Abstract
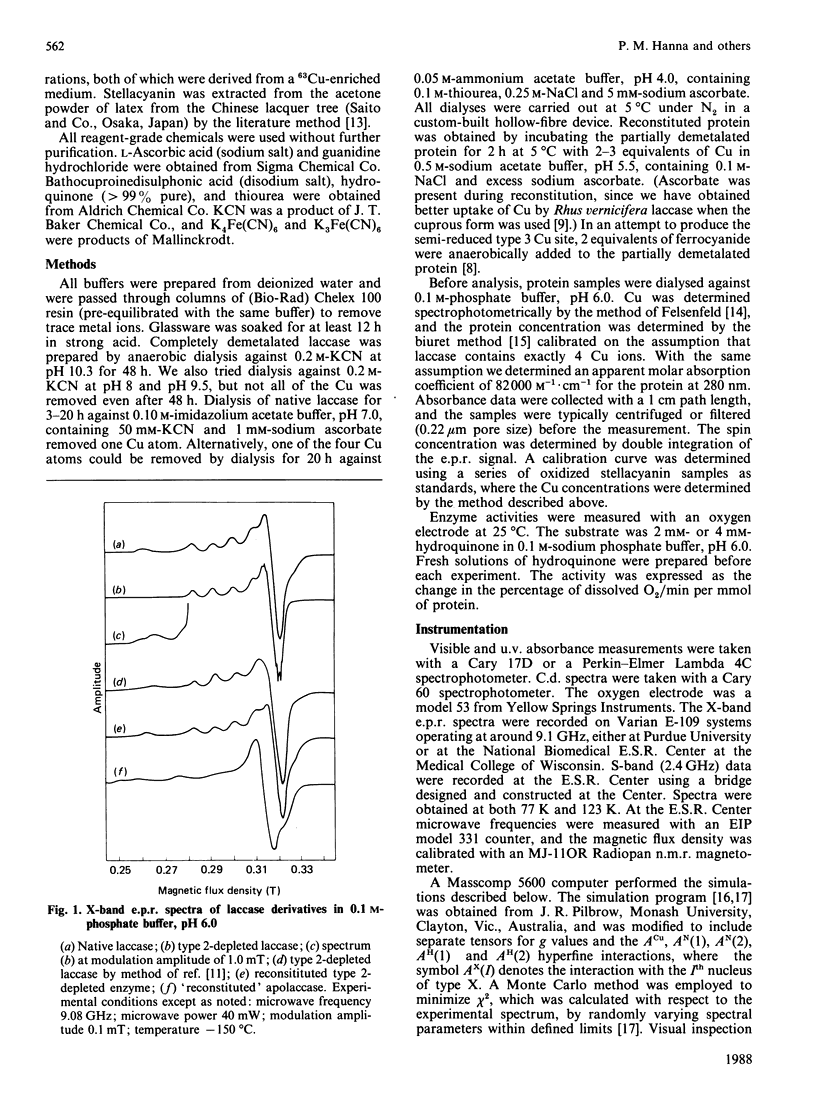
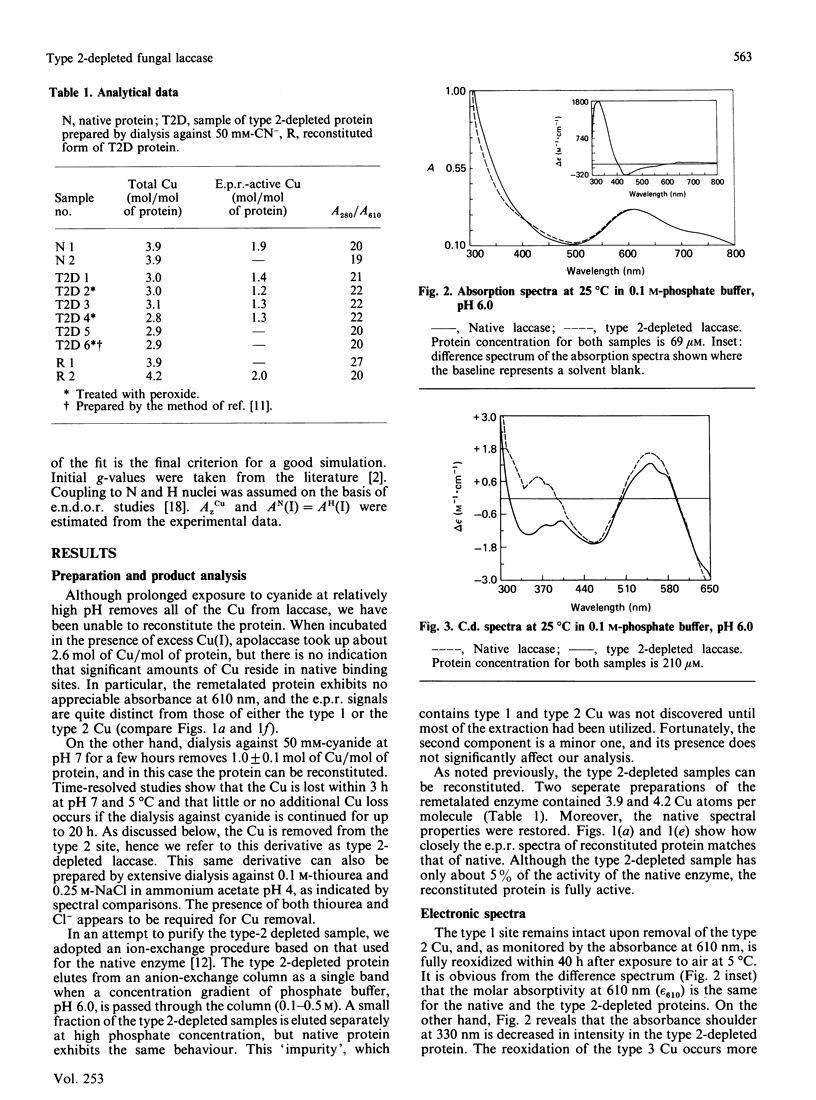
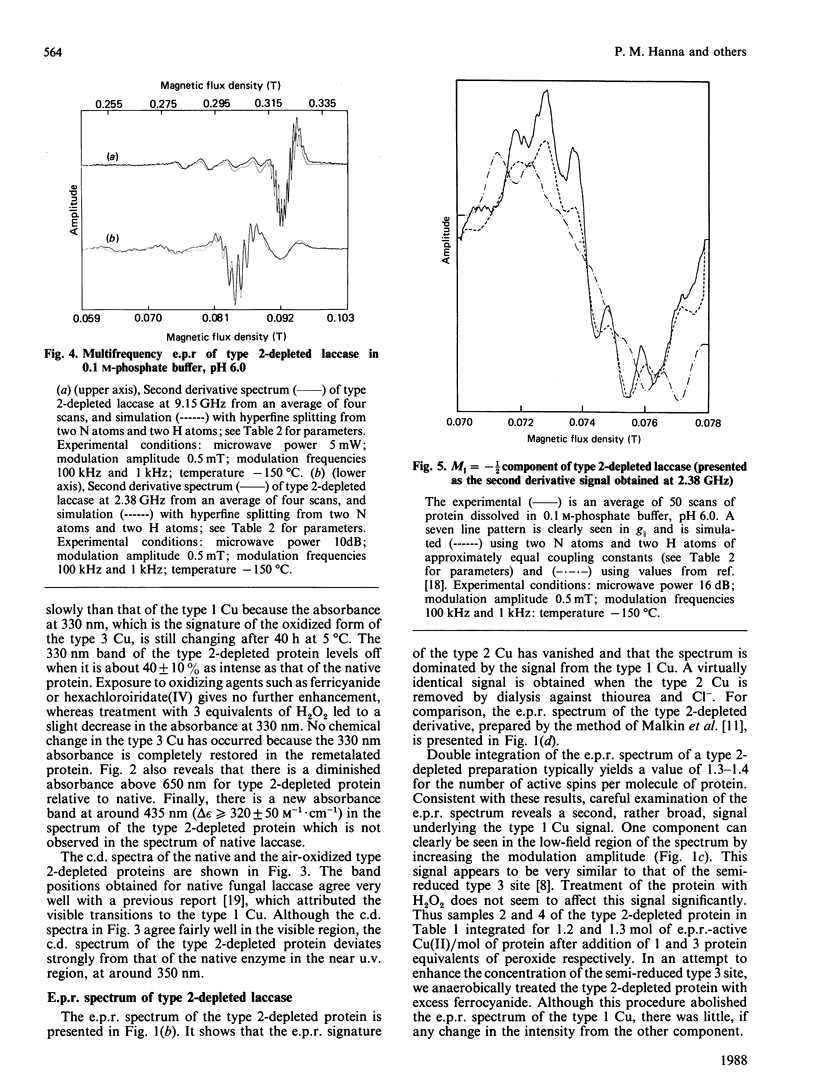
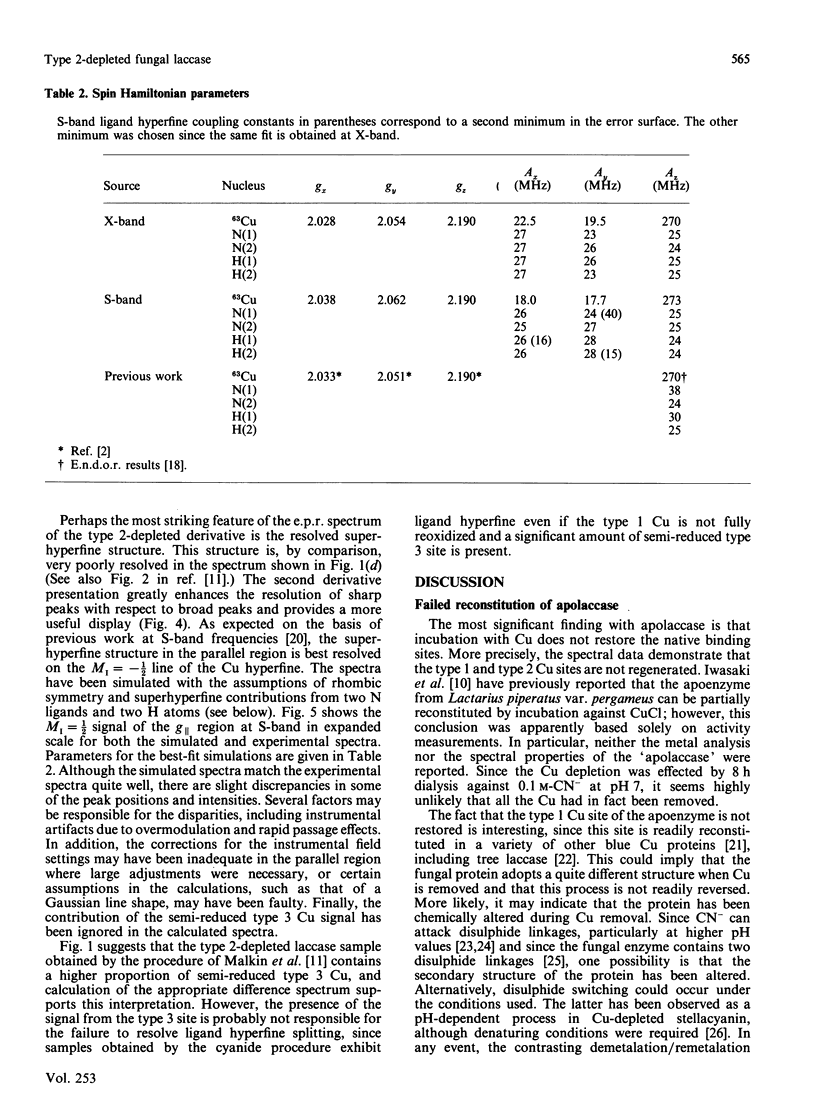
Although copper is quantitatively removed from fungal laccase (Polyporus versicolor) by extended dialysis against high concentrations of cyanide, we have been unable to reconstitute the protein by addition of Cu(I) ions. However, two new methods for reversibly removing the type 2 Cu centre have been developed. The visible absorption at 610 nm, which is attributable to type 1 Cu, is unaffected by the procedure, but the absorbance of the type 3 Cu at 330 nm is decreased by 60 +/- 10%. The decrease is due, at least in part, to partial reduction of the binuclear type 3 centre, although there may be some change in the molar absorptivity of the oxidized chromophore as well. The change in the c.d. spectrum that occurs at approx. 350 nm may be explained in the same way, but it may also reflect the loss of a signal due to the type 2 Cu. Upon removal of the type 2 Cu an absorbance increase appears at approx. 435 nm, and it is assigned to the semi-reduced form of the type 3 pair. In the e.p.r. spectrum of the type 2-depleted enzyme the type 1 Cu signal exhibits well-resolved ligand hyperfine splitting, which can be simulated on the basis of contributions from two N and two H nuclei (AH congruent to AN congruent to 25 MHz). The H atoms are assumed to be attached to the beta-carbon of the covalently bonded cysteine ligand. A signal from a semi-reduced form(s) of the type 3 site can also be resolved in the spectrum of the type 2-depleted enzyme, and on the basis of the second integral of the e.p.r. spectrum 40% of the type 3 pairs are believed to be in a partially reduced state. The semi-reduced type 3 site is remarkably stable and is not readily oxidized by H2O2 or IrCl6(2-) or reduced by Fe(CN)6(4-). Intramolecular electron transfer is apparently quite slow in at least some forms of the type 2-depleted enzyme, and this may explain why the activity is at best 5% of that of the native enzyme. Full activity returns when type 2 copper is restored.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andréasson L. E., Brändén R., Malmström B. G., Vänngård T. An intermediate in the reaction of reduced laccase with oxygen. FEBS Lett. 1973 May 15;32(1):187–189. doi: 10.1016/0014-5793(73)80768-9. [DOI] [PubMed] [Google Scholar]
- Andréasson L. E., Brändén R., Reinhammar B. Kinetic studies of Rhus vernicifera laccase. Evidence for multi-electron transfer and an oxygen intermediate in the reoxidation reaction. Biochim Biophys Acta. 1976 Jul 8;438(2):370–379. doi: 10.1016/0005-2744(76)90254-0. [DOI] [PubMed] [Google Scholar]
- Andréasson L. E., Reinhammar B. The mechanism of electron transfer in laccase-catalysed reactions. Biochim Biophys Acta. 1979 May 10;568(1):145–156. doi: 10.1016/0005-2744(79)90282-1. [DOI] [PubMed] [Google Scholar]
- Briving C., Gandvik E. K., Nyman P. O. Structural studies around cysteine and cystine residues in the "blue" oxidase fungal laccase B. Similarity in amino acid sequence with ceruloplasmin. Biochem Biophys Res Commun. 1980 Mar 28;93(2):454–461. doi: 10.1016/0006-291x(80)91099-2. [DOI] [PubMed] [Google Scholar]
- Brändén R., Deinum J. Type 2 copper (II) as a component of the dioxygen reducing site in laccase: evidence from EPR experiments with 17O. FEBS Lett. 1977 Feb 1;73(2):144–146. doi: 10.1016/0014-5793(77)80967-8. [DOI] [PubMed] [Google Scholar]
- Brändén R., Malmström B. G., Vänngård T. The interaction of fungal laccase with hydrogen peroxide and the removal of fluoride from the inhibited enzyme. Eur J Biochem. 1971 Jan;18(2):238–241. doi: 10.1111/j.1432-1033.1971.tb01236.x. [DOI] [PubMed] [Google Scholar]
- Engeseth H. R., Hermodson M. A., McMillin D. R. A new assignment of the disulfide linkage in stellacyanin. FEBS Lett. 1984 Jun 11;171(2):257–261. doi: 10.1016/0014-5793(84)80499-8. [DOI] [PubMed] [Google Scholar]
- FELSENFELD G. The determination of cuprous ion in copper proteins. Arch Biochem Biophys. 1960 Apr;87:247–251. doi: 10.1016/0003-9861(60)90168-5. [DOI] [PubMed] [Google Scholar]
- Falk K. E., Reinhammar B. Visible and near-infrared circular dichroism of some blue copper proteins. Biochim Biophys Acta. 1972 Nov 28;285(1):84–90. doi: 10.1016/0005-2795(72)90182-1. [DOI] [PubMed] [Google Scholar]
- Frank P., Farver O., Pecht I. The Type 3 copper site is intact but labile in Type 2-depleted laccase. J Biol Chem. 1983 Sep 25;258(18):11112–11117. [PubMed] [Google Scholar]
- Fåhraeus G., Reinhammar B. Large scale production and purification of laccase from cultures of the fungus Polyporus versicolor and some properties of laccase A. Acta Chem Scand. 1967;21(9):2367–2378. doi: 10.3891/acta.chem.scand.21-2367. [DOI] [PubMed] [Google Scholar]
- GOA J. A micro biuret method for protein determination; determination of total protein in cerebrospinal fluid. Scand J Clin Lab Invest. 1953;5(3):218–222. doi: 10.3109/00365515309094189. [DOI] [PubMed] [Google Scholar]
- Graziani M. T., Morpurgo L., Rotilio G., Mondovì B. Selective removal of type 2 copper from Rhus vernicifera laccase. FEBS Lett. 1976 Nov;70(1):87–90. doi: 10.1016/0014-5793(76)80732-6. [DOI] [PubMed] [Google Scholar]
- Hahn J. E., Co M. S., Spira D. J., Hodgson K. O., Solomon E. I. Quantitative Cu(I) determination using X-ray absorption edge spectroscopy: oxidation of the reduced binuclear copper site in type 2 depleted Rhus laccase. Biochem Biophys Res Commun. 1983 Apr 29;112(2):737–745. doi: 10.1016/0006-291x(83)91524-3. [DOI] [PubMed] [Google Scholar]
- Iwasaki H., Matsubara T., Mori T. A fungal laccase, its properties and reconstitution from its protein and copper. J Biochem. 1967 Jun;61(6):814–816. doi: 10.1093/oxfordjournals.jbchem.a128618. [DOI] [PubMed] [Google Scholar]
- Malkin R., Malmström B. G. The state and function of copper in biological systems. Adv Enzymol Relat Areas Mol Biol. 1970;33:177–244. doi: 10.1002/9780470122785.ch4. [DOI] [PubMed] [Google Scholar]
- Malkin R., Malmström B. G., Vänngård T. Spectroscopic differentiation of the electron-accepting sites in fungal laccase. Association of a near ultraviolet band with a two electron-accepting unit. Eur J Biochem. 1969 Sep;10(2):324–329. doi: 10.1111/j.1432-1033.1969.tb00693.x. [DOI] [PubMed] [Google Scholar]
- Malkin R., Malmström B. G., Vänngård T. The reversible removal of one specific copper(II) from fungal laccase. Eur J Biochem. 1969 Jan;7(2):253–259. doi: 10.1111/j.1432-1033.1969.tb19600.x. [DOI] [PubMed] [Google Scholar]
- Morpurgo L., Graziani M. T., Finazzi-Agrò A., Rotilio G., Mondovì B. Optical properties of japanese-lacquer-tree (Rhus vernicifera) laccase depleted of type 2 copper(II). Involvement of type-2 copper(II) in the 330nm chromophore. Biochem J. 1980 May 1;187(2):361–366. doi: 10.1042/bj1870361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penner-Hahn J. E., Hedman B., Hodgson K. O., Spira D. J., Solomon E. I. On the spectral features associated with peroxide reactivity of the coupled binuclear copper active site in type 2 depleted and native Rhus laccase. Biochem Biophys Res Commun. 1984 Mar 15;119(2):567–574. doi: 10.1016/s0006-291x(84)80286-7. [DOI] [PubMed] [Google Scholar]
- Rakhit G., Antholine W. E., Froncisz W., Hyde J. S., Pilbrow J. R., Sinclair G. R., Sarkar B. Direct evidence of nitrogen coupling in the copper(II) complex of bovine serum albumin by S-band electron spin resonance technique. J Inorg Biochem. 1985 Nov;25(3):217–224. doi: 10.1016/0162-0134(85)80015-5. [DOI] [PubMed] [Google Scholar]
- Reinhammar B., Malkin R., Jensen P., Karlsson B., Andréasson L. E., Aasa R., Vänngård T., Malmström B. G. A new copper(II) electron paramagnetic resonance signal in two laccases and in cytochrome c oxidase. J Biol Chem. 1980 Jun 10;255(11):5000–5003. [PubMed] [Google Scholar]
- Reinhammar B., Oda Y. Spectroscopic and catalytic properties of Rhus vernicifera laccase depleted in type 2 copper. J Inorg Biochem. 1979 Oct;11(2):115–127. doi: 10.1016/s0162-0134(00)80177-4. [DOI] [PubMed] [Google Scholar]
- Reinhammar B. Purification and properties of laccase and stellacyanin from Rhus vernicifera. Biochim Biophys Acta. 1970 Apr 7;205(1):35–47. doi: 10.1016/0005-2728(70)90059-9. [DOI] [PubMed] [Google Scholar]
- Spande T. F., Witkop B., Degani Y., Patchornik A. Selective cleavage and modification of peptides and proteins. Adv Protein Chem. 1970;24:97–260. doi: 10.1016/s0065-3233(08)60242-9. [DOI] [PubMed] [Google Scholar]


