Abstract
Introduction
Despite an improved understanding of its pathogenesis, dry eye disease (DED) remains relatively underestimated and its treatment challenging. A better alignment between the clinical evaluation and the patient self-assessment also requires capturing the whole patient experience of DED. This project aimed to unveil this experience through narrative medicine (NM).
Methods
The project involved 38 expert centres in Italy and one in San Marino, targeting adult patients with DED, their informal caregivers and their treating ophthalmologists. Written narratives and sociodemographic and quality of life (QoL)-related data were anonymously collected through the project’s webpage. Narratives were analysed through MAXQDA (VERBI Software, Berlin, Germany), NM classifications and content analysis.
Results
A total of 171 patients with DED, 37 informal caregivers and 81 ophthalmologists participated in the research. DED was defined as a disabling condition by 19% of patients and 35% of caregivers; 70% of patients reported that a therapeutic alliance is an integral part of DED treatment and 32% hope for more effective therapies. Forty-four per cent of patients assessed their own QoL as good; however, DED emerged as importantly impacting work performance and social events. DED physical, emotional and economic burden and the cruciality of a trusting care relationship represent the main themes that emerged across all narratives, while empathy and effective treatment are among the factors favouring coping with DED.
Conclusion
This project marked a pioneering initiative investigating the lived experience of patients with DED through NM, simultaneously involving all viewpoints involved in the care pathway. NM enabled the unveiling of factors favouring the ability to cope with DED and its associated QoL implications and provided valuable insights to improve the therapeutic alliance.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40123-024-01033-7.
Keywords: Dry eye disease, Patient experience, Narrative medicine, Quality of life, Coping strategies
Key Summary Points
| Why carry out this study? |
| Dry eye disease (DED) is a prevalent, multifactorial condition that can severely affect patients’ quality of life (QoL), daily functioning and work productivity, and is associated with increased mental health burden. |
| Despite advancements in understanding DED, a better alignment is needed between clinical evaluation and patient self-assessment. Since qualitative issues are crucial to obtain a deeper comprehension of DED management, narrative medicine (NM) constitutes an innovative approach for investigating the issues of living with DED to complement clinical data with patient-reported information. |
| What was learned from the study? |
| Patients with DED may face disbelief about their limitations and a lack of understanding regarding symptom severity. DED underestimation by healthcare professionals may exacerbate anxiety and depression in patients. |
| DED is perceived as a disabling condition by a significant portion of patients and caregivers, underlining the need for greater recognition and support. |
| A therapeutic alliance between patients and healthcare professionals is crucial, with empathy and effective communication being fundamental in enabling patients to cope with DED. |
| DED carries a considerable economic burden, both in terms of expenses for the care pathway (e.g. treatments of eye specialist visits not covered by the Italian national healthcare system) and in terms of work performance. Patients highlighted the need for more affordable and accessible therapies. |
| NM provided a pioneering overview of the nature of living with DED in Italy. It revealed lesser-known practical, emotional, and social issues related to DED; furthermore, it underlined the importance of fostering empathy in care relationships and enhancing DED awareness to improve therapeutic alliances and, therefore, patient compliance and outcomes in the care pathway. |
| Overall, this research improved our understanding of DED by integrating the perspectives of patients, caregivers, and treating ophthalmologists, and offers insights to improve the therapeutic alliance and quality of care in DED. |
Introduction
Dry eye disease (DED) is a prevalent, multifactorial, chronic and progressive condition of the ocular surface, characterised by the loss of homeostasis of the tear film [1, 2]. A summary of available data suggested that DED prevalence ranges from 6% to 34% of the adult population worldwide, with a higher prevalence observed among the elderly [3, 4]; some of the variation in observed prevalence between studies is thought to be due to differences in the definition of DED used.
The onset and development of DED are influenced by both intrinsic (e.g. ageing, autoimmunity, drying medications) and extrinsic factors (e.g. desiccating environment, exposure) [5, 6]. Female sex, prolonged screen use, contact lens usage, and hypertension remain among the most common risk factors [7–10], while other contributing factors include ageing, lifestyle, medication usage, Sjögren’s syndrome, chronic inflammation and oxidative stress [11].
DED symptoms—including irritation, stinging, dryness, ocular fatigue and fluctuating visual disturbances [3]—can severely affect patients’ quality of life (QoL), daily activities and work productivity [12–16] and are associated with increased levels of anxiety and depression [17]. The socioeconomic costs of DED can be high, in terms of both direct (medical fees and treatment costs) and indirect (work absenteeism and impaired productivity) costs, with the greatest economic impact arising from indirect costs [18, 19].
Despite advances in understanding the pathogenesis, diagnosis and treatment of DED remain challenging [20]. Specifically, diagnosis is complicated by the multifactorial nature of DED, often leading to disparities between diagnostic tests and patient-reported symptoms [21, 22]. As reported in the literature, the impact of DED on patients’ lives can be evaluated by assessing symptoms and QoL. A standardised classification system that combines objective measurements with subjective assessment has been proposed to inform treatment strategies [2, 23]; however, QoL questionnaires may not fully capture the complete disease experience, as they may fail to integrate quantitative data with semantic meaning. Therefore, obtaining qualitative and patient-reported data is crucial to ensure comprehensive DED evaluation and management [24].
Narrative research has been endorsed by the World Health Organisation for investigating the subjective experience of a medical condition [25] within a real-world context [26] to complement quantitative and clinical data. Specifically, narrative medicine (NM), based on illness narratives [27], seeks to integrate the biomedical domain, focusing on the disease, with the personal (illness) and social experience (sickness) of a condition [28]. NM embraces the viewpoints of all individuals involved in the care pathway and thereby addresses feasible interventions for a particular disorder [29], enhancing the quality of clinical care, as evidenced by previous research [30–32].
Aim
The Dry Eye: Narrative Medicine for Ophthalmology (DINAMO) project collected and analysed narratives of patients with DED, their informal caregivers and their treating ophthalmologists. The research aimed (a) to unveil practical, emotional, and social challenges associated with DED, along with patients’ coping strategies and QoL; (b) to involve caregivers’ and ophthalmologists’ experiences to gain a deeper understanding of informal caregiving and the care relationship in DED; and (c) to offer valuable insights that contribute to a deeper understanding of DED and to advance clinical practices for its management.
Through NM, this project provided a pioneering overview of living with DED in Italy, integrating the viewpoints of all the actors involved in the care pathway.
Methods
The project was conducted between July 2022 and February 2023 across 38 Italian centres with expertise in DED as well as one hospital-based centre in San Marino (Supplementary Material Supplement 1). The project was designed to target adult patients with DED, their informal caregivers and their treating ophthalmologists.
In July and September 2023, ophthalmologists from these centres participated in a webinar conducted by researchers from ISTUD (Istituto Studi Direzionali), Healthcare Area. The webinar aimed to train these clinicians in NM and to discuss the project aim, design, and investigation tools. Afterwards, these ophthalmologists were invited to engage patients with DED and their informal caregivers to join the project by accessing the webpage https://www.medicinanarrativa.eu/dinamo. However, participation in the research was entirely voluntary.
A diagnosis of DED at any stage (mild, moderate, severe) and without a minimum follow-up period after diagnosis, or being an informal caregiver of a person diagnosed with DED, were the sole inclusion criteria for patients and caregivers, respectively. However, proficiency in Italian writing and a basic level of computer literacy were essential for participation in the project. In the event that participants lacked either of these abilities, they were entitled to request assistance.
Narrative and Data Collection
Written narratives were collected anonymously via the Alchemer platform (LLC, CO, USA), which participants accessed anonymously through the project webpage. Researchers adhered to the Web Content Accessibility Guidelines 2.1 [33] to ensure survey accessibility; no sensitive data were requested.
Upon accessing the online platform, patients and caregivers were requested to answer a sociodemographic and QoL survey and to write their narrative supported by an illness plot [34], characterised by evocative words. This encouraged individual expression and was chronologically structured to guide the narrative and to identify changes over time [35]. The experience of the treating ophthalmologists was gathered on the same platform through the parallel chart [36], i.e. a personal block, parallel to the clinical chart, to note their own considerations and emotions related to the care relationship in plain language. The patients described in these parallel charts could not include patients participating in the project. Overall, these investigation tools (Supplement 2) addressed two common aspects: (a) the personal, emotional and care experience of DED from the onset of symptoms; and (b) the QoL perception and the current daily life and care pathway with DED.
Data collection tools were designed by two ISTUD researchers with different academic backgrounds and subsequently reviewed by the project steering committee, which included three internationally experienced DED ophthalmologists, to reduce any potential cognitive bias.
Ethical Considerations
The project was performed according to the Declaration of Helsinki and approved by the Ethical Committee of the Policlinico Campus Bio-Medico (Rome, Italy) in October 2022 (protocol ID PAR 68.22 OSS). Participants provided web-based informed consent before accessing the online survey and the narrative collection and after being briefed on the project purposes and personal data handling procedures, according to the General Data Protection Regulation of the European Union (EU) 2016/679 [37] and the Italian Law 196/2003 [38]. All web-based informed consent forms included the publication of anonymised responses and narratives. Furthermore, participants were informed that the online platform used in the research operates on a European server that adheres to EU regulations, thereby precluding the collection of potentially sensitive digital data.
Narrative and Data Analysis
ISTUD researchers employed descriptive statistical techniques to analyse the sociodemographic data. Answering survey questions or filling in fields in the illness plots and parallel charts was not mandatory and non-respondents were considered as a separate category.
Open interpretive coding was used to identify and analyse the emerging contents and themes in all narratives. Narratives were entered into MAXQDA (VERBI Software, Berlin, Germany) qualitative analysis software [39] for coding and content analysis [40]. To ensure consistency across team members, researchers collectively coded ten narratives for each group of participants; this process allowed for the identification and resolution of any discrepancies in interpretation across team members. Subsequently, the remaining narratives were independently coded by researchers, with regular meetings scheduled to address any ambiguity and peer debriefings conducted to limit any potential interpretation bias.
Furthermore, narratives were retrospectively classified in accordance with the following: (a) Kleinman’s classification [28] identification of disease-, illness-, and sickness-related aspects in narratives; (b) Launer and Robinson’s classification [41] distinguishing between stable narratives, where the lack of coping strategies determines a lack of future perspective, progressive narratives, in which coping strategies allow a future perspective, and regressive narratives, in which the lack of coping strategies leads to a worse future compared with the initial situation. Strategies for coping [42] with DED were identified within narratives.
The steering committee discussed the results to address the emerging issues and data interpretation collectively. The research team followed the ‘Consolidated criteria for reporting qualitative research’ (COREQ) reporting guidelines [43].
Results
A total of 171 patients diagnosed with DED and 37 informal caregivers participated in the research; 163 patient narratives and 35 caregiver narratives were identified as suitable for the analysis. Eighty-one ophthalmologists wrote 111 parallel charts.
Seventy-eight per cent of patients were female, with a median age of 58 years (range 24–89); patients described in parallel charts were similar in terms of prevalence of female sex (77%) and median age (56 years, range 13–84). Table 1 provides sociodemographic data of participants and patients described in the parallel charts, including non-responders as a separate category.
Table 1.
Sociodemographic data of participants
| Patients with DED (N = 171) | Caregivers (N = 37) | Treating ophthalmologists (N = 81) | Patients in parallel charts (N = 113) | |
|---|---|---|---|---|
| Gender | ||||
| Female | 134 (78%) | 24 (65%) | 44 (54%) | 87 (77%) |
| Male | 31 (18%) | 11 (30%) | 37 (46%) | 25 (22%) |
| Not specified | 6 (4%) | 2 (6%) | – | 1 (1%) |
| Age, years (range) | 58 (24–89) | 48.5 (19–80) | 39.5 (25–72) | 56 (13–84) |
| Nationality* | ||||
| Italian | 159 (93%) | 37 (100%) | 80 (99%) | 105 (93%) |
| Other EU country | 4 (2%) | – | – | 2 (2%) |
| Extra-EU country | 2 (1%) | – | 1 (1%) | 3 (3%) |
| Not specified | 6 (4%) | – | – | 3 (3%) |
| Geographic residence | ||||
| Northern Italy | 54 (32%) | 16 (43%) | 25 (31%) | 38 (34%) |
| Central Italy | 46 (27%) | 7 (19%) | 19 (23%) | 21 (19%) |
| Southern Italy | 69 (40%) | 13 (35%) | 35 (43%) | 50 (44%) |
| San Marino | 2 (1%) | 1 (3%) | 2 (2%) | 4 (4%) |
| Education | ||||
| Elementary school | 8 (5%) | 1 (3%) | – | 8 (7%) |
| Middle school | 21 (12%) | 3 (8%) | – | 18 (16%) |
| High school | 72 (42%) | 15 (41%) | – | 39 (35%) |
| Bachelor or higher degree | 62 (36%) | 16 (43%) | – | 29 (26%) |
| Not specified | 8 (5%) | 2 (5%) | – | 19 (17%) |
| Employment status | ||||
| Student | 5 (3%) | 2 (6%) | – | 3 (3%) |
| Working | 86 (50%) | 24 (65%) | – | 52 (47%) |
| Not working | 14 (8%) | 2 (5%) | – | 10 (9%) |
| Retired | 51 (30%) | 7 (19%) | – | 4 (4%) |
| Other | 7 (4%) | – | – | – |
| Not specified | 8 (5%) | 2 (5%) | – | 7 (6%) |
| Marital state | ||||
| Single | 28 (16%) | 9 (24%) | – | 15 (13%) |
| Married or cohabiting | 113 (66%) | 21 (57%) | – | 76 (67%) |
| Separated | 9 (5%) | 2 (5%) | – | 5 (4%) |
| Widow | 13 (8%) | 3 (8%) | – | 6 (5%) |
| Not specified | 8 (5%) | 2 (5%) | – | 11 (10%) |
| Professional activity, years (range) | – | – | 10 (1–45) | – |
| Workplace | ||||
| Hospital | – | – | 54 (67%) | – |
| University hospital | – | – | 16 (20%) | – |
| Other | – | – | 11 (14%) | – |
Data are presented as N (%) or median (range)
Patients were recruited by ophthalmologists at Italian centres, and while almost all were resident in Italy, the nationalities of patients extended beyond Italy to other EU countries (‘other EU’) and countries outside the EU (‘extra-EU’)
DED dry eye disease, EU European Union
The results are presented with a focus on (1) the DED care pathway from early symptoms to current management and treatment; (2) patients’ and caregivers’ perception of QoL, encompassing the impact of DED on daily life and its associated economic burden; (3) the DED illness experience as analysed through Kleinman’s classification and the main themes that emerged; and (4) factors contributing to coping with DED for patients, caregivers and ophthalmologists as identified through Launer and Robinson’s classification.
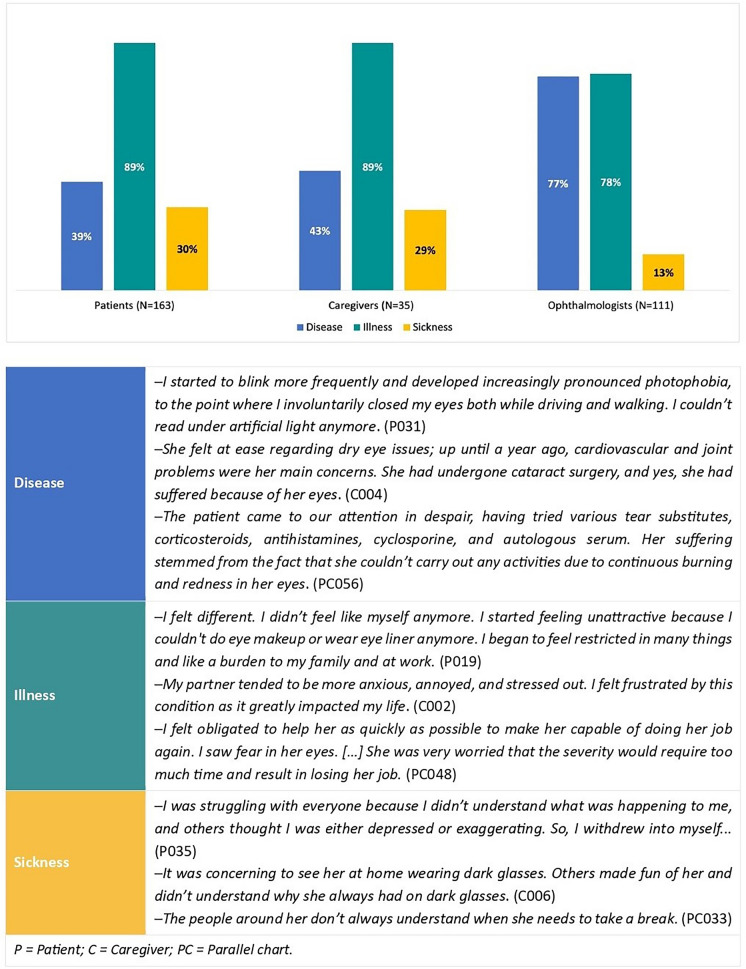
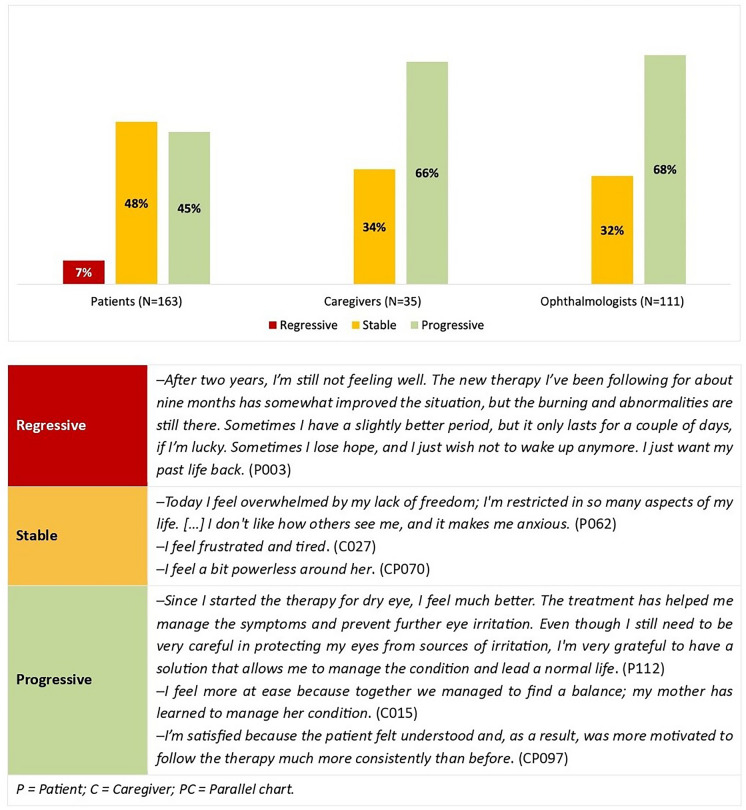
Narratives informed (1) to (4), while both narratives and quantitative survey data were used to investigate (1) and (2). Supporting quotes are provided in Figs. 3 and 4 and Tables 2 and 3; three narratives are available in English within Supplement 3. To mitigate reidentification risks, different codes were used compared with those used during the initial data collection to identify participants.
Fig. 3.
Narratives analysed according Kleinman’s classification
Fig. 4.
Narratives analysed according to Launer and Robinson’s classification
Table 2.
Shared themes among patients, caregivers and ophthalmologists: quotes from narratives
| DED physical impact |
‘Before, I had never felt any issues with my eyes, I wasn’t even aware of them. There were no warning signs; it all happened suddenly one morning when I woke up. The night before, everything was fine… But that morning, I felt something strange in my eyes, a new sensation of heaviness and discomfort. My days were marked by discomfort, pain, burning, sensitivity to light and blurry vision, especially in the evenings. At home, I used various eye drops several times a day. I was bothered by the kitchen light during dinner and by the TV light in the evenings. I tried to explain my symptoms to others, but unless you experience them yourself, it’s hard to understand, like with all illnesses.’ (P008) ‘Mom complained about some eye discomfort, especially feeling like there was a foreign body inside, like having sand in her eyes, causing a lot of irritation and burning sensation, accompanied by headaches.’ (C014) ‘The patient told me she had always experienced some discomfort in her eyes since she was a child, around the time she was diagnosed with atopic dermatitis, a condition known to also cause alterations in the eye surface. However, for about a year, the eye discomfort intensified to become a real issue, especially when she went out during autumn–winter with cold air, which triggered uncontrollable tearing. As she wiped and rubbed her eyes, they became red, irritated, with a persistent sensation of a foreign body. Even though it didn’t happen all the time, the patient said she truly couldn’t bear this discomfort anymore, and it even embarrassed her because the eye makeup often didn’t hold up, resulting in what she described as terrible outcomes.’ (PC035) |
| Daily use of eye drops |
‘I wake up early in the morning. I wash my face, but most importantly, I use cotton pads soaked in warm water to clean my eyes. I put a few drops of cortisone eye drops on the inside of the lower eyelids and, after an hour, I use a state-of-the-art eye drop for treating dry eyes. Throughout the day now, I go about my activities regularly, often using lubricating eye drops for relief. In the evening, when I return home, I dedicate almost an hour to eye care, using warm compresses for twenty minutes, an eye mask for ten minutes and cotton pads soaked in warm water to remove any remaining debris for another ten minutes. Before going to sleep, I apply a few drops of cortisone eye drops on the inside of the lower eyelids (like the morning routine). Thanks to the consistency of these measures, I have gradually resumed applying makeup, using specific natural, anti-allergic eyeshadows.’ (P015) ‘Her days were marked by schedules for instilling eye drops, which provided her with some minimal relief.’ (C002) ‘I started her on tear substitutes until we found the right one for her, together with steroid eye drops in varying dosages.’ (PC071) |
| DED emotional and social impact |
‘At first, they didn’t understand at home. Then they tried to be as supportive as they could. I couldn’t carry out normal daily activities anymore, so my children and my mother helped me. My partner, with whom I had been for eight years, started becoming impatient and angry with me. He said it was all dependent on me, that I shouldn’t dwell on it and that I should go out and try. He was bothered that he had to accompany me to appointments and that we were no longer socially active. Eventually, out of desperation, I ended this relationship, as it was only worsening my psychological condition.’ (P019) ‘At first, she was calm because the discomfort was minimal, but as the problem worsened, she began to complain and sometimes felt demoralised.’ (C032) ‘Her life had changed enormously; she had started avoiding going out with friends because she felt uncomfortable in crowded places.’ (PC042) |
| DED economic and work impact |
‘I want my treatment to be reimbursed by the national health system. Sjögren’s syndrome patients have a constant need for eye lubrication, unfortunately eye drops are very expensive and not everyone can afford it.’ (P068) ‘She was heavily restricted in her activities. I noticed she struggled to use the computer and read books, and she no longer felt confident driving at night. With the arrival of the pandemic, she had to teach her students in front of a screen, and I returned home from the place where I was studying to help her with her computer-related tasks. Moreover, she avoided going out, especially on windy days. Over time, she began delegating all tasks to me.’ (C015) ‘She had recently become a widow and was forced to support herself by working long hours on the computer. However, she could no longer do so, affecting her ability to provide for herself.’ (PC048) |
| Importance of care relationship |
‘I was lucky to be followed by competent specialists who understand me emotionally and support me medically.’ (P096) ‘Doctors are crucial not only for the treatments but also for the information and explanations they provide for these kinds of issues.’ (C036) ‘I dedicated a lot of myself to this person. A friendship developed, and above all, trust. Thanks to this trust, even the ocular therapy worked.’ (PC046) |
DED dry eye disease, C caregiver, P patient, PC parallel chart
Table 3.
Factors favouring coping with DED experience and care: quotes from narratives
| Proper diagnosis and DED understanding |
‘I became aware of the problem and tried to address it with the doctor.’ (P046) ‘Today I feel more at peace because together we managed to find a balance, and my mother has learned to manage her condition.’ (C015) ‘People felt satisfied and relieved because they finally had a diagnosis and a proper treatment.’ (PC045) |
| Empathetic care relationship |
‘I am happy to have found good and helpful professionals who did not underestimate even the smallest details.’ (P092) ‘The current treatment seems to be showing positive signs. Our current eye doctor is compassionate. He wants to help us. He’s empathetic, listens to my mom, lets her talk and tries to reassure her. He’s honest. I think he’s the first doctor truly worthy of his role: he’s someone who is there for those who suffer, has a big heart, and when he diagnosed my mother, he cried with her.’ (C017) ‘The treatment of dry eye involves a multifactorial approach that addresses on the one hand, the problem occurring on the ocular surface, but also involves explaining to the patient what is happening, why the therapy that appears complex and invasive at first can then gradually be modified as the clinical picture changes, and demonstrating that the doctor can create a therapeutic alliance with the patient, so that the problems created by the disease can be addressed and resolved together. In a word, it is crucial that empathy is established between doctor and patient.’ (PC095) |
| Effective therapies and strategies to achieve balance |
‘The doctors were truly a miracle for me; after a few months of treatment, I was truly reborn.’ (P054) ‘The doctors are highly competent, and the treatments are tolerable and effective.’ (C035) ‘We both had to have the patience to look for the right product for her; I tried to encourage her not to give up and to continue the therapy. Sometimes the results are not immediate, and I tried to make her understand that this does not mean that the therapy does not work, but that it takes perseverance and dedication to get lasting relief from the symptoms.’ (PC033) |
DED dry eye disease, C caregiver, P patient, PC parallel chart
Sixty-five per cent of patients, 46% of caregivers and 47% of ophthalmologists described writing as a positive experience (‘I am extremely happy to have shared my experience before and after learning about having dry eye. Many people like me, especially women, suffer. I have met other women my age who did not know it is a disease and endured and worsened to avoid bothering others’—P083). Specifically, writing was beneficial for 30% of patients, 39% of caregivers (‘Living with someone with dry eye is not always easy; you often feel helpless […]. Collecting data for research is invaluable’—C014) and 22% of ophthalmologists. Nonetheless, it remained a challenging experience for 5% of both patients and ophthalmologists (‘I usually talk about patients’ diseases from a purely technical rather than psychological standpoint, despite always considering the impact on the quality of life’—PC028).
DED from Early Symptoms to Current Management
Patients reported experiencing DED onset, on average, at 46 years (± 17). Thirty-eight per cent of patients reported having moderate DED and 27% severe DED; 14% stated that they were not informed about their DED stage. A total of 98 patients (57%) suffered from other conditions, among them, arthritis (11/98), hypertension (22/98), diseases involving the thyroid (12/98) and Sjögren’s syndrome (13/98). Only 10% underwent surgery to reduce myopia. In 62% of the cases, the ophthalmologist was the first addressed healthcare professional, followed by the general practitioner (17%). Eighty-seven per cent reported using tear-replacement eye drops and 42% other treatment strategies to manage DED.
Twenty-two per cent of patients reported having experienced eye issues before DED onset. At diagnosis, 31% stated that they did not know about DED (‘I had no idea what this disease was’—P013), while 21% felt disbelief or discouragement (‘I was very sad because I realised that it was not healing and that I had to live with it’—P035).
DED was defined as a bothersome condition in 45% of patient and 21% of caregiver narratives; moreover, it emerged as manageable (23%) or torturous (13%) in patient narratives and underestimated (29%) or impactful (9%) in caregiver narratives. Notably, patients and caregivers both defined DED as a disabling disease in 19% and 35% of narratives, respectively (‘This disease is severely disabling and should be properly recognised by law. Besides this, it also heavily affects the economic situation of the sufferer’—P019; ‘DED is disabling, unrecognised, unknown, and not fully understood’—C006).
In narratives, 70% of patients reported that therapeutic alliance is an integral part of treatment; 11% emphasised the need for constancy in therapies and 14% reported ineffective treatments. Four per cent highlighted their economic burden (‘Treatments are rather expensive, because they are not covered by the national healthcare system’—P123). Specifically, in the survey, an average annual cost of €1505 for DED treatment and care (e.g. visits with eye specialists or therapies not covered by the Italian national healthcare system) emerged for patients with severe DED, €630 for patients with moderate DED and €398 for patients with mild DED. Forty-two per cent of patients reported they had undergone other procedures, such as photobiomodulation and tear plugs.
Seventy-eight per cent of caregivers reported that they were satisfied with the current DED management, while 13% stated that it remains challenging.
Concerning DED therapies in general, 30% of ophthalmologists described them as good, effective or undergoing innovations. For 16% of ophthalmologists, DED treatment remains critical, while for 9% it should be tailored to each patient’s ocular condition. Additionally, 26% of ophthalmologists believed that DED awareness should be raised among colleagues and patients. In parallel charts, a tailored treatment strategy (37%) and being at the patient’s side (31%) were indicated as crucial actions in DED management.
Concerning the future of DED care, 32% of patients stated a need for more effective or definitive therapy (‘I wish I had a more effective and lasting solution’—P101), with an additional 6% wishing for a therapy requiring fewer administrations (‘I would like to reduce the number of administrations’—P001); conversely, only 5% seek actions such as heightened awareness, cost-effective therapies and disease exemptions. Twenty-two per cent of caregivers advocated for increased research efforts and improved access to treatments (‘I would like research to be funded, for there to be expert centres in every city and above all for more information to be conveyed in the most appropriate way for everyone’—C006), 29% of ophthalmologists highlighted the importance of more effective treatments and 21% advocated attentive listening within the care pathway (‘I would like the way of dealing with this disease to be increasingly based on empathy and the relationship between doctors and patients to be constructive so as to create a true complicity aimed at combatting the disease and its consequences’—PC096).
QoL Perception and DED Impact on Daily Life
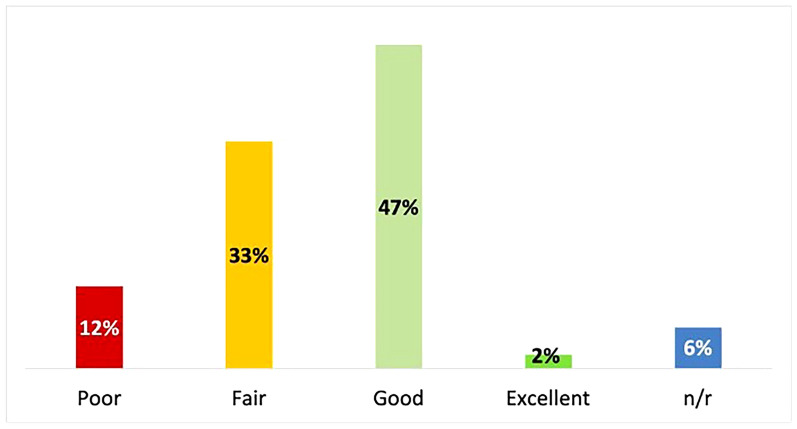
Overall, 47% of patients assessed their QoL as good, 33% as fair and 12% as poor (Fig. 1); 42% of caregivers rated the patients’ QoL as good, while 25% rated it as fair and 17% as poor. Patients reported experiencing the most discomfort due to DED in the evening (53%), followed by the morning (44%), with an additional 16% reporting discomfort at night.
Fig. 1.
Quality of life as self-assessed by patients with dry eye disease (N = 171)
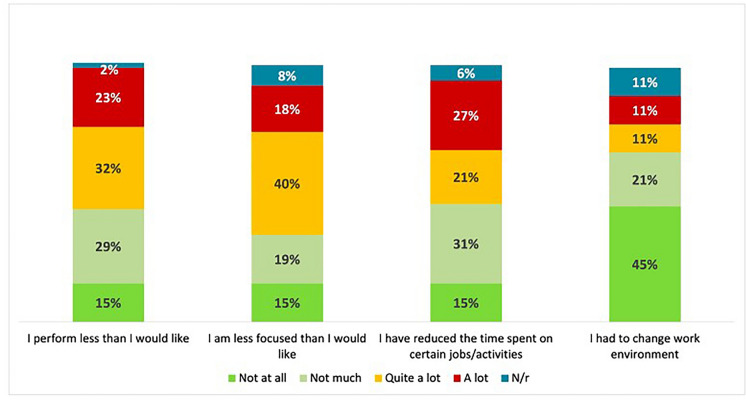
In the survey, DED symptoms were identified as a significant factor affecting work performance, with patients reporting difficulties in reading for extended periods, particularly on digital screens, or needing to interrupt their activities (‘I can’t read for long, especially at the computer. I need to moisten my eyes often and must take many breaks’—P129). Patients also reported difficulties in concentrating during tasks (‘I always feel unfocused’—P047) and indicated that they had less time to complete specific jobs and activities, particularly those who had to work long hours in front of digital screens (Fig. 2).
Fig. 2.
Dry eye disease impact on work performance and activities as self-assessed by patients (N = 171)
Although an improvement in activities was reported in 23% of patient narratives (‘My activities have greatly improved in quality and quantity’—P092) compared with the onset of DED, 10% of patients stated that many of these activities require specific attention; nonetheless, 30% still reported severe limitations in their activities (‘I always feel limited: driving used to give me great independence both in my daily life and on even short holidays […]. I still can’t read or even watch films’—P032). Furthermore, 15% and 27% of patients indicated that DED has a ‘high (relevant)’ or ‘quite (somewhat)’ impact, respectively, on social engagements and events.
DED Experience in NM Classifications and Content Analysis
Patient narratives were mostly characterised by illness elements (89%) [28], which were also predominant in caregiver narratives (89%) and parallel charts (78%). The use of technical and scientific language to describe DED, which characterised 77% of the parallel charts and 43% of caregiver narratives, was less present in patients’ narratives (39%). Sickness elements emerged in 30% of patient narratives and 29% of caregiver narratives, while they were notably less present in the parallel charts (13%; Fig. 3).
The physical, emotional, social and economic impacts of DED emerged from content analysis as the main themes shared among participants’ narratives, together with the daily use of eye drops and the importance of a trusting care relationship (Table 2).
Strategies and Factors to Cope with DED
As shown in Fig. 4, following the Launer and Robinson’s classification [41], 48% of patient narratives were classified as stable, as were 34% of caregiver narratives and 32% of parallel charts. Progressive narratives were prevalent among caregivers (66%) and ophthalmologists (68%), but slightly less present among patients (45%). Notably, regressive narratives were present only among patients (7%).
The coping factors [42] that allow a patient narrative to progress included the following: (a) reaching an understanding of DED, with the support of an ophthalmologist; (b) a solid care relationship and an empathetic attitude by the ophthalmologist; (c) even partially effective treatment strategies; and (d) understanding and support from close people, together with the possibility to talk openly about DED. Caregivers shared (a), (b) and (c) with patients, highlighting the importance for patients to reach a balance in living with DED. For ophthalmologists, building a therapeutic alliance with patients and feeling improved in their professional skills constituted the main coping factors (Table 3).
Discussion
The DINAMO project marked a pioneering NM initiative in Italy dedicated specifically to DED. It engaged a substantial number of Italian centres, investigating simultaneously the perspectives of patients, informal caregivers and treating ophthalmologists.
The analysis of patient narratives revealed a distinct focus on sickness [28] compared with caregiver narratives and parallel charts, highlighting critical aspects of living with DED that risk being overlooked. Primarily, patients frequently encounter a lack of understanding of the disease, with their acquaintances frequently doubting the severity of their symptoms and the hindrance that DED causes in their daily activities. Furthermore, healthcare professionals may also occasionally underestimate DED, as evidenced in the literature [22], which may result in an increase in patient anxiety and depression as they seek solutions for their condition.
Moreover, many patients perceive DED as a disabling condition. Caregivers echo this perception, emphasising how DED is underestimated in terms of its impact on patients’ QoL and activities. As highlighted in the literature [44], DED—especially moderate to severe DED—is associated with pain, limitations in daily activities, and poor general health, often leading to depression [2]; severe DED impact on patient QoL could be comparable to that of angina [45].
DED perception as a disabling condition raises several considerations. Firstly, it draws attention to the economic burden patients and caregivers endure to adhere to the optimal therapeutic strategies agreed upon with healthcare professionals. In addition to the economic cost of therapies and treatments with eye specialists not covered by the Italian national healthcare system, patients are also affected in the workplace, where DED symptoms can lead to a relevant loss of productivity [46]. Although they do not set out specific claims, in narratives patient and caregivers call for legislative changes to recognise DED as a disabling disease, also requesting support to address this economic burden, for example by reducing the costs of more advanced therapies.
Secondly, while patient and caregiver narratives vividly illustrate DED as a disabling illness, this aspect is not as prominently evident in parallel charts, indicating a significant misalignment among patients and treating ophthalmologists. As for other eye conditions [32], the significant impact on QoL, the economic burden and the chronicity of the condition should require an important commitment from ophthalmologists towards people suffering from DED. Ophthalmologists should be aware of this discrepancy and address it while acknowledging the physical, emotional, and social burden of DED for more comprehensive and effective care. To establish a therapeutic alliance with patients suffering from this predominantly symptomatic condition, ophthalmologists must consider not only the improvement of objective symptoms but also the promotion of patient wellbeing and QoL, as discussed in the literature [24].
Thirdly, narratives categorised as regressive [41] are prevalent among patients alone. This indicates the need to promote coping mechanisms beyond the severity level of DED, including building an effective therapeutic relationship, facilitating understanding and patient awareness of DED and ensuring patients feel understood. As highlighted in narratives from ophthalmologists and the literature [44], personalised treatment in accordance with the needs expressed by patients is crucial and has the potential to revolutionise clinical practice, while building an effective therapeutic alliance requires empathetic dialogue, creating a supportive environment for patients. Considering the subjective nature of DED symptoms, physicians should understand their patients not solely based on objective eye features but also within their personal context [47].
Since DED symptoms may not always align with the objective disease picture, promoting a strong empathetic doctor–patient relationship becomes crucial. Empathy, furthermore, emerges from narratives as a significant coping mechanism for both patients and caregivers.
This research has some limitations. First, patients were included regardless of specific clinical characteristics. Second, as participation in the project was voluntary, researchers could not quantify agreements between patients, caregivers and ophthalmologists. Third, the project patient sample only included those patients who had expressed a willingness to narrate their experience during follow-up at expert centres; consequently, the situation of other patients without access to these centres and/or who did not choose to share their experience may be different, and even more critical. Fourth, no validated questionnaires were used to collect QoL data; however, the absence of validated scales for QoL assessment allowed the capture of a more spontaneous and qualitative experience of the disease through narratives.
This project represents an initial step toward a more comprehensive understanding of how DED is experienced by patients, caregivers, and treating ophthalmologists throughout the care pathway. Narratives revealed lesser-known aspects of living with DED, including the lack of understanding from others and its perception as a disabling condition. Furthermore, they underlined the importance of gaining insight into coping mechanisms, such as fostering empathy in care relationship and enhancing personal and societal DED awareness. These factors are of critical importance to strengthen the therapeutic alliance, thereby enabling personalised treatment and improving patient adherence.
Conclusion
The project investigated the practical, emotional and social issues of DED as experienced by patients, their informal caregivers and treating ophthalmologists, simultaneously addressing and integrating these viewpoints. Their comparison provided preliminary insights useful for DED management, specifically concerning the care relationship and disease awareness. NM unveiled factors that favour the ability to cope with DED and its associated QoL implications and provided valuable insights to improve the therapeutic alliance.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors thank participant of the study for their involvement and time contributed to the study.
Declarations
Funding
The study was funded by Bausch + Lomb. Bausch + Lomb also provided funding for publications costs, including the journal’s Rapid Service Fee.
Medical Writing, Editorial, and Other Assistance
Bausch + Lomb provided financial support for this publication, including writing and editorial assistance by Lee-Jay Bannister PhD (Illuminate Medical, UK).
Author Contributions
Pasquale Aragona, Linda Landini, Maria G Marini, Alessandra Fiorencis, Maurizio Rolando, and Stefano Bonini contributed to study conception and design. Pasquale Aragona, Stefano Barabino, Ertugrul Akbas, Robert Ryan, Linda Landini, Maria G Marini, Alessandra Fiorencis, Antonietta Cappuccio, Andrea Leonardi, Antonio Vercesi, Rino Frisina, Francesco Bandello, Luigi Berchicci, Emanuela Aragona, Francesco Semeraro, Vito Romano, Igor Di Carlo, Michele Reibaldi, Andrea Ghilardi, Stefano De Cillà, Giorgio Marchini, Daniele Tognetto, Luigi Fontana, Piera Versura, Domenico D’Eliseo, Alessandro Mularoni, Carlo Cagini, Rita Mencucci, Marco Coassin, Antonio Di Zazzo, Stanislao Rizzo, Romina Fasciani, Luca Gualdi, Andrea Cusumano, Leopoldo Spadea, Emily Cantera, Vincenzo Scorcia, Giuseppe Giannaccare, Pasquale Rosa, Salvatore Troisi, Antonio Provenzano, Francesca Simonelli, Michele Marullo, Lorenza Ciracì, Ciro Costagliola, Vito Primavera, Caterina Gagliano, Antonio Pinna, Alessio Giovanni, Boscia Francesco, Aldo Gelso, Leonardo Mastropasqua, Enza Bonfiglio, Maurizio Rolando, Stefano Bonini contributed to data collection. Maria G Marini, Alessandra Fiorencis, and Antonietta Cappuccio performed data analysis; all authors contributed to data validation and critical interpretation of research findings. Alessandra Fiorencis and Antonietta Cappuccio provided a first draft of the manuscript; all authors contributed to a critical revision and approved the final submitted version of the manuscript.
Conflict of Interest
Ertugrul Akbas, Linda Landini, and Robert Ryan were employees of Bausch + Lomb at the time of this study and development of the manuscript. Maria G Marini, Alessandra Fiorencis, Antonietta Cappuccio were employees of ISTUD S.r.l., Healthcare Area at the time of this study and development of the manuscript. Pasquale Aragona, Stefano Barabino, Andrea Leonardi, Antonio Vercesi, Rino Frisina, Francesco Bandello, Luigi Berchicci, Emanuela Aragona, Francesco Semeraro, Vito Romano, Igor Di Carlo, Michele Reibaldi, Andrea Ghilardi, Stefano De Cillà, Giorgio Marchini, Daniele Tognetto, Luigi Fontana, Piera Versura, Domenico D’Eliseo, Alessandro Mularoni, Carlo Cagini, Rita Mencucci, Marco Coassin, Antonio Di Zazzo, Stanislao Rizzo, Romina Fasciani, Luca Gualdi, Andrea Cusumano, Leopoldo Spadea, Emily Cantera, Vincenzo Scorcia, Giuseppe Giannaccare, Pasquale Rosa, Salvatore Troisi, Antonio Provenzano, Francesca Simonelli, Michele Marullo, Lorenza Ciracì, Ciro Costagliola, Vito Primavera, Caterina Gagliano, Antonio Pinna, Alessio Giovanni, Boscia Francesco, Aldo Gelso, Leonardo Mastropasqua, Enza Bonfiglio, Maurizio Rolando, Stefano Bonini report no conflicts associated with this work.
Ethical Approval
The project was performed according to the Declaration of Helsinki and approved by the Ethical Committee of the Policlinico Campus Bio-Medico (Rome, Italy) in October 2022 (protocol ID PAR 68.22 OSS). Participants provided web-based informed consent before accessing the online survey and the narrative collection and after being briefed on the project purposes and personal data handling procedures, according to the General Data Protection Regulation of the European Union (EU) 2016/679 and the Italian Law 196/2003. All web-based informed consent forms included the publication of anonymised responses and narratives. Furthermore, participants were informed that the online platform used in the research operates on a European server that adheres to EU regulations, thereby precluding the collection of potentially sensitive digital data.
Data Availability
All data generated during this study are included in this published article and supplementary information files. All datasets used and analysed during the research are available in Italian from the corresponding author. Please contact the corresponding author for any data clarification questions.
Footnotes
Prior Presentation: Summary results from this study were presented at the European Dry Eye Society Congress (EuDEC), June 20–22, 2024, Madrid, Spain; Poster FP-05.
Contributor Information
Pasquale Aragona, Email: pasquale.aragona@unime.it.
Stefano Barabino, Email: stebarabi@gmail.com.
Maurizio Rolando, Email: maurizio.rolando@gmail.com.
Stefano Bonini, Email: S.Bonini@policlinicocampus.it.
References
- 1.Nelson JD, Craig JP, Akpek EK, et al. TFOS DEWS II introduction. Ocul Surf. 2017;15(3):269–75. 10.1016/j.jtos.2017.05.005. [DOI] [PubMed] [Google Scholar]
- 2.Craig JP, Nichols KK, Akpek EK, et al. TFOS DEWS II definition and classification report. Ocul Surf. 2017;15(3):276–83. 10.1016/j.jtos.2017.05.008. [DOI] [PubMed] [Google Scholar]
- 3.The epidemiology of dry eye disease: report of the Epidemiology Subcommittee of the International Dry Eye WorkShop (2007). Ocul Surf. 2007;5(2):93–107. 10.1016/s1542-0124(12)70082-4. [DOI] [PubMed]
- 4.Baudouin C, Aragona P, Van Setten G, et al. Diagnosing the severity of dry eye: a clear and practical algorithm. Br J Ophthalmol. 2014;98(9):1168–76. 10.1136/bjophthalmol-2013-304619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baudouin C, Irkeç M, Messmer EM, et al. Clinical impact of inflammation in dry eye disease: proceedings of the ODISSEY group meeting. Acta Ophthalmol. 2018;96(2):111–9. 10.1111/aos.13436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Messmer EM. The pathophysiology, diagnosis, and treatment of dry eye disease. Dtsch Arztebl Int. 2015;112(5):71–81. 10.3238/arztebl.2015.0071. (quiz 82). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Talens-Estarelles C, Sanchis-Jurado V, Esteve-Taboada JJ, Pons ÁM, García-Lázaro S. How do different digital displays affect the ocular surface? Optom Vis Sci. 2020;97(12):1070–9. 10.1097/OPX.0000000000001616. [DOI] [PubMed] [Google Scholar]
- 8.Talens-Estarelles C, García-Marqués JV, Cervino A, García-Lázaro S. Use of digital displays and ocular surface alterations: a review. Ocul Surf. 2021;19:252–65. 10.1016/j.jtos.2020.10.001. [DOI] [PubMed] [Google Scholar]
- 9.Stapleton F, Alves M, Bunya VY, et al. TFOS DEWS II epidemiology report. Ocul Surf. 2017;15(3):334–65. 10.1016/j.jtos.2017.05.003. [DOI] [PubMed] [Google Scholar]
- 10.de Paiva CS. Effects of aging in dry eye. Int Ophthalmol Clin. 2017;57(2):47–64. 10.1097/IIO.0000000000000170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hasan ZAIY. Dry eye syndrome risk factors: a systemic review. Saudi J Ophthalmol. 2022;35(2):131–9. 10.4103/1319-4534.337849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nichols KK. Patient-reported symptoms in dry dye disease. Ocul Surf. 2006;4(3):137–45. 10.1016/s1542-0124(12)70040-x. [DOI] [PubMed] [Google Scholar]
- 13.Friedman NJ. Impact of dry eye disease and treatment on quality of life. Curr Opin Ophthalmol. 2010;21(4):310–6. 10.1097/ICU.0b013e32833a8c15. [DOI] [PubMed] [Google Scholar]
- 14.Miljanović B, Dana R, Sullivan DA, Schaumberg DA. Impact of dry eye syndrome on vision-related quality of life. Am J Ophthalmol. 2007;143(3):409–15. 10.1016/j.ajo.2006.11.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McDonald M, Patel DA, Keith MS, Snedecor SJ. Economic and humanistic burden of dry eye disease in Europe, North America, and Asia: a systematic literature review. Ocul Surf. 2016;14(2):144–67. 10.1016/j.jtos.2015.11.002. [DOI] [PubMed] [Google Scholar]
- 16.Barabino S, Labetoulle M, Rolando M, Messmer EM. Understanding symptoms and quality of life in patients with dry eye syndrome. Ocul Surf. 2016;14(3):365–76. 10.1016/j.jtos.2016.04.005. [DOI] [PubMed] [Google Scholar]
- 17.Li M, Gong L, Sun X, Chapin WJ. Anxiety and depression in patients with dry eye syndrome. Curr Eye Res. 2011;36(1):1–7. 10.3109/02713683.2010.519850. [DOI] [PubMed] [Google Scholar]
- 18.Morthen KM, Magno MS, Utheim TP, Hammond CJ, Vehof J. The work-related burden of dry eye. Ocul Surf. 2023;28:30–6. [DOI] [PubMed] [Google Scholar]
- 19.Chan C, Ziai S, Myageri V, Burns JG, Prokopich CL. Economic burden and loss of quality of life from dry eye disease in Canada. BMJ Open Ophthalmol. 2021;6(1):e000709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heidari M, Noorizadeh F, Wu K, Inomata T, Mashaghi A. Dry eye disease: emerging approaches to disease analysis and therapy. J Clin Med. 2019;8(9):1439. 10.3390/jcm8091439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barabino S, Aragona P, di Zazzo A, Rolando M, Società Italiana di Dacriologia e Superficie Oculare. Updated definition and classification of dry eye disease: renewed proposals using the nominal group and Delphi techniques. Eur J Ophthalmol. 2021;31(1):42–8. 10.1177/1120672120960586. [DOI] [PubMed] [Google Scholar]
- 22.Nichols KK, Nichols JJ, Mitchell GL. The lack of association between signs and symptoms in patients with dry eye disease. Cornea. 2004;23(8):762–70. 10.1097/01.ico.0000133997.07144.9e. [DOI] [PubMed] [Google Scholar]
- 23.Behrens A, Doyle JJ, Stern L, et al. Dysfunctional tear syndrome: a Delphi approach to treatment recommendations. Cornea. 2006;25(8):900–7. 10.1097/01.ico.0000214802.40313.fa. [DOI] [PubMed] [Google Scholar]
- 24.Lin CW, Lin MY, Huang JW, Wang TJ, Lin IC. Impact of dry eye disease treatment on patient quality of life. Front Med (Lausanne). 2024;28(11):1305579. 10.3389/fmed.2024.1305579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pierret J. The illness experience: state of knowledge and perspectives for research. Sociol Health Illn. 2003;25:4–22. 10.1111/1467-9566.t01-1-00337. [DOI] [PubMed] [Google Scholar]
- 26.Greenhalgh T. Cultural contexts of health: the use of narrative research in the health sector. Copenhagen: WHO Regional Office for Europe; 2016. [PubMed] [Google Scholar]
- 27.Marini MG. Narrative medicine: bridging the gap between evidence-based care and medical humanities. London: Springer International; 2016. [Google Scholar]
- 28.Kleinman A. The illness narrative, suffering and healing the human condition. New York: Basic Book; 1989. [Google Scholar]
- 29.Greenhalgh T, Hurwitz B. Why study narrative? BMJ. 1999;318(7175):48–50. 10.1136/bmj.318.7175.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marini MG. Languages of care in narrative medicine: words, space and time in the healthcare ecosystem. London: Springer International; 2019. [Google Scholar]
- 31.Simonelli F, Sodi A, Falsini B, et al. Narrative medicine to investigate the quality of life and emotional impact of inherited retinal disorders through the perspectives of patients, caregivers and clinicians: an Italian multicentre project. BMJ Open. 2022;12(9):e061080. 10.1136/bmjopen-2022-061080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Midena E, Varano M, Pilotto E, et al. Real-life patient journey in neovascular age-related macular degeneration: a narrative medicine analysis in the Italian setting. Eye (London). 2022;36(1):182–92. 10.1038/s41433-021-01470-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.W3C Recommendation. Web Content Accessibility Guidelines (WCAG) 2.1. [Internet]. https://www.w3.org/TR/WCAG21/. Accessed 21 Sept 2023.
- 34.Reid K, Soundy A. A qualitative study examining the illness narrative master plots of people with head and neck cancer. Behav Sci (Basel). 2019;9(10):110. 10.3390/bs9100110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Peeters B, Marini M. Narrative medicine across languages and cultures: using minimal English for increased comparability of patients’ narratives. In: Goddard C, editor. Minimal English for a global world: improved communication using fewer words. Basingstoke: Palgrave Macmillan; 2018. p. 259–86. [Google Scholar]
- 36.Charon R. The patient-physician relationship. Narrative medicine: a model for empathy, reflection, profession, and trust. JAMA. 2001;286(15):1897–902. 10.1001/jama.286.15.1897. [DOI] [PubMed] [Google Scholar]
- 37.Personal data code protection. Legislat. Decree no. 196 of 30 June 2003. Italian Off J. 2003; 174(Suppl 123).
- 38.Official Journal of the European Union L 119. Regulation (EU) 2016/679 of the European Parliament and of the Council of 27 April 2016 on the protection of natural persons with regard to the processing of personal data and on the free movement of such data and repealing Directive 95/46/EC (General Data Protection Regulation). [Internet]. 2016. https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32016R0679.
- 39.VERBI Software. MAXQDA 2022. Berlin: VERBI Software, 2021.
- 40.Hsieh H-F, Shannon SE. Three approaches to qualitative content analysis. Qual Health Res. 2005;15(9):1277–88. 10.1177/1049732305276687. [DOI] [PubMed] [Google Scholar]
- 41.Launer J. New stories for old: narrative-based primary care in Great Britain. Fam Syst Health. 2006;24(3):336–44. 10.1037/1091-7527.24.3.336. [Google Scholar]
- 42.Carver CS, Scheier MF, Weintraub JK. Assessing coping strategies: a theoretically based approach. J Pers Soc Psychol. 1989;56(2):267–83. 10.1037/0022-3514.56.2.267. [DOI] [PubMed] [Google Scholar]
- 43.Tong A, Sainsbury P, Craig J. Consolidated criteria for reporting qualitative research (COREQ): a 32-item checklist for interviews and focus groups. Int J Qual Health Care. 2007;19(6):349–57. 10.1093/intqhc/mzm042. [DOI] [PubMed] [Google Scholar]
- 44.Messmer E, Chan C, Asbell P, Johnson G, Sloesen B, Cook N. Comparing the needs and preferences of patients with moderate and severe dry eye symptoms across four countries. BMJ Open Ophthalmol. 2019;4(1):e000360. 10.1136/bmjophth-2019-000360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schiffman RM, Walt JG, Jacobsen G, Doyle JJ, Lebovics G, Sumner W. Utility assessment among patients with dry eye disease. Ophthalmology. 2003;110(7):1412–9. 10.1016/S0161-6420(03)00462-7. [DOI] [PubMed] [Google Scholar]
- 46.Patel VD, Watanabe JH, Strauss JA, Dubey AT. Work productivity loss in patients with dry eye disease: an online survey. Curr Med Res Opin. 2011;27(5):1041–8. 10.1185/03007995.2011.566264. [DOI] [PubMed] [Google Scholar]
- 47.Aragona P, Giannaccare G, Mencucci R, et al. The management of dry eye disease: proceedings of Italian Dry Eye Consensus Group using the Delphi method. J Clin Med. 2022;11(21):6437. 10.3390/jcm11216437. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated during this study are included in this published article and supplementary information files. All datasets used and analysed during the research are available in Italian from the corresponding author. Please contact the corresponding author for any data clarification questions.