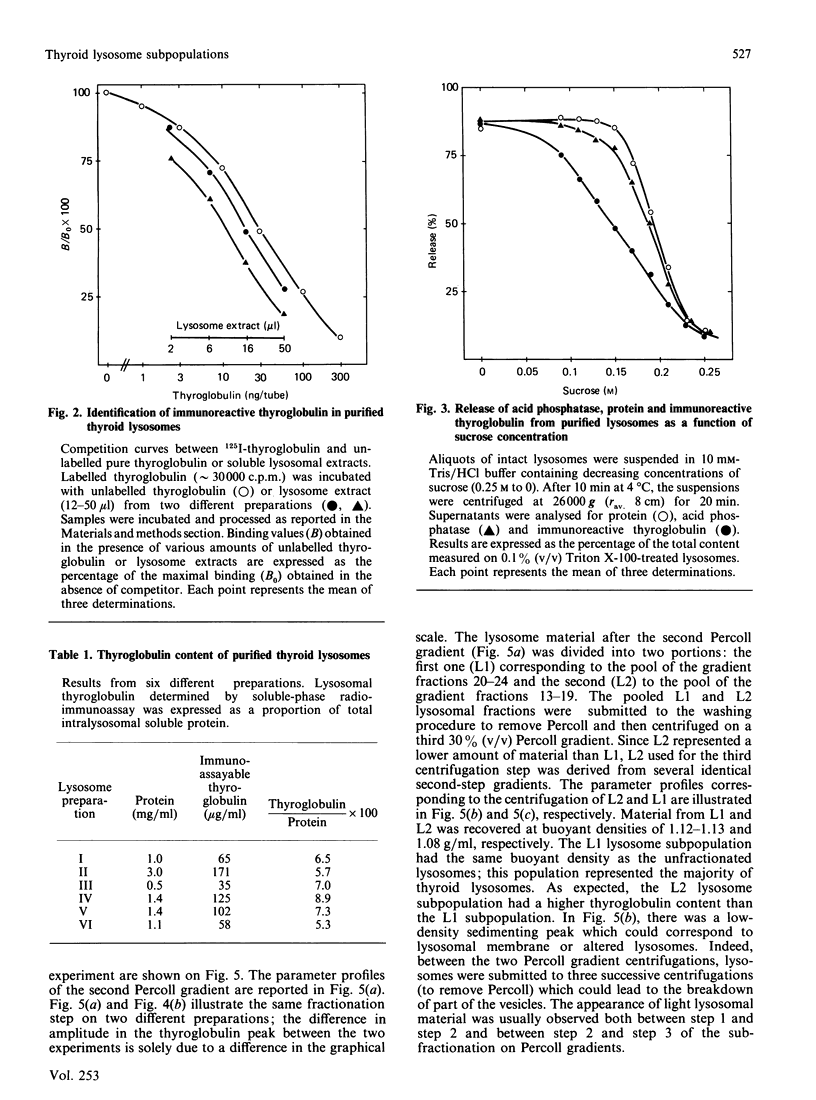
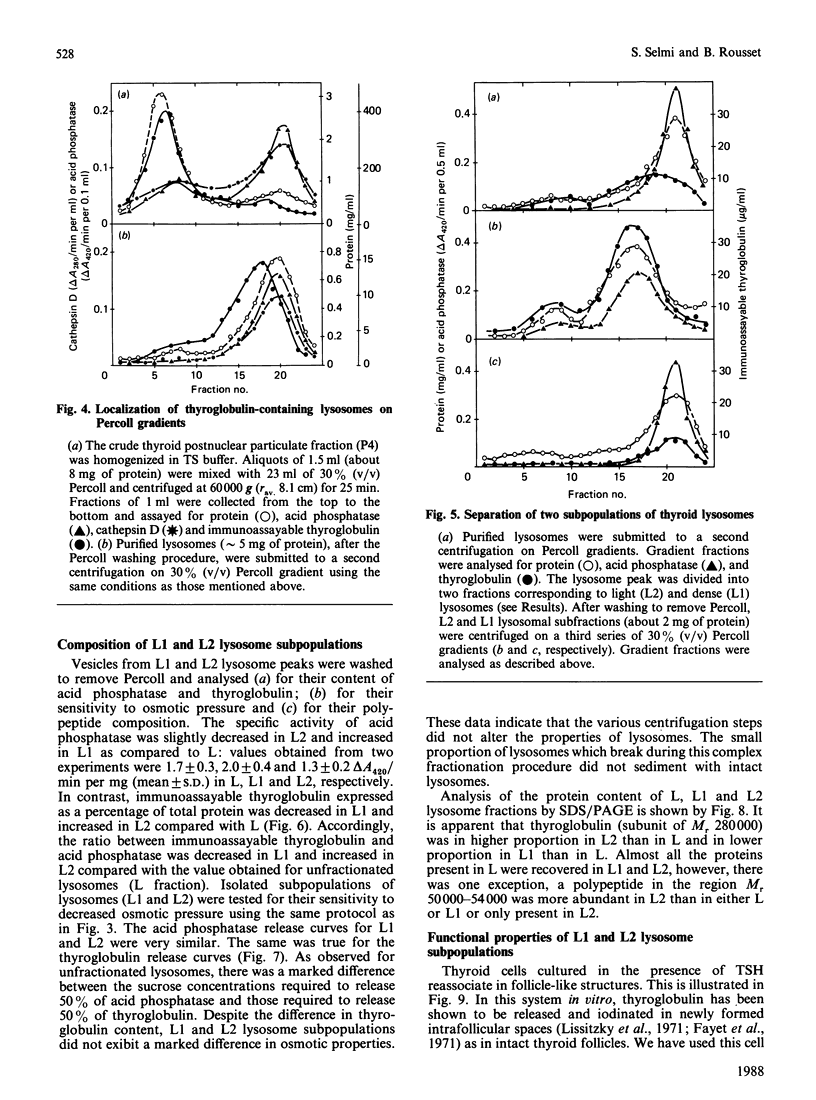
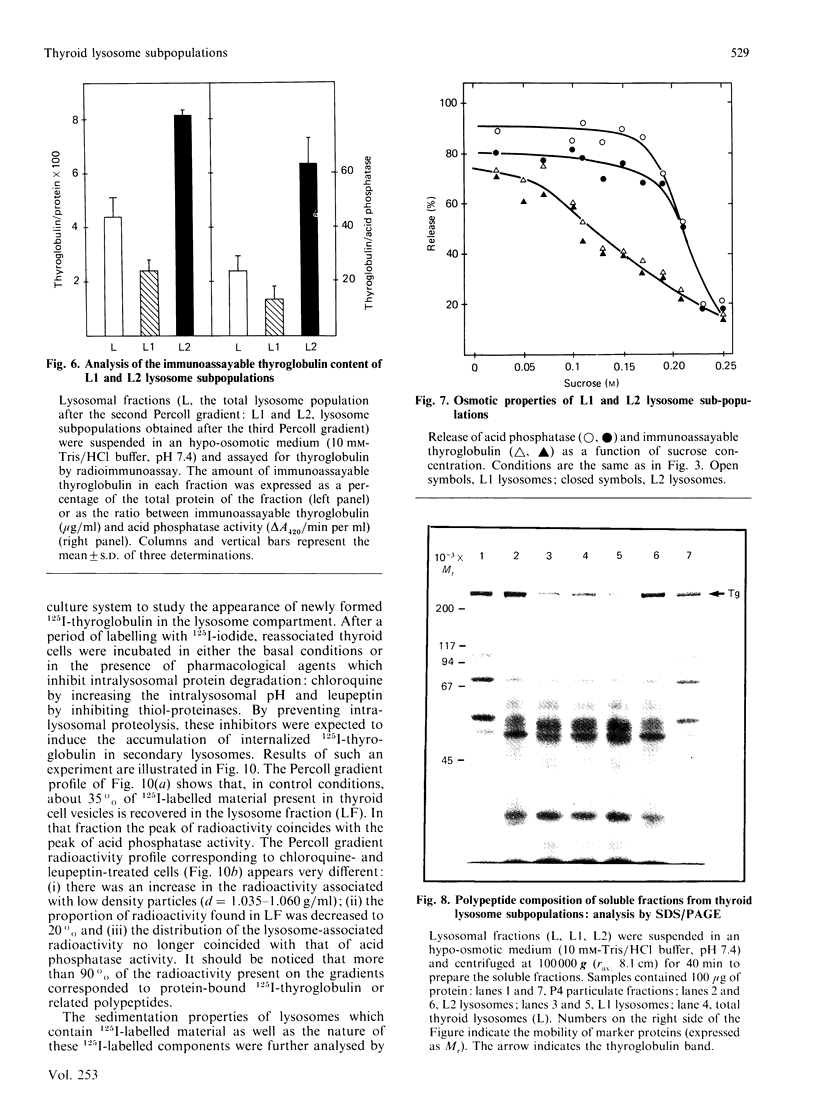
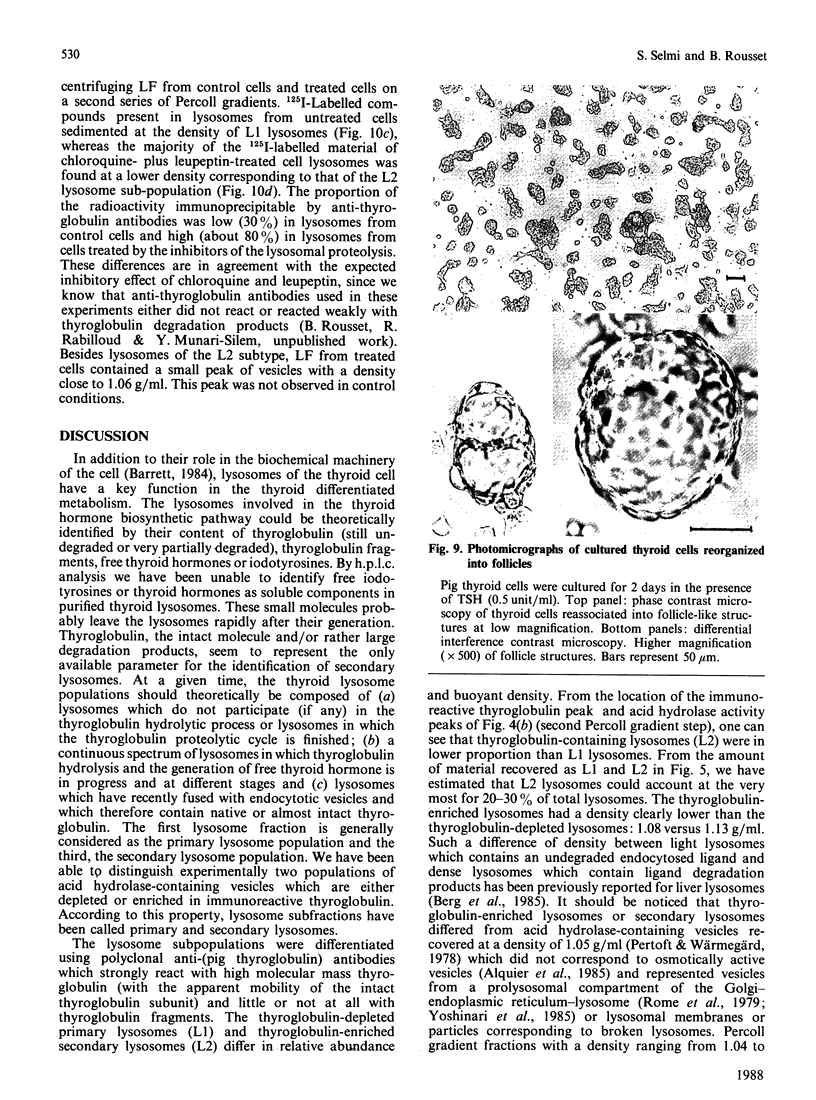
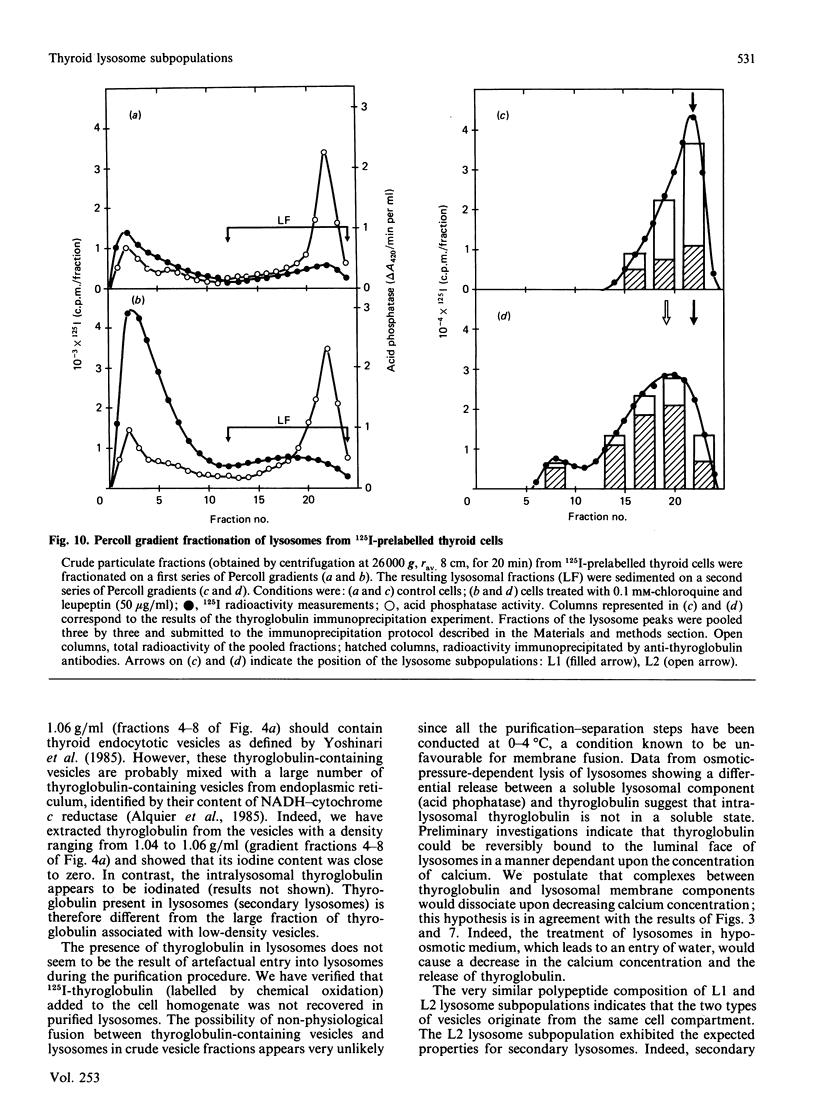
Using a combination of differential centrifugation and isopycnic centrifugation in Percoll gradients, we obtained a highly purified preparation of thyroid lysosomes [Alquier, Guenin, Munari-Silem, Audebet & Rousset (1985) Biochem. J. 232, 529-537] in which we identified thyroglobulin. From this observation, we postulated that the isolated lysosome population could be composed of primary lysosomes and of secondary lysosomes resulting from the fusion of lysosomes with thyroglobulin-containing vesicles. In the present study, we have tried to characterize these lysosome populations by (a) subfractionation of purified lysosomes using iterative centrifugation on Percoll gradients and (b) by functional studies on cultured thyroid cells. Thyroglobulin analysed by soluble phase radioimmunoassay, Western blotting or immunoprecipitation was used as a marker of secondary lysosomes. The total lysosome population separated from other cell organelles on a first gradient was centrifuged on a second Percoll gradient. Resedimented lysosomes were recovered as a slightly asymmetrical peak under which the distribution patterns of acid hydrolase activities and immunoreactive thyroglobulin did not superimpose. This lysosomal material (L) was separated into two fractions: a light (thyroglobulin-enriched) fraction (L2) and a dense fraction (L1). L1 and L2 subfractions centrifuged on a third series of Percoll gradients were recovered as symmetrical peaks at buoyant densities of 1.12-1.13 and 1.08 g/ml, respectively. In each case, protein and acid hydrolase activities were superimposable. The specific activity of acid phosphatase was slightly lower in L2 than in L1. In contrast, the immunoassayable thyroglobulin content of L2 was about 4-fold higher than that of L1. The overall polypeptide composition of L, L1 and L2 analysed by polyacrylamide-gel electrophoresis was very similar, except for thyroglobulin which was more abundant in L2 than in either L or L1. The functional relationship between L1 and L2 lysosome subpopulations has been studied in cultured thyroid cells reassociated into follicles. Thyroid cells, prelabelled with 125I-iodide to generate 125I-thyroglobulin, were incubated in the absence of in the presence of inhibitors of intralysosomal proteolysis. The fate of 125I-thyroglobulin, and especially its appearance in the lysosomal compartment, was studied by Percoll gradient fractionation and immunoprecipitation. Treatment of prelabelled thyroid cells with chloroquine and leupeptin induced the accumulation of immunoprecipitable 125I-thyroglobulin into a lysosome fraction corresponding to the L2 subpopulation. In control cells, in which intralysosomal proteolysis was n
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alquier C., Guenin P., Munari-Silem Y., Audebet C., Rousset B. Isolation of pig thyroid lysosomes. Biochemical and morphological characterization. Biochem J. 1985 Dec 1;232(2):529–537. doi: 10.1042/bj2320529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett A. J. Proteolytic and other metabolic pathways in lysosomes. Biochem Soc Trans. 1984 Dec;12(6):899–902. doi: 10.1042/bst0120899. [DOI] [PubMed] [Google Scholar]
- Berg T., Kindberg G. M., Ford T., Blomhoff R. Intracellular transport of asialoglycoproteins in rat hepatocytes. Evidence for two subpopulations of lysosomes. Exp Cell Res. 1985 Dec;161(2):285–296. doi: 10.1016/0014-4827(85)90086-2. [DOI] [PubMed] [Google Scholar]
- Dean R. T. Modes of access of macromolecules to the lysosomal interior. Biochem Soc Trans. 1984 Dec;12(6):911–913. doi: 10.1042/bst0120911. [DOI] [PubMed] [Google Scholar]
- Durrieu C., Bernier-Valentin F., Rousset B. Binding of glyceraldehyde 3-phosphate dehydrogenase to microtubules. Mol Cell Biochem. 1987 Mar;74(1):55–65. doi: 10.1007/BF00221912. [DOI] [PubMed] [Google Scholar]
- Fayet G., Michel-Béchet M., Lissitzky S. Thyrotrophin-induced aggregation and reorganization into follicles of isolated porcine-thyroid cells in culture. 2. Ultrastructural studies. Eur J Biochem. 1971 Dec 22;24(1):100–111. doi: 10.1111/j.1432-1033.1971.tb19659.x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lissitzky S., Fayet G., Giraud A., Verrier B., Torresani J. Thyrotrophin-induced aggregation and reorganization into follicles of isolated porcine-thyroid cells. 1. Mechanism of action of thyrotrophin and metabolic properties. Eur J Biochem. 1971 Dec 22;24(1):88–99. doi: 10.1111/j.1432-1033.1971.tb19658.x. [DOI] [PubMed] [Google Scholar]
- Lloyd J. B. Penetration of small molecules across the lysosome membrane: the 'classical' view. Biochem Soc Trans. 1984 Dec;12(6):906–906. doi: 10.1042/bst0120906. [DOI] [PubMed] [Google Scholar]
- Munari-Silem Y., Champier J., Riou J. P., Audebet C., Rabilloud R., Rousset B. Cyclic AMP-dependent phosphorylation of high molecular mass proteins in pig thyroid cells. Mol Cell Endocrinol. 1986 Mar;44(3):251–260. doi: 10.1016/0303-7207(86)90131-0. [DOI] [PubMed] [Google Scholar]
- Pertoft H., Wärmegård B., Hök M. Heterogeneity of lysosomes originating from rat liver parenchymal cells. Metabolic relationship of subpopulations separated by density-gradient centrifugation. Biochem J. 1978 Jul 15;174(1):309–317. doi: 10.1042/bj1740309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rome L. H., Garvin A. J., Allietta M. M., Neufeld E. F. Two species of lysosomal organelles in cultured human fibroblasts. Cell. 1979 May;17(1):143–153. doi: 10.1016/0092-8674(79)90302-7. [DOI] [PubMed] [Google Scholar]
- Rousset B., Poncet C., Dumont J. E., Mornex R. Intracellular and extracellular sites of iodination in dispersed hog thyroid cells. Biochem J. 1980 Dec 15;192(3):801–812. doi: 10.1042/bj1920801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousset B., Poncet C., Mornex R. Evidence for a secretion of thyroxine by isolated hog thyroid cells. Biochim Biophys Acta. 1976 Jul 21;437(2):543–561. doi: 10.1016/0304-4165(76)90022-2. [DOI] [PubMed] [Google Scholar]
- Rousset B., Wolff J. Lactoperoxidase-tubulin interactions. J Biol Chem. 1980 Mar 25;255(6):2514–2523. [PubMed] [Google Scholar]
- Yoshinari M., Taurog A., Krupp P. P. Purification of thyroid lysosomes by colloidal silica density gradient centrifugation. Endocrinology. 1985 Aug;117(2):580–590. doi: 10.1210/endo-117-2-580. [DOI] [PubMed] [Google Scholar]