Abstract
Background:
We aimed to investigating the sex-specific and age-specific melanoma mortality trends observed on the territory of Serbia between 2000 and 2021.
Methods:
This population-based study used data from the Statistical Office of the Republic of Serbia database during the period 2000–2021. The calculation of the gender and age-standardized rates (ASR) was performed. We used a regression analysis complete with linear trend model.
Results:
The mean ASR was 1.77 per 100,000 people, meaning that male mortality rates (2.24 per 100,000) was higher than female mortality rates (1.34 per 100,000). During the observation period, a rising trend in mortality from melanoma skin cancer was reported. Observed by gender, the change of melanoma mortality trend was significant in men (P=0.021), but not in women (P=0.747). The annual growth rate of ASRs values was 1.43%. A increase in the melanoma mortality rate was observed since 2000 by 2.44% annually in males and by 2.79% annually in females. Mortality rates were increasing in both sexes as they aged, and the greatest number of deaths was recorded in the group of those aged 80 yr or above (16.25 per 100,000 for men; 10.45 per 100,000 for women).
Conclusion:
Our study findings underline the importance of launching more effective public health awareness campaigns to educate people about the dangers of melanoma and its symptoms’ detection along with establishing a diagnosis at an early stage of the disease, especially among male patients and those at an advanced age.
Keywords: Malignant melanoma, Mortality, Age-standardized rates, Population-based study
Introduction
Malignant melanoma poses a significant global health challenge due to its rising incidence, high mortality rates, and the escalating costs associated with treating advanced stages of the disease (1, 2).
As the deadliest form of skin cancer, melanoma is responsible for approximately 80% of all skin cancer-related deaths (3). Nearly 85% of new melanoma cases are diagnosed in economically developed countries, where it is anticipated to become the sixth most commonly diagnosed cancer (4).
The incidence rates of melanoma have shown a continuous increase over the past five decades (1, 5, 6). The number of new cases of melanoma has surged by over 40% in just the last decade. Global cancer data indicates that nearly 200,000 cases of melanoma were diagnosed in 2008, a figure that soared to over 287,000 worldwide by 2018 (7). There was a projected future increase of more than 50% in new melanoma cases by 2040, rising from 325,000 in 2020 to 510,000 in 2040. Similarly, melanoma-related deaths are expected to rise by approximately 68%, from 57,000 in 2020 to 96,000 in 2040 (1).
The increasing incidence of cutaneous melanoma is associated with a rise in the number of deaths. In 2020, the GLOBOCAN database (1, 8) reported 324,635 cases of melanoma worldwide (173,844 males, 150,791 females), with 57,043 reported deaths (32,385 males, 24,658 females). Consequently, melanoma is confirmed as the 22nd leading cause of death among all malignant tumors, with an age-standardized rate (ASR) of 0.56/100,000 (1, 8, 9).
In the same year, Europe reported almost 145,000 new cases of melanoma and 27,000 deaths from the disease, accounting for 47% of all global deaths. Melanoma represents 4% of all new cancer diagnoses and 1.3% of cancer-related deaths in the EU-27. Thus, globally, melanoma ranks as the fifth most prevalent cancer compared to other malignancies and is considered one of the 15 most frequent causes of death in the EU-27 (10).
The process of melanocytes transforming into malignant cells is complex and involves genetic and environmental factors as well as their interactions. Risk factors for melanoma include unprotected UV exposure (from natural sunlight or indoor tanning), race and skin phenotype, early-onset photosensitivity reactions (resulting from intense solar radiation exposure), presence of atypical or dysplastic nevi, immunodeficiency, personal history of melanoma or other skin cancers, and family history of melanoma (2, 4, 11, 12). There is a lack of comprehensive studies examining trends in melanoma mortality in Serbia. The aim of our current population-based analysis is to investigate temporal patterns of melanoma mortality influenced by sex and age in Serbia from 2000 to 2021 and compare the data with international findings.
We aimed to investigate sex-specific and age-specific trends in melanoma mortality observed in Serbia between 2000 and 2021.
Methods
Data sources
The study was classified as a population-based one, which included epidemiological data and their retrospective analysis. This particular study comprised primary cutaneous melanoma-diagnosed patients (The International Statistical Classification of Diseases, the 10th Revision (ICD-10-CM) code C43), whose data were obtained by the Statistical Office of the Republic of Serbia during the period 2000–2021 (unpublished data). Data on deaths were obtained from individual statistical “Notification of Deaths” (special statistical form – DEM 2) reports, which have been regularly submitted, to the Statistical Office of the Republic of Serbia by health institutions. Only licensed medical practitioners, forensic physicians or pathologists have the permission to provide a “Notification of Deaths”.
The assessment was performed by extracting the skin melanoma death figures from the database which were further classified based on sex and 18 age groups (0–4, 5–9, . . . 80–84, and ≥85 years). We used age and sex-specific data on the number and composition of the Serbian population (not taking into consideration the Autonomous Province of Kosovo and Metohija) collected based on the 2002 and 2011 population censuses; for inter-censal years, we used the numbers estimated by the Statistical Office of the Republic of Serbia.
Statistical analysis
What followed was the detailed calculation of crude, age-specific and age-standardized mortality rates. The age and sex-adjusted rates were expressed and shown as deaths per 100,000 inhabitants. The calculation of the ASRs was performed by using direct method. The rates were computed by using the Segi’s population (13). The ASRs calculations were based on the age groups consisting of five-year-olds. ASRs were not shown for the age subgroups (<24 years) with less than five cases of deaths from malignant melanoma per year in each of the quinquennium. We employed regression analysis using a linear trend model to examine current trends in malignant melanoma mortality. The annual percentage change (APC) in the mortality rate of melanoma was calculated by comparing the percentage changes observed between the adjusted rates for two consecutive years. Additionally, the average annual percentage change (AAPC) was calculated as the average mean of these changes during the observation period. Confidence intervals (CI) for the mean values of age-adjusted and age-specific mortality rates were generated with a stated probability of 95%. Bilateral P values were observed and considered statistically significant if they were below 0.05.
Results
During the period from 2000 to 2021, 5,291 inhabitants in Serbia died from melanoma (3,102 men and 2,189 women). The annual number of deaths during the analyzed period increased from 181 (2000) to 267 (2021). The number of recorded deaths was greater among men (58.63%) than women (41.37%).
Taking into consideration the fact that the mean ASR was 1.77 per 100,000 people, it was observed that male mortality rates (2.24 per 100,000) were higher than female mortality rates (1.34 per 100,000). Regarding the age-standardized mortality rates, they varied from 1.66 to 2.87 per 100,000 among male individuals and from 0.80 to 1.62 per 100,000 among the female ones. The use of the total standard population size was needed in order to show the number of cases, the crude mortality rate, and age-standardized incidence rates per 100,000 persons (Table 1).
Table 1:
Melanoma Mortality in Serbia, excluding the Autonomous Province of Kosovo and Metohija, in the period 2000–2021, by sex
| Year | Total | Men | Women | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| N | Crude rate | ASR | N | Crude rate | ASR | N | Crude rate | ASR | |
| 2000 | 181 | 2.41 | 1.51 | 105 | 2.87 | 1.87 | 76 | 1.97 | 1.19 |
| 2001 | 207 | 2.76 | 1.70 | 120 | 3.29 | 2.13 | 87 | 2.26 | 1.35 |
| 2002 | 194 | 2.59 | 1.61 | 98 | 2.69 | 1.69 | 96 | 2.49 | 1.57 |
| 2003 | 182 | 2.43 | 1.57 | 101 | 2.78 | 1.83 | 81 | 2.11 | 1.32 |
| 2004 | 206 | 2.76 | 1.66 | 111 | 3.06 | 1.94 | 95 | 2.48 | 1.42 |
| 2005 | 223 | 3.00 | 1.77 | 127 | 3.51 | 2.15 | 96 | 1.28 | 0.80 |
| 2006 | 208 | 2.81 | 1.71 | 114 | 3.16 | 1.99 | 94 | 2.47 | 1.45 |
| 2007 | 238 | 3.22 | 1.79 | 130 | 3.62 | 2.21 | 108 | 2.85 | 1.44 |
| 2008 | 258 | 3.51 | 1.92 | 160 | 4.48 | 2.54 | 98 | 2.60 | 1.40 |
| 2009 | 259 | 3.54 | 1.97 | 151 | 4.24 | 2.54 | 108 | 2.87 | 1.46 |
| 2010 | 253 | 3.47 | 1.83 | 150 | 4.23 | 2.34 | 103 | 2.57 | 1.40 |
| 2011 | 232 | 3.21 | 1.70 | 145 | 4.11 | 2.35 | 87 | 2.34 | 1.17 |
| 2012 | 273 | 3.79 | 1.98 | 157 | 4.48 | 2.44 | 116 | 3.14 | 1.62 |
| 2013 | 247 | 3.45 | 1.76 | 153 | 4.38 | 2.27 | 94 | 2.56 | 1.36 |
| 2014 | 264 | 3.70 | 1.86 | 149 | 4.29 | 2.30 | 115 | 3.14 | 1.49 |
| 2015 | 300 | 4.23 | 2.05 | 190 | 5.50 | 2.87 | 110 | 3.02 | 1.37 |
| 2016 | 271 | 3.84 | 1.87 | 159 | 4.63 | 2.40 | 112 | 3.09 | 1.44 |
| 2017 | 274 | 3.90 | 1.88 | 166 | 4.85 | 2.48 | 108 | 3.00 | 1.36 |
| 2018 | 269 | 3.85 | 1.78 | 159 | 4.67 | 2.32 | 110 | 3.07 | 1.33 |
| 2019 | 283 | 4.07 | 1.90 | 175 | 5.17 | 2.67 | 108 | 3.03 | 1.22 |
| 2020 | 202 | 2.93 | 1.33 | 117 | 3.48 | 1.66 | 85 | 2.40 | 1.07 |
| 2021 | 267 | 3.91 | 1.75 | 165 | 4.96 | 2.34 | 102 | 2.91 | 1.27 |
| Overall | 5291 | 3.34 | 1.77 | 3102 | 4.02 | 2.24 | 2189 | 2.62 | 1.34 |
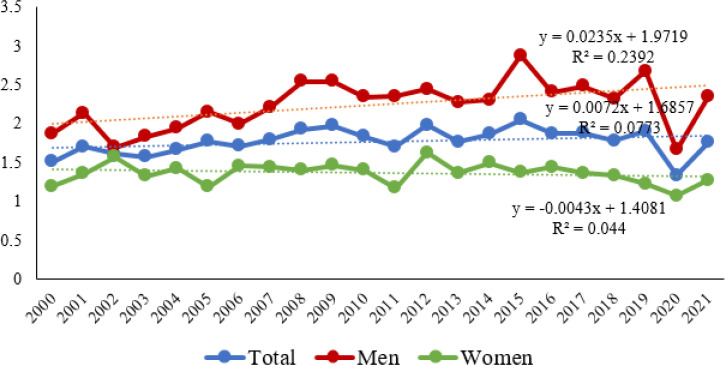
During the observation period, a rising trend in mortality from malignant melanoma was reported (y=0.0072x + 1.6857). As regards gender differences in mortality trends of males and females, mortality trends were found to be increasing among males (y=0.0235x + 1.9719), whereas they were found to be decreasing among females diagnosed with malignant melanoma (y=−0. 0043x + 1.4081) (Fig. 1). The change of melanoma mortality trend was significant in men (P=0.021), but not in women (P=0.747) as regards the total number of people diagnosed with melanoma (p=0.269).
Fig. 1:
Trends of age-standardized malignant melanoma mortality rates in Serbia by sex, in the period 2000–2021
The annual growth rate of ASRs values was 1.43% (95% CI: −4.14 – 7.01). A substantial increase in the melanoma mortality rate has been observed since 2000 by 2.44% (95% confidence interval (CI: −5.13 – 9.99) annually in males and by 2.79% (95% CI: −8.33 – 13.92) annually in females. Among men, the greatest percentage rise in the number of deaths was recorded in 2021 (+40.96), whereas among women, the greatest percentage rise in the number of deaths was recorded in 2006 (+81.25) (Table 2).
Table 2:
Changes in age-standardized death rates (ASDRs) between 2000 and 2021 for malignant melanoma in Serbia
| Year | Male | Female | ||||
|---|---|---|---|---|---|---|
| Change in ASDR | Change in ASDR | |||||
| ASR | Absolute | Percentage | ASR | Absolute | Percentage | |
| 2000 | 1.87 | 1.19 | ||||
| 2001 | 2.13 | +0.26 | +13.90 | 1.35 | +0.16 | +13.45 |
| 2002 | 1.69 | −0.44 | −20.66 | 1.57 | +0.22 | +16.30 |
| 2003 | 1.83 | +0.14 | +8.28 | 1.32 | −0.25 | −15.92 |
| 2004 | 1.94 | +0.11 | +6.01 | 1.42 | +0.10 | +7.58 |
| 2005 | 2.15 | +0.21 | +10.82 | 0.80 | −0.62 | −43.66 |
| 2006 | 1.99 | −0.16 | −7.44 | 1.45 | +0.65 | +81.25 |
| 2007 | 2.21 | +0.22 | +11.06 | 1.44 | −0.01 | −0.69 |
| 2008 | 2.54 | +0.33 | +14.93 | 1.40 | −0.04 | −2.78 |
| 2009 | 2.54 | 0.00 | 0.00 | 1.46 | +0.06 | +4.29 |
| 2010 | 2.34 | −0.20 | −7.87 | 1.40 | −0.06 | −4.11 |
| 2011 | 2.35 | +0.01 | +0.43 | 1.17 | −0.23 | −16.43 |
| 2012 | 2.44 | +0.09 | +3.83 | 1.62 | +0.45 | +38.46 |
| 2013 | 2.27 | −0.17 | −6.97 | 1.36 | −0.26 | −16.05 |
| 2014 | 2.30 | +0.03 | +1.32 | 1.49 | +0.13 | +9.56 |
| 2015 | 2.87 | +0.57 | 24.78 | 1.37 | −0.12 | −8.05 |
| 2016 | 2.40 | −0.47 | −16.38 | 1.44 | +0.07 | +5.11 |
| 2017 | 2.48 | +0.08 | +3.33 | 1.36 | −0.08 | −5.56 |
| 2018 | 2.32 | −0.16 | −6.45 | 1.33 | −0.03 | −2.21 |
| 2019 | 2.67 | +0.35 | +15.09 | 1.22 | −0.11 | −8.27 |
| 2020 | 1.66 | −1.01 | −37.83 | 1.07 | −0.15 | −12.30 |
| 2021 | 2.34 | +0.68 | +40.96 | 1.27 | 0.20 | +18.69 |
Mortality rates were observed to be increasing in both males and females as they were getting older, meaning that the greatest number of deaths was recorded in the group of those aged 80 or above (16.25 per 100,000 for male individuals; 10.45 per 100,000 for female individuals) (Table 3).
Table 3:
The average age-standardized mortality rates and linear trend of melanoma malignum in Serbia, in 2000–2021
| Age | Age-specific rates* | Linear trend | R2 | p | Average annual percentage change (95% CI) |
|---|---|---|---|---|---|
|
| |||||
| (yr) | (per 100,000) | ||||
| Men | |||||
| 25 - 29 | 0.40 | † | |||
| 30 - 34 | 1.09 | † | |||
| 35 -39 | 1.65 | † | |||
| 40 -44 | 1.98 | † | |||
| 45 - 49 | 3.35 | ||||
| 50 - 54 | 4.83 | y = 172.36 − 0.43·x | 0.046 | 11.59 (−11.11 – 34.29) | |
| 55 - 59 | 6.36 | † | |||
| 60 - 64 | 7.93 | † | |||
| 65 - 69 | 10.23 | y = 11.88 + 0.46·x | 0.217 | 0.030 | 6.16 (−9.25 – 21.57) |
| 70 - 74 | 12.61 | y = 18.09 + 0.72·x | 0.519 | 0.000 | 8.37 (−8.33 – 25.07) |
| 75 - 79 | 14.69 | y = 9.03 + 0.64·x | 0.407 | 0.002 | 10.99 (−6.64 – 28.63) |
| 80 + | 16.25 | y = 8.42 + 0.79·x | 0.617 | 0.000 | 15.76 (−0.83 – 32.35) |
| Women | |||||
| 25 - 29 | 0.41 | † | |||
| 30 - 34 | 1.00 | y = 7.74 − 0.63·x | 0.401 | 0.001 | 15.81 (−34.10 – 65,70) |
| 35 -39 | 1.19 | † | 0.035 | 36.89 (−10.00 – 83.77) | |
| 40 -44 | 1.74 | † | |||
| 45 - 49 | 2.44 | † | |||
| 50 - 54 | 2.69 | † | |||
| 55 - 59 | 3.76 | † | |||
| 60 - 64 | 3.60 | † | |||
| 65 - 69 | 5.38 | † | |||
| 70 - 74 | 6.17 | y = 7.96 + 0.52·x | 0.268 | 0.012 | 13.36 (−12.36 – 33.39) |
| 75 - 79 | 7.34 | y = 3.47 + 0.50·x | 0.252 | 0.012 | 9.19 (−7.95 – 26.34) |
| 80+ | 10.45 | y = 2.62 + 0.44·x | 0.195 | 0.032 | 19.17 (−11.90 – 50.25) |
Mortality rates among individuals younger than 25 years were low for both men and women. The highest increase in the number of death cases among males was observed in the 80 and above age group, with an average annual percent rise of 15.76 (95% CI: −0.83 – 32.35; P=0.000). Conversely, the highest increase in the number of death cases among females was observed in the 35 to 39 age group, with an average annual percent rise of 36.89 (95% CI: −10.00 – 83.77; P=0.035).
Discussion
Melanoma mortality rates in Serbia exhibit differing trends between men and women, with a declining trend observed for women and a rising trend for men. Despite the overall increase in incidence rates, melanoma mortality rates have been reported to rise over recent decades (6,14,15). However, the increase in mortality rates appears to be slowing down or even ceasing completely in some regions, possibly due to earlier detection and the availability of modern life-prolonging treatments (16,17). The variability in melanoma mortality trends is influenced by factors such as geography (variations in atmospheric gas absorption, altitude, latitude, and season), ethnicity, age, and gender (18,19). Melanoma is more common in individuals with fair skin compared to those with darker skin (lifetime risk: 2.4% vs. 0.1%) (5). The lower incidence in dark-skinned populations is associated with a protective phenotype against UV sensitivity. Regions with higher altitudes and lower latitudes have been associated with a higher number of melanoma deaths. Until the 1980s, mortality rates continued to increase in regions with high-risk factors such as New Zealand, Australia, North America, and Europe (20).
The most significant risk factors for skin cancer, which are unlikely to be modified, include age and gender. Our study revealed a correlation between higher mortality rates in both male and female individuals diagnosed with melanoma and their age, with the greatest number of deaths recorded in the group of patients aged 80 yr or above. It is well established that melanoma mortality increases with age, primarily due to the gradual accumulation of skin melanoma risk factors over the course of life. A study examining melanoma mortality trends in the EU-28 for the period 1960–2020 found higher death rates of melanoma in the 75-and-older age group for both males and females (10). Incidence rates of malignant melanoma are higher among older populations, reaching their peak in individuals aged 70 to 80 years (21). The average age at diagnosis of melanoma is 65 yr; with two-thirds of all new cases, occurring in individuals aged 55 to 84 yr. The higher prevalence of melanoma among the elderly may be attributed to their significant exposure to UV radiation from the sun, leading to an increased risk of developing melanoma with each subsequent decade of cumulative exposure to UV radiation from the sun (22).
Although individuals under 40 are reported to have lower melanoma incidence rates, melanoma remains one of the most prevalent forms of cancer diagnosed in adolescents and young adults worldwide (20, 23). In the USA, malignant melanoma is considered the second leading cancer affecting females aged 20 to 29 (23), possibly due in part to the frequent use of tanning beds by women, which increases their risk of developing melanoma (24). These findings underscore the importance of raising awareness about the health risks associated with exposure to UV radiation from indoor tanning devices and promoting a shift in attitudes toward sun tanning among adolescents and young adults. After the age of 40, there is a reversal of incidence rates between the sexes, with men demonstrating higher incidence rates of melanoma. Furthermore, for individuals older than 75 years, the incidence rate of skin cancer is nearly three times higher in men than in women (25).
Melanoma was reported to be more common in men than in women worldwide (10, 26), which is consistent with our research findings. A recent study analyzing trends in melanoma death rates for 31 countries between 1985 and 2015 revealed that males had a higher melanoma mortality rate compared to females in all countries included in the study except for the Czech Republic. The countries with the highest increase in melanoma mortality rates included the Republic of Korea (+535.3%), Ireland (+115.5%), and Croatia (+91.2%). Conversely, several countries showed reduced or stable melanoma mortality rates in women during the follow-up period, with Israel, the Czech Republic, and Australia reporting the largest declines (−23.4%, −15.5%, and −10.3%, respectively) (14). Consistent with trends observed in most countries, our study demonstrated higher melanoma mortality rates in men, with male mortality increasing more rapidly compared to female mortality.
The underlying causes of such gender differences are still not clearly understood. However, several hypotheses that could contribute to explaining these findings may be presented. These are primarily related to behavioral factors specific to gender, such as men’s less attention to sun protection and self-examination of the skin (27). Some arguments strongly suggest that outdoor employment is more frequently associated with men, who are therefore more likely to be exposed to UV rays without adequate sunscreen protection (15, 28). In contrast, women seem to be more concerned about protecting themselves from the sun’s UV rays, regardless of their tendency to sunbathe intentionally (29).
Gender differences also exist in terms of the anatomical localization of the lesion, which may contribute to lower mortality in women. Specifically, the most common localization of melanoma in females is the lower and upper extremities, accounting for 38% and 25% of cases of melanoma, respectively (30). This localization may facilitate the detection of tumors at an early stage in two ways. Firstly, the choice of clothing along with exposed parts of their body makes it easier for females to spot any recently developed or growing lesions located in the lower extremities. In contrast, the back region, which is the most frequent location where skin cancer develops in men (accounting for 41% of melanoma cases), is not easily accessible for self-examination, leading to later diagnosis in men (15). The disparities observed in terms of gender-specific anatomical distribution are often attributed to differences in behavior related to sun protection between males and females. Differences in mortality rates can partly be explained by genetic and hormonal differences between the sexes (24).
Conclusion
The global incidence of melanoma is on the rise, accompanied by increasing trends in melanoma mortality. Our study highlights the necessity of implementing more effective public health awareness campaigns to educate individuals about the dangers of melanoma, the recognition of its symptoms and cancerous changes, and the importance of early diagnosis, particularly among male patients and those at an advanced age. Primary prevention and early detection, coupled with accessible modern therapy for all patients, are crucial elements for effectively reducing melanoma incidence and mortality rates, despite its ongoing global threat.
Journalism Ethics considerations
Ethical issues (including plagiarism, informed consent, misconduct, data fabrication and/or falsification, double publication and/or submission, and redundancy) were observed by the authors.
Acknowledgements
No finical support was received.
Footnotes
Conflict of Interest
The authors declare that there is no conflict of interest.
References
- 1.Arnold M, Singh D, Laversanne M, et al. (2022). Global Burden of Cutaneous Melanoma in 2020 and Projections to 2040. JAMA Dermatol, 158 (5): 495–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dziankowska-Zaborszczyk E, Maniecka-Bryła I, Pikala M. (2022). Mortality Trends Due to Skin Melanoma in Poland in the Years 2000–2020. Int J Environ Res Public Health, 19 (23): 16118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Erdei E, Torres SM. (2010). A new understanding in the epidemiology of melanoma. Expert Rev Anticancer Ther, 10 (11): 1811–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Erdmann F, Lortet-Tieulent J, Schüz J, et al. (2013). International trends in the incidence of malignant melanoma 1953–2008--are recent generations at higher or lower risk? Int J Cancer, 132 (2): 385–400. [DOI] [PubMed] [Google Scholar]
- 5.Lopes J, Rodrigues CMP, Gaspar MM, Reis CP. (2022). Melanoma Management: From Epidemiology to Treatment and Latest Advances. Cancers (Basel), 14 (19): 4652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guy GP, Thomas CC, Thompson T, et al. (2015). Vital signs: Melanoma incidence and mortality trends and projections – United States, 1982–2030. MMWR Morb Mortal Wkly Rep, 64 (21): 591–6. [PMC free article] [PubMed] [Google Scholar]
- 7.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. (2018). Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin, 68, 394–424. [DOI] [PubMed] [Google Scholar]
- 8.Saginala K, Barsouk A, Aluru JS, Rawla P, Barsouk A. (2021). Epidemiology of melanoma. Med Sci (Basel), 9 (4): 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.International Agency for Research on Cancer . Cancer Tomorrow. Estimated Number of Deaths from 2020 to 2040 of Melanoma of Skin. Available from: https://gco.iarc.fr/tomorrow/en/dataviz/isotype?cancers=16_17&single_unit=5000&group_cancers=1&multiple_cancers=1&types=1 [cited August 2023].
- 10.Koczkodaj P, Sulkowska U, Didkowska J, Rutkowski P, Mańczuk M. (2023). Melanoma Mortality Trends in 28 European Countries: A Retrospective Analysis for the Years 1960–2020. Cancers (Basel), 15 (5): 1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gandini S, Sera F, Cattaruzza MS, et al. (2005). Meta-analysis of risk factors for cutaneous melanoma: I. Common and atypical naevi. Eur J Cancer, 41 (1): 28–44. [DOI] [PubMed] [Google Scholar]
- 12.Memon A, Bannister P, Rogers I, et al. (2021). Changing epidemiology and age-specific incidence of cutaneous malignant melanoma in England: An analysis of the national cancer registration data by age, gender and anatomical site, 1981–2018. Lancet Reg Health Eur, 2: 100024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Segi M. (1960). Cancer Mortality for Selected Sites in 24 Countries (1950–1957). Sendai: Department of Public Health, Tohoku University of Medicine. https://search.worldcat.org/title/36669244 [Google Scholar]
- 14.Yang DD, Salciccioli JD, Marshall DC, Sheri A, Shalhoub J. (2020). Trends in malignant melanoma mortality in 31 countries from 1985 to 2015. Br J Dermatol, 183: 1056–1064. [DOI] [PubMed] [Google Scholar]
- 15.Eriksson H, Lyth J, Månsson-Brahme E, et al. (2014). Later stage at diagnosis and worse survival in cutaneous malignant melanoma among men living alone: a nationwide population-based study from Sweden. J Clin Oncol, 32 (13): 1356–64. [DOI] [PubMed] [Google Scholar]
- 16.Berk-Krauss J, Stein JA, Weber J, Polsky D, Geller AC. (2020). New Systematic Therapies and Trends in Cutaneous Melanoma Deaths among US Whites, 1986–2016. Am J Public Health, 110 (5): 731–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gutiérrez-González E, López-Abente G, Aragonés N, et al. (2019). Trends in Mortality from Cutaneous Malignant Melanoma in Spain (1982–2016): Sex-Specific Age-Cohort-Period Effects. J Eur Acad Dermatol Venereol, 33 (8): 1522–8. [DOI] [PubMed] [Google Scholar]
- 18.Ward-Peterson M, Acuna JM, Alkhalifah MK, et al. (2016). Association between race/ethnicity and survival of melanoma patients in the United States over 3 decades: A secondary analysis of SEER data. Medicine, 95 (17): e3315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sneyd MJ, Cox B. (2013). A comparison of trends in melanoma mortality in New Zealand and Australia: The two countries with the highest melanoma incidence and mortality in the world. BMC Cancer, 13: 372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Garbe C, Leiter U. (2009). Melanoma epidemiology and trends. Clin Dermatol, 27 (1): 3–9. [DOI] [PubMed] [Google Scholar]
- 21.Ward WH, Farma JM. (2017). Cutaneous Melanoma: Etiology and Therapy. Brisbane (AU): Codon Publications. [PubMed] [Google Scholar]
- 22.Armstrong BK. (2004). How sun exposure causes skin cancer: An epidemiological perspective in: Hill D, Elwood JM, English D. Prevention of Skin Cancer. Dordrecht, The Netherlands: Kluwer Academic Publishers, pp. 89–116. [Google Scholar]
- 23.Watson M, Geller AC, Tucker MA, Guy GP, Jr, Weinstock MA. (2016). Melanoma burden and recent trends among non-Hispanic whites aged 15–49years, United States. Prev Med, 91: 294–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pantovic M, Djordjevic O, Radevic S, Bankovic D, Parezanovic Ilic K, Radovanovic S. (2023). Mortality of malignant melanoma in Central Serbia, in the period 1999–2015. Dermatol Pract Concept, 13 (1): e2023008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rastrelli M, Tropea S, Rossi CR, Alaibac M. (2014). Melanoma: Epidemiology, risk factors, pathogenesis, diagnosis and classification. In Vivo, 28: 1005–1011. [PubMed] [Google Scholar]
- 26.Arnold M, de Vries E, Whiteman DC, et al. (2018). Global burden of cutaneous melanoma attributable to ultraviolet radiation in 2012. Int J Cancer, 143 (6): 1305–1314. [DOI] [PubMed] [Google Scholar]
- 27.Olsen CM, Thompson JF, Pandeya N, Whiteman DC. (2020). Evaluation of sex-specific incidence of melanoma. JAMA Dermatol, 156 (5): 553–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Robsahm TE, Bergva G, Hestvik UE, Møller B. (2013). Sex differences in rising trends of cutaneous malignant melanoma in Norway, 1954–2008. Melanoma Res, 23 (1): 70–8. [DOI] [PubMed] [Google Scholar]
- 29.Boniol M, Autier P, Boyle P, Gandini S. (2012). Cutaneous melanoma attributable to sunbed use: Systematic review and meta-analysis. BMJ, 345: e4757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sung H, Siegel RL, Rosenberg PS, Jemal A. (2019). Emerging cancer trends among young adults in the USA: analysis of a population-based cancer registry. Lancet Public Health, 4 (3): e137–e147. [DOI] [PubMed] [Google Scholar]