Abstract
Sjögren’s disease (SjD), characterized by circulating autoantibodies and exocrine gland inflammation, is typically diagnosed in women over 50 years of age. However, the contribution of age to SjD pathogenesis is unclear. C57BL/6 female mice at different ages were studied to investigate how aging influences the dynamics of salivary gland inflammation. Salivary glands were characterized for immune cell infiltration, inflammatory gene expression, and saliva production. At 8 months, gene expression of several chemokines involved in immune cell trafficking was significantly elevated. At this age, age-associated B cells (ABCs), a unique subset of B cells expressing the myeloid markers CD11b and/or CD11c, were preferentially enriched in the salivary glands compared to other organs like the spleen or liver. The salivary gland ABCs increased with age and positively correlated with increased CD4 T follicular helper cells. By 14 months, lymphocytic foci of well-organized T and B cells spontaneously developed in the salivary glands. In addition, the mice progressively developed high titers of serum autoantibodies. A subset of aged mice developed salivary gland dysfunction mimicking SjD patients. Our data demonstrates that aging is a significant confounding factor for SjD. Thus, aged female C57BL/6 mice are more appropriate and a valuable preclinical model for investigating SjD pathogenesis and novel therapeutic interventions.
Supplementary Information
The online version contains supplementary material available at 10.1007/s11357-024-01159-3.
Keywords: Age-associated B cells, Aging, Autoimmunity, Mice, Salivary glands, Sjögren’s disease
Introduction
Sjögren’s disease (SjD) is a chronic autoimmune disorder targeting multiple organ systems [1]. The disease has a strong gender bias, predominantly affecting women over men at a 9:1 ratio [2]. The most common symptom is oral and ocular dryness caused by reduced saliva and tear production. Oral dryness in SjD is linked to difficulty in speech and swallowing food, microbial overgrowth leading to bad breath, dental decay, and tooth loss [3]. Dry eyes in SjD patients lead to a constant sensation of particulate material in the eyes, corneal damage, pain, and, in severe cases, vision loss [4]. The central nervous system involvement drives debilitating fatigue, brain fog, and depression [5]. In rare cases (< 5%), patients develop life-threatening lymphomas [6]. Overall, the disease is responsible for reducing the quality of life and presents itself as a significant economic burden for the patients and their families [7]. Although genetics influence SjD susceptibility, other factors like epigenetics, sex, and environmental interactions play significant roles in disease pathogenesis [8].
SjD is more common at older ages and is typically diagnosed in or after the fifth decade of life, with the median age of diagnosis being 52 years [2]. The diagnostic hallmarks of SjD are the presence of lymphocytic foci in minor salivary gland biopsies and high-titer autoantibodies to Ro60/SSA, La/SSB, and Ro52/TRIM21 proteins. It is interesting to note that autoantibodies are detectable in serum several years before the appearance of clinical symptoms and disease diagnosis [9]. However, the reasons for this delay between loss of tolerance and manifestation of clinical signs are unclear. How aging influences SjD development and progression has yet to be thoroughly interrogated, and this represents a significant gap in our knowledge of the disease.
B cells play a critical role in SjD pathogenesis, generating autoantibodies found in the systemic circulation and locally in the salivary glands. B cells contribute prominently to the lymphocytic foci observed in salivary gland biopsies from SjD patients. The organization of B cells within these foci into germinal center–like structures has been linked to poor disease prognosis [10]. In 2011, a unique population of B cells expressing myeloid markers was shown to accumulate in the spleens of older mice and peripheral blood of older humans [11, 12], and they were designated as age-associated B cells (ABCs). ABCs are a heterogeneous B cell subset that lack CD21 and CD23 and express myeloid markers like CD11b and/or CD11c. The activation requirements of ABCs are distinct from conventional B cells. ABCs respond poorly to B cell receptor or CD40 ligation but expand following stimulation with toll-like receptor (TLR) 7 and TLR9 agonists. Some ABCs expressing the transcription factor T-bet can produce autoantibodies and are seen in lupus-prone mice and patients with autoimmunity [13]. ABC expansion occurs preferentially in females, suggestive of their pathogenic contribution to female-dominant autoimmune diseases.
In SjD patients, peripheral blood shows an elevation of autoreactive CD21−/low B cells unresponsive to CD40-mediated stimulation [14]. Moreover, CD21−/low B cells expressing FcRL4, CD11c, and T-bet were recovered from minor labial salivary gland biopsies from SjD patients [15]. In mice, repeated treatment with a TLR7 agonist was associated with an expansion of T-bet + B cells in the spleen and an accelerated onset of SjD-like disease in NOD.B10Sn-H-2b mice [16]. These reports suggested a potential pathogenic role for ABCs in salivary gland manifestations in SjD. Whether the entry of ABCs into salivary glands is a cause or consequence of lymphocytic focus formation and what mechanisms are involved in glandular infiltration are not known. To address these issues, we investigated salivary glands from aged C57BL/6 (B6) mice in the present study. Previous reports have demonstrated that aged B6 mice spontaneously develop lymphocytic infiltrates in their salivary [17, 18] and lacrimal [19] glands and autoantibodies in circulation [20]. Our study shows that early inflammatory changes in the salivary glands by 8 months of age precede lymphocytic focus formation. Further, our data demonstrate that ABC infiltration into the salivary glands inextricably links aging with loss of immune tolerance and exocrine gland inflammation.
Methods
Mice
Female C57BL/6N and C57BL/6J (B6) mice were obtained at different ages from the Aged Rodent Colony of the National Institute of Aging, the Jackson Laboratory (Bar Harbor, ME), and the Charles River Laboratories (Wilmington, MA). The mice were housed in a specific pathogen-free facility in the Oklahoma Medical Research Foundation vivarium (Supplementary Table S1). Mice were also bred in the OMRF facility from breeding pairs obtained from the Jackson Laboratory. All mice had unrestricted access to food (5053 PicoLab rodent Diet 20) and water. All animal experiments were approved by the Institutional Animal Care and Use Committee and followed the National Institutes of Health guidelines for the care and use of laboratory animals.
Tail bleeds were collected to determine serum autoantibody titers. Pilocarpine-induced saliva production was measured to evaluate salivary gland function [21]. Mice were euthanized at 3, 8–9, 13–14, and 17–26 months of age. The mice were perfused with PBS before tissue collection. Submandibular salivary glands were harvested, cut into multiple pieces, and processed for gene expression analysis, histopathology, immunostaining, and flow cytometry as described previously [22, 23]. In some experiments, the spleen and liver were harvested and processed for immune cell analysis by flow cytometry.
Salivary gland histopathology
Formalin-fixed and paraffin-embedded salivary gland Sections (5 µm thickness) were stained with hematoxylin and eosin and evaluated for inflammation [24]. High-resolution images were obtained with a Zeiss AxioScan 7 digital pathology scanner, and image analysis was performed using QuPath software [25]. An aggregate of > 50 lymphocytes within the glandular parenchyma was considered a focus. The sizes of lymphocytic foci and the total area of the salivary gland parenchyma were measured. The results are expressed as the area of inflammation (%) calculated as (area with lymphocytic foci / total glandular area) × 100. A total area of 7.58 ± 0.59 sq mm (mean ± SEM) was evaluated for each sample.
Immunofluorescence staining
Immune cell infiltrates were studied in salivary gland pieces fixed in 1% paraformaldehyde-lysine-periodate and processed as previously described [26]. The fluorochrome-conjugated monoclonal antibodies to MHC II, CD3, CD4, CD8a, and B220 were used for staining (Supplementary Table S2). For T-bet, the sections were incubated with a rabbit anti-T-bet antibody followed by a fluorochrome-conjugated donkey anti-rabbit IgG antibody. DAPI was used for nuclear staining, and the tissues were mounted in Prolong Gold (Invitrogen, Waltham, MA). Images were captured using the LSM710 confocal microscope with Zen software (Carl Zeiss Microscopy LLC, White Plains, NY).
Gene expression analyses
Snap-frozen salivary gland pieces in liquid nitrogen were used for gene expression analysis. RNA was isolated using the RNeasy mini kit (Qiagen, Germantown, MD), and gene expression was studied using the nCounter mouse myeloid cell panel with probes for 734 genes and 20 internal reference genes (Nanostring Technologies, Seattle, WA). Gene expression analysis was performed using the ROSALIND analysis software (ROSALIND, San Diego, CA). Ingenuity Pathway Analysis (Qiagen) was also performed using differentially expressed genes.
Flow cytometry
The salivary glands, livers, and spleens were minced and digested with collagenase D (1 mg/ml) and DNase (0.1 mg/ml) (Sigma-Aldrich, St Louis, MO) to obtain single-cell suspensions. Using standard protocols, cells were stained with fluorochrome-conjugated antibodies (Supplementary Table S2). Zombie Aqua fixable viability kit (Biolegend, San Diego, CA) was used for live/dead cell discrimination. Sample acquisition was performed on a 5-Laser Cytek Aurora spectral flow cytometer using Spectroflo software (Cytek Biosciences, Fremont, CA). FlowJo software (Becton Dickenson, Ashland, OR) was used for data analysis. Dimensionality reduction was performed with the Uniform Manifold Approximation and Projection (UMAP) plug-in, and the X-shift algorithm was used for clustering B cell subsets.
Serum antibody analysis
Serum antibodies to nuclear and cytoplasmic antigens were evaluated by indirect immunofluorescence staining of HeLa cells, as described previously [24]. Serum samples were serially diluted starting at 1:50 and tested for reactivity. FITC-conjugated goat F(ab)2 anti-mouse IgG (Jackson Immunoresearch) was used to detect bound antibodies. Nuclei were stained with DAPI and mounted in ProLong Gold (Invitrogen). Images were captured at fixed exposure on a Zeiss Axiovert fluorescence microscope using AxioVision software. The results are expressed as endpoint titers (highest serum dilution generating a signal over the negative control). Serial dilutions of pooled serum from nephritic MRL.lpr/lpr mice were used as a positive control. All experiments used a pooled serum sample from young (8-week) C57BL/6 mice at 1:50 dilution as a negative control.
Statistical analysis
All statistical analyses were performed using Prism 9.4 software (GraphPad, San Diego, CA). Gaussian distribution was assessed for each dataset using a normality test. Student’s t-test was used to compare differences between two groups when data followed a Gaussian distribution. A two-way analysis of variance (ANOVA) followed by Sidak’s post-test was used for multiple comparisons. The non-parametric Mann–Whitney test determined differences between two groups for non-Gaussian distributions. Kruskal–Wallis test, followed by Dunn’s post-test, was used for multiple comparisons. The Spearman method was used to determine correlation coefficients. A p-value of less than 0.05 was considered statistically significant and is shown in each figure.
Results
Hierarchical recruitment of adaptive immune cells into aging salivary glands is preceded by changes in chemokine gene expression in B6 mice
Submandibular salivary glands harvested from 3-, 8-, 13-, and > 17-month-old C57BL/6N (open circles) or C57BL/6J (filled circles) female mice were evaluated for the formation of lymphocytic foci (defined as aggregation of > 50 lymphocytes), and the area of inflammation was quantified (Fig. 1A). None of the mice showed lymphocytic foci at 3 months (Fig. 1B, F). Mild inflammation was first detected at 8–9 months of age in 3/14 mice. By 13 months, the glands showed lymphocytic foci in the salivary gland parenchyma (Fig. 1C–E). The foci were of varying sizes and were present in the peri-vascular and peri-ductal regions extending into the acini. The incidence of inflammation significantly increased with age, progressing from 6/10 mice at 13 months and 13/17 mice at > 17 months. Immunostaining showed that the lymphocytic foci comprised T cells (CD4 and CD8) and B cells (Fig. 1G). Large foci showed well-organized, discrete areas of T cells (both CD4 and CD8) and B cells (Fig. 1H). Increased MHC II expression was seen in the foci, corresponding to the B cell areas (Supplementary Figure S1).
Fig. 1.
Salivary gland inflammation in aging C57BL/6 mice. (A) The area of inflammation in salivary glands from C57BL/6N (open circles) and C57BL/6J (closed circles) at different ages was calculated as (the size of lymphocytic foci/total area of the salivary gland section) × 100. Each data point represents one mouse. The difference between the groups was determined by the Kruskal–Wallis one-way ANOVA and Dunn’s post-test for multiple comparisons. ns, not significant; * p < 0.05; ****p < 0.0001. (B–E) Representative histopathology images of H&E stained sections from a normal salivary gland in 3-month-old (B) and inflamed glands in 13-month-old mice (C–E) showing peri-vascular and per-ductal lymphocytic foci (arrows) of different sizes invading the surrounding acini and ducts. Scale bar = 100 µm. (F–H) Representative immunofluorescence images of salivary gland sections stained with antibodies to CD4, CD8, and B220 at 3 months (F) and at 13 months, showing a small focus (G) and a large focus (H). The large focus shows distinct areas of T and B cells, suggesting organization into germinal center. Scale bars = 50 µm
The pathology of salivary gland inflammation was similar in both B6 substrains. However, the C57BL/6N and C57BL/6 J have numerous phenotypic and genotypic differences [27]. Therefore, all gene expression and flow cytometry analyses presented in this study are restricted to C57BL/6N mice, which is also the substrain provided by the National Institute of Aging, Aged Rodent Colony, and is widely used in aging research.
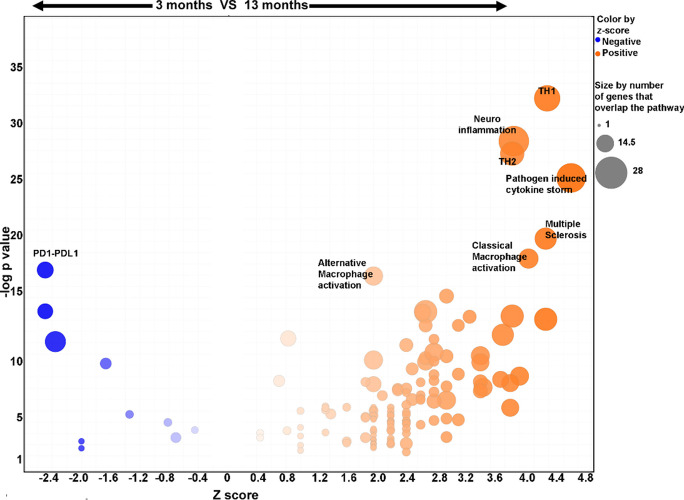
To investigate the mechanisms for the onset of inflammation, RNA was isolated from submandibular glands of mice at 3, 8, and 13 months (n = 4/grp), and gene expression was studied using the nCounter myeloid gene expression probe set. Hierarchical clustering and principal component analysis showed age-dependent gene expression patterns in 11/12 mice. One mouse in the 13-month age group fell between the 8- and 13-month groups (Supplementary Figure S2A and S2B). This pattern was replicated in the hierarchical clustering based on differential gene expression (DEG) (Fig. 2A–C). Significant changes in DEG were noted between 3- and 8-month salivary glands, with 23/734 upregulated and 9/734 downregulated genes (Fig. 2D). Despite the absence of lymphocytic foci, 8-month-old salivary glands showed increased expression of chemokines (Cxcl9, Cxcl11, Ccl5, Ccl8) and monocyte/macrophage-related genes (Trem2, Ldlr, Cebpd). At 13 months, there was a further increase in the number of genes and the extent of fold change in DEG (Fig. 2E, F). Notably, the most significant gains occurred in chemokine expression and MHC II, suggesting immune cell–related inflammation. These results indicate that increased pro-inflammatory gene expression precedes lymphocytic focus formation in the salivary glands. Furthermore, the ingenuity pathway analysis of DEG in the salivary glands at 3 and 13 months showed enrichment in pathways suggestive of T cell and macrophage activation and elevated cytokine production (Fig. 3).
Fig. 2.
Gene expression analysis in salivary glands of mice at 3-, 8-, and 13-months of age. (A–C) Heat maps showing hierarchical clustering of DEG in salivary glands from 3-, 8-, and 13-month-old female mice, n = 4/group. Each row represents a gene, and each column represents a mouse. Based on gene expression patterns, mice from each group cluster together except for one mouse at 13 months of age. (D–F) Volcano plots showing DEG in salivary glands from mice at different ages. The DEG showing maximal fold changes and highest significance are labeled
Fig. 3.
Ingenuity pathway analysis of DEG from salivary glands at 3 and 13 months of age. The Z scores and p values for each pathway are shown. The top 7 signaling pathways with positive Z scores indicating enrichment at 13 months are labeled. The most significantly downregulated PD1-PDL1 pathway with a negative Z score at 13 months is shown
Commensurate with the increased chemokine expression, flow cytometry analysis of the salivary glands at 3, 8, 13, and > 17 months showed a progressive expansion of the adaptive immune cells, specifically in CD4 T, CD8 T, and B cell populations (Fig. 4A). The UMAP multicolor plot overlays and gating strategy for salivary gland immune cell analysis are shown in Supplementary Figure S3. Compared to 3 months, 13-month-old salivary glands showed a significant increase in CD4 (3.99 ×) and CD8 (4.99 ×) T cells. The most dramatic change was in the B cells, with a 33.5 × increase (Fig. 4B). The CD4 T, CD8 T, and B cell increases persisted at 17–26 months. The CD4 T cells showed a slight (1.75 ×) but significant drop at 17–26 months compared to 13–14 months. However, the cause for this relative reduction of salivary gland CD4 T cells at 17–26 months is unclear. Regardless, the spontaneous salivary gland inflammation in aging B6 mice shows characteristics similar to the lymphocytic infiltration in minor labial gland biopsies from SjD patients [28].
Fig. 4.
Flow cytometry analysis of immune cells infiltrating the salivary glands at different ages. (A) UMAP plots identifying CD4 T, CD8 T, Ly6C monocyte, B cell, and macrophage/dendritic cell clusters at 3-, 8-, and 13-months. Each plot represents 50,000 CD45 + cells from 5 mice with 10,000 CD45 + cells per mouse. (B) Frequency of different immune cell types in salivary glands at 3 months (n = 5), 8–9 months (n = 5), 13–14 months (n = 10), and 17–26 months (n = 10) of age. * p < 0.05; ** p < 0.01; ***p < 0.001,****p < 0.0001
Age-associated B cells (ABCs) accumulate within lymphocytic infiltrates in aging salivary glands
CD19 + B220 + B cells were analyzed for CD21, CD23, CD11b, and CD11c expression to characterize B cell subsets infiltrating the salivary glands (Fig. 5A). The gating for B cell subsets in the spleen at 13 months (Supplementary Figure S4) was used to define follicular B cells (CD23 + CD21 +), marginal zone B cells (CD23negCD21hi), and ABCs (CD21negCD23neg B cells expressing CD11b/CD11c). ABCs were detected in the salivary glands at 8 months and progressively increased in frequency at 13 and > 17 months of age (Fig. 5B). Thus, ABCs were enriched in the salivary glands before the formation of large lymphocytic foci. Interestingly, salivary gland sections from aged mice showed that ABCs (T-bet + CD19 +) were present within the lymphocytic focus (Supplementary Figure S5).
Fig. 5.
Changes in age-associated B cells (ABCs) and T follicular helper (Tfh) cells in salivary glands of aged mice. ABCs (A, B) and Tfh (C, D) cells from salivary glands at 3 months (n = 5), 8–9 months (n = 5), 13–14 months (n = 10), and 17–26 months (n = 10) of age. (A) Gating strategy showing CD23 + CD21 + follicular cells (Fo) and CD21negCD23neg double negative (DN) cells within the B cell gate. ABCs were identified as the DN cells expressing either CD11b and/or CD11c. (B) Frequency of ABCs within the CD45 + gate. (C) Gating strategy showing CD4 and CD8 T cells within the CD45 + gate. Tfh were identified as CD4 T cells expressing CD279 (PD1) and CD278 (ICOS). (D) Frequency of Tfh within the CD45 + gate. (E). Correlation between frequencies of ABCs and Tfh in salivary glands determined by the Spearman method. Each data point represents one mouse. In (B) and (D), the difference between the groups was determined by the Kruskal–Wallis one-way ANOVA and Dunn’s post-test for multiple comparisons. *p < 0.05; ** p < 0.01; ***p < 0.001
The expansion and differentiation of ABCs into antibody-producing cells may occur with or without help from T follicular helper (Tfh) cells in a germinal center–dependent or independent fashion [29]. To determine the presence of Tfh cells, CD4 cells in the salivary glands were interrogated for CD278 (ICOS) and CD279 (PD-1) expression (Fig. 5C). Tfh cells were present in the glands by 8 months and increased significantly with age (Fig. 5D). Indeed, a strong positive correlation existed between ABCs and CD4 Tfh frequencies (Fig. 5E).
To determine whether the ABCs-CD4 Tfh correlation was indicative of a germinal center interaction, salivary gland B cells were analyzed for GL-7 expression. At 13–14 months, GL-7 positive cells constituted 1.67 ± 0.19% (mean ± SEM, n = 10) of the B cell subset. A considerable fraction of the GL-7 + germinal center B cells (26.02 ± 3.88%) were CD21negCD23negCD11b + CD11c ± (Supplementary Figure S6). Thus, tertiary lymphoid follicle–like structures in the salivary glands consisted of a mixture of ABC and non-ABC germinal center B cells.
The aging salivary glands provide a favorable environment for ABC recruitment
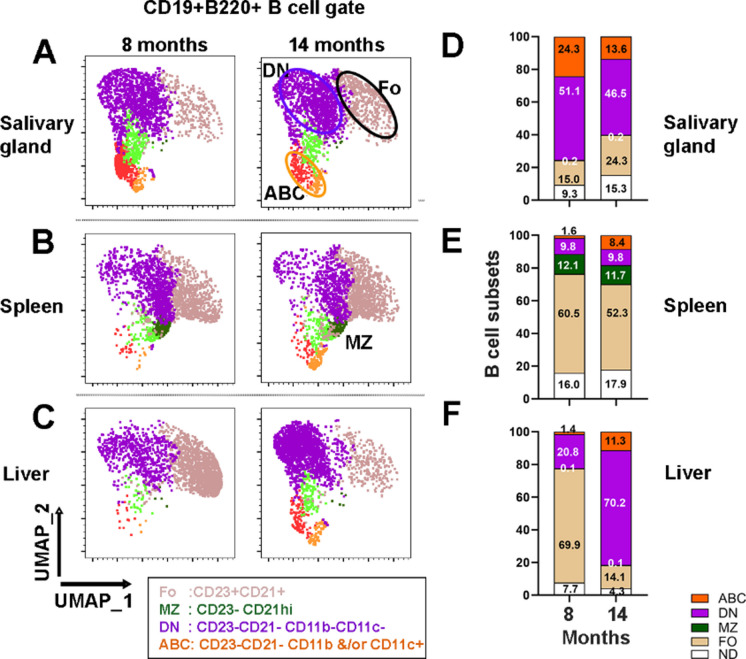
To investigate whether the ABC infiltration was unique to the salivary glands, B cells in the liver and spleen were studied at 8 and 14 months of age (Fig. 6). To compare the different B cell subsets, B cells were clustered using UMAP and X-shift algorithms based on CD23, CD21, CD11c, and CD11b expression (Fig. 6A–C, Supplementary Figure S7). At 8 months, the composition of B cell subsets in the salivary glands differed from the spleen and liver (Fig. 6D and E). A significant fraction of B cells in the salivary glands were ABCs (24.3 ± 3.54) compared to the spleen (1.62 ± 0.27) or liver (1.39 ± 0.21; p < 0.0001, n = 5). At 14 months, the ABC fractions were comparable between the different organs. At 8 months, the salivary glands also showed higher DN (CD21negCD23neg) and lower follicular cell subsets (p < 0.0001) than the spleen and liver (Fig. 6D–F). Thus, ABCs and DN cells were enriched in the salivary gland before the formation of lymphocytic foci.
Fig. 6.
Analysis of B cell subsets at 8 and 14 months of age. UMAP plots of salivary gland (A), spleen (B), and liver (C). CD19 + B220 + B cells clustering based on CD21, CD23, CD11c, and CD11b expression. Multicolor plot overlays are shown in Supplementary Figure S7. Clusters were defined by the X-Shift clustering algorithm. Each plot represents 2,500 B cells from 5 mice, with 500 B cells per mouse. CD23negCD21hi marginal zone (MZ) cells were seen predominantly in the spleen and rarely in other organs. Frequencies of the different B cell subsets in salivary glands (D), spleen (E), and liver (F) at 8 and 14 months. ABC age-associated B cells, DN double negative, MZ marginal zone, FO follicular, ND not determined
Aged C57BL/6 mice spontaneously develop high titers of autoantibodies in serum
Loss of tolerance with spontaneous development of autoantibodies has been reported in aged B6 mice [20]. In the present study, sera collected during sacrifice were tested for antinuclear antibodies (ANA) in an indirect immunofluorescence assay (Fig. 7). Aged mice showed antibodies with heterogeneous specificities to nuclear and cytoplasmic antigens (Fig. 7B–D). Endpoint titers showed significant elevation in serum autoantibody with increasing age (Fig. 7E). The autoantibody titers in some of the B6 mice were comparable to a high-titer pooled serum from MRL.lpr/lpr mice with lupus nephritis. These results demonstrate that with aging, there is a loss of immunological tolerance to self-antigens in the B6 mice.
Fig. 7.
Autoantibodies to nuclear and cytoplasmic antigens in aged C57BL/6 mice. The top panel shows autoantibody reactivity in sera of C57BL/6 females, and the bottom panel shows overlay with nuclear stain (DAPI). (A) Fluorescence intensity with a negative control serum at 1:50 dilution. (B–D) Different reactivity patterns to the nuclear membrane (B), homogenous nuclear (C), and cytoplasmic antigens (D). Scale bar = 20 µm. (E) Endpoint titers were determined by serial dilution. ♦: represents endpoint titer of pooled sera from autoimmune MRL.lpr/lpr mice. The difference between the groups was determined by the Kruskal–Wallis one-way ANOVA and Dunn’s post-test for multiple comparisons. ns, not significant; *p < 0.05
Age-associated salivary gland dysfunction may be independent of inflammation
Salivary gland dysfunction, as measured by pilocarpine-induced saliva production, was reduced in 1/5 mice at 13 months and 4/10 mice at 17–26 months of age (Fig. 8). However, salivary function did not show a statistically significant drop between the groups. Further, the drop in saliva volumes did not correlate with the severity of inflammation (Fig. 8), suggesting a significant reserve of functional tissue in the salivary glands. In contrast, tear production in aging B6 mice showed increased tear production despite severe lacrimal gland inflammation [19], suggesting differences in functional outcomes between salivary and lacrimal glands. Overall, our results show that aging in B6 female mice results in salivary gland inflammation and systemic autoimmunity, with some mice progressing to glandular dysfunction, all critical characteristics of SjD.
Fig. 8.
Salivary gland function and correlation with inflammation in aged mice. Left panel. Pilocarpine-induced saliva in mice at different ages. The cut-off was calculated as mean-2SD of the control mice at 3 months of age. Right panel. Correlation between the area of inflammation and amount of saliva produced as determined by the Spearman method
Discussion
The clinical diagnosis of SjD occurs most frequently in the 5th–6th decade of life, with symptoms worsening with age. This human life phase is equivalent to 14–18month-old mice [30]. Our results show that by 14 months, female B6 mice spontaneously develop well-organized lymphocytic foci within the salivary glands. No significant differences were noted in salivary gland inflammation between C57BL/6J and C57BL/6N mouse strains, and the kinetics of salivary gland inflammation and autoantibody development in our study were comparable with other reports in B6 mice [17, 18]. Collectively, these data establish that in B6 mice, aging of the exocrine glands facilitates adaptive immune cell infiltration.
Our study shows that by 8 months of age, corresponding to the 4th decade in humans, the salivary glands from B6 mice underwent inflammatory changes. The chemokine Cxcl11 showed significant upregulation. CXCL11 is a high-affinity ligand for CXCR3, a chemokine receptor on CD4 Th1 and CD8 effector T cells [31]. The CXCL11-CXCR3 axis is essential for recruiting activated CD4 and CD8 T cells and setting up a pro-inflammatory amplification loop for lymphocytic infiltration. The salivary glands of SjD patients have been shown to express CXCL11 [32], and CXCL11 levels were elevated in the tear film and on the ocular surface in SjD patients [33]. The study showing that disrupting CXCR3 function in NOD mice significantly influenced the development of SjD-like disease further highlights the importance of the CXCL11-CXCR3 axis in SjD [34]. The mechanisms involved in elevated CXCL11 expression in salivary glands of aging mice are unknown. Previous studies have suggested that in age-related macular degeneration, advanced glycation end products affect CXCL11 expression in the retinal pigmental epithelium [35]. Whether advanced glycation end products accumulate in aging salivary glands and influence CXCL11 expression by salivary gland epithelial cells will be investigated in the future.
Salivary gland gene expression in 3 months to 8 and 13 months showed a progressive increase in several pro-inflammatory chemokines and cytokines. Notable among these is Cxcl13, which is involved in aged adipose B cell recruitment and the formation of fat-associated lymphoid clusters in the visceral fatty tissue [36]. In the salivary glands, the increased CXCL13 may play a role in the organization of the lymphocytic foci and germinal center formation.
Chemokines produced by aged salivary epithelial cells have been implicated in inducing pro-inflammatory cytokines and T cell migration [18]. In this report, the authors suggested that aging salivary glands accumulated PD1 + senescent T cells. Although, in our study, we did not specifically investigate T cell senescence, by 14 months, our results showed that PD1-PDL1 signaling pathway associated with T cell exhaustion and/or senescence was downregulated in the salivary glands of aging mice (Fig. 3). Further, our study showed gene expression patterns with significantly increased Th1 and Th2 T cell function, classical and alternative macrophage activation, and cytokine storm signaling pathways (Fig. 3).
Our study demonstrates that ABCs are present in the salivary glands of aging B6 mice. At 8 months of age, the frequency of ABCs in salivary glands was higher than that of the spleen and liver. The mechanisms responsible for the early enrichment of ABCs in salivary glands are unknown. However, given that the engagement of TLR7 plays a vital role in ABC expansion, the possibility exists that endogenous ligands in the salivary glands sustain or expand the ABC population. Salivary gland epithelial cells have been shown to secrete exosomes loaded with SjD-associated Ro-RNP antigens [37]. The small cytoplasmic RNA associated with the Ro-RNP activates TLR7 [38] and might be involved in the expansion of ABCs in the salivary gland. Additionally, our study noted a strong correlation between the frequencies of ABCs and Tfh in the salivary glands of aging mice. Collectively, these data suggest that in addition to TLR7 engagement, Tfh also might be involved in expanding the ABCs population in salivary glands.
The salivary gland ABCs in aged mice consisted of heterogeneous populations that were CD11b + , CD11c + , or CD11b + CD11c + (Fig. 5A). A population of innate-like CD11b + B1 cells has been extensively studied in pleural and peritoneal cavities. Following activation, innate-like B1 cells produce large amounts of natural IgM antibodies and can migrate into the spleen [39]. However, the exit from the peritoneal cavity is associated with the loss of CD11b expression [40]. Whether some of the salivary gland CD11b + B cells are recent innate-like B1 migrants is unclear. Further phenotyping will provide insights into the functional differences between the different subsets.
Heterogeneity within ABC populations is reported in various autoimmune and infectious diseases [41, 42], yet how it affects SjD is unknown. In SjD patients, the CD21−/low B cells elevated in peripheral blood showed immunoglobulin gene rearrangement indicative of memory B cells and expressed antibodies reactive with nuclear and cytoplasmic autoantigens. In minor labial gland biopsies from SjD patients, a heterogeneous population of FcRL4 + B cells expressed Ki67, indicating actively dividing cells [43]. A fraction of the FcRL4 + B cells within the peri-ductal infiltrates were CD11c + Tbet + and bonafide ABCs [15]. Thus, the pathogenic contribution of ABCs in SjD may range from autoantibody production to lymphomagenesis. Indeed, in a mouse model of SjD, ABCs isolated from spleens and activated in vitro secreted antibodies reactive with multiple self-antigens [44].
The number of mouse models that recapitulate some features of SjD is abundant in the literature [45]. However, a lack of consideration of age as a confounding factor is a significant caveat in most models. Some of the more popular models on non-obese diabetes (NOD) genetic background develop salivary gland inflammation as early as 10–12 weeks of age [46], corresponding to a 20–30-year-old human being. Since female B6 mice develop severe age-dependent exocrine gland inflammation and some functional loss, mimicking SjD patients, aged mice would be more appropriate preclinical models for testing immunomodulating therapies.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors thank the OMRF Imaging Core and Flow Cytometry Core for their assistance. In addition, we thank the Laboratory for Molecular Biology and Cytometry Research at Oklahoma University Health Sciences Center, which provided Nanostring gene expression service. The authors thank the Aged Rodent Colony Resource of the National Institute of Aging for providing C57BL/6N mice at different ages.
Author contribution
HB designed and performed the experiments, analyzed the data, and wrote the manuscript. JD, MP, MM, and JP performed the experiments and analyzed the data. MP, MM, and JP edited the manuscript. USD conceived the idea, designed the experiments, and wrote the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Institute of Dental and Craniofacial Research, grant number DE032911, and Institutional Funds from the Oklahoma Medical Research Foundation. The flow cytometry equipment was supported by the Shared Instrumentation Grant 1S10OD028479-01.
Data Availability
Data will be made available upon reasonable request.
Declarations
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Harini Bagavant and Justyna Durslewicz contributed equally to this work.
Contributor Information
Harini Bagavant, Email: harini-bagavant@omrf.org.
Umesh S. Deshmukh, Email: umesh-deshmukh@omrf.org
References
- 1.Mariette X, Criswell LA. Primary Sjögren’s syndrome. N Engl J Med. 2018;378(10):931–9. 10.1056/NEJMc1804598. [DOI] [PubMed] [Google Scholar]
- 2.Retamozo S, Acar-Denizli N, Horváth IF, Ng WF, Rasmussen A, Dong X, Li X, Baldini C, Olsson P, Priori R, Seror R, Gottenberg JE, Kruize AA, Hernandez-Molina G, Vissink A, Sandhya P, Armagan B, Quartuccio L, Sebastian A, Praprotnik S, Bartoloni E, Kwok SK, Kvarnstrom M, Rischmueller M, Soláns-Laqué R, Sene D, Pasoto SG, Suzuki Y, Isenberg DA, Valim V, Nordmark G, Nakamura H, FernandesMoçaTrevisani V, Hofauer B, Sisó-Almirall A, Giacomelli R, Devauchelle-Pensec V, Bombardieri M, Atzeni F, Hammenfors D, Maure B, Carsons SE, Gheita T, Sánchez-Berná I, López-Dupla M, Morel J, Inanç N, Fonseca-Aizpuru E, Morcillo C, Vollenweider C, Melchor S, Vázquez M, Díaz-Cuiza E, Consani-Fernández S, de-Miguel-Campo B, Szántó A, Bombardieri S, Gattamelata A, Hinrichs A, Sánchez-Guerrero J, Danda D, Kilic L, De Vita S, Wiland P, Gerli R, Park SH, Wahren-Herlenius M, Bootsma H, Mariette X, Ramos-Casals M, Brito-Zerón P. Influence of the age at diagnosis in the disease expression of primary Sjögren syndrome. Analysis of 12,753 patients from the Sjögren Big Data Consortium. Clin Exp Rheumatol. 2021;39 Suppl 133(6):166–74. 10.55563/clinexprheumatol/egnd1i. [DOI] [PubMed] [Google Scholar]
- 3.Turner MD. Hyposalivation and xerostomia: etiology, complications, and medical management. Dent Clin North Am. 2016;60(2):435–43. 10.1016/j.cden.2015.11.003. [DOI] [PubMed] [Google Scholar]
- 4.Bjordal O, Norheim KB, Rødahl E, Jonsson R, Omdal R. Primary Sjögren’s syndrome and the eye. Surv Ophthalmol. 2020;65(2):119–32. 10.1016/j.survophthal.2019.10.004. [DOI] [PubMed] [Google Scholar]
- 5.Goulabchand R, Castille E, Navucet S, Etchecopar-Etchart D, Matos A, Maria A, Gutierrez LA, Le Quellec A, de Champfleur NM, Gabelle A, Guilpain P. The interplay between cognition, depression, anxiety, and sleep in primary Sjogren’s syndrome patients. Sci Rep. 2022;12(1):13176. 10.1038/s41598-022-17354-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brito-Zerón P, Acar-Denizli N, Ng WF, Zeher M, Rasmussen A, Mandl T, Seror R, Li X, Baldini C, Gottenberg JE, Danda D, Quartuccio L, Priori R, Hernandez-Molina G, Armagan B, Kruize AA, Kwok SK, Kvarnström M, Praprotnik S, Sène D, Bartoloni E, Solans R, Rischmueller M, Suzuki Y, Isenberg DA, Valim V, Wiland P, Nordmark G, Fraile G, Bootsma H, Nakamura T, Giacomelli R, Devauchelle-Pensec V, Knopf A, Bombardieri M, Trevisani VF, Hammenfors D, Pasoto SG, Retamozo S, Gheita TA, Atzeni F, Morel J, Vollenveider C, Horvath IF, Sivils KL, Olsson P, De Vita S, Sánchez-Guerrero J, Kilic L, Wahren-Herlenius M, Mariette X, Ramos-Casals M. How immunological profile drives clinical phenotype of primary Sjögren’s syndrome at diagnosis: analysis of 10,500 patients (Sjögren Big Data Project). Clin Exp Rheumatol. 2018;36 Suppl 112(3):102–12. [PubMed] [Google Scholar]
- 7.Perera S, Ma L, Punwaney R, Ramachandran S. Clinical and cost burden of primary Sjögren’s syndrome: descriptive analysis using a US administrative claims database. J Health Econ Outcomes Res. 2018;5(2):150–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thorlacius GE, Björk A, Wahren-Herlenius M. Genetics and epigenetics of primary Sjögren syndrome: implications for future therapies. Nat Rev Rheumatol. 2023;19(5):288–306. 10.1038/s41584-023-00932-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Theander E, Jonsson R, Sjöström B, Brokstad K, Olsson P, Henriksson G. Prediction of Sjögren’s syndrome years before diagnosis and identification of patients with early onset and severe disease course by autoantibody profiling. Arthritis Rheumatol. 2015;67(9):2427–36. 10.1002/art.39214. [DOI] [PubMed] [Google Scholar]
- 10.Theander E, Vasaitis L, Baecklund E, Nordmark G, Warfvinge G, Liedholm R, Brokstad K, Jonsson R, Jonsson MV. Lymphoid organisation in labial salivary gland biopsies is a possible predictor for the development of malignant lymphoma in primary Sjögren’s syndrome. Ann Rheum Dis. 2011;70(8):1363–8. 10.1136/ard.2010.144782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hao Y, O’Neill P, Naradikian MS, Scholz JL, Cancro MP. A B-cell subset uniquely responsive to innate stimuli accumulates in aged mice. Blood. 2011;118:1294–304. 10.1182/blood-2011-01-330530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rubtsov AV, Rubtsova K, Fischer A, Meehan RT, Gillis JZ, Kappler JW, Marrack P. Toll-like receptor 7 (TLR7)-driven accumulation of a novel CD11c+ B-cell population is important for the development of autoimmunity. Blood. 2011;118(5):1305–15. 10.1182/blood-2011-01-331462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rubtsova K, Rubtsov AV, Thurman JM, Mennona JM, Kappler JW, Marrack P. B cells expressing the transcription factor T-bet drive lupus-like autoimmunity. J Clin Invest. 2017;127(4):1392–404. 10.1172/JCI91250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saadoun D, Terrier B, Bannock J, Vazquez T, Massad C, Kang I, Joly F, Rosenzwajg M, Sene D, Benech P, Musset L, Klatzmann D, Meffre E, Cacoub P. Expansion of autoreactive unresponsive CD21-/low B cells in Sjögren’s syndrome-associated lymphoproliferation. Arthritis Rheum. 2013;65(4):1085–96. 10.1002/art.37828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Verstappen GM, Ice JA, Bootsma H, Pringle S, Haacke EA, de Lange K, van der Vries GB, Hickey P, Vissink A, Spijkervet FKL, Lessard CJ, Kroese FGM. Gene expression profiling of epithelium-associated FcRL4+ B cells in primary Sjögren’s syndrome reveals a pathogenic signature. J Autoimmun. 2020;109:102439. 10.1016/j.jaut.2020.102439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Punnanitinont A, Kasperek EM, Kiripolsky J, Zhu C, Miecznikowski JC, Kramer JM. TLR7 agonism accelerates disease in a mouse model of primary Sjögren’s syndrome and drives expansion of T-bet+ B cells. Front Immunol. 2022;13:1034336. 10.3389/fimmu.2022.1034336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hayashi Y, Utsuyama M, Kurashima C, Hirokawa K. Spontaneous development of organ-specific autoimmune lesions in aged C57BL/6 mice. Clin Exp Immunol. 1989;78(1):120–6. [PMC free article] [PubMed] [Google Scholar]
- 18.Kurosawa M, Shikama Y, Furukawa M, Arakaki R, Ishimaru N, Matsushita K. Chemokines upregulated in epithelial cells control senescence-associated T cell accumulation in salivary glands of aged and Sjögren’s Syndrome model mice. Int J Mol Sci. 2021;22(5):2302. 10.3390/ijms22052302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Galletti JG, Scholand KK, Trujillo-Vargas CM, Yu Z, Mauduit O, Delcroix V, Makarenkova HP, de Paiva CS. Ectopic lymphoid structures in the aged lacrimal glands. Clin Immunol. 2023;248:109251. 10.1016/j.clim.2023.109251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nusser A, Nuber N, Wirz OF, Rolink H, Andersson J, Rolink A. The development of autoimmune features in aging mice is closely associated with alterations of the peripheral CD4+ T-cell compartment. Eur J Immunol. 2014;44(10):2893–902. 10.1002/eji.201344408. [DOI] [PubMed] [Google Scholar]
- 21.Bagavant H, Trzeciak M, Papinska J, Biswas I, Dunkleberger ML, Sosnowska A, Deshmukh US. A method for the measurement of salivary gland function in mice. J Vis Exp. 2018;131:57203. 10.3791/57203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Papinska J, Bagavant H, Gmyrek GB, Sroka M, Tummala S, Fitzgerald KA, Deshmukh US. Activation of stimulator of interferon genes (STING) and Sjögren syndrome. J Dent Res. 2018;97(8):893–900. 10.1177/0022034518760855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bagavant H, Trzeciak M, Biswas I, Papinska JA, Cizio K, Deshmukh US. Antibody deposition on vascular endothelial cells contributes to localized inflammation in salivary glands. J Oral Pathol Med. 2022;51(7):674–7. 10.1111/jop.13330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bagavant H, Deshmukh US. Protocols for experimental Sjögren’s Syndrome. Curr Protoc Immunol. 2020;131(1):e114. 10.1002/cpim.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bankhead P, Loughrey MB, Fernández JA, Dombrowski Y, McArt DG, Dunne PD, McQuaid S, Gray RT, Murray LJ, Coleman HG, James JA, Salto-Tellez M, Hamilton PW. QuPath: open source software for digital pathology image analysis. Sci Rep. 2017;7(1):16878. 10.1038/s41598-017-17204-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bagavant H, Cizio K, Araszkiewicz AM, Papinska JA, Garman L, Li C, Pezant N, Drake WP, Montgomery CG, Deshmukh US. Systemic immune response to vimentin and granuloma formation in a model of pulmonary sarcoidosis. J Transl Autoimmun. 2022;5:100153. 10.1016/j.jtauto.2022.100153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mekada K, Yoshiki A. Substrains matter in phenotyping of C57BL/6 mice. Exp Anim. 2021;70(2):145–60. 10.1538/expanim.20-0158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bharaj TK, Aqrawi LA, Fromreide S, Jonsson R, Brun JG, Appel S, Skarstein K. Inflammatory stratification in primary Sjögren’s syndrome reveals novel immune cell alterations in patients’ minor salivary glands. Front Immunol. 2021;12:701581. 10.3389/fimmu.2021.701581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Phalke S, Rivera-Correa J, Jenkins D, Flores Castro D, Giannopoulou E, Pernis AB. Molecular mechanisms controlling age-associated B cells in autoimmunity. Immunol Rev. 2022;307(1):79–100. 10.1111/imr.13068. [DOI] [PubMed] [Google Scholar]
- 30.Flurkey K, Currer JM, Harrison DE. Mouse models in aging research. The mouse in biomedical research. 2nd edition. 2007;3:637–72.
- 31.Groom JR, Luster AD. CXCR3 in T cell function. Exp Cell Res. 2011;317(5):620–31. 10.1016/j.yexcr.2010.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ogawa N, Kawanami T, Shimoyama K, Ping L, Sugai S. Expression of interferon-inducible T cell alpha chemoattractant (CXCL11) in the salivary glands of patients with Sjögren’s syndrome. Clin Immunol. 2004;112(3):235–8. 10.1016/j.clim.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 33.Yoon KC, Park CS, You IC, Choi HJ, Lee KH, Im SK, Park HY, Pflugfelder SC. Expression of CXCL9, -10, -11, and CXCR3 in the tear film and ocular surface of patients with dry eye syndrome. Invest Ophthalmol Vis Sci. 2010;51(2):643–50. 10.1167/iovs.09-3425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhou J, Yu Q. Disruption of CXCR3 function impedes the development of Sjögren’s syndrome-like xerostomia in non-obese diabetic mice. Lab Invest. 2018;98(5):620–8. 10.1038/s41374-017-0013-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lin T, Walker GB, Kurji K, Fang E, Law G, Prasad SS, Kojic L, Cao S, White V, Cui JZ, Matsubara JA. Parainflammation associated with advanced glycation endproduct stimulation of RPE in vitro: implications for age-related degenerative diseases of the eye. Cytokine. 2013;62(3):369–81. 10.1016/j.cyto.2013.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bénézech C, Luu NT, Walker JA, Kruglov AA, Loo Y, Nakamura K, Zhang Y, Nayar S, Jones LH, Flores-Langarica A, McIntosh A, Marshall J, Barone F, Besra G, Miles K, Allen JE, Gray M, Kollias G, Cunningham AF, Withers DR, Toellner KM, Jones ND, Veldhoen M, Nedospasov SA, McKenzie ANJ, Caamaño JH. Inflammation-induced formation of fat-associated lymphoid clusters. Nat Immunol. 2015;16(8):819–28. 10.1038/ni.3215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kapsogeorgou EK, Abu-Helu RF, Moutsopoulos HM, Manoussakis MN. Salivary gland epithelial cell exosomes: A source of autoantigenic ribonucleoproteins. Arthritis Rheum. 2005;52(5):1517–21. 10.1002/art.21005. [DOI] [PubMed] [Google Scholar]
- 38.Kelly KM, Zhuang H, Nacionales DC, Scumpia PO, Lyons R, Akaogi J, Lee P, Williams B, Yamamoto M, Akira S, Satoh M, Reeves WH. “Endogenous adjuvant” activity of the RNA components of lupus autoantigens Sm/RNP and Ro 60. Arthritis Rheum. 2006;54(5):1557–67. 10.1002/art.21819. [DOI] [PubMed] [Google Scholar]
- 39.Baumgarth N. The double life of a B-1 cell: self-reactivity selects for protective effector functions. Nat Rev Immunol. 2011;11(1):34–46. 10.1038/nri2901. [DOI] [PubMed] [Google Scholar]
- 40.Yang Y, Tung JW, Ghosn EE, Herzenberg LA, Herzenberg LA. Division and differentiation of natural antibody-producing cells in mouse spleen. Proc Natl Acad Sci U S A. 2007;104(11):4542–6. 10.1073/pnas.0700001104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mouat IC, Goldberg E, Horwitz MS. Age-associated B cells in autoimmune diseases. Cell Mol Life Sci. 2022;79(8):402. 10.1007/s00018-022-04433-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li ZY, Cai ML, Qin Y, Chen Z. Age/autoimmunity-associated B cells in inflammatory arthritis: an emerging therapeutic target. Front Immunol. 2023;14:1103307. 10.3389/fimmu.2023.1103307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Haacke EA, Bootsma H, Spijkervet FKL, Visser A, Vissink A, Kluin PM, Kroese FGM. FcRL4+ B-cells in salivary glands of primary Sjögren’s syndrome patients. J Autoimmun. 2017;81:90–8. 10.1016/j.jaut.2017.03.012. [DOI] [PubMed] [Google Scholar]
- 44.Punnanitinont A, Kasperek EM, Zhu C, Yu G, Miecznikowski JC, Kramer JM. TLR7 activation of age-associated B cells mediates disease in a mouse model of primary Sjögren’s disease. J Leukoc Biol. 2023;135. 10.1093/jleuko/qiad135. [DOI] [PMC free article] [PubMed]
- 45.Kaklamanos A, Goules AV, Tzioufas AG. Experimental models of Sjögren’s syndrome: differences and similarities with human disease. Clin Exp Rheumatol. 2022;40(12):2398–412. 10.55563/clinexprheumatol/d4cx78. [DOI] [PubMed] [Google Scholar]
- 46.Allushi B, Bagavant H, Papinska J, Deshmukh US. Hyperglycemia and salivary gland dysfunction in the non-obese diabetic mouse: caveats for preclinical studies in Sjögren’s syndrome. Sci Rep. 2019;9(1):17969. 10.1038/s41598-019-54410-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available upon reasonable request.