Abstract
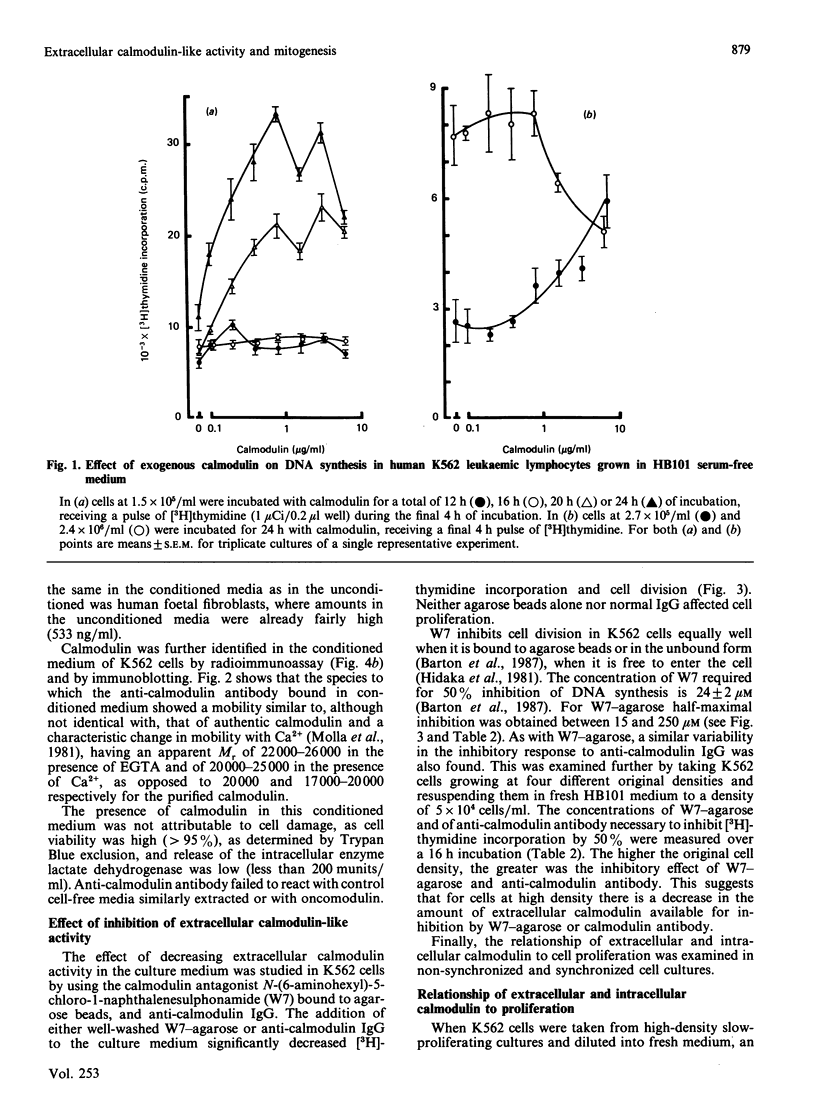
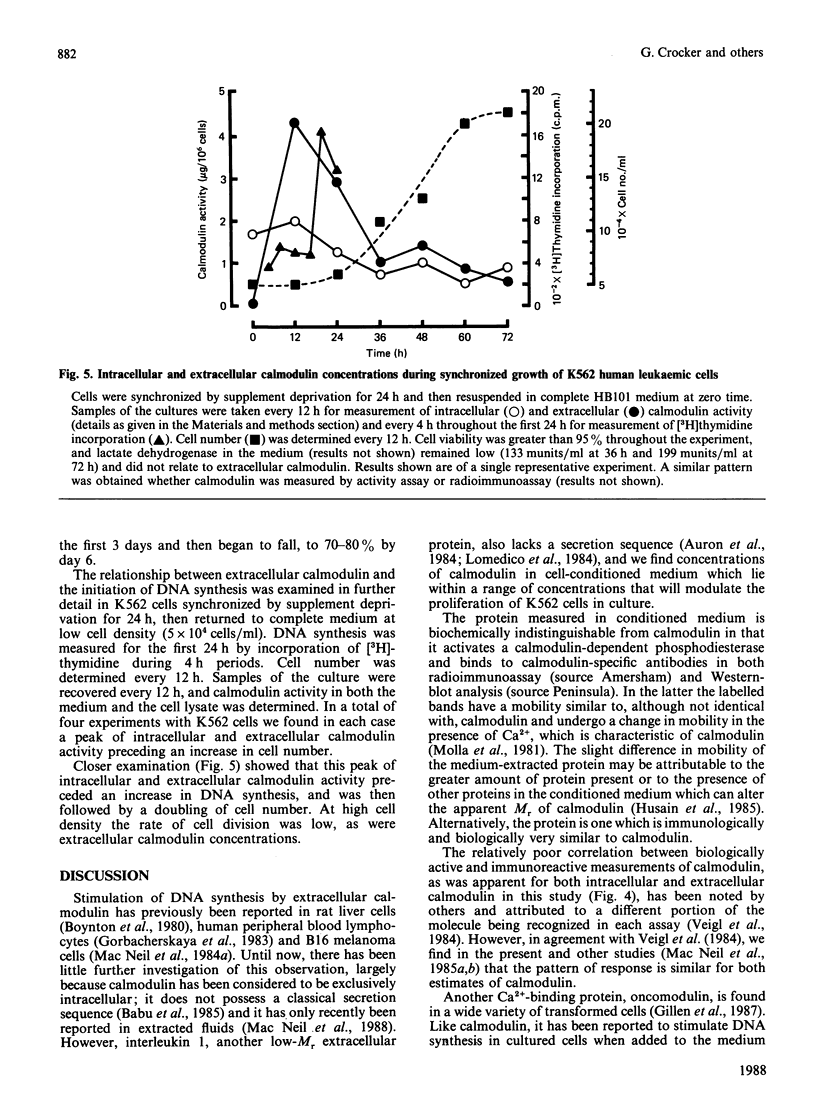
1. Addition of extracellular pure pig brain calmodulin was found to modulate DNA synthesis and cell proliferation in K562 human leukaemic lymphocytes. At lower cell densities calmodulin significantly stimulated [3H]thymidine uptake; at higher densities it decreased it. 2. A protein biochemically indistinguishable from calmodulin was detected in the cell-conditioned media of rapidly dividing K562 cells. The concentration of calmodulin-like activity found in the conditioned media of these and a range of other normal and neoplastic cells (250-1636 ng/ml) was of the same order as would stimulate DNA synthesis in subconfluent cells. 3. Amounts of extracellular calmodulin-like activity and immunoreactivity varied during cell growth from low to high density, a peak of extracellular calmodulin preceding DNA synthesis in synchronized K562 cells. Extracellular calmodulin concentrations did not correlate with the presence of lactate dehydrogenase in the medium. 4. Inhibition of extracellular calmodulin activity by calmodulin antagonist immobilized on agarose beads, or by antibody to calmodulin, significantly decreased DNA synthesis. 5. These data strongly suggest that calmodulin or a very closely related protein can influence mitosis through an extracellular mechanism.
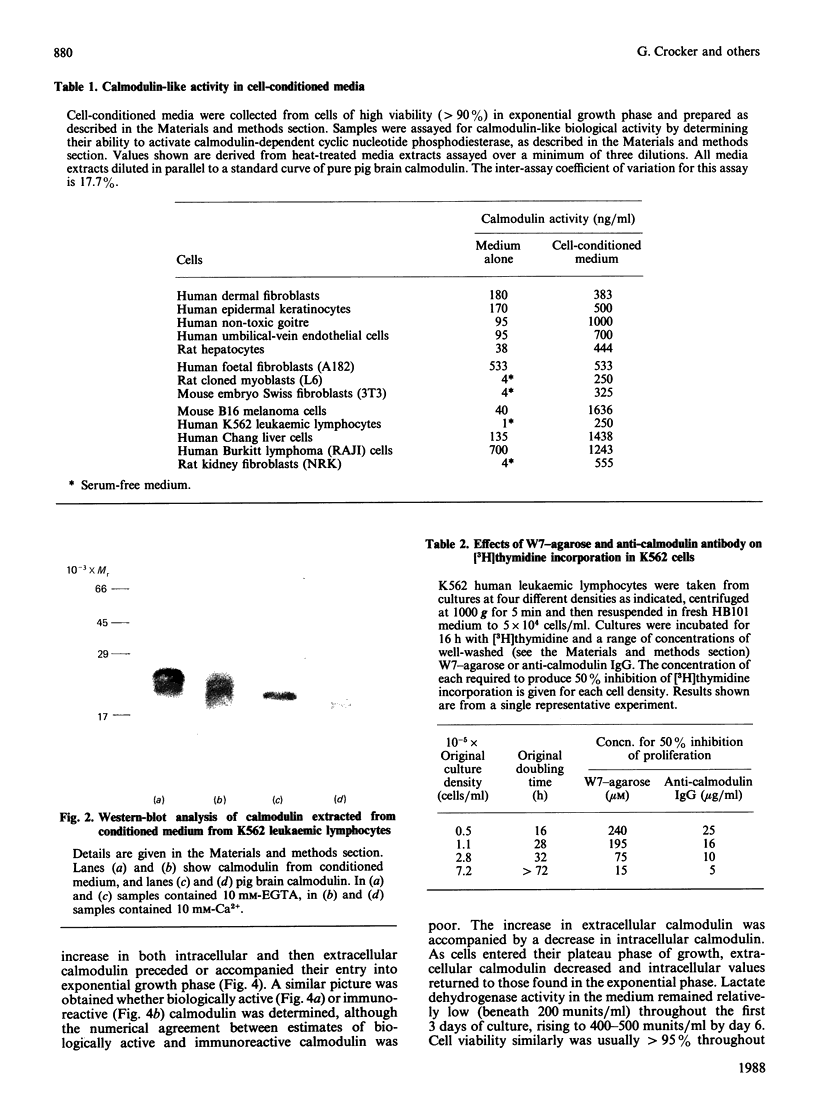
Full text
PDF

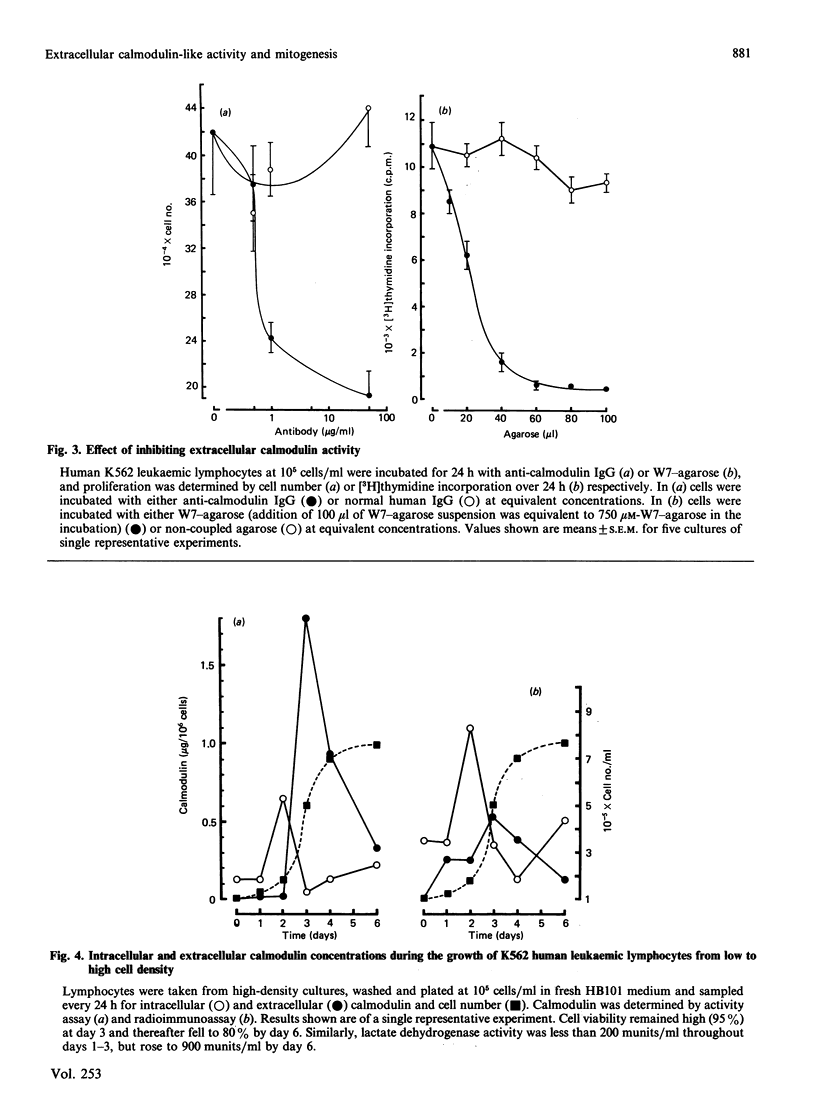


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auron P. E., Webb A. C., Rosenwasser L. J., Mucci S. F., Rich A., Wolff S. M., Dinarello C. A. Nucleotide sequence of human monocyte interleukin 1 precursor cDNA. Proc Natl Acad Sci U S A. 1984 Dec;81(24):7907–7911. doi: 10.1073/pnas.81.24.7907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babu Y. S., Sack J. S., Greenhough T. J., Bugg C. E., Means A. R., Cook W. J. Three-dimensional structure of calmodulin. Nature. 1985 May 2;315(6014):37–40. doi: 10.1038/315037a0. [DOI] [PubMed] [Google Scholar]
- Barton C. H., Blackburn G. M., Rees R., Bleehen S. S., Senior J. H., Munro D. S., MacNeil S. A comparative study of the anti-proliferative effects of calmodulin antagonists in cultured cells--W7 derivatives of improved cytostatic potential. Carcinogenesis. 1987 Jul;8(7):919–923. doi: 10.1093/carcin/8.7.919. [DOI] [PubMed] [Google Scholar]
- Bodine P. V., Tupper J. T. Calmodulin antagonists decrease the binding of epidermal growth factor to transformed, but not to normal, human fibroblasts. Biochem J. 1984 Mar 1;218(2):629–632. doi: 10.1042/bj2180629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boynton A. L., MacManus J. P., Whitfield J. F. Stimulation of liver cell DNA synthesis by oncomodulin, an MW 11 500 calcium-binding protein from hepatoma. Exp Cell Res. 1982 Apr;138(2):454–457. doi: 10.1016/0014-4827(82)90198-7. [DOI] [PubMed] [Google Scholar]
- Boynton A. L., Whitfield J. F., MacManus J. P. Calmodulin stimulates DNA synthesis by rat liver cells. Biochem Biophys Res Commun. 1980 Jul 31;95(2):745–749. doi: 10.1016/0006-291x(80)90849-9. [DOI] [PubMed] [Google Scholar]
- Brewer L. M., Durkin J. P., MacManus J. P. Immunocytochemical detection of oncomodulin in tumor tissue. J Histochem Cytochem. 1984 Oct;32(10):1009–1016. doi: 10.1177/32.10.6384359. [DOI] [PubMed] [Google Scholar]
- Chafouleas J. G., Bolton W. E., Hidaka H., Boyd A. E., 3rd, Means A. R. Calmodulin and the cell cycle: involvement in regulation of cell-cycle progression. Cell. 1982 Jan;28(1):41–50. doi: 10.1016/0092-8674(82)90373-7. [DOI] [PubMed] [Google Scholar]
- Chafouleas J. G., Lagacé L., Bolton W. E., Boyd A. E., 3rd, Means A. R. Changes in calmodulin and its mRNA accompany reentry of quiescent (G0) cells into the cell cycle. Cell. 1984 Jan;36(1):73–81. doi: 10.1016/0092-8674(84)90075-8. [DOI] [PubMed] [Google Scholar]
- Colca J. R., DeWald D. B., Pearson J. D., Palazuk B. J., Laurino J. P., McDonald J. M. Insulin stimulates the phosphorylation of calmodulin in intact adipocytes. J Biol Chem. 1987 Aug 25;262(24):11399–11402. [PubMed] [Google Scholar]
- Dorée M., Picard A., Cavadore J. C., Le Peuch C., Demaille J. G. Calmodulin antagonists and hormonal control of meiosis in starfish oocytes. Exp Cell Res. 1982 May;139(1):135–144. doi: 10.1016/0014-4827(82)90327-5. [DOI] [PubMed] [Google Scholar]
- Durham A. C., Walton J. M. Calcium ions and the control of proliferation in normal and cancer cells. Biosci Rep. 1982 Jan;2(1):15–30. doi: 10.1007/BF01142195. [DOI] [PubMed] [Google Scholar]
- Gillen M. F., Banville D., Rutledge R. G., Narang S., Seligy V. L., Whitfield J. F., MacManus J. P. A complete complementary DNA for the oncodevelopmental calcium-binding protein, oncomodulin. J Biol Chem. 1987 Apr 15;262(11):5308–5312. [PubMed] [Google Scholar]
- Graves C. B., Gale R. D., Laurino J. P., McDonald J. M. The insulin receptor and calmodulin. Calmodulin enhances insulin-mediated receptor kinase activity and insulin stimulates phosphorylation of calmodulin. J Biol Chem. 1986 Aug 5;261(22):10429–10438. [PubMed] [Google Scholar]
- Gregoriou M., Rees A. R. Studies on the structure and function of the epidermal-growth-factor receptor. Biochem Soc Trans. 1984 Apr;12(2):160–165. doi: 10.1042/bst0120160. [DOI] [PubMed] [Google Scholar]
- Hidaka H., Sasaki Y., Tanaka T., Endo T., Ohno S., Fujii Y., Nagata T. N-(6-aminohexyl)-5-chloro-1-naphthalenesulfonamide, a calmodulin antagonist, inhibits cell proliferation. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4354–4357. doi: 10.1073/pnas.78.7.4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husain A., Howlett G. J., Sawyer W. H. Analysis of the calcium-dependent interaction of calmodulin with bovine serum albumin. Anal Biochem. 1985 Mar;145(2):217–221. doi: 10.1016/0003-2697(85)90352-5. [DOI] [PubMed] [Google Scholar]
- Jones A., Boynton A. L., MacManus J. P., Whitfield J. F. Ca-calmodulin mediates the DNA-synthetic response of calcium-deprived liver cells to the tumor promoter TPA. Exp Cell Res. 1982 Mar;138(1):87–93. doi: 10.1016/0014-4827(82)90094-5. [DOI] [PubMed] [Google Scholar]
- Kakiuchi S., Sobue K., Yamazaki R., Kambayashi J., Sakon M., Kosaki G. Lack of tissue specificity of calmodulin: a rapid and high-yield purification method. FEBS Lett. 1981 Apr 20;126(2):203–207. doi: 10.1016/0014-5793(81)80242-6. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lomedico P. T., Gubler U., Hellmann C. P., Dukovich M., Giri J. G., Pan Y. C., Collier K., Semionow R., Chua A. O., Mizel S. B. Cloning and expression of murine interleukin-1 cDNA in Escherichia coli. 1984 Nov 29-Dec 5Nature. 312(5993):458–462. doi: 10.1038/312458a0. [DOI] [PubMed] [Google Scholar]
- Mac Neil S., Dawson R., Tucker W. F., Clegg A., Platts A., Rees R. C. The calmodulin content of normal and leukaemic lymphocytes. Biosci Rep. 1985 Sep;5(9):721–727. doi: 10.1007/BF01119869. [DOI] [PubMed] [Google Scholar]
- Mac Neil S., Tucker W. F., Dawson R. A., Bleehen S. S., Tomlinson S. The calmodulin content of the epidermis in psoriasis. Clin Sci (Lond) 1985 Dec;69(6):681–688. doi: 10.1042/cs0690681. [DOI] [PubMed] [Google Scholar]
- Mac Neil S., Walker S. W., Seid J., Tomlinson S. Calmodulin in human serum and the specific release of calmodulin from calmodulin-rich platelets. Biosci Rep. 1984 Aug;4(8):643–650. doi: 10.1007/BF01121017. [DOI] [PubMed] [Google Scholar]
- Mac Neil S., Walker S. W., Senior H. J., Bleehen S. S., Tomlinson S. Effects of extracellular calmodulin and calmodulin antagonists on B16 melanoma cell growth. J Invest Dermatol. 1984 Jul;83(1):15–19. doi: 10.1111/1523-1747.ep12261637. [DOI] [PubMed] [Google Scholar]
- Molla A., Kilhoffer M. C., Ferraz C., Audemard E., Walsh M. P., Demaille J. G. Octopus calmodulin. The trimethyllysyl residue is not required for myosin light chain kinase activation. J Biol Chem. 1981 Jan 10;256(1):15–18. [PubMed] [Google Scholar]
- Mooibroek M. J., Michiel D. F., Wang J. H. Clathrin light chains are calcium-binding proteins. J Biol Chem. 1987 Jan 5;262(1):25–28. [PubMed] [Google Scholar]
- Moolenaar W. H., Defize L. H., de Laat S. W. Calcium in the action of growth factors. Ciba Found Symp. 1986;122:212–231. doi: 10.1002/9780470513347.ch13. [DOI] [PubMed] [Google Scholar]
- Rasmussen C. D., Means A. R. Calmodulin is involved in regulation of cell proliferation. EMBO J. 1987 Dec 20;6(13):3961–3968. doi: 10.1002/j.1460-2075.1987.tb02738.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selinfreund R., Lin P. H., Cooper J. L., Wharton W. Effects of phenothiazines on binding and processing of epidermal growth factor in 3T3 cells. Am J Physiol. 1986 Dec;251(6 Pt 1):C904–C911. doi: 10.1152/ajpcell.1986.251.6.C904. [DOI] [PubMed] [Google Scholar]
- Tomlinson S., MacNeil S., Walker S. W., Ollis C. A., Merritt J. E., Brown B. L. Calmodulin and cell function. Clin Sci (Lond) 1984 May;66(5):497–507. doi: 10.1042/cs0660497. [DOI] [PubMed] [Google Scholar]
- Veigl M. L., Vanaman T. C., Sedwick W. D. Calcium and calmodulin in cell growth and transformation. Biochim Biophys Acta. 1984;738(1-2):21–48. doi: 10.1016/0304-419x(84)90018-0. [DOI] [PubMed] [Google Scholar]
- Wong P. Y., Cheung W. Y. Calmodulin stimulates human platelet phospholipase A2. Biochem Biophys Res Commun. 1979 Sep 27;90(2):473–480. doi: 10.1016/0006-291x(79)91259-2. [DOI] [PubMed] [Google Scholar]
- Wong P. Y., Lee W. H., Chao P. H., Cheung W. Y. The role of calmodulin in prostaglandin metabolism. Ann N Y Acad Sci. 1980;356:179–189. doi: 10.1111/j.1749-6632.1980.tb29610.x. [DOI] [PubMed] [Google Scholar]