Abstract
Introduction
The progression patterns, dispositions, and outcomes of patients with advanced hepatocellular carcinoma (HCC) who achieved durable responses with immunotherapy remain poorly characterized.
Methods
Patients with advanced HCC who received immune checkpoint inhibitor (ICI)-based immunotherapy and achieved durable responses were retrospectively included. A durable response was defined as partial response (PR) or stable disease (SD) per RECIST 1.1 for more than 8 months after initiation of immunotherapy. Oligoprogression and polyprogression were defined as progression at ≤3 and >3 lesions, respectively.
Results
A total of 91 durable responders (63 PR and 28 SD) were identified. The majority had chronic viral hepatitis (n = 69, 75.8%). Forty-seven (51.6%) and 44 (48.4%) patients received the index immunotherapy as first-line and second- or beyond-line therapy, respectively. Fifty-four (59.3%) patients subsequently developed progression, with a predominant pattern of oligoprogression (66.7%). The median overall survival (OS) was 46.2 months (95% CI: 34.1–58.3). For patients with subsequent progression, employment of locoregional therapy (LRT) for progression was associated with prolonged OS (univariate analysis: hazard ratio [HR] 0.397, p = 0.009; multivariate analysis: HR 0.363, p = 0.050). Patients with oligoprogression who received LRT showed longer median OS than those who did not (48.4 vs. 20.5 months, p < 0.001). In contrast, the median OS of patients with polyprogression who received LRT was not different from those without LRT (27.7 vs. 25.5 months, p = 0.794).
Conclusion
Approximately 60% of the post-immunotherapy durable responders of HCC subsequently develop progression. Proactive LRT may further rescue patients who develop subsequent oligoprogression. Prospective studies are mandatory to clarify the proper management of durable responders with subsequent progression.
Keywords: Hepatocellular carcinoma, Immunotherapy, Durable response, Locoregional therapy, Oligoprogression
Introduction
Immune checkpoint inhibitors (ICIs) exhibit novel response patterns that are less commonly observed with cytotoxic chemotherapy or targeted therapies [1]. One of the novel response patterns is a durable response, predominantly characterized in patients with lung cancer and melanoma [2–4]. Durable responses have also been noted in patients with hepatocellular carcinoma (HCC) receiving ICI-based immunotherapies [5, 6]. Approximately 30% of patients receiving atezolizumab-bevacizumab in IMbrave150 trial experienced a partial response (PR) or stable disease (SD) for more than 6 months, with half of them eventually developing progression [7]. However, the progression patterns, associated dispositions, and long-term outcomes of these durable responders were less characterized in clinical trials and previous scientific literature.
Locoregional therapies (LRTs), including resection, ablation, liver transplant, transarterial chemoembolization (TACE), transarterial radioembolization (TARE), stereotactic body radiation therapy, and hepatic arterial infusional chemotherapy (HAIC), have been commonly employed in the real-world practice, especially in Asia-Pacific regions, for the treatment of patients with Barcelona Clinic Liver Cancer (BCLC) stage C HCC [8]. With the high local tumor control rates and immune-modulatory potentials [9–15], LRT may play novel roles in the era of immunotherapy. First, LRT may eradicate residual tumors after successful conversion immunotherapy, prevent immune escape, and potentially provide a cure to patients [16–19]. Second, LRT may rescue patients who developed subsequent progression after the initial response to immunotherapy, particularly in the absence of effective subsequent systemic therapy and in case of limited progression, referred to as “oligoprogression” [20–23]. Lastly, LRT in combination with immunotherapy may promote antitumor immune responses and further improve the efficacy of immunotherapy [15, 24]. Nevertheless, the survival benefits of LRTs with curative or palliative intent in the era of immunotherapy have yet to be defined. This retrospective multicenter study aimed to characterize the progression patterns, subsequent therapies with a particular focus on LRT, and outcomes in a large cohort of real-world durable responders with HCC following immunotherapy.
Methods
Study Design
This retrospective study was conducted by the Taiwan Liver Cancer Association Research Group in compliance with all applicable ethical standards and was approved by the Research Ethics Committee of each participating hospital. Patients with unresectable HCC who received ICI-based immunotherapy (referred to as the index immunotherapy) between August 2015 and March 2021 and achieved durable responses from 8 major referral hospitals were included for analysis. In this study, the term “index immunotherapy” refers to the specific immunotherapy regimen that resulted in the durable response being analyzed. Computed tomography or magnet resonance images were performed every 8–12 weeks according to the local guidelines, and the response was evaluated by individual treating physicians. A durable response is defined as PR or SD per Response Evaluation Criteria in Solid Tumors version 1.1 (RECIST 1.1) for more than 8 months after initiation of the index immunotherapy. The data cutoff point was Oct 31, 2023. Retrospective medical record reviews were performed to collect the following data: age, gender, underlying viral hepatitis status, Child-Pugh classification, performance status, BCLC stage, extrahepatic spread, vascular invasion, serum alpha-fetoprotein (AFP) level, types of immunotherapies, previous line of systemic therapies, best tumor response, progression patterns, subsequent therapies, LRTs after the index immunotherapy, and death. LRTs include surgery, ablation, TACE, TARE, radiotherapy, and HAIC. The decision of LRT was made on the judgment of the treating physicians. Oligoprogression and polyprogression were defined as progression at ≤3 and >3 lesions, respectively. Data were de-identified for all statistical analyses.
Statistical Analysis
Overall survival (OS) was defined as the time from the initiation of the index immunotherapy to death of any causes. The Kaplan-Meier method was employed to analyze the OS curve, and the median survival time was reported with a 95% confidence interval (95% CI). The log-rank test was utilized to determine whether there were survival discrepancies among different patient subgroups. Univariate and multivariate analyses were conducted using the Cox proportional hazard model. The χ2 test was employed to examine demographic differences between subgroups. All p values were based on a two-sided hypothesis, and those of <0.05 were considered statistically significant. All analyses were performed using SPSS software (version 29; International Business Machines Corporation).
Results
Patient Characteristics
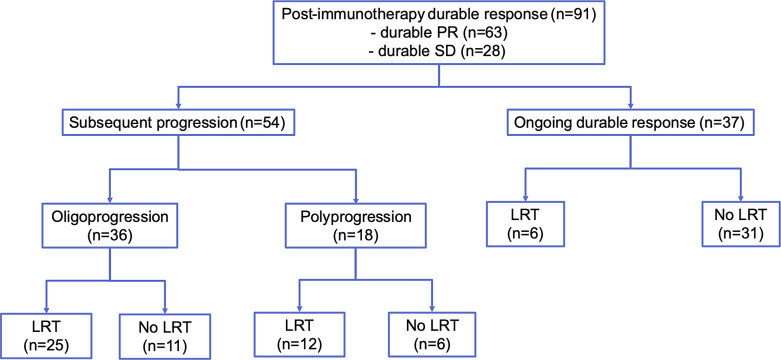
A total of 91 durable responders were identified, with 63 classified as PR and 28 as SD (Fig. 1). The patient characteristics are shown in Table 1. The median age was 62.0 years, and the majority were male (n = 69, 75.8%). Chronic viral hepatitis was prevalent among most patients (n = 69, 75.8%), along with Child-Pugh class A liver function (n = 81, 89%), ECOG performance status ≤2 (n = 83, 91.2%), and BCLC stage C (n = 81, 89%). Forty-one (45.1%) and 58 (63.7%) patients had vascular invasion and extrahepatic spread, respectively. Thirty-four (37.4%) patients had serum AFP levels ≥400 ng/mL. Forty-seven (51.6%) patients received the index immunotherapies as first-line therapy, while 44 (48.4%) patients received them as second-line therapy or beyond. The types of ICI-based immunotherapy included anti-programmed cell death-1 (PD-1)/programmed cell death ligand 1 (PD-L1) monoclonal antibody (mAb) plus anti-vascular endothelial growth factor (VEGF) mAb (n = 32, 35.2%), anti-PD-1/PD-L1 mAb alone (n = 24, 26.4%), anti-PD-1/PD-L1 mAb plus multitarget kinase inhibitor (MKI) (n = 23, 25.3%), anti-PD-1/PD-L1 mAb plus anti-cytotoxic T-lymphocyte antigen 4 (CTLA-4) mAb (n = 4, 4.4%), and others (n = 8, 8.8%).
Fig. 1.
Patient flowchart. The patient flowchart summarizes the progression patterns and LRT utilization in the entire cohort of durable responders.
Table 1.
Patient characteristics of all patients with durable responses
| N | % | |
|---|---|---|
| Total | 91 | 100.0 |
| Age, years | ||
| Mean (SD) | 61.9 (10.8) | |
| Median (IQR) | 62.0 (57–70) | |
| Gender | ||
| Male | 69 | 75.8 |
| Female | 22 | 24.2 |
| Etiology | ||
| Viral (HBV or HCV) | 69 | 75.8 |
| Nonviral | 22 | 24.2 |
| Child-Pugh class | ||
| A | 81 | 89.0 |
| B | 8 | 8.8 |
| C | 2 | 2.2 |
| ECOG performance status | ||
| ≤2 | 83 | 91.2 |
| >2 | 8 | 8.8 |
| BCLC stage | ||
| B | 10 | 11.0 |
| C | 81 | 89.0 |
| Extrahepatic spread and/or vascular invasion | ||
| Vascular invasion | 41 | 45.1 |
| Extrahepatic spread | 58 | 63.7 |
| AFP ≥400 ng/mL | 34 | 37.4 |
| Prior line of systemic therapy | ||
| 0 | 47 | 51.6 |
| 1 or above | 44 | 48.4 |
| Types of ICIs | ||
| Anti-PD-1/PD-L1mAb alone | 24 | 26.4 |
| Anti-PD-1/PD-L1 mAb + anti-CTLA-4 mAb | 4 | 4.4 |
| Anti-PD-1/PD-L1 mAb + anti-VEGF mAb | 32 | 35.2 |
| Anti-PD-1/PD-L1 mAb + MKI | 23 | 25.3 |
| Othersa | 8 | 8.8 |
| Best response (RECIST 1.1) | ||
| PR | 63 | 69.2 |
| SD | 28 | 30.8 |
| Employment of LRT after the index immunotherapy | ||
| Yes | 43 | 47.3 |
| No | 48 | 52.7 |
AFP, alpha-fetoprotein; BCLC, Barcelona Clinic Liver Cancer; CTLA-4, cytotoxic T-lymphocyte-associated antigen 4; ECOG, Eastern Cooperative Oncology Group; HBV, hepatitis B virus; HCV, hepatitis C virus; ICI, immune checkpoint inhibitor; IQR, interquartile range; mAb, monoclonal antibody; MKI, multikinase inhibitors; LRT, locoregional therapy; PD-1, programmed death-1; PD-L1, programmed death ligand 1; RECIST 1.1, response evaluation criteria in solid tumors 1.1; SD, standard deviation; VEGF, vascular endothelial growth factor; y, year.
aOther types of ICIs included anti-CTLA-4 alone, anti-PD-1 plus anti-ALK-1, anti-PD-L1 plus anti-glypican-3, anti-PD-1 plus anti-LAG-3 antibodies.
Subsequent Progression and Disposition
During a median follow-up time of 36.3 months (IQR: 20.8–46.2 months), 54 patients (59.3%), comprising 31 with initial PR and 23 with initial SD, experienced subsequent progression. Notably, subsequent progression was more common in patients with durable SD than those with durable PR (86.5 vs. 57.4%, p = 0.005). Baseline patient characteristics were generally similar between patients with subsequent progression and those with ongoing durable responses, except for serum AFP level and ECOG status (online suppl. Table 1; for all online suppl. material, see https://doi.org/10.1159/000536549). Significantly fewer patients with subsequent progression had serum AFP ≥400 ng/mL before immunotherapy compared to those with ongoing durable responses (27.8 vs. 51.4%, p = 0.028).
Among those patients with subsequent progression, 36 patients (66.7%) had oligoprogression, and 18 patients (33.3%) had polyprogression. Baseline patient characteristics and AFP responses within the first 4 weeks after treatment initiation were generally similar between patients with oligoprogression and those with polyprogression except for types of immunotherapies (online suppl. Table 2). Their subsequent therapies are presented in Table 2. Twenty-eight (51.9%) patients switched to a different systemic therapy, comprising 11 patients on lenvatinib, 7 on sorafenib, 2 each on regorafenib and ramucirumab, 1 on cabozantinib, 2 on systemic chemotherapy, and 3 on different immunotherapy combinations. Meanwhile, 15 (27.8%) patients continued the index immunotherapy, and 11 (20.4%) patients did not receive any systemic therapy afterward. Thirty-seven (68.5%) patients received LRTs. The most employed LRT for hepatic progression alone was TACE (5 for oligoprogression and 4 for polyprogression), followed by ablation (5 for oligoprogression), TACE + ablation (1 for oligoprogression and 3 for polyprogression), and radiotherapy (2 for oligoprogression). In contrast, the most employed LRT for extrahepatic progression alone was radiotherapy (3 for oligoprogression and 3 for polyprogression), followed by metastasectomy (4 for oligoprogression) and a combination of metastasectomy plus radiotherapy (1 for polyprogression). Six patients received LRT for concomitant hepatic and extrahepatic progression with varied approaches (Table 2). The employment rates of either systemic therapy or LRT were not different between patients with oligoprogression and those with polyprogression.
Table 2.
Dispositions of patients with subsequent progression
| Treatment after progression | All progression | Oligoprogression | Polyprogression | p value | |||
|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | ||
| 54 | 100 | 36 | 100 | 18 | 100 | ||
| Systemic therapy | 0.733 | ||||||
| No | 11 | 20.4 | 8 | 22.2 | 3 | 16.7 | |
| Yes | 43 | 79.6 | 28 | 77.8 | 15 | 83.3 | |
| Continuing the index immunotherapy | 15 | 27.8 | 10 | 27.8 | 5 | 27.8 | |
| Switching to a different systemic therapy | 28a | 51.9 | 18 | 50.0 | 10 | 55.6 | |
| LRT | >0.999 | ||||||
| No | 17 | 31.5 | 11 | 30.6 | 6 | 33.3 | |
| Yes | 37 | 68.5 | 25 | 69.4 | 12 | 66.7 | |
| For hepatic progression | 20 | 37.0 | 13 | 36.1 | 7 | 38.9 | |
| Hepatectomy | 0 | 0 | 0 | 0 | 0 | 0 | |
| Ablation alone | 5 | 9.3 | 5 | 13.9 | 0 | 0 | |
| TACE alone | 9 | 16.7 | 5 | 13.9 | 4 | 22.2 | |
| TACE + ablation | 4 | 7.4 | 1 | 2.8 | 3 | 16.7 | |
| Radiotherapy | 2 | 3.7 | 2 | 5.6 | 0 | 0 | |
| For extrahepatic progression | 11 | 20.4 | 7 | 19.4 | 4 | 22.2 | |
| Metastasectomy | 4 | 7.4 | 4 | 11.1 | 0 | 0 | |
| Radiotherapy | 6 | 11.1 | 3 | 8.3 | 3 | 16.7 | |
| Metastasectomy + radiotherapy | 1 | 1.9 | 0 | 0 | 1 | 5.6 | |
| For concurrent hepatic and extrahepatic progression | 6 | 11.1 | 5 | 13.9 | 1 | 5.6 | |
| Ablation (hepatic) + radiotherapy (extrahepatic) | 1 | 9.1 | 1 | 2.8 | 0 | 0 | |
| Hepatectomy + radiotherapy (extrahepatic) | 1 | 9.1 | 1 | 2.8 | 0 | 0 | |
| Radiotherapy (hepatic + extrahepatic) | 1 | 9.1 | 1 | 2.8 | 0 | 0 | |
| TACE (hepatic) + surgery (extrahepatic) | 2 | 18.2 | 2 | 5.6 | 0 | 0 | |
| TACE + TARE + HAIC (hepatic) + RT (extrahepatic) | 1 | 9.1 | 0 | 0 | 1 | 5.6 | |
TACE, transarterial chemoembolization; TARE, transarterial radioembolization; HAIC, hepatic arterial infusional chemotherapy.
aEleven patients were treated with lenvatinib, 7 with sorafenib, 2 each with regorafenib and ramucirumab, 1 with cabozantinib, 2 with systemic chemotherapy, and 3 with different immunotherapy combinations.
For patients with ongoing durable responses, all patients (n = 37, 100%) continued the index immunotherapy during the follow-up period. Three patients received LRT to the responding hepatic lesions, including hepatectomy (n = 1), TACE (n = 1), and radiotherapy (n = 1); 3 patients received LRT for responding extrahepatic lesions, including metastasectomy (n = 2) and radiotherapy (n = 1).
Survival and Risk Factors for Death
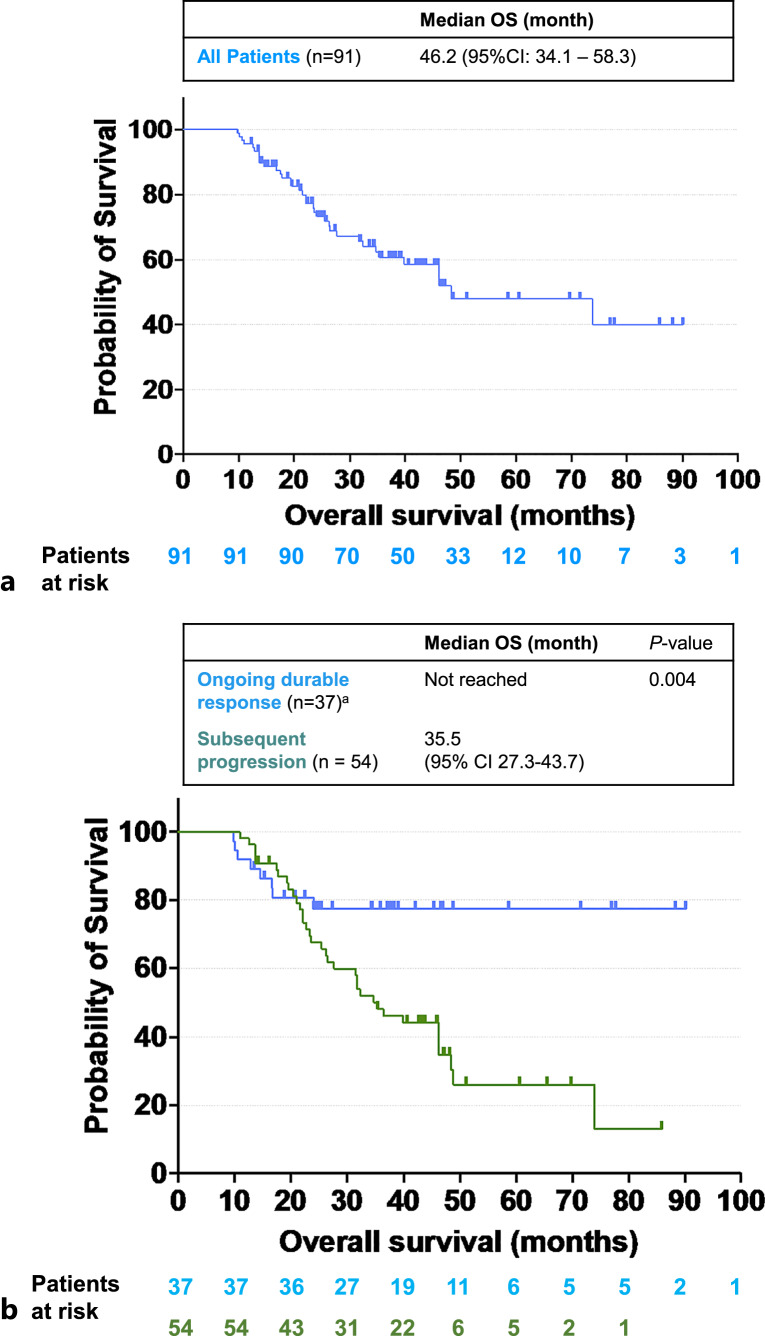
For the entire group (n = 91), the median OS was 46.2 months (95% CI: 34.1–58.3) (Fig. 2a). The median OS of those with subsequent progression was 35.5 months (95% CI: 27.3–43.7), while the median OS of patients with ongoing durable response was not reached (Fig. 2b). The median OS was statistically different between patients who subsequently developed progression and patients who experienced ongoing durable response (p = 0.004). Macrovascular invasion was found to be associated with shorter survival in univariate analysis (hazard ratio [HR] 2.047, p = 0.02) but not in multivariate analysis (HR 1.983, p = 0.056). Subsequent progression was found to be associated with shorter survival in both univariate analysis (HR 2.915, p = 0.007) and multivariate analysis (HR 4.910, p < 0.001). Employment of LRT after the index immunotherapy was associated with longer survival in multivariate analysis (HR 0.341, p = 0.004) but not in the univariate analysis (HR 0.940, p = 0.840) (Table 3a).
Fig. 2.
OS of all durable responders. a Kaplan-Meier OS curve of the entire cohort of patients who exhibited a durable response. b Kaplan-Meier OS curves depicting a comparison between 2 subgroups: patients who experienced ongoing durable responses and those who subsequently developed progression. OS, overall survival; CI, confidence interval. a There were 8 deaths noted in the ongoing durable response group. Six patients died of infection/pneumonia, and 1 each died of severe gastrointestinal bleeding and complications of cirrhosis.
Table 3.
Univariate and multivariate analyses of risk factors associated with death
| Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|
| HR | p value | HR | p value | |
| a All durable responders | ||||
| Age | 1.001 | 0.966 | 0.988 | 0.370 |
| Male | 1.156 | 0.690 | 0.979 | 0.959 |
| Viral etiology associated HCC | 0.889 | 0.732 | 0.711 | 0.393 |
| Macrovascular invasion | 2.047 | 0.020* | 1.983 | 0.056 |
| Extrahepatic spread | 0.664 | 0.193 | 0.980 | 0.956 |
| AFP >400 ng/mL | 0.666 | 0.235 | 0.648 | 0.280 |
| Child-Pugh class A | 0.613 | 0.312 | 0.563 | 0.265 |
| No prior systemic therapy | 0.896 | 0.723 | 1.352 | 0.375 |
| Subsequent progression | 2.915 | 0.007* | 4.910 | <0.001* |
| LRT after the index immunotherapy | 0.940 | 0.840 | 0.341 | 0.004* |
| b Durable responders with subsequent progression | ||||
| Agea | 0.988 | 0.406 | 0.988 | 0.433 |
| Male | 0.799 | 0.582 | 1.129 | 0.819 |
| Viral etiology associated HCC | 0.795 | 0.548 | 0.496 | 0.189 |
| Macrovascular invasiona | 1.754 | 0.101 | 1.645 | 0.293 |
| Extrahepatic spreada | 0.615 | 0.167 | 0.741 | 0.514 |
| AFP >400 ng/mLa | 0.976 | 0.950 | 0.605 | 0.351 |
| Oligoprogressiona | 0.678 | 0.275 | 0.477 | 0.141 |
| LRT after the index immunotherapy | 0.397 | 0.009* | 0.363 | 0.050* |
| Systemic therapy after progression | 0.110 | 0.685 | ||
| Switching to a different systemic therapy | 1 | 1 | ||
| Continuing the index immunotherapy | 0.406 | 0.054 | 0.609 | 0.386 |
| No more systemic therapy | 0.518 | 0.231 | 0.880 | 0.847 |
AFP, alpha-fetoprotein; HCC, hepatocellular carcinoma; HR, hazard ratio.
aAnalyzed based on the data upon progression.
*Denote p value < 0.05 which is considered statistically significant.
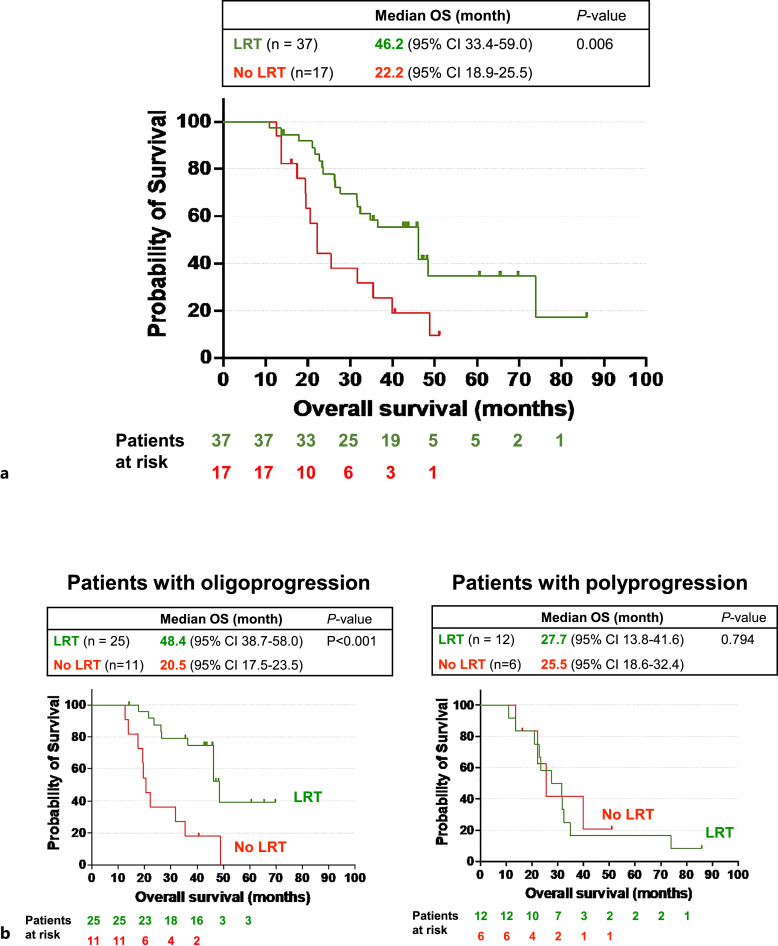
Among those patients with subsequent progression (n = 54), LRT for progression but not progression pattern (oligoprogression vs. polyprogression) nor systemic therapy after progression was an independent risk factor for death through the Cox proportional hazard analysis (Table 3b). LRT for progression was associated with longer survival in both univariate analysis (HR 0.397, p = 0.009) and multivariate analysis (HR 0.363, p = 0.050).
The clinical characteristics of patients who subsequently progressed and received LRT or those who did not receive LRT were similar either at baseline or upon progression (Table 4). The median OS was longer for patients who received LRT than those who did not (46.2 vs. 22.2 months, p = 0.006) (Fig. 3a). Eleven (29.7%) out of 37 patients who received LRT achieved complete response with LRT. The OS of the patients who did not achieve complete response with LRT was still longer than those who did not receive LRT for progression (online suppl. Fig. 1). Furthermore, patients with oligoprogression who received LRT showed a significantly longer median OS than those who did not (48.4 vs. 20.5 months, p < 0.001). However, the median OS of patients with polyprogression who received LRT was not different from those without LRT (27.7 vs. 25.5 months, p = 0.794) (Fig. 3b). Among those patients who experienced ongoing durable responses, the median OS was not statistically different between patients who received LRT and those who did not (p = 0.687) (online suppl. Fig. 2).
Table 4.
Patient characteristics of patient with subsequent progression according to the use of LRT
| Receiving LRT | Not receiving LRT | p value | |||
|---|---|---|---|---|---|
| Baseline characteristics (when receiving the index immunotherapy) | |||||
| N | % | N | % | ||
| Total | 37 | 100.0 | 17 | 100.0 | |
| Age, years | 0.598 | ||||
| Mean (SD) | 62.0 (10.1) | 63.8 (14.3) | |||
| Median (IQR) | 61.0 (56.5–67.5) | 67 (57.0–74.5) | |||
| Gender | |||||
| Male | 30 | 81.1 | 13 | 76.5 | 0.696 |
| Female | 7 | 18.9 | 4 | 23.5 | |
| Etiology | |||||
| Viral (HBV or HCV) | 27 | 73.0 | 14 | 82.35 | 0.512 |
| Nonviral | 10 | 27.0 | 3 | 17.65 | |
| Child-Pugh class | |||||
| A | 34 | 91.9 | 15 | 88.2 | 0.321 |
| B | 3 | 8.1 | 1 | 5.9 | |
| C | 0 | 0 | 1 | 5.9 | |
| ECOG | |||||
| ≤2 | 37 | 100.0 | 16 | 94.1 | 0.315 |
| >2 | 0 | 0.0 | 1 | 5.9 | |
| BCLC stage | |||||
| B | 3 | 8.1 | 2 | 11.8 | 0.645 |
| C | 34 | 91.9 | 15 | 88.2 | |
| Vascular invasion | 17 | 45.9 | 9 | 52.9 | 0.633 |
| Extrahepatic spread | 26 | 70.3 | 8 | 47.1 | 0.101 |
| AFP >400 ng/mL | 10 | 27.0 | 5 | 29.4 | 0.856 |
| Prior line of systemic Therapy | |||||
| 0 | 18 | 48.6 | 7 | 58.8 | 0.609 |
| 1 or above | 19 | 51.4 | 10 | 41.2 | |
| Types of ICIs | |||||
| Anti-PD-1/PD-L1mAb alone | 13 | 35.1 | 5 | 29.4 | 0.856 |
| Anti-PD-1/PD-L1 mAb + anti-CTLA-4 mAb | 2 | 5.4 | 0 | 0.0 | |
| Anti-PD-1/PD-L1 mAb + anti-VEGF mAb | 10 | 27.0 | 5 | 29.0 | |
| Anti-PD-1/PD-L1 mAb + MKI | 10 | 27.0 | 6 | 35.3 | |
| Othersa | 2 | 5.4 | 1 | 5.9 | |
| Clinical characteristics upon progression | |||||
|---|---|---|---|---|---|
| N | % | N | % | p value | |
| Child-Pugh class | |||||
| A | 34 | 91.9 | 12 | 70.6 | 0.092 |
| B | 3 | 8.1 | 5 | 29.4 | |
| C | 0 | 0 | 0 | 0 | |
| ECOG | |||||
| ≤2 | 34 | 91.9 | 15 | 88.2 | 0.645 |
| >2 | 3 | 8.1 | 2 | 11.8 | |
| Vascular invasion | 13 | 35.1 | 5 | 29.4 | 0.763 |
| Extrahepatic spread | 21 | 56.8 | 12 | 70.6 | 0.383 |
| AFP >400 ng/mL | 8 | 21.6 | 5 | 29.4 | 0.738 |
AFP, alpha-fetoprotein; BCLC, Barcelona Clinic Liver Cancer; CTLA-4, cytotoxic T-lymphocyte-associated antigen 4; ECOG, Eastern Cooperative Oncology Group; HBV, hepatitis B virus; HCV, hepatitis C virus; ICI, immune checkpoint inhibitor; IQR, interquartile range; mAb, monoclonal antibody; MKI, multikinase inhibitors; PD-1, programmed death-1; PD-L1, programmed death ligand 1; RECIST 1.1, response evaluation criteria in solid tumors 1.1; SD, standard deviation; VEGF, vascular endothelial growth factor; y, year.
aOther types of ICIs include anti-CTLA-4 alone, anti-PD-1 plus anti-ALK-1, anti-PD-L1 plus anti-glypican-3, anti-PD-1 plus anti-LAG-3 antibodies.
Fig. 3.
OS of durable responders who subsequently developed progression. a Kaplan-Meier overall survival curves depicting a comparison between 2 subgroups: patients with subsequent progression who received locoregional therapy (LRT) and the corresponding subgroup who did not receive LRT. b Left panel: Kaplan-Meier OS curves depicting a comparison between 2 subgroups: patients who developed subsequent oligoprogression and received LRT, and the corresponding subgroup who did not receive LRT; right panel: Kaplan-Meier OS curves illustrating a comparison between 2 subgroups: patients who subsequently developed polyprogression and received LRT and the corresponding subgroup who did not receive LRT. OS, overall survival; CI, confidence interval.
Discussion
To the best of our knowledge, this study is the first to characterize the subsequent progression patterns, dispositions, and long-term outcomes of patients who had unresectable HCC and achieved durable responses (defined as PR or SD lasting for more than 8 months) under ICI-based immunotherapy in the real-world setting. The findings of this study demonstrate that these durable responders had remarkably longer OS with a median of 46.2 months, despite approximately half of them eventually developing progression, primarily in the form of oligoprogression. Furthermore, employment of LRT following the index immunotherapy was associated with prolonged OS, and this survival advantage was predominantly observed in patients receiving LRT for oligoprogression rather than those receiving LRT for polyprogression or ongoing durable response.
Subsequent Progression in Durable Responders
The precise rate at which patients with advanced HCC experience subsequent progression after the initial response to immunotherapy remains uncertain. In a post hoc analysis of data from the atezolizumab-bevacizumab arm of the IMbrave150 trial, durable responders were defined as patients with PR or SD lasting for more than 6 months. Among these durable responders, 54 (59.3%) out of 97 experienced subsequent progression during a median follow-up period of 17.6 months [7]. Specifically, 41.7% of patients with durable PR and 47.9% of those with durable SD experienced subsequent progression. In contrast, the current real-world study revealed that 54 (59.3%) out of 91 durable responders (57.4% of patients with durable PR and 86.5% of patients with durable SD) experienced subsequent progression during a median follow-up period of 36.3 months. Although the definitions of durable response, types of immunotherapies, and patient populations differed between the 2 studies, the data indicate that approximately 60% of durable responders develop subsequent progression despite initial disease control for at least 6–8 months under immunotherapy. Currently, no clinicopathological characteristics have been identified to predict subsequent progression. Therefore, it is of great interest that pathological specimen analysis of subsequently progressed lesions may help researchers gain insight into immune escape mechanisms and develop novel strategies to overcome resistance.
LRT for Subsequent Progression
This study revealed significant survival advantages associated with LRT for progression in durable responders who subsequently developed progression despite 70.3% of them having extrahepatic diseases at the time of progression (Table 3b, 4). Notably, the survival benefits of LRT were primarily observed in patients who received LRT for oligoprogression (Fig. 3b). The median OS for patients who received LRT for subsequent oligoprogression is 48.4 months (95% CI: 38.7–58.0), implicating the crucial role of LRT in addressing acquired immunotherapy resistance in HCC. These findings align with a growing concept that aggressive LRTs, with or without systemic therapy, can improve survival in patients with different types of metastatic cancers experiencing oligoprogression following systemic therapies, including immunotherapy [20, 25–38].
In this study, the 3 most employed LRTs for oligoprogression were TACE alone (n = 5) and ablation alone (n = 5), followed by metastasectomy (n = 4). Many patients received various combinations of these LRTs in our study, which underscores the commonly adopted and practiced concepts of utilizing LRTs along with systemic therapy in the Asia-Pacific region's real-world practice. While the role of radiotherapy [25, 34, 36, 37], metastasectomy [21, 26, 30], and ablation [28, 38] in treating oligoprogression has been extensively investigated in other cancer types, the role of TACE for oligoprogression in HCC had not been reported before. Nevertheless, it is reasonable to expect that judicious TACE without compromising liver reserve may contribute to prolonged OS in cases of hepatic oligoprogression.
Systemic Therapy for Subsequent Progression
Systemic therapy switch is usually considered to manage subsequent progression following prior systemic therapy failure, particularly in the scenario of polyprogression, while LRT primarily serves a palliative role, aiming to relieve symptoms. However, the current study found that switching to a different systemic therapy was less common than the employment of LRT in durable responders who experienced subsequent progression, regardless of progression patterns (all progression: 51.9% switched systemic therapy vs. 68.5% received LRT; oligoprogression: 50.0 vs. 69.4%; polyprogression: 55.6 vs. 66.7%) (Table 2). The lower rate of switching to a different systemic therapy in this study can be attributed to the limited availability of subsequent systemic treatment options for patients who no longer responded to ICI-based immunotherapy, particularly when 53.7% of them had already received at least one line of systemic therapy, predominantly as MKI (online suppl. Table 1). On the other hand, the higher rate of LRT for subsequent progression is consistent with the preexisting preference and extensive experiences in employing liver-directed LRT to treat advanced-stage HCC in the Asia-Pacific region [8]. Additionally, recent advancements in minimally invasive metastasectomy of lung metastases and stereotactic body radiation therapy contribute to the increased utilization of LRT for extrahepatic progression.
Furthermore, this study demonstrates that switching to a different systemic therapy did not provide any survival benefit for durable responders who encountered subsequent progression (Table 3b). In contrast, a global retrospective real-world study revealed that subsequent therapy (n = 165), compared to best supportive care (n = 152), is associated with prolonged OS in HCC patients who discontinued ICI-based immunotherapy primarily due to progressive disease [39]. The subsequent therapy following immunotherapy discontinuation included not only systemic therapies (MKIs/immunotherapy/chemotherapy: 66.1/12.7/5.5%) but also LRTs (radiotherapy/TACE or TARE/radiotherapy/ablation: 17/11.5/3.6/2.4%). Therefore, the OS benefits from subsequent therapy in that study were most likely derived from systemic therapies rather than LRTs. Another retrospective single-institute study demonstrated that subsequent MKI, compared to no subsequent MKI, was also associated with improved OS in multivariate analysis (HR 0.412, p = 0.0043) in 77 HCC patients who received immunotherapy [40]. The varying impacts of subsequent systemic therapies on OS observed in these studies may be attributed to different patient populations (durable PR or SD to immunotherapy vs. any response to immunotherapy), varying proportions of prior MKI failure, and various patient selection biases.
Limitations
Our study has several limitations that require acknowledgment. First, it is crucial to recognize that our analyses are retrospective in nature, which introduces the possibility of information bias, including selection bias, recall bias, or misclassification bias. Second, there is considerable heterogeneity in the immunotherapy combinations received by the durable responders, as well as significant variation in the types of subsequent LRTs utilized. Third, the limited size of our study population prevented us from conducting adequately powered comparisons between the different subgroups. Moreover, comprehensive information regarding treatment toxicities and co-medication, such as antibiotics, corticosteroids, immunosuppressants, or proton pump inhibitors, which can potentially impact the response to ICIs, was not fully available. Despite these limitations, our study’s strength lies in being the first investigation, to the best of our knowledge, that comprehensively characterizes the progression patterns, dispositions, and outcomes of real-world durable responders with advanced HCC treated with immunotherapies.
In conclusion, patients with advanced HCC who experienced durable responses after initiation of immunotherapy have excellent clinical outcomes. For this group of patients, proactive LRTs may further benefit or rescue patients who developed subsequent oligoprogression. Prospective studies are mandatory to clarify the proper management of durable responders with subsequent progression.
Acknowledgment
We would like to acknowledge the service provided by the administrative office of the Taiwan Liver Cancer Association.
Statement of Ethics
The study protocol was reviewed and approved by the Research Ethics Committee of National Taiwan University Hospital/National Taiwan University Cancer Center (approval number 202109071RINC), Taipei Veterans General Hospital (approval number TPEVGH IRB No.: 2023-08-010CC), E-Da Cancer Hospital (approval number EMRP-110-091), Chang Gung Memorial Hospital Linkou Branch (approval number 202002147B0C501), National Health Research Institute/National Cheng Kung University Hospital (approval number A-ER-112-215), Tri-Service General Hospital (approval number IRB-C202105167), National Taiwan University Hospital Yunlin Branch (approval number 202212015RINC), respectively, for each participating hospital. This is a retrospective clinical study and was exempted from individual patient informed consent. The raw data were first extracted from each participating hospital, and patients’ identities, including names, screening IDs, patient IDs, and mobile phone numbers, were de-identified before the statistical analyses of aggregated de-identified data.
Conflict of Interest Statement
The authors declare no conflicts of interest.
Funding Sources
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author Contributions
Study design and concept: Tsung-Hao Liu, Ying-Chun Shen, and Ann-Lii Cheng; data collection and data interpretation and review and approval of the manuscript submission: Tsung-Hao Liu, San-Chi Chen, Kun-Ming Rau, Li-Chun Lu, Po-Ting Lin, Yung-Yeh Su, Wei Teng, Shiue-Wei Lai, Ren-Hua Yeh, Tsui-Mai Kao, Pei-Chang Lee, Chi-Jung Wu, Chien-Hung Chen, Chih-Hung-Hsu, Shi-Ming Lin, Yi-Hsiang Huang, Li-Tzong Chen, Ann-Lii Cheng, and Ying-Chun Shen; and study analysis and manuscript writing: Tsung-Hao Liu and Ying-Chun Shen.
Funding Statement
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Data Availability Statement
For ethical reasons, the data are not publicly available. The datasets generated and analyzed in this study are available from the corresponding author upon reasonable request. Further inquiries can be directed to the corresponding author.
Supplementary Material.
Supplementary Material.
Supplementary Material.
References
- 1. Borcoman E, Kanjanapan Y, Champiat S, Kato S, Servois V, Kurzrock R, et al. Novel patterns of response under immunotherapy. Ann Oncol. 2019;30(3):385–96. [DOI] [PubMed] [Google Scholar]
- 2. Gettinger S, Horn L, Jackman D, Spigel D, Antonia S, Hellmann M, et al. Five-year follow-up of nivolumab in previously treated advanced non-small-cell lung cancer: results from the ca209-003 study. J Clin Oncol. 2018;36(17):1675–84. [DOI] [PubMed] [Google Scholar]
- 3. Pons-Tostivint E, Latouche A, Vaflard P, Ricci F, Loirat D, Hescot S, et al. Comparative analysis of durable responses on immune checkpoint inhibitors versus other systemic therapies: a pooled analysis of phase III trials. Jco Precis Oncol. 2019;3:1–10. [DOI] [PubMed] [Google Scholar]
- 4. Wolchok JD, Chiarion-Sileni V, Gonzalez R, Grob JJ, Rutkowski P, Lao CD, et al. Long-term outcomes with nivolumab plus ipilimumab or nivolumab alone versus ipilimumab in patients with advanced melanoma. J Clin Oncol. 2022;40(2):127–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. El-Khoueiry AB, Sangro B, Yau T, Crocenzi TS, Kudo M, Hsu C, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet. 2017;389(10088):2492–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yau T, Kang YK, Kim TY, El-Khoueiry AB, Santoro A, Sangro B, et al. Efficacy and safety of nivolumab plus ipilimumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib: the CheckMate 040 randomized clinical trial. JAMA Oncol. 2020;6(11):e204564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Shen YC, Liu TH, Lu LC, Nicholas A, Hernandez S, Galle PR, et al. CLINICOPATHOLOGICAL characteristics and OUTCOME OF hepatocellular CARCINOMA patients WHO achieved durable partial response or stable disease status IN IMbrave150 study. Hepatology. 2022;76:S1417–8. [Google Scholar]
- 8. Park JW, Chen M, Colombo M, Roberts LR, Schwartz M, Chen PJ, et al. Global patterns of hepatocellular carcinoma management from diagnosis to death: the BRIDGE Study. Liver Int. 2015;35(9):2155–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dong BW, Zhang J, Liang P, Yu XL, Su L, Yu DJ, et al. Sequential pathological and immunologic analysis of percutaneous microwave coagulation therapy of hepatocellular carcinoma. Int J Hyperthermia. 2003;19(2):119–33. [DOI] [PubMed] [Google Scholar]
- 10. Kroemer G, Galluzzi L, Kepp O, Zitvogel L. Immunogenic cell death in cancer therapy. Annu Rev Immunol. 2013;31:51–72. [DOI] [PubMed] [Google Scholar]
- 11. Craciun L, de Wind R, Demetter P, Lucidi V, Bohlok A, Michiels S, et al. Retrospective analysis of the immunogenic effects of intra-arterial locoregional therapies in hepatocellular carcinoma: a rationale for combining selective internal radiation therapy (SIRT) and immunotherapy. BMC Cancer. 2020;20(1):135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fucikova J, Kepp O, Kasikova L, Petroni G, Yamazaki T, Liu P, et al. Detection of immunogenic cell death and its relevance for cancer therapy. Cell Death Dis. 2020;11(11):1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhu H, Shan Y, Ge K, Lu J, Kong W, Jia C. Oxaliplatin induces immunogenic cell death in hepatocellular carcinoma cells and synergizes with immune checkpoint blockade therapy. Cell Oncol. 2020;43(6):1203–14. [DOI] [PubMed] [Google Scholar]
- 14. Leuchte K, Staib E, Thelen M, Gödel P, Lechner A, Zentis P, et al. Microwave ablation enhances tumor-specific immune response in patients with hepatocellular carcinoma. Cancer immunology, immunotherapy: CII. Cancer Immunol Immunother. 2021;70(4):893–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Llovet JM, De Baere T, Kulik L, Haber PK, Greten TF, Meyer T, et al. Locoregional therapies in the era of molecular and immune treatments for hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. 2021;18(5):293–313. [DOI] [PubMed] [Google Scholar]
- 16. Kudo M. Changing the treatment paradigm for hepatocellular carcinoma using atezolizumab plus bevacizumab combination therapy. Cancers. 2021(21):13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Katariya NN, Lizaola-Mayo BC, Chascsa DM, Giorgakis E, Aqel BA, Moss AA, et al. Immune checkpoint inhibitors as therapy to down-stage hepatocellular carcinoma prior to liver transplantation. Cancers. 2022;14(9):2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kudo M. Atezolizumab plus bevacizumab followed by curative conversion (ABC conversion) in patients with unresectable, TACE-unsuitable intermediate-stage hepatocellular carcinoma. Liver Cancer. 2022;11(5):399–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kudo M, Aoki T, Ueshima K, Tsuchiya K, Morita M, Chishina H, et al. Achievement of complete response and drug-free status by atezolizumab plus bevacizumab combined with or without curative conversion in patients with transarterial chemoembolization-unsuitable, intermediate-stage hepatocellular carcinoma: a multicenter proof-of-concept study. Liver Cancer. 2023;12(4):321–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Klemen ND, Wang M, Feingold PL, Cooper K, Pavri SN, Han D, et al. Patterns of failure after immunotherapy with checkpoint inhibitors predict durable progression-free survival after local therapy for metastatic melanoma. J Immunother Cancer. 2019;7(1):196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Medina BD, Choi BH, Rodogiannis KG, Moran U, Shapiro RL, Pavlick A, et al. Metastasectomy for melanoma is associated with improved overall survival in responders to targeted molecular or immunotherapy. J Surg Oncol. 2020;122(3):555–61. [DOI] [PubMed] [Google Scholar]
- 22. Wang Y, Lu LC, Guan Y, Ho MC, Lu S, Spahn J, et al. Atezolizumab plus bevacizumab combination enables an unresectable hepatocellular carcinoma resectable and links immune exclusion and tumor dedifferentiation to acquired resistance. Exp Hematol Oncol. 2021;10(1):45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lee HY, Kim SH, Lee HY, Lee YM, Han JY, Cho H, et al. Successful treatment of induced oligometastasis and repeated oligoprogression of advanced lung adenocarcinoma with immunotherapy and radiotherapy. Thorac Cancer. 2022;13(13):1998–2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Dendy MS, Ludwig JM, Stein SM, Kim HS. Locoregional therapy, immunotherapy and the combination in hepatocellular carcinoma: future directions. Liver Cancer. 2019;8(5):326–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kissel M, Martel-Lafay I, Lequesne J, Faivre JC, Le Péchoux C, Stefan D, et al. Stereotactic ablative radiotherapy and systemic treatments for extracerebral oligometastases, oligorecurrence, oligopersistence and oligoprogression from lung cancer. BMC Cancer. 2019;19(1):1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bello DM, Panageas KS, Hollmann T, Shoushtari AN, Momtaz P, Chapman PB, et al. Survival outcomes after metastasectomy in melanoma patients categorized by response to checkpoint blockade. Ann Surg Oncol. 2020;27(4):1180–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Comito F, Leslie I, Boos L, Furness A, Pickering L, Turajlic S, et al. Oligoprogression after checkpoint inhibition in metastatic melanoma treated with locoregional therapy: a single-center retrospective analysis. J Immunother. 2020;43(8):250–5. [DOI] [PubMed] [Google Scholar]
- 28. Jairam V, Park HS, Decker RH. Local ablative therapies for oligometastatic and oligoprogressive non-small cell lung cancer. Cancer J. 2020;26(2):129–36. [DOI] [PubMed] [Google Scholar]
- 29. Deek MP, Phillips RM, Tran PT. Local therapies in oligometastatic and oligoprogressive prostate cancer. Semin Radiat Oncol. 2021;31(3):242–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Joosten PJM, de Langen AJ, van der Noort V, Monkhorst K, Klomp HM, Veenhof A, et al. The role of surgery in the treatment of oligoprogression after systemic treatment for advanced non-small cell lung cancer. Lung Cancer. 2021;161:141–51. [DOI] [PubMed] [Google Scholar]
- 31. Prelaj A, Pircher CC, Massa G, Martelli V, Corrao G, Lo Russo G, et al. Beyond first-line immunotherapy: potential therapeutic strategies based on different pattern progressions: oligo and systemic progression. Cancers. 2021;13(6):1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Versluis JM, Hendriks AM, Weppler AM, Brown LJ, de Joode K, Suijkerbuijk KPM, et al. The role of local therapy in the treatment of solitary melanoma progression on immune checkpoint inhibition: A multicentre retrospective analysis. Eur J Cancer. 2021;151:72–83. [DOI] [PubMed] [Google Scholar]
- 33. Chang JY, Verma V. Optimize local therapy for oligometastatic and oligoprogressive non-small cell lung cancer to enhance survival. J Natl Compr Canc Netw. 2022;20(5):531–9. [DOI] [PubMed] [Google Scholar]
- 34. Damen PJJ, Suijkerbuijk KPM, VAN Lindert ASR, Eppinga WSC, El Sharouni SY, Verhoeff JJC. Long-term local control and overall survival after radiotherapy in oligoprogressive patients during treatment with checkpoint inhibitors. Anticancer Res. 2022;42(10):4795–804. [DOI] [PubMed] [Google Scholar]
- 35. Kim H, Venkatesulu BP, McMillan MT, Verma V, Lin SH, Chang JY, et al. Local therapy for oligoprogressive disease: a systematic review of prospective trials. Int J Radiat Oncol Biol Phys. 2022;114(4):676–83. [DOI] [PubMed] [Google Scholar]
- 36. Mahmood U, Huynh MA, Killoran JH, Qian JM, Bent EH, Aizer AA, et al. Retrospective review of outcomes after radiation therapy for oligoprogressive disease on immune checkpoint blockade. Int J Radiat Oncol Biol Phys. 2022;114(4):666–75. [DOI] [PubMed] [Google Scholar]
- 37. Yaney A, Stevens A, Monk P, Martin D, Diaz DA, Wang SJ. Radiotherapy in oligometastatic, oligorecurrent and oligoprogressive prostate cancer: a mini-review. Front Oncol. 2022;12:932637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ozluk AA, Karateke M, Sanli UA, Karaca B. Efficacy of local ablative therapies in patients with solid tumors treated with immune checkpoint inhibitors and oligoprogression: a single-center analysis. Melanoma Res. 2023;33:417–21. [DOI] [PubMed] [Google Scholar]
- 39. Sharma R, Pillai A, Marron TU, Fessas P, Saeed A, Jun T, et al. Patterns and outcomes of subsequent therapy after immune checkpoint inhibitor discontinuation in HCC. Hepatol Commun. 2022;6(7):1776–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Armstrong S, Roy T, Singh B, Kulasekaran M, Shaukat F, Geng X, et al. TKIs beyond immunotherapy predict improved survival in advanced HCC. J Cancer Res Clin Oncol. 2023;149(6):2559–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
For ethical reasons, the data are not publicly available. The datasets generated and analyzed in this study are available from the corresponding author upon reasonable request. Further inquiries can be directed to the corresponding author.