Abstract
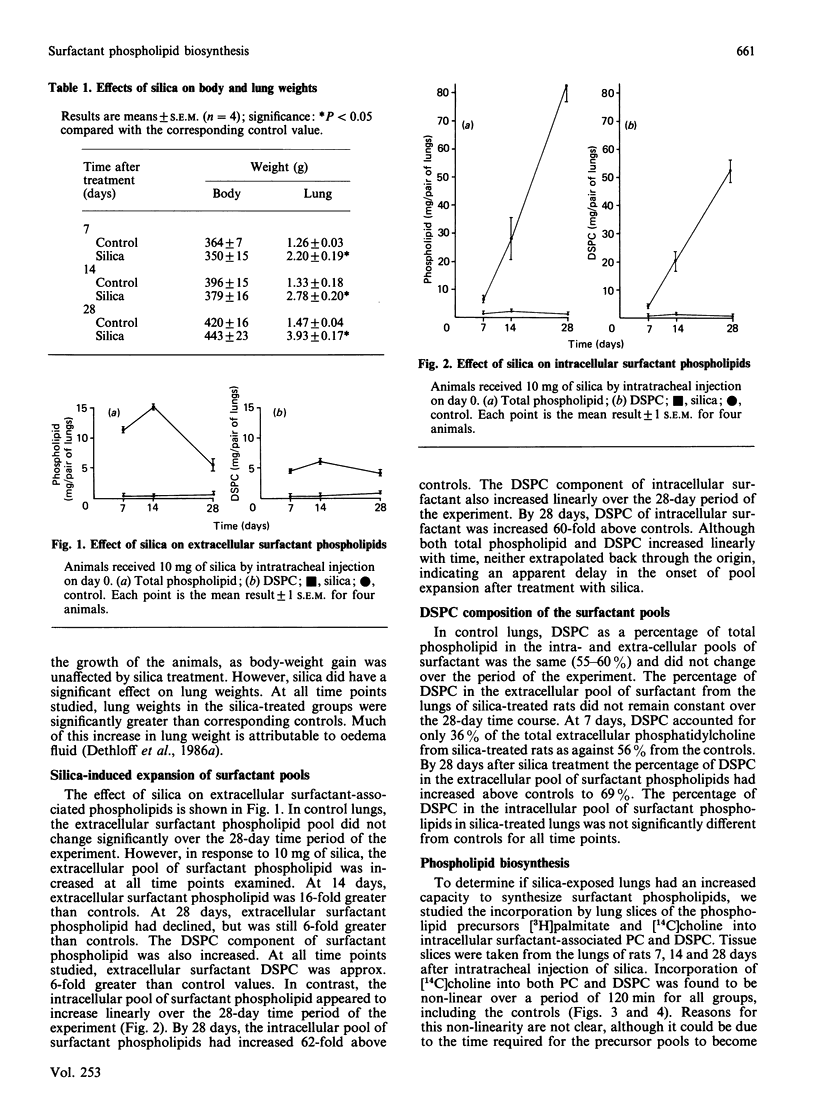
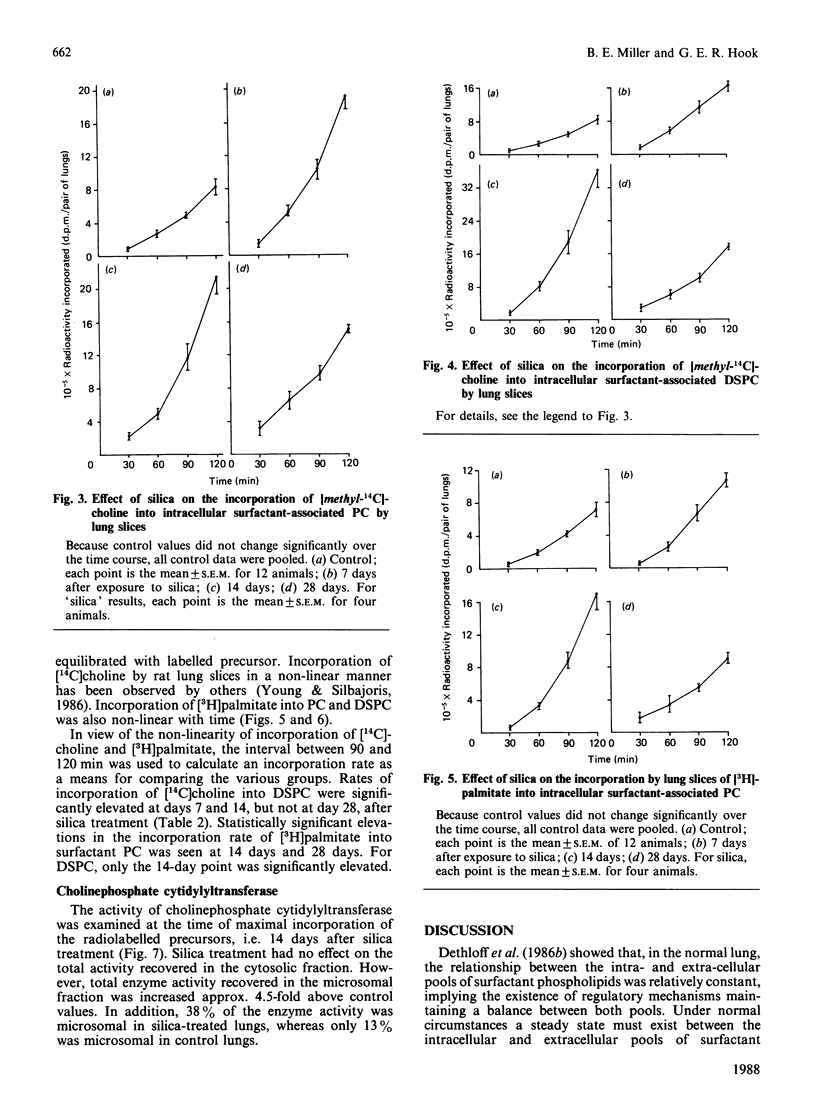
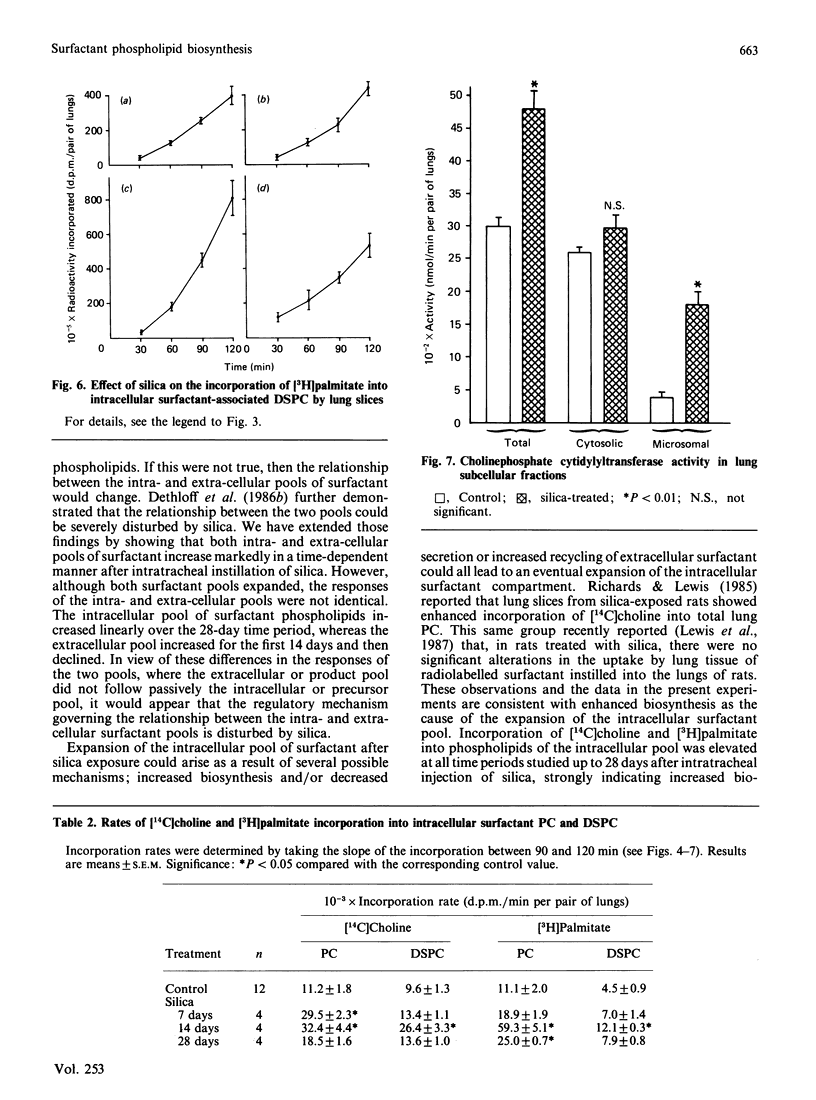
The effects of intratracheally instilled silica (10 mg/rat) on the biosynthesis of surfactant phospholipids was investigated in the lungs of rats. The sizes of the intracellular and extracellular pools of surfactant phospholipids were measured 7, 14 and 28 days after silica exposure. The ability of lung slices to incorporate [14C]choline and [3H]palmitate into surfactant phosphatidylcholine (PC) and disaturated phosphatidylcholine (DSPC) was also investigated. Both intra- and extra-cellular pools of surfactant phospholipids were increased by silica treatment. The intracellular pool increased linearly over the 28-day time period, ultimately reaching a size 62-fold greater than controls. The extracellular pool also increased, but showed a pattern different from that of the intracellular pool. The extracellular pool increased non-linearly up to 14 days, and then declined. At its maximum, the extracellular pool was increased 16-fold over the control. The ability of lung slices to incorporate phospholipid precursors into surfactant-associated PC and DSPC was elevated at all time periods. The rate of incorporation of [14C]choline into surfactant PC and DSPC was maximal at 14 days and was nearly 3-fold greater than the rate in controls. The rate of incorporation of [3H]palmitate was also maximal at 14 days, approx. 5-fold above controls for PC and 3-fold for DSPC. At this same time point, the microsomal activity of cholinephosphate cytidylyltransferase was increased 4.5-fold above controls, but cytosolic activity was not significantly affected by silica treatment. These data indicate that biosynthesis of surfactant PC is elevated after treatment of lungs with silica and that this increased biosynthesis probably underlies the expansion of the intra- and extra-cellular pools of surfactant phospholipids.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chen R. F. Removal of fatty acids from serum albumin by charcoal treatment. J Biol Chem. 1967 Jan 25;242(2):173–181. [PubMed] [Google Scholar]
- Dethloff L. A., Gilmore L. B., Brody A. R., Hook G. E. Induction of intra- and extra-cellular phospholipids in the lungs of rats exposed to silica. Biochem J. 1986 Jan 1;233(1):111–118. doi: 10.1042/bj2330111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dethloff L. A., Gilmore L. B., Gladen B. C., George G., Chhabra R. S., Hook G. E. Effects of silica on the composition of the pulmonary extracellular lining. Toxicol Appl Pharmacol. 1986 Jun 15;84(1):66–83. doi: 10.1016/0041-008x(86)90417-5. [DOI] [PubMed] [Google Scholar]
- Dethloff L. A., Gilmore L. B., Hook G. E. The relationship between intra- and extra-cellular surfactant phospholipids in the lungs of rabbits and the effects of silica-induced lung injury. Biochem J. 1986 Oct 1;239(1):59–67. doi: 10.1042/bj2390059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Gabor S., Zugravu E., Kováts A., Böhm B., Andrasoni D. Effects of quartz on lung surfactant. Environ Res. 1978 Jul;16(1-3):443–448. doi: 10.1016/0013-9351(78)90177-9. [DOI] [PubMed] [Google Scholar]
- Gilfillan A. M., Chu A. J., Smart D. A., Rooney S. A. Single plate separation of lung phospholipids including disaturated phosphatidylcholine. J Lipid Res. 1983 Dec;24(12):1651–1656. [PubMed] [Google Scholar]
- Heppleston A. G., Fletcher K., Wyatt I. Changes in the composition of lung lipids and the "turnover" of dipalmitoyl lecithin in experimental alveolar lipo-proteinosis induced by inhaled quartz. Br J Exp Pathol. 1974 Aug;55(4):384–395. [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lewis R. W., Harwood J. L., Richards R. J. A method for preparing radiolabelled rat pulmonary surfactant. Biochem J. 1986 Apr 1;235(1):75–79. doi: 10.1042/bj2350075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis R. W., Harwood J. L., Richards R. J. The fate of instilled pulmonary surfactant in normal and quartz-treated rats. Biochem J. 1987 May 1;243(3):679–685. doi: 10.1042/bj2430679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason R. J., Nellenbogen J., Clements J. A. Isolation of disaturated phosphatidylcholine with osmium tetroxide. J Lipid Res. 1976 May;17(3):281–284. [PubMed] [Google Scholar]
- Miller B. E., Chapin R. E., Pinkerton K. E., Gilmore L. B., Maronpot R. R., Hook G. E. Quantitation of silica-induced type II cell hyperplasia by using alkaline phosphatase histochemistry in glycol methacrylate embedded lung. Exp Lung Res. 1987;12(2):135–148. doi: 10.3109/01902148709062837. [DOI] [PubMed] [Google Scholar]
- Miller B. E., Dethloff L. A., Gladen B. C., Hook G. E. Progression of type II cell hypertrophy and hyperplasia during silica-induced pulmonary inflammation. Lab Invest. 1987 Nov;57(5):546–554. [PubMed] [Google Scholar]
- Miller B. E., Dethloff L. A., Hook G. E. Silica-induced hypertrophy of type II cells in the lungs of rats. Lab Invest. 1986 Aug;55(2):153–163. [PubMed] [Google Scholar]
- O'Neil J. J., Sanford R. L., Wasserman S., Tierney D. F. Metabolism in rat lung tissue slices: technical factors. J Appl Physiol Respir Environ Exerc Physiol. 1977 Nov;43(5):902–906. doi: 10.1152/jappl.1977.43.5.902. [DOI] [PubMed] [Google Scholar]
- Post M., Batenburg J. J., Smith B. T., Van Golde L. M. Pool sizes of precursors for phosphatidylcholine formation in adult rat lung type II cells. Biochim Biophys Acta. 1984 Oct 4;795(3):552–557. doi: 10.1016/0005-2760(84)90185-1. [DOI] [PubMed] [Google Scholar]
- Richards R. J., Curtis C. G. Biochemical and cellular mechanisms of dust-induced lung fibrosis. Environ Health Perspect. 1984 Apr;55:393–416. doi: 10.1289/ehp.8455393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards R. J., Lewis R. Surfactant changes in experimentally induced disease. Biochem Soc Trans. 1985 Dec;13(6):1084–1087. doi: 10.1042/bst0131084. [DOI] [PubMed] [Google Scholar]
- Smith F. B., Kikkawa Y. The type II epithelial cells of the lung. III. Lecithin synthesis: a comparison with pulmonary macrophages. Lab Invest. 1978 Jan;38(1):45–51. [PubMed] [Google Scholar]
- Young S. L., Kremers S. A., Apple J. S., Crapo J. D., Brumley G. W. Rat lung surfactant kinetics biochemical and morphometric correlation. J Appl Physiol Respir Environ Exerc Physiol. 1981 Aug;51(2):248–253. doi: 10.1152/jappl.1981.51.2.248. [DOI] [PubMed] [Google Scholar]
- Young S. L., Silbajoris R. Dexamethasone increases adult rat lung surfactant lipids. J Appl Physiol (1985) 1986 May;60(5):1665–1672. doi: 10.1152/jappl.1986.60.5.1665. [DOI] [PubMed] [Google Scholar]


