Abstract
Abstract
Aging-induced aortic stiffness has been associated with altered fatty acid metabolism. We studied aortic stiffness using cardiac magnetic resonance (CMR)-assessed ventriculo-arterial coupling (VAC) and novel aortic (AO) global longitudinal strain (GLS) combined with targeted metabolomic profiling. Among community older adults without cardiovascular disease, VAC was calculated as aortic pulse wave velocity (PWV), a marker of arterial stiffness, divided by left ventricular (LV) GLS. AOGLS was the maximum absolute strain measured by tracking the phasic distance between brachiocephalic artery origin and aortic annulus. In 194 subjects (71 ± 8.6 years; 88 women), AOGLS (mean 5.6 ± 2.1%) was associated with PWV (R = −0.3644, p < 0.0001), LVGLS (R = 0.2756, p = 0.0001) and VAC (R = −0.3742, p <0.0001). Stiff aorta denoted by low AOGLS <4.26% (25th percentile) was associated with age (OR 1.13, 95% CI 1.04–1.24, p = 0.007), body mass index (OR 1.12, 95% CI 1.01–1.25, p = 0.03), heart rate (OR 1.04, 95% CI 1.01–1.06, p = 0.011) and metabolites of medium-chain fatty acid oxidation: C8 (OR 1.005, p = 0.026), C10 (OR 1.003, p = 0.036), C12 (OR 1.013, p = 0.028), C12:2-OH/C10:2-DC (OR 1.084, p = 0.032) and C16-OH (OR 0.82, p = 0.006). VAC was associated with changes in long-chain hydroxyl and dicarboxyl carnitines. Multivariable models that included acyl-carnitine metabolites, but not amino acids, significantly increased the discrimination over clinical risk factors for prediction of AOGLS (AUC [area-under-curve] 0.73 to 0.81, p = 0.037) and VAC (AUC 0.78 to 0.87, p = 0.0044). Low AO GLS and high VAC were associated with altered medium-chain and long-chain fatty acid oxidation, respectively, which may identify early metabolic perturbations in aging-associated aortic stiffening.
Trial registration
ClinicalTrials.gov Identifier: NCT02791139
Supplementary Information
The online version contains supplementary material available at 10.1007/s11357-024-01127-x.
Keywords: Aorta, Strain, Stiffness, Metabolomics, Aging, Cardiovascular
Introduction
Aortic stiffness is widely acknowledged as a marker of vascular aging. This implies that aortic stiffness should be a rational target for clinical trials into human aging or longevity medicine. However, in contrast to the wealth of the knowledge about aortic stiffness and its strong position as a hallmark of human cardiovascular aging, there is far less work on how to modify vascular aging. Understanding molecular perturbations that occur beneath the clinical phenomenon of aortic stiffness may advance more research into vascular aging therapeutics.
In the area of cardiovascular risk stratification, metabolomics is increasingly used to complement traditional risk-scoring systems to provide finer cardiovascular risk stratification, for more mechanistic prediction of cardiac events in cardiovascular disease cohorts [1–3]. In line with disrupted energy pathways that characterize aging, alterations in fuel metabolism including mitochondrial dysfunction and/or defects in fatty acid β-oxidation [4] have been described in aortic stiffness. Specifically, using a target metabolomics approach, our prior work detected associations between arterial stiffness as measured by applanation tonometry and acyl-carnitines [4]. When used in conjunction with modern human imaging techniques [5], signals from metabolomics provide an opportunity to use blood-based strategies, targeted towards specific metabolic pathways to intervene on vascular aging.
On the other hand, our understanding about the aorta is incomplete without considering the mechanical connection between the AO and the left ventricle (LV). Firstly, movement of the aortic valve plane arising from LV contraction causes longitudinal movement of the ascending aorta [6, 7]. Secondly, longitudinal movement of the aorta occurs at the proximal aorta [6, 7] but also at the brachiocephalic trunk as well. These longitudinal strains on the aorta are unaccounted for by current conventional markers, including aortic pulse wave velocity (PWV) [8], diameter [9], distensibility [10] and aortic area change [10, 11], all of which assess stiffness solely by examining circumferential changes within the aorta.
Considering current drawbacks in aortic assessment, we developed a novel parameter, known as AO global longitudinal strain (GLS), to assess longitudinal strain of the ascending aorta that considers both the longitudinal movement of the aortic valve and the brachiocephalic trunk. We hypothesize that AO GLS would represent a more comprehensive marker of aortic strain. Apart from being correlated to PWV, we further hypothesize that mechanically, AO GLS would correlate with ventriculoarterial coupling of the LV. We also test the hypothesis that targeted metabolomics profiling involving acylcarnitines and amino acids which have been observed in association with changes in LV function in older adults [12] will characterize specific metabolic pathways associated with AO strain functions and ventriculoarterial coupling in these fuel-dependent tissues, AO and LV.
Methods
Study population
The subjects were recruited from the Cardiac Aging Study (CAS), which was previously described [13]. The current study cohort consisted of men and women who participated in the baseline CAS examination and who had no self-reported history of physician-diagnosed cardiovascular disease (such as coronary heart disease or stroke) or cancer. Written informed consent was obtained from participants upon enrolment. The local institutional review board (2014/628/C) had approved the study protocol.
Cardiac magnetic resonance (CMR) acquisition and analysis
The CMR acquisition protocol was previously published [13].
AO and LV global longitudinal strain
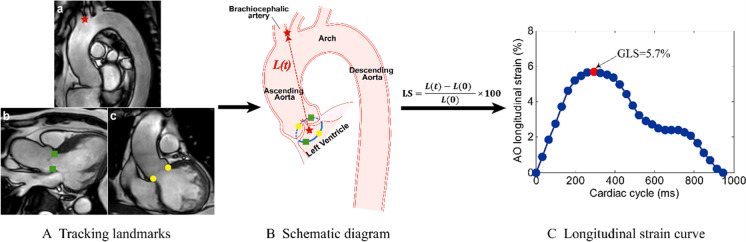
We used an in-house semi-automatic algorithm to evaluate the ascending AO GLS by feature tracking the displacement of the aortic valve relative to the origin of the brachiocephalic artery. The movement of the aortic annulus and the origin of the brachiocephalic artery from the aorta was automatically tracked over the cardiac cycle in Arch, LVOT and coronal LVOT views (Fig. 1a–c). The trajectory of the centroid derived from four aortic annular points was simulated (Fig. 1). The distance (L) from the brachiocephalic artery to the centroid was calculated throughout the cardiac cycle. Longitudinal strain at a time point (t) relative to the initial time point (time 0) at end-diastole was calculated as (L(t) − L(0))*100/L(0), and the AO GLS was defined as the maximal absolute strain value. Based on cine four-chamber CMR images, the LV endocardium was tracked by one reader (X.D.Z.) blinded to all participant characteristics, and LV GLS was automatically obtained by using dedicated QStrain software (Version 2.0, Medis BV, Leiden, The Netherlands). As LV GLS was negative and the calculated direction was different from AO GLS, we took their absolute values for a simple interpretation. Twenty random subjects were selected to assess the intra- and inter-observer variability of AO GLS performed by two independent operators (H.Z.Z. and S.L.). Feature tracking was performed twice with an interval of 1 month by the first operator (H.Z.Z.) to assess intra-observer variability. Correlation and Bland-Altman analyses were conducted to investigate intra- and inter-observer variabilities (Supplementary Figure S1).
Fig. 1.
Measurement method of ascending aorta (AO) global longitudinal strain (GLS). A. Tracking the landmarks on the brachiocephalic artery from the aorta (a, red star), left ventricular outflow tract (LVOT) (b, green squares) and coronal LVOT (b, yellow dots) views. B. Schematic diagram of feature tracking. The trajectory of the centroid (red star) derived from four aortic annular points was calculated. The distance (L(t), the red dotted line) between two red stars was automatically tracked throughout the cardiac cycle in 3D space. C. Longitudinal strain (LS) curve. LS at a time point (t) relative to the initial time point (time 0) at end-diastole was calculated as (L(t) − L(0))*100/L(0), and the AO GLS was defined as the maximal absolute strain value
Aortic arch PWV
Aortic arch PWV (AA PWV) was assessed by 2D phase-contrast CMR in a segment that includes part of the ascending aorta, the aortic arch and the proximal descending aorta. The contours of the ascending and descending aorta were automatically detected in all phases of the cardiac cycle by using the MASS software (Version 2109EXP, Leiden University Medical Center, Leiden, The Netherlands). The transit time (Δt) between two velocity-time curves of the ascending and descending aorta was automatically calculated by using the half-max method of MASS software. The length between the ascending and descending aorta was measured manually along the aortic intra-luminal center-line from the arch view. The AA PWV was calculated by dividing the length into the transit time (Δt) (Supplementary Figure S2a-c). Correlation and Bland-Altman analyses were conducted to investigate intra- and inter-observer variabilities (Supplementary Figure S3).
Metabolomic profiling
In brief, serum samples (50 μl) were spiked with 10 μl deuterium-labeled amino acid mixture and diluted with 400 μl methanol. After centrifugation of the mixture at 17,000 g for 5 min at 4 °C, the supernatant fraction was collected (10 μl) for amino acid analysis. A pooled quality control (QC) sample was prepared by mixing equal amounts (10 μl) of each extracted serum sample. Extraction and measurement of amino acid panels (quantified in units of μM) were performed as previously described [12]. Extraction and measurement of acyl-carnitine were performed as previously described [14] (more details in Supplementary).
Statistical analysis
All variables are displayed as mean ± SD for continuous data and frequency and percentage for categorical data. Cardiovascular risk factor 2 (CVRF2) was defined as the presence of any two or more cardiovascular risk factors (hypertension, dyslipidemia, ever smoked). We defined aorta low as the AO GLS was below than 25th percentile [15] of the cohort. Low AO GLS indicated high aortic stiffness, while higher PWV indicated high aortic stiffness. The ratio of AA PWV to LV GLS was used to assess ventriculo-arterial coupling (VAC) [16]. We defined high AA PWV/LV GLS as the VAC was higher than the average of the cohort. The associations between AO GLS and AA PWV and LV GLS were assessed by Pearson correlation. We analyzed 88 metabolites comprising 71 acyl-carnitine metabolites and 17 amino acid metabolites. The list of measured metabolites is presented in Supplementary Table A.
Univariate logistic regression of clinical factors was performed between the high AO GLS (low aortic stiffness) group and low AO GLS (high aortic stiffness) group. The odds ratio (OR) and the 95% confidence interval (CI) were presented. All clinical risk factors that show OR with p value <0.05 for predicting low AO GLS (high aortic stiffness) in univariate analysis were further included in multivariable logistic regression models. Receiver operating characteristic (ROC) analysis was performed to assess the clinical utility of clinical risk factors for predicting low AO GLS (high aortic stiffness). The association between metabolites and AO GLS was identified in three steps. Firstly, univariate logistic regression of metabolites for aorta low was conducted respectively to identify significant metabolites with p value <0.05. Secondly, multivariable logistic regression adjusting for significant clinical risk factors was performed for each metabolite that showed an association with p value <0.05 in univariate analysis. Thirdly, significant clinical factors in univariate analysis and significant metabolites with p value <0.05 in multivariable analysis were further included in multivariable logistic regression. Using ROC analysis, we compared models with clinical factors with models that included significant metabolites to predict low AO GLS (high aortic stiffness). Similarly, multivariable logistic regression was performed for VAC using significant clinical factors in the univariate analysis and significant acyl-carnitines with p value <0.05 in multivariable analysis. ROC analysis compared models with clinical factors with models that included significant metabolites to VAC. All statistical analyses were performed using STATA 18 (College Station, TX, USA). For all analyses, a two-tailed p value of <0.05 was considered significant.
Results
We analyzed a total of 194 participants (mean age 71±8.6 years, 88 women) that have all available CMR image views to obtain the AO GLS and AA PWV. The cutoff value 4.26% of AO GLS was identified to distinguish low versus high AO GLS. Table 1 shows clinical characteristics and AO GLS of participants in the overall cohort, and high versus low AO GLS. The average AO GLS is 5.6 ± 2.1% in the overall cohort. Hypertension (55.7%), diabetes mellitus (48.5%), dyslipidemia (20.6%) and smoking (15.0%) were the most common associated comorbidities of participants; 24.7% of participants had two or more risk factors (dyslipidemia, hypertension, smoking). Systolic and diastolic blood pressures were 147 ± 16 mmHg and 74 ± 11 mmHg respectively in this study sample.
Table 1.
Baseline clinical characteristics between AO GLS high group and AO GLS low group and univariate logistic regression for predicting low AO GLS
| Parameters | Overall (n = 194) | High AO GLS (n = 145) | Low AO GLS (n = 49) | OR (95% CI) | p value |
|---|---|---|---|---|---|
| Age (year) | 71 ± 8.6 | 70 ± 9.5 | 73 ± 4.0 | 1.13 (1.04, 1.23) | 0.006 |
| Female, n (%) | 88 (45.4%) | 71 (49.0%) | 17 (34.7%) | 0.55 (0.28, 1.09) | 0.085 |
| Weight (kg) | 60 ± 10.2 | 59 ± 9.4 | 62 ± 12.0 | 1.03 (1.0, 1.06) | 0.055 |
| Height (cm) | 160 ± 8.1 | 160 ± 8.1 | 160 ± 8.1 | 1.00 (0.96, 1.04) | 0.99 |
| Body surface area (m2) | 1.6 ± 0.2 | 1.6 ± 0.2 | 1.6 ± 0.2 | 4.47 (0.56, 36) | 0.16 |
| Body mass index (kg/m2) | 24 ± 3.4 | 23 ± 3.3 | 24 ± 3.5 | 1.11 (1.006, 1.22) | 0.038 |
| Systolic blood pressure (mmHg) | 147 ± 16.4 | 146 ± 17.4 | 148 ± 13.2 | 1.01 (0.99, 1.03) | 0.48 |
| Diastolic blood pressure (mmHg) | 74 ± 10.8 | 74 ± 10.7 | 77 ± 10.7 | 1.03 (1.0, 1.06) | 0.068 |
| Heart rate (bpm) | 74 ± 13.3 | 73 ± 13.5 | 78 ± 11.9 | 1.03 (1.004, 1.05) | 0.024 |
| Hypertension, n (%) | 108 (55.7) | 73 (50.3) | 35 (71.4) | 2.47 (1.22, 4.97) | 0.012 |
| Dyslipidemia, n (%) | 40 (20.6) | 27 (18.6) | 13 (26.5) | 1.58 (0.74, 3.37) | 0.24 |
| Diabetes mellitus, n (%) | 94 (48.5) | 71 (49.0) | 23 (46.9) | 0.92 (0.48, 1.76) | 0.81 |
| Smoking, n (%) | 29 (15.0) | 16 (11.0) | 13 (26.5) | 2.91 (1.28, 6.61) | 0.011 |
| CVRF2, n (%) | 48 (24.7) | 30 (20.7) | 18 (36.7) | 2.23 (1.10, 4.51) | 0.026 |
| LV GLS (%) | −21 ± 2.8 | −21 ± 2.8 | −19 ± 2.4 | 1.33 (1.16, 1.53) | <0.0001 |
| AA PWV (m/s) | 8.9 ± 3.0 | 8.6 ± 2.7 | 9.7 ± 3.6 | 1.12 (1.01, 1.25) | 0.0303 |
| VAC | −0.44 ± 0.19 | −0.42 ± 0.17 | −0.51 ± 0.22 | 0.08 (0.01, 0.43) | 0.0026 |
| AO GLS (%) | 5.6 ± 2.1 | 6.3 ± 1.9 | 3.5 ± 0.6 |
AO GLS was negatively associated with AA PWV (r =−0.3644, p < 0.0001) (Supplementary Figure S4A). AA PWV in low AO GLS (high aortic stiffness) was higher than high AO GLS (low aortic stiffness) group (9.7 ± 3.6 m/s vs 8.6 ± 2.7 m/s, p = 0.027). AO GLS was positively associated with LV GLS (r =0.2756, p = 0.0001) (Supplementary Figure S4B).
LV GLS in the low AO GLS (high aortic stiffness) group was lower than the high AO GLS (low aortic stiffness) group (19 ± 2.4% vs 21 ± 2.8%, p < 0.0001). AO GLS was negatively associated with VAC (r =0.3742, p < 0.0001).
Univariate logistic regression revealed that the determinants of low AO GLS (high aortic stiffness) were age, body mass index (BMI), heart rate, hypertension, smoking and CVRF2. We only included CVRF2 into the multivariable model as CVRF2 contained hypertension and smoking. Multivariable logistic regression showed that low AO GLS (high aortic stiffness) was associated with older age (OR: 1.13, 95% CI: 1.03–1.24), higher BMI (OR: 1.12, 95% CI: 1.01–1.25) and elevated heart rate (OR: 1.04, 95% CI: 1.01–1.06) (Supplementary Table S1: clinical model 1). The area under the ROC curve (AUC) was 0.73 in this model (clinical model 1).
Amino acids associated with low AO GLS are displayed in Table S2. Low AO GLS (high aortic stiffness) was associated with isoleucine (OR: 1.006, 95% CI: 1.0003–1.012), ornithine (OR: 1.01, 95% CI: 1.0005–1.022) and proline (OR: 1.005, 95% CI: 1.0004–1.009) in univariate logistic regression. After adjusting for age, BMI, heart rate and CVRF2, these amino acids showed no significant association with low AO GLS (high aortic stiffness) (Supplementary Table S2). These amino acids were not statistically significant in the multivariable logistic regression model combined with significant clinical risk factors (Supplementary Table S3). Levels of the identified metabolites are summarized in Supplementary Table S4.
Table 2 shows the results of univariate logistic regression of acyl-carnitines for low AO GLS (high aortic stiffness) and multivariable analysis of significant acyl-carnitines adjusting for age, BMI, heart rate and CVRF2. Low AO GLS (high aortic stiffness) was associated with C8, C10, C12, C12:2-OH/C10:2-DC, C14, C16:1 and C16-OH in univariate analysis (p < 0.05 for all). After adjusting for significant clinical risk factors, low AO GLS (high aortic stiffness) remained associated with C8 (OR: 1.005, 95% CI: 1.0006–1.01), C10 (OR: 1.003, 95% CI: 1.0002–1.006), C12 (OR: 1.013, 95% CI: 1.001–1.025), C12:2-OH/C10:2-DC (OR: 1.084, 95% CI: 1.007–1.17) and C16-OH (OR: 0.82, 95% CI: 0.71–0.94).
Table 2.
Logistic regression of acyl-carnitines for predicting low AO GLS
| Acyl-carnitines | Unadjusted | Adjusted* | Model characteristics* | Model D) | Model characteristics (Model D) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | p value | OR (95% CI) | p value | N | p value | Pseudo R2 | OR (95% CI) | p value | N p value Pseudo R2 | |
| C8 | 1.005 (1.001–1.009) | 0.013 | 1.005 (1.0006–1.01) | 0.026 | 194 | <0.0001 | 0.14 | 1.003 (1.0–1.011) | 0.52 | 192 <0.0001 0.22 |
| C10 | 1.003 (1.0003–1.006) | 0.028 | 1.003 (1.0002–1.006) | 0.036 | 192 | <0.0001 | 0.13 | - | - | |
| C12 | 1.013 (1.002–1.024) | 0.016 | 1.013 (1.001–1.025) | 0.028 | 194 | <0.0001 | 0.14 | 1.013 (0.99–1.036) | 0.27 | |
| C12:2-OH/C10:2-DC | 1.077 (1.006–1.15) | 0.033 | 1.084 (1.007–1.17) | 0.032 | 194 | <0.0001 | 0.14 | 1.076 (0.99–1.17) | 0.077 | |
| C14 | 1.046 (1.004–1.09) | 0.034 | 1.043 (0.997–1.091) | 0.066 | 194 | <0.0001 | 0.13 | |||
| C16:1 | 1.047 (1.005–1.092) | 0.030 | 1.041 (0.994–1.090) | 0.091 | 194 | <0.0001 | 0.13 | |||
| C16-OH | 0.88 (0.78–0.995) | 0.042 | 0.82 (0.71–0.94) | 0.006 | 194 | <0.0001 | 0.16 | 0.76 (0.64–0.90) | 0.001 | |
BMI body mass index, CVRF2 cardiovascular risk factor ≥2 (hypertension dyslipidaemia, ever smoked), AO aorta, GLS global longitudinal strain, OR odds ratio
*Multivariable analysis adjusted for age, BMI, heart rate, CVRF2 (dyslipidaemia, hypertension, smoking)
We evaluated the AUC of adding each significant metabolite identified from the multivariable adjusted analysis to the clinical model 1 (Fig. 2). Model E included all significant metabolites (AUC 0.8057), while model D had the highest AUC (AUC 0.8062). Compared to the baseline clinical model 1, both model E (p = 0.0286) and model D (p = 0.0374) had incrementally improved the AUC for predicting low AO GLS (Fig. 3A). Overall, AO GLS associated mostly with changes in medium-chain acyl-carnitines, implicating this part of the fatty acid oxidation machinery in aortic function.
Fig. 2.
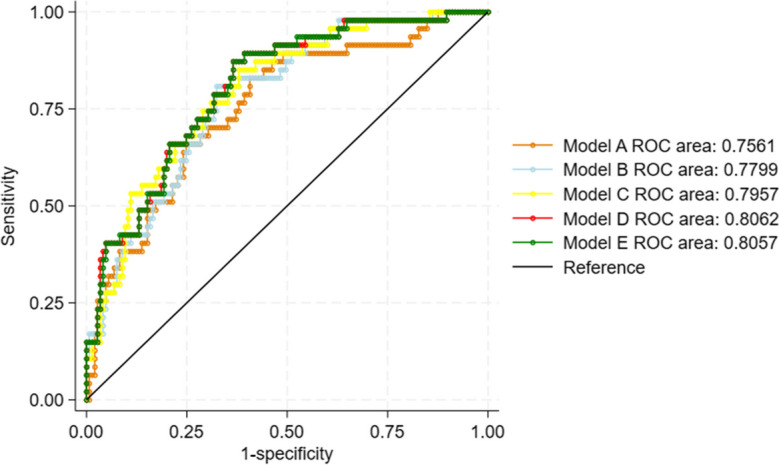
Depiction of AUC based on significant metabolites for predicting low AO GLS. Model A: Clinical model 1 and C16-OH; Model B: Clinical model 1 and C16-OH and C8; Model C: Clinical model 1 and C16-OH and C8 and C12; Model D: Clinical model 1 and C16-OH and C8 and C12 and C12:2-OH/C10:2-DC; Model E: Clinical model 1 and C16-OH and C8 and C12 and C12:2-OH/C10:2-DC and C10
Fig. 3.
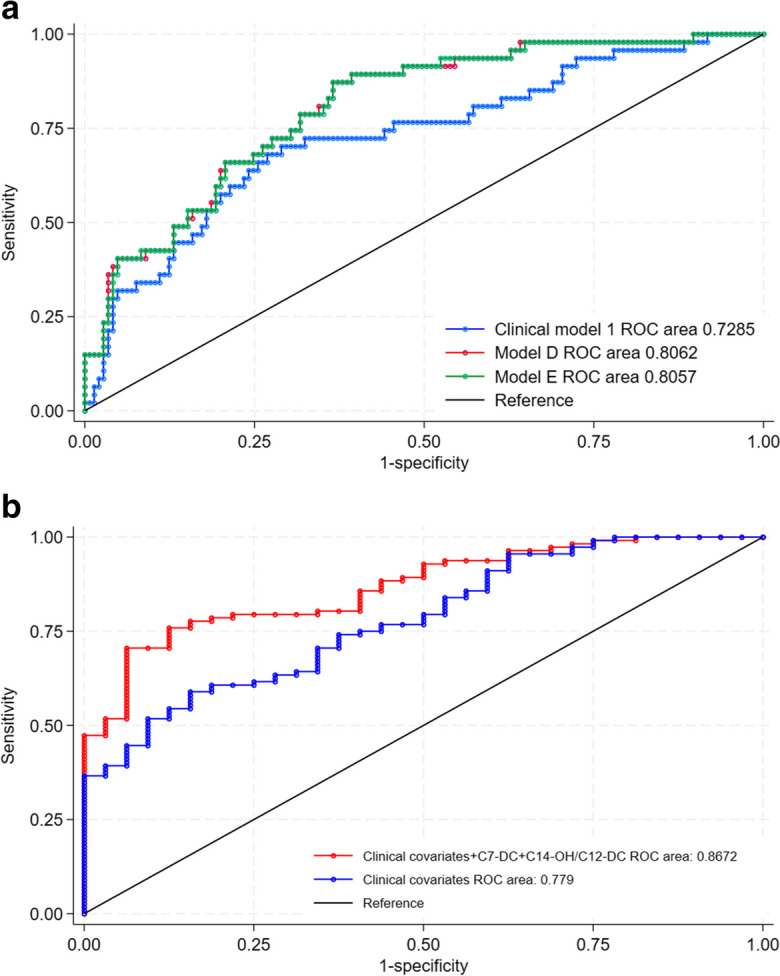
Comparison of AUC between models. A: Clinical model 1 versus model D (p = 0.0374) and between clinical model 1 versus model E (p = 0.028), for predicting low AO GLS; B: Clinical covariates versus clinical covariates and metabolites, for predicting VAC (p = 0.0044)
In contrast, VAC was associated with changes in long-chain hydroxyl and dicarboxyl carnitines (Table 3). These differences may point to other aspects of fatty acid handling that are important in ventriculoarterial interactions. The AUC of a model that contained significant metabolites and clinical covariates was 0.87 and was significantly higher than a model containing clinical covariates alone (p = 0.0044) (Fig. 3B).
Table 3.
Multivariable logistic regression of acyl-carnitines for predicting VAC
| Acyl-carnitines | Unadjusted | Adjusted* | Model characteristics* | Adjusted (clinical + metabolites) | Model characteristics | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | p value | OR (95% CI) | p value | N | p value | Pseudo R2 | OR (95% CI) | p value | N | p value | Pseudo R2 | |
| C4-OH | 1.057 (1.01–1.11) | 0.015 | 1.068 (1.01–1.13) | 0.019 | 194 | <0.0001 | 0.19 | - | - | 144 | <0.0001 | 0.33 |
| C8-OH/C6-DC | 1.017 (1.0002–1.03) | 0.047 | 1.023 (1.004–1.04) | 0.020 | 194 | <0.0001 | 0.19 | - | - | |||
| C10:3 | 1.020 (1.003–1.04) | 0.024 | 1.020 (1.0004–1.04) | 0.046 | 194 | <0.0001 | 0.18 | - | - | |||
| C10:2 | 1.059 (1.005–1.17) | 0.033 | 1.058 (1.0004–1.12) | 0.048 | 158 | <0.0001 | 0.20 | - | - | |||
| C7-DC | 1.069 (1.02–1.12) | 0.005 | 1.082 (1.02–1.14) | 0.005 | 174 | <0.0001 | 0.22 | 1.067 (1.0–1.14) | 0.060 | |||
| C8-DC | 1.035 (1.002–1.07) | 0.038 | 1.038 (1.002–1.08) | 0.039 | 194 | <0.0001 | 0.18 | - | - | |||
| C122 | 1.10 (1.007–1.21) | 0.035 | 1.11 (1.0002–1.22) | 0.050 | 194 | <0.0001 | 0.18 | - | - | |||
| C14-OH/C12-DC | 1.26 (1.09–1.45) | 0.002 | 1.39 (1.15–1.67) | 0.001 | 194 | <0.0001 | 0.23 | 1.40 (1.10–1.78) | 0.006 | |||
| C16:2-OH | 1.31 (1.05–1.63) | 0.016 | 1.41 (1.09–1.82) | 0.009 | 194 | <0.0001 | 0.19 | - | - | |||
| C16:1-OH/C14:1-DC | 1.26 (1.04–1.53) | 0.019 | 1.35 (1.07–1.71) | 0.011 | 194 | <0.0001 | 0.19 | - | - | |||
| C16-OH | 1.30 (1.13–1.51) | <0.0001 | 1.30 (1.10–1.52) | 0.002 | 194 | <0.0001 | 0.21 | - | - | |||
| C18:1-OH/C16:1-DC | 1.37 (1.09–1.72) | 0.008 | 1.40 (1.07–1.82) | 0.014 | 194 | <0.0001 | 0.19 | - | - | |||
*Multivariable analysis adjusted for clinical covariates of age, systolic blood pressure (SBP), diastolic blood pressure (DBP), female, diabetes
Discussion
Longitudinal stretching of the ascending aorta was assessed by a novel index, AO GLS, via conventional cine CMR images, utilizing longitudinal movement of the aortic valve and aortic arch simultaneously. We characterized AO GLS by clinical risk factors and metabolites representative of aging in a human cohort. Low AO GLS (high aortic stiffness) was associated with aging, elevated heart rate, higher BMI, hypertension, smoking, medium- and long-chain acyl-carnitine and amino acid metabolites. AO GLS characterizes the aging human aorta in a more in-depth manner. By extending aortic observations into the LV, our results provide novel insights into fuel oxidation processes that occur in these critical tissues.
AO GLS and ventriculo-arterial coupling
The ascending aorta is anatomically and mechanically connected to the LV and aortic arch, which plays a pivotal role in buffering pulsatile stress from ventricular contraction and transmitting blood to the periphery [17]. The constant mechanical and physiologic interplay between the heart and arterial system is known as VAC, which can be assessed by the ratio of PWV to LV GLS18. The absolute PWV/GLS ratio was higher in hypertensives than in controls. The PWV/GLS ratio has been a superior marker of VAC compared to the traditional echocardiographic derived Ea/Ees [18]. Longitudinal strain of ascending aorta had been a novel imaging parameter for assessing aortic stiffness [6]. In this study, we observed that AO GLS was correlated with the absolute AA PWV/ LV GLS ratio among older adults, indicative of the mechanical value of AO GLS as a biomarker of the central aortic system that is linked to the heart.
AA PWV is a crucial quantitative parameter that has been widely used to assess the bioelastic function of the aorta and serves as a pathogenic marker in cardiovascular disease. Consistent with the partial results of Vanessa et al. [6], we also observed a negative correlation between AO GLS and AA PWV. The difference in tracking site and direction of stiffness may be the reasons for the low correlation coefficient.
LV GLS, a sensitive marker of myocardial function, has been demonstrated to be an independent predictor of prognosis for a variety of cardiac and vascular diseases, such as hypertension, heart failure, aortic stenosis and coarctation of the aorta [19]. Longitudinal strain was independently related to the aortic root dilation rate and aortic events in vascular diseases [7]. We observed a positive correlation between AO GLS and LV GLS, which further confirmed the longitudinal interplay between the ascending aorta and the LV [20].
AO GLS and metabolomics
Circulating metabolites have been associated with the development of aortic stiffness assessed by PWV in vascular aging [4, 21]. However, beyond changes in aortic distensibility, restricted longitudinal stretching of cells and fibrous tissue in the aortic wall occurs as well, resulting in aortic stiffness in the longitudinal direction. In our present study, we integrate advanced CMR imaging with serum metabolomic signals to assess the features of longitudinal aortic stiffness and metabolite patterns in older adults. We observed links between AO GLS and circulating acyl-carnitines and amino acids.
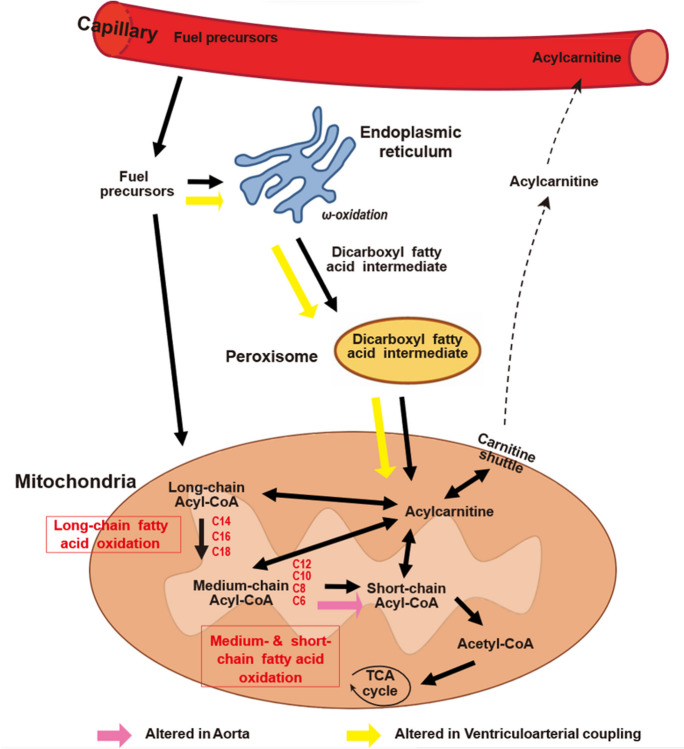
Acyl-carnitines are intermediates in the mitochondrial oxidation of fatty acid fuels [22]. Higher circulating levels of medium- and long-chain acyl-carnitines have been associated with increased arterial stiffness in patients with cardiovascular diseases [23], type 2 diabetes [24] or in older subjects without cardiovascular disease [4]. Our study found that higher levels of medium-chain acyl-carnitines were associated with low AO GLS and thus high aortic longitudinal stiffness. Our previous studies examining arterial stiffness as assessed by PWV also noted this positive correlation between medium/long chain acyl-carnitines and vascular stiffness [4]. We hypothesized that the increased accumulation of these metabolic intermediates is a result of changes in mitochondrial oxidation of fatty acids. However, in this study we also noted an inverse relationship between C16-OH and AO GLS which differs from the trends noted in our previous studies. C16-OH represents a hydroxylated intermediate of palmitate metabolism which can be either be generated during the beta oxidation spiral or by the process of alpha oxidation, which occurs mainly in peroxisomes instead of the mitochondria [25–28]. In either case the negative association between C16-OH carnitine and AO GLS suggests a divergence in tissue handling of shorter fatty acid fuels (i.e. C8, C10 and C12) which accumulate in the subjects with low AO GLS and longer chain fuels (C16) which are decreased in low AO GLS. The findings suggest that defects in medium chain fatty acid oxidation may be linked to impaired longitudinal aortic function. Our assessment of fuel metabolism and ventricular function found a similar pattern where medium chain acyl-carnitines were higher in patients with high VAC. In contrast, long chain fatty acid intermediates, which are frequently linked to left ventricular diseases, were positively associated with high VAC. The difference in relationship between long chain fatty acid oxidation and aortic versus ventricular function highlights potential differences in the response of these different tissues to changes in fuel metabolism (Fig. 4).
Fig. 4.
Schematic representation of mitochondrial pathways indicated by aortic longitudinal strain and ventriculo-arterial coupling. From the blood stream, fuel precursors enter the cell and undergo oxidative processes resulting in the production of acetyl-CoA. Fuel precursors enter the mitochondria and may undergo beta oxidation with progressive chain shortening leading to generation of acetyl CoA. Long chain fatty acids may also enter the endoplasmic reticulum to undergo alpha and omega-oxidation, which yields dicarboxyl and hydroxyl intermediates. Dicarboxyl and hydroxyl fatty acids CoAs enter the peroxisome to undergo chain shortening before eventually moving to the mitochondria for beta oxidation. Integrating advanced CMR imaging with serum metabolomics, levels of medium-chain acyl-carnitines (C8, C10, C12, C12:2-OH/C10:2-DC) were associated with aortic longitudinal strain, suggesting alterations in medium chain fatty acid oxidation as part of the process which alters aortic longitudinal strain. Accumulations of long chain dicarboxyl and hydroxyl fatty acid intermediates seen in association with ventriculo-arterial coupling may indicate disruption of alpha and omega oxidation in the left ventricle
Amino acid profiling was included in our targeted metabolomic approach as branched-chain amino acids have been implicated in aortic stiffness via inflammatory and oxidative stress pathways [29], including perturbations in lipid metabolism [30]. Despite observations between isoleucine, ornithine and proline, with AO GLS at the univariate level, we did not observe significant associations between amino acid metabolites and AO GLS at the multivariable level. However, isoleucine, one of the branched-chain amino acids (BCAAs), has been linked to aortic stiffness by pulse wave velocity [31]. As branched chain amino acids (BCAA), isoleucine and leucine are also frequently found altered in metabolic diseases including heart failure [32]. The lack of association after adjustments for clinical variables between these amino acid metabolites and AO GLS in our sample may be multifactorial but could be related to inherent characteristics within our sample of participants without cardiovascular disease, compared to studies that have included heart failure patients with advanced cardiovascular disease.
For risk stratification, circulating metabolites augmented the ability of traditional risk factors to predict aortic stiffness in older adults. This upstream phenomenon observed in our community older adults expands the ability of metabolomics to act as a biomarker for cardiovascular risk prediction, such as in disease cohorts [23]. In a study of older adults at higher cardiovascular risk, medium- and long-chain acyl-carnitines improved the prediction accuracy of cardiovascular events [33]. Medium-chain acyl-carnitine (C8 and C12:2-OH/C10:2-DC) and C16-OH had also been previously shown predictive of aortic stiffness assessed by non-CMR technique [4].
Finally, our observations add to the body of literature linking mitochondrial pathways to human aging, specifically towards vascular aging in the aorta and in coupling to the left ventricle. These findings may be used to inform on therapeutic strategies that alter vascular and myocardial metabolic remodeling, such as in promoting fatty acid utilization in the myocardium [34], or in ameliorating arterial stiffness [35].
Limitations
We acknowledge limitations to our study. The sample size of the analyzed cohort was relatively small despite statistically significant associations. Despite low sample power, these participants were from a low-risk community cohort for which observed relationships are likely to be underestimated, rather than over-estimated. Although these metabolites represent a small portion of the human metabolome, they report on critical pathways for cellular metabolism. With cross-sectional data alone it is difficult to differentiate between causation, consequence and association. Longitudinal follow-up data and clinical outcomes are needed to rationalize these findings. However, the associations between imaging biomarkers and metabolomics identified in this study are novel, and these markers have been associated with cardiovascular disease in individual studies as previously discussed. It is thus reasonable that there exist plausible relationships between early imaging findings and these metabolic processes which deserve further inquiry. Participants were not specifically instructed to perform an overnight fast prior to serum sampling. Non-fasting serum samples may produce analytic differences compared to other cohorts that use fasting samples. However, our practice is aligned with other cohort studies that have found that fasting did not contribute to variability in the measurement of most metabolites [36]. We present AO GLS obtained by this method as a novel imaging biomarker that may be relevant for human aging studies. Without prior reference values, we used the 25th percentile to define high aortic stiffness albeit with promising results. Future replication and corroboration of this method would be helpful to validate these cutoffs.
Conclusions
AO GLS, a novel parameter of aortic stiffness, studied in tandem with ventriculo-arterial coupling, was associated with aging and cardiovascular risk factors among a community cohort of older adults. Integrating advanced CMR imaging with serum metabolomics, levels of medium-chain acyl-carnitines were associated with aortic longitudinal strain, suggesting alterations in medium chain fatty acid oxidation as part of the process which alters aortic longitudinal strain. Accumulations of long chain dicarboxyl and hydroxyl fatty acid intermediates seen in association with ventriculo-arterial coupling may indicate disruption of alpha and omega oxidation in the left ventricle. AO GLS may be a novel risk stratification tool to identify early metabolic perturbations associated with aortic stiffness in human aging.
Supplementary information
(DOCX 504 kb)
Funding
The Cardiac Ageing Study has received funding support from the National Medical Research Council (MOH-000153; HLCA21Jan-0052; MOH-001193) and NMRC/OFIRG/0018/2016, Hong Leong Foundation. H.Z.Z. is supported by Graduate Research and Innovation Projects (No. YC2020-B055).
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Hongzhou Zhang and Shuang Leng contributed equally.
Liang Zhong and Angela S. Koh are joint senior authors.
Contributor Information
Liang Zhong, Email: zhong.liang@duke-nus.edu.sg.
Angela S. Koh, Email: angela.koh.s.m@singhealth.com.sg
References
- 1.Hilvo M, Meikle PJ, Pedersen ER, Tell GS, Dhar I, Brenner H, Schottker B, Laaperi M, Kauhanen D, Koistinen KM, Jylha A, Huynh K, Mellett NA, Tonkin AM, Sullivan DR, Simes J, Nestel P, Koenig W, Rothenbacher D, et al. Development and validation of a ceramide- and phospholipid-based cardiovascular risk estimation score for coronary artery disease patients. Eur Heart J. 2020;41:371–80. [DOI] [PubMed] [Google Scholar]
- 2.Paynter NP, Balasubramanian R, Giulianini F, Wang DD, Tinker LF, Gopal S, Deik AA, Bullock K, Pierce KA, Scott J, Martinez-Gonzalez MA, Estruch R, Manson JE, Cook NR, Albert CM, Clish CB, Rexrode KM. Metabolic predictors of incident coronary heart disease in women. Circulation. 2018;137:841–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Carvalho LP, Tan SH, Ow GS, Tang Z, Ching J, Kovalik JP, Poh SC, Chin CT, Richards AM, Martinez EC, Troughton RW, Fong AY, Yan BP, Seneviratna A, Sorokin V, Summers SA, Kuznetsov VA, Chan MY. Plasma ceramides as prognostic biomarkers and their arterial and myocardial tissue correlates in acute myocardial infarction. JACC Basic Transl Sci. 2018;3:163–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koh AS, Gao F, Liu J, Fridianto KT, Ching J, Tan RS, Wong JI, Chua SJ, Leng S, Zhong L, Keng BM, Huang FQ, Yuan JM, Koh WP, Kovalik JP. Metabolomic profile of arterial stiffness in aged adults. Diab Vasc Dis Res. 2018;15:74–80. [DOI] [PubMed] [Google Scholar]
- 5.Koh AS, Kovalik JP. Metabolomics and cardiovascular imaging: a combined approach for cardiovascular ageing. ESC Heart Fail. 2021;8:1738–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bell V, Mitchell WA, Sigurethsson S, Westenberg JJ, Gotal JD, Torjesen AA, Aspelund T, Launer LJ, de Roos A, Gudnason V, Harris TB, Mitchell GF. Longitudinal and circumferential strain of the proximal aorta. J Am Heart Assoc. 2014;3:e001536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guala A, Teixido-Tura G, Rodriguez-Palomares J, Ruiz-Munoz A, Dux-Santoy L, Villalva N, Granato C, Galian L, Gutierrez L, Gonzalez-Alujas T, Sanchez V, Forteza A, Garcia-Dorado D, Evangelista A. Proximal aorta longitudinal strain predicts aortic root dilation rate and aortic events in Marfan syndrome. Eur Heart J. 2019;40:2047–55. [DOI] [PubMed] [Google Scholar]
- 8.Soulat G, Gencer U, Kachenoura N, Villemain O, Messas E, Boutouyrie P, Laurent S, Mousseaux E. Changes in segmental pulse wave velocity of the thoracic aorta with age and left ventricular remodelling. An MRI 4D flow study. J Hypertens. 2020;38:118–26. [DOI] [PubMed] [Google Scholar]
- 9.Plonek T, Rylski B, Nawrocki P, Beyersdorf F, Jasinski M, Kuliczkowski W. Systolic stretching of the ascending aorta. Arch Med Sci. 2021;17:25–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Voges I, Jerosch-Herold M, Hedderich J, Pardun E, Hart C, Gabbert DD, Hansen JH, Petko C, Kramer HH, Rickers C. Normal values of aortic dimensions, distensibility, and pulse wave velocity in children and young adults: a cross-sectional study. J Cardiovasc Magn Reson. 2012;14:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shang Q, Sarikouch S, Patel S, Schuster A, Steinmetz M, Ou P, Danford DA, Beerbaum P, Kutty S. Assessment of ventriculo-vascular properties in repaired coarctation using cardiac magnetic resonance-derived aortic, left atrial and left ventricular strain. Eur Radiol. 2017;27:167–77. [DOI] [PubMed] [Google Scholar]
- 12.Kovalik JP, Zhao X, Gao F, Leng S, Chow V, Chew H, Teo LLY, Tan RS, Ewe SH, Tan HC, Wee HN, Lee LS, Ching J, Keng BMH, Koh WP, Zhong L, Koh AS. Amino acid differences between diabetic older adults and non-diabetic older adults and their associations with cardiovascular function. J Mol Cell Cardiol. 2021;158:63–71. 10.1016/j.yjmcc.2021.05.009.:63-71. [DOI] [PubMed] [Google Scholar]
- 13.Koh AS, Gao F, Leng S, Kovalik JP, Zhao X, Tan RS, Fridianto KT, Ching J, Chua SJ, Yuan JM, Koh WP, Zhong L. Dissecting clinical and metabolomics associations of left atrial phasic function by cardiac magnetic resonance feature tracking. Sci Rep. 2018;8:8138–26456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gao F, Kovalik JP, Zhao X, Chow VJ, Chew H, Teo LL, Tan RS, Leng S, Ewe SH, Tan HC, Tan TY, Lee LS, Ching J, Keng BM, Zhong L, Koh WP, Koh AS. Exacerbation of cardiovascular ageing by diabetes mellitus and its associations with acyl-carnitines. Aging (Albany NY). 2021;13:14785–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wei J, Palta P, Meyer ML, Kucharska-Newton A, Pence BW, Aiello AE, Power MC, Walker KA, Sharrett AR, Tanaka H, Jack CR, Mosley TH, Reid RI, Reyes DA, Heiss G. Aortic stiffness and white matter microstructural integrity assessed by diffusion tensor imaging: the ARIC-NCS. J Am Heart Assoc. 2020;9:e014868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pugliese NR, Balletti A, Armenia S, De Biase N, Faita F, Mengozzi A, Paneni F, Ruschitzka F, Virdis A, Ghiadoni L, Taddei S, Williams B, Antonini-Canterin F, Masi S. Ventricular-arterial coupling derived from proximal aortic stiffness and aerobic capacity across the heart failure spectrum. JACC Cardiovasc Imaging. 2022;15:1545–59. [DOI] [PubMed] [Google Scholar]
- 17.O'Rourke MF, Hashimoto J. Mechanical factors in arterial aging: a clinical perspective. J Am Coll Cardiol. 2007;50:1–13. [DOI] [PubMed] [Google Scholar]
- 18.Saeed S, Holm H, Nilsson PM. Ventricular-arterial coupling: definition, pathophysiology and therapeutic targets in cardiovascular disease. Expert Rev Cardiovasc Ther. 2021;19:753–61. [DOI] [PubMed] [Google Scholar]
- 19.Ikonomidis I, Aboyans V, Blacher J, Brodmann M, Brutsaert DL, Chirinos JA, De Carlo M, Delgado V, Lancellotti P, Lekakis J, Mohty D, Nihoyannopoulos P, Parissis J, Rizzoni D, Ruschitzka F, Seferovic P, Stabile E, Tousoulis D, Vinereanu D, et al. The role of ventricular-arterial coupling in cardiac disease and heart failure: assessment, clinical implications and therapeutic interventions. A consensus document of the European Society of Cardiology Working Group on Aorta & Peripheral Vascular Diseases, European Association of Cardiovascular Imaging, and Heart Failure Association. Eur J Heart Fail. 2019;21:402–24. [DOI] [PubMed] [Google Scholar]
- 20.Safar ME, Thomas F, Blacher J, Nzietchueng R, Bureau JM, Pannier B, Benetos A. Metabolic syndrome and age-related progression of aortic stiffness. J Am Coll Cardiol. 2006;47:72–5. [DOI] [PubMed] [Google Scholar]
- 21.Paapstel K, Kals J. Metabolomics of arterial stiffness. Metabolites. 2022;12:370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jarrell ZR, Smith MR, Hu X, Orr M, Liu KH, Quyyumi AA, Jones DP, Go YM. Plasma acylcarnitine levels increase with healthy aging. Aging (Albany NY). 2020;12:13555–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shah SH, Sun JL, Stevens RD, Bain JR, Muehlbauer MJ, Pieper KS, Haynes C, Hauser ER, Kraus WE, Granger CB, Newgard CB, Califf RM, Newby LK. Baseline metabolomic profiles predict cardiovascular events in patients at risk for coronary artery disease. Am Heart J. 2012;163:844–50. [DOI] [PubMed] [Google Scholar]
- 24.Ha CY, Kim JY, Paik JK, Kim OY, Paik YH, Lee EJ, Lee JH. The association of specific metabolites of lipid metabolism with markers of oxidative stress, inflammation and arterial stiffness in men with newly diagnosed type 2 diabetes. Clin Endocrinol (Oxf). 2012;76:674–82. [DOI] [PubMed] [Google Scholar]
- 25.Lodhi IJ, Wei X, Semenkovich CF. Lipoexpediency: de novo lipogenesis as a metabolic signal transmitter. Trends Endocrinol Metab. 2011;22:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reddy JK, Hashimoto T. Peroxisomal beta-oxidation and peroxisome proliferator-activated receptor alpha: an adaptive metabolic system. Annu Rev Nutr. 2001;21:193–230. [DOI] [PubMed] [Google Scholar]
- 27.Wanders RJ, Komen J, Kemp S. Fatty acid omega-oxidation as a rescue pathway for fatty acid oxidation disorders in humans. FEBS J. 2011;278:182–94. [DOI] [PubMed] [Google Scholar]
- 28.Wanders RJ, Waterham HR. Biochemistry of mammalian peroxisomes revisited. Annu Rev Biochem. 2006;75:295–332. [DOI] [PubMed] [Google Scholar]
- 29.Zhenyukh O, Gonzalez-Amor M, Rodrigues-Diez RR, Esteban V, Ruiz-Ortega M, Salaices M, Mas S, Briones AM, Egido J. Branched-chain amino acids promote endothelial dysfunction through increased reactive oxygen species generation and inflammation. J Cell Mol Med. 2018;22:4948–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang FH, Liu J, Deng QJ, Qi Y, Wang M, Wang Y, Zhang XG, Zhao D. Association between plasma essential amino acids and atherogenic lipid profile in a Chinese population: a cross-sectional study. Atherosclerosis. 2019;286:7–13. [DOI] [PubMed] [Google Scholar]
- 31.Jiang Y, Zhang K, Zhu Z, Cui M, An Y, Wang Y, Suo C, Fan M, Jin L, Tian W, Chen X. Associations between serum metabolites and subclinical atherosclerosis in a Chinese population: the Taizhou Imaging Study. Aging (Albany NY). 2020;12:15302–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hiraiwa H, Okumura T, Kondo T, Kato T, Kazama S, Ishihara T, Iwata E, Shimojo M, Kondo S, Aoki S, Kanzaki Y, Tanimura D, Sano H, Awaji Y, Yamada S, Murohara T. Usefulness of the plasma branched-chain amino acid/aromatic amino acid ratio for predicting future cardiac events in patients with heart failure. J Cardiol. 2020;75:689–96. [DOI] [PubMed] [Google Scholar]
- 33.Rizza S, Copetti M, Rossi C, Cianfarani MA, Zucchelli M, Luzi A, Pecchioli C, Porzio O, Di CG, Urbani A, Pellegrini F, Federici M. Metabolomics signature improves the prediction of cardiovascular events in elderly subjects. Atherosclerosis. 2014;232:260–4. [DOI] [PubMed] [Google Scholar]
- 34.Xie S, Zhang M, Shi W, Xing Y, Huang Y, Fang WX, Liu SQ, Chen MY, Zhang T, Chen S, Zeng X, Wang S, Deng W, Tang Q. Long-term activation of glucagon-like peptide-1 receptor by dulaglutide prevents diabetic heart failure and metabolic remodeling in type 2 diabetes. J Am Heart Assoc. 2022;11:e026728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lambadiari V, Pavlidis G, Kousathana F, Varoudi M, Vlastos D, Maratou E, Georgiou D, Andreadou I, Parissis J, Triantafyllidi H, Lekakis J, Iliodromitis E, Dimitriadis G, Ikonomidis I. Effects of 6-month treatment with the glucagon like peptide-1 analogue liraglutide on arterial stiffness, left ventricular myocardial deformation and oxidative stress in subjects with newly diagnosed type 2 diabetes. Cardiovasc Diabetol. 2018;17:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Townsend MK, Bao Y, Poole EM, Bertrand KA, Kraft P, Wolpin BM, Clish CB, Tworoger SS. Impact of pre-analytic blood sample collection factors on metabolomics. Cancer Epidemiol Biomarkers Prev. 2016;25:823–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 504 kb)