Abstract
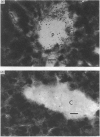
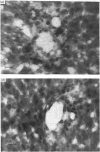
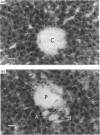
Autoradiographs of tissue slices from livers perfused with 1 x 10(-9) M-1-O-[3H]octadecyl-2-acetyl-sn-glycero-3-phosphocholine ([ 3H]18:0-sn-3-AGEPC) indicate that binding of this agonist is localized in the portal venules in anterograde perfused livers, and in the central venules in retrograde perfused livers. The pattern of silver grains in anterograde perfused liver was not affected significantly by prior exposure to 100-fold excesses of unlabelled 16:0- or 18:0-sn-3-AGEPC, 16:0-sn-1-AGEPC, or a 1000-fold excess of U.66985. [3H]18:0-sn-3-lyso-GEPC produced the same pattern of binding as the acetylated analogue. Measurement of glucose release stimulated by 16:0-sn-3-AGEPC demonstrated that the retrograde perfused liver was nearly 1000-fold less sensitive to this compound than the anterograde perfused liver. Exposure of the livers to bovine serum albumin prior to 5 x 10(-11) M-[3H]18:0-sn-3-AGEPC resulted in inhibition of stimulated glucose release, and decreased both the amount of label retained in the livers and the amount of silver grains over the portal sinusoidal cells without affecting the amount of grains seen over all other regions of the liver. Glucose release from primary monolayer cultures of hepatocytes or suspensions of liver slices was not stimulated by 16:0-sn-3-AGEPC. The results suggest that specific binding of [3H]18:0-sn-3-AGEPC is restricted to the portal side of the liver microvasculature, the majority of binding is nonspecific, and the biological response to AGEPC requires an intact and perfused vasculature.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Buxton D. B., Fisher R. A., Hanahan D. J., Olson M. S. Platelet-activating factor-mediated vasoconstriction and glycogenolysis in the perfused rat liver. J Biol Chem. 1986 Jan 15;261(2):644–649. [PubMed] [Google Scholar]
- Buxton D. B., Hanahan D. J., Olson M. S. Specific antagonists of platelet activating factor-mediated vasoconstriction and glycogenolysis in the perfused rat liver. Biochem Pharmacol. 1986 Mar 15;35(6):893–897. doi: 10.1016/0006-2952(86)90073-0. [DOI] [PubMed] [Google Scholar]
- Buxton D. B., Shukla S. D., Hanahan D. J., Olson M. S. Stimulation of hepatic glycogenolysis by acetylglyceryl ether phosphorylcholine. J Biol Chem. 1984 Feb 10;259(3):1468–1471. [PubMed] [Google Scholar]
- Charest R., Prpić V., Exton J. H., Blackmore P. F. Stimulation of inositol trisphosphate formation in hepatocytes by vasopressin, adrenaline and angiotensin II and its relationship to changes in cytosolic free Ca2+. Biochem J. 1985 Apr 1;227(1):79–90. doi: 10.1042/bj2270079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher R. A., Shukla S. D., Debuysere M. S., Hanahan D. J., Olson M. S. The effect of acetylglyceryl ether phosphorylcholine on glycogenolysis and phosphatidylinositol 4,5-bisphosphate metabolism in rat hepatocytes. J Biol Chem. 1984 Jul 25;259(14):8685–8688. [PubMed] [Google Scholar]
- Hill C. E., Olson M. S. Stimulation of uric acid release from the perfused rat liver by platelet activating factor or potassium. Biochem J. 1987 Oct 1;247(1):207–214. doi: 10.1042/bj2470207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill C. E., Pryor J. S., Olson M. S., Dawson A. P. Potassium-mediated stimulation of hepatic glycogenolysis. J Biol Chem. 1987 May 15;262(14):6623–6627. [PubMed] [Google Scholar]
- Hwang S. B., Lam M. H., Shen T. Y. Specific binding sites for platelet activating factor in human lung tissues. Biochem Biophys Res Commun. 1985 Apr 30;128(2):972–979. doi: 10.1016/0006-291x(85)90142-1. [DOI] [PubMed] [Google Scholar]
- Ison E. J., Sheridan P. J. Autoradiography of diffusible substances--a practical approach. Am J Med Technol. 1981 Jan;47(1):38–42. [PubMed] [Google Scholar]
- Kramp W., Pieroni G., Pinckard R. N., Hanahan D. J. Observations on the critical micellar concentration of 1-O-alkyl-2-acetyl-sn-glycero-3-phosphocholine and a series of its homologs and analogs. Chem Phys Lipids. 1984 May;35(1):49–62. doi: 10.1016/0009-3084(84)90032-x. [DOI] [PubMed] [Google Scholar]
- McCuskey R. S. A dynamic and static study of hepatic arterioles and hepatic sphincters. Am J Anat. 1966 Nov;119(3):455–477. doi: 10.1002/aja.1001190307. [DOI] [PubMed] [Google Scholar]
- Mendlovic F., Corvera S., García-Sáinz J. A. Possible involvement of cyclooxygenase products in the actions of platelet-activating factor and of lipoxygenase products in the vascular effects of epinephrine in perfused rat liver. Biochem Biophys Res Commun. 1984 Sep 17;123(2):507–514. doi: 10.1016/0006-291x(84)90259-6. [DOI] [PubMed] [Google Scholar]
- Miwa M., Hill C., Kumar R., Sugatani J., Olson M. S., Hanahan D. J. Occurrence of an endogenous inhibitor of platelet-activating factor in rat liver. J Biol Chem. 1987 Jan 15;262(2):527–530. [PubMed] [Google Scholar]
- Praaning-Van Dalen D. P., Knook D. L. Quantitative determination of in vivo endocytosis by rat liver Kupffer and endothelial cells facilitated by an improved cell isolation method. FEBS Lett. 1982 May 17;141(2):229–232. doi: 10.1016/0014-5793(82)80054-9. [DOI] [PubMed] [Google Scholar]
- Scholz R., Hansen W., Thurman R. G. Interaction of mixed-function oxidation with biosynthetic processes. 1. Inhibition of gluconeogenesis by aminopyrine in perfused rat liver. Eur J Biochem. 1973 Sep 21;38(1):64–72. doi: 10.1111/j.1432-1033.1973.tb03034.x. [DOI] [PubMed] [Google Scholar]
- Shukla S. D., Buxton D. B., Olson M. S., Hanahan D. J. Acetylglyceryl ether phosphorylcholine. A potent activator of hepatic phosphoinositide metabolism and glycogenolysis. J Biol Chem. 1983 Sep 10;258(17):10212–10214. [PubMed] [Google Scholar]
- Widmann J. J., Cotran R. S., Fahimi H. D. Mononuclear phagocytes (Kupffer cells) and endothelial cells. Identification of two functional cell types in rat liver sinusoids by endogenous peroxidase activity. J Cell Biol. 1972 Jan;52(1):159–170. doi: 10.1083/jcb.52.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisse E. An ultrastructural characterization of the endothelial cell in the rat liver sinusoid under normal and various experimental conditions, as a contribution to the distinction between endothelial and Kupffer cells. J Ultrastruct Res. 1972 Mar;38(5):528–562. doi: 10.1016/0022-5320(72)90089-5. [DOI] [PubMed] [Google Scholar]
- Yamashita M., Homma H., Inoue K., Nojima S. The metabolism of platelet activating factor in platelets and plasma of various animals. J Toxicol Sci. 1983 Aug;8(3):177–188. doi: 10.2131/jts.8.177. [DOI] [PubMed] [Google Scholar]
- Yokota S. Functional differences between sinusoidal endothelial cells and interlobular or central vein endothelium in rat liver. Anat Rec. 1985 May;212(1):74–80. doi: 10.1002/ar.1092120111. [DOI] [PubMed] [Google Scholar]