Abstract
Hepatocellular carcinoma (HCC) is the leading cause of cancer-related mortality in Taiwan. The Taiwan Liver Cancer Association and the Gastroenterological Society of Taiwan established HCC management consensus guidelines in 2016 and updated them in 2023. Current recommendations focus on addressing critical issues in HCC management, including surveillance, diagnosis, systemic treatment, and posttreatment monitoring. For surveillance and diagnosis, we updated the guidelines to include the role of protein induced by vitamin K absence or antagonist II (PIVKA-II) and gadoxetic acid (Gd-EOB-DTPA)-enhanced magnetic resonance imaging (MRI) in detecting HCCs. For systemic treatment, the updated guidelines summarize the multiple choices available for targeted therapy, immune checkpoint inhibitors, and a combination of both, especially for those carcinomas refractory to or unsuitable for transarterial chemoembolization. We have added a new section, posttreatment monitoring, that describes the important roles of PIVKA-II and EOB-MRI after HCC therapy, including surgery, locoregional therapy, and systemic treatment. Through this update of the management consensus guidelines, patients with HCC may benefit from optimal diagnosis, therapeutic modalities, and posttreatment monitoring.
Keywords: Diagnosis, Guideline, Hepatocellular carcinoma, Posttreatment monitoring, Surveillance, Systemic treatment
Introduction
Hepatocellular carcinoma (HCC) is the third leading cause of cancer-related deaths worldwide [1]. In 2020, the crude rate of HCC was 46.61 per 100,000 person-years, the fifth highest among all cancers; the crude mortality rate was 32.99 per 100,000 person-years, the second highest among all cancers in Taiwan [2]. Despite improvements in hepatitis control and management through vaccination and antiviral agents, HCC remains a critical public health concern. To assist clinical physicians in managing patients with HCC, the Taiwan Liver Cancer Association (TLCA) and Gastroenterological Society of Taiwan (GEST) first published management consensus guidelines in 2016 and updated them once in 2020. Due to the emergence of new data, the Taiwan Liver Cancer Association decided to update the consensus guidelines regarding surveillance, diagnosis, and systemic treatment, and add a new section on the posttreatment monitoring of HCCs. The target audience of the updated guidelines encompasses healthcare professionals, including gastroenterologists, transplant hepatologists, medical oncologists, and pharmacists as well as policy makers involved in the decision making process according to evidence-based data in Taiwan and on a global scale. The current updated guidelines focus on provide guidance on surveillance, diagnosis, systemic treatment, and posttreatment monitoring. Regarding comprehensive management strategies including surgery, local ablation, selective internal radiation therapy (SIRT), hepatic arterial infusion chemotherapy (HAIC), and radiation therapy, audiences may refer to the previous TLCA guidelines, published in 2016 [3].
Methodology
The updated guidelines incorporate evidence from systematic reviews of the literature based on clinical practice guidelines from various medical societies, clinical trials, meta-analyses of results, expert opinions, and real-world data. When developing guidelines in North America and Britain, experts rated the level of evidence for each recommendation based on combined experience [4]. The strengths of recommendations were categorized into three levels: strong, moderate, and considerable (Table 1). A strong recommendation reflects a high level of confidence of the expert group regarding a specific clinical practice and that most, if not all, target users should adopt the recommendation. Moderate recommendation reflects a moderate level of confidence of the expert group in a specific clinical practice, and while most target users should adopt the recommendation, the joint decision made by the physician and patient should be considered in clinical practice. Considerable recommendation reflects limited confidence of the expert group in a specific clinical practice; the recommendation should be conditionally applied to the target group with an emphasis on joint decision making by the physician and patient. Although some references were only real-world reports and not randomized controlled trials or comparative studies with lower levels of evidence, they were still valuable for clinical practice, with 100% agreement between experts.
Table 1.
Level of evidence and recommendations
| Level | Definition |
|---|---|
| Evidence | |
| 1 | At least one well-designed RCT |
| 1a | Meta-analysis of RCTs |
| 1b | At least one RCT |
| 2 | Comparative studies: non-RCT, but with well-designed cohort or case-control studies (prospective or retrospective), and outcomes research |
| 3 | Noncomparative studies: case series, case report, or not well-designed clinical studies |
| 4 | Opinion of respected authorities, descriptive epidemiology, or report of expert committee |
| Recommendation | |
| A | Strongly recommended |
| B | Moderate recommended |
| C | Considerable, but insufficient evidence |
RCT, randomized controlled trial.
The guidelines were updated by two teams comprising experts from various fields, including epidemiology, hepatology, surgery, medical oncology, radiation oncology, and diagnostic and interventional radiology. First, they were assigned into two expert teams: diagnosis group (7 experts, including epidemiologists and diagnostic radiologists) and systemic treatment group (22 experts, including hepatologists, surgeons, medical oncologists, radiation oncologists, and interventional radiologists). The initial draft statements were discussed, debated, and agreed upon by each expert team. Then, the statements were sent to all of the experts for further discussion and refinement. The coordinators of the expert teams presented the refined statement at the committee meeting on March 3, 2023, in Taipei; subsequently, all experts were invited to vote for the revised statements via secret ballots, including the level of evidence and recommendation [4–6]. The voting results were listed as percentages of agreement votes after each statement. After this meeting, the coordinators revised the statements and recommendation according to the experts’ suggestions and presented the refined statement at the annual TLCA meeting held in Kaohsiung, Taiwan, on April 22, 2023.
Consensus Statements
Section 1: Surveillance
Statement 1-1: Surveillance for HCCs should be performed using both ultrasonography and tumor markers such as alpha-fetoprotein (AFP) and/or PIVKA-II in clinical settings for HCC surveillance (A: 100%; E: 2; R: B).
Ultrasonography is a feasible and noninvasive modality for HCC screening. According to established guidelines, ultrasonography is the recommended tool for HCC surveillance. Serological test findings or tumor markers – such as AFP, Lens culinaris agglutinin-reactive fraction of AFP, and PIVKA-II/des-gamma-carboxy prothrombin (PIVKA-II/DCP) – are often elevated in individuals with HCC, and the prevalence of these markers increases with disease progression [7–9]. Employing a combination of multiple tumor markers can enhance accuracy of diagnostic tests in detecting small HCC lesions. The guidelines provided by the Japan Society of Hepatology advocate the combined use of ultrasonography and AFP, Lens culinaris agglutinin-reactive fraction of AFP, and PIVKA-II/DCP measurements for effective surveillance [10]. These markers can be conveniently and repeatedly measured, and monitoring their temporal changes is valuable for detecting and diagnosing HCCs. By carefully selecting an appropriate combination of the three tumor markers, their diagnostic performance can be further improved. Ultrasonography has limitations in identifying small tumors in patients with cirrhosis; however, the combination of serum AFP and/or PIVKA-II/DCP testing with ultrasonography can effectively identify additional tumors that might be missed on ultrasonography alone [8]. Therefore, the committee recommends combining the assessment of serum AFP and/or PIVKA-II/DCP levels with ultrasonography to augment screening efficacy for HCC surveillance.
Statement 1-2: Regular screening methods can be combined with dynamic computed tomography (CT) or magnetic resonance imaging (MRI) or gadoxetic acid (Gd-EOB-DTPA)-enhanced MRI (EOB-MRI) every 6–12 months for extremely high-risk patients and/or patients whose liver is difficult to image with ultrasonography due to liver atrophy, severe obesity, or postoperative deformity (A: 100%; E: 1; R: A).
Guidelines from various associations suggest regular surveillance of high-risk patients with chronic hepatitis B, hepatitis C, and nonviral cirrhosis [5, 6, 11, 12]. High-risk patients, particularly those diagnosed with chronic hepatitis B- or C-related cirrhosis, are classified into an extremely high-risk category [11]. The Taiwanese consensus guidelines recommend screening every 6 months using ultrasonography and serum AFP levels as tumor markers [6]. A general consensus across various liver cancer associations’ states that the duration between ultrasonography examinations for patients diagnosed with cirrhosis should be shortened to 3–4 months [10, 13, 14]. At instances where detecting HCC by ultrasonography becomes challenging due to factors such as liver atrophy, severe obesity, or postoperative deformity, alternative imaging modalities like dynamic CT or MRI can be employed. Gadoxetic acid (Gd-EOB-DTPA), an advanced MRI contrast agent, enables hepatocyte-phase imaging and has shown potential in identifying liver nodules at a high risk of developing into HCC, particularly in patients with hepatitis B or C [15].
The use of multiphase CT and dynamic MRI for extensive surveillance in the early detection of HCC is generally considered not cost-effective because of the high expenses. However, it is recognized that CT and MRI demonstrate superior sensitivity and specificity compared to ultrasonography. The argument against the cost-effectiveness of surveillance by using CT and MRI primarily not arises from their diagnostic capabilities but the high cost of the tests. Therefore, noninvasive laboratory and imaging evaluations are crucial for identifying individuals at risk of advanced fibrosis and cirrhosis and ensuring timely medical attention through HCC screening programs. Current guidelines recommend a combination of ultrasonography, AFP, and PIVKA-II for HCC screening, suggesting alternative modalities like dynamic CT, MRI, or EOB-MRI for high-risk groups in whom liver ultrasonography is challenging. Recent improvements in survival rates of HCC patients are partially due to early detection via these monitoring programs, with current data supporting the cost-effectiveness of HCC screening for both high- and medium-risk cirrhosis patients [16–18]. However, the necessity for ongoing research and application of these findings for personalized HCC screening and risk stratification are imperative. Therefore, combining regular screening methods with dynamic CT, MRI, or EOB-MRI is recommended for extremely high-risk patients or those for whom ultrasonography is challenging.
Statement 1-3: Kupffer-phase contrast-enhanced ultrasound (CEUS) with Sonazoid, combined with the reinjection technique, can also be recommended as a primary test for HCC patients who have cirrhosis with very coarse liver parenchyma and renal dysfunction, if a liver nodule larger than 1 cm is detected by B-Mode ultrasonography (A: 100%; E: 2; R: B).
CEUS facilitates the intravascular administration of microbubbles, referred to as ultrasonography contrast agents, thus enabling the real-time imaging of hepatic blood flow. The Kupffer phase can be determined using an advanced ultrasonography contrast agent, known as Sonazoid; Sonazoid is utilized in defect reperfusion imaging for HCC diagnosis. Sonazoid-enhanced CEUS demonstrates a diagnostic efficacy comparable to dynamic CT in identifying malignant liver tumors [19]. In a recent study, Sonazoid-enhanced CEUS exhibited heightened sensitivity in detecting smaller HCCs among patients who have liver cirrhosis with very coarse liver parenchyma compared with unenhanced ultrasonography [20]. In particular, CEUS has proven to be a valuable noninvasive and efficient tool for diagnosing focal liver lesions in individuals at an elevated risk of developing liver malignancies [21]. CEUS is especially beneficial for patients with renal dysfunction, as the contrast agents are not excreted by the kidneys. It is recommended as the primary test for cirrhotic patients with very coarse liver parenchyma and renal dysfunction, to be conducted every 3–6 months. For screening HCC in such patients, Kupffer-phase CEUS, which utilizes Sonazoid and a reinjection technique, is specifically advised [6, 20].
Section 2: Diagnosis
Statement 2-1: For high-risk or extremely high-risk patients, nodules smaller than 1 cm in which malignancy cannot be confirmed should be followed up with ultrasonography, AFP, and/or PIVKA-II at 3- to 6-month intervals (A: 100%; E: 2; R: A).
A systematic review and meta-analysis revealed a pooled estimate of approximately 4.6 months for the tumor volume doubling time in HCC [22]. However, considerable heterogeneity was observed among the included studies, with reported tumor volume doubling times ranging from 2.2 to 11.3 months [22]. Consequently, the expert panel advocated the follow-up imaging of nodules smaller than 1 cm at 3- to 6-month intervals [3, 11]. Ultrasonography is effective in detecting early stage HCC; however, the diagnostic accuracy of PIVKA-II/DCP and AFP levels alone in diagnosing small HCCs remains inconclusive. In other words, the results indicate that either marker may be more accurate in terms of sensitivity, specificity, and/or AUC [7, 23–25]. However, combining both markers with optimized cutoff levels enhances the detection of small HCCs. In a nested control study conducted within the Hepatitis C Antiviral Long-Term Treatment Against Cirrhosis Trial (HALT-C), combined AFP and PIVKA-II/DCP led to increased sensitivity of 91% at month 0 and 73% at month 12, albeit at the cost of specificity, which was reduced to 74% and 71%, respectively [26]. Although further evidence is needed, the combination of PIVKA-II/DCP with ultrasonography and AFP for HCC surveillance, including small HCCs, has the potential to improve sensitivity while compromising specificity [26, 27]. Moreover, its role in detecting AFP-negative HCCs is expected to increase as more patients undergo treatment for hepatitis-related HCC.
Statement 2-2: Liver nodules larger than 1 cm should be investigated using dynamic imaging (multidetector CT [MDCT], MRI, CEUS, or EOB-MRI) (A: 100%; E: 2; R: B).
Statement 2-3: For nodules larger than 1 cm in patients with cirrhosis or chronic hepatitis B or C, characteristic vascular patterns on a four-phase CEUS, MDCT, MRI, or EOB-MRI image could be diagnosed without biopsy. However, obtaining tissue proof is encouraged (A: 100%; E: 2; R: A).
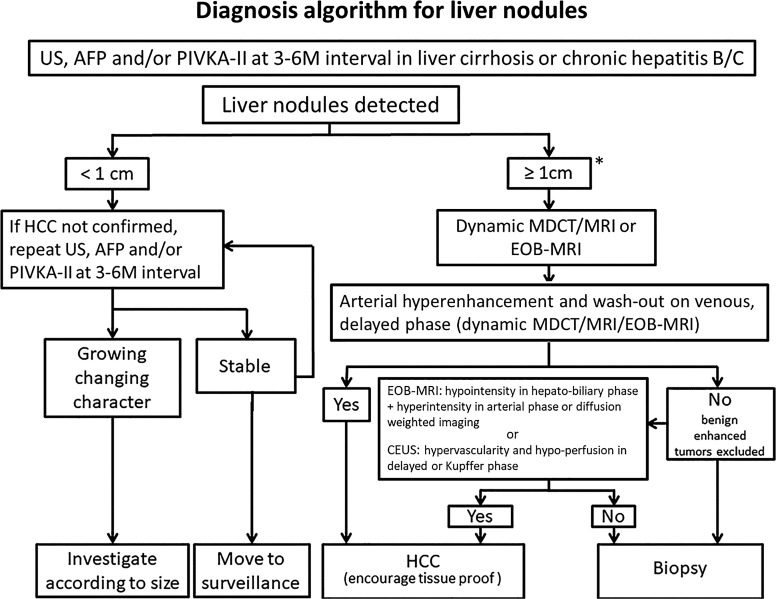
According to the guidelines provided by the American Association for the Study of Liver Diseases, European Association for the Study of the Liver, and Taiwan Liver Cancer Association, investigating focal liver lesions larger than 1 cm as potential HCCs is recommended [5, 6, 28]. To assess the enhancement patterns of these nodules, a multiphase contrast-enhanced imaging study, such as MDCT or MRI, is necessary. A systematic review and meta-analysis indicated that CEUS, dynamic MDCT, and dynamic MRI demonstrated comparable levels of sensitivity and specificity in diagnosing HCCs [29]. Nevertheless, for HCC detection, EOB-MRI exhibits higher sensitivity than non-liver-specific contrast MRI (79% vs. 69%) but similar specificity (96% vs. 94%) [30]. However, biopsies remain the gold standard for confirming HCC. In cases where the vascular pattern is not characteristic, or in patients with non-cirrhotic livers or without chronic hepatitis B or C, biopsy may be performed. For lesions lacking a distinctive vascular pattern, characterized by arterial hyperenhancement and washout in the venous or delayed phases on dynamic MDCT/MRI or EOB-MRI, and for those deemed unsuitable for biopsy, hypointensity in the hepatobiliary phase of EOB-MRI or hypoperfusion in the Kupffer phase of CEUS should be considered as an alternative diagnostic approach [31] (Fig. 1). The diagnostic algorithms employed in Taiwan incorporate EOB-MRI and CEUS because of their respective advantages [6].
Statement 2-4: If the biopsy is negative for HCC, patients should be followed up for 2 years using ultrasonography, CT, MRI or CEUS, AFP, and/or PIVKA-II every 3–6 months until the nodule either disappears, enlarges, or displays the diagnostic characteristics of HCC (A: 100%; E: 2; R: A).
If nodules larger than 1 cm cannot be definitively identified through imaging studies and the pathological diagnoses are inconclusive, closely monitoring the patient using ultrasonography, CT, MRI, or CEUS every 3–6 months for up to 2 years is recommended [32]. Monitoring should continue until the nodule resolves, increases in size, or exhibits diagnostic features indicative of HCC. PIVKA-II/DCP has been included in this statement as a biomarker for the early detection of HCC. The simultaneous use of AFP and PIVKA-II/DCP has shown potential in enhancing the sensitivity of detecting early stage HCC [7, 8, 26].
Fig. 1.
Diagnostic algorithm for liver nodules. AFP, alpha-fetoprotein; CEUS, contrast-enhanced ultrasound; EOB-MRI, gadoxetic acid (Gd-EOB-DTPA)-enhanced magnetic resonance imaging; HCC, hepatocellular carcinoma; MDCT, multidetector computed tomography; MRI, magnetic resonance imaging; PIVKA-II, Protein induced by Vitamin K absence or antagonists-II; US, ultrasound. *Kupffer-phase contrast-enhanced ultrasound (CEUS) with Sonazoid, combined with the reinjection technique, can also be recommended as a primary test for HCC patients who have cirrhosis with very coarse liver parenchyma and renal dysfunction, if a liver nodule ≥1 cm is detected by B-Mode ultrasonography (Statement 1–3).
Role of Liver Tumor Biopsy
The aspect of determining of whether a definitive diagnosis of HCC should rely exclusively on histopathological methods, such as biopsy, engages with core issues in medical diagnostics and patient management. Histopathology, which involves microscopic tissue examination by a pathologist, has traditionally been upheld as the diagnostic “gold standard” for various cancers, including HCC [33]. Its diagnostic primacy is attributed to its ability to facilitate the direct visualization of cancerous cells and to yield extensive data concerning a tumor’s histological grade, probable origin, and diverse cellular features. Recent decades have witnessed substantial advancements in imaging technologies, including MRI, CT, and ultrasonography. These techniques have increasingly demonstrated a capacity to detect the hallmark characteristics of HCC, particularly within patient cohorts presenting with pre-existing cirrhosis or other predisposing factors. In numerous instances, the imaging results are sufficiently definitive to obviate biopsy, especially when considering the potential hazards associated with such an invasive procedure. Biopsy procedures, while informative, are not devoid of risk. Specifically, hepatic interventions entail a risk of hemorrhage, infection, and neoplastic seeding – the latter involving tumor cell dissemination along the tract of the biopsy needle [34]. Patients with cirrhosis may exhibit heightened complication risks. Furthermore, a biopsy may only provide insight into the part of the tumor sampled, which poses a limitation in heterogeneous neoplasms with regions exhibiting variable cellular characteristics potentially escaping detection. Such heterogeneity can result in an underrepresentation of the tumor’s aggression or impact on therapeutic decision making. Occasionally, treatment strategies remain unaffected by biopsy findings, especially when imaging studies strongly indicate HCC and the patient history includes relevant risk factors. In these scenarios, the evaluation of risks versus benefits may favor the nonperformance of a biopsy. Thus, while the role of biopsy in confirming histopathological diagnoses remains foundational, the decision to employ this diagnostic tool must be personalized, while considering the entirety of clinical evidence and imaging data. The contemporary medical paradigm mandates that patient care decisions be comprehensive, rooted in evidence, and customized to each patient’s specific requirements.
Statement 2-5: CEUS demonstrates superior sensitivity in detecting arterial hypervascularity and is better able to demonstrate rapid washout for non-HCC malignancy and very late washout for HCC than dynamic CT or dynamic MRI (A: 100%; E: 2; R: B).
CEUS provides real-time observations and offers several advantages over CT and MRI [5, 31, 35]. CEUS enables both morphological and temporal enhancement patterns to be captured, enabling the differential diagnosis of liver lesions. In particular, arterial hyperenhancement can be identified using CEUS regardless of the timing, thus overcoming the timing limitations of CT and MRI. In perfusion studies using CEUS, intrahepatic cholangiocarcinoma exhibited a significantly shorter and more pronounced washout than that of HCC [36–38]. Moreover, the morphological patterns in the arterial phase of intrahepatic cholangiocarcinoma (characterized by peripheral rim-like enhancement) differ from those of HCC (exhibiting global enhancement) [39, 40]. Sonazoid-enhanced ultrasonography, which offers a longer observation time, may have higher sensitivity and accuracy than those of dynamic CT [41, 42]. Additionally, CEUS can assist in the histological differentiation of HCC, with poorly differentiated HCCs demonstrating short washout times and well-differentiated HCCs demonstrating long washout times during the Kupffer phase [42, 43].
Statement 2-6: EOB-MRI can detect the earliest initial changes in HCCs, including high-grade dysplastic nodules (HGDNs) and early HCCs (A: 100%; E: 2; R: B).
Gd-EOB-DTPA is a contrast agent used in EOB-MRI that exhibits specific uptake by hepatocytes [11, 31]. Within the context of EOB-MRI, the presence of a hypoenhancing lesion is a distinct characteristic of malignancies [44, 45]. Early HCCs and high-grade dysplastic nodules (HGDNs) manifest as hypovascular nodules that lack arterial enhancement or portal venous washout, resulting in hypointensity during the hepatocyte phase. T2-weighted imaging or diffusion-weighted imaging can aid in distinguishing an early HCC from an HGDN by highlighting their hyperintensities. Hypovascular hypointense nodules detected on EOB-MRI are associated with an increased likelihood of progression to hypervascular HCCs [46]. EOB-MRI hepatobiliary phase images are more effective than those of dynamic MDCT in detecting hypovascular hepatocellular nodules in patients at high risk of HCC [47]. A diagnostic algorithm that incorporates the hepatobiliary phase, arterial phase, and diffusion-weighted imaging of EOB-MRI demonstrated substantial sensitivity and specificity for diagnosing overt HCCs, early HCCs, and HGDNs [48].
Statement 2–7: EOB-MRI can improve the evaluation of tumor burden and tumor staging, and optimize the therapeutic options and clinical outcome (A: 100%; E: 2; R: B).
The sensitivity of EOB-MRI exceeds that of multiphase MRI in detecting small or early stage HCCs. Specifically, EOB-MRI demonstrates higher sensitivity than non-gadoxetic acid-enhanced MRI for HCCs measuring less than 2 cm [49–51]. Incorporating hepatocyte-phase imaging into EOB-MRI enables the identification of hypovascular HCCs. Additionally, integrating EOB-MRI into the diagnostic process enhances the evaluation of the tumor burden, contributes to the identification of stage migration, and provides valuable guidance for treatment planning. Patients with preprocedural non-hepatocellular hyperplastic nodules detected using EOB-MRI have an increased risk of HCC recurrence after surgical resection or radiofrequency ablation (RFA). Employing EOB-MRI prior to intervention to detect non-hepatocellular hyperplastic nodule may assist in determining curative treatment options for HCCs [52–55]. EOB-MRI can detect previously unnoticed HCCs in 16% of the patients, thereby improving treatment outcomes [56]. By optimizing treatment through EOB-MRI, overall survival (OS) rates can be enhanced and the risk of HCC recurrence can be reduced [55, 57].
Statement 2-8: Combined interpretation of the dynamic and hepatobiliary phases of EOB-MRI with diffusion-weighted imaging can improve the diagnostic accuracy of MRI in detecting HCC (A: 100%; E: 2; R: B).
The pathogenesis of HCC involves an increase in cellularity, which can be identified using diffusion-weighted MRI with restricted diffusion [58]. The integration of this technique with conventional multiphase MRI enhances the diagnostic performance of HCC. Furthermore, administering liver-specific contrast agents such as Gd-EOB-DTPA or gadobenate dimeglumine during the hepatocyte phase of MRI enhances the sensitivity in detecting small HCCs [50, 59]. The combined use of diffusion-weighted imaging and liver-specific contrast agents further improves diagnostic accuracy and sensitivity in the detection of HCCs [57, 60].
The severity of cirrhosis negatively impacts the visualization of HCC during Gd-EOB-DTPA-enhanced MRI. To enhance the detection of HCC in cirrhosis patients undergoing MRI, the timing of the hepatobiliary phase delay time (HBP-DT) must be optimized. The optimal HBP-DT for detecting HCC in patients with Child-Pugh class A (CP-A) and class B (CP-B) cirrhosis is 10 min post-contrast (DT-10). However, for patients with CP-C cirrhosis, a delay time of 15 min or longer (DT-15) is recommended to improve the visualization of HCC [61].
Statement 2-9: Preoperative EOB-MRI may support prediction of microvascular invasion and is more effective than dynamic CT in detecting additional HCC nodules, leading to improved recurrence-free survival of patients following hepatic resection (A: 100%; E: 2; R: A).
Numerous studies have examined the use of preoperative MRI to predict the occurrence of microvascular invasion and recurrence in patients diagnosed with HCC. The independent predictors of microvascular invasion include arterial peritumoral enhancement, non-smooth tumor margins, and peritumoral hypointensity in the hepatobiliary phase [62]. A scoring system that incorporated five EOB-MRI features and serum AFP levels to accurately predict the presence of microvascular invasion and postoperative survival has been developed [63]. The signal intensity ratio of peritumoral tissue-to-normal liver during the arterial phase is associated with microvascular invasion and pathological grade and predicts the rate of recurrence-free survival [64]. Compared to dynamic CT, EOB-MRI demonstrates superior effectiveness in identifying suitable surgical candidates at a reduced risk of early recurrence following hepatic resection [65]. Further assessment using MRI with gadoxetic acid after CT results in an increased detection rate of HCC nodules, modification of treatment plans, low rate of HCC recurrence, and decreased overall mortality [56]. Changes in preoperative tumor stage between dynamic CT and EOB-MRI are associated with CT-defined early stages, albumin-bilirubin grades, high log AFP levels, and early recurrence [66]. Preoperative EOB-MRI combined with simultaneous treatment contributes to extended recurrence-free survival following hepatic resection [67]. Therefore, EOB-MRI has demonstrated superiority over dynamic CT in identifying appropriate surgical candidates who have a reduced risk of early recurrence. Moreover, preoperative MRI can contribute to enhanced recurrence-free survival in patients undergoing curative hepatic resection. The suggested diagnostic algorithm for liver nodules is shown in Figure 1.
Section 3: Systemic Treatment
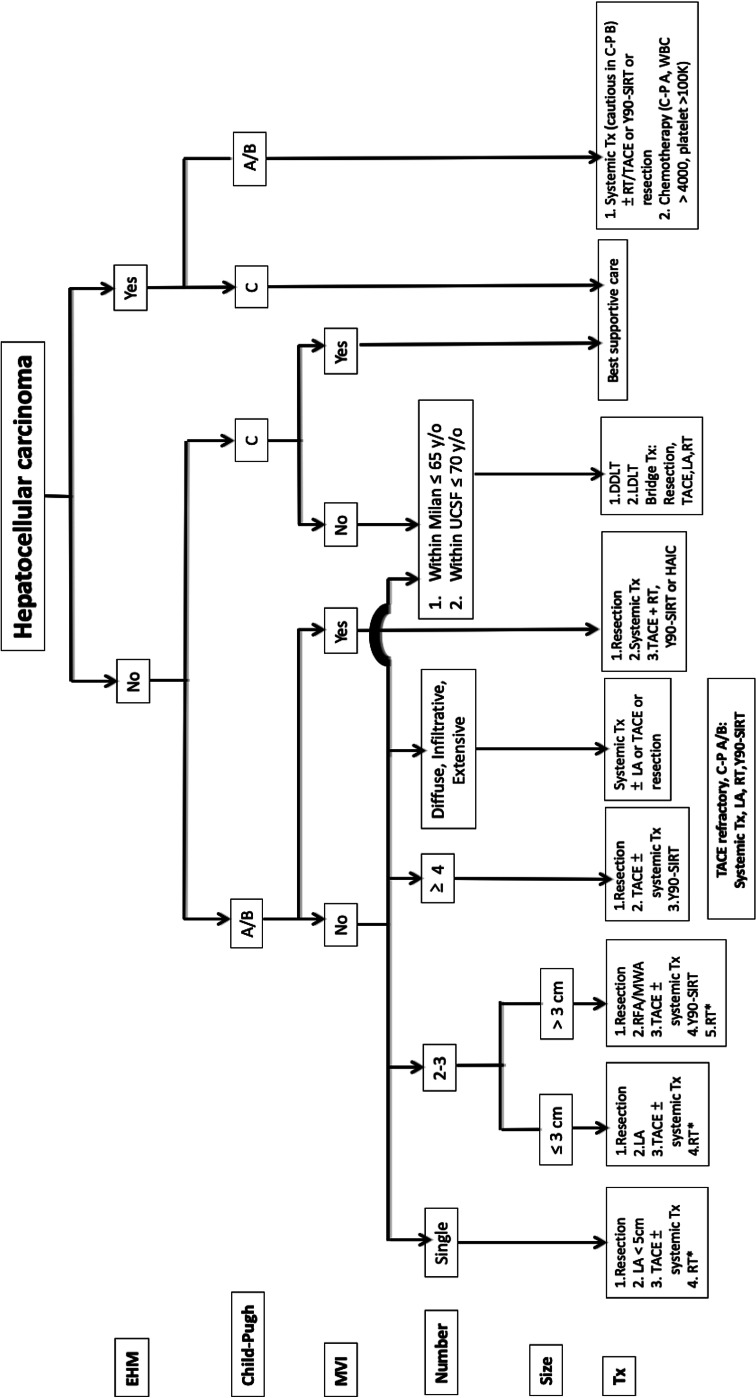
The BCLC staging system is currently widely used worldwide. However, the BCLC system is a treatment selection staging method intended for decision making purposes. Further refinement of the treatment algorithm is still needed, particularly in the Asia-Pacific region, emerging evidence supports the use of aggressive treatment, especially of multi-modalities for patients with portal vein tumor thrombosis and with large tumor burden. The original TLCA treatment algorithm was based on other Asian guidelines [10, 68] and five factors: (1) extrahepatic metastasis, (2) liver function, (3) macrovascular invasion, (4) number of tumors, and (5) tumor size. We have updated the treatment algorithm for HCC and shown in Figure 2. Systemic therapies include multikinase inhibitors and immune checkpoint inhibitors. Detailed statements regarding systemic therapy are as follows.
Fig. 2.
Treatment algorithms for hepatocellular carcinoma. C–P, Child-Pugh; DDLT, deceased donor liver transplantation; EHM, extrahepatic metastasis; HAIC, hepatic arterial infusion of chemotherapy; LA, local ablation (including PEI, RFA, and MWA); LDLT, living donor liver transplantation; MWA, microwave ablation; MVI, macrovascular invasion; PEI, percutaneous ethanol injection; RFA, radiofrequency ablation; RT, radiotherapy; SIRT, selective internal radiation therapy; TACE, transarterial chemoembolization; Tx, therapy; UCSF, University of California, San Francisco. *When the above treatments are infeasible.
Statement 3-1: TACE alone or TACE combined with sorafenib/lenvatinib can be considered in unresectable intermediate stage HCCs within up-to-11 criteria, Child-Pugh class A liver function, an Eastern Cooperative Oncology Group performance status of 1 or less, no vascular invasion, and no extrahepatic spread (A: 100%; E: 2; R: B).
For patients with intermediate stage HCC, TACE remains the standard treatment. Multiple kinase inhibitors exhibit antitumor effects, affect tumor vasculature [69–71], and inhibit vascular endothelial growth factor after TACE [72]; therefore, the concomitant use of multiple kinase inhibitors can enhance the efficacy of TACE and suppress the progression of residual tumors and appearance of new intrahepatic lesions. Based on these concepts, various clinical trials [73–77] have been conducted. With the exception of two trials – the TACTICS [78] and LAUNCH [79] trials – the results are not encouraging. The TACTICS trial was a randomized, open-label phase 2 trial comparing sorafenib plus TACE with TACE alone. It did not demonstrate a significant OS benefit of TACE plus sorafenib over TACE alone (36.2 vs. 30.8 months; hazard ratio [HR], 0.861; 95% confidence interval [CI], 0.607–1.223; p = 0.40). However, TACE-specific progression-free survival (PFS) was significantly better in the TACE plus sorafenib group than in the TACE alone group (22.8 vs. 13.5 months; HR, 0.661; 95% CI: 0.466–0.938; p = 0.02). Additionally, both PFS and OS benefit were identified in TACE plus sorafenib patients with tumor burden beyond up-to-seven criteria (PFS: 22.1 vs. 9.0 months; OS: 36.3 vs. 25.0 months).
The LAUNCH trial was a randomized, open-label phase 3 trial comparing lenvatinib plus TACE (LEN-TACE group) and lenvatinib monotherapy (LEN group). The median OS was significantly longer in the LEN-TACE group than in the LEN group (17.8 vs. 11.5 months; HR, 0.45; p < 0.001). The median PFS was 10.6 months in the LEN-TACE group and 6.4 months in the LEN group (HR, 0.43; p < 0.001). Patients in the LEN-TACE group had a higher objective response rate (ORR) according to the modified response evaluation criteria in the Response Evaluation Criteria in Solid Tumors (RECIST) (54.1% vs. 25.0%; p < 0.001). The phase 2, single-arm TACTICS-L trial [80] enrolled 62 patients who received a combination of lenvatinib and TACE and identified the best ORR and complete response (CR) rates were 88.7% and 66.1%, respectively. The primary endpoint of median PFS was 28.0 months and the secondary endpoint of median OS was not attained, indicating promising therapeutic efficacy.
Conventional definitions of tumor burden are not optimal for every patient with intermediate-stage HCC. The new seven-eleven criteria have excellent discriminative power for predicting radiological response and survival in patients with intermediate-stage HCC undergoing TACE [81]. Both the TACTICS and LAUNCH trials enrolled patients with a single tumor (≤10.0 cm) or multiple tumors (≤10 foci). Therefore, we propose that a combination of TACE with or without sorafenib or lenvatinib can be considered for treating intermediate-stage HCCs with up-to-eleven criteria.
Four randomized phase 3 clinical trials of a combination of TACE and immune checkpoint inhibitor-based therapy are underway (Table 2): EMERALD-1 (NCT03778957) [82], a three-arm global clinical trial comparing TACE followed by durvalumab plus bevacizumab, TACE followed by durvalumab, and TACE followed by placebo; LEAP-012 (NCT04246177) [83], investigating TACE plus combination treatment with pembrolizumab and lenvatinib; CheckMate 74W (NCT04340193) [84], investigating nivolumab plus ipilimumab in combination with TACE; and TALENTACE (NCT04712643) [85], investigating atezolizumab plus bevacizumab in combination with TACE.
Table 2.
Summary of active phase 3 clinical trials of a combination of TACE and immune checkpoint inhibitor-based therapy
| Trial name (Registration No.) | Characteristics | Endpoint | ||
|---|---|---|---|---|
| arm | patient, n | primary | secondary | |
| EMERALD-1 [81] (NCT03778957) | A: TACE followed by durvalumab | 600 | PFS: arm A versus arm C by RECIST v1.1 | 1. PFS: arm B versus arm C |
| B: TACE followed by durvalumab plus Bevacizumab | 2. OS | |||
| C: TACE followed by placebo | 3. Health-related quality of life measures | |||
| 4. Safety | ||||
| LEAP-012 [82] (NCT04246177) | A: lenvatinib plus pembrolizumab plus TACE | 950 | OS and PFS by RECIST v1.1 | 1. PFS, ORR, DCR, DOR, and TTP by modified RECIST |
| B: placebo plus TACE | 2. ORR, DCR, DOR, and TTP by RECIST v1.1 | |||
| 3. Safety | ||||
| CheckMate 74W [83] (NCT04340193) | A: nivolumab plus ipilimumab plus TACE | 765 | 1. TTTP | 1. TTTP and OS: arm B versus arm C |
| B: nivolumab plus placebo plus TACE | 2. OS: arm A versus arm C | 2. Event-free survival | ||
| C: placebo plus TACE | 3. PFS | |||
| TALENTACE [84] (NCT04712643) | A: TACE plus atezolizumab plus bevacizumab | 342 | 1. Independent review committee-determined TACE-PFS (randomization to unTACEable progression, TACE failure/refractoriness, or death) | 1. Investigator-determined TACE-PFS |
| 2. OS | 2. Time to unTACEable progression, progression, MVI, EHS, and MVI/EHS | |||
| B: TACE alone | 3. ORR, DOR, patient-reported outcomes, AE | |||
AE, adverse events; CI, confidence interval; DCR, disease control rate; DOR, duration of response; EHS, extrahepatic spread; HR, hazard ratio; MVI, microvascular invasion; ORR, objective response rate; OS, overall survival; PFS, progression-free survival; TACE, transarterial chemoembolization TTP, time to progression; TTTP, time to TACE progression; RECIST, Response Evaluation Criteria in Solid Tumors.
Statement 3-2: Systemic therapy may be considered in patients with HCC refractory to or unsuitable for TACE (A: 100%; E: 1; R: A).
Based on several treatment guidelines, systemic therapy is recommended for patients who are not eligible for effective TACE [10, 86–88]. Repeated TACE in a patient who has become refractory to the treatment leads to impaired liver function and, consequently, poor prognosis [89]. The Japan Society of Hepatology (JSH) criteria [10] define TACE refractoriness as failure to control the tumor in the target lesion or the appearance of new lesions after two or more consecutive TACE sessions, continuous elevation of tumor markers, appearance of vascular invasion, or extrahepatic spread. Switch-to [89] or add-on [90] sorafenib after TACE refractoriness is more likely to improve OS and reduce the incidence of disease progression. Updated data from the IMbrave150 study, revealed that 130 (39%) patients treated with atezolizumab plus bevacizumab showed a survival-benefit trend over sorafenib (19.0 vs. 14.4 months; HR, 0.71; 95% CI: 0.50–1.02) after TACE refractoriness [91]. The JSH criteria also define TACE unsuitability to include the following three clinical conditions: likely to develop TACE failure/refractoriness, likely to progress to CP B liver function after TACE, or unlikely to respond to TACE. Apart from tumor burden, radiologic patterns also determine the outcomes of initial and subsequent TACE [92]. Since TACE does not benefit patients with confluent multinodular or infiltrative type lesions, they are deemed unsuitable for TACE. Systemic therapy should be considered for patients with unfavorable radiologic patterns. Lenvatinib treatment followed by additional selective TACE could improve the efficacy of TACE and prolong survival [93, 94]; hence, it may be recommended for patients with HCC who are refractory to or unsuitable for TACE.
Statement 3-3: Atezolizumab plus bevacizumab is recommended as the preferred choice for treatment-naïve patients with Child-Pugh class A liver function, Eastern Cooperative Oncology Group performance status of 2 or less, and HCC that is unresectable and not amenable to locoregional therapy. Otherwise, tremelimumab plus durvalumab is an alternative choice in case of a high risk of bleeding. Sorafenib and lenvatinib are recommended as alternative choices if contraindications for immunotherapy are present (A: 100%; E: 1; R: A).
The combination of atezolizumab plus bevacizumab is currently the first-choice first-line treatment according to different guidelines [12, 87, 88, 95–98] if no contraindications, such as bleeding risk, are present. In one study, after a median 15.6 months of follow-up, the median OS was 19.2 months (95% CI: 17.0–23.7) with atezolizumab plus bevacizumab and 13.4 months (95% CI: 11.4–16.9) with sorafenib (HR, 0.66; 95% CI: 0.52–0.85; p < 0.001). The median PFS was 6.9 (95% CI: 5.7–8.6) and 4.3 (95% CI: 4.0–5.6) months in the respective treatment groups (HR: 0.65; 95% CI: 0.53–0.81; p < 0.001). Confirmed objective responses occurred in 30% (95% CI: 25–35) treated with atezolizumab plus bevacizumab versus 11% (95% CI: 7–17) treated with sorafenib, according to RECIST 1.1. Treatment-related grade 3/4 adverse events occurred in 43% and 46% of safety evaluable patients in the respective groups [91]. In addition to the superior survival benefit of atezolizumab plus bevacizumab treatment to that of sorafenib, prespecified analyses of patient-reported outcomes data from the IMbrave150 trial revealed clinically meaningful benefits in terms of quality of life, functioning, and disease symptoms for the former regimen [99, 100].
Recently, data from the phase 3 HIMALAYA trial [101] revealed that a single priming dose of tremelimumab (anti-cytotoxic T lymphocyte-associated antigen 4) added to durvalumab (anti-programmed cell death ligand-1) (STRIDE) provided a statistically significant survival benefit compared to sorafenib and that durvalumab as a monotherapy is not inferior to sorafenib as a first-line treatment. The median OS was 16.4 months (95% CI: 14.2–19.6) with STRIDE, 16.6 months (95% CI: 14.1–19.1) with durvalumab, and 13.8 months (95% CI: 12.3–16.1) with sorafenib. The OS HR for STRIDE versus sorafenib was 0.78 (96.02% CI: 0.65–0.93; p < 0.0035). OS with durvalumab monotherapy was non-inferior to sorafenib (HR: 0.86; 95.67% CI: 0.73–1.03; non-inferiority margin, 1.08). The median PFS was not significantly different among all three groups. Grade 3/4 treatment-emergent adverse events occurred in 50.5% of the patients on STRIDE, 37.1% on durvalumab, and 52.4% on sorafenib. Immune-mediated events requiring treatment with high-dose glucocorticoids occurred in 20.1%, 9.5%, and 1.9% patients receiving STRIDE, durvalumab, and sorafenib, respectively. The most common immune-mediated events were hepatic events, diarrhea/colitis, and dermatitis/rash.
Atezolizumab plus bevacizumab treatment has not been directly evaluated against tremelimumab plus durvalumab. Durvalumab plus tremelimumab is another preferred option for patients in the first-line setting, particularly for patients who are not candidates for anti-VEGF therapy, such as those with a high risk of bleeding. Sorafenib [102] and lenvatinib [103] are recommended as alternative choices if contraindications for immunotherapy, such as arterial hypertension, cardiovascular disease, and prior autoimmune conditions, are present [96].
Recently, tislelizumab (an anti-programmed cell death-1 monoclonal antibody) demonstrated non-inferior OS to sorafenib (HR, 0.85; p = 0.0398) in the first-line phase 3 RATIONALE 301 study [104]. Other combinations of antiangiogenic targeted therapy and PD-1/PD-L1 blockade, such as sintilimab plus IBI305 (bevacizumab biosimilar) (ORIENT-32) [105] showed a significant OS (HR, 0.57; p < 0.0001) and PFS (HR, 0.56; p < 0.0001) benefit versus sorafenib for Chinese patients with unresectable, HBV-associated HCC. A combination of cabozantinib and atezolizumab (the COSMIC-312 trial) demonstrated a significant benefit in PFS (HR: 0.63) but no survival benefit compared to sorafenib [106]. The combination treatment of lenvatinib and pembrolizumab in the phase 3 LEAP-002 trial failed to achieve its primary endpoint; that is, the OS and PFS were not significantly higher than that associated with lenvatinib monotherapy [107]. Another phase 3 trial (CARES-310) [108] demonstrated superiority of combined camrelizumab and rivoceranib to sorafenib in terms of OS (HR: 0.62; p < 0.001) and PFS (HR, 0.52; p < 0.001) (Table 3).
Table 3.
Overview of phase-3 first-line clinical trials for systemic therapies in advanced-stage HCC
| Trial name, year | Arms | OS, median (95% CI), months | HR of OS (95% CI) | PFS, median (95% CI), months | HR of PFS (95% CI) | ORR by RECIST 1.1, % |
|---|---|---|---|---|---|---|
| SHARP [101] (2008) | Sorafenib | 10.7 (9.4–13.3) | 0.69 (0.55–0.87) | 5.5 (4.1–6.9) | 0.58 (0.45–0.74) | 2.0 |
| Placebo | 7.9 (6.8–9.1) | 2.8 (2.7–3.9) | 1.0 | |||
| REFLECT [102] (2018) | Lenvatinib | 13.6 (12.1–14.9) | 0.92 (0.79–1.06) | 7.4 (6.9–8.8) | 0.66 (0.57–0.77) | 18.8 |
| Placebo | 12.3 (10.4–13.9) | 3.7 (3.6–4.6) | 6.5 | |||
| IMbrave150 [98] (2020) | Atezolizumab plus bevacizumab | 19.2 (17.0–23.7) | 0.66 (0.52–0.85) | 6.9 (5.7–8.6) | 0.65 (0.53–0.81) | 27.3 |
| Sorafenib | 13.4 (11.4–16.9) | 4.3 (4.0–5.6) | 11.9 | |||
| ORIENT-32 [104] (2021) | Sintilimab plus bevacizumab biosimilar | Not reached | 0.57 (0.43–0.75) | 4.6 (4.1–5.7) | 0.56 (0.46–0.70) | 21.0 |
| Sorafenib | 10.4 (8.5-not reached) | 2.8 (2.7–3.2) | 7.0 | |||
| HIMALAYA [100] (2022) | A: Tremelimumab plus durvalumab | 16.4 (14.2–19.6) | A versus C: 0.78 (0.65–0.92) | 3.8 (3.7–5.3) | A versus C: 0.90 (0.77–1.05) | 20.1 |
| B: Durvalumab | 16.6 (14.1–19.1) | B versus C: 0.86 (0.73–1.03) | 3.7 (3.2–3.8) | B versus C: 1.02 (0.88–1.19) | 17.0 | |
| C: Sorafenib | 13.8 (12.3–16.1) | 4.1 (3.8–5.5) | 5.1 | |||
| RATIONALE-301 [103] (2022) | Tislelizumab | 15.9 | 0.85 (0.71–1.02) | 2.2 | 1.1 (0.92–1.33) | 14.3 |
| Sorafenib | 14.1 | 3.6 | 5.4 | |||
| COSMIC-312 [105] (2022) | Cabozantinib plus atezolizumab | 15.4 (96% CI: 13.7–17.7) | 0.90 (0.69–1.18) | 6.8 (99% CI: 5.6–8.3) | 0.63 (0.44–0.91) | 11 |
| Sorafenib | 15.5 (96% CI: 12.1-not reached) | 4.2 (99% CI: 2.8–7.0) | 4 | |||
| LEAP-002 [106] (2022) | Lenvatinib plus pembrolizumab | 21.2 (19.0–23.6) | 0.84 (0.71–0.99) | 8.2 (6.4–8.4) | 0.87 (0.73–1.02) | 26.1 |
| Lenvatinib plus placebo | 19.0 (17.2–21.7) | 8.0 (6.3–8.2) | 17.5 | |||
| CARES-310 [107] (2023) | Camrelizumab plus rivoceranib | 22.1 (19.1–27.2) | 0.62 (0.49–0.80) | 5.6 (5.5–6.3) | 0.52 (0.41–0.65) | 25 |
| Sorafenib | 15.2 (13.0–18.5) | 3.7 (2.8–3.7) | 6 |
CI, confidence interval; HR, hazard ratio; ORR, objective response rate; OS, overall survival; PFS, progression-free survival.
Statement 3-4: Treatment with sorafenib or lenvatinib or atezolizumab plus bevacizumab may be recommended for selected patients with HCC and Child-Pugh class B liver function, whose tumors are unresectable and not amenable to locoregional therapy (A: 100%; E: 2; R: C).
No large randomized clinical trial has tested the feasibility and efficacy of any systemic therapy in patients with advanced HCC and CP B status. In the GIDEON observational registry study, 666 patients were administered sorafenib for advanced HCC with CP-B liver function. The type and incidence of adverse events and incidence of drug-related adverse events leading to discontinuation were similar between the CP-A and CP-B patients; however, the median OS was shorter in the CP-B patients [109]. A retrospective study [110] of data from the REFLECT trial evaluated patients who were administered lenvatinib therapy and deteriorated to a CP-B status within 8 weeks after randomization. Patients with CP-B versus CP-A classifications had ORRs of 28.3% and 42.9%, respectively. The median PFS and OS were 3.7 months (95% CI: 1.8–7.4) and 6.8 months (95% CI: 2.6–10.3) in the CP-B subgroup versus 6.5 months (95% CI: 5.6–7.4) and 13.3 months (95% CI: 11.6–16.1) in the CP-A subgroup, respectively. The ORR in these patients was 28.3% (no CRs were observed). These results suggest that patients with unresectable HCC whose liver function deteriorates to CP-B after initiation of therapy may continue to be administered lenvatinib. A global multicenter retrospective study [111] enrolled 48 patients with CP-B receiving atezolizumab plus bevacizumab treatment. The study showed comparable rates of immune-related adverse effects (IrAEs). The median OS was 6.7 months (95% CI: 4.3–15.6), whereas the median PFS was 3.4 months (95% CI: 2.6–4.2), although shorter than that in CP-A patients. The ORR and disease control rate (DCR) were 25% and 73%, respectively, with no significant differences observed across the CP classes. In this setting, shared decision making is particularly important to weigh the safety profile against the likely modest clinical benefits observed in well-selected patients.
Statement 3-5: Treatment with regorafenib, cabozantinib, and ramucirumab (when AFP ≥400 ng/mL) extends survival of patients with HCC and Child-Pugh class A liver function, whose tumors are unresectable and not amenable to locoregional therapy when the tumors have progressed after sorafenib or lenvatinib treatment (A: 100%; E: 1, R: A for post-sorafenib; E: 3, R: B for post-lenvatinib).
The multikinase inhibitors regorafenib and cabozantinib as well as the monoclonal antibody ramucirumab are antiangiogenic targeted therapies. In phase 3 clinical trials, all three have been proven to prolong OS in patients with advanced HCC for whom sorafenib treatment failed [112–114]. No large randomized clinical trial has tested the feasibility and efficacy of any systemic therapy in patients for whom lenvatinib treatment failed, although the above regimens are recommended by several international guidelines [12, 87, 95, 96]. Two small-scale retrospective studies [115, 116] enrolled patients who were administered regorafenib after lenvatinib treatment: the ORR and DCR were 10.7–13.6% and 36.3–60.7%, respectively. Two other small-scale retrospective studies [117, 118] enrolled patients who were administered ramucirumab after lenvatinib treatment, and the ORR and DCR were 0–3.8% and 42.3–80.0%, respectively. The efficacy of both agents was similar to that observed in clinical trials with sorafenib as first-line therapy [112, 114].
Statement 3-6: Immunotherapy, such as nivolumab plus ipilimumab and pembrolizumab, can be considered for patients who are intolerant of or have progressed after sorafenib or lenvatinib treatment (A: 100%; E: 2, R: B for post-sorafenib; E: 3, R: B for post-lenvatinib).
Two large single-arm studies have demonstrated the efficacy and safety of the anti-programmed cell death 1 (PD1) antibodies nivolumab and pembrolizumab in patients with Child-Pugh class A liver function and advanced HCC in whom sorafenib treatment failed [119, 120]. However, the Food and Drug Administrations of both the USA and Taiwan opposed nivolumab monotherapy for second-line advanced HCC due to its failure in the confirmatory CheckMate 459 trial [121]. Another study tested multiple regimens combining nivolumab and ipilimumab, an anticytotoxic T-lymphocyte protein-4 antibody. All regimens demonstrated a respectable response rate at the expense of increased toxicity compared to nivolumab treatment alone [122]. A phase 3 clinical trial that compared pembrolizumab treatment with a placebo failed to achieve its primary endpoints, as the OS and PFS not reach statistical significance, possibly because its statistical hypothesis was overly aggressive; however, it demonstrated consistent tumor response rates, response duration, and OS [123]. In addition, another phase 3 study revealed that pembrolizumab treatment significantly prolonged the OS, PFS, and ORR compared to placebo in Asian patients with advanced HCC [124]. In conclusion, immunotherapy using either nivolumab plus ipilimumab or pembrolizumab alone, can be considered for patients who are intolerant of or have progressed during treatment with approved multiple kinase inhibitors [12, 98].
Section 4: Posttreatment Monitoring
We have added a new section to address posttreatment monitoring. To effectively monitor and identify the tumor status in patients undergoing anticancer therapy for HCC, serum tumor markers and imaging modalities can be utilized. The detailed statements regarding treatment monitoring are as follows.
Statement 4-1: AFP and PIVKA-II markers may be effective factors for selecting patients who will benefit from liver transplantation. Serial measurements of both AFP and PIVKA-II might be helpful for monitoring early diagnosis of tumor recurrence after resection and liver transplantation (A: 100%; E: 2; R: A).
Several studies have investigated the role of AFP and PIVKA-II/DCP levels as risk factors for HCC recurrence after resection and liver transplantation [27, 125]. In one study involving 688 patients, preoperative levels of AFP and PIVKA-II/DCP were identified as significant risk factors for posttransplantation HCC recurrence. AFP and PIVKA-II/DCP showed better predictive capability when used in combination than when used individually. Patients classified as low-risk patients (AFP + PIVKA-II ≤300) had a 5-year OS rate of 47.8% and a recurrence-free survival rate of 53.4% [126]. Another study involving 155 patients found that elevated AFP or PIVKA-II/DCP levels indicated recurrence after surgical resection. Serial measurements of AFP and PIVKA-II/DCP levels showed significant variations from the initial diagnosis to recurrence [127]. Furthermore, a study of 120 patients found that preoperative PIVKA-II/DCP levels correlated with tumor characteristics and had higher sensitivity (88.8%) than AFP (59.2%) in detecting recurrent HCC after liver transplantation. Combining AFP and PIVKA-II/DCP increased the sensitivity to 92.5% and elevated PIVKA-II/DCP levels were the most common initial sign of HCC recurrence after transplantation [128].
Statement 4-2: AFP and PIVKA-II response may be useful prognostic predictors for prolonged clinical outcomes after curative RFA and TACE for HCC (A: 100%; E: 2; R: A).
The short half-life of PIVKA-II/DCP within 48 h after RFA indicates its favorable role in diagnostics. Moreover, the shorter half-life of PIVKA-II/DCP is associated with a higher disease-free survival during the 12-month follow-up period [129]. A study that focused on patients with HCC who underwent RFA identified several risk factors for mortality, including low serum albumin levels, high PIVKA-II/DCP levels, and the presence of multiple nodules. Patients with PIVKA-II/DCP levels equal to or exceeding 100 mAU/mL exhibited low survival rates [130]. In a retrospective study involving 327 patients who underwent chemoembolization, both AFP and PIVKA-II/DCP responders demonstrated superior outcomes in terms of disease progression and OS to nonresponders. This finding suggests that response to PIVKA-II/DCP and AFP can serve as a valuable surrogate endpoint for evaluating clinical outcomes in patients with HCC undergoing chemoembolization [131]. Although PIVKA-II/DCP shows promise for predicting prognosis following locoregional therapy, further evidence is necessary to demonstrate its clinical utility in posttreatment surveillance.
Statement 4-3: Postablation EOB-MRI enables early assessment of RFA effectiveness in the majority of HCC nodules, with the tumor size as an independent predictive factor for local tumor progression. Postablation EOB-MRI performs better than MDCT in detecting recurrent hypervascular HCCs (A: 100%; E: 2; R: B).
A total of 124 patients with HCC underwent RFA treatment and follow-up examinations using either enhanced MRI with EOB-MRI or enhanced CT. The ablation margin (AM) was categorized into three grades: AM (+), AM-zero, and AM (−). AM (+) nodules demonstrated lower cumulative rates of local tumor progression than AM-zero nodules. EOB-MRI allowed early assessment of RFA effectiveness in the majority of HCC nodules. No instances of local tumor progression were observed in patients with AM (+) nodules on EOB-MRI during the 3-year follow-up period [132]. In a separate retrospective study, magnetic resonance fusion imaging facilitated the evaluation of treatment outcomes in a significantly higher proportion of HCC patients than did CT fusion imaging (86 of 92 patients [93.5%] vs. 62 of 92 patients [67.4%]; p < 0.05) [133]. The integration of pre- and postablation Gd-EOB-DTPA-MRI enables accurate treatment assessment in cases where CT fusion imaging lacks information. Gd-EOB-DTPA-enhanced MRI demonstrates higher diagnostic accuracy and sensitivity than dynamic MDCT for detecting recurrent hypervascular HCCs [133, 134]. Therefore, it may be a superior tool for monitoring patients after RFA.
Statement 4-4: Monitoring the serum levels of AFP and PIVKA-II before and after HCC treatment may be helpful for assessing and predicting prognosis, survival, and effectiveness of immunotherapy and systemic therapy (A: 100%; E: 2; R: A).
In an analysis of a cohort of 62 patients diagnosed with HCC who were treated with atezolizumab and bevacizumab in combination with molecularly targeted agents, the correlation between the response to the PIVKA-II/DCP assay – measured by the decrease in levels after 1 month of treatment – and extended PFS was more significant in responders than in nonresponders (5.8 months vs. 3.8 months; p = 0.0205) [135]. These findings indicate that PIVKA-II/DCP can effectively stratify PFS in patients with advanced HCC. Moreover, the response rate to PD-1 blockade was positively associated with a reduction in AFP levels greater than 50% and PIVKA-II/DCP levels greater than 50% (p < 0.001 and p = 0.003, respectively). Patients with HCC who achieved a >50% reduction in AFP and PIVKA-II/DCP levels demonstrated significantly improved PFS (p < 0.001 and p = 0.021, respectively). Additionally, reductions greater than 50% in AFP and PIVKA-II/DCP levels were positively correlated with increased OS (p = 0.003 and p = 0.006, respectively) [136]. Therefore, early reduction in AFP and PIVKA-II/DCP levels could be considered a potential predictor of the effectiveness of PD-1 blockade in patients with HCC.
Conclusion
In summary, we have updated the Taiwan Liver Cancer Association guidelines regarding surveillance, diagnosis, and systemic treatment of HCCs and added a new section on posttreatment monitoring of these carcinomas. For surveillance and diagnosis, we updated the roles of PIVKA-II/DCP and EOB-MRI in detecting HCCs. The tumor marker PIVKA-II/DCP showed higher sensitivity than that of AFP in detecting early HCCs. AFP plus PIVKA-II/DCP showed greater sensitivity and specificity than either biomarker alone. Moreover, combining these biomarkers with ultrasonography improves the detection rate of early HCC in clinical practice. We have witnessed relevant advances in the systemic treatment of HCC in the last 3 years. Furthermore, four oral multi-tyrosine kinase inhibitors (sorafenib, lenvatinib, regorafenib, and cabozantinib), one antiangiogenic antibody (ramucirumab), and four immune checkpoint inhibitors for administration alone or in combination (atezolizumab in combination with bevacizumab, ipilimumab in combination with nivolumab, and pembrolizumab in monotherapy) are licensed for use in Taiwan as well as in other countries. In addition, tremelimumab in combination with durvalumab demonstrated a statistically significant improvement in OS versus sorafenib in 2022. The roles of PIVKA-II/DPC and EOB-MRI in managing patients who receive anticancer therapies, including surgery, RFA, TACE, and systemic treatment, were highlighted. Prolonged survival is expected in most patients with sensitive tumors and well-preserved liver function that renders them suitable for sequential therapies.
Acknowledgments
This consensus update was conducted by the expert committee. All consensus statements were contributed by two expert groups and the following experts (*coordinators of each expert group). The TLCA was grateful to Prof. Ann-Lii Cheng’s suggestions and all the members of the expert committee for their contributions (listed in alphabetical order of family name):
Diagnosis group: Kuan-Yang Chen*, Yangming branch, Taipei City Hospital, Taipei, Taiwan; Jui-Ting Hu, Cathay General Hospital, Taipei, Taiwan; Po-Chin Liang, National Taiwan University Hospital, Taipei, Taiwan; Ja-Der Liang, National Taiwan University Hospital, Taipei, Taiwan; Chih-Lin Lin, Renai branch, Taipei City Hospital, Taipei, Taiwan; Shen-Yung Wang, MacKay Memorial Hospital, Taipei, Taiwan; and Chih-Horng Wu, National Taiwan University Hospital, Taipei, Taiwan.
Systemic treatment group: Shi-Ming Lin*, Chang Gung Memorial Hospital, Linkou, Taiwan; Ching-Wei Chang, MacKay Memorial Hospital, Taipei, Taiwan; Gar-Yang Chau, Taipei Veterans General Hospital, Taipei, Taiwan; Chien-Hung Chen, National Taiwan University Hospital, Taipei, Taiwan; San-Chi Chen, Taipei Veterans General Hospital, Taipei, Taiwan; Chia-Hsien Chen, National Taiwan University Hospital, Taipei, Taiwan; Pin-Nan Cheng, National Cheng Kung University Hospital, Tainan, Taiwan; Chia-Yen Dai, Kaohsiung Medical University Hospital, Kaohsiung Medical University, Kaohsiung, Taiwan; Chia-Hsun Hsieh, New Taipei Municipal TuCheng Hospital, New Taipei City, Taiwan; Yi-Hsiang Huang, Taipei Veterans General Hospital, Taipei, Taiwan; Kai-Wen Huang, National Taiwan University Hospital, Taipei, Taiwan; Bing-Shen Huang, Chang Gung Memorial Hospital, Linkou; Hsueh-Chou Lai, China Medical University Hospital, Taichung, Taiwan; Wei-Chen Lee, Chang Gung Memorial Hospital, Linkou, Taiwan; Yu-Min Lin, Shin Kong Wu Ho-Su Memorial Hospital, Taipei; Chun-Jen Liu, National Taiwan University Hospital, Taipei, Taiwan; Sheng-Nan Lu, Kaohsiung Chang Gung Memorial Hospital, Kaohsiung, Taiwan; Yu-Yun Shao, National Taiwan University Hospital, Taipei, Taiwan; Yu-Lueng Shin, Medical Affairs Bureau, Ministry of National Defense, Taiwan; Chung-Kwe Wang, Kang Ning Hospital, Taipei, Taiwan; Chia-Chi Wang, Taipei Tzu Chi Hospital, Taipei, Taiwan; and Sheng-Shun Yang, Taichung Veterans General Hospital, Taichung, Taiwan.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
This consensus update was funded by the Taiwan Liver Cancer Association (TLCA).
Author Contributions
Wei Teng and Hung-Wei Wang drafted the manuscript. Shi-Ming Lin was responsible for the study concept, design, supervision, and manuscript revision.
Funding Statement
This consensus update was funded by the Taiwan Liver Cancer Association (TLCA).
References
- 1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49. [DOI] [PubMed] [Google Scholar]
- 2. Health Promotion Administraion. Ministry of health and welfare . Taiwan: cancer registry annual report. 2020. Available from: https://www.hpa.gov.tw/Pages/List.aspx?nodeidZ269. (Accessed June 7, 2023). [Google Scholar]
- 3. Surveillance group; Diagnosis group; Staging group; Surgery group; Local ablation group; TACE/TARE/HAI group, et al. Management consensus guideline for hepatocellular carcinoma: 2016 updated by the Taiwan Liver Cancer Association and the Gastroenterological Society of Taiwan. J Formos Med Assoc. 2018;117(5):381–403. [DOI] [PubMed] [Google Scholar]
- 4. Shekelle PG, Woolf SH, Eccles M, Grimshaw J. Clinical guidelines: developing guidelines. BMJ. 1999;318(7183):593–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. European Association for the Study of the Liver Electronic address easloffice@easlofficeeu; European Association for the Study of the Liver . EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69(1):182–236. [DOI] [PubMed] [Google Scholar]
- 6. Shao YY, Wang SY, Lin SM; Diagnosis Group; Systemic Therapy Group . Management consensus guideline for hepatocellular carcinoma: 2020 update on surveillance, diagnosis, and systemic treatment by the Taiwan Liver Cancer Association and the Gastroenterological Society of Taiwan. J Formos Med Assoc. 2021;120(4):1051–60. [DOI] [PubMed] [Google Scholar]
- 7. Chen H, Chen S, Li S, Chen Z, Zhu X, Dai M, et al. Combining des-gamma-carboxyprothrombin and alpha-fetoprotein for hepatocellular carcinoma diagnosing: an update meta-analysis and validation study. Oncotarget. 2017;8(52):90390–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Caviglia GP, Ribaldone DG, Abate ML, Ciancio A, Pellicano R, Smedile A, et al. Performance of protein induced by vitamin K absence or antagonist-II assessed by chemiluminescence enzyme immunoassay for hepatocellular carcinoma detection: a meta-analysis. Scand J Gastroenterol. 2018;53(6):734–40. [DOI] [PubMed] [Google Scholar]
- 9. Chan HLY, Vogel A, Berg T, De Toni EN, Kudo M, Trojan J, et al. Performance evaluation of the Elecsys PIVKA-II and Elecsys AFP assays for hepatocellular carcinoma diagnosis. JGH Open. 2022;6(5):292–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kudo M, Kawamura Y, Hasegawa K, Tateishi R, Kariyama K, Shiina S, et al. Management of hepatocellular carcinoma in Japan: JSH consensus statements and recommendations 2021 update. Liver Cancer. 2021;10(3):181–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hasegawa K, Takemura N, Yamashita T, Watadani T, Kaibori M, Kubo S, et al. Clinical practice guidelines for hepatocellular carcinoma: the Japan society of hepatology 2021 version (5th JSH-HCC guidelines). Hepatol Res. 2023;53(5):383–90. [DOI] [PubMed] [Google Scholar]
- 12. Singal AG, Llovet JM, Yarchoan M, Mehta N, Heimbach JK, Dawson LA, et al. AASLD practice guidance on prevention, diagnosis, and treatment of hepatocellular carcinoma. Hepatology. 2023;78(6):1922–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Korean Liver Cancer Association (KLCA); National Cancer Center (NCC) Korea . 2022 KLCA-NCC Korea practice guidelines for the management of hepatocellular carcinoma. J Liver Cancer. 2023;23(1):1–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Singal AG, Sanduzzi-Zamparelli M, Nahon P, Ronot M, Hoshida Y, Rich N, et al. International Liver Cancer Association (ILCA) white paper on hepatocellular carcinoma risk stratification and surveillance. J Hepatol. 2023;79(1):226–39. [DOI] [PubMed] [Google Scholar]
- 15. Komatsu N, Motosugi U, Maekawa S, Shindo K, Sakamoto M, Sato M, et al. Hepatocellular carcinoma risk assessment using gadoxetic acid-enhanced hepatocyte phase magnetic resonance imaging. Hepatol Res. 2014;44(13):1339–46. [DOI] [PubMed] [Google Scholar]
- 16. Goossens N, Singal AG, King LY, Andersson KL, Fuchs BC, Besa C, et al. Cost-effectiveness of risk score-stratified hepatocellular carcinoma screening in patients with cirrhosis. Clin Transl Gastroenterol. 2017;8(6):e101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kim SY, An J, Lim YS, Han S, Lee JY, Byun JH, et al. MRI with liver-specific contrast for surveillance of patients with cirrhosis at high risk of hepatocellular carcinoma. JAMA Oncol. 2017;3(4):456–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Taouli B, Ba-Ssalamah A, Chapiro J, Chhatwal J, Fowler K, Kang TW, et al. Consensus report from the 10th global forum for liver magnetic resonance imaging: developments in HCC management. Eur Radiol. 2023;33(12):9152–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hatanaka K, Kudo M, Minami Y, Maekawa K. Sonazoid-enhanced ultrasonography for diagnosis of hepatic malignancies: comparison with contrast-enhanced CT. Oncology. 2008;75(Suppl 1):42–7. [DOI] [PubMed] [Google Scholar]
- 20. Kudo M, Ueshima K, Osaki Y, Hirooka M, Imai Y, Aso K, et al. B-mode ultrasonography versus contrast-enhanced ultrasonography for surveillance of hepatocellular carcinoma: a prospective multicenter randomized controlled trial. Liver Cancer. 2019;8(4):271–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bartolotta TV, Randazzo A, Bruno E, Taibbi A. Focal liver lesions in cirrhosis: role of contrast-enhanced ultrasonography. World J Radiol. 2022;14(4):70–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nathani P, Gopal P, Rich N, Yopp A, Yokoo T, John B, et al. Hepatocellular carcinoma tumour volume doubling time: a systematic review and meta-analysis. Gut. 2021;70(2):401–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Marrero JA, Feng Z, Wang Y, Nguyen MH, Befeler AS, Roberts LR, et al. Alpha-fetoprotein, des-gamma carboxyprothrombin, and lectin-bound alpha-fetoprotein in early hepatocellular carcinoma. Gastroenterology. 2009;137(1):110–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Xing H, Zheng YJ, Han J, Zhang H, Li ZL, Lau WY, et al. Protein induced by vitamin K absence or antagonist-II versus alpha-fetoprotein in the diagnosis of hepatocellular carcinoma: a systematic review with meta-analysis. Hepatobiliary Pancreat Dis Int. 2018;17(6):487–95. [DOI] [PubMed] [Google Scholar]
- 25. Singal AG, Hoshida Y, Pinato DJ, Marrero J, Nault JC, Paradis V, et al. International liver cancer association (ILCA) white paper on biomarker development for hepatocellular carcinoma. Gastroenterology. 2021;160(7):2572–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lok AS, Sterling RK, Everhart JE, Wright EC, Hoefs JC, Di Bisceglie AM, et al. Des-gamma-carboxy prothrombin and alpha-fetoprotein as biomarkers for the early detection of hepatocellular carcinoma. Gastroenterology. 2010;138(2):493–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kim DY, Toan BN, Tan CK, Hasan I, Setiawan L, Yu ML, et al. Utility of combining PIVKA-II and AFP in the surveillance and monitoring of hepatocellular carcinoma in the Asia-Pacific region. Clin Mol Hepatol. 2023;29(2):277–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Marrero JA, Kulik LM, Sirlin CB, Zhu AX, Finn RS, Abecassis MM, et al. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American association for the study of liver diseases. Hepatology. 2018;68(2):723–50. [DOI] [PubMed] [Google Scholar]
- 29. Chou R, Cuevas C, Fu R, Devine B, Wasson N, Ginsburg A, et al. Imaging techniques for the diagnosis of hepatocellular carcinoma: a systematic review and meta-analysis. Ann Intern Med. 2015;162(10):697–711. [DOI] [PubMed] [Google Scholar]
- 30. Lee S, Kim SS, Chang DR, Kim H, Kim MJ. Comparison of LI-RADS 2018 and KLCA-NCC 2018 for noninvasive diagnosis of hepatocellular carcinoma using magnetic resonance imaging. Clin Mol Hepatol. 2020;26(3):340–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Omata M, Cheng AL, Kokudo N, Kudo M, Lee JM, Jia J, et al. Asia-Pacific clinical practice guidelines on the management of hepatocellular carcinoma: a 2017 update. Hepatol Int. 2017;11(4):317–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sheu JC, Sung JL, Chen DS, Yang PM, Lai MY, Lee CS, et al. Growth rate of asymptomatic hepatocellular carcinoma and its clinical implications. Gastroenterology. 1985;89(2):259–66. [DOI] [PubMed] [Google Scholar]
- 33. Ducreux M, Abou-Alfa GK, Bekaii-Saab T, Berlin J, Cervantes A, de Baere T, et al. The management of hepatocellular carcinoma. Current expert opinion and recommendations derived from the 24th ESMO/World Congress on Gastrointestinal Cancer, Barcelona, 2022. ESMO Open. 2023;8(3):101567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Thomaides-Brears HB, Alkhouri N, Allende D, Harisinghani M, Noureddin M, Reau NS, et al. Incidence of complications from percutaneous biopsy in chronic liver disease: a systematic review and meta-analysis. Dig Dis Sci. 2022;67(7):3366–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hanna RF, Miloushev VZ, Tang A, Finklestone LA, Brejt SZ, Sandhu RS, et al. Comparative 13-year meta-analysis of the sensitivity and positive predictive value of ultrasound, CT, and MRI for detecting hepatocellular carcinoma. Abdom Radiol. 2016;41(1):71–90. [DOI] [PubMed] [Google Scholar]
- 36. Strobel D, Seitz K, Blank W, Schuler A, Dietrich C, von Herbay A, et al. Contrast-enhanced ultrasound for the characterization of focal liver lesions--diagnostic accuracy in clinical practice (DEGUM multicenter trial). Ultraschall Med. 2008;29(5):499–505. [DOI] [PubMed] [Google Scholar]
- 37. Wildner D, Pfeifer L, Goertz RS, Bernatik T, Sturm J, Neurath MF, et al. Dynamic contrast-enhanced ultrasound (DCE-US) for the characterization of hepatocellular carcinoma and cholangiocellular carcinoma. Ultraschall Med. 2014;35(6):522–7. [DOI] [PubMed] [Google Scholar]
- 38. Liu GJ, Wang W, Lu MD, Xie XY, Xu HX, Xu ZF, et al. Contrast-enhanced ultrasound for the characterization of hepatocellular carcinoma and intrahepatic cholangiocarcinoma. Liver Cancer. 2015;4(4):241–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Vilana R, Forner A, Bianchi L, Garcia-Criado A, Rimola J, de Lope CR, et al. Intrahepatic peripheral cholangiocarcinoma in cirrhosis patients may display a vascular pattern similar to hepatocellular carcinoma on contrast-enhanced ultrasound. Hepatology. 2010;51(6):2020–9. [DOI] [PubMed] [Google Scholar]
- 40. Bohle W, Clemens PU, Heubach T, Zoller WG. Contrast-enhanced ultrasound (CEUS) for differentiating between hepatocellular and cholangiocellular carcinoma. Ultraschall Med. 2012;33(7):E191–5. [DOI] [PubMed] [Google Scholar]
- 41. Hwang JA, Jeong WK, Min JH, Kim YY, Heo NH, Lim HK. Sonazoid-enhanced ultrasonography: comparison with CT/MRI Liver Imaging Reporting and Data System in patients with suspected hepatocellular carcinoma. Ultrasonography. 2021;40(4):486–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Jeong WK, Kang HJ, Choi SH, Park MS, Yu MH, Kim B, et al. Diagnosing hepatocellular carcinoma using sonazoid contrast-enhanced ultrasonography: 2023 guidelines from the Korean society of radiology and the Korean society of abdominal radiology. Korean J Radiol. 2023;24(6):482–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Feng Y, Qin XC, Luo Y, Li YZ, Zhou X. Efficacy of contrast-enhanced ultrasound washout rate in predicting hepatocellular carcinoma differentiation. Ultrasound Med Biol. 2015;41(6):1553–60. [DOI] [PubMed] [Google Scholar]
- 44. Briani C, Di Pietropaolo M, Marignani M, Carbonetti F, Begini P, David V, et al. Non-hypervascular hypointense nodules at gadoxetic acid MRI: hepatocellular carcinoma risk assessment with emphasis on the role of diffusion-weighted imaging. J Gastrointest Cancer. 2018;49(3):302–10. [DOI] [PubMed] [Google Scholar]
- 45. Li Y, Chen J, Weng S, Yan C, Ye R, Zhu Y, et al. Hepatobiliary phase hypointensity on gadobenate dimeglumine-enhanced magnetic resonance imaging may improve the diagnosis of hepatocellular carcinoma. Ann Transl Med. 2021;9(1):55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kanefuji T, Takano T, Suda T, Akazawa K, Yokoo T, Kamimura H, et al. Factors predicting aggressiveness of non-hypervascular hepatic nodules detected on hepatobiliary phase of gadolinium ethoxybenzyl diethylene-triamine-pentaacetic-acid magnetic resonance imaging. World J Gastroenterol. 2015;21(15):4583–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Morimoto N, Miura K, Watanabe S, Tsukui M, Takaoka Y, Nomoto H, et al. Usefulness of Gd-EOB-DTPA-enhanced MRI for evaluating the potential for early development of hepatocellular carcinoma after HCV eradication by direct-acting antiviral treatment. J Rural Med. 2019;14(1):78–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Renzulli M, Biselli M, Brocchi S, Granito A, Vasuri F, Tovoli F, et al. New hallmark of hepatocellular carcinoma, early hepatocellular carcinoma and high-grade dysplastic nodules on Gd-EOB-DTPA MRI in patients with cirrhosis: a new diagnostic algorithm. Gut. 2018;67(9):1674–82. [DOI] [PubMed] [Google Scholar]
- 49. Golfieri R, Renzulli M, Lucidi V, Corcioni B, Trevisani F, Bolondi L. Contribution of the hepatobiliary phase of Gd-EOB-DTPA-enhanced MRI to Dynamic MRI in the detection of hypovascular small (≤ 2 cm) HCC in cirrhosis. Eur Radiol. 2011;21(6):1233–42. [DOI] [PubMed] [Google Scholar]
- 50. Liu X, Jiang H, Chen J, Zhou Y, Huang Z, Song B. Gadoxetic acid disodium-enhanced magnetic resonance imaging outperformed multidetector computed tomography in diagnosing small hepatocellular carcinoma: a meta-analysis. Liver Transpl. 2017;23(12):1505–18. [DOI] [PubMed] [Google Scholar]
- 51. Yinzhong W, Xiaoxue T, Jinhui T, Pengcheng Y, Xiaoying L, Junqiang L. Is gadoxetic acid disodium (Gd-EOB-DTPA)-Enhanced magnetic resonance imaging an accurate diagnostic method for hepatocellular carcinoma? A systematic review with meta-analysis. Curr Med Imaging. 2022;18(6):633–47. [DOI] [PubMed] [Google Scholar]
- 52. Toyoda H, Kumada T, Tada T, Niinomi T, Ito T, Sone Y, et al. Non-hypervascular hypointense nodules detected by Gd-EOB-DTPA-enhanced MRI are a risk factor for recurrence of HCC after hepatectomy. J Hepatol. 2013;58(6):1174–80. [DOI] [PubMed] [Google Scholar]
- 53. Lee DH, Lee JM, Lee JY, Kim SH, Kim JH, Yoon JH, et al. Non-hypervascular hepatobiliary phase hypointense nodules on gadoxetic acid-enhanced MRI: risk of HCC recurrence after radiofrequency ablation. J Hepatol. 2015;62(5):1122–30. [DOI] [PubMed] [Google Scholar]
- 54. Suh CH, Kim KW, Pyo J, Lee J, Kim SY, Park SH. Hypervascular transformation of hypovascular hypointense nodules in the hepatobiliary phase of gadoxetic acid-enhanced MRI: a systematic review and meta-analysis. AJR Am J Roentgenol. 2017;209(4):781–9. [DOI] [PubMed] [Google Scholar]
- 55. Zhang Y, Wei H, Song B. Magnetic resonance imaging for treatment response evaluation and prognostication of hepatocellular carcinoma after thermal ablation. Insights Imaging. 2023;14(1):87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kim HD, Lim YS, Han S, An J, Kim GA, Kim SY, et al. Evaluation of early-stage hepatocellular carcinoma by magnetic resonance imaging with gadoxetic acid detects additional lesions and increases overall survival. Gastroenterology. 2015;148(7):1371–82. [DOI] [PubMed] [Google Scholar]
- 57. Tan CH, Chou SC, Inmutto N, Ma K, Sheng R, Shi Y, et al. Gadoxetate-enhanced MRI as a diagnostic tool in the management of hepatocellular carcinoma: report from a 2020 asia-pacific multidisciplinary expert meeting. Korean J Radiol. 2022;23(7):697–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Choi JY, Lee JM, Sirlin CB. CT and MR imaging diagnosis and staging of hepatocellular carcinoma: part I. Development, growth, and spread: key pathologic and imaging aspects. Radiology. 2014;272(3):635–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Ding Y, Rao SX, Wang WT, Chen CZ, Li RC, Zeng M. Comparison of gadoxetic acid versus gadopentetate dimeglumine for the detection of hepatocellular carcinoma at 1.5 T using the liver imaging reporting and data system (LI-RADS v.2017). Cancer Imag. 2018;18(1):48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Park MJ, Kim YK, Lee MW, Lee WJ, Kim YS, Kim SH, et al. Small hepatocellular carcinomas: improved sensitivity by combining gadoxetic acid-enhanced and diffusion-weighted MR imaging patterns. Radiology. 2012;264(3):761–70. [DOI] [PubMed] [Google Scholar]
- 61. Wu JW, Yu YC, Qu XL, Zhang Y, Gao H. Optimization of hepatobiliary phase delay time of Gd-EOB-DTPA-enhanced magnetic resonance imaging for identification of hepatocellular carcinoma in patients with cirrhosis of different degrees of severity. World J Gastroenterol. 2018;24(3):415–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Lee S, Kim SH, Lee JE, Sinn DH, Park CK. Preoperative gadoxetic acid-enhanced MRI for predicting microvascular invasion in patients with single hepatocellular carcinoma. J Hepatol. 2017;67(3):526–34. [DOI] [PubMed] [Google Scholar]
- 63. Jiang H, Wei J, Fu F, Wei H, Qin Y, Duan T, et al. Predicting microvascular invasion in hepatocellular carcinoma: a dual-institution study on gadoxetate disodium-enhanced MRI. Liver Int. 2022;42(5):1158–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Wang LL, Li JF, Lei JQ, Guo SL, Li JK, Xu YS, et al. The value of the signal intensity of peritumoral tissue on Gd-EOB-DTPA dynamic enhanced MRI in assessment of microvascular invasion and pathological grade of hepatocellular carcinoma. Medicine. 2021;100(20):e25804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Shim JH, Han S, Shin YM, Lee YJ, Lee SG, Kim KM, et al. Prognostic performance of preoperative gadoxetic acid-enhanced MRI in resectable hepatocellular carcinoma. J Magn Reson Imaging. 2015;41(4):1115–23. [DOI] [PubMed] [Google Scholar]
- 66. Wu CH, Lee YH, Liang PC, Hu RH, Shih TT, Ho MC. Predictors of changes in preoperative tumor stage between dynamic computed tomography and gadoxetate disodium-enhanced magnetic resonance imaging for hepatocellular carcinoma. J Formos Med Assoc. 2022;121(8):1550–9. [DOI] [PubMed] [Google Scholar]
- 67. Matsuda M, Ichikawa T, Amemiya H, Maki A, Watanabe M, Kawaida H, et al. Preoperative gadoxetic Acid-enhanced MRI and simultaneous treatment of early hepatocellular carcinoma prolonged recurrence-free survival of progressed hepatocellular carcinoma patients after hepatic resection. HPB Surg. 2014;2014:641685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Yau T, Tang VY, Yao TJ, Fan ST, Lo CM, Poon RT. Development of Hong Kong Liver Cancer staging system with treatment stratification for patients with hepatocellular carcinoma. Gastroenterology. 2014;146(7):1691–700 e3. [DOI] [PubMed] [Google Scholar]
- 69. Willett CG, Boucher Y, di Tomaso E, Duda DG, Munn LL, Tong RT, et al. Direct evidence that the VEGF-specific antibody bevacizumab has antivascular effects in human rectal cancer. Nat Med. 2004;10(2):145–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Jain RK. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science. 2005;307(5706):58–62. [DOI] [PubMed] [Google Scholar]
- 71. Kano MR, Komuta Y, Iwata C, Oka M, Shirai YT, Morishita Y, et al. Comparison of the effects of the kinase inhibitors imatinib, sorafenib, and transforming growth factor-beta receptor inhibitor on extravasation of nanoparticles from neovasculature. Cancer Sci. 2009;100(1):173–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Jia ZZ, Jiang GM, Feng YL. Serum HIF-1alpha and VEGF levels pre- and post-TACE in patients with primary liver cancer. Chin Med Sci J. 2011;26(3):158–62. [DOI] [PubMed] [Google Scholar]
- 73. Kudo M, Imanaka K, Chida N, Nakachi K, Tak WY, Takayama T, et al. Phase III study of sorafenib after transarterial chemoembolisation in Japanese and Korean patients with unresectable hepatocellular carcinoma. Eur J Cancer. 2011;47(14):2117–27. [DOI] [PubMed] [Google Scholar]
- 74. Kudo M, Han G, Finn RS, Poon RT, Blanc JF, Yan L, et al. Brivanib as adjuvant therapy to transarterial chemoembolization in patients with hepatocellular carcinoma: a randomized phase III trial. Hepatology. 2014;60(5):1697–707. [DOI] [PubMed] [Google Scholar]
- 75. Lencioni R, Llovet JM, Han G, Tak WY, Yang J, Guglielmi A, et al. Sorafenib or placebo plus TACE with doxorubicin-eluting beads for intermediate stage HCC: the SPACE trial. J Hepatol. 2016;64(5):1090–8. [DOI] [PubMed] [Google Scholar]
- 76. Meyer T, Fox R, Ma YT, Ross PJ, James MW, Sturgess R, et al. Sorafenib in combination with transarterial chemoembolisation in patients with unresectable hepatocellular carcinoma (TACE 2): a randomised placebo-controlled, double-blind, phase 3 trial. Lancet Gastroenterol Hepatol. 2017;2(8):565–75. [DOI] [PubMed] [Google Scholar]
- 77. Kudo M, Cheng AL, Park JW, Park JH, Liang PC, Hidaka H, et al. Orantinib versus placebo combined with transcatheter arterial chemoembolisation in patients with unresectable hepatocellular carcinoma (ORIENTAL): a randomised, double-blind, placebo-controlled, multicentre, phase 3 study. Lancet Gastroenterol Hepatol. 2018;3(1):37–46. [DOI] [PubMed] [Google Scholar]
- 78. Kudo M, Ueshima K, Ikeda M, Torimura T, Tanabe N, Aikata H, et al. Final results of TACTICS: a randomized, prospective trial comparing transarterial chemoembolization plus sorafenib to transarterial chemoembolization alone in patients with unresectable hepatocellular carcinoma. Liver Cancer. 2022;11(4):354–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Peng Z, Fan W, Zhu B, Wang G, Sun J, Xiao C, et al. Lenvatinib combined with transarterial chemoembolization as first-line treatment for advanced hepatocellular carcinoma: a phase III, randomized clinical trial (LAUNCH). J Clin Oncol. 2023;41(1):117–27. [DOI] [PubMed] [Google Scholar]
- 80. Kudo M, Ueshima K, Saeki I, Ishikawa T, Inaba Y, Morimoto N, et al. A phase 2, prospective, multicenter, single-arm trial of transarterial chemoembolization therapy in combination strategy with lenvatinib in patients with unresectable intermediate-stage hepatocellular carcinoma: TACTICS-L trial. Liver Cancer. 2023;13:99–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Hung YW, Lee IC, Chi CT, Lee RC, Liu CA, Chiu NC, et al. Redefining tumor burden in patients with intermediate-stage hepatocellular carcinoma: the seven-eleven criteria. Liver Cancer. 2021;10(6):629–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Sangro B, Kudo M, Qin S, Ren Z, Chan S, Joseph E, et al. P-347 A phase 3, randomized, double-blind, placebo-controlled study of transarterial chemoembolization combined with durvalumab or durvalumab plus bevacizumab therapy in patients with locoregional hepatocellular carcinoma: EMERALD-1. Ann Oncol. 2020;31:S202–S203. [Google Scholar]
- 83. Ogasawara S, Llovet J, El-Khoueiry A, Vogel A, Madoff D, Finn R, et al. P-107 LEAP-012: a randomized, double-blind, phase 3 study of pembrolizumab plus lenvatinib in combination with transarterial chemoembolization (TACE) in patients with intermediate-stage hepatocellular carcinoma not amenable to curative treatment. Ann Oncol. 2020;31:S124–5. [Google Scholar]
- 84. Sangro BHJ, Harding JJ, Johnson M, Palmer DH, Edeline J, Abou-Alfa GK, et al. A phase III, double-blind, randomized study of nivolumab (NIVO) and ipilimumab (IPI), nivo monotherapy or placebo plus transarterial chemoembolization (TACE) in patients with intermediate-stage hepatocellular carcinoma (HCC). J Clin Oncol. 2021;39(3_suppl l):TPS349. [Google Scholar]
- 85. Kudo MGY, Guo Y, Hua Y, Zhao M, Xing W, Zhang, Y, et al. TALENTACE: a phase III, open-label, randomized study of on-demand transarterial chemoembolization combined with atezolizumab + bevacizumab or on-demand transarterial chemoembolization alone in patients with untreated hepatocellular carcinoma. J Clin Oncol. 2022;40(4_Suppl l):TPS487. [Google Scholar]
- 86. Kudo M, Han KH, Ye SL, Zhou J, Huang YH, Lin SM, et al. A changing paradigm for the treatment of intermediate-stage hepatocellular carcinoma: asia-pacific primary liver cancer expert consensus statements. Liver Cancer. 2020;9(3):245–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Vogel A, Martinelli E; ESMO Guidelines Committee Electronic address clinicalguidelines@esmo.org; ESMO Guidelines Committee . Updated treatment recommendations for hepatocellular carcinoma (HCC) from the ESMO Clinical Practice Guidelines. Ann Oncol. 2021;32(6):801–5. [DOI] [PubMed] [Google Scholar]
- 88. Reig M, Forner A, Rimola J, Ferrer-Fabrega J, Burrel M, Garcia-Criado A, et al. BCLC strategy for prognosis prediction and treatment recommendation: the 2022 update. J Hepatol. 2022;76(3):681–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Ogasawara S, Chiba T, Ooka Y, Kanogawa N, Motoyama T, Suzuki E, et al. Efficacy of sorafenib in intermediate-stage hepatocellular carcinoma patients refractory to transarterial chemoembolization. Oncology. 2014;87(6):330–41. [DOI] [PubMed] [Google Scholar]
- 90. Lin PT, Teng W, Jeng WJ, Hsieh YC, Hung CF, Huang CH, et al. Add-on sorafenib is beneficial for hepatocellular carcinoma patients with transarterial chemoembolization refractoriness: a real-world experience. Eur J Gastroenterol Hepatol. 2020;32(9):1192–9. [DOI] [PubMed] [Google Scholar]
- 91. Cheng AL, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, et al. Updated efficacy and safety data from IMbrave150: atezolizumab plus bevacizumab vs. sorafenib for unresectable hepatocellular carcinoma. J Hepatol. 2022;76(4):862–73. [DOI] [PubMed] [Google Scholar]
- 92. Hung YW, Lee IC, Chi CT, Lee RC, Liu CA, Chiu NC, et al. Radiologic patterns determine the outcomes of initial and subsequent transarterial chemoembolization in intermediate-stage hepatocellular carcinoma. Liver Cancer. 2023;13:29–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Kudo M. A new treatment option for intermediate-stage hepatocellular carcinoma with high tumor burden: initial lenvatinib therapy with subsequent selective TACE. Liver Cancer. 2019;8(5):299–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Kudo M, Ueshima K, Chan S, Minami T, Chishina H, Aoki T, et al. Lenvatinib as an initial treatment in patients with intermediate-stage hepatocellular carcinoma beyond up-to-seven criteria and child-pugh a liver function: a proof-of-concept study. Cancers. 2019;11(8):1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Gordan JD, Kennedy EB, Abou-Alfa GK, Beg MS, Brower ST, Gade TP, et al. Systemic therapy for advanced hepatocellular carcinoma: ASCO guideline. J Clin Oncol. 2020;38(36):4317–45. [DOI] [PubMed] [Google Scholar]
- 96. Bruix J, Chan SL, Galle PR, Rimassa L, Sangro B. Systemic treatment of hepatocellular carcinoma: an EASL position paper. J Hepatol. 2021;75(4):960–74. [DOI] [PubMed] [Google Scholar]
- 97. National Comprehensive Cancer Network . Hepatobiliary cancer (version 5.2022). 2022. Available from: https://www.nccn.org/professionals/physician_gls/pdf/hepatobiliary.pdf. (Accessed January 13, 2023). [Google Scholar]
- 98. Su GL, Altayar O, O’Shea R, Shah R, Estfan B, Wenzell C, et al. AGA clinical practice guideline on systemic therapy for hepatocellular carcinoma. Gastroenterology. 2022;162(3):920–34. [DOI] [PubMed] [Google Scholar]
- 99. Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382(20):1894–905. [DOI] [PubMed] [Google Scholar]
- 100. Galle PR, Finn RS, Qin S, Ikeda M, Zhu AX, Kim TY, et al. Patient-reported outcomes with atezolizumab plus bevacizumab versus sorafenib in patients with unresectable hepatocellular carcinoma (IMbrave150): an open-label, randomised, phase 3 trial. Lancet Oncol. 2021;22(7):991–1001. [DOI] [PubMed] [Google Scholar]
- 101. Abou-Alfa GK, Lau G, Kudo M, Chan SL, Kelley RK, Furuse J, et al. Tremelimumab plus durvalumab in unresectable hepatocellular carcinoma. NEJM Evid. 2022;1(8):EVIDoa2100070. [DOI] [PubMed] [Google Scholar]
- 102. Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359(4):378–90. [DOI] [PubMed] [Google Scholar]
- 103. Kudo M, Finn RS, Qin S, Han KH, Ikeda K, Piscaglia F, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018;391(10126):1163–73. [DOI] [PubMed] [Google Scholar]
- 104. Qin SKM, Kudo M, Meyer T, Finn R, Vogel A, Bai Y, et al. LBA36 Final analysis of RATIONALE-301: randomized, phase III study of tislelizumab versus sorafenib as first-line treatment for unresectable hepatocellular carcinoma. Ann Oncol. 2022;33(Suppl 7):S1402–3. [Google Scholar]
- 105. Ren Z, Xu J, Bai Y, Xu A, Cang S, Du C, et al. Sintilimab plus a bevacizumab biosimilar (IBI305) versus sorafenib in unresectable hepatocellular carcinoma (ORIENT-32): a randomised, open-label, phase 2-3 study. Lancet Oncol. 2021;22(7):977–90. [DOI] [PubMed] [Google Scholar]
- 106. Kelley RK, Yau T, Cheng AL, Kaseb A, Qin S, Zhu AX, et al. VP10-2021: cabozantinib (C) plus atezolizumab (A) versus sorafenib (S) as first-line systemic treatment for advanced hepatocellular carcinoma (aHCC): results from the randomized phase III COSMIC-312 trial. Ann Oncol. 2022;33(1):114–6. [Google Scholar]
- 107. Finn R, Km PPM, Meyer T, Qin S, Ikeda M, Xu R, et al. Primary results from the phase III LEAP-002 study: lenvatinib plus pembrolizumab versus lenvatinib as first- line (1L) therapy for advanced hepatocellular carcinoma (aHCC). Ann Oncol. 2022;33(Suppl l_7):S808–69. [Google Scholar]
- 108. Qin S, Chan SL, Gu S, Bai Y, Ren Z, Lin X, et al. Camrelizumab plus rivoceranib versus sorafenib as first-line therapy for unresectable hepatocellular carcinoma (CARES-310): a randomised, open-label, international phase 3 study. Lancet. 2023;402(10408):1133–46. [DOI] [PubMed] [Google Scholar]
- 109. Marrero JA, Kudo M, Venook AP, Ye SL, Bronowicki JP, Chen XP, et al. Observational registry of sorafenib use in clinical practice across Child-Pugh subgroups: the GIDEON study. J Hepatol. 2016;65(6):1140–7. [DOI] [PubMed] [Google Scholar]
- 110. Huynh J, Cho MT, Kim EJ, Ren M, Ramji Z, Vogel A. Lenvatinib in patients with unresectable hepatocellular carcinoma who progressed to Child-Pugh B liver function. Ther Adv Med Oncol. 2022;14:17588359221116608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. D’Alessio A, Fulgenzi CAM, Nishida N, Schonlein M, von Felden J, Schulze K, et al. Preliminary evidence of safety and tolerability of atezolizumab plus bevacizumab in patients with hepatocellular carcinoma and Child-Pugh A and B cirrhosis: a real-world study. Hepatology. 2022;76(4):1000–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Bruix J, Qin S, Merle P, Granito A, Huang YH, Bodoky G, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;389(10064):56–66. [DOI] [PubMed] [Google Scholar]
- 113. Abou-Alfa GK, Meyer T, Cheng AL, El-Khoueiry AB, Rimassa L, Ryoo BY, et al. Cabozantinib in patients with advanced and progressing hepatocellular carcinoma. N Engl J Med. 2018;379(1):54–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Zhu AX, Kang YK, Yen CJ, Finn RS, Galle PR, Llovet JM, et al. Ramucirumab after sorafenib in patients with advanced hepatocellular carcinoma and increased α-fetoprotein concentrations (REACH-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019;20(2):282–96. [DOI] [PubMed] [Google Scholar]
- 115. Koroki K, Kanogawa N, Maruta S, Ogasawara S, Iino Y, Obu M, et al. Posttreatment after lenvatinib in patients with advanced hepatocellular carcinoma. Liver Cancer. 2021;10(5):473–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Zhai J, Liu J, Fu Z, Bai S, Li X, Qu Z, et al. Comparison of the safety and prognosis of sequential regorafenib after sorafenib and lenvatinib treatment failure in patients with unresectable hepatocellular carcinoma: a retrospective cohort study. J Gastrointest Oncol. 2022;13(3):1278–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Kuzuya T, Ishigami M, Ito T, Ishizu Y, Honda T, Ishikawa T, et al. Initial experience of ramucirumab treatment after lenvatinib failure for patients with advanced hepatocellular carcinoma. Anticancer Res. 2020;40(4):2089–93. [DOI] [PubMed] [Google Scholar]
- 118. Hiraoka A, Kumada T, Tada T, Ogawa C, Tani J, Fukunishi S, et al. Therapeutic efficacy of ramucirumab after lenvatinib for post-progression treatment of unresectable hepatocellular carcinoma. Gastroenterol Rep. 2021;9(2):133–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. El-Khoueiry AB, Sangro B, Yau T, Crocenzi TS, Kudo M, Hsu C, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet. 2017;389(10088):2492–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Zhu AX, Finn RS, Edeline J, Cattan S, Ogasawara S, Palmer D, et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a non-randomised, open-label phase 2 trial. Lancet Oncol. 2018;19(7):940–52. [DOI] [PubMed] [Google Scholar]
- 121. Yau T, Park JW, Finn RS, Cheng AL, Mathurin P, Edeline J, et al. Nivolumab versus sorafenib in advanced hepatocellular carcinoma (CheckMate 459): a randomised, multicentre, open-label, phase 3 trial. Lancet Oncol. 2022;23(1):77–90. [DOI] [PubMed] [Google Scholar]
- 122. Yau TKY, Kang YK, Kim TY, El-Khoueiry AB, Santoro A, Sangro B, et al. Nivolumab (NIVO) + ipilimumab (IPI) combination therapy in patients (pts) with advanced hepatocellular carcinoma (aHCC): results from CheckMate 040. J Clin Oncol. 2019;37(15_Suppl l):4012. [Google Scholar]
- 123. Finn RS, Ryoo BY, Merle P, Kudo M, Bouattour M, Lim HY, et al. Pembrolizumab as second-line therapy in patients with advanced hepatocellular carcinoma in KEYNOTE-240: a randomized, double-blind, phase III trial. J Clin Oncol. 2020;38(3):193–202. [DOI] [PubMed] [Google Scholar]
- 124. Qin S, Chen Z, Fang W, Ren Z, Xu R, Ryoo BY, et al. Pembrolizumab versus placebo as second-line therapy in patients from Asia with advanced hepatocellular carcinoma: a randomized, double-blind, phase III trial. J Clin Oncol. 2022;1(41):1434–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Wang MD, Sun LY, Qian GJ, Li C, Gu LH, Yao LQ, et al. Prothrombin induced by vitamin K Absence-II versus alpha-fetoprotein in detection of both resectable hepatocellular carcinoma and early recurrence after curative liver resection: a retrospective cohort study. Int J Surg. 2022;105:106843. [DOI] [PubMed] [Google Scholar]
- 126. Lee HW, Song GW, Lee SG, Kim JM, Joh JW, Han DH, et al. Patient selection by tumor markers in liver transplantation for advanced hepatocellular carcinoma. Liver Transpl. 2018;24(9):1243–51. [DOI] [PubMed] [Google Scholar]
- 127. Kim DY, Paik YH, Ahn SH, Youn YJ, Choi JW, Kim JK, et al. PIVKA-II is a useful tumor marker for recurrent hepatocellular carcinoma after surgical resection. Oncology. 2007;72(Suppl 1):52–7. [DOI] [PubMed] [Google Scholar]
- 128. Park MS, Lee KW, Kim H, Choi YR, Hong G, Yi NJ, et al. Usefulness of PIVKA-II after living-donor liver transplantation for hepatocellular carcinoma. Transpl Proc. 2017;49(5):1109–13. [DOI] [PubMed] [Google Scholar]
- 129. Yao CC, Wang JH, Chen CH, Hung CH, Yen YH, Kee KM, et al. Short half-life of des-gamma-carboxy prothrombin is a superior factor for early prediction of outcomes of hepatocellular carcinoma treated with radiofrequency ablation. Diagnostics. 2023;13(4):696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Takahashi S, Kudo M, Chung H, Inoue T, Ishikawa E, Kitai S, et al. PIVKA-II is the best prognostic predictor in patients with hepatocellular carcinoma after radiofrequency ablation therapy. Oncology. 2008;75(Suppl 1):91–8. [DOI] [PubMed] [Google Scholar]
- 131. Park WH, Shim JH, Han SB, Won HJ, Shin YM, Kim KM, et al. Clinical utility of des-gamma-carboxyprothrombin kinetics as a complement to radiologic response in patients with hepatocellular carcinoma undergoing transarterial chemoembolization. J Vasc Interv Radiol. 2012;23(7):927–36. [DOI] [PubMed] [Google Scholar]
- 132. Koda M, Tokunaga S, Okamoto T, Hodozuka M, Miyoshi K, Kishina M, et al. Clinical usefulness of the ablative margin assessed by magnetic resonance imaging with Gd-EOB-DTPA for radiofrequency ablation of hepatocellular carcinoma. J Hepatol. 2015;63(6):1360–7. [DOI] [PubMed] [Google Scholar]
- 133. Makino Y, Imai Y, Igura T, Hori M, Fukuda K, Sawai Y, et al. Comparative evaluation of three-dimensional Gd-EOB-DTPA-enhanced MR fusion imaging with CT fusion imaging in the assessment of treatment effect of radiofrequency ablation of hepatocellular carcinoma. Abdom Imaging. 2015;40(1):102–11. [DOI] [PubMed] [Google Scholar]
- 134. Imai Y, Katayama K, Hori M, Yakushijin T, Fujimoto K, Itoh T, et al. Prospective comparison of Gd-EOB-DTPA-Enhanced MRI with dynamic CT for detecting recurrence of HCC after radiofrequency ablation. Liver Cancer. 2017;6(4):349–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Kaneko S, Kurosaki M, Tsuchiya K, Yasui Y, Hayakawa Y, Inada K, et al. Clinical evaluation of Elecsys PIVKA-II for patients with advanced hepatocellular carcinoma. PLoS One. 2022;17(3):e0265235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Sun X, Mei J, Lin W, Yang Z, Peng W, Chen J, et al. Reductions in AFP and PIVKA-II can predict the efficiency of anti-PD-1 immunotherapy in HCC patients. BMC Cancer. 2021;21(1):775. [DOI] [PMC free article] [PubMed] [Google Scholar]