Abstract
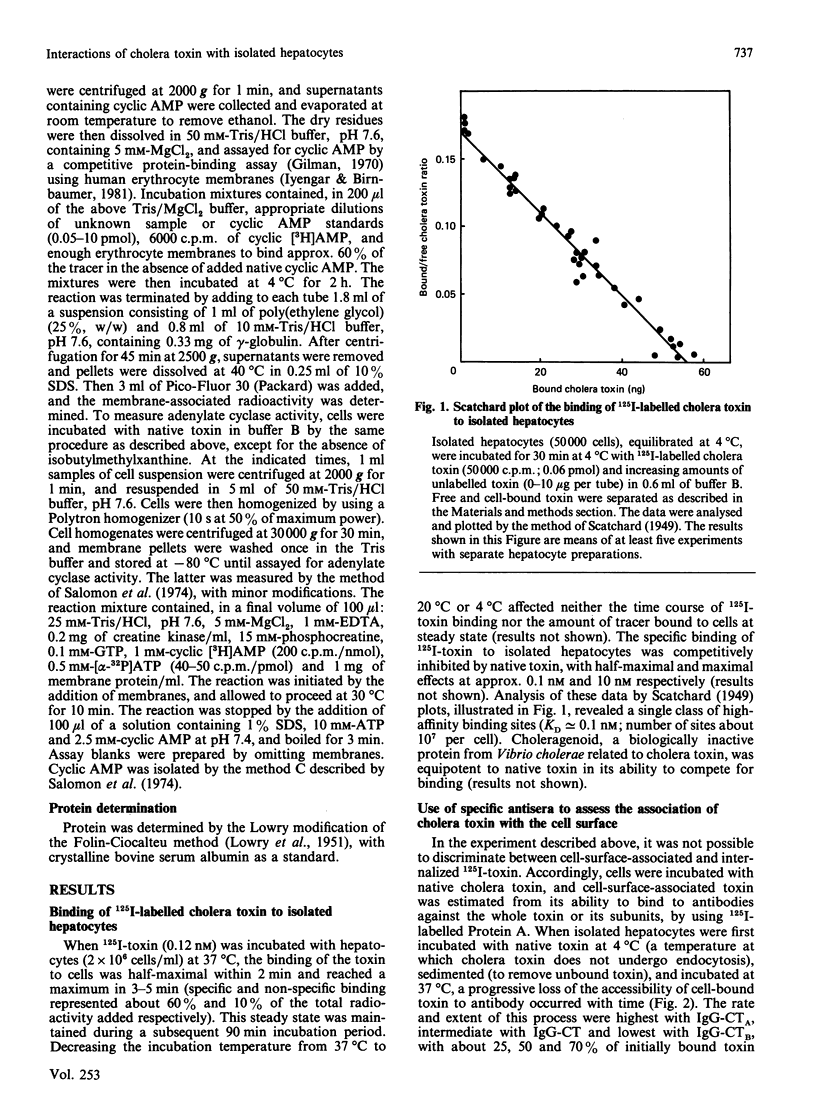
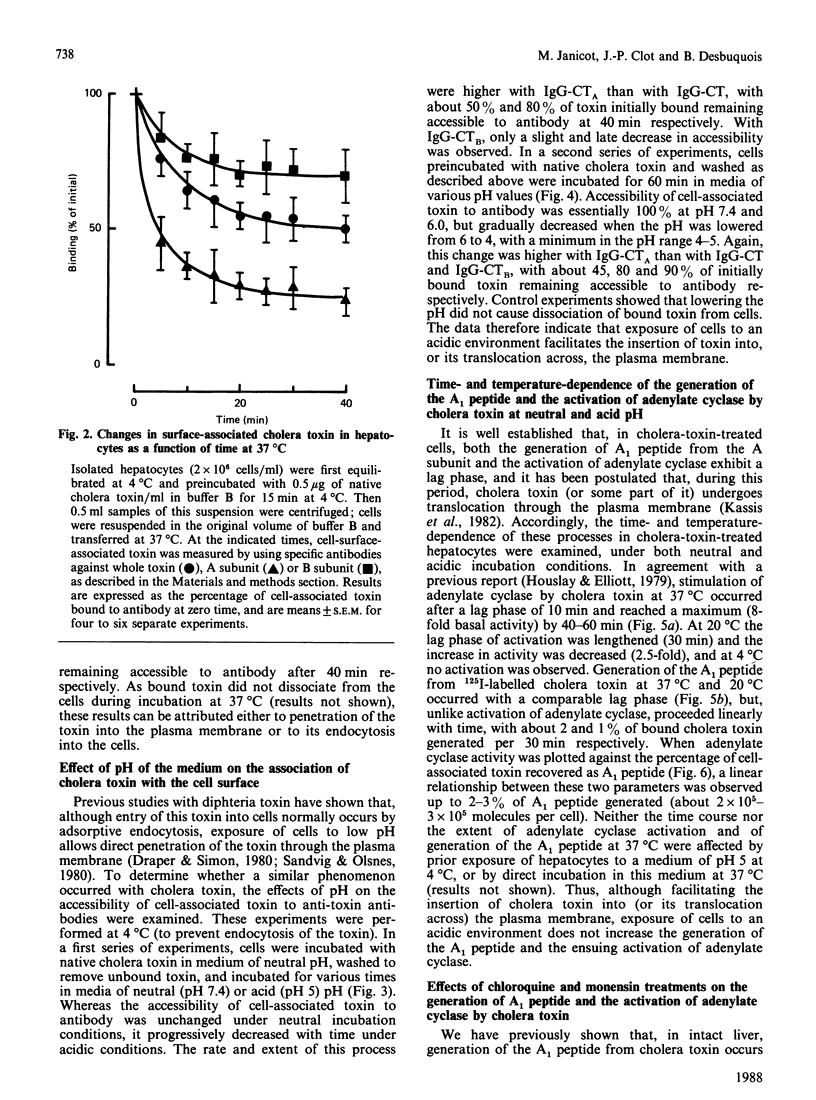
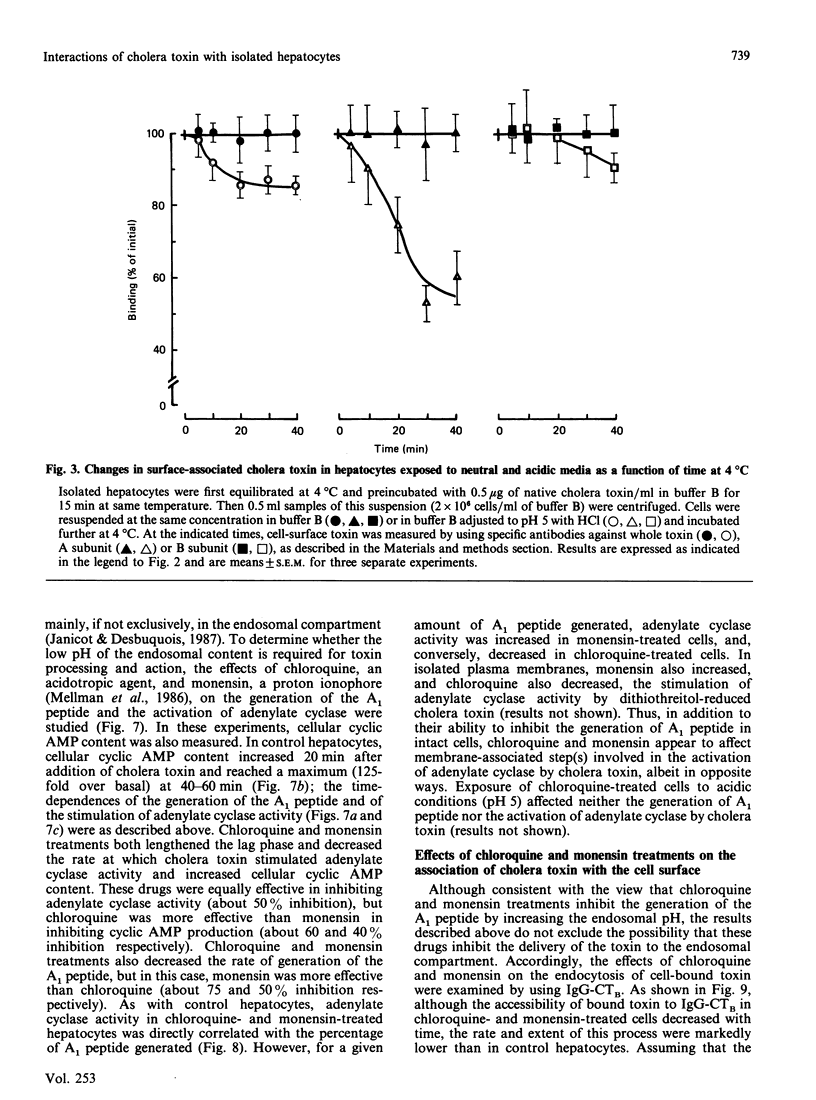
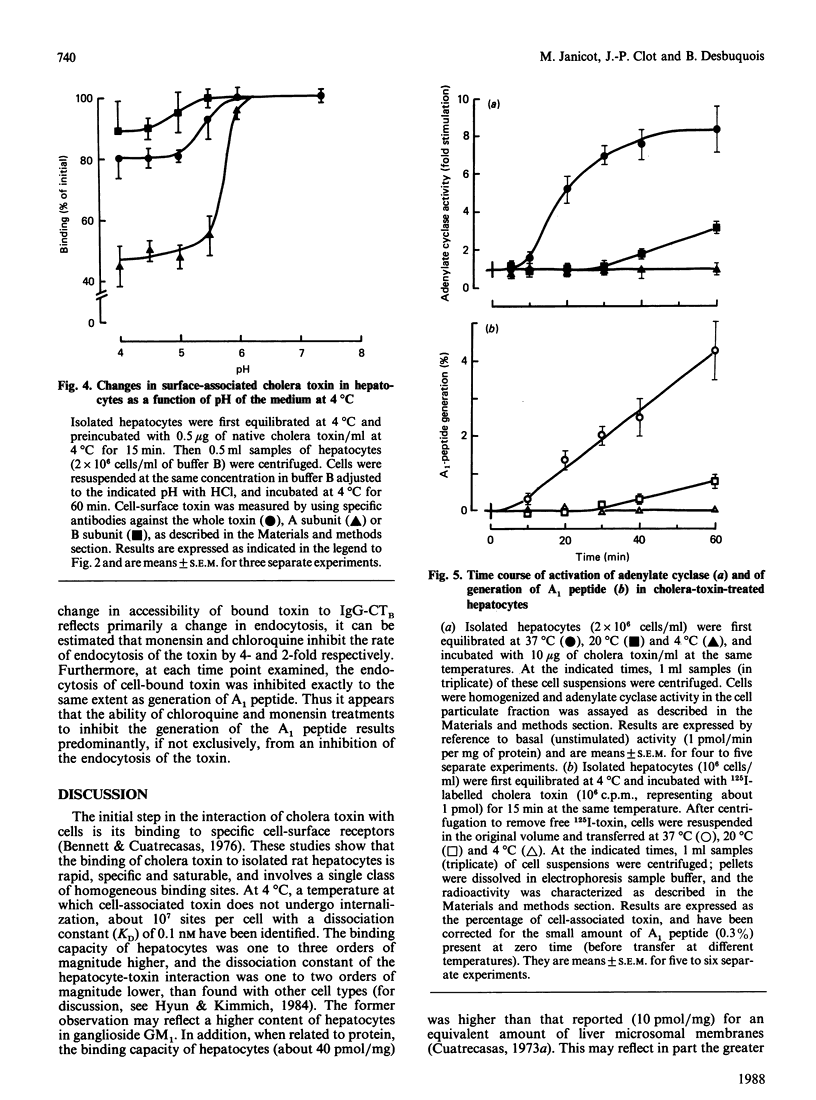
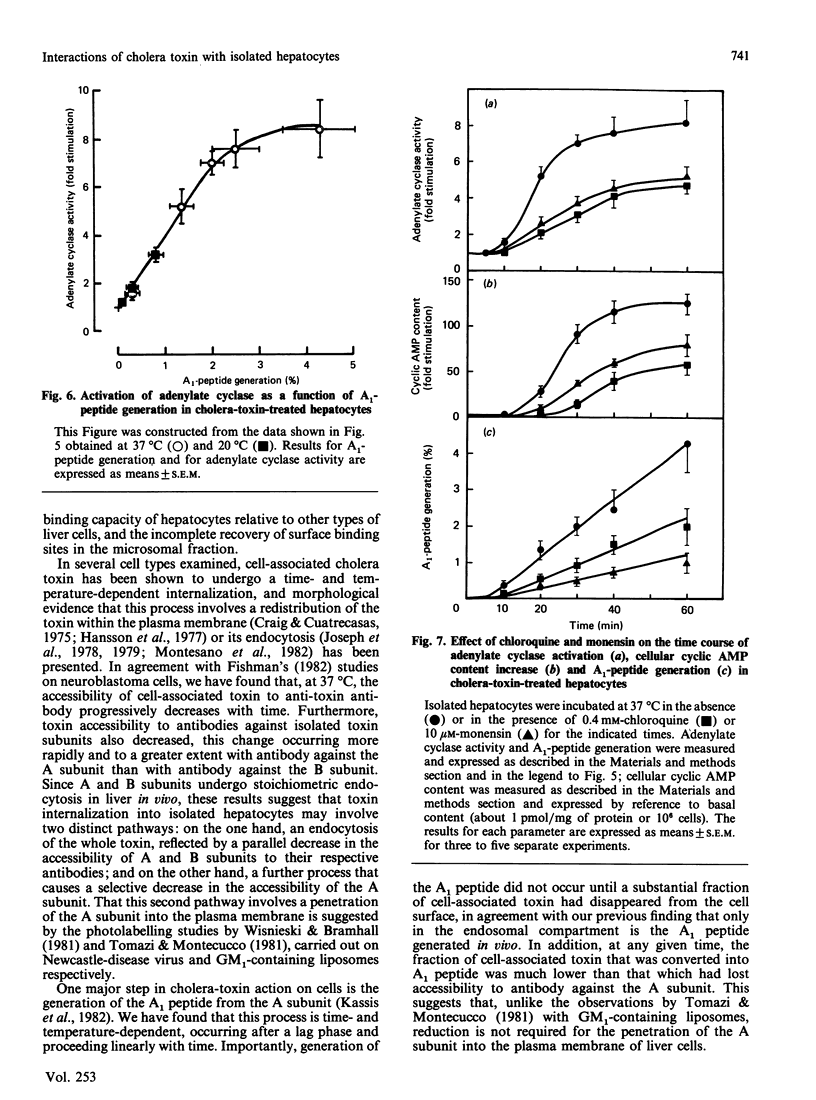
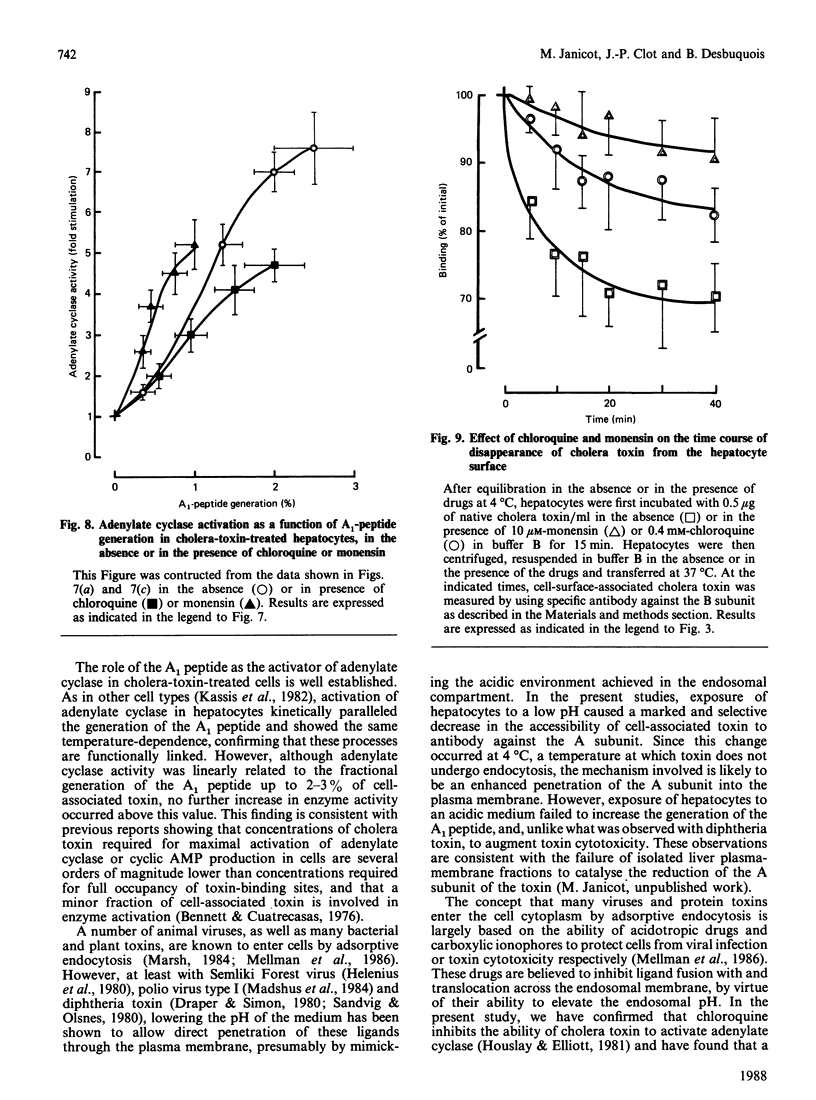
The major steps in cholera-toxin action, i.e. binding, internalization, generation of A1 peptide and activation of adenylate cyclase, were examined in isolated hepatocytes. The binding of toxin involves a single class of high-affinity sites (KD congruent to 0.1 nM; Bmax. congruent to 10(7) sites/cell). At 37 degrees C, cell-associated toxin is progressively internalized, as judged by the loss of its accessibility to antibodies against whole toxin, A and B subunits (about 50, 75 and 30% of initially bound toxin after 40 min respectively). Two distinct pathways are involved in this process: endocytosis of the whole toxin, and selective penetration of the A subunit into the plasma membrane. Exposure of hepatocytes to an acidic medium (pH 5) results in a rapid and marked disappearance of the A subunit from the cell surface. Generation of A1 peptide and activation of adenylate cyclase by the toxin occur after a lag phase (10 min at 37 degrees C), and increase with time in a parallel manner up to 2-3% A1 peptide generated; they are unaffected by exposure of cells to an acidic medium. Chloroquine and monensin, which elevate the pH in acidic organelles, inhibit by 2-4-fold both the generation of A1 peptide and the activation of adenylate cyclase. Unexpectedly, these drugs also inhibit the internalization of the toxin. These results suggest that an acidic pH facilitates the penetration of A subunit into the plasma membrane and presumably the endosomal membrane as well, and that endocytosis of cholera toxin is required for generation of A1 peptide and activation of adenylate cyclase.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cassel D., Pfeuffer T. Mechanism of cholera toxin action: covalent modification of the guanyl nucleotide-binding protein of the adenylate cyclase system. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2669–2673. doi: 10.1073/pnas.75.6.2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig S. W., Cuatrecasas P. Mobility of cholera toxin receptors on rat lymphocyte membranes. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3844–3848. doi: 10.1073/pnas.72.10.3844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuatrecasas P. Gangliosides and membrane receptors for cholera toxin. Biochemistry. 1973 Aug 28;12(18):3558–3566. doi: 10.1021/bi00742a032. [DOI] [PubMed] [Google Scholar]
- Cuatrecasas P. Interaction of Vibrio cholerae enterotoxin with cell membranes. Biochemistry. 1973 Aug 28;12(18):3547–3558. doi: 10.1021/bi00742a031. [DOI] [PubMed] [Google Scholar]
- Draper R. K., Simon M. I. The entry of diphtheria toxin into the mammalian cell cytoplasm: evidence for lysosomal involvement. J Cell Biol. 1980 Dec;87(3 Pt 1):849–854. doi: 10.1083/jcb.87.3.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishman P. H. Internalization and degradation of cholera toxin by cultured cells: relationship to toxin action. J Cell Biol. 1982 Jun;93(3):860–865. doi: 10.1083/jcb.93.3.860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill D. M., Meren R. ADP-ribosylation of membrane proteins catalyzed by cholera toxin: basis of the activation of adenylate cyclase. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3050–3054. doi: 10.1073/pnas.75.7.3050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill D. M. Multiple roles of erythrocyte supernatant in the activation of adenylate cyclase by Vibrio cholerae toxin in vitro. J Infect Dis. 1976 Mar;133 (Suppl):55–63. doi: 10.1093/infdis/133.supplement_1.s55. [DOI] [PubMed] [Google Scholar]
- Gilman A. G. A protein binding assay for adenosine 3':5'-cyclic monophosphate. Proc Natl Acad Sci U S A. 1970 Sep;67(1):305–312. doi: 10.1073/pnas.67.1.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson H. A., Holmgren J., Svennerholm L. Ultrastructural localization of cell membrane GM1 ganglioside by cholera toxin. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3782–3786. doi: 10.1073/pnas.74.9.3782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helenius A., Kartenbeck J., Simons K., Fries E. On the entry of Semliki forest virus into BHK-21 cells. J Cell Biol. 1980 Feb;84(2):404–420. doi: 10.1083/jcb.84.2.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmgren J. Actions of cholera toxin and the prevention and treatment of cholera. Nature. 1981 Jul 30;292(5822):413–417. doi: 10.1038/292413a0. [DOI] [PubMed] [Google Scholar]
- Houslay M. D., Elliott K. R. Cholera toxin mediated activation of adenylate cyclase in intact rat hepatocytes. FEBS Lett. 1979 Aug 15;104(2):359–363. doi: 10.1016/0014-5793(79)80852-2. [DOI] [PubMed] [Google Scholar]
- Houslay M. D., Elliott K. R. Is the receptor-mediated endocytosis of cholera toxin A pre-requisite for its activation of adenylate cyclase in intact rat hepatocytes? FEBS Lett. 1981 Jun 15;128(2):289–292. doi: 10.1016/0014-5793(81)80101-9. [DOI] [PubMed] [Google Scholar]
- Hyun C. S., Kimmich G. A. Interaction of cholera toxin and Escherichia coli enterotoxin with isolated intestinal epithelial cells. Am J Physiol. 1984 Dec;247(6 Pt 1):G623–G631. doi: 10.1152/ajpgi.1984.247.6.G623. [DOI] [PubMed] [Google Scholar]
- Janicot M., Desbuquois B. Fate of injected 125I-labeled cholera toxin taken up by rat liver in vivo. Generation of the active A1 peptide in the endosomal compartment. Eur J Biochem. 1987 Mar 2;163(2):433–442. doi: 10.1111/j.1432-1033.1987.tb10816.x. [DOI] [PubMed] [Google Scholar]
- Joseph K. C., Kim S. U., Stieber A., Gonatas N. K. Endocytosis of cholera toxin into neuronal GERL. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2815–2819. doi: 10.1073/pnas.75.6.2815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph K. C., Stieber A., Gonatas N. K. Endocytosis of cholera toxin in GERL-like structures of murine neuroblastoma cells pretreated with GM1 ganglioside. Cholera toxin internalization into Neuroblastoma GERL. J Cell Biol. 1979 Jun;81(3):543–554. doi: 10.1083/jcb.81.3.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassis S., Hagmann J., Fishman P. H., Chang P. P., Moss J. Mechanism of action of cholera toxin on intact cells. Generation of A1 peptide and activation of adenylate cyclase. J Biol Chem. 1982 Oct 25;257(20):12148–12152. [PubMed] [Google Scholar]
- Keusch G. T., Jacewicz M. Primary amines and chloroquine inhibit cytotoxic responses to Shigella toxin and permit late antibody rescue of toxin treated cells. Biochem Biophys Res Commun. 1984 May 31;121(1):69–76. doi: 10.1016/0006-291x(84)90689-2. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Madshus I. H., Olsnes S., Sandvig K. Mechanism of entry into the cytosol of poliovirus type 1: requirement for low pH. J Cell Biol. 1984 Apr;98(4):1194–1200. doi: 10.1083/jcb.98.4.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh M. The entry of enveloped viruses into cells by endocytosis. Biochem J. 1984 Feb 15;218(1):1–10. doi: 10.1042/bj2180001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh M., Wellsteed J., Kern H., Harms E., Helenius A. Monensin inhibits Semliki Forest virus penetration into culture cells. Proc Natl Acad Sci U S A. 1982 Sep;79(17):5297–5301. doi: 10.1073/pnas.79.17.5297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellman I., Fuchs R., Helenius A. Acidification of the endocytic and exocytic pathways. Annu Rev Biochem. 1986;55:663–700. doi: 10.1146/annurev.bi.55.070186.003311. [DOI] [PubMed] [Google Scholar]
- Montesano R., Roth J., Robert A., Orci L. Non-coated membrane invaginations are involved in binding and internalization of cholera and tetanus toxins. Nature. 1982 Apr 15;296(5858):651–653. doi: 10.1038/296651a0. [DOI] [PubMed] [Google Scholar]
- Moss J., Stanley S. J., Lin M. C. NAD glycohydrolase and ADP-ribosyltransferase activities are intrinsic to the A1 peptide of choleragen. J Biol Chem. 1979 Dec 10;254(23):11993–11999. [PubMed] [Google Scholar]
- Moss J., Vaughan M. Activation of adenylate cyclase by choleragen. Annu Rev Biochem. 1979;48:581–600. doi: 10.1146/annurev.bi.48.070179.003053. [DOI] [PubMed] [Google Scholar]
- Postel-Vinay M. C., Desbuquois B. Interaction of human growth hormone with isolated rat liver cells. Endocrinology. 1977 Jan;100(1):209–215. doi: 10.1210/endo-100-1-209. [DOI] [PubMed] [Google Scholar]
- Salomon Y., Londos C., Rodbell M. A highly sensitive adenylate cyclase assay. Anal Biochem. 1974 Apr;58(2):541–548. doi: 10.1016/0003-2697(74)90222-x. [DOI] [PubMed] [Google Scholar]
- Sandvig K., Olsnes S. Diphtheria toxin entry into cells is facilitated by low pH. J Cell Biol. 1980 Dec;87(3 Pt 1):828–832. doi: 10.1083/jcb.87.3.828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandvig K., Olsnes S., Pihl A. Inhibitory effect of ammonium chloride and chloroquine on the entry of the toxic lectin modeccin into HeLa cells. Biochem Biophys Res Commun. 1979 Sep 27;90(2):648–655. doi: 10.1016/0006-291x(79)91284-1. [DOI] [PubMed] [Google Scholar]
- Schlegel R., Willingham M., Pastan I. Monensin blocks endocytosis of vesicular stomatitis virus. Biochem Biophys Res Commun. 1981 Oct 15;102(3):992–998. doi: 10.1016/0006-291x(81)91636-3. [DOI] [PubMed] [Google Scholar]
- Seglen P. O. Preparation of rat liver cells. 3. Enzymatic requirements for tissue dispersion. Exp Cell Res. 1973 Dec;82(2):391–398. doi: 10.1016/0014-4827(73)90357-1. [DOI] [PubMed] [Google Scholar]
- Sorimachi K., Yasumura Y., Okayasu T. Inhibitory effect of chloroquine on concanavalin A internalization into cell monolayer culture. Jpn J Exp Med. 1985 Apr;55(2):53–59. [PubMed] [Google Scholar]
- Tomasi M., Montecucco C. Lipid insertion of cholera toxin after binding to GM1-containing liposomes. J Biol Chem. 1981 Nov 10;256(21):11177–11181. [PubMed] [Google Scholar]
- Wilcox D. K., Kitson R. P., Widnell C. C. Inhibition of pinocytosis in rat embryo fibroblasts treated with monensin. J Cell Biol. 1982 Mar;92(3):859–864. doi: 10.1083/jcb.92.3.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisnieski B. J., Bramhall J. S. Photolabelling of cholera toxin subunits during membrane penetration. Nature. 1981 Jan 22;289(5795):319–321. doi: 10.1038/289319a0. [DOI] [PubMed] [Google Scholar]


