Abstract
Histones and polyamines nick the phosphodiester bond 3' to AP (apurinic/apyrimidinic) sites in DNA by inducing a beta-elimination reaction, which can be followed by delta-elimination. These beta- and delta-elimination reactions might be important for the repair of AP sites in chromatin DNA in either of two ways. In one pathway, after the phosphodiester bond 5' to the AP site has been hydrolysed with an AP endonuclease, the 5'-terminal base-free sugar 5'-phosphate is released by beta-elimination. The one-nucleotide gap limited by 3'-OH and 5'-phosphate ends is then closed by DNA polymerase-beta and DNA ligase. We have shown in vitro that such a repair is possible. In the other pathway, the nicking 3' to the AP site by beta-elimination occurs first. We have shown that the 3'-terminal base-free sugar so produced cannot be released by the chromatin AP endonuclease from rat liver. But it can be released by delta-elimination, leaving a gap limited by 3'-phosphate and 5'-phosphate. After conversion of the 3'-phosphate into a 3'-OH group by the chromatin 3'-phosphatase, there will be the same one-nucleotide gap, limited by 3'-OH and 5'-phosphate, as that formed by the successive actions of the AP endonuclease and the beta-elimination catalyst in the first pathway.
Full text
PDF

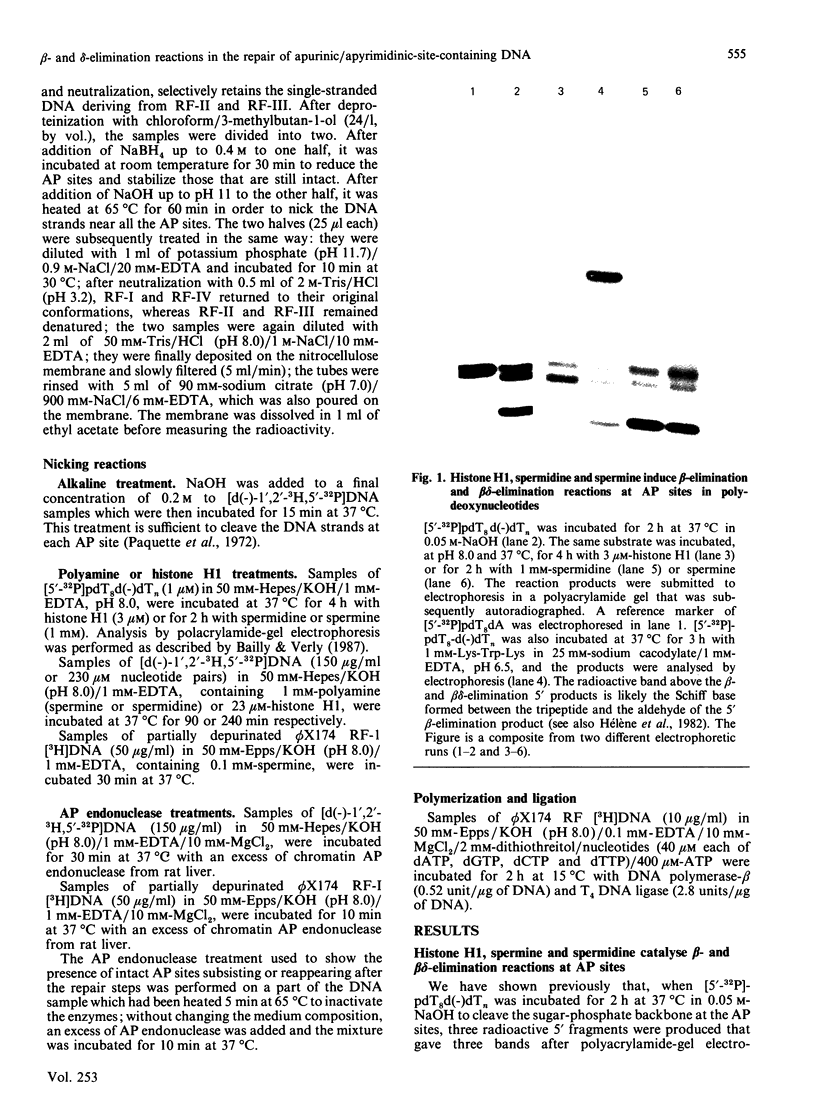

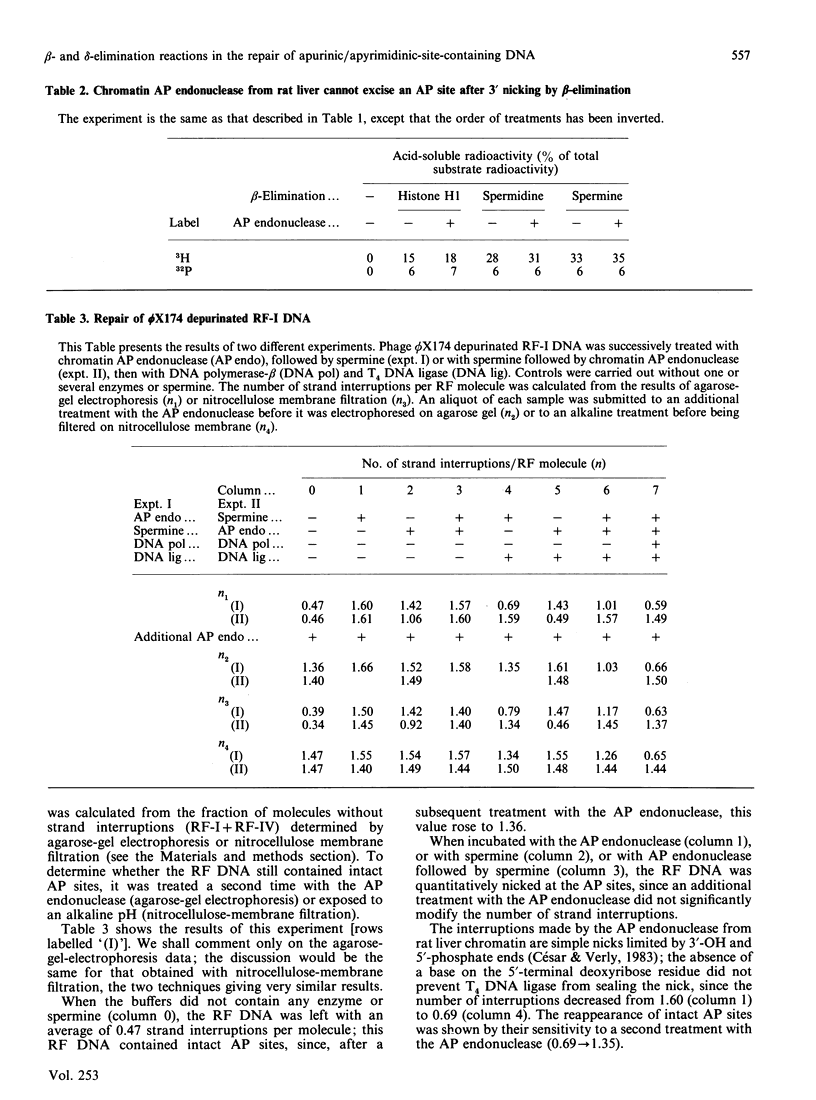


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Axelrod N. Transcription of bacteriophage phi-X174 in vitro: selective initiation with oligonucleotides. J Mol Biol. 1976 Dec 25;108(4):753–770. doi: 10.1016/s0022-2836(76)80115-5. [DOI] [PubMed] [Google Scholar]
- Bailly V., Verly W. G. Escherichia coli endonuclease III is not an endonuclease but a beta-elimination catalyst. Biochem J. 1987 Mar 1;242(2):565–572. doi: 10.1042/bj2420565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailly V., Verly W. G. The excision of AP sites by the 3'-5' exonuclease activity of the Klenow fragment of Escherichia coli DNA polymerase I. FEBS Lett. 1984 Dec 10;178(2):223–227. doi: 10.1016/0014-5793(84)80605-5. [DOI] [PubMed] [Google Scholar]
- Behmoaras T., Toulmé J. J., Hélène C. A tryptophan-containing peptide recognizes and cleaves DNA at apurinic sites. Nature. 1981 Aug 27;292(5826):858–859. doi: 10.1038/292858a0. [DOI] [PubMed] [Google Scholar]
- Clements J. E., Rogers S. G., Weiss B. A DNase for apurinic/apyrimidinic sites associated with exonuclease III of Hemophilus influenzae. J Biol Chem. 1978 May 10;253(9):2990–2999. [PubMed] [Google Scholar]
- César R., Verly W. G. The apurinic/apyrimidinic endodeoxyribonuclease of rat-liver chromatin. Eur J Biochem. 1983 Jan 1;129(3):509–517. doi: 10.1111/j.1432-1033.1983.tb07077.x. [DOI] [PubMed] [Google Scholar]
- Frechette A., Huletsky A., Aubin R. J., de Murcia G., Mandel P., Lord A., Grondin G., Poirier G. G. Poly(ADP-ribosyl)ation of chromatin: kinetics of relaxation and its effect on chromatin solubility. Can J Biochem Cell Biol. 1985 Jul;63(7):764–773. doi: 10.1139/o85-096. [DOI] [PubMed] [Google Scholar]
- Goffin C., Bailly V., Verly W. G. Nicks 3' or 5' to AP sites or to mispaired bases, and one-nucleotide gaps can be sealed by T4 DNA ligase. Nucleic Acids Res. 1987 Nov 11;15(21):8755–8771. doi: 10.1093/nar/15.21.8755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goffin C., Verly W. G. Excision of apurinic sites from DNA with enzymes isolated from rat-liver chromatin. Eur J Biochem. 1982 Oct;127(3):619–623. doi: 10.1111/j.1432-1033.1982.tb06917.x. [DOI] [PubMed] [Google Scholar]
- Goffin C., Verly W. G. Repair of depurinated DNA with enzymes from rat liver chromatin. Biochem J. 1984 May 15;220(1):133–137. doi: 10.1042/bj2200133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gossard F., Verly W. G. Properties of the main endonuclease specific for apurinic sites of Escherichia coli (endonuclease VI). Mechanism of apurinic site excision from DNA. Eur J Biochem. 1978 Jan 16;82(2):321–332. doi: 10.1111/j.1432-1033.1978.tb12026.x. [DOI] [PubMed] [Google Scholar]
- Grondal-Zocchi G., Verly W. G. Deoxyribonuclease IV from rat liver chromatin and the excision of apurinic sites from depurinated DNA. Biochem J. 1985 Jan 15;225(2):535–542. doi: 10.1042/bj2250535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habraken Y., Verly W. G. The DNA 3'-phosphatase and 5'-hydroxyl kinase of rat liver chromatin. FEBS Lett. 1983 Aug 22;160(1-2):46–50. doi: 10.1016/0014-5793(83)80933-8. [DOI] [PubMed] [Google Scholar]
- Helene C., Toulme J. J., Behmoaras T., Cazenave C. Mechanisms for the recognition of chemically-modified DNA by peptides and proteins. Biochimie. 1982 Aug-Sep;64(8-9):697–705. doi: 10.1016/s0300-9084(82)80113-2. [DOI] [PubMed] [Google Scholar]
- Henner W. D., Grunberg S. M., Haseltine W. A. Sites and structure of gamma radiation-induced DNA strand breaks. J Biol Chem. 1982 Oct 10;257(19):11750–11754. [PubMed] [Google Scholar]
- Lindahl T., Andersson A. Rate of chain breakage at apurinic sites in double-stranded deoxyribonucleic acid. Biochemistry. 1972 Sep 12;11(19):3618–3623. doi: 10.1021/bi00769a019. [DOI] [PubMed] [Google Scholar]
- Lindahl T., Gally J. A., Edelman G. M. Deoxyribonuclease IV: a new exonuclease from mammalian tissues. Proc Natl Acad Sci U S A. 1969 Feb;62(2):597–603. doi: 10.1073/pnas.62.2.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Male R., Fosse V. M., Kleppe K. Polyamine-induced hydrolysis of apurinic sites in DNA and nucleosomes. Nucleic Acids Res. 1982 Oct 25;10(20):6305–6318. doi: 10.1093/nar/10.20.6305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDONALD M. R., KAUFMANN B. P. The degradation by ribonuclease of substrates other than ribonucleic acid. J Histochem Cytochem. 1954 Sep;2(5):387–394. doi: 10.1177/2.5.387. [DOI] [PubMed] [Google Scholar]
- Mosbaugh D. W., Linn S. Excision repair and DNA synthesis with a combination of HeLa DNA polymerase beta and DNase V. J Biol Chem. 1983 Jan 10;258(1):108–118. [PubMed] [Google Scholar]
- Mosbaugh D. W., Linn S. Further characterization of human fibroblast apurinic/apyrimidinic DNA endonucleases. The definition of two mechanistic classes of enzyme. J Biol Chem. 1980 Dec 25;255(24):11743–11752. [PubMed] [Google Scholar]
- Mosbaugh D. W., Meyer R. R. Interaction of mammalian deoxyribonuclease V, a double strand 3' to 5' and 5' to 3' exonuclease, with deoxyribonucleic acid polymerase-beta from the Novikoff hepatoma. J Biol Chem. 1980 Nov 10;255(21):10239–10247. [PubMed] [Google Scholar]
- Paquette Y., Crine P., Verly W. G. Properties of the endonuclease for depurinated DNA from Escherichia coli. Can J Biochem. 1972 Nov;50(11):1199–1209. doi: 10.1139/o72-163. [DOI] [PubMed] [Google Scholar]
- Pierre J., Laval J. Specific nicking of DNA at apurinic sites by peptides containing aromatic residues. J Biol Chem. 1981 Oct 25;256(20):10217–10220. [PubMed] [Google Scholar]
- RICHARDSON C. C., SCHILDKRAUT C. L., APOSHIAN H. V., KORNBERG A. ENZYMATIC SYNTHESIS OF DEOXYRIBONUCLEIC ACID. XIV. FURTHER PURIFICATION AND PROPERTIES OF DEOXYRIBONUCLEIC ACID POLYMERASE OF ESCHERICHIA COLI. J Biol Chem. 1964 Jan;239:222–232. [PubMed] [Google Scholar]
- Richards R. G., Shaw B. R. In situ protamine release: a versatile sample preparation method for the electrophoretic analysis of nuclear proteins on acid/urea-based gels. Anal Biochem. 1982 Mar 15;121(1):69–82. doi: 10.1016/0003-2697(82)90558-9. [DOI] [PubMed] [Google Scholar]
- TAMM C., SHAPIRO H. S., CHARGAFF E. Correlation between the action of pancreatic desoxyribonuclease and the nature of its substrates. J Biol Chem. 1952 Nov;199(1):313–327. [PubMed] [Google Scholar]
- Thibodeau L., Verly W. G. Cellular localization of the apurinic/apyrimidinic endodeoxyribonucleases in rat liver. Eur J Biochem. 1980 Jun;107(2):555–563. doi: 10.1111/j.1432-1033.1980.tb06063.x. [DOI] [PubMed] [Google Scholar]
- Thibodeau L., Verly W. G. Purification and properties of a plant endonuclease specific for apurinic sites. J Biol Chem. 1977 May 25;252(10):3304–3309. [PubMed] [Google Scholar]
- Verly W. G., Paquette Y. An endonuclease for depurinated DNA in rat liver. Can J Biochem. 1973 Jul;51(7):1003–1009. doi: 10.1139/o73-130. [DOI] [PubMed] [Google Scholar]
- Verly W. G., Paquette Y., Thibodeau L. Nuclease for DNA apurinic sites may be involved in the maintenance of DNA in normal cells. Nat New Biol. 1973 Jul 18;244(133):67–69. doi: 10.1038/newbio244067a0. [DOI] [PubMed] [Google Scholar]
- van der Westhuyzen D. R., von Holt C. A new procedure for the isolation and fractionation of histones. FEBS Lett. 1971 May 20;14(5):333–337. doi: 10.1016/0014-5793(71)80294-6. [DOI] [PubMed] [Google Scholar]