Abstract
Following the invasion by the pine wood nematode (PWN) into north‐east China, a notable disparity in susceptibility was observed among Pinaceae species. Larix olgensis exhibited marked resilience and suffered minimal fatalities, while Pinus koraiensis experienced significant mortality due to PWN infection. Our research demonstrated that the PWNs in L. olgensis showed a 13.43% reduction in lipid content compared to P. koraiensis (p < 0.05), which was attributable to the accumulation of caffeic acid in L. olgensis. This reduction in lipid content was correlated with a decreased overwintering survival of PWNs. The diminished lipid reserves were associated with substantial stunting in PWNs, including reduced body length and maximum body width. The result suggests that lower lipid content is a major factor contributing to the lower overwintering survival rate of PWNs in L. olgensis induced by caffeic acid. Through verification tests, we concluded that the minimal fatalities observed in L. olgensis could be attributed to the reduced overwintering survival of PWNs, a consequence of caffeic acid‐induced stunting. This study provides valuable insights into PWN–host interactions and suggests that targeting caffeic acid biosynthesis pathways could be a potential strategy for managing PWN in forest ecosystems.
Keywords: Bursaphelenchus xylophilus, caffeic acid, Larix olgensis, lipid, overwintering survival
The pine wood nematode (PWN) invasion in north‐east China devastated Pinus koraiensis, but Larix olgensis showed resilience due to its high levels of caffeic acid, which disrupt PWN lipid metabolism and hinder PWN winter survival.
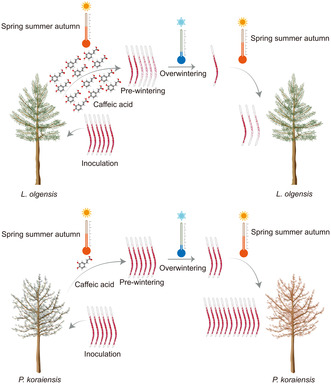
1. INTRODUCTION
The pine wood nematode (Bursaphelenchus xylophilus; PWN), responsible for pine wilt disease (PWD), has inflicted considerable damage on conifer forests globally (Futai, 2013; Jones et al., 2013). PWNs have been documented in various regions, including Asia, North America, and Europe, and the impact of PWNs on local coniferous ecosystems has been significant (Mamiya, 1988; Mota et al., 1999; Robertson et al., 2011; Ye, 2019). Notably, the severity of PWN infection in Asia surpasses that in North America, highlighting a geographical discrepancy in disease impact (Bergdahl, 1988; Wingfield et al., 1982). The initial identification of PWNs in China was reported in 1982 (Cheng et al., 1983; Yu et al., 2011). By 2021, PWNs had affected approximately 1.72 million ha, resulting in the loss of over 14 million trees in China alone (Li et al., 2022). Recent announcements by the China State Forestry and Grassland Administration (no. 6 of 2022) indicate that PWNs have expanded their host range to include species such as Larix spp., Pinus koraiensis, and Pinus sylvestris var. mongolica, particularly in the Liaoning and Jilin provinces of north‐east China. These areas are known for their harsh winter conditions, with temperatures often reaching below −20°C. The emergence of PWNs in north‐east China has led to significant changes in host species, vector insects, and local ecosystems, complicating efforts to control the spread of PWNs. Despite over four decades of research into PWNs, the mechanisms of its overwintering in different hosts within the cold climates of north‐east China remain a burgeoning field of study. A comprehensive understanding of how PWNs survive low temperatures across various host species is critical for developing effective management strategies and mitigating the spread of PWNs.
In north‐east China, the cold climate significantly shortens the reproductive period for PWNs, leading to most pine trees not exhibiting visible symptoms of infection until the subsequent year, a phenomenon known as ‘over‐year death’ (Futai, 2013; Zhang et al., 2022). Therefore, the ability of PWNs to overwinter and their survival rate directly determines the initial PWN population in pine trees the following year, which in turn affects whether the trees will exhibit symptoms in the subsequent year. Low temperatures can profoundly affect the physiological processes of organisms, including the collapse of ion homeostasis (Tomcala et al., 2006), protein denaturation (Bischof et al., 2002), and impaired membrane function (Drobnis et al., 1993). The transition of lipid structures into a more rigid gel form at low temperatures, which impairs membrane functions, can be mitigated by increased lipid unsaturation, lowering the temperature at which this transition occurs (Bai et al., 2021; Hayward et al., 2007; Liu et al., 2019; Savory et al., 2011). A key adaptation for PWN survival in low‐temperature environments is the development of third‐stage dispersal juveniles (DJ3), which are characterized by a significant increase in lipid content (Chen, Zhang, Li, & Wang, 2021; Chen, Zhang, Li, Wang, Jiang, & Wang, 2021; Mamiya, 1983; Zhao et al., 2020). The physiological responses to cold exposure in DJ3 of PWNs include an increase in unsaturated lipid and glycerol content (Liu et al., 2019). Research on Caenorhabditis elegans has demonstrated that the accumulation of cryoprotectant glycerol, resulting from lipid hydrolysis regulated by the cAMP‐protein kinase A (cAMP‐PKA) pathway, enhances tolerance to low temperatures (Liu et al., 2017), using lipids as a substrate for these physiological processes. Therefore, lipid accumulation is a vital strategy for PWNs to withstand low temperatures, serving as a crucial source of energy. In the context of C. elegans, for example, lipid reserves become a critical energy source after dauer formation, a stage during which the nematode ceases feeding (Riddle & Albert, 1997). This underscores the importance of lipids for nematode survival, especially under extreme conditions such as low temperatures.
Since the 1970s, afforestation and reforestation programmes have been actively implemented in China. Due to the expansion of the forest plantation area (from 12.7 × 106 ha in the late 1970s to 23.1 × 106 ha in 1994–1998), approximately 4 × 108 tonnes of carbon were sequestered (Fang et al., 2001). Larix forests, comprising fast‐growing tree species, were among the species promoted by government policies for reforestation (Zhang et al., 2000). Beyond their ecological benefit of carbon sequestration, Larix species are also significant timber resources in China. A previous study indicates that Larix species cover approximately 23% (9.2 × 106 ha) of the area dedicated to the five main afforestation types (Larix spp., Pinus tabuliformis, Pinus massoniana, Cunninghamia lanceolata, Populus spp.) in China. Furthermore, the stem volume of Larix species constituted 40% (872 × 106 m3) of the total stem volume of these five afforestation types (Zhao & Zhou, 2005). Larix species are widely distributed in north‐east China, with Liaoning Province alone accounting for 6.45 × 105 ha. Unfortunately, instances of mortality in Larix olgensis, Larix kaempferi, and Larix gmelinii var. principis‐rupprechtii due to PWN infection have been reported in Fushun, Liaoning, China (Yu et al., 2019).
Both L. olgensis and P. koraiensis have been reported to be naturally infected with PWNs in Liaoning, China (Yu et al., 2019; Yu & Wu, 2018). Consistent with the susceptibility observed in P. koraiensis in Korea (Han et al., 2008), P. koraiensis in Liaoning, China, exhibits a high susceptibility to PWNs (Zhang et al., 2022). Compared to P. koraiensis, L. olgensis showed a significantly lower probability of exhibiting symptoms under the same conditions, indicating a higher level of resistance in L. olgensis. It was discovered that the lipid content in PWNs isolated from L. olgensis was significantly lower than that in PWNs isolated from P. koraiensis. Moreover, PWNs with high lipid content are capable of surviving overwintering. Morphological, transcriptomic, and metabolomic along with in vitro validation tests revealed that the stunting and reduced lipid content in PWNs were induced by caffeic acid secreted by L. olgensis. This decreased the overwintering survival rate of PWNs in L. olgensis, further revealing the limited mortality in L. olgensis to PWN infection. This study enhances the understanding of the interaction mechanism between PWNs and L. olgensis in the middle temperate zone. It lays a crucial groundwork for developing strategies aimed at breeding and selecting PWN‐resistant varieties, potentially curbing the spread of PWNs in low‐temperate regions.
2. RESULTS
2.1. The overwintering survival rate of PWNs in resistant L. olgensis was found to be low
Feeding marks attributed to Monochamus saltuarius, the insect vector for PWNs, were observed on both L. olgensis and P. koraiensis, indicating its ability to feed on both species. Nevertheless, the likelihood of the two species exhibiting symptoms of PWD differed significantly. Of the P. koraiensis trees, 33.6% exhibited symptoms, with needles turning chlorotic or reddish‐brown (Figure 1a,b). However, no L. olgensis trees in the same sampling plot displayed symptoms. This suggests that L. olgensis exhibits resistance to PWNs when infected naturally through transmission by M. saltuarius.
FIGURE 1.
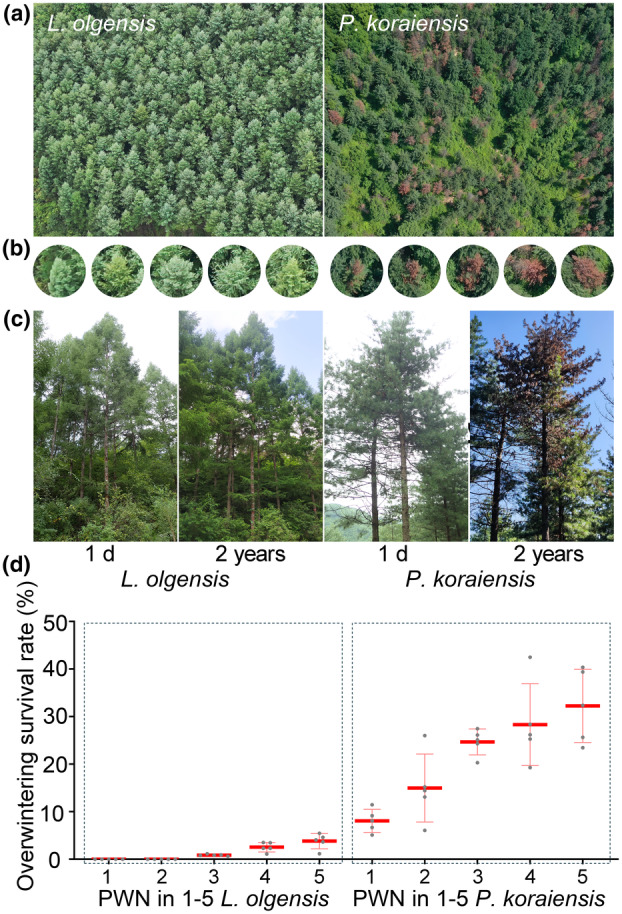
Resistance of Larix olgensis to pine wood nematodes (PWNs) in natural infection and inoculation scenarios. (a) Aerial view depicting PWN‐infected L. olgensis and Pinus koraiensis. (b) Close‐up of individual L. olgensis and P. koraiensis under natural PWN infection. (c) Assessment of L. olgensis and P. koraiensis 1 day and 2 years after artificial PWN inoculation. (d) Overwintering survival rates of PWNs in one to five individuals of L. olgensis and P. koraiensis.
The evaluation of host resistance to PWNs was carried out by artificially inoculating 30‐year‐old L. olgensis and P. koraiensis trees. The results indicated that L. olgensis exhibited resistance to PWNs (Figure 1c). Two years after inoculation, 58.3% of the P. koraiensis trees showed symptoms, with needles turning yellow or reddish‐brown. In contrast, the needles of the 70 inoculated L. olgensis trees remained green without any noticeable symptoms throughout the observation period. These findings suggest that mature L. olgensis trees exhibit resistance to PWNs when subjected to artificial inoculation.
To elucidate the correlation between host resistance and the overwintering survival rate of PWNs, PWNs from different hosts were quantitatively analysed. Significant differences in the overwintering survival rates of PWNs between L. olgensis and P. koraiensis were observed (Figure 1d). Specifically, the average overwintering survival rate of PWNs in L. olgensis was 1.5%, compared to 21.7% in P. koraiensis (p < 0.01). These findings suggest that L. olgensis presents a challenging environment for PWN proliferation.
2.2. Stunted PWNs were observed during prewintering in L. olgensis
The impact of the challenging environment in L. olgensis on PWN proliferation was investigated through morphological measurements (Table S1, Figure 2). PWNs isolated from P. koraiensis were the control group (CK) for the phytophagous phase, and PWNs isolated from Botrytis cinerea were the CK for the mycophagous phase. The body sizes of fourth‐stage propagative juveniles (J4), DJ3, male, and female nematodes isolated from L. olgensis were significantly smaller compared to those from P. koraiensis and B. cinerea (Figure 2a). The body length (L), maximum body width (MW), and median bulb width (MBW) of PWNs were measured. The L and MW for J4, DJ3, male, and female nematodes from L. olgensis were smaller than those of PWNs at equivalent stages from P. koraiensis and B. cinerea (Figure 2b). No significant differences in L or MW were detected at the second‐stage propagative juvenile (J2) or third‐stage propagative juvenile (J3) level under various culture conditions. Consistently, the MBW did not significantly vary among the different culture conditions (Figure 2b). These results indicate that PWN growth was hindered in L. olgensis compared with P. koraiensis and B. cinerea.
FIGURE 2.
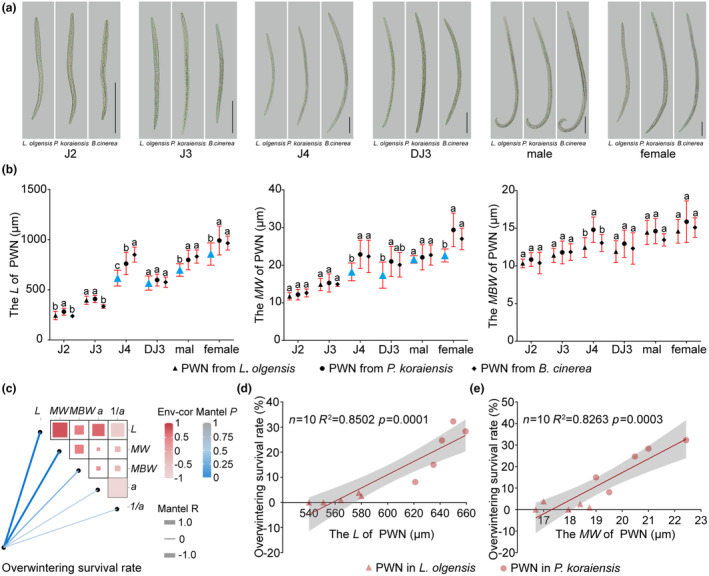
Growth inhibition of pine wood nematodes (PWNs) in Larix olgensis prewintering. (a) Microscopic examination of J2, J3, J4, DJ3, male, and female nematodes extracted from L. olgensis and Pinus koraiensis prewintering samples, with PWN from Botrytis cinerea as the control (CK) for mycophagous phase. Scale bars represent 100 μm. (b) Comparison of length (L), maximum body width (MW), and median bulb width (MBW) of prewintering PWN from L. olgensis, P. koraiensis, and B. cinerea. (c) Correlation analysis between overwintering survival rates and morphometric parameters, as well as correlations among different morphometric measurements. (d) Correlation analysis between overwintering survival rates and L. (e) Correlation analysis between overwintering survival rates and MW.
Correlation analysis demonstrated that L and MW of prewintering PWN were positively correlated with the PWN overwintering survival rate (Figure 2c), with correlation coefficients of 0.85 and 0.83, respectively (Figure 2d,e,p <0.05). The correlation coefficient between the MBW and the overwintering survival rate was 0.45 (p < 0.05). The parameters a (L/MW) and 1/a (MW/L) were calculated from L and MW. No significant correlation was detected between a and the overwintering survival rate, or between 1/a and the overwintering survival rate. These results suggest that the observed stunted growth of PWNs was associated with a lower overwintering survival rate.
2.3. The expression of genes involved in lipid degradation in PWNs from L. olgensis was promoted
Transcriptomic analyses were conducted to explore the causes behind differences in the L and MW of PWNs isolated from L. olgensis and P. koraiensis. The analyses revealed that 486 genes were upregulated and 1957 genes were downregulated in PWNs from L. olgensis compared to those from P. koraiensis. Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analysis was performed on differentially expressed genes (DEGs) (Figure 3a). The pathways identified were classified into metabolism, genetic information processing, environmental information processing, cellular processes, and organismal systems. Within the metabolism category, lipid metabolism emerged as the secondary classification with the largest number of genes, in addition to global and overview maps. This finding highlighted the importance of focusing on lipid metabolism in our study.
FIGURE 3.
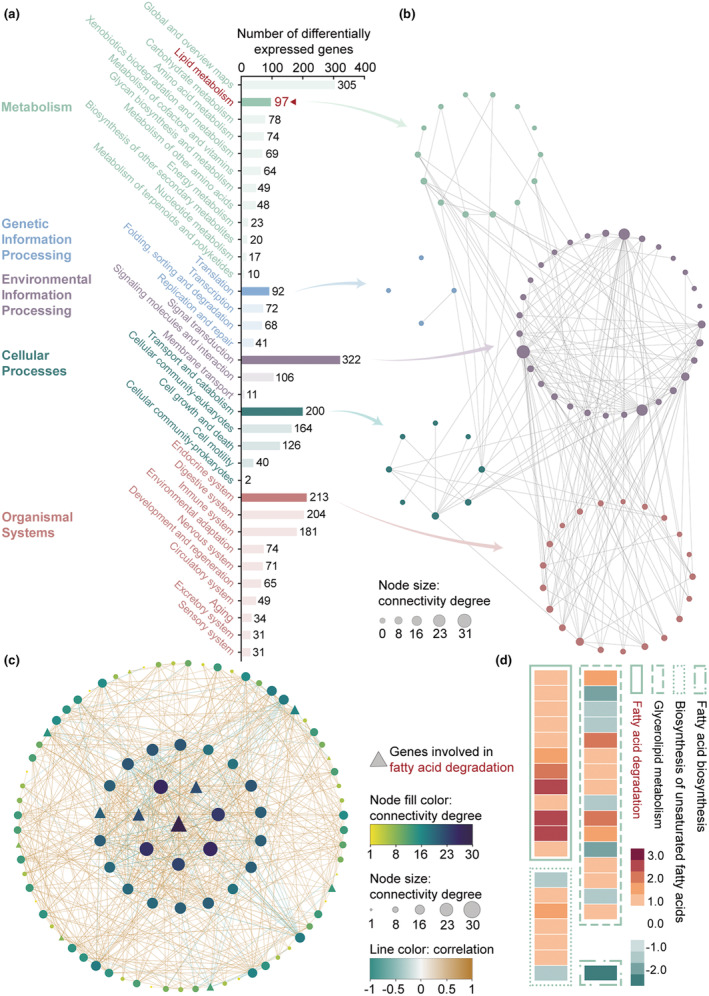
Gene expression involved in lipid degradation of pine wood nematodes (PWNs) from Larix olgensis is promoted. (a) KEGG enrichment analysis of differentially expressed genes (DEGs) in PWNs from L. olgensis relative to PWNs from Pinus koraiensis. In metabolism, in addition to global and overview maps, lipid metabolism has the largest number of genes. (b) The pathway connection analysis. The secondary classifications with the largest number of genes in each primary classification are selected for pathway connection analysis. The nodes represent KEGG pathways, and the node size represents the connectivity degree. (c) Network for the genes enriched in lipid metabolism. The central position triangle node is the gene with the highest node degree score, which is regarded as the hub gene. Three of the top eight genes in the connectivity degree score are involved in fatty acid degradation (the triangle nodes). (d) Expression of genes involved in fatty acid degradation, glycerolipid metabolism, biosynthesis of unsaturated fatty acid, and fatty acid biosynthesis. Genes involved in fatty acid degradation (lipid degradation) are all upregulated. Glycerolipid metabolism, biosynthesis of unsaturated fatty acid, and fatty acid biosynthesis involve lipid synthesis.
For each primary classification, the secondary classification containing the largest number of genes was selected for pathway connection analysis (Figure 3b). In the signal transduction category, pathways associated with lipid metabolism were identified, such as the mTOR signalling pathway (ko04150), the cAMP signalling pathway (ko04024), and the AMPK signalling pathway (ko04152), which have been validated by previous studies. Additionally, lipid metabolism was connected to the PPAR signalling pathway (ko03320) and other hormone‐related pathways within the organismal systems category. These findings suggest that lipid metabolism in PWNs from L. olgensis is influenced by regulation of the aforementioned signalling pathways.
The correlations among 97 lipid metabolism‐related genes were analysed, leading to the exclusion of two genes due to their low correlation with the others (Figure 3c). Among the 95 remaining genes, BXY_0492900, which is represented by the central triangle in Figure 3c, was identified as a hub gene with the highest connectivity degree, involved in fatty acid degradation (ko00071). Of the top eight genes in terms of connectivity degree, three were associated with fatty acid degradation (indicated by triangles). In addition, all the genes involved in fatty acid degradation were significantly upregulated (Figure 3d). Lipid degradation primarily entails the hydrolysis of triglycerides to glycerol and fatty acids, with the fatty acids then being broken down to generate energy (Zechner et al., 2012). The upregulation of genes associated with fatty acid degradation indicates the modifications in lipid metabolism in PWNs from L. olgensis; specifically, these findings suggest a promotion in lipid degradation. The expression of genes related to lipid synthesis, including those involved in glycerolipid metabolism (ko00561), biosynthesis of unsaturated fatty acids (ko01040), and fatty acid biosynthesis (ko00061), was examined. Among the 24 genes involved in lipid synthesis, nine were found to be downregulated, while 15 were upregulated. These upregulated genes associated with lipid synthesis could be induced by lipid degradation.
2.4. Low lipid content stunted the growth and impaired the survival of PWNs
Lipid staining of PWNs under different culture conditions revealed significant differences. Specifically, prewintering PWNs isolated from P. koraiensis exhibited extensive red lipid droplets, similar to PWNs cultured on B. cinerea. In contrast, prewintering PWNs isolated from L. olgensis exhibited noticeably fewer lipid droplets. Furthermore, PWNs isolated from L. olgensis consistently showed a reduced lipid content (Figure 4a,b).
FIGURE 4.
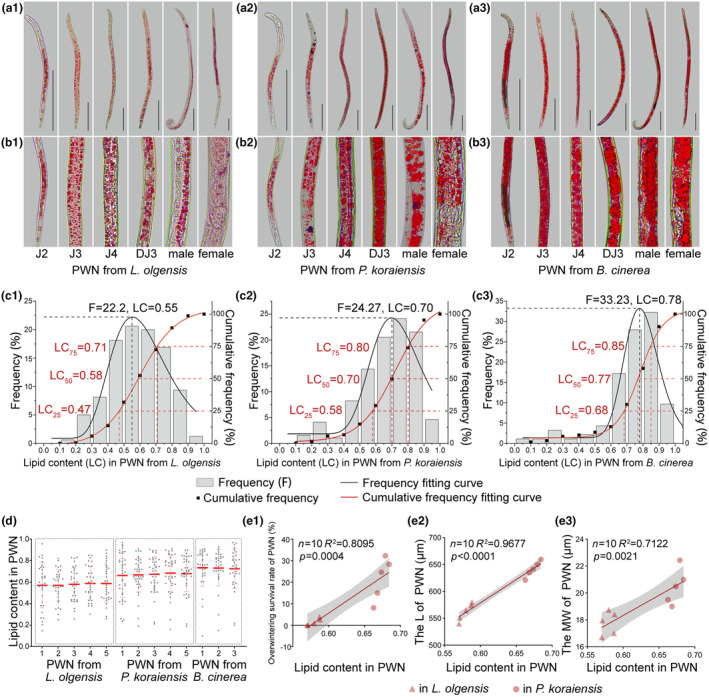
Low lipid content stunts the growth of pine wood nematodes (PWNs) isolated from Larix olgensis prewintering. (a) Lipid staining of PWNs cultured from L. olgensis, Pinus koraiensis or Botrytis cinerea. (b) Magnified view of lipid staining in PWNs cultured under different conditions. (c) Frequency distribution histogram and cumulative frequency curve illustrating the variation in lipid content (LC) of PWNs cultured under different conditions. The grey bars represent frequency (left y‐axis), while the black points represent cumulative frequency (right y‐axis). The red line represents the frequency fitting curve, and the black line represents the cumulative frequency fitting curve. (d) Quantification of lipid content in PWNs cultured under different conditions. (e) Correlation analyses between lipid content and overwintering survival rate, length (L), and maximum body width (MW).
The distribution of the lipid content in PWNs varied significantly with host species, with notably lower levels observed in populations in L. olgensis (Figure 4c). A total of 75.6% of the lipid content in PWNs from L. olgensis was distributed between 0.4 and 0.8. However, 75.9% of the lipid content in PWN from P. koraiensis was distributed between 0.5 and 0.9. A total of 77.4% of the lipid content in PWNs from B. cinerea was distributed between 0.6 and 0.9. The fitting results indicated that at the maximum frequency, the lipid content in PWNs from L. olgensis was 0.55, which was less than that in the PWNs from P. koraiensis (0.70) and B. cinerea (0.78). These results indicate that the lipid content in PWNs from L. olgensis was centred around 0.55, whereas the lipid content in PWNs from P. koraiensis and B. cinerea was centred around 0.70 and 0.78, respectively.
The cumulative frequency of lipid content in PWNs from different hosts was analysed (Figure 4c). The analysis separated the percentage of PWNs falling below specific lipid content thresholds, revealing a clear gradient in lipid content levels from L. olgensis to P. koraiensis and B. cinerea. A lower proportion of individuals with high lipid content was observed in PWNs from L. olgensis compared to those from other conditions. The cumulative frequency fitting curve further indicated that 75% of the PWNs from L. olgensis had a lipid content less than 0.71, while the lipid contents in PWNs from P. koraiensis and B. cinerea were 0.80 and 0.85, respectively. Fifty percent of PWNs from L. olgensis had lipid contents less than 0.58, whereas the lipid contents in PWNs from P. koraiensis and B. cinerea were 0.70 and 0.77, respectively. Twenty‐five percent of PWNs from L. olgensis had lipid contents less than 0.47, whereas the lipid contents in PWNs from P. koraiensis and B. cinerea were 0.58 and 0.68, respectively (Figure 4c). PWNs isolated from L. olgensis exhibited a distinct trait, with a low proportion (26.2%) of high‐lipid individuals (lipid content above 0.70), differing significantly from the PWNs from P. koraiensis (>50.0%) or B. cinerea (>69.2%).
The lipid content in PWNs isolated from L. olgensis (0.58) was lower than that in PWNs from P. koraiensis (0.67) and B. cinerea (0.73, Figure 4d). This disparity suggests an insufficient lipid content in prewintering PWNs from L. olgensis. Further analysis revealed a significant positive correlation between the overwintering survival rate of PWNs and lipid content (R 2 = 0.8095, p = 0.0004). Additionally, both the L and MW of the PWNs were positively correlated with lipid content (R 2 = 0.97, p < 0.0001, and R 2 = 0.71, p = 0.002, respectively). These correlations indicate that PWNs isolated from L. olgensis experienced stunted growth due to their low lipid content (Figure 4e), which adversely affected their survival through winter.
2.5. Caffeic acid levels in L. olgensis increased significantly following PWN inoculation
To investigate the cause of the reduced lipid content in PWNs from L. olgensis, the metabolite profiles of L. olgensis and P. koraiensis were compared. In L. olgensis, 135 metabolites were upregulated at 15 days post‐inoculation (dpi), and 203 metabolites were upregulated at 30 dpi. In contrast, fewer metabolites were upregulated in P. koraiensis, with 74 at 15 dpi and 153 at 30 dpi. Thirty‐eight metabolites were upregulated in L. olgensis but downregulated or unchanged in P. koraiensis at both 15 and 30 dpi (Figure 5a–c).
FIGURE 5.
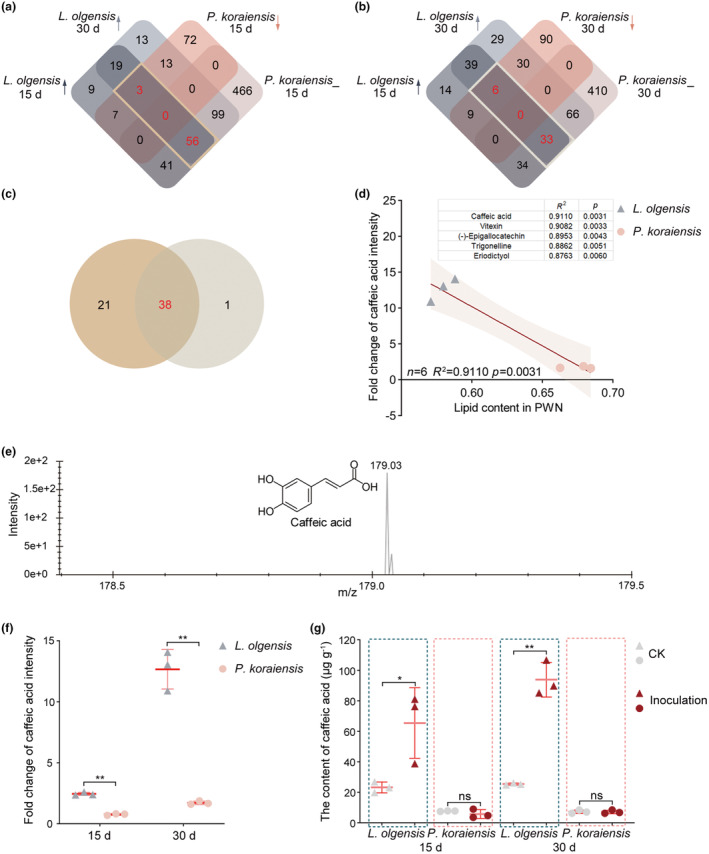
Upregulation of caffeic acid in Larix olgensis following pine wood nematode (PWN) inoculation. (a) Intersection of metabolite lists upregulated in L. olgensis at 15 and 30 days post‐inoculation (dpi) and downregulated or unchanged in Pinus koraiensis at 15 dpi. (b) Intersection of metabolite lists upregulated in L. olgensis at 15 and 30 dpi and downregulated or unchanged in P. koraiensis at 30 dpi. (c) Differential metabolite screening of upregulated metabolites in L. olgensis while downregulated or unchanged in P. koraiensis at both 15 and 30 dpi after PWN inoculation. (d) Correlation analysis between lipid content in PWN and fold change of metabolite intensity at corresponding time. Top five metabolites selected from 38 screened metabolites for exhibition. (e) Peak diagram and structural formula of caffeic acid. (f) Fold change of caffeic acid intensity determined in L. olgensis and P. koraiensis at 15 and 30 dpi using untargeted metabolomics. (g) HPLC analysis determining the caffeic acid content in L. olgensis and P. koraiensis at 15 and 30 dpi after PWN inoculation. CK, not inoculated.
Of the 38 differentially expressed metabolites, the five most strongly correlated with lipid content are shown in Figure 5d. Caffeic acid (3,4‐dihydroxycinnamic acid) was identified as having the greatest correlation with PWN lipid content (R 2 = 0.91, p = 0.003). The peak diagram and the structural formula of caffeic acid are shown in Figure 5e. In addition, the results showed that the fold change in caffeic acid intensity in L. olgensis increased over the inoculation period and was greater than that in P. koraiensis at both 15 and 30 dpi (Figure 5f). HPLC analyses confirmed a significant increase in caffeic acid in L. olgensis at both 15 and 30 dpi after PWN inoculation (Figure 5g, Figure S1). In contrast, P. koraiensis did not exhibit a significant change in caffeic acid content after inoculation. At 15 dpi, the mean caffeic acid content in L. olgensis was 65.45 μg/g, significantly greater than the 5.75 μg/g observed in P. koraiensis. By 30 dpi, the mean caffeic acid content in L. olgensis rose to 93.93 μg/g, compared to 7.14 μg/g in P. koraiensis.
The significant increase in caffeic acid after PWN inoculation suggests a possible role in the immune response of L. olgensis against PWN, which may contribute to the observed reduction in PWN lipid content. The inhibitory impact of caffeic acid on PWN lipid metabolism was further substantiated.
2.6. Greater caffeic acid concentration linked to reduced lipid content in PWNs
Validation experiments demonstrated that PWNs treated with caffeic acid displayed fewer lipid droplets compared to the negative control (CK, 0 μg/mL) (Figure 6a–d). The lipid content in PWNs decreased as the concentration of caffeic acid increased (Figure 6e). No significant difference in lipid content was observed between PWNs treated with caffeic acid at concentrations below 25 μg/mL (10 and 25 μg/mL) and the CK. However, PWNs treated with concentrations of caffeic acid exceeding 25 μg/mL (50, 75, and 100 μg/mL) had significantly lower lipid contents compared to the CK. Correlation analysis revealed a negative correlation between the lipid content in PWNs and the concentration of caffeic acid (R 2 = 0.55, p < 0.0001), indicating that greater concentrations of caffeic acid reduce the lipid content of PWN.
FIGURE 6.
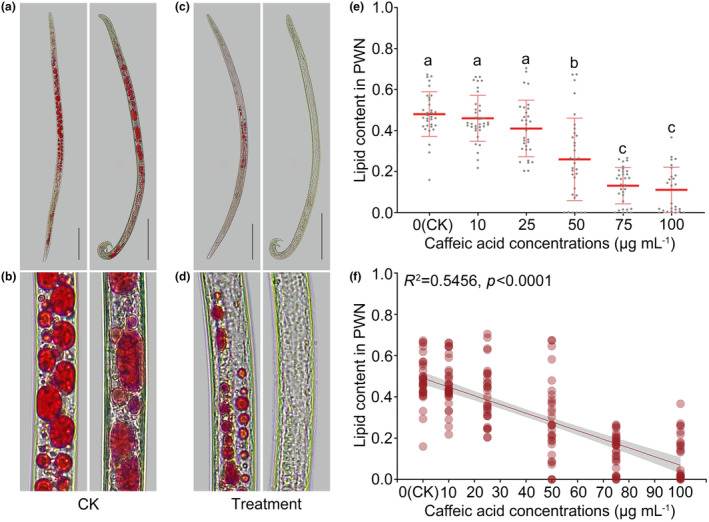
Greater concentration of caffeic acid reduces the lipid content of pine wood nematodes (PWNs). (a) Lipid staining of control (CK) treated with 0 μg/mL caffeic acid. (b) Magnified view of lipid staining in the CK treated with 0 μg/mL caffeic acid. (c) Lipid staining of PWNs treated with 100 μg/mL caffeic acid. (d) Magnified view of lipid staining in PWNs treated with 100 μg/mL caffeic acid. (e) Quantification of lipid content in PWNs treated with varying concentrations of caffeic acid. (f) Correlation analysis between lipid content in PWNs and the concentration of caffeic acid treatment. Scale bars represent 100 μm.
2.7. Low lipid content impairs PWN survival at low temperatures
PWNs that had decreased lipid content after caffeic acid treatment were exposed to low‐temperature conditions (8°C) to evaluate their survival rate. The survival rate of PWNs decreased as the concentration of caffeic acid increased (Figure 7a). Correlation analyses revealed a negative correlation between the survival rate of PWNs at low temperatures and the concentration of caffeic acid (R 2 = 0.60, p = 0.0001, Figure 7b). Additionally, there was a positive correlation between the survival rate of PWNs at low temperatures and their lipid content (R 2 = 0.55, p = 0.0004, Figure 7c). These findings suggest that greater concentrations of caffeic acid decrease PWNs' survival under low‐temperature conditions by reducing their lipid content.
FIGURE 7.
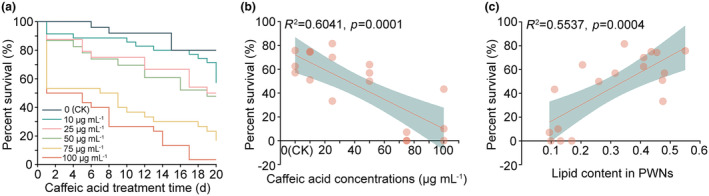
Caffeic acid reduced the survival of pine wood nematodes (PWNs) at low temperature by reducing lipid content. (a) Survival curve of PWNs treated with 0 (CK), 10, 25, 50, 75, and 100 μg/mL of caffeic acid under low temperature. (b) Correlation analysis between PWN survival rate under low temperature and concentrations of caffeic acid treatment. (c) Correlation analysis between PWN survival rate under low temperature and lipid content in PWNs.
3. DISCUSSION
3.1. Caffeic acid as a game changer: Lipid metabolism and PWN survival
The profound impact of the PWN on Pinaceae species, particularly P. koraiensis, along with the notable resilience of L. olgensis, provides insights into the intricate interactions between invasive pests and hosts. Our study uses a multidisciplinary approach, incorporating morphological, transcriptomic, and metabolomic analyses, to investigate the intricate dynamics of interactions between the PWNs and hosts.
In north‐east China, PWNs face formidable challenges due to the harsh climate. Firstly, exacerbated by the harsh climate, the effective reproductive period of the PWN is shortened, significantly curbing the population growth of the PWN. This explains why PWNs can cause the death of P. massoniana and P. thunbergii in warm southern China within a year, but not P. koraiensis in north‐east China. Secondly, the harsh winter in north‐east China can significantly reduce the PWN population. Despite the natural suppression by the harsh climate in north‐east China, P. koraiensis is still declining annually due to PWN infection. Notably, only a negligible number of L. olgensis in these forests were visibly affected by the PWN, despite being potential hosts. How the resilience of L. olgensis combined with the natural suppression by the harsh climate works together to resist PWNs posed a scientific puzzle.
Our research aimed to unravel this mystery through a rigorous 4‐year inoculation trial involving 180 mature Pinaceae trees that were 30 years old. We discovered that L. olgensis has evolved from being a passive victim to an active defender against PWNs, primarily by secreting high levels of caffeic acid (Figure 8). This chemical defence mechanism disrupts the lipid metabolism of PWNs, severely stunting their growth and impairing their ability to survive the harsh winter.
FIGURE 8.
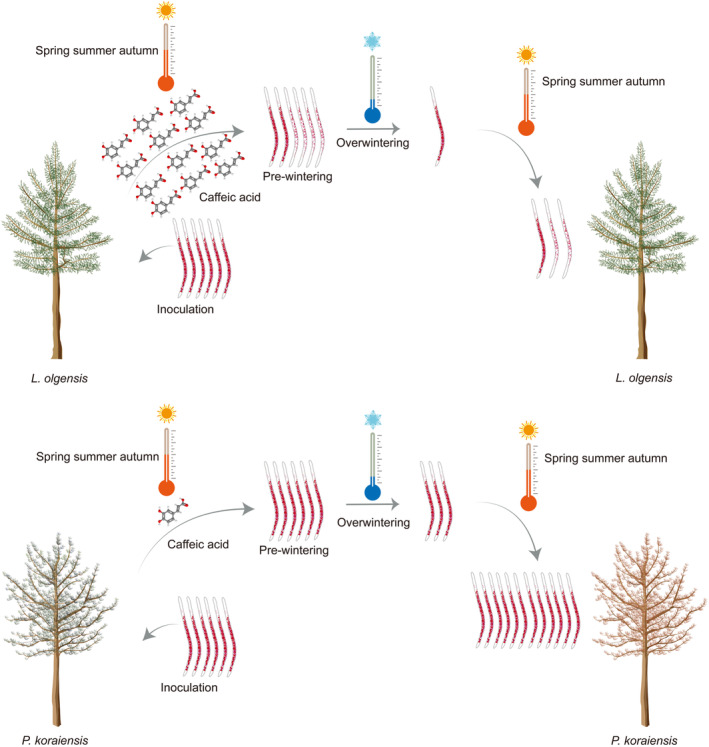
Mechanism of the limited mortality in Larix olgensis after pine wood nematode (PWN) infection. High levels of caffeic acid in L. olgensis significantly diminish the overwintering survival of the PWNs by decreasing their lipid content, correlating with the limited mortality observed in L. olgensis following PWN infection. In contrast, Pinus koraiensis does not produce sufficient caffeic acid, which fails to diminish PWN overwintering survival through disruption of lipid metabolism. Consequently, the PWN population in P. koraiensis reaches a critical threshold more quickly, leading to increased mortality in the subsequent year.
Further insights were also gained from a comprehensive 5‐year field survey (natural infection), confirming that mortality due to PWN infection was not observed in the surveyed L. olgensis. These findings underscore the effectiveness of the L. olgensis's unique biochemical strategy. Caffeic acid‐induced stunting significantly reduced the overwintering survival rate of PWNs in L. olgensis, providing insight into the limited susceptibility of L. olgensis to PWN infection. In contrast, the neighbouring P. koraiensis trees have suffered extensively due to their inability to produce sufficient caffeic acid to thwart the PWN. This deficiency allows the PWNs to flourish within P. koraiensis, often reaching a lethal population threshold within just 1–2 years.
This study represents a significant milestone in the large‐scale inoculation of 30‐year‐old Pinaceae trees, a task made challenging by quarantine regulations and the substantial resources required to manage pathogens. Conducting field trials involving 180 trees was intricate, necessitating meticulous control measures to mitigate potential ecological harm. Despite these challenges, the 4‐year experiment was successfully concluded without causing proliferation or ecological disasters, thereby providing novel insights into the behaviour of PWNs and setting a standard for future research in forest pathology under stringent quarantine constraints.
This study not only highlights the stark differences in species resilience against the PWN but also underscores the potential of leveraging naturally occurring chemical defences as a strategy for managing PWNs. By harnessing the L. olgensis's innate resistance mechanism, more effective and ecologically friendly strategies can be developed to protect vulnerable pine species, ultimately preserving forest health and biodiversity.
3.2. The role of lipids in cold tolerance and overwintering survival of PWNs
Lipids play crucial roles in energy storage and thermogenesis and serve as the primary regulators of energy balance and nutritional homeostasis (Rosen & Spiegelman, 2014). Studies have demonstrated larger individuals of insects (within a species) with larger lipid storage can survive for longer (Sinclair & Marshall, 2018), which was also confirmed in our research. The L and MW of PWN were positively correlated with their overwintering survival rate. Larger PWNs from P. koraiensis, which had larger lipid storage, exhibited stronger cold tolerance. In contrast, it was difficult for PWNs in L. olgensis to survive in winter. Lipids confer cold tolerance to nematodes through three main mechanisms. The first function is used to synthesize unsaturated fatty acids (Lee et al., 2019; Svensk et al., 2013). The second function is as substrates for the formation of cryoprotectants (Liu et al., 2017). The third function is to provide energy for organisms. Lipid content is essential not only for individual survival but also for species reproduction. The fat‐5 fat‐6 double mutants of C. elegans exhibit reduced survival in the absence of food. The fat‐6 fat‐7 double mutants have decreased lipid content, exhibiting slow growth and reduced fertility (Brock et al., 2007). These findings reinforce our results that PWNs with decreased lipid content and stunted growth struggled to survive and reproduce in L. olgensis over an extended period.
The persistence of many populations has been linked to the success of overwintering (Lynch et al., 2014), and lipids are a key component of overwintering success and post‐winter fitness (Sinclair, 2015), both of which were further confirmed in combination in this study. Due to reduced lipid content, there was a significant decrease in the population amount during each overwintering. Reductions in PWN populations may make it difficult for the PWN population to reproduce to the threshold that causes L. olgensis to show symptoms.
3.3. The host strategy: L. olgensis caffeic acid and lipid reduction in PWNs
Plants have exploited their metabolic systems to produce natural chemicals to adapt to challenging ecosystems (Weng, 2014). Plant polyphenols not only possess direct antimicrobial properties (Bhattacharya et al., 2010; Polturak et al., 2023) but also reduce lipid content (Nwakiban Atchan et al., 2022) by inhibiting triglyceride accumulation, stimulating lipolysis, and fatty acid β‐oxidation (Cao et al., 2022). Caffeic acid is a polyphenol (Cao et al., 2022) that suppresses lipid accumulation by promoting lipolysis and β‐oxidation (Kong et al., 2022). Phosphorylation of AMPK increases after caffeic acid treatment, leading to promotion of lipolysis. Furthermore, caffeic acid reduces triglyceride content and inhibits lipid synthase activities via sterol regulatory element‐binding protein 1c (Liao et al., 2013, 2014). Our study found that higher caffeic acid content in L. olgensis reduced the lipid content in PWNs. It was found that the caffeic acid content in L. olgensis increased with the time of interaction with PWNs, which was the host strategy to cope with PWN infection. The effect of P. koraiensis on reducing lipid content in PWNs was not significant, one reason being that the caffeic acid content in P. koraiensis was deficient. In the in vitro validation test, no significant difference in lipid content was observed between PWNs treated with 10 μg/mL of caffeic acid (similar to the content of caffeic acid in P. koraiensis) and the CK (0 μg/mL, Figure 6e). PWNs treated with 75 and 100 μg/mL of caffeic acid (similar to the content of caffeic acid in L. olgensis) had significantly lower lipid content compared to those treated with 10 μg/mL of caffeic acid and the CK (0 μg/mL). At present, the mechanism by which caffeic acid reduces lipid content in PWN needs to be further clarified. Based on the transcriptome results, caffeic acid reduced lipid content in PWNs by promoting fatty acid degradation. The mTOR, cAMP, AMPK, and PPAR signalling pathways were potentially involved in the regulation of caffeic acid‐promoted fatty acid degradation in PWNs from L. olgensis. Our upcoming research will focus on investigating the regulatory mechanism of fatty acid degradation by caffeic acid in PWNs and exploring the practical application of caffeic acid in screening for resistance breeding.
It should be emphasized that, based on five biological replicates, no significant mortality was observed in PWNs treated with caffeic acid (100 μg/mL) for 24 h at 25°C. Additionally, downregulated metabolites in L. olgensis were screened (Tables S2 and S3), including procyanidin C1, neryl rhamnosyl‐glucoside, and procyanidin B2. The downregulated metabolites were found to be predominantly flavonoids. Flavonoids accounted for 30.6% of the downregulated metabolites at 15 dpi and 32.3% at 30 dpi. Whether the downregulation of these metabolites contributes to L. olgensis resistance and the mechanisms involved need to be further investigated.
4. EXPERIMENTAL PROCEDURES
4.1. Investigation of the resistance of different hosts to PWN
Resistance of L. olgensis and P. koraiensis to PWNs was investigated under natural infection. The experimental site was selected as Dahuofang Forest Farm in Fushun, Liaoning, China. The plots of L. olgensis and P. koraiensis are located in adjacent subcompartments of the same compartment. The distance between the two plots is within the flight capacity of M. saltuarius. The probability of showing symptoms in the two plots was calculated according to formula (1):
| (1) |
where p is the probability of showing symptoms of hosts, A S is the amount of hosts showing symptoms, and A t is the total amount of hosts.
Resistances of the two hosts to PWNs were further investigated under artificial inoculation. PWNs were collected from diseased P. koraiensis in Fushun and cultured on the fungus B. cinerea at 25°C in an incubator without light. The selected inoculation plots are situated at an elevation of 220–300 m, with an eastward orientation in Dahuofang Forest Farm (41°59′ N, 124°17′ E). The first inoculation assay was conducted in August–September 2020, simulating infection by M. saltuarius during its autumn emergence. PWNs were inoculated into 70 healthy 30‐year‐old L. olgensis and P. koraiensis originating from the plantation forests. The selected trees appeared healthy, no PWNs were isolated, and no DNA amplification of PWNs was detected by molecular detection (Figures S2 and S3, Table S4). Ten wounds were drilled on each tree using an electric drill. Two hundred microlitres of distilled water containing 5000 PWNs (a mixture of PWNs with the ratio of female to male to juvenile approximately 1:1:2) was pipetted into each wound and sealed for a total of 50,000 PWNs per tree. The success of inoculation was determined by isolating PWNs from inoculated trees at 7 dpi. Twenty healthy 30‐year‐old L. olgensis and P. koraiensis trees were treated with distilled water as negative controls. The symptoms of both hosts were monitored, and the probability of showing symptoms after PWN inoculation was calculated according to Equation (1). A second inoculation assay was conducted in June 2021, simulating infection by M. saltuarius during its summer emergence to verify consistency. The two inoculation assays yielded consistent results.
Prewintering and overwintering, PWNs in inoculated L. olgensis and P. koraiensis were isolated to calculate the PWN overwintering survival rate. Prewintering period was estimated to be 16–20 October, overwintering period was estimated to be 11–15 April based on the 2015–2019 Fushun temperatures data (Figure S4). As illustrated in Figure S5, the wooden discs of varying parts from inoculated trees were collected. The PWNs in wooden discs were isolated using a Baermann funnel. The overwintering survival rate was calculated after counting the amount of PWNs. Equation (2) for calculating the overwintering survival rate is as follows:
| (2) |
where r is the overwintering survival rate, N p is the amount of PWNs prewintering, and N o is the amount of PWNs after overwintering.
4.2. Comparison of PWN morphology from different hosts
Prewintering PWNs were isolated from L. olgensis and P. koraiensis. PWNs isolated from B. cinerea were the CK for the mycophagous phase. Morphometric measurements were performed on J2, J3, J4, DJ3, male, and female nematodes. The L, MW, and MBW of PWNs were measured using a BX51 microscope (Olympus) with an ocular micrometer and converted to actual lengths. PWNs were photographed using the BX51 microscope. Parameter a (L/MW) and 1/a (MW/L) were calculated through L and MW.
4.3. Gene expression differences in PWNs from different hosts
PWNs from five L. olgensis individuals and five P. koraiensis individuals were isolated prewintering. The wooden strips of the sample were split extremely finely to rapidly isolate the PWNs. Total RNA of PWNs was extracted using TRIzol (Invitrogen). The RNA quality was evaluated by agarose gel electrophoresis and a NanoDrop spectrophotometer (Thermo Scientific). Transcriptome sequencing was performed according to the BGISEQ‐500 standard protocol (BGI). Raw data were filtered using SOAPnuke. Clean reads were then mapped to reference sequences using HISAT2.
DEGs were screened on the basis of log2(PWN L. olgensis /PWN P. koraiensis ) > 1 (false discovery rate [FDR] < 0.05, upregulated) or log2(PWN L. olgensis /PWN P. koraiensis ) < −1 (FDR <0.05, downregulated). KEGG enrichment analysis (https://www.kegg.jp/) was performed on the screened DEGs. In addition to metabolism, the secondary classification with the highest gene number was selected for pathway connection analysis. The pathway connection analysis was performed among lipid metabolism, translation, signal transduction, transport and catabolism, and endocrine system. Lipid metabolism gene connection analysis was performed based on gene correlations >0.7 or < −0.7, p < 0.05. The hub gene was screened through connectivity degree calculated by Cytoscape v. 3.9.1.
4.4. Lipid content determination of PWNs from different hosts
Lipids in nematodes are stored mainly as triglycerides and other neutral lipids. Therefore, we focused on the role of neutral lipids in this study. The Oil‐Red‐O staining method was employed to visualize the neutral lipid in PWNs isolated from L. olgensis and P. koraiensis prewintering (Leagene) (Chen, Zhang, Li, & Wang, 2021). PWNs isolated from B. cinerea were the control for the mycophagous phase. The stained PWNs were photographed using a BX51 microscope (Olympus). ImageJ was used to quantify the Oil‐Red‐O intensity.
4.5. Differential metabolite screening in different hosts
A liquid chromatography‐mass spectrometry (LC–MS) system was used for untargeted metabolomic analysis (Di Guida et al., 2016). We speculated that metabolites produced by L. olgensis in early defences would inhibit the PWNs, as indicated by the 15 and 30 dpi PWN amount results (data not shown). Therefore, the metabolite intensity of L. olgensis and P. koraiensis was measured at 15 and 30 dpi. Differential metabolite screening was based on inoculation/CK fold change >2 or <0.5 and p < 0.05 (Yang & Lv, 2022). The differential metabolites were screened, of which the intensity was upregulated in L. olgensis, with downregulated or no significant change in P. koraiensis at both 15 and 30 dpi after inoculation. The correlation between the fold change of metabolite intensity and lipid content was analysed by Graphpad Prism v. 10.1.2, and caffeic acid with the highest correlation was selected for further investigation. The caffeic acid content in L. olgensis and P. koraiensis at 15 and 30 dpi was further determined by HPLC.
4.6. Evaluating the effect of caffeic acid on reducing the lipid content in PWNs
Stock solutions of caffeic acid were prepared by dissolving it in dimethylsulphoxide (DMSO). Working solutions were obtained by diluting the stock solutions with M9 buffer. Treatments were performed in 48‐well polystyrene culture plates in the dark at 25°C and 53%–58% RH. Based on the HPLC analysis of the caffeic acid content in the trees, the concentration of the treatment was set at 10, 25, 50, 75, and 100 μg/mL. PWNs treated with the same concentration of DMSO (0.5% vol/vol) was the CK (0 μg/mL caffeic acid). Sixty PWNs per well were treated in 200 μL solution for 14 days. The pH of the treatment solutions was 6.77–6.82, and the pH of CK solution was 6.79–6.83. The pH of the treatments and CK solutions exhibited no significant differences and remained consistently near neutral. Three biological replicates were performed in the experiment. The staining and quantification of the PWN lipid content were conducted as described above.
4.7. Determination of the low‐temperature survival rate of PWNs after caffeic acid treatment
PWNs treated with 10, 25, 50, 75, and 100 μg/mL of caffeic acid were the treatments, and PWNs treated with DMSO (0.5% vol/vol) was the CK (0 μg/mL caffeic acid). Treatment was performed in 48‐well polystyrene culture plates in the dark at 25°C, 60 PWNs per well were treated for 14 days. After 14 days of treatment, PWNs were transferred to an incubator at 8°C. The survival rate was monitored daily for the next 20 days. Three biological replicates were performed.
4.8. Harmless treatment
All experiments involving PWNs in this study were conducted in Liaoning. All materials associated with PWNs underwent harmless treatment, and trees inoculated with PWNs were handled in compliance with regulatory requirements. No spread of PWNs was observed, and no ecological disturbances were reported, during ongoing field surveys and testing.
4.9. Data statistics and text editing
Graphpad Prism v. 10.1.2 was used to analyse the correlations. Origin was used to calculate the frequency. The Shapiro–Wilk test was used to test normality, and the Levene test was used to test the uniformity of variance. One‐way analysis of variance (ANOVA) or t test was used for data analyses. The LSD posterior was used to compare differences among the groups. DeepL Write was used to improve the readability.
Supporting information
Figure S1. HPLC chromatogram of caffeic acid in Larix olgensis and Pinus koraiensis at 15 and 30 days post‐inoculation (dpi) after pine wood nematode (PWN) inoculation. (a) HPLC chromatogram of caffeic acid in L. olgensis control (CK) at 15 dpi. (b) HPLC chromatogram of caffeic acid in L. olgensis at 15 dpi. (c) HPLC chromatogram of caffeic acid in P. koraiensis CK at 15 dpi. (d) HPLC chromatogram of caffeic acid in P. koraiensis at 15 dpi. (e) HPLC chromatogram of caffeic acid in L. olgensis CK at 30 dpi. (f) HPLC chromatogram of caffeic acid in L. olgensis at 30 dpi. (g) HPLC chromatogram of caffeic acid in P. koraiensis CK at 30 dpi. (h) HPLC chromatogram of caffeic acid in P. koraiensis at 30 dpi.
Figure S2. Quantitative PCR assay for pine wood nematode detection.
Figure S3. Quantitative PCR assay for pine wood nematode detection.
Figure S4. The 5‐year daily mean temperature from 2015 to 2019.
Figure S5. Sampling diagram.
Table S1. Morphology comparison of pine wood nematodes from different culture conditions.
Table S2. The five metabolites with the lowest fold change in intensity among the Larix olgensis downregulated metabolites at 15 days post‐inoculation.
Table S3. The five metabolites with the lowest fold change in intensity among the Larix olgensis downregulated metabolites at 30 days post‐inoculation.
Table S4. The primers used in the quantitative PCR assay for pine wood nematode detection.
ACKNOWLEDGEMENTS
This work was supported by the National Natural Science Foundation of China (31971656). The authors declare no competing interests.
Wang, J. , Chen, Q. , Xu, B. , Yu, Q. , Shen, Y. , Wu, H. et al. (2024) Caffeic acid: A game changer in pine wood nematode overwintering survival. Molecular Plant Pathology, 25, e70018. Available from: 10.1111/mpp.70018
DATA AVAILABILITY STATEMENT
The datasets are available in the NCBI repository, at https://www.ncbi.nlm.nih.gov/genbank/, with accession numbers SRR28675111 and SRR28675112; BioProject ID PRJNA1100174.
REFERENCES
- Bai, J. , Farias‐Pereira, R. , Jang, M. , Zhang, Y. , Lee, S.M. , Kim, Y.S. et al. (2021) Azelaic acid promotes Caenorhabditis elegans longevity at low temperature via an increase in fatty acid desaturation. Pharmaceutical Research, 38, 15–26. [DOI] [PubMed] [Google Scholar]
- Bergdahl, D.R. (1988) Impact of pinewood nematode in North America: present and future. Journal of Nematology, 20, 260–265. [PMC free article] [PubMed] [Google Scholar]
- Bhattacharya, A. , Sood, P. & Citovsky, V. (2010) The roles of plant phenolics in defence and communication during Agrobacterium and Rhizobium infection. Molecular Plant Pathology, 11, 705–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bischof, J.C. , Wolkers, W.F. , Tsvetkova, N.M. , Oliver, A.E. & Crowe, J.H. (2002) Lipid and protein changes due to freezing in Dunning AT‐1 cells. Cryobiology, 45, 22–32. [DOI] [PubMed] [Google Scholar]
- Brock, T.J. , Browse, J. & Watts, J.L. (2007) Fatty acid desaturation and the regulation of adiposity in Caenorhabditis elegans . Genetics, 176, 865–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao, Y. , Han, S. , Lu, H. , Luo, Y. , Guo, T. , Wu, Q. et al. (2022) Targeting mTOR signaling by dietary polyphenols in obesity prevention. Nutrients, 14, 5171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, Q. , Zhang, R. , Li, D. & Wang, F. (2021) Genetic characteristics of Bursaphelenchus xylophilus third‐stage dispersal juveniles. Scientific Reports, 11, 3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, Q. , Zhang, R. , Li, D. , Wang, F. , Jiang, S. & Wang, J. (2021) Trehalose in pine wood nematode participates in DJ3 formation and confers resistance to low‐temperature stress. BMC Genomics, 22, 524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng, H. , Lin, M. , Li, W. & Fang, Z. (1983) Pine wilt disease on black pine in Nanjing. Forest Pest and Disease, 4, 1–5. [Google Scholar]
- Di Guida, R. , Engel, J. , Allwood, J.W. , Weber, R.J. , Jones, M.R. , Sommer, U. et al. (2016) Non‐targeted UHPLC‐MS metabolomic data processing methods: a comparative investigation of normalisation, missing value imputation, transformation and scaling. Metabolomics, 12, 93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drobnis, E.Z. , Crowe, L.M. , Berger, T. , Anchordoguy, T.J. , Overstreet, J.W. & Crowe, J.H. (1993) Cold shock damage is due to lipid phase transitions in cell membranes: a demonstration using sperm as a model. Journal of Experimental Zoology, 265, 432–437. [DOI] [PubMed] [Google Scholar]
- Fang, J.Y. , Chen, A.P. , Peng, C.H. & Zhao, S.Q. (2001) Changes in forest biomass carbon storage in China between 1949 and 1998. Science, 292, 2320–2322. [DOI] [PubMed] [Google Scholar]
- Futai, K. (2013) Pine wood nematode, Bursaphelenchus xylophilus . Annual Review of Phytopathology, 51, 61–83. [DOI] [PubMed] [Google Scholar]
- Han, H. , Chung, Y.J. & Shin, S.C. (2008) First report of pine wilt disease on Pinus koraiensis in Korea. Plant Disease, 92, 1251. [DOI] [PubMed] [Google Scholar]
- Hayward, S.A. , Murray, P.A. , Gracey, A.Y. & Cossins, A.R. (2007) Beyond the lipid hypothesis: mechanisms underlying phenotypic plasticity in inducible cold tolerance. Advances in Experimental Medicine and Biology, 594, 132–142. [DOI] [PubMed] [Google Scholar]
- Jones, J.T. , Haegeman, A. , Danchin, E.G.J. , Gaur, H.S. , Johannes, H. , Jones, M.G.K. et al. (2013) Top 10 plant‐parasitic nematodes in molecular plant pathology. Molecular Plant Pathology, 14, 946–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong, L. , Zhang, W. , Liu, S. , Zhong, Z. & Zheng, G. (2022) Quercetin, engelitin and caffeic acid of Smilax china L. polyphenols, stimulate 3T3‐L1 adipocytes to brown‐like adipocytes via β3‐AR/AMPK signaling pathway. Plant Foods for Human Nutrition, 77, 529–537. [DOI] [PubMed] [Google Scholar]
- Lee, D. , An, S.W.A. , Jung, Y. , Yamaoka, Y. , Ryu, Y. , Goh, G.Y.S. et al. (2019) MDT‐15/MED15 permits longevity at low temperature via enhancing lipidostasis and proteostasis. PLoS Biology, 17, e3000415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, S. , Sun, H. , Zhou, Y. , Li, X. , Yu, Z. & Dong, Z. (2022) The 2021 of major forest pests in China and the forecast of their occurrence trend in 2022. Forest Pest and Disease, 41, 44–47. [Google Scholar]
- Liao, C. , Ou, T. , Wu, C. & Wang, C. (2013) Prevention of diet‐induced hyperlipidemia and obesity by caffeic acid in C57BL/6 mice through regulation of hepatic lipogenesis gene expression. Journal of Agricultural and Food Chemistry, 61, 11082–11088. [DOI] [PubMed] [Google Scholar]
- Liao, C.‐C. , Ou, T.T. , Huang, H. , Wang, C. & Wang, C. (2014) The inhibition of oleic acid induced hepatic lipogenesis and the promotion of lipolysis by caffeic acid via up‐regulation of AMP‐activated kinase. Journal of the Science of Food and Agriculture, 94, 1154–1162. [DOI] [PubMed] [Google Scholar]
- Liu, F. , Xiao, Y. , Ji, X. , Zhang, K. & Zou, C. (2017) The cAMP‐PKA pathway‐mediated fat mobilization is required for cold tolerance in C. elegans . Scientific Reports, 7, 638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, Z. , Li, Y. , Pan, L. , Meng, F. & Zhang, X. (2019) Cold adaptive potential of pine wood nematodes overwintering in plant hosts. Biology Open, 8, bio041616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch, H.J. , Rhainds, M. , Calabrese, J.M. , Cantrell, S. , Cosner, C. & Fagan, W.F. (2014) How climate extremes—not means—define a species' geographic range boundary via a demographic tipping point. Ecological Monographs, 84, 131–149. [Google Scholar]
- Mamiya, Y. (1983) Pathology of the pine wilt disease caused by Bursaphelenchus xylophilus . Annual Review of Phytopathology, 21, 201–220. [DOI] [PubMed] [Google Scholar]
- Mamiya, Y. (1988) History of pine wilt disease in Japan. Journal of Nematology, 20, 219–226. [PMC free article] [PubMed] [Google Scholar]
- Mota, M.M. , Braasch, H. , Bravo, M.A. , Penas, A.C. , Burgermeister, W. , Metge, K. et al. (1999) First report of Bursaphelenchus xylophilus in Portugal and in Europe. Nematology, 1, 727–734. [Google Scholar]
- Nwakiban Atchan, A.P. , Shivashankara, S.T. , Piazza, S. , Tchamgoue, A.D. , Beretta, G. , Dell'Agli, M. et al. (2022) Polyphenol‐rich extracts of Xylopia and Aframomum species show metabolic benefits by lowering hepatic lipid accumulation in diet‐induced obese mice. ACS Omega, 7, 11914–11928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polturak, G.A.‐O. , Misra, R.A.‐O. , El‐Demerdash, A.A.‐O. , Owen, C.A.‐O. , Steed, A. , McDonald, H.P. et al. (2023) Discovery of isoflavone phytoalexins in wheat reveals an alternative route to isoflavonoid biosynthesis. Nature Communications, 14, 6977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddle, D.L. & Albert, P.S. (1997) Genetic and environmental regulation of dauer larva development. In: Riddle, D.L. , Blumenthal, T. , Meyer, B.J. & Priess, J.R. (Eds.) C. elegans II, 2nd edition. New York, NY: Cold Spring Harbor Laboratory Press. Chapter 26. [PubMed] [Google Scholar]
- Robertson, L. , Cobacho Arcos, S. , Escuer, M. , Santiago Merino, R. , Esparrago, G. , Abelleira, A. et al. (2011) Incidence of the pinewood nematode Bursaphelenchus xylophlius Steiner & Buhrer, 1934 (Nickle, 1970) in Spain. Nematology, 13, 755–757. [Google Scholar]
- Rosen, E.D. & Spiegelman, B.M. (2014) What we talk about when we talk about fat. Cell, 156, 20–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savory, F.R. , Sait, S.M. & Hope, I.A. (2011) DAF‐16 and Δ9 desaturase genes promote cold tolerance in long‐lived Caenorhabditis elegans age‐1 mutants. PLoS One, 6, e24550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair, B.J. (2015) Linking energetics and overwintering in temperate insects. Journal of Thermal Biology, 54, 5–11. [DOI] [PubMed] [Google Scholar]
- Sinclair, B.J. & Marshall, K.E. (2018) The many roles of fats in overwintering insects. Journal of Experimental Biology, 221, jeb161836. [DOI] [PubMed] [Google Scholar]
- Svensk, E. , Ståhlman, M. , Andersson, C.H. , Johansson, M. , Borén, J. & Pilon, M. (2013) PAQR‐2 regulates fatty acid desaturation during cold adaptation in C. elegans . PLoS Genetics, 9, e1003801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomcala, A. , Tollarová, M. , Overgaard, J. , Simek, P. & Kostál, V. (2006) Seasonal acquisition of chill tolerance and restructuring of membrane glycerophospholipids in an overwintering insect: triggering by low temperature, desiccation and diapause progression. Journal of Experimental Biology, 209, 4102–4114. [DOI] [PubMed] [Google Scholar]
- Weng, J.‐K. (2014) The evolutionary paths towards complexity: a metabolic perspective. New Phytologist, 201, 1141–1149. [DOI] [PubMed] [Google Scholar]
- Wingfield, M.J. , Blanchette, R.A. , Nicholls, T.H. & Robbins, K. (1982) The pine wood nematode: a comparison of the situation in the United States and Japan. Canadian Journal of Forest Research, 12, 71–75. [Google Scholar]
- Yang, F. & Lv, G. (2022) Combined analysis of transcriptome and metabolome reveals the molecular mechanism and candidate genes of Haloxylon drought tolerance. Frontiers in Plant Science, 13, 1020367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye, J.R. (2019) Epidemic status of pine wilt disease in China and its prevention and control techniques and counter measures. Scientia Silvae Sinica, 55, 1–10. [Google Scholar]
- Yu, H. & Wu, H. (2018) New host and vectors of pine wood nematodes were found in Liaoning province. Forest Pest and Disease, 37, 61. [Google Scholar]
- Yu, H. , Wu, H. , Zhang, X. , Wang, L. , Zhang, X. & Song, Y. (2019) Preliminary study on Larix spp. infected by Bursaphelenchus xylophilus in natural environment. Forest Pest and Disease, 38, 7–10. [Google Scholar]
- Yu, M. , Xu, X. & Ding, P. (2011) Economic loss versus ecological gain: the outbreaks of invaded pinewood nematode in China. Biological Invasions, 13, 1283–1290. [Google Scholar]
- Zechner, R. , Zimmermann, R. , Thomas, O.E. , Kohlwein, S.D. , Haemmerle, G. , Lass, A. et al. (2012) FAT SIGNALS‐lipases and lipolysis in lipid metabolism and signaling. Cell Metabolism, 15, 279–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, P. , Shao, G. , Zhao, G. , Master, D.C.L. , Parker, G.R., Jr. , Dunning, J.B. et al. (2000) China's forest policy for the 21st century. Science, 288, 2135–2136. [DOI] [PubMed] [Google Scholar]
- Zhang, R. , Wang, J. , Xia, R. , Li, D. & Wang, F. (2022) Antioxidant processes involving epicatechin decreased symptoms of pine wilt disease. Frontiers in Plant Science, 13, 1015970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, L. , Wei, W. , Kulhavy, D. , Zhang, X. & Sun, J. (2020) Low temperature induces two growth‐arrested stages and change of secondary metabolites in Bursaphelenchus xylophilus . Nematology, 9, 663–670. [Google Scholar]
- Zhao, M. & Zhou, G.S. (2005) Estimation of biomass and net primary productivity of major planted forests in China based on forest inventory data. Forest Ecology and Management, 207, 295–313. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. HPLC chromatogram of caffeic acid in Larix olgensis and Pinus koraiensis at 15 and 30 days post‐inoculation (dpi) after pine wood nematode (PWN) inoculation. (a) HPLC chromatogram of caffeic acid in L. olgensis control (CK) at 15 dpi. (b) HPLC chromatogram of caffeic acid in L. olgensis at 15 dpi. (c) HPLC chromatogram of caffeic acid in P. koraiensis CK at 15 dpi. (d) HPLC chromatogram of caffeic acid in P. koraiensis at 15 dpi. (e) HPLC chromatogram of caffeic acid in L. olgensis CK at 30 dpi. (f) HPLC chromatogram of caffeic acid in L. olgensis at 30 dpi. (g) HPLC chromatogram of caffeic acid in P. koraiensis CK at 30 dpi. (h) HPLC chromatogram of caffeic acid in P. koraiensis at 30 dpi.
Figure S2. Quantitative PCR assay for pine wood nematode detection.
Figure S3. Quantitative PCR assay for pine wood nematode detection.
Figure S4. The 5‐year daily mean temperature from 2015 to 2019.
Figure S5. Sampling diagram.
Table S1. Morphology comparison of pine wood nematodes from different culture conditions.
Table S2. The five metabolites with the lowest fold change in intensity among the Larix olgensis downregulated metabolites at 15 days post‐inoculation.
Table S3. The five metabolites with the lowest fold change in intensity among the Larix olgensis downregulated metabolites at 30 days post‐inoculation.
Table S4. The primers used in the quantitative PCR assay for pine wood nematode detection.
Data Availability Statement
The datasets are available in the NCBI repository, at https://www.ncbi.nlm.nih.gov/genbank/, with accession numbers SRR28675111 and SRR28675112; BioProject ID PRJNA1100174.


