Abstract
Purpose
Age-related macular degeneration (AMD) is a leading cause of irreversible vision loss. Documentation of the disease’s description and treatment experience of Canadian patients is limited but of interest given the aging population and resultant implications for healthcare systems. A mixed-methods study was conducted to understand the challenges experienced by patients living in Canada with AMD to identify areas of need and for potential reform.
Patients and Methods
Canadian residents with wet or dry AMD were eligible for participation in an online survey and one-on-one telephone interview regarding their disease experience. Participants were recruited via a not-for-profit stakeholder organization and an ophthalmology clinic. Surveys were completed from January–June 2020 and interviews were conducted from November–December 2020, with findings reported using descriptive statistics and thematic analysis, respectively.
Results
Findings from 303 survey responses and 20 interviews were analyzed. Most participants indicated their vision loss negatively impacts their ability to conduct daily activities (eg, self care, driving) and leads to constant worry, loneliness, and/or isolation. Participants frequently reported requiring caregiver support, often for eye appointment travel or everyday tasks. Regardless of AMD type, participants reported having several appointments each year, and that time spent travelling to/from and waiting at appointments and related costs were considerable. Although participants receiving anti-vascular endothelial growth factor injections valued treatment, the experience added additional burden related to anxiety, fear, pain, and even greater reliance on caregivers. Many participants indicated they felt poorly informed about their disease and treatment options, particularly at diagnosis, which increased their emotional burden.
Conclusion
Patients with AMD living in Canada experience a significant and persistent mental, physical, and financial burden as a direct result of their disease. Improvements to provision of disease-related information, support of daily activities and appointment attendance, and the overall treatment experience could substantially enhance outcomes among the growing population of patients with AMD.
Keywords: age-related macular degeneration, Canada, burden of illness, disease experience, survey, interview
Introduction
Age-related macular degeneration (AMD) is a degenerative eye disease caused by pathologic changes to the layers of the retina, retinal pigment epithelium, and choroidal vasculature, usually in the macula.1–3 Later stages of the condition can be classified as either “wet” or “dry”, which vary in presentation and management.1 The hallmark characteristic of wet AMD is choroidal neovascularization, an aberrant process of angiogenesis controlled by growth factors such as vascular endothelial growth factor (VEGF).4 Although not curative, anti-VEGF injections represent the standard of care for these patients;4 left untreated, more than half become visually impaired or blind within three years of disease onset.1
Given the advancing average age of the global population and prolonged life expectancies, AMD represents a critical concern for healthcare systems.5,6 The disease is a leading cause of blindness among adults aged 50 years and older worldwide;7 in Canada, the condition affected approximately 2.5 million individuals in 2019, with almost 180,000 experiencing vision loss.8 It is estimated that by 2050, more than 4.2 million Canadians will be impacted by AMD.8
Not surprisingly, patients with AMD experience daily, multifaceted challenges related to functional and psychological impairment, socio-economic status, and provider- and system-level barriers.9–11 For example, treatment with anti-VEGF injections can improve vision and vision-related quality of life (VRQoL), yet also exacerbate AMD disease burden as a result of costs and other factors.11–13 Additionally, availability of high-quality, implementable, brand-agnostic AMD information that can be understood and accessed by patients is reported to be limited.14,15 Although these challenges are generally well recognized, there remains a paucity of recent data detailing the experience of patients with AMD living across Canada. Moreover, several previously published burden of illness studies have included relatively small numbers of patients (often n ≤160) and/or have not investigated the spectrum of hurdles associated with daily functioning and well-being, eye appointments, treatment, and informational needs that are encountered by patients with wet and/or dry AMD.16–22 Given the continuously growing AMD population, it is critical to understand the specific perceptions and experiences of patients to better support their evolving needs. Therefore, the goal of this study was to 1) comprehensively understand the physical, psychosocial, and practical challenges experienced by Canadian patients with lived AMD experience, and 2) identify areas of need and for potential reform from medical, health policy, and social care perspectives.
Material and Methods
Study Design & Participants
The study was conducted as part of the Valuation and Interpretation of Experiences of Wet Age-related Macular Degeneration (VIEW AMD) research project. A mixed-methods design was employed that included surveys and one-on-one telephone interviews. Given that age is the foremost risk factor for AMD and that, by definition, more advanced age is requisite for diagnosis,23 Canadians aged ≥41 years living with wet or dry AMD were eligible for study enrolment. Participants were required to be Canadian residents at the time of survey administration, with fluency in English and/or French; interviews were conducted in English only. Exclusion criteria were minimized to ensure the sample was reflective of and generalizable to the Canadian landscape.
Survey Characteristics
A team of researchers and ophthalmologists developed the survey, which was available in electronic and paper formats to support completion by individuals with low vision. The survey included a total of 49 multiple choice and Likert scale of agreement questions regarding participant demographics, medical history, treatment history and satisfaction, educational needs, and the impact of AMD on functional ability and emotional well-being (see Figure S1). Some questions were adapted from the Brief – Impact of Vision Impairment (B-IVI) questionnaire (n = 9 questions/topics), a patient-reported outcomes measure designed to assess the quality of life (QoL) of patients with eye conditions that has been validated in the AMD population.24 Potential participants were invited directly through the Fighting Blindness Canada (FBC) patient database, advertising on the FBC website and social media channels, and at a physician clinic located in Windsor, Ontario, Canada through a study coordinator. Survey completion occurred between January and June of 2020.
One-on-One Interviews
During survey completion, all participants were invited to take part in a one-on-one interview to gather additional qualitative data about their AMD experience. Among those in agreement, a heterogeneous sample was selected for participation allowing representation across a range of perceptions with respect to QoL impact, treatment experience, and geographic location. Caregivers were permitted to participate in the interviews in a supportive manner with patient permission. Each interview was conducted by a trained facilitator and ranged from 45 to 60 minutes in length. A semi-structured guide provided a framework for the discussions, which evolved on the basis of survey responses (see Figure S2); follow-up questions were permitted. With participant permission, audio recordings were captured for later transcription and analysis. All interviews were conducted in November and December of 2020.
Data Analysis & Reporting
All information, including AMD diagnosis, was self-reported by participants. Survey responses were analyzed using STATA version 10.0 (2007, StataCorp, LP, College Station, Texas, USA) and reported using descriptive statistics. Treatment and appointment experience outcomes were analyzed separately for participants receiving anti-VEGF eye injections for AMD at the time of survey completion and for those not receiving such injections. A sub-analysis was undertaken to understand differences between urban and rural participants overall and within the injected/non-injected groups.
Interview transcripts were analyzed using conventional content analysis and themes were elicited both within and across interviews. Two individuals independently coded the transcripts and developed a coding dictionary, which was modified as necessary to capture the relationship between codes and development of sub-codes. Disagreements were resolved through discussion until consensus was reached. Once coding was completed, the codes were imported into qualitative analysis software (QSR NVivo, Version 8.0, Lumivero, Denver, Colorado, USA), with pattern analysis and an iterative process conducted until themes emerged from the data.
Ethics
The study was conducted in compliance with the principles for medical research involving human subjects per the Declaration of Helsinki, as well as those specified by the Advarra Institutional Review Board (protocol number PRO00037096). All participants provided informed consent for study participation, which included the publication of anonymized responses and direct quotations. Participation was voluntary and compensation was provided (Amazon gift cards: $20 for survey respondents and $50 for interviewees). No personal identifiers were collected except for email addresses and telephone numbers, and this information was removed from the dataset with a randomized four-digit number assigned to each participant. Full participant names were not used in audio recordings or transcripts.
Results
A total of 303 participants responded to the survey, of whom 20 participated in one-on-one telephone interviews.
Survey Results
Participant Demographics
Among participants with available data, most were female (158/288 [54.9%]) and aged between 61 and 80 years (117/286 [40.9%]) (Table 1). Almost half of participants were living in the province of Ontario (145/303 [47.9%]) and the vast majority lived in an urban setting (276/303 [91.1%]). More than half of participants (185/303 [61.1%]) reported living with a spouse/partner. About 62% (178/288) of participants reporting on employment status were retired, while 14.9% (43/288) stated they worked full time. Approximately one-third of participants (96/303 [31.7%]) reported having wet AMD in both eyes, 40 (13.2%) had wet AMD in one eye only, 43 (14.2%) had wet AMD in one eye and dry AMD in the other eye, and 116 (38.3%) had dry AMD only in one or both eyes. Eight participants (2.6%) did not know their AMD type.
Table 1.
Baseline Characteristics of Survey Participants
| Characteristic | n (%) |
|---|---|
| Age (n = 286) | |
| Mean (SD) | 67.1 (13.3) |
| 41–60 years | 112 (39.2) |
| 61–80 years | 117 (40.9) |
| Over 80 years | 57 (19.9) |
| Biological Sex (n = 288) | |
| Female | 158 (54.9) |
| Male | 129 (44.8) |
| Intersex | 1 (0.3) |
| Province (n = 303) | |
| Ontario | 145 (47.9) |
| British Columbia | 61 (20.1) |
| Alberta | 32 (10.6) |
| Quebec | 23 (7.6) |
| Manitoba | 10 (3.3) |
| Nova Scotia | 10 (3.3) |
| Newfoundland | 9 (3.0) |
| New Brunswick | 4 (1.3) |
| Northwest Territories | 1 (0.3) |
| Prince Edward Island | 3 (1.0) |
| Saskatchewan | 4 (1.3) |
| Nunavut | 1 (0.3) |
| Location (n = 303) | |
| Urban | 276 (91.1) |
| Rural | 27 (8.9) |
| Type of AMD (n = 303) | |
| Wet AMD in both eyes | 96 (31.7) |
| Dry AMD in both eyes | 58 (19.1) |
| Dry AMD in one eye | 58 (19.1) |
| Wet AMD in one eye | 40 (13.2) |
| Wet AMD in one eye and dry AMD in the other eye | 43 (14.2) |
| Does not know AMD type | 8 (2.6) |
| Other Household Members (n = 303)a | |
| Partner/spouse | 185 (61.1) |
| My child(ren) | 61 (20.1) |
| No one | 53 (17.5) |
| Family member(s) other than partner and child | 24 (7.9) |
| I live in a retirement home | 20 (6.6) |
| Roommate/friend | 10 (3.3) |
| I live in a nursing home/long-term care facility | 2 (0.7) |
| Employment Status (n = 288) | |
| Retired | 178 (61.8) |
| Employed, working full-time | 43 (14.9) |
| Employed, working part-time | 32 (11.1) |
| Homemaker | 18 (6.3) |
| Not employed, looking for work | 8 (2.8) |
| Unemployed due to illness or disability | 6 (2.1) |
| Taking care of a family member | 2 (0.7) |
| Other | 1 (0.3)b |
Note: categories may not sum to 100% because of rounding. aParticipants could select more than one response. bOther employment status: in training for a new career.
Abbreviations: AMD, age-related macular degeneration; SD, standard deviation.
General AMD Experience
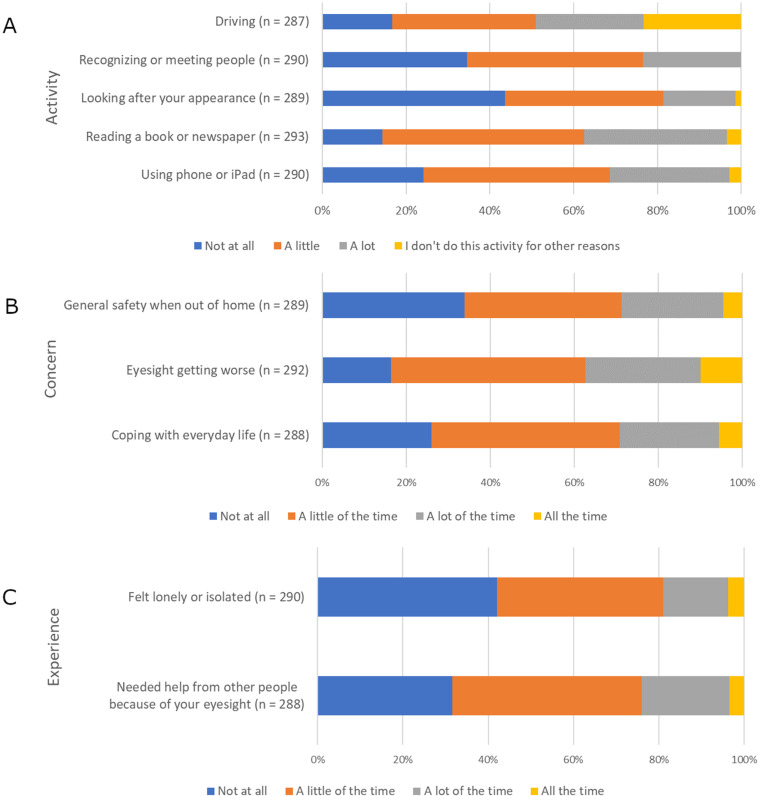
The survey results emphasized a profound physical, psychological, and social impact of AMD on patients. The majority of participants (~60%-80%) reported that common daily activities such as reading, self care, recognizing or meeting people, and driving were negatively affected by their vision loss (Figure 1A). Approximately one-third reported that sight-related concerns, such as coping with everyday life, worsening eyesight, or general outside safety, were on their mind a lot or constantly (Figure 1B). When considering the previous month, about 70% of participants reported needing help from others because of their eyesight and more than 50% reported feeling lonely or isolated (Figure 1C). About three-quarters of participants (231/296 [78.0%]) expressed worry that their condition might worsen (Table 2). Other frequent worries and challenges included inability to perform daily activities (122/296 [41.2%]), long wait times for appointments (93/296 [31.4%]), and explaining their condition to family and friends (91/296 [30.7%]).
Figure 1.
Impact of AMD on daily activities (A), concerns (B), and experiences (C) over the past month.
Abbreviations: AMD, age-related macular degeneration.
Table 2.
Challenges Associated with AMD (n = 296)
| Challenge | n (%) |
|---|---|
| Worry that my condition might worsen in the future | 231 (78.0) |
| Not being able to do the daily activities I used to | 122 (41.2) |
| The long wait times for appointments | 93 (31.4) |
| Explaining my condition to family and friends | 91 (30.7) |
| Lack of social support | 86 (29.1) |
| Finding answers to my questions about my condition | 66 (22.3) |
| Socializing | 65 (22.0) |
| Other | 34 (11.5)a |
Note: participants could select more than one response. aReading smaller print; hobbies; driving/night driving; doing fine work; eye tiredness (though possibly old-age–related); gradual sight deterioration; needing lots of light to read/do work; early retirement due to vision loss and resultant financial impact; everything impacted except sleeping; doctor does not take it seriously; unable to recognize people’s faces; peoples’ lack of understanding of AMD and vision-related capabilities; working.
Abbreviation: AMD, age-related macular degeneration.
AMD Treatment Experience
A total of 244 of the 303 survey participants (80.5%) reported having experience with injections for AMD, with first injections having occurred most frequently between one and five years prior (87/244; 35.7%) (see Table S1). Of the participants with eye injection experience, 221 (90.6%) indicated they were receiving injections at the time of survey completion. The 23 participants (9.4%) who reported not receiving injections at the time of survey completion cited various reasons, including the Coronavirus disease 2019 (COVID-19), stable AMD, the injections did not help or were no longer effective, dry eye, and AMD management with diet or vitamins, among others.
Experience of Participants Receiving Eye Injections for AMD
Among the 215 participants who reported on eye injection scheduling in the previous year, 94 (43.7%) were scheduled to receive between one and three eye injections, while 54 (25.1%) had more than six injections scheduled (see Table S2). Sixty-one of 219 participants (27.9%) reported canceling or delaying an appointment in this timeframe. Reasons for cancellation or delay varied but most frequently included inability to get to the appointment (with or without help; 44/60 participants [73.3%]) (Table 3). Almost half of responding participants (100/219 [45.7%]) reported spending 31 to 60 minutes traveling one-way to their injection appointment; 8.2% (18/219) spent 120 minutes or longer. Travel to injection appointments was reported to be very easy, easy, or neither easy nor difficult by almost all participants (199/219 [90.9%]). The total time spent at injection appointments was less than 120 minutes for more than three-quarters of responding participants (169/218 [77.5%]).
Table 3.
Reasons for AMD Appointment Cancellation or Delay
| Reason | Injected Patientsa N = 60 |
Non-injected Patients N = 10 |
|---|---|---|
| n (%) | n (%) | |
| Unable to find someone to take me to the appointment | 26 (43.3) | 1 (10.0) |
| Unable to travel to appointment | 18 (30.0) | 3 (30.0) |
| Could not afford attending the appointment | 14 (23.3) | 0 (0) |
| Too busy to attend appointment | 13 (21.7) | 2 (20.0) |
| Did not know how important the injection was to my sight | 11 (18.3) | N/A |
| Scared to receive the injection | 11 (18.3) | N/A |
| Did not find previous injections helpful | 7 (11.7) | N/A |
| I forgot about the appointment | 3 (5.0) | 0 (0) |
| I was not feeling well | 7 (11.7) | 1 (10.0) |
| Other | 11 (18.3)b | 7 (70.0)c |
Note: participants could select more than one response. aQuestion referred to AMD injection appointments only for this group, not general AMD appointments. bCOVID-19, hospitalization, vacation, retinal detachment requiring several surgeries. cCOVID-19, physician unavailable.
Abbreviations: AMD, age-related macular degeneration; COVID-19, Coronavirus disease 2019; N/A, not applicable.
Bevacizumab was the most frequently reported injection drug type (63/219 participants [28.8%]); ranibizumab (50 [22.8%]) and aflibercept (46 [21.0%]) were the next most commonly reported therapies (see Table S3). Twenty-six participants (11.9%) did not know the name of the drug they were receiving. Just over half of reporting participants (113/218 [51.8%]) described their AMD injections as “slightly painful”, while about one in five (46/218 [21.1%]) classified them as “painful” or “extremely painful”. Approximately 80% of participants (169/217) stated that their injection eye pain lingered into the evening after administration. Post-injection, about half of patients (101/215 [47.0%]) reported their vision was blurry for one to three hours, while 25.6% (55/215) indicated this effect lasted four to six hours (see Table S4). All reporting participants (n = 180) indicated an inability to conduct one or more of their regular activities after their AMD injection, with watching television and reading ability being impaired in approximately half of participants and driving and preparing meals being impaired in about one third (Table 4).
Table 4.
Activities Patients are Unable to Perform After an Injection (n = 180)
| Activity | n (%) |
|---|---|
| Watch television | 89 (49.4) |
| Read | 83 (46.1) |
| Drive | 60 (33.3) |
| Prepare meals | 52 (28.9) |
| Provide care to family members | 24 (13.3) |
| Work | 25 (13.9) |
| None of the above activities | 0 |
Note: participants could select more than one response.
Approximately 86% (185/216) of participants reported having someone help them with their eye appointments, most commonly a spouse (115/216 [53.2%]) and/or other family member (52/216 [24.1%]). Over half indicated that such help included support with everyday tasks after appointments (100/183 [54.6%]), waiting with them at appointments (101/183 [55.2%]), and/or travel to/from appointments (108/183 [59.0%]) (Table 5). Among participants who reported on difficult aspects of their eye injection appointments (n = 215), anxiety or fear about the injection was most common (82 [38.1%]) (Table 6), while about one-quarter to one-third of participants found long wait times at the appointment (75 [34.9%]), the cost of travel to/from the appointment (55 [25.6%]), or finding support with travel to/from appointments (51 [23.7%]) to be difficult. Still, approximately one in five (48 [22.3%]) of these participants reported that their appointments were not difficult. Despite the challenges experienced by some participants, three-quarters (167/219 [76.3%]) indicated they were satisfied or very satisfied with their injections. Almost three-quarters (161/220 [73.2%]) reported that the injections helped them avoid losing more eyesight, and just under half stated the injections improved their eyesight (98/220 [44.5%]) (see Table S5). However, 17.3% of participants (38/220) reported the injections had no effect or that they were unsure of the effect.
Table 5.
Types of Help Provided by Others Related to Eye Appointments
| Help Provided | Injected Patients N = 183 |
Non-injected Patients N = 50 |
|---|---|---|
| n (%) | n (%) | |
| Help me after the injections/appointments with everyday tasks | 100 (54.6) | 16 (32.0) |
| Wait with me at the appointment | 101 (55.2) | 34 (68.0) |
| Travel with me or drive me to/from the appointment | 108 (59.0) | 40 (80.0) |
| Take care of things at home while I am away | 55 (30.1) | 7 (14.0) |
| Physical support at my appointment | 47 (25.7) | 10 (20.0) |
| Other | 3 (1.6)a | 4 (8.0)b |
Note: participants could select more than one response. aEmotional support; help with eye drops. bFinancial; handling paperwork; second set of ears; help with daughter; shopping/cooking.
Table 6.
Difficult Aspects of Eye Appointments
| Injected Patients N = 215 |
Non-injected Patients N = 82 |
|
|---|---|---|
| Reason | n (%) | n (%) |
| Anxiety or fear about the injection | 82 (38.1) | N/A |
| Long waiting time at the appointment | 75 (34.9) | 19 (23.2) |
| Cost of travel to/from the appointment | 55 (25.6) | 10 (12.2) |
| Finding someone to drive me to/from the appointment | 51 (23.7) | 18 (22.0) |
| Finding someone to help me with my daily tasks after the injection | 48 (22.3) | N/A |
| I do not find any part difficult | 48 (22.3) | 45 (54.9) |
| Scratchiness or pain in my eye after the appointment | 45 (20.9) | N/A |
| Taking time off work to attend | 30 (14.0) | 1 (1.2) |
| Other | 8 (3.7)a | 10 (12.2)b |
Note: participants could select more than one response. aSide effects; time commitment and planning; transport and support needs; sitting alone waiting. bLong appointment wait times; short appointment duration; unreliable transit; appointment navigation; post-appointment mobility; stress.
Abbreviations: N/A, not applicable.
Experience of Participants with AMD Not Receiving Eye Injections
The healthcare experience of participants not receiving eye injections for AMD at the time of survey completion (N = 83) showed both similarities and differences to that of participants receiving injections. Of these participants, most (53/82 [64.6%]) reported having one to three eye appointments scheduled in the previous year (see Table S6). Only 10 participants reported delaying or cancelling these appointments (Table 3), most commonly because they were unable to travel to the appointment (3 [30.0%]) or too busy to attend (2 [20.0%]). Travel time to appointments was 60 minutes or less one-way for three-quarters of reporting participants (61/81 [75.3%]), though some reported a duration of 120 minutes or more (10/81 [12.3%]). The total time spent at each appointment was <60 minutes for 50.6% (41/81) of participants, 60 to 120 minutes for 38.3% (31/81), and 120 to 240 minutes for 11.1% (9/81).
Many non-injected participants reported receiving help with their eye appointments, which was most often provided by their spouse (32/82 [39.0%]) and/or another family member (28/82 [34.1%]). The types of help provided by these individuals varied but most frequently included support with travel to/from appointments (40/50 [80.0%]) and/or waiting at appointments with the participant (34/50 [68.0%]); one-third of participants (16/50 [32.0%]) received help after their appointments with everyday tasks (Table 5). Still, 30 participants (36.6%) indicated that no one helps them with their appointments. The majority of participants did not find their appointments difficult (45/82 [54.9%]), though some noted long wait times (19/82 [23.2%]) and/or challenges finding someone to take them to/from appointments (18/82 [22.0%]) (Table 6). Overall, more than half of participants (46/83 [55.4%]) reported satisfaction with their eye appointments, while approximately 13% (11/83) were dissatisfied or very dissatisfied.
Experience of Rural versus Urban Participants
Comparisons of responses from participants living in rural (n = 27) versus urban (n = 276) Canadian centres revealed a limited number of differences. Compared with urban participants, the primary source of AMD information for rural participants was more likely to be their family physician (30.0% vs 20.0%, respectively) and less likely to be an ophthalmologist (59.3% vs 74.6%). Still, other than the ophthalmologist, the internet was the preferred source of information for participants in both groups (64.5–74.1%). Among the 23 rural and 196 urban participants receiving eye injections, approximately three-times as many rural participants traveled more than 60 minutes one-way to their appointments (60.9% vs 18.9%), and nearly four-times as many rural participants also found such travel difficult (26.1% vs 7.1%). Rural participants were more likely to list cost of travel to their injection appointment as a challenge compared with those from urban areas (9/22 [40.9%] vs 46/194 [23.7%]).
Informational Needs and Preferences
Many of the 303 participants indicated that upon initial diagnosis of AMD, information related to the need to attend doctor’s appointments (192 [63.4%]), what to expect with the condition (203 [67.0%]), and vitamin supplementation (165 [54.5%]) was important (Table 7). Approximately 40% of participants additionally reported that receiving information at later appointments (to provide time to think, develop questions) and understanding how to access support services and information on coping with vision loss would be important. For more than half of the 303 participants, key informational topics of interest included how to prevent their eyes from worsening (227 [74.9%]), predictors of worsening (185 [61.1%]), treatment effectiveness based on photos (179 [59.1%]), and new AMD research (184 [60.7%]) (see Table S7). Genetic testing and its relationship to the benefits of certain vitamins was also of interest to many participants (136 [44.9%]).
Table 7.
Important Information to Receive Upon AMD Diagnosis (n = 303)
| Information | n (%) |
|---|---|
| Importance of going to doctor’s appointments | 192 (63.4) |
| What to expect with this condition | 203 (67.0) |
| Information about vitamins | 165 (54.5) |
| Patients should receive more information at later appointments, so they have time to think about what they’d like to know | 127 (41.9) |
| How to access support services and information on coping with vision loss | 121 (39.9) |
| Other | 16 (5.3)a |
Note: participants could select more than one response. aResearch; stem cells/treatments; disease progression; importance of family support; nutrition; risk of conversion to wet AMD; low vision assistive technology; good explanation of diagnosis; available resources.
Abbreviation: AMD, age-related macular degeneration.
For most participants (222/303 [73.3%]), the primary source of AMD information was their ophthalmologist; however, information was also sought online (eg, FBC and Canadian National Institute for the Blind websites) and from other types of healthcare practitioners (HCPs) (Table 8). Aside from their ophthalmologist, most participants (198/303 [65.3%]) indicated they preferred online sources of information; pamphlets and informational videos were preferred by about one-third each (see Table S8). Almost all participants (288/301 [95.7%]) indicated interest in learning about new treatment options for AMD.
Table 8.
Primary Sources of AMD Information (n = 303)
| Source | n (%) |
|---|---|
| My ophthalmologist | 222 (73.3) |
| FBC website | 127 (41.9) |
| My optometrist | 115 (38.0) |
| My family physician | 63 (20.8) |
| Family/friends | 51 (16.8) |
| CNIB website | 60 (19.8) |
| Other | 33 (10.9)a |
Note: participants could select more than one response. aOther sources of information: Google, Macular Society, Mayo Clinic, CCB, Berkley clinic newsletter, retinal specialist, I-Deal program at senior center, FBC conference, television, Dr. Randall Wong’s blog.
Abbreviations: AMD, age-related macular degeneration; CCB, Canadian Council of the Blind; CNIB, Canadian National Institute for the Blind; FBC, Fighting Blindness Canada.
Interview Findings: Thematic Analysis
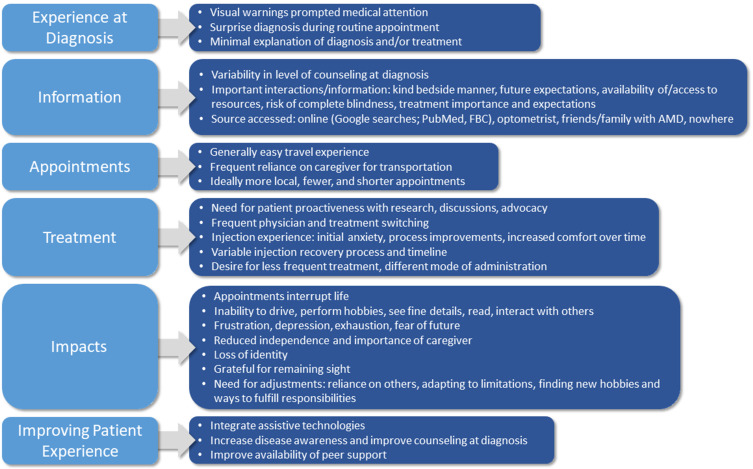
Analysis of the interview transcripts revealed several key themes related to the AMD experience and provided further breadth to the survey findings. Themes included participants’ experience at diagnosis, information provision and needs, experience with appointments, treatment considerations, the physical and emotional impact of both diagnosis and the condition, as well as recommendations to improve the experience of individuals with AMD. Details on these themes are presented below and in Figure 2.
Figure 2.
Themes and details identified in analysis of interview transcripts. N = 20 participants.
Abbreviations: AMD, age-related macular degeneration; FBC, Fighting Blindness Canada.
Experience at Diagnosis
Several interview participants stated that their AMD was discovered during a routine optometrist appointment while they were still asymptomatic, while others recalled specific visual deteriorations that caused them to see their optometrist or ophthalmologist. For participants without symptoms, diagnosis of AMD was surprising, and few were previously familiar with the condition. Some of these participants initially reacted with fear, while others were generally unbothered given their lack of visual symptoms.
It was just my annual checkup, and [the optometrist] informed me at that time. I didn’t notice there was anything wrong, and it was several years later that I started to notice a decline in my vision.
Information Provision & Needs
Many participants described being informed of their condition in brief, curt terms by physicians, which made coming to terms with their illness especially difficult. Many also expressed dissatisfaction with their initial appointment because they received little information about AMD and its treatment. In most cases, this increased participant fear as they felt poorly equipped to understand and manage what was to come. Participants who did receive counselling on their condition often reported being shown pictures and given explanations of the etiology and pathophysiology of AMD. These participants reported being fairly satisfied with the information received and the manner in which it was provided. Several participants spoke of their physicians explaining possible progression pathways of AMD and being honest about future uncertainty. These participants noted it was comforting to know possible options and outcomes and that the trajectory was not necessarily dire (eg, complete blindness). They stated that information about how treatment slows down sight loss and what to expect with injections and side effects was helpful. Overall, however, such counseling was reported to be limited, leading to participant stress and anxiety.
Helpful if you’re told everything that’s going to happen so you can prepare mentally. Explained in clear language. Doctors don’t have time or will to explain. There is no information. It’s up to the patient to start digging. And it’s not that easy.
Several participants stated that after diagnosis, they were unaware of the various aids available to help individuals with low vision conduct activities of daily living. This led to increased fear about the progression of AMD and their inability to cope. The participants underscored that knowledge that such supports and technologies are available would be beneficial. Similarly, provision of or direction to multiple credible resources of AMD information, which participants could refer to when they felt ready, was considered ideal.
Experience with Appointments
Most participants reported that getting to their ophthalmology appointments was fairly easy, regardless of travel time. This was especially the case for those with caregiver support. Several participants acknowledged that if they did not have a supportive caregiver, attending appointments would be much more challenging. Participants who lived alone or without family nearby appeared to have more difficulty attending appointments and experienced stress associated with finding transportation. Some participants mentioned that more local and fewer appointments and shorter wait times would be favorable.
I have a very great support [caregiver]. It’s something I don’t think I could live without. And I feel ill for those who have to.
AMD Treatment
Some participants stated they were not informed about options for AMD injections, although it should be noted that only one type may have been available at the time of such discussions. Some participants noted being proactive, conducting their own research about treatment options to inform discussions with their physician and to advocate for new therapies. Several participants spoke of not being satisfied with their initial ophthalmologist and switching to another with whom they felt communication was improved. The participants also mentioned treatment switching, which was primarily physician-initiated and related to poor response, funding issues, or a new option becoming available.
I do not think [my doctor] gave me any choices. She said, “This is the newest [treatment], it’s having really good results, let’s try it and see what happens”.
Across participants, feelings at first injection varied from resignation to extreme anxiety, though several commented that the experience was better than expected. Some participants stated that different injection protocols were encountered during care with different HCPs, some of which exacerbated stress and pain. These participants indicated that certain techniques may render the process more manageable (eg, use of topical gel or injected anesthetic, reduction of post-injection eye drop requirements, shorter in-clinic wait times) and should be considered where feasible. The participants further indicated that comfort with injections generally increased over time, although improved comfort did not fully mitigate anxiety and fear. Recovery processes and time were reported to vary considerably across participants, sometimes leading into the next morning, and coping mechanisms (eg, resting, relying on others) were often employed.
I feel a lot of anxiety before an appointment. I feel anxiety even the day before because I just really detest it.
Although several participants mentioned being satisfied with their injections, others expressed a desire for fewer treatments or none at all. Some participants rationalized that a benefit of frequent treatment was consistent monitoring, which increased confidence that any changes in vision would be identified. Several participants greatly disliked their injections and wished for an option with a different mode of administration (eg, eye drops or oral medication).
Impact of AMD
The participants indicated that AMD negatively impacted their ability to conduct multiple aspects of daily living, including driving, hobbies, reading, seeing fine details, and interactions with others. They noted that AMD appointments interrupt not only their own life but that of their caregiver. Although many participants spoke of trying to be grateful for the vision they had left and appreciative of the activities they can still do, they also expressed frustration, depression, fear, and exhaustion related to their disease. The participants flagged worries about the future and what their life purpose will be when they can no longer perform the activities they currently enjoy and find fulfilling.
My eyes are getting progressively worse. I’m fortunate that I’m comfortable, we have a home, my partner is caring and loving, but the loss of independence has been horrible. I haven’t been able to drive for 3-4 years because I don’t trust myself.
Participants with more progressed AMD and poorer vision spoke about the importance of a supportive caregiver who assumed driving duties and many responsibilities around the home. Although such support was considered invaluable, a loss of independence was felt by participants. Those who lived on their own or whose partner had difficulty coming to terms with the participant’s vision loss were most acutely affected by the inability to drive and the lack of freedom to be mobile without planning. Most participants considered loss of their driver’s license to be the most difficult AMD-related challenge and felt like a burden to others upon whom they relied for transportation.
Improving the Patient Experience
I do not think people think of eye diseases blinding you, it’s usually an accident. And if I say to people, “I have AMD”, they have no idea what I am talking about. I think what’s needed is a bigger voice.
The participants provided numerous recommendations regarding ways in which the AMD patient experience can be improved. They stated that increased public education is needed, including outreach highlighting the importance of routine screening and monitoring for progression. The participants mentioned a need for lists of aids and assistive technologies for individuals with poor vision, as well as names of online stores that sell such products. They emphasized the importance of peer support and being linked with family members or other patients with AMD to discuss experiences and expectations. Finally, the participants further underscored the need for improved patient counseling at diagnosis, which should include more time and information provided in clear and accessible language.
In the course of being diagnosed, [it] would be great to be able to talk to someone whose gone through it and tell me how they adapted, and if they did.
Discussion
Although the general burden of AMD and its treatment is well described in the literature, a comprehensive assessment of the experience of both anti-VEGF–injected and non-injected patients located across Canada is lacking. Such information is of high interest considering that AMD is a primary cause of vision loss among individuals aged ≥50 years, the advancing age of the Canadian population, and the potential implications for healthcare and socioeconomic systems. This large, qualitative, mixed-methods study provides helpful details regarding the impact of wet and dry AMD on both patients and caregivers, shedding light on challenges and needs that in some cases are encountered on a daily basis.
The study results clearly show the negative influence of AMD on common and necessary activities performed by patients, such as reading, self care, and driving. The impact of the disease on driving ability was regarded as particularly deleterious, resulting in loss of independence and even identity for some participants. The ability of the participants to conduct hobbies was also diminished, a concern given the self-worth and fulfillment attributed to such activities. The participants indicated that sight-related concerns were frequently top of mind, including those related to the uncertain progression of AMD and the burden placed upon family and other caregivers. Regardless of whether or not anti-VEGF injections were being received, the participants reported attending multiple appointments and requiring help because of their poor vision. Such assistance was frequently provided in relation to traveling to and from appointments. Inability to attend appointments was a major reason for appointment delay or cancellation, underscoring a key area in which support could help maximize adherence to anti-VEGF injections, facilitate earlier recognition of disease progression, and improve clinical outcomes.13,21,25 Other difficulties associated with appointments included the need for extended travel and time commitments; even though many participants were retired, time requirements and financial implications remained problematic. Given increased limitations in vision and functionality, the participants also often required considerable support immediately after their injection appointments.
As expected, the physical impairment, loss of independence, and treatment experience associated with AMD translated into a significant emotional burden for patients. Participants expressed persistent worry, anxiety, fear, and depression related to progressive vision loss and other repercussions of the disease; feelings of loneliness and isolation were also identified. Fear and anxiety were expressed predominantly in relation to anti-VEGF injections, particularly in the lead up to the treatment appointment. Implementation of processes proven to mitigate issues surrounding injection procedures, such as thorough use of anesthetic, were recommended. Other options may include ensuring extra staff members are present during the injection, use of a neck pillow, verbal warnings prior to injection, hand holding, and performing bilateral injections on the same day.26 Such considerations may be of particular interest given that novel therapies for geographic atrophy in late-stage AMD, which are also administered via intravitreal injection, will soon be available in Canada.27 Notably, almost one-third of study participants reported receiving bevacizumab, an anti-VEGF injection therapy that is not publicly funded in Ontario, the province in which almost half of participants resided. This finding may relate to variations in coverage of bevacizumab across Canada and potentially the age of some patients in this subset (eg, <65 years with private insurance coverage).
Although the injected and non-injected participants were asked slightly different questions regarding whether they were “satisfied” with their AMD experience (ie, satisfied with injections or with appointments, respectively), the difference in responses between groups was notable, at 76.3% versus 56.6%, respectively. This finding was not unexpected: evidence indicates that injected patients tend to feel satisfied with their therapy;28,29 in contrast, given their lack of treatment options and the need for a “watch and wait” approach, non-injected patients may experience feelings of helplessness and therefore less overall satisfaction with their AMD experience.
In a previously published study of trends in low vision care in Ontario, Canada between 2009 and 2015, differences in access of vision rehabilitation services (including those for AMD) were found to vary on the basis of patient age, sex, and geographic location.30 In the current study, few differences in AMD experiences were identified between rural and urban participants. As expected, rural participants reported increased travel time for appointments; however, this did not appear to translate into a worse overall disease experience. The rural participants also appeared to have greater confidence in and a better relationship with their general practitioner than their ophthalmologist, a situation that may arise given the need for greater involvement of the general practitioner in non-urban locations.
A recurring theme throughout the surveys and interviews was the participants’ need for additional information, particularly at the time of diagnosis of AMD. The survey results showed that although 179 participants reported having wet AMD in one or both eyes, many more (n = 221) reported receiving anti-VEGF injections at the time of survey completion; additionally, 8 participants did not know their AMD type. These findings emphasize a need for improved education regarding AMD diagnosis. The participants indicated that information on disease expectations, prevention and prediction of progression, treatment options, the injection experience, assistive devices, and ways to connect with other patients living with AMD would be useful. However, this information was inconsistently provided, leaving them feeling ill-informed about their condition. The participants also highlighted the importance of receiving information throughout their treatment journey as it affords opportunity to reflect on their diagnosis, conduct additional research, and formulate questions for future HCP discussions. More than half of participants indicated a preference for obtaining information online. Although not unexpected, this may be of concern, as evidence indicates that online sources can be problematic in terms of the quality and reliability of AMD information.14,15
Other Canadian publications on the disease-related burden of AMD have typically focused on smaller groups of patients with wet AMD and have varied considerably in terms of evaluated outcomes. Similar to the current evaluation, a multinational (including Canada) qualitative study by Giocanti-Aurégan et al conducted semi-structured interviews with 49 wet AMD patients, 47 AMD caregivers, and 62 retina specialists to understand the real-world experience associated with anti-VEGF treatment.18 As in the current analysis, most patients in this study reported disruption to their routines related to their injections. Barriers to treatment adherence were also reported and categorized as tolerability, clinical factors, logistical parameters (eg, visit frequency, travel logistics, wait times, financial burden), and human factors (eg, fear/anxiety, education). Other Canadian studies have examined risk factors and outcomes associated with delayed AMD follow-up21 and fear associated with the COVID-19 pandemic among patients with wet AMD,22 or perceptions of metamorphopsia and VRQoL in AMD.16 Additionally, in 2007, a burden-of-illness study evaluated self-reported outcomes among Canadian patients with wet AMD, reporting reduced functioning and QoL that resulted in high healthcare resource utilization and patient support costs.20
Some strengths and limitations of the study should be acknowledged. Participant recruitment occurred via two settings (registry and clinic), which helped ensure responses were derived from a diverse cohort of patients and reduced bias related to patient perceptions and proactiveness about their condition. Additionally, the mixed-methods design supported a granular and rich understanding of the Canadian AMD experience, while the sample size was sufficiently large to permit a thorough examination of outcomes. One limitation of the study was its potential for non-response bias, as patients who chose to participate were more likely to be receiving regular care. This is emphasized by the fact that some participants were recruited at a treatment clinic. Furthermore, individuals with considerable vision loss due to AMD may not have been receptive or able (or lacked assistance) to complete the online or paper surveys. Another limitation is that the participants self-reported their AMD diagnosis—it is possible that patients may have lacked information or confidence to answer certain questions properly, such as the name of their condition, their AMD type (ie, wet vs dry), or the drug they were receiving. Similarly, self-reporting may have impacted the consistency of patient responses across questions. Administration of a validated questionnaire on burden or QoL, for example, may have helped mitigate variations related to self-reporting and other factors. Additionally, given the study was a Canada-wide survey and no national standard of care exists related to patient consent, information sheets, testing, and use of local anesthetic, participant experiences were likely to vary between clinics. Finally, it should be noted that data collection occurred during the height of the COVID-19 pandemic, before vaccinations were available in Canada. It is therefore possible that the pandemic exacerbated some findings of this study.22,31
Conclusion
As one of the largest and most comprehensive analyses of its kind, this burden of illness study reveals the heterogeneous yet substantial physical, emotional, practical, and social impacts of AMD and its care as reported by Canadian patients. The findings reflect the lived AMD experience and highlight numerous opportunities for improvement of healthcare encounters. Such improvements may include expanded provision of high-quality disease- and treatment-related information, which will help patients set expectations, increase comfort, mitigate fear, and improve ability to participate in shared decision-making. Increased support with appointment travel requirements also appears to be critical, considering extensive reliance on caregivers and the relatively high number of cancelled or delayed appointments and resultant clinical implications. Additionally, although participants appeared to value AMD treatment, the anti-VEGF injections were associated with inconvenience, pain, and considerable emotional burden. These findings may inform considerations related to new injection therapies that will soon be available for patients with dry AMD and geographic atrophy. Efforts are also needed to increase injection education, implement techniques that reduce stress and alleviate pain, and evaluate more convenient therapeutic options. Healthcare practitioner and regulator awareness of these needs may support the introduction of novel programs, policies, and guides to address them, which could significantly improve outcomes and emotional well-being among the growing Canadian population of patients with AMD.
Acknowledgments
The authors wish to thank Dana L. Anger of WRITRIX Medical Communications Inc. for assistance with medical writing and journal submission requirements, which was funded by Hoffmann-La Roche Limited.
Funding Statement
Coordination and financial support for study design, development, implementation, and data analysis were provided by Fighting Blindness Canada. Financial support for manuscript development (medical writing) and publication was provided by Hoffmann-La Roche Limited.
Data Sharing Statement
The anonymized datasets generated during this study are available upon reasonable request to the corresponding author.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all of these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
Dr Larissa Moniz reports grants to Fighting Blindness Canada from Hoffmann-La Roche, Bayer, Novartis, Biogen, Apellis, and Astellas, during the conduct of this study. All authors report no other conflicts of interest in this work.
References
- 1.National Institute for Health and Care Excellence (NICE). Age-related macular degeneration: diagnosis and management. NICE Guideline, No. 82. London. 2018. Available from: https://www.ncbi.nlm.nih.gov/books/NBK481428/. Accessed October 5, 2023. [PubMed] [Google Scholar]
- 2.Fleckenstein M, Keenan TDL, Guymer RH, et al. Age-related macular degeneration. Nat Rev Dis Primers. 2021;7(1):31. [DOI] [PubMed] [Google Scholar]
- 3.Thomas CJ, Mirza RG, Gill MK. Age-related macular degeneration. Med Clin North Am. 2021;105(3):473–491. [DOI] [PubMed] [Google Scholar]
- 4.Canadian Agency for Drugs and Technologies in Health (CADTH). Aflibercept (Eylea): treatment of Neovascular (Wet) Age-Related Macular Degeneration (wAMD). Ottawa. 2015. Available from: https://www.ncbi.nlm.nih.gov/books/NBK349613/. Accessed October 10, 2023. [PubMed]
- 5.Chaudhuri M, Hassan Y, Bakka Vemana PPS, Bellary Pattanashetty MS, Abdin ZU, Siddiqui HF. Age-related macular degeneration: an exponentially emerging imminent threat of visual impairment and irreversible blindness. Cureus. 2023;15(5):e39624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wong WL, Su X, Li X, et al. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Glob Health. 2014;2(2):e106–16. [DOI] [PubMed] [Google Scholar]
- 7.Flaxman SR, Bourne RRA, Resnikoff S, et al. Global causes of blindness and distance vision impairment 1990-2020: a systematic review and meta-analysis. Lancet Glob Health. 2017;5(12):e1221–e1234. [DOI] [PubMed] [Google Scholar]
- 8.Deloitte Access Economics. The cost of vision loss and blindness in Canada. Canadian Council of the Blind. 2021. Available from: https://www.fightingblindness.ca/wp-content/uploads/2021/12/Deloitte-Cost-of-vision-loss-and-blindness-in-Canada-report-May-2021.pdf. Accessed October 5, 2023.
- 9.Taylor DJ, Hobby AE, Binns AM, Crabb DP. How does age-related macular degeneration affect real-world visual ability and quality of life? A systematic review. BMJ Open. 2016;6(12):e011504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gale RP, Finger RP, Eldem B, et al. The management of neovascular age-related macular degeneration: a systematic literature review of patient-reported outcomes, patient mental health and caregiver burden. Acta Ophthalmol. 2023;101(1):e26–e42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Choi A, Nawash BS, Du K, Ong J, Chhablani J. Barriers to care in neovascular age-related macular degeneration: current understanding, developments, and future directions. Surv Ophthalmol. 2024;69(1):160–164. [DOI] [PubMed] [Google Scholar]
- 12.Finger RP, Daien V, Eldem BM, et al. Anti-vascular endothelial growth factor in neovascular age-related macular degeneration – a systematic review of the impact of anti-VEGF on patient outcomes and healthcare systems. BMC Ophthalmology. 2020;20(1):294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shahzad H, Mahmood S, McGee S, et al. Non-adherence and non-persistence to intravitreal anti-vascular endothelial growth factor (anti-VEGF) therapy: a systematic review and meta-analysis. Syst Rev. 2023;12(1):92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kloosterboer A, Yannuzzi N, Topilow N, Patel N, Kuriyan A, Sridhar J. Assessing the quality, content, and readability of freely available online information for patients regarding age-related macular degeneration. Semin Ophthalmol. 2021;36(5–6):400–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang E, Kalloniatis M, Ly A. Assessment of patient education materials for age-related macular degeneration. Ophthalmic Physiol Opt. 2022;42(4):839–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu K, Gupta V, Bae S, Sharma S. Metamorphopsia and vision-related quality of life among patients with age-related macular degeneration. Can J Ophthalmol. 2018;53(2):168–172. [DOI] [PubMed] [Google Scholar]
- 17.Święch A, Dolar-Szczasny J, Wróbel-Dudzińska D, Kosior-Jarecka E, Mackiewicz J. Quality of life among patients from urban and rural areas with advanced age-related macular degeneration assessed using the NEI-VFQ-25. Ann Agric Environ Med. 2021;28(2):243–249. [DOI] [PubMed] [Google Scholar]
- 18.Giocanti-Aurégan A, García-Layana A, Peto T, et al. Drivers of and barriers to adherence to neovascular age-related macular degeneration and diabetic macular edema treatment management plans: a multi-national qualitative study. Patient Prefer Adherence. 2022;16:587–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reitan G, Kjellevold Haugen IB, Andersen K, Bragadottir R, Bindesbøll C. Through the eyes of patients: understanding treatment burden of intravitreal anti-VEGF injections for nAMD patients in Norway. Clin Ophthalmol. 2023;17:1465–1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cruess A, Zlateva G, Xu X, Rochon S. Burden of illness of neovascular age-related macular degeneration in Canada. Can J Ophthalmol. 2007;42(6):836–843. [DOI] [PubMed] [Google Scholar]
- 21.Rozon JP, Hébert M, Laverdière C, et al. Delayed follow-up in patients with neovascular age-related macular degeneration treated under universal health coverage: risk factors and visual outcomes. Retina. 2022;42(9):1693–1701. [DOI] [PubMed] [Google Scholar]
- 22.Rozon JP, Hébert M, Bourgault S, et al. Fear associated with COVID-19 in patients with neovascular age-related macular degeneration. Clin Ophthalmol. 2021;15:1153–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McCannel CA, Kim SJ. Section 12: retina and Vitreous. In: 2021-2022 Basic and Clinical Science Course. American Academy of Ophthalmology; 2021. [Google Scholar]
- 24.Fenwick EK, Man RE, Rees G, Keeffe J, Wong TY, Lamoureux EL. Reducing respondent burden: validation of the Brief Impact of Vision Impairment questionnaire. Qual Life Res. 2017;26(2):479–488. [DOI] [PubMed] [Google Scholar]
- 25.Okada M, Mitchell P, Finger RP, et al. Nonadherence or nonpersistence to intravitreal injection therapy for neovascular age-related macular degeneration: a mixed-methods systematic review. Ophthalmol. 2021;128(2):234–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gomez J, Koozekanani DD, Feng AZ, et al. Strategies for improving patient comfort during intravitreal injections: results from a survey-based study. Ophthalmol Ther. 2016;5(2):183–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Khan H, Aziz AA, Sulahria H, et al. Emerging treatment options for geographic atrophy (GA) secondary to age-related macular degeneration. Clin Ophthalmol. 2023;17:321–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Calles-Monar PS, Sanabria MR, Alonso-Tarancon AM, Coco-Martin RM, Mayo-Iscar A. Modifiable determinants of satisfaction with intravitreal treatment in patients with neovascular age-related macular degeneration. Drugs Aging. 2022;39(5):355–366. [DOI] [PubMed] [Google Scholar]
- 29.Gohil R, Crosby-Nwaobi R, Forbes A, Burton BJ, Hykin P, Sivaprasad S. Treatment satisfaction of patients undergoing ranibizumab therapy for neovascular age-related macular degeneration in a real-life setting. Patient Prefer Adherence. 2016;10:949–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Basilious A, Basilious A, Mao A, Hutnik CML. Trends in low vision care provided by ophthalmologists in Ontario between 2009 and 2015. Can J Ophthalmol. 2019;54(2):229–236. [DOI] [PubMed] [Google Scholar]
- 31.Gordon KD The impact of the COVID-19 pandemic on Canadians who are blind, deaf-blind, and partially-sighted. 2020. Available from: https://ccbnational.net/shaggy/wp-content/uploads/2020/05/COVID-19-Survey-Report-Final-wb.pdf. Accessed October 5, 2023.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The anonymized datasets generated during this study are available upon reasonable request to the corresponding author.