Abstract
Vulvovaginal candidiasis (VVC) is a prevalent gynecological infection characterized by high incidence and recurrent episodes, causing significant distress in women. This study aims to assess the effectiveness of different clotrimazole and fluconazole treatment regimens for severe vulvovaginal candidiasis (SVVC). A retrospective analysis was conducted on 1303 cases of SVVC among first-time visitors to the gynecology outpatient department at Peking University Shenzhen Hospital between January 2013 and December 2022. Vaginal secretions were systematically collected for fungal culture, with species identification conducted using Chromogenic culture medium and API Candida test reagents. Mycological cure rates were assessed at days 7–14, days 25–35, and day 35 to 6 months after treatment. The three-dose clotrimazole regimen demonstrated significantly higher mycological cure rates (85.7%, 80.0% and 74.6% at three follow-up periods, respectively) compared to the two-dose clotrimazole regimen (76.0%, 61.6%, and 59.8%, all P < 0.05). The three-dose fluconazole regimen showed no significant difference to three-dose clotrimazole regimen, with cure rates of 82.8%, 79.3%, and 75.9% (all P > 0.05). The two-dose fluconazole regimen had cure rates of 74.3%, 56.4% and 51.1%, with no significant difference from two-dose clotrimazole regimen at days 7–14 and 25–35, but lower than three-dose fluconazole regimen at days 25–35 and 35 to 6 months. The three-dose clotrimazole regimen demonstrated higher cure rates in Candida albicans and non-albicans Candida SVVC cases than two-dose regimen. These findings suggest that three-dose antifungal regimens may be more efficacious than two-dose regimens for SVVC. The three-dose clotrimazole regimen could serve as a promising alternative for SVVC management.
Keywords: Severe vulvovaginal candidiasis, Clotrimazole, Fluconazole
Introduction
The epidemiological data collection and accurate diagnosis of vulvovaginal candidiasis (VVC), present significant challenges. It is estimated that approximately 70–75% of women will experience episodes of VVC during their lifetime. Globally, the incidence of VVC ranges from 12.1 to 57.3%. A notable 40–50% of those affected are likely to experience recurrent episodes, predominantly among women aged 20–40 [1, 2]. VVC is not only a significant public health concern but also imposes considerable socio-economic burdens, substantially impacting women's psychological well-being. VVC increases stress in women's lives, decreases self-esteem and confidence, fosters social anxiety, and affects overall life satisfaction [3]. In high-income countries, it is estimated that the economic burden caused by lost productivity due to recurrent vulvovaginal candidiasis (RVVC) alone can reach as high as $14.39 billion annually [4]. During pregnancy, VVC can significantly increase the rate of miscarriages and hinder placental development, ultimately leading to adverse pregnancy outcomes [5]. Without proper treatment, VVC can result in various complications, such as pelvic inflammatory disease (PID), infertility, ectopic pregnancies, pelvic abscesses, miscarriages, and menstrual irregularities. Therefore, prevention, early diagnosis, and timely standardized treatment of VVC are paramount.
The main clinical goal of VVC treatment is to treat vaginal mucosal inflammation and eliminate the microorganisms causing the disease, while mycological cure occurs 4–7 days after treatment initiation [6].The sensitivity of all strains to fluconazole decreased significantly [7]. Fluconazole is one of the most commonly used drugs in the treatment of VVC. However, due to the results of long-term use, drug-resistance cases of Candida albicans and non-Candida albicans have also been reported. Patients with oral drug preparations may also develop allergies, hepatotoxicity and headaches, and interactions with other drugs may reduce the efficacy of fluconazole [6]. Fluconazole resistance was most common (6%) among drug-resistant Candida albicans strains [8]. Therefore, it is suggested that fluconazole should not be the absolutely choice for RVVC treatment. Recent and repeated exposure to fluconazole is considered the primary reason for fluconazole-resistant VVC. Unnecessary and inappropriate use of fluconazole should be avoided. After discontinuing of fluconazole, resistant Candida albicans strains have been shown to revert to susceptibility over time [9, 10]. Hence, treatment regimens primarily based on local medications for VVC, reducing fluconazole resistance and limiting exposure to more antifungal drugs, are beneficial.
VVC can be classified into uncomplicated VVC and complicated VVC, which includes RVVC, severe vulvovaginal candidiasis (SVVC), and VVC caused by non-albicans Candida (NAC) species [11]. SVVC is defined as VVC with extensive vulvar erythema, edema, excoriation, and fissure formation, with a VVC score of ≥ 7 [11–13]. Current domestic and international guidelines have recommended using two doses of fluconazole 150 mg (on the first and fourth day) for SVVC [11]. The Infectious Diseases Society of America (IDSA) guideline in 2016 and the American College of Obstetricians and Gynecologists (ACOG) guideline in 2020 recommended for the treatment of SVVC use of fluconazole 150 mg every 72 h for a total of two or three doses [14, 15]. However, there is a lack of research and literature evidence supporting the specific differences in efficiency between the three-dose and two-dose regimens, both domestically and internationally.
Patients and Methods
Study Design and Participants
This real-world study involved patients with severe vulvovaginal candidiasis (SVVC) treated in the Gynecology Outpatient Department of Peking University Shenzhen Hospital between January 1, 2013, and December 31, 2022. General information and vaginal secretion specimens of the patients were collected. The study protocol received approval from the hospital's ethical review committee (No. 2014-002), and informed consent was obtained from all participants. After obtaining informed consent, SVVC patients were divided into four groups based on the medication regimen, clotrimazole vaginal tablets (Canesten, 500 mg/tablet, Bayer Medical and Healthcare Co., Ltd.) and oral fluconazole capsule (Diflucan, 150 mg/tablet, Pfizer Pharmaceutical Co., Ltd.).
These four groups included the following regimens, two doses of clotrimazole vaginal suppository 500 mg with a 72-h interval on days 1 and 4 (two-dose clotrimazole group), two doses of oral fluconazole 150 mg with a 72-h interval on days 1 and 4 (two-dose fluconazole group), three doses of clotrimazole vaginal suppository 500 mg with a 72-h interval on days 1, 4 and 7 (three-dose clotrimazole group), and three doses of oral fluconazole 150 mg with a 72-h interval on days 1, 4 and 7 (three-dose fluconazole group).
Patients with SVVC were followed up at three different time intervals: days 7–14 after the initial visit (short-term efficacy), days 25–35 (long-term efficacy), and day 35 to 6 months.
During the follow-up period, the vaginal secretions of the patients were collected for fungal cultures according to the standard procedure.
Cases of SVVC mixed infected by more than one strain were excluded, the number of strains was equal to the number of cases.
Definition of VVC Cases
A VVC case was defined as the presence of clinical symptoms, detection of spores and pseudohyphae on a microscopic examination of vaginal secretions treated with 10% potassium hydroxide, as well as positive fungal cultures.
The severity of each symptom and sign (including itching, burning, discharge, and erythema) was graded on the following scale: 0 = absent, 1 = mild, 2 = moderate, 3 = severe. Patients with a severity score of 7 or higher were designated as having SVVC [12, 13].
Sample Data Collection
Patients were scheduled for follow-up visits at the gynecology outpatient department on days 7–14, days 25–35, and day 35 to 6 months post-initial treatment for data collection and vaginal secretion sampling.
Mycological cure was defined as negative results in fungal cultures indicative of cure, whereas positive results were indicated treatment failure.
Vaginal secretion cultures were performed in the Gynecology Laboratory of Peking University Shenzhen Hospital. Swabs were cultured on Sabouraud's dextrose agar at 30 °C for 48 h, after which individual colonies were collected. Separate colonies were initially identified as Candida albicans or NAC using Chromogenic agar medium (CHROMagar, France), with further identification of NAC species using the API 20C AUX system (BioMérieux S.A., France).
Statistical Analysis
Statistical analysis was performed using the chi-squared test and Fisher's exact test to compare follow-up mycological cure rates and assess treatment efficacy, with P < 0.05 indicating statistical significance. Statistical analysis and figure generation were conducted using SPSS 26.0 (IBM Corp., Armonk, NY, USA) and GraphPad Prism (version 8.2.1, GraphPad Software, San Diego, California, USA).
Results
Patient Characteristics and Candida strains
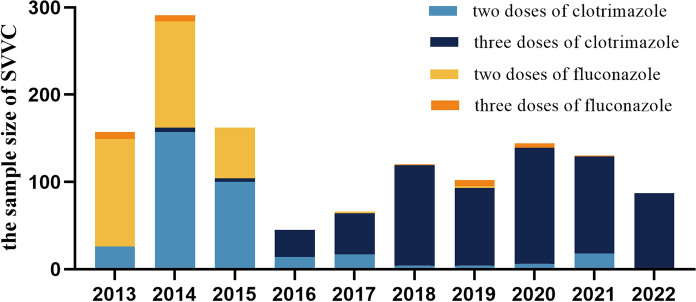
This investigation encompassed a comprehensive study of 1303 cases of SVVC treated at the gynecology outpatient department of Peking University Shenzhen Hospital from 2013 to 2022 (Fig. 1). The distribution of SVVC cases for each treatment regimen is as follows, two-dose clotrimazole (346/1303, 26.5%), three-dose clotrimazole (621/1303, 47.7%), two-dose fluconazole (307/1303, 23.6%) and three-dose fluconazole (29/1303, 2.2%).
Fig. 1.
Distribution of SVVC samples at Peking University Shenzhen Hospital, 2013–2022
The average age of patients was 31.57 (± 7.09) years. SVVC cases were diagnosed throughout the entire decade, the number of cases was relatively low in some years. The use of the three-dose clotrimazole regimen gradually increased after 2016. A total of 9 Candida species were identified, including C. albicans (1093/1303, 83.9%), C. glabrata (143/1303, 10.9%), C. parapsilosis (32/1303, 2.4%), C. tropicalis (12/1303, 0.9%), C. krusei (12/1303, 0.9%), Saccharomyces cerevisiae (brewer's yeast) (6/1303, 0.5%), C. kefyr (3/1303, 0.3%), C. membranifaciens (1/1303, 0.1%) and C. utilis (1/1303, 0.1%).
Treatment Outcomes of Two Dosages and Three Dosages
The three-dose regimen of clotrimazole or fluconazole groups showed higher mycological cure rates than two-dose regimen groups. There was no significant difference between three-doses regimen of clotrimazole and fluconazole, and no significant difference between the two-doses (Table 1).
Table 1.
Mycological Follow-up Cure Rate of the Three-dose Regimen and the Two-dose Regimen
| Follow-up efficacy | Clotrimazole | Fluconazole | ||
|---|---|---|---|---|
| Three-dose regimen (%) | Two-dose regimen (%) | Three-dose regimen (%) | Two-dose regimen (%) | |
|
Days 7–14 (short-term efficacy) |
532 (85.7%) | 263 (76.0%)* | 24 (82.8%) | 228 (74.3%) |
|
Days 25–35 (long-term efficacy) |
497 (80.0%) | 213 (61.6%)* | 23 (79.3%) | 173 (56.4%)# |
| Day 35 to 6 month | 463 (74.6%) | 207 (59.8%)* | 22 (75.9%) | 157 (51.1%)#,§ |
*Compared with three-dose regimen of clotrimazole, P < 0.05
#Compared with three-dose regimen of fluconazole, P < 0.05
§Compared with two-dose regimen of clotrimazole, P < 0.05
Treatment Outcomes for Candida albicans and NAC SVVC
Candida albicans (CA) SVVC showed a higher mycological cure rates than NAC SVVC, and the trends among the different regimens were similar to the overall results. Three-dose regimens of clotrimazole or fluconazole groups showed a higher mycological cure rates than two-dose regimen groups in the CA and NAC groups, respectively. Candida glabrata (CG) SVVC showed a lower mycological cure rates than NAC compared to CG SVVC. The three-dose clotrimazole regimen showed a higher mycological cure rate than the two-dose regimen groups in CG and NAC other than CG groups, respectively. The three-dose fluconazole regimen for NAC SVVC was not statistically significant because the sample size was too small (Table 2).
Table 2.
Mycological Follow-up Cure Rate of the Candida albicans SVVC, non-albicans Candida SVVC, Candida glabrata SVVC and NAC other than CG SVVC
| Regimens | Follow-up efficacy | CA (%) | NAC (%) | CG (%) | NAC other than CG (%) |
|---|---|---|---|---|---|
| Three-dose clotrimazole | Days 7–14 | 475(89.5%) | 57 (63.3%)* | 33 (51.6%) | 24 (92.3%)† |
| Days 25–35 | 444 (83.6%) | 53 (58.9%)* | 30 (46.9%) | 23 (88.5%)† | |
| Day 35 to 6 month | 411 (77.4%) | 52 (57.8%)* | 29 (45.3%) | 23 (88.5%)† | |
| Two-dose clotrimazole | Days 7–14 | 231 (84.9%) | 32 (43.2%)§ | 13 (27.1%)† | 19 (73.1%)‡ |
| Days 25–35 | 184 (67.6%)* | 29 (39.2%)§ | 138 (27.1%)† | 16 (61.5%)‡ | |
| Day 35 to 6 month | 178 (65.4%)* | 29 (39.2%)§ | 13 (27.1%)† | 16 (61.5%)‡ | |
| Three-dose fluconazole | Days 7–14 | 24 (92.3%) | 0(-) | 0 (-) | – |
| Days 25–35 | 23 (88.5%) | 0(-) | 0 (-) | – | |
| Day 35 to 6 month | 22 (84.6%) | 0(-) | 0 (-) | – | |
| Two-dose fluconazole | Days 7–14 | 210 (79.5%) | 18 (41.9%) | 10 (35.7%) | 8 (53.3%) |
| Days 25–35 | 160 (60.6%)# | 13 (30.2%) | 7 (25.0%) | 6 (40.0%) | |
| Day 35 to 6 month | 144 (54.5%)# | 13 (30.2%) | 7 (25.0%) | 6 (40.0%) |
*Compared with the CA three-dose regimen of clotrimazole, P < 0.05
#Compared with the CA three-dose regimen of fluconazole, P < 0.05
¶Compared with the CA two-dose regimen of clotrimazole, P < 0.05
§Compared with the NAC three-dose regimen of clotrimazole, P < 0.05
†Compared with the CG three-dose regimen of clotrimazole, P < 0.05
‡Compared with the CG two-dose regimen of clotrimazole, P < 0.05
Discussion
This study evaluated the efficacy of three-dose versus two-dose antifungal treatment of SVVC. It was found that the mycological cure rate of three-dose antifungal drugs was higher than that of two doses, especially the comprehensive mycological cure rate of clotrimazole is better than that of fluconazole. The three-dose fluconazole regimen seems to demonstrate slightly higher mycological cure rate than that of three-dose clotrimzole regimen on day 35 to 6 month. However, numerous factors can influence SVVC treatment outcomes, and further research is needed to investigate the impact of various factors on SVVC treatment effectiveness. Currently, clinical practice involves both overuse and underuse of treatment, resulting in low cure rates and recurrent infections, as well as increasing antifungal drug resistance. Standard therapies for VVC include azole antifungal drugs such as clotrimazole, fluconazole, voriconazole, itraconazole, ketoconazole and miconazole, which inhibit ergosterol synthesis of ergosterols in fungal cell membrane. Notably, fluconazole and clotrimazole have diverse therapeutic dosages and regimens for VVC treatment.
Compared with clotrimazole, fluconazole has been reported more frequently in all types of VVC. Two 1990s studies showed that in single-dose clotrimazole 500 mg vaginal tablets in the treatment of uncomplicated VVC, the mycological cure rate was 72% (214 cases, United Kingdom). In clotrimazole, 200 mg vaginal tablets for three consecutive days for the treatment of acute VVC, the clinical cure rate of clotrimazole was 100% (95 cases, United States) [16, 17]. In recent years, there has been limited large-scale research data on the specific clinical and mycological cure rates of clotrimazole in VVC. Fan et al. reported that in the randomized controlled trial (RCT) study of SVVC, the mycological cure rate of the two-dose 150 mg fluconazole was 84.0% on days 7–14 and 69.7% on days 30–35 follow-up. The clinical cure rate was similar to the mycological cure rate (577 cases, China) [18]. In the prospective case–control study by Li et al., the clinical cure rate and mycological cure rate of two-dose 150 mg fluconazole were 75.8% and 71.2% respectively on days 7–14 follow-up, and the clinical cure rate and mycological cure rate were 56.1% and 53.0% respectively on days 30–35 follow-up. Local irritation was the main adverse reaction related to vaginal administration, while systemic side effects were related to fluconazole (140 cases, China) [19]. Fluconazole is also widely used in RVVC. The mycological cure rate of fluconazole was 73.8% after initial treatment, 72.7% at the end of maintenance treatment, and 82.19% after six months of treatment. The mycological cure rate of fluconazole in RVVC caused by Candida albicans was 81.8% and that of RVVC caused by Candida glabrata was 12.5% (293 cases, China) [20]. Fluconazole can also treat most VVC caused by infection with NAC strains. Powell et al. reported that fluconazole as initial treatment was effective in 60% of C. glabrata and 81% of C. parapsilosis patients, and that symptom improvement in 52.7%, 66.7%, and 57.1% of C. glabrata, C. parapsilosis, and C. tropicalis cases (108 cases, United States) [21]. In a study of acute VVC, 84.7% were clinically cured, 80.5% were mycological cured in 150 mg oral fluconazole, and 83.3% were clinically cured and 70% were mycological cured in 200 mg clotrimazole vaginal use for seven days (142 cases, Iran) [22]. In a phase II clinical study of acute VVC, the cure rate of the fluconazole group was 62.5%, and that of different doses and regimens of oteseconazole (VT-1161) was 75.0–85.7% (55 cases, United States) [23]. At present, oral medications are the most commonly used in the treatment of VVC, but they are contraindicated during pregnancy and may have common limitations in terms of side effects and toxicity, the incidence of fluconazole allergy rate is not clear, but if angioedema or severe rash occurs, vaginal table is preferred [10, 24]. A review by Denison et al. showed that oral anti-fungal treatment probably improves short-term and long-term mycological cure rate over intra-vaginal for uncomplicated VVC. Still, the certainty of this evidence is low [25]. C. glabrata and C. krusei infections are usually resistant to fluconazole, and pregnant women should avoid fluconazole as given cases report fetal craniofacial and heart abnormalities. Still, the current data on abortion are contradictory [19]. An RCT study of SVVC reported that the clinical cure rates of two-dose 500 mg clotrimazole and two-dose 150 mg fluconazole were 88.7% and 89.1%, mycological cure rates were 78.3% and 73.6% on days 7–14 follow-up, and on days 30–35 follow-up the clinical cure rates were 71.9% and 78.0%, the mycological cure rates were 54.4% and 56.0% (240 cases, China). In most cases, local treatment of vaginal infections is as effective as oral treatment, with higher local drug concentrations, fewer drug interactions and adverse reactions [26, 27]. A study of a series of products with different formulations of clotrimazole and fluconazole reported that more than 90% of patients showed improvement in symptoms after treatment, 42% felt improvement within four hours after the first use, 76%-88% of symptoms were relieved within one day, and the overall rate of symptom relief of oral drugs was slightly longer than that of vaginal preparations (475 cases, United Kingdom and Canada) [28]. The high systemic toxicity of some antifungal drugs and the potential to disrupt vaginal microecology has prompted the exploration of the vaginal mucosa as a way of drug administration. Compared with oral administration, vaginal mucosal administration avoids the first-pass effect of the liver and systemic pre-elimination in the gastrointestinal tract. The vaginal mucosa also has a dense vascular network and a larger surface area, allowing for rapid drug absorption. At present, the commonly used local drugs in the drug market include clotrimazole, nystatin, ketoconazole and so on. Moreover, due to the current indiscriminate use of antifungal drugs, some fungi have developed resistance, which makes it challenging to control recurring infections and leads to further opportunities for antifungal drug use, forming a vicious cycle. Therefore, it is necessary to develop more effective treatments or drugs.
For VVC caused by NAC, non-fluconazole antifungal drugs may be a good choice. It is believed that the abuse of both over-the-counter and prescription antifungal drugs may lead to selective NAC VVC. In a study of 785 VVC strains, 99 strains (12.6%) were fluconazole-resistant, most of which were NAC. One of the main mechanisms of drug resistance is the overexpression of efflux pump-related genes, resulting in reduced drug concentrations inside fungal cells [29]. Candida albicans can usually be isolated from the vulva or vaginal specimens of up to 20% of asymptomatic reproductive-age women. As the vaginal defense mechanisms do not work quickly, the presence of yeast cells in the vagina may not represent actual colonization, but rather a transitional or subclinical infection. During asymptomatic intervals between clinical symptoms, yeast may persist in the vagina or vulva even after antifungal treatment. Thus, many recurrences may result from the continuous presence of yeast strains after long-term antifungal treatment rather than from exogenous reinfection. In fact, in over two-thirds of recurrent cases, the strains isolated before and after treatment were the same [26]. Therefore, increasing the dose of antifungal drug can enhance the chance of fungal eradication under higher drug load, may reduce the recurrence rate of SVVC, reduce the exposure chance of antifungal drugs, and may decrease antifungal drug resistance indirectly.
The single center, lack of RCT and clotrimazole combination regimen were not included in the analysis were limitations of this study. The correlation between antifungal drug sensitivity and clinical results of VVC is inconsistent. In the current research, the resistance of candida to fluconazole were rare, and there is no difference in the proportion of strains resistant to fluconazole between cured patients and failed patients [30, 31]. Therefore, this study did not introduce the results of antifungal drug sensitivity. Various factors, including patient hormone levels, pregnancy, microbial flora, age, enzyme activity, pH, content and composition of cervical vaginal fluid may affect the transport and distribution of drugs on the vaginal mucosa. The physicochemical properties of clotrimazole will also affect drug transport, thereby affecting effectiveness [32]. Other factors that influence clotrimazole in the treatment of SVVC need to be further explored. Currently, continuous monitoring of epidemic strains and drug sensitivity is not only an epidemiological problem, but a necessary step to guide appropriate clinical practice. For refractory cases, individualized treatment plans should be based on the basic situation of each patient and the results of drug sensitivity in vitro.
Conclusion
The three-dose antifungal regimen demonstrates a higher mycological cure rate than the two-dose regimen. Candida albicans remains the most prevalent species in SVVC, followed by Candida glabrata. The mycological cure rate of Candida albicans SVVC and NAC SVVC were similar to the overall results. In Candida glabrata SVVC, the three-dose clotrimazole regimen shows a higher cure rate than the two-dose regimen, but overall cure rates remain modest. This study is based on real-world data, and it is hoped that future RCT will further investigate the efficacy of three-dose antifungal therapy and explore the influencing factors of treatment failure. Therefore, with the gradual increase of clinical resistance to fluconazole, the three-dose clotrimazole regimen emerges as a promising alternative for treating SVVC.
Author’s Contributions
Zhansong Xiao, Yuxia Zhu, Liting Huang and Yiheng Liang contributed to the data collection. Zhansong Xiao wrote the manuscript and conducted data analysis for the study. Shangrong Fan and Xiaowei Zhang supervised and evaluated the manuscript revision and read and approved the submitted version.
Funding
This research was supported by the Shenzhen Science and Technology Innovation Commission (JCYJ20190809101409603), Science and Technology Planning Project of Shenzhen Municipality (JCYJ20200109140614667 and JCYJ20220530160206014), the National Natural Science Foundation of China (82171676 and 82201793), and Scientific Research Foundation of Peking University Shenzhen hospital (KYQD2022111 and KYQD2021090).
Declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sobel JD. Vulvovaginal candidosis. Lancet. 2007;369(9577):1961–71. [DOI] [PubMed] [Google Scholar]
- 2.Gonçalves B, Ferreira C, Alves CT, Henriques M, Azeredo J, Silva S. Vulvovaginal candidiasis: epidemiology, microbiology and risk factors. Crit Rev Microbiol. 2016;42(6):905–27. [DOI] [PubMed] [Google Scholar]
- 3.Sobel JD, Nyirjesy P. Oteseconazole: an advance in treatment of recurrent vulvovaginal candidiasis. Fut Microbiol. 2021;16:1453–61. [DOI] [PubMed] [Google Scholar]
- 4.Denning DW, Kneale M, Sobel JD, Rautemaa-Richardson R. Global burden of recurrent vulvovaginal candidiasis: a systematic review. Lancet Infect Dis. 2018;18(11):e339–47. [DOI] [PubMed] [Google Scholar]
- 5.Dong Z, Fan C, Hou W, Rui C, Wang X, Fan Y, et al. Vaginal exposure to Candida albicans during early gestation results in adverse pregnancy outcomes via inhibiting placental development. Front Microbiol. 2022;12: 816161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Conte J, Parize AL, Caon T. Advanced solid formulations for vulvovaginal candidiasis. Pharm Res. 2023;40(2):593–610. [DOI] [PubMed] [Google Scholar]
- 7.Wang FJ, Zhang D, Liu ZH, Wu WX, Bai HH, Dong HY. Species distribution and in vitro antifungal susceptibility of vulvovaginal candida isolates in China. Chin Med J. 2016;129(10):1161–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kan S, Song N, Pang Q, Mei H, Zheng H, Li D, et al. In vitro antifungal activity of azoles and other antifungal agents against pathogenic yeasts from vulvovaginal candidiasis in China. Mycopathologia. 2023;188(1–2):99–109. [DOI] [PubMed] [Google Scholar]
- 9.Sobel JD, Sebastian S, Boikov DA. A longitudinal study on fluconazole resistance in Candida albicans vaginal isolates. Mycoses. 2023;66(7):563–5. [DOI] [PubMed] [Google Scholar]
- 10.Marchaim D, Lemanek L, Bheemreddy S, Kaye KS, Sobel JD. Fluconazole-resistant Candida albicans vulvovaginitis. Obstet Gynecol. 2012;120(6):1407–14. [DOI] [PubMed] [Google Scholar]
- 11.Workowski KA, Bachmann LH, Chan PA, Johnston CM, Muzny CA, Park I, et al. Sexually transmitted infections treatment guidelines, 2021. MMWR Recomm Rep. 2021;70(4):1–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sobel JD, Kapernick PS, Zervos M, Reed BD, Hooton T, Soper D, et al. Treatment of complicated Candida vaginitis: comparison of single and sequential doses of fluconazole. Am J Obstet Gynecol. 2001;185(2):363–9. [DOI] [PubMed] [Google Scholar]
- 13.Wang X, Wang W, Li J, An R, Chen L, Lin J, et al. Efficacy and safety of oral ibrexafungerp in Chinese patients with vulvovaginal candidiasis: a phase III, randomized, double-blind study. Infection. 2024; 3. [DOI] [PMC free article] [PubMed]
- 14.Pappas PG, Kauffman CA, Andes DR, Clancy CJ, Marr KA, Ostrosky-Zeichner L, et al. Clinical practice guideline for the management of candidiasis: 2016 update by the infectious diseases society of America. Clin Infect Dis. 2016;62(4):e1-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Paavonen JA, Brunham RC. Vaginitis in nonpregnant patients: ACOG practice bulletin number 215. Obstet Gynecol. 2020;135(5):1229–30. [DOI] [PubMed] [Google Scholar]
- 16.Tobin JM, Loo P, Granger SE. Treatment of vaginal candidosis: a comparative study of the efficacy and acceptability of itraconazole and clotrimazole. Genitourin Med. 1992;68(1):36–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stein GE, Mummaw N. Placebo-controlled trial of itraconazole for treatment of acute vaginal candidiasis. Antimicrob Agents Chemother. 1993;37(1):89–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fan S, Liu X, Liang Y. Miconazole nitrate vaginal suppository 1200 mg versus oral fluconazole 150 mg in treating severe vulvovaginal candidiasis. Gynecol Obstet Invest. 2015;80(2):113–8. [DOI] [PubMed] [Google Scholar]
- 19.Li T, Zhu Y, Fan S, Liu X, Xu H, Liang Y. A randomized clinical trial of the efficacy and safety of terconazole vaginal suppository versus oral fluconazole for treating severe vulvovaginal candidiasis. Med Mycol. 2015;53(5):455–61. [DOI] [PubMed] [Google Scholar]
- 20.Fan S, Liu X, Wu C, Xu L, Li J. Vaginal nystatin versus oral fluconazole for the treatment for recurrent vulvovaginal candidiasis. Mycopathologia. 2015;179(1–2):95–101. [DOI] [PubMed] [Google Scholar]
- 21.Powell AM, Gracely E, Nyirjesy P. Non-albicans Candida Vulvovaginitis: treatment experience at a tertiary care vaginitis center. J Low Genit Tract Dis. 2016;20(1):85–9. [DOI] [PubMed] [Google Scholar]
- 22.Sekhavat L, Tabatabaii A, Tezerjani FZ. Oral fluconazole 150 mg single dose versus intra-vaginal clotrimazole treatment of acute vulvovaginal candidiasis. J Infect Public Health. 2011;4(4):195–9. [DOI] [PubMed] [Google Scholar]
- 23.Brand SR, Sobel JD, Nyirjesy P, Ghannoum MA, Schotzinger RJ, Degenhardt TP. A randomized phase 2 study of VT-1161 for the treatment of acute vulvovaginal candidiasis. Clin Infect Dis. 2021;73(7):e1518–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marnach ML, Wygant JN, Casey PM. Evaluation and management of vaginitis. Mayo Clin Proc. 2022;97(2):347–58. [DOI] [PubMed] [Google Scholar]
- 25.Denison HJ, Worswick J, Bond CM, Grimshaw JM, Mayhew A, Gnani RS. Oral versus intra-vaginal imidazole and triazole anti-fungal treatment of uncomplicated vulvovaginal candidiasis (thrush). Cochrane Database Syst Rev. 2020;8(8):CD002845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Palmeira-de-Oliveira R, Palmeira-de-Oliveira A, Martinez-de-Oliveira J. New strategies for local treatment of vaginal infections. Adv Drug Deliv Rev. 2015;92:105–22. [DOI] [PubMed] [Google Scholar]
- 27.Zhou X, Li T, Fan S, Zhu Y, Liu X, Guo X, et al. The efficacy and safety of clotrimazole vaginal tablet vs oral fluconazole in treating severe vulvovaginal candidiasis. Mycoses. 2016;59(7):419–28. [DOI] [PubMed] [Google Scholar]
- 28.Zhang L, De Salvo R, Ehret A, Young K, Trapp S. Vulvovaginal candidiasis: a real-world evidence study of the perceived benefits of Canesten®. SAGE Open Med. 2022;10:20503121221085436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jafarzadeh L, Ranjbar M, Nazari T, Naeimi Eshkaleti M, Aghaei Gharehbolagh S, Sobel JD, et al. Vulvovaginal candidiasis: an overview of mycological, clinical, and immunological aspects. J Obstet Gynaecol Res. 2022;48(7):1546–60. [DOI] [PubMed] [Google Scholar]
- 30.Fan SR, Liu XP. In vitro miconazole susceptibility and clinical outcome in vulvovaginal candidiasis. Int J Gynaecol Obstet. 2007;97(3):207–8. [DOI] [PubMed] [Google Scholar]
- 31.Fan SR, Liu XP. In vitro fluconazole and nystatin susceptibility and clinical outcome in complicated vulvovaginal candidosis. Mycoses. 2011;54(6):501–5. [DOI] [PubMed] [Google Scholar]
- 32.Sofi HS, Abdal-Hay A, Ivanovski S, Zhang YS, Sheikh FA. Electrospun nanofibers for the delivery of active drugs through nasal, oral and vaginal mucosa: current status and future perspectives. Mater Sci Eng C Mater Biol Appl. 2020;111: 110756. [DOI] [PubMed] [Google Scholar]