Abstract
Purpose
Serum cytokine alterations are associated with increased Staphylococcus aureus bacteremia (SAB) mortality. Unfortunately, clinical use of these cytokines is uncommon due to limited availability and high cost. Complete blood count (CBC) with differential reflects the host immune response, and CBC subgroup parameters may have prognostic value in SAB. We sought to determine the association between CBC subgroup parameters on the day of index blood culture and 30-day all-cause mortality in SAB patients.
Methods
We conducted a retrospective study of adult SAB patients with infectious diseases consultation to evaluate the discriminatory capacity of CBC subgroup parameters in predicting SAB mortality. Clinical and microbiological data were collected, including severity of illness and CBC subgroup parameters, on the day of index blood culture. The primary outcome was 30-day all-cause mortality. A multivariable logistic regression model was used to determine the association between patient-level variables and mortality.
Results
A total of 119 patients were included. The overall 30-day all-cause mortality rate was 10.1%. The median neutrophil-to-lymphocyte count ratio (NLCR) among survivors was 13.6 vs 23.2 among non-survivors (p = .007). Median lymphocyte count among survivors was 0.9 x 103 cells/μL vs 0.6 x 103 cells/μL among non-survivors (p = .031). Median platelet count was higher among survivors than non-survivors (239 x 103 cells/μL vs 171 x 103 cells/μL, respectively; p = .018). All other CBC subgroup parameters were similar between the two groups. Known SAB mortality predictors, including age, were also associated with increased mortality. Lower lymphocyte count was independently associated with increased mortality (adjusted odds ratio [aOR] 0.236, 95% confidence interval [CI] 0.064–0.872), as was higher PITT bacteremia score (aOR 2.439, 95% CI 1.565–3.803).
Conclusions
CBC subgroup parameters may have prognostic value in SAB. Additional study is warranted to further ascertain the prognostic value of these readily available laboratory values.
Keywords: S. aureus bacteremia, host response, complete blood count, CBC subgroup parameters, clinical outcomes
Patient-Friendly Recap
Staphylococcus aureus is a type of bacteria carried by humans that can cause life-threatening infections in the blood, despite treatment with antibiotics.
We looked at common blood cell values on the first day of S. aureus infection in patients to find patterns between the values and patient survival.
We found that infected patients with a higher lymphocyte count had a higher chance of survival (lymphocytes are a type of white blood cell that helps fight infection), but more research is needed to confirm our findings.
Staphylococcus aureus is one of the leading causes of bacteremia and has been associated with a high incidence of mortality.1,2 Mortality rates have remained between 10–20% even with the availability of multiple staphylococcal antibiotics and improved application of treatment principles.2 Staphylococcus aureus bacteremia (SAB) remains a leading cause of infectious disease-related morbidity and mortality, affecting patients across the spectrum of age and comorbidity. While antibiotic treatments have advanced, there remains a lack of readily available prognostic tools for clinician use. Analyses of host inflammatory response to SAB and their correlation with clinical outcomes, including mortality and persistence of bacteremia, have identified several potential prognostic markers for SAB that have the potential to better inform SAB treatment and improve SAB treatment outcomes. These include an elevated interleukin-10 (IL-10), which has been associated with increased mortality.2–4 An increase in the pro-inflammatory marker, interleukin-1β (IL-1β), has been associated with a reduction in prolonged bacteremia.4,5 The relationship between the pro-inflammatory tumor necrosis factor α (TNF α) and SAB outcomes has been studied with variable results.2–4 The high cost and limited availability of tests for these inflammatory markers in the clinical laboratory limit their use in routine patient care. The readily available complete blood count (CBC) panel is routinely ordered as a measure of the host inflammatory response for patients across the continuum of care. CBC subgroup parameters reported on the panel are affected by anti- and pro-inflammatory responses, suggesting that the results of a CBC could correlate with outcomes in patients with SAB; this would make them a potential prognostic tool readily available to patients and clinicians with access to a clinical laboratory.2,3
Leukocytosis with acute infection is commonly characterized by elevated neutrophils and reduced lymphocytes. Lymphopenia (lymphocyte count < 1 x 109 cells/L) has been associated with more severe infections and worse outcomes.6 The “neutrophil-lymphocyte count ratio” (NLCR) has been studied as a predictor of severity of disease and outcomes.6–13 The NLCR was associated with disease severity in oncologic and cardiovascular diseases.12,13 Emerging data suggest that the NLCR can predict the presence and outcomes of infectious diseases such as community acquired pneumonia and endocarditis.8,10 Leukocytosis, thrombocytopenia, lymphopenia, and eosinopenia have also been associated with worse SAB outcomes.14,15 An increased red blood cell distribution width in patients with bacteremia was associated with increased mortality as well.16
CBC subgroup parameters may have prognostic value in SAB and, if so, would represent a readily available alternative to cytokine measurements, with potential to impact patient care using commonly available laboratory testing equipment. The primary objective of this study was to determine the association between the NLCR on the day of index blood culture and 30-day all-cause mortality in SAB patients. We hypothesized that a high NLCR would be associated with an increased SAB mortality. Secondary objectives included ascertaining the associations among SAB mortality and leukocytosis, thrombocytopenia, lymphopenia, eosinopenia, and red blood cell distribution width.
METHODS
Design
This was a retrospective, multicenter, observational cohort study of SAB patients at 16 sites in a single U.S. healthcare system between June 1 and November 16, 2016. Patients ≥ 18 years of age with S. aureus who recovered from at least one blood culture bottle and for whom antibiotic therapy for S. aureus was initiated for ≥ 72 hours were eligible for study inclusion. For patients with more than one episode of SAB, only the index SAB was included for analysis.
Patients who may have altered CBC subgroup parameters at baseline, including patients with hematologic malignancy, hematologic disorders such as HIV or hemophilia, or treatment-induced neutropenia, were excluded. Patients with recent chemotherapy or radiation therapy, receiving immunosuppression therapy (including glucocorticoid therapy of more than 10 mg prednisone-equivalents daily), or receiving immunomodulation therapy such as biologic immunomodulators were excluded due to potential for an altered immune response with these therapies. Pregnant patients or patients with other immunocompromising conditions, including solid organ transplant, bone marrow transplant, and/or HIV, were also excluded. Patients lacking all CBC subgroup parameters from the same day as the index S. aureus blood culture were excluded. Additionally, patients who did not have an infectious diseases consult were excluded, as only patients who followed the best practices of the internal SAB bundle were to be evaluated.17
This study was reviewed and approved by the Aurora Health Care Research Subject Protection Program. This research was performed in accordance with the Declaration of Helsinki and conforms to the STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) recommendations for reporting observational studies.18
Data Collection
A database of electronic medical records was queried to identify patients with a positive blood culture for S. aureus. Retrospective electronic medical record review was then used to collect each patient’s age, gender, and comorbid conditions. Relevant comorbid conditions included hospitalization within the last year, intravenous drug use, chronic kidney disease with or without need for dialysis, chronic liver disease, diabetes, chronic lung disease, and chronic heart failure. Cultures and sensitivities were collected, including automated minimum inhibitory concentrations for all antibiotics tested. The bacteremia source was classified into three groups based on confirmed or suspected foci, as previously described by Rose et al:4 i) non-catheter primary endovascular, encompassing endocarditis (based on Modified Duke Criteria: either two major criteria or one major plus three minor criteria) and unknown or presumed endovascular sources; ii) secondary to another primary non-endovascular focus of infection (eg, lung, soft tissue, bone/joint); or iii) catheter source of infection. Clinical outcomes were measured including in-hospital mortality and 30-day all-cause mortality.
The PITT bacteremia score was calculated as a validated marker of disease severity. PITT bacteremia scores were calculated using scoring system variables for temperature, acute hypotensive events, receipt of mechanical ventilation, occurrence of cardiac arrest, and alteration in mental status reported within 24 hours of positive blood cultures.19 The CBC panel and differential type (automated or manual) from the day of the first positive S. aureus blood culture were used to record the white blood cell count, neutrophil count, lymphocyte count, NLCR, eosinophil count, platelet count, and red cell distribution width.
Statistical Analysis
The primary outcome was 30-day all-cause mortality after documented SAB. Categorical variables were expressed as numbers, percentages, and rates. Continuous variables were expressed as the mean, standard deviation (SD) or median, and the interquartile range (IQR), as appropriate. Categorical variables were compared using the chi-squared test or Fisher’s exact test, and continuous variables were compared using a two-sample t-test or the Wilcoxon rank sum test.
A forward, stepwise multivariable logistic regression model was utilized to establish the prognostic value of CBC subgroup parameters and 30-day all-cause mortality. The PITT bacteremia score and CBC subgroup parameters were analyzed as continuous variables to preserve power and minimize the risk of false positive associations that can occur when dichotomizing continuous data.20 Independent variables associated with 30-day all-cause mortality at an alpha of ≤ 0.05 in the univariable analysis were eligible for entry into the multivariable logistic regression model. Independent variables associated with 30-day all-cause mortality with an alpha of ≤ 0.10 were retained in the final model. The area under the receiver operating characteristic (ROC) curve was used to determine the discriminatory power of these measurements and their predictive value. All tests were 2-tailed, and a p-value of < 0.05 was considered statistically significant. SAS version 9.4 (SAS Institute, Cary, NC) was used for all analyses.
RESULTS
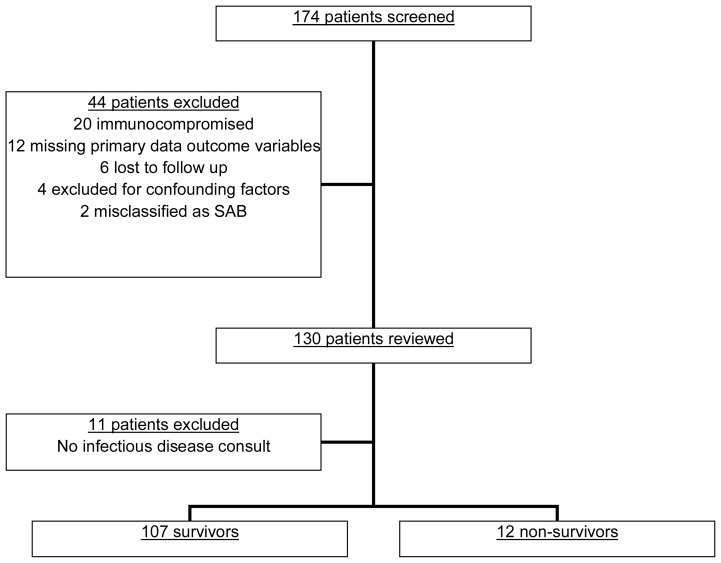
A total of 174 patients were screened, and 44 patients were excluded from the study (Figure 1). An additional 11 patients lacked an infectious diseases consultation and were excluded, leaving a final cohort of 119 patients. The overall 30-day all-cause mortality rate was 10.1%. Survivors were slightly younger (59 vs 70 years; p = .203) and had a lower incidence of congestive heart failure (23.4% vs 50%; p = .076). There were no differences in age, sex, ethnicity, prior hospitalizations, or other comorbid conditions between survivors and non-survivors (Table 1). The majority of SAB was caused by MSSA (68.9%). All patients received appropriate antibiotics within 24 hours of positive blood cultures.
Figure 1.
Patient eligibility screening.
Table 1.
Comparison of Those Who Died Within 30 Days vs. Those Who Surviveda
| Survival (n=107) | Mortality (n=12) | p-value | |
|---|---|---|---|
| Age | 59 (45–73) | 70 (49–76) | 0.203 |
| Male Gender | 64 (59.8) | 7 (58.3) | >0.999 |
| Caucasian | 92 (86.0) | 11 (91.7) | >0.999 |
| Prior Hospitalization | 53 (49.5) | 6 (50.0) | >0.999 |
| Comorbid Conditions | |||
| Immunocompromising Condition | 14 (13.1) | 3 (25.0) | 0.376 |
| Diabetes | 49 (45.8) | 5 (41.7) | >0.999 |
| Injection Drug Use | 18 (16.8) | 3 (25.0) | 0.442 |
| Chronic Lung Disease | 18(16.8) | 2 (16.7) | >0.999 |
| Renal Disease | 60 (56.1) | 8 (66.7) | 0.552 |
| Hemodialysis | 13 (12.2) | 1 (8.3) | >0.999 |
| Liver Disease | 16 (15.0) | 3 (25.0) | 0.404 |
| Congestive Heart Failure | 25 (23.4) | 6 (50.0) | 0.076 |
| Charlson Comorbidity Index | 4 (2–7) | 5 (2.5–7.0) | 0.512 |
| Methicillin-sensitive Staphylococcus aureus | 75 (70.1) | 7 (58.3) | 0.512 |
| Time to Blood Culture Positivity, Hours | 18 (15–21) | 14 (12–19) | 0.041 |
| Bacteremia Source | 0.047 | ||
| Endovascular | 11 (10.3) | 4 (33.3) | |
| Catheter or Secondary, Non-Endovascular | 96 (89.7) | 8 (66.7) | |
| PITT Bacteremia Score | 1 (0–2) | 4 (2–4.5) | <0.001 |
| Catheter or Prosthesis | 34 (31.8) | 2 (16.7) | 0.343 |
| Complete Blood Count Variables, Day of First Positive Blood Culture | |||
| White Blood Cell Count | 14.2 (10.6–20.2) | 17.8 (12.2–21.4) | 0.312 |
| Absolute Neutrophil Count | 12 (8.5–17.5) | 16.1 (11.4–19.3) | 0.173 |
| Lymphocyte Count | 0.9 (0.6–1.5) | 0.6 (0.3–0.9) | 0.030 |
| Neutrophil to Lymphocyte Count Ratio | 13.6 (8.4–21.3) | 23.2 (17.1–46.5) | 0.007 |
| Platelet Count | 239 (166–306) | 171 (114–195) | 0.018 |
| Eosinophil Count | 0 (0–0.1) | 0 (0–0) | 0.433 |
| Red Blood Cell Distribution Width | 14.2 (13.2–15.3) | 14.3 (13.3–17.5) | 0.740 |
| Automated Differential | 91 (85.0) | 9 (75.0) | 0.404 |
All data presented as No. (%) or median (interquartile range).
Survivors had a longer median time to blood culture positivity (18 hours vs 14 hours; p = .041) and a lower median PITT bacteremia score (1 vs 4; p < .001). Patients with an endovascular source of infection were more likely to die compared to patients with a secondary, non-endovascular or catheter source of infection (26.7% vs 8.3%; p = .047).
The CBC subgroup parameters on the day of positive blood cultures are reported in Table 1. The median NLCR for survivors was 13.6 compared to 23.2 for non-survivors (p = .007). The median absolute lymphocyte count was higher among survivors (0.9 x 103 cells/μL vs 0.6 x 103 cells/μL; p = .031). Finally, survivors had a higher absolute platelet count than survivors (239 x 103 cells/μL vs 171 x 103 cells/μL; p = .018).
CBC subgroup parameters were compared between those with endovascular and non-endovascular SAB sources. The only significant difference was platelet count with a lower absolute platelet count among those with an endovascular source of infection (190 x 103 cells/μL vs 239 x 103 cells/μL; p = .041).
After controlling for other patient-level characteristics, lower absolute lymphocyte count was an independent predictor of mortality (adjusted odds ratio [aOR], 0.236; 95% CI 0.064–0.872; Table 2), indicating that a lower absolute lymphocyte count increased the likelihood of mortality in the study population. Higher PITT bacteremia score was also an independent predictor of mortality (aOR, 2.439; 95% CI 1.565–3.803). Age was not an independent predictor of mortality in this study (aOR, 1.043; 95% CI 0.994–1.094). The final multivariable model was able to distinguish between mortality and survival with a good area under the ROC curve of 0.9046 (95% CI 0.8279–0.9814; p < .001), and the final model did not reach significance with the Hosmer-Lemeshow goodness-of-fit test (final model; p = .834), indicating that there was no evidence of a lack of model fit. All but two non-survivors had an absolute lymphocyte count of 1.0 x 103 cells/μL or less (1.1 x 103 cells/μL and 3.9 x 103 cells/μL).
Table 2.
Association Between Potential Predictor Variables and Mortality Among Patients With S. aureus Bloodstream Infection (n=119)a
| Variable, on the day of first positive blood culture | Adjusted OR | 95% CI | p-value |
|---|---|---|---|
| PITT bacteremia score | 2.439 | 1.565–3.803 | <0.001 |
| Lymphocyte count, day of first positive blood culture | 0.236 | 0.064–0.872 | 0.031 |
| Age | 1.043 | 0.994–1.094 | 0.081 |
The final multivariable model was able to distinguish between mortality and survival with a good area under the receiver operating characteristics curve of 0.9046 (95% CI 0.8279–0.9814; p < .001).
The final model did not reach significance with the Hosmer-Lemeshow goodness-of-fit test (final model; p = 0.834), indicating that the overall model fit is good.
DISCUSSION
In this study, a lower absolute lymphocyte count and higher PITT bacteremia score were independently associated with increased 30-day all-cause mortality among SAB patients. A decreased platelet count and increased NLCR were associated with SAB mortality but not when adjusting for other independent variables. Other variables associated with SAB mortality, such as older age, decreased time to detection of S. aureus in blood cultures, and the PITT bacteremia score, were observed in this study population, which is consistent with existing data.21,22
Lower lymphocyte counts among SAB non-survivors may be attributable to a dysregulated host response and/or S. aureus virulence. A decreased platelet count may also reflect disease severity, which has been suggested previously to be a result of S. aureus virulence.15 A prior study found eosinopenia was a significant marker of bacteremia in critically ill patients.14 Another study found eosinopenia persistence, and an elevated NLCR presaged poor clinical outcomes, including mortality, in patients with bacteremia or fungemia.6 In the current study, eosinopenia alone was not associated with increased SAB mortality. This may be a result of the smaller study sample and narrow range of eosinophil counts in the study population.
We examined the NLCR as an alternative measure of host immune response in SAB based on its prognostic value in other, similar infectious processes. Greenberg et al examined the NLCR on the first day of SAB, finding that non-survivors had a significantly higher ratio than survivors (27 vs 15, p = .01).3 While NLCRs were numerically higher among non-survivors in our study, only the absolute lymphocyte count, not the NLCR, was independently associated with SAB mortality in our cohort, despite the impact of lymphocyte count on the NLCR. This observation suggests that the readily available absolute lymphocyte count may have a prognostic role in SAB.
It is possible that CBC subgroup parameter changes reflect cytokine alterations described in previous SAB studies.4,5 One proposed mechanism for the increase in the anti-inflammatory cytokine, IL-10, includes immune stimulation by S. aureus peptidoglycan. Rose et al reported a correlation between SAB inoculum and IL-10 serum concentration.4 A higher S. aureus inoculum may contribute to greater toxin production, worsening a patient’s prognosis. Increased IL-10 was also more frequently seen in patients with an endovascular SAB source. IL-10 modulates CD8+ T lymphocytes in acute viral infections; specifically, higher IL-10 levels initiate contraction of antiviral T lymphocyte populations.23 Future studies should examine whether elevated IL-10 in SAB is associated with the observation of a reduced absolute lymphocyte count in those with higher mortality.
CBC subgroup parameters are inexpensive and routinely collected from hospitalized patients suspected of having an acute infection such as bacteremia. In the absence of serum cytokine measurements, using the absolute lymphocyte count as a prognostic tool for SAB patients may be appropriate for infectious diseases physicians and antimicrobial stewards working to optimize SAB treatment and triage. Practically, an absolute lymphocyte count of 1.0 x 103 cells/μL or less may be a reasonable threshold to use if applying the results of this study to clinical decisions, for example, identifying patients at higher risk of mortality for whom escalation of care may be prudent. Lymphopenia is often defined as an absolute lymphocyte count of less than 1.0 x 103 cells/μL, and only two non-survivors had a lymphocyte count greater than 1.0 x 103 cells/μL. However, this threshold remains speculative for clinical use, requiring additional testing and validation.
This study has several limitations. The retrospective design is subject to bias and unmeasured confounding. However, we performed a multivariable analysis that identified known predictors of SAB mortality in addition to a lower absolute lymphocyte count. Given that treatment for SAB can be longer than 4 weeks, it is possible that we missed poor outcomes beyond the 30-day period. High NLCR has been associated with poor outcomes in several disease states, so it is possible that the observed association in previous studies between NLCR and poor SAB outcomes is due to underlying illness beyond SAB. Similarly, we did not include a non-SAB treatment arm, so lymphopenia could have also been due to underlying degree of illness. There was variation in how the CBC differential was performed (Table 1). Values obtained by automated and manual methods may differ slightly, obscuring interpretation of our results. The smaller sample size and single health-system design may limit the generalizability of our findings. Internal validity should be high, but all 16 hospitals were part of the healthcare system with the same SAB treatment bundle and antimicrobial stewardship surveillance. Most patients (67%) had MSSA, which is consistent with lower rates of MRSA in recent years.24 However, MRSA is associated with a higher mortality rate than MSSA, so our findings may be less generalizable to patients with MRSA bacteremia. Finally, beyond antibiotic sensitivities, we neither collected nor determined any additional characteristics of these S. aureus isolates.
CONCLUSIONS
S. aureus bacteremia remains a leading cause of infectious disease-related morbidity and mortality, affecting patients across the spectrum of age and comorbidity. While antibiotic treatments have advanced, there remains a lack of readily available prognostic tools for clinician use. In this study, we identified the prognostic value of the absolute lymphocyte count for SAB mortality. Additional study, with a larger sample size and a comparison of CBC subgroup parameters to cytokines known to be prognostic in SAB, is warranted to further ascertain the prognostic value of these readily available laboratory values.
Acknowledgments
This work was presented at the 2020 Society of Critical Care Medicine meeting, abstract number 578.
Footnotes
Author Contributions: Study design: All. Data acquisition or analysis: All. Manuscript drafting: All. Critical revision: All.
Conflicts of Interest: None.
References
- 1.van Hal SJ, Jensen SO, Vaska VL, et al. Predictors of mortality in Staphylococcus aureus bacteremia. Clin Microbiol Rev. 2012;25:362–86. doi: 10.1128/CMR.05022-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Minejima E, Bensman J, She RC, et al. A dysregulated balance of proinflammatory and anti-inflammatory host cytokine response early during therapy predicts persistence and mortality in Staphylococcus aureus bacteremia. Crit Care Med. 2016;44:671–9. doi: 10.1097/CCM.0000000000001465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Greenberg JA, Hrusch CL, Jaffery MR, et al. Distinct T-helper cell responses to Staphylococcus aureus bacteremia reflect immunologic comorbidities and correlate with mortality. Crit Care. 2018;22:107. doi: 10.1186/s13054-018-2025-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rose WE, Shukla SK, Berti AD, et al. Increased endovascular Staphylococcus aureus inoculum is the link between elevated serum interleukin 10 concentrations and mortality in patients with bacteremia. Clin Infect Dis. 2017;64:1406–12. doi: 10.1093/cid/cix157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rose WE, Eickhoff JC, Shukla SK, et al. Elevated serum interleukin-10 at time of hospital admission is predictive of mortality in patients with Staphylococcus aureus bacteremia. J Infect Dis. 2012;206:1604–11. doi: 10.1093/infdis/jis552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Jager CPC, van Wijk PTL, Mathoera RB, et al. Lymphocytopenia and neutrophil-lymphocyte count ratio predict bacteremia better than conventional infection markers in an emergency care unit. Crit Care. 2010;14:R192. doi: 10.1186/cc9309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Terradas R, Grau S, Blanch J, et al. Eosinophil count and neutrophil-lymphocyte count ratio as prognostic markers in patients with bacteremia: a retrospective cohort study PLoS One 20127e42860 10.1371/journal.pone.0042860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Jager CPC, Wever PC, Gemen EFA, et al. The neutrophil-lymphocyte count ratio in patients with community-acquired pneumonia PLoS One 20127e46561 10.1371/journal.pone.0046561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lowsby R, Gomes C, Jarman I, et al. Neutrophil to lymphocyte count ratio as an early indicator of blood stream infection in the emergency department. Emerg Med J. 2015;32:531–4. doi: 10.1136/emermed-2014-204071. [DOI] [PubMed] [Google Scholar]
- 10.Turak O, Özcan F, Işleyen A, et al. Usefulness of neutrophil-to-lymphocyte ratio to predict in-hospital outcomes in infective endocarditis. Can J Cardiol. 2013;29:1672–8. doi: 10.1016/j.cjca.2013.05.005. [DOI] [PubMed] [Google Scholar]
- 11.Yang M, Li L, Su N, et al. Dynamic monitoring of the neutrophil/lymphocyte ratio could predict the prognosis of patients with bloodstream infection. Chinese Critical Care Medicine. 2015;27:471–6. doi: 10.3760/cma.j.issn.2095-4352.2015.06.011. [DOI] [PubMed] [Google Scholar]
- 12.Howard R, Kanetsky PA, Egan KM. Exploring the prognostic value of the neutrophil-to-lymphocyte ratio in cancer. Sci Rep. 2019;9:19673. doi: 10.1038/s41598-019-56218-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tamaki S, Nagai Y, Shutta R, et al. Combination of neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios as a novel predictor of cardiac death in patients with acute decompensated heart failure with preserved left ventricular ejection fraction: a multicenter study. J Am Heart Assoc. 2023;12:e026326. doi: 10.1161/JAHA.122.026326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ho KM, Towler SC. A comparison of eosinopenia and C-reactive protein as a marker of bloodstream infections in critically ill patients: a case control study. Anaesth Intensive Care. 2009;37:450–6. doi: 10.1177/0310057X0903700319. [DOI] [PubMed] [Google Scholar]
- 15.Gafter-Gvili A, Mansur N, Bivas A, et al. Thrombocytopenia in Staphylococcus aureus bacteremia: risk factors and prognostic importance. Mayo Clin Proc. 2011;86:389–96. doi: 10.4065/mcp.2010.0705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yeh CF, Chen KF, Ye JR, et al. Derivation of a clinical prediction rule for bloodstream infection mortality of patients visiting the emergency department based on predisposition, infection, response, and organ dysfunction concept. J Microbiol Immunol Infect. 2014;47:469–77. doi: 10.1016/j.jmii.2013.06.012. [DOI] [PubMed] [Google Scholar]
- 17.López-Cortés LE, del Toro MD, Gálvez-Acebal J, et al. Impact of an evidence-based bundle intervention in the quality-of-care management and outcome of Staphylococcus aureus bacteremia. Clin Infect Dis. 2013;57:1225–33. doi: 10.1093/cid/cit499. [DOI] [PubMed] [Google Scholar]
- 18.von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Prev Med. 2007;45:247–51. doi: 10.1016/j.ypmed.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 19.Al-Hasan MN, Baddour LM. Resilience of the Pitt bacteremia score: 3 decades and counting. Clin Infect Dis. 2020;70:1834–6. doi: 10.1093/cid/ciz535. [DOI] [PubMed] [Google Scholar]
- 20.Altman DG, Royston P. The cost of dichotomising continuous variables. BMJ. 2006;332:1080. doi: 10.1136/bmj.332.7549.1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Siméon S, Le Moing V, Tubiana S, et al. Time to blood culture positivity: an independent predictor of infective endocarditis and mortality in patients with Staphylococcus aureus bacteremia. Clin Microbiol Infect. 2019;25:481–8. doi: 10.1016/j.cmi.2018.07.015. [DOI] [PubMed] [Google Scholar]
- 22.Liao CH, Chen SY, Huang YT, et al. Outcome of patients with meticillin-resistant Staphylococcus aureus bacteraemia at an emergency department of a medical centre in Taiwan. Int J Antimicrob Agents. 2008;32:326–32. doi: 10.1016/j.ijantimicag.2008.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rojas JM, Avia M, Martín V, et al. IL-10: a multifunctional cytokine in viral infections. J Immunol Res. 2017;2017:6104054. doi: 10.1155/2017/6104054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jackson KA, Gokhale RH, Nadle J, et al. Public health importance of invasive methicillin-sensitive Staphylococcus aureus infections: surveillance in 8 US counties, 2016. Clin Infect Dis. 2020;70:1021–8. doi: 10.1093/cid/ciz323. [DOI] [PMC free article] [PubMed] [Google Scholar]