Abstract
For the outcome of a hepatitis B virus (HBV) infection, the viral L envelope protein with its pre-S domain performs pivotal functions by mediating attachment of HBV to liver cells, envelopment of viral capsids, release of (sub)viral particles, regulation of supercoiled DNA amplification, and transcriptional transactivation. To assess its multiple functions and host-protein assistance involved, we initiated a two-hybrid screen using the L-specific pre-S1 domain as bait. With this approach, we have identified γ2-adaptin, a putative member of the clathrin adaptor proteins responsible for protein sorting and trafficking, as a specific binding partner of L protein. Evidence for a physical interaction between L protein and γ2-adaptin was also demonstrated by affinity chromatography and coimmunoprecipitation, and the binding sites were mapped to the L-specific pre-S1 domain and the γ2-adaptin-specific ear domain. The specificity of the interaction was further sustained by the failure of γ1-adaptin, a closely related γ2-adaptin homologue, to associate with L protein. Analysis of an L mutant protein indicates that the L–γ2-adaptin interaction strictly depends on the pre-S1 domain of transmembrane L protein oriented to the cytosol and thus appears to occur in the cytosolic environment. Interestingly, coexpression of the two interacting partners in transfected cells resulted in recruitment of γ2-adaptin by L protein onto cis-Golgi-like structures, strongly indicating that the association is physiologically relevant. Together, the results suggest a role for γ2-adaptin in L-mediated processes of viral biogenesis and/or pathogenesis, such as facilitating and guiding HBV assembly.
The hepatitis B virus (HBV) is a small, enveloped DNA virus of the hepadnavirus family that causes acute and chronic liver infection. HBV remains a major worldwide health problem, as there is no generally effective therapy available for the estimated ∼300 million chronic carriers who face increased risk for developing hepatocellular carcinoma. Understanding the HBV life cycle and, importantly, the host cell protein interactions involved is thus a vital prerequisite for the development of antiviral concepts.
The HBV virion is a double-shelled sphere with an inner nucleocapsid and an outer lipoprotein envelope containing three distinct but related viral proteins, the large L, middle M, and small S envelope proteins (10, 23). All three envelope proteins are encoded by a single open reading frame of the viral genome by means of three different start codons that are spaced at intervals of 108 (or 119, depending on the subtype) and 55 codons. Accordingly, the 226-amino acid (aa) sequence of the S protein is repeated at the C termini of the M and L proteins, which carry the additional pre-S2 domain or pre-S2 and pre-S1 domains, respectively (see Fig. 1) (10, 23).
FIG. 1.
Domain structure and transmembrane topology of the HBV L protein. (A) Schematic representation of L protein consisting of the pre-S1, pre-S2, and S domains. Numbers below the domains refer to the corresponding amino acid positions; ¥ indicates the used N-glycosylation site. (B) Proposed split mixed topology of L protein in the (post)ER membrane and the virion envelope. Upon cotranslational membrane insertion, the pre-S1 and pre-S2 domains of L protein are initially located on the cytosolic side of the membrane (i-pre-S; left model); during maturation, about half of the L molecules posttranslationally translocate the pre-S domain to the luminal space (e-pre-S; right model), thereby leading to the dual topology. (C) Bait protein constructs for the yeast two-hybrid screen carrying the entire or parts of the pre-S1 domain of L protein. Below the rectangles, the corresponding amino acid positions of L protein fused to GAL4 BD are denoted. To measure GAL4 AD-independent transcriptional activation potentials of the bait constructs, transformed yeast cells were assayed for β-galactosidase reporter gene expression. Their transactivation properties are presented by percentage conversion of the relative value to a positive transactivation control provided by the supplier of the two-hybrid system.
Central to virogenesis is the large L envelope protein, as it has been shown to play manifold roles in the viral life cycle. The multifunctional nature of L protein is related to its unique topogenic properties, since it adopts two transmembrane topologies by disposing its N-terminal pre-S (pre-S1 plus pre-S2) domain to both the cytosolic (internal of the virion envelope [i-pre-S]) and luminal (external of the virion envelope [e-pre-S]) sides of the (post)endoplasmic reticulum (ER) membrane (see Fig. 1) (4, 25, 27). This split topology is achieved by a novel process of partial posttranslational translocation (22, 27) and appears to maximize the functions of L protein. In the early steps of infection, L protein with pre-S domains exposed to the external side (e-pre-S) is functionally important for hepatocyte receptor binding and intracellular uptake of virions, likely proceeding via receptor-mediated endocytosis (1, 18, 20, 24, 36). In the late stages of infection, L protein with pre-S domains disposed to the cytosolic side (i-pre-S) of intracellular membranes is essential for virus assembly, establishing a physical interaction of the viral envelope with preformed cytosolic nucleocapsids before the budding event (2, 3, 5). Though this event has not been unequivocally defined, HBV is thought to mature by budding into intraluminal cisternae of post-ER-premedial-Golgi compartments and to exit the cell by the constitutive secretory pathway (14, 15, 29, 39). The i-pre-S isoform of L protein has also been implicated to regulate viral replication by controlling amplification of the viral supercoiled DNA genome (32). Independent of the membrane topology, other crucial roles have been assigned for L protein that may contribute to the normal infection cycle and possibly also to viral pathogenesis. These include L-mediated transactivation of a variety of promoter elements and L-dependent intracellular retention and accumulation of the HBV envelope proteins (6, 11, 16), all of which can affect host cell physiology.
While it is true that the various functions described above are performed by the L protein, in a closer view one can easily find out that these roles are mostly, if not solely, performed by the pre-S domain of L protein. To assess its diverse functions, we initiated a two-hybrid screen to identify binding partners of L protein, especially those that interact with its pre-S domain. Among different clones recovered, here we identified γ2-adaptin, a novel clathrin adaptor-related protein, as a cellular target of L protein. Adaptor protein (AP) complexes, like AP-1 and AP-2, are instrumental in intracellular membrane trafficking, involving budding, transport, and fusion of transport vesicles (17, 31). While AP-1 mediates trafficking of cargo proteins from the trans-Golgi network (TGN) to the late endocytic pathway, AP-2 is involved in endocytosis from the plasma membrane. Each AP complex consists of heterotetrameric adaptor proteins that mediate the recruitment of clathrin to the vesicle budding site by interacting with sorting signals within the cytosolic domains of selected membrane proteins. AP-1 is made of two large subunits, γ1- and β1-adaptin, one medium subunit, μl, and one small subunit, ς1, whereas AP-2 consists of β2, α, μ2, and ς2 chains (17, 31). γ2-Adaptin, the L-interacting partner discovered in this work, closely resembles γ1-adaptin in both primary sequence and bipartite domain structure but appears to serve a separate yet unknown function distinct from that of AP-1 (21, 33). To confirm and characterize the interaction between L protein and γ2-adaptin, we have used a number of in vitro and in vivo techniques. Based on these results, we propose models for the assistance of the cellular sorting and trafficking machinery in HBV biogenesis.
MATERIALS AND METHODS
Yeast two-hybrid screening.
A MATCHMAKER human liver cDNA library fused with the yeast GAL4 activation domain (AD) in plasmid pACT2 was purchased from Clontech Laboratories and screened according to the manufacturer's instructions. The constructs used as bait were the entire pre-S1 region of the HBV (subtype ayw) L protein (L1-108), the N-terminal part of pre-S1 (L1-70), the C-terminal part of pre-S1 (L44-108), and an internally truncated pre-S1 region (L1-44,70-108). These baits were created by excision of the corresponding L gene restriction fragments from pNI2.L (see below) or existing pNI2.L derivatives and insertion into the SmaI site of plasmid pAS2-1 (Clontech) carrying the GAL4 binding domain (BD). Transformed yeast strain Y190 was allowed to grow for 8 days in synthetic medium lacking Trp, Leu, and His and then replica-plated and assayed for β-galactosidase activity. Positive colonies were grown in medium containing cycloheximide and were tested for loss of the pAS2-1.pre-S1 constructs and then reassayed for β-galactosidase activity. Only those colonies that tested negative were analyzed further by using yeast mating and semiquantitative filter assay for β-galactosidase activity, as instructed by the supplier of the two-hybrid system (Clontech). For quantification of the filter assay, a defined number of yeast cells was analyzed. Plasmid DNA isolated from yeast clones was then transformed into Escherichia coli HB101 and analyzed by sequencing.
Plasmid construction.
Mammalian expression vectors carrying the HBV L gene (pNI2.L) or the S gene (pNI2.S) under the transcriptional control of the human metallothionein IIA promoter have been described (27). For tagging of the HBV envelope proteins with an influenza virus hemagglutinin (HA) epitope, the HA-specific amino acid sequence YPYDVPDYASL was fused in frame to the C termini of the L (L-HA) and S (S-HA) proteins by site-directed mutagenesis using a recombinant M13mp19.HBV bacteriophage (22) and the antisense oligonucleotide 5′-GTTTTGTTAGGGTTTACAAGCTAGCGTAATCGGTAACATCGTATGGGTAAATGTATACCCAAAG-3′ (the HA-specific sequence is underlined). Construction of the mutant Ile-9::L, carrying the signal sequence from human interleukin-9 (MLLAMVLTSALLLCSVAG) fused to the N terminus of the L gene, was done by PCR using the oligonucleotide 5′-ACAAGAT CTACAGCATGCTTCTGGCCATGGTCCTTACCTCTGCCCTGCTCCTGT GCTCCGTGGCAGGCGGGCAGAATCTTTCC-3′ (the interleukin-9-specific sequence is underlined) as the mutagenic primer.
Expression vectors for γ1-adaptin (pcDNA3-HAγ1) and γ2-adaptin (pcDNA3-HA-γ2) which contain, respectively, either the human γ1-adaptin or γ2-adaptin cDNA derived from hepatoma HepG2 cells with an N-terminal HA tag preceded by the cytomegalovirus promoter were kindly provided by K. Nakayama, Tsukuba University, Tsukuba, Japan (33). For immunofluorescence analysis, plasmid pcDNA3-γ2, carrying the untagged γ2-adaptin gene (33), was additionally employed. To construct C-terminally truncated variants of γ2-adaptin, an XhoI-ApaI (nucleotide [nt] 1613 to nt 2455; numbering as referred to the γ2-cDNA; GenBank accession number AB015318) restriction fragment or a BglII-ApaI (nt 1926 to nt 2455) fragment was excised from plasmid pcDNA3-HA-γ2, thereby generating γ2Δ528-785 and γ2Δ627-785 mutants which lacked the last 257 or 158 aa of γ2-adaptin, respectively.
Transient transfection, immunoprecipitation, and Western blotting.
For ectopic expression of the HBV envelope and γ-adaptin proteins, transient transfection of COS-7 cells or HuH-7 cells, a human hepatoma cell line, was used. Therefore, 5 × 106 COS-7 cells were transfected with 12 μg of plasmid DNA by electroporation (for cotransfection, 12 μg of each DNA was used), whereas HuH-7 cells were transfected with 20 μg of plasmid DNA by the calcium phosphate precipitation technique. Three days posttransfection, cells were washed twice in phosphate-buffered saline (PBS) and lysed with 1 ml of 2% CHAPS {3-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulfonate}–HBS (50 mM HEPES [pH 7.5], 200 mM NaCl) and supplemented with antipain (1.5 μg/ml), pepstatin (2 μg/ml), chymostatin (3 μg/ml), and aprotinin (10 μg/ml) for 30 min on ice. Proper protein expression was monitored by gel electrophoresis of cleared cellular lysates and subsequent immunoblotting with specific antibodies. Enzymatic N-deglycosylation of proteins with PNGase F was done as described previously (22). To prevent N-linked glycosylation during protein synthesis, cells were pretreated with tunicamycin (10 μg/ml; Sigma) for 2 h before transfection, and the drug was maintained during the transient expression period of 24 h. For coimmunoprecipitation, an S-specific polyclonal antiserum was used which recognizes epitopes within the S region of the HBV envelope proteins (27). To this aim, 1 ml of lysate was incubated for 3 h at 4°C (with rocking) with 100 μl of a 10% suspension of protein G-agarose that had been precoated with 7 μl of the S-specific antiserum. Immune complexes were washed three times with 0.5% CHAPS–HBS and once with 125 mM Tris (pH 6.8) prior to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and Western blotting to nitrocellulose membranes. The blot was incubated with a mouse monoclonal antibody (MAb) against the HA epitope (BabCO) and peroxidase-labeled secondary antibody and detected by enhanced chemiluminescence, as instructed by the manufacturer (Amersham). Alternatively, immunoblotting was performed with the mouse MAb H166 (Abbott), specific for the S region of the HBV envelope proteins.
Affinity capture assay.
For recombinant expression of a histidine-tagged pre-S1 polypeptide in E. coli M15pREP4 cells, plasmid pQE11-preS1 was constructed by inserting a BglII-EcoRI (nt 2839 to nt 3182) fragment of the L gene into the BamHI-EcoRI sites of the pQE11 vector (Qiagen). As a control construct, plasmid pQE11-E7 carrying fragments of the E7 gene of human papillomavirus type 33 with an N-terminal six-His tag was used. After induction with 1 mM isopropyl-β-d-thiogalactopyranoside, bacteria were harvested by centrifugation and lysed with solubilization buffer (SB; 100 mM NaPi, 10 mM Tris [pH 8.0]) supplemented with 0.5% Triton X-100 by using a Branson sonifier. For purification and immobilization of the His-tagged polypeptides, the cleared lysates were incubated with Ni2+-nitrilotriacetic acid agarose (Ni-NTA) in SB buffer–10 mM imidazole for 2 h at 4°C with rocking. The Ni-NTA resin was subsequently washed four times with SB buffer–20 mM imidazole and three times with PBS. For in vitro binding assays, Ni-NTA beads loaded with about 37 μg of His-tagged proteins were equilibrated in PBS containing 2% bovine serum albumin for 2 h at 4°C with mild agitation. The slurry was then incubated for 3 h at 4°C with 0.5 ml of lysate of COS-7 cells which had been transfected with the γ2-adaptin or γ1-adaptin expression vectors, pcDNA3-HA-γ2 or pcDNA3-HA-γ1, respectively, and lysed as outlined above. To remove nonspecific bound proteins, beads were collected by centrifugation and washed two times with PBS and three times with PBS–0.1% Tween 20, followed by three washes with PBS. The His-tagged proteins together with bound proteins of the lysate were then eluted with 1 M imidazole in SB buffer. The eluates were separated by SDS-PAGE and subjected to immunoblotting using the HA-specific antibody.
Immunofluorescence microscopy.
Transiently transfected COS-7 and HuH-7 cells were grown on coverslips for 48 h to 90% confluence. Cells were fixed and permeabilized with ice-cold methanol containing 2 mM EGTA for 15 min at −20°C. For staining, cells were incubated with the mouse anti-HA MAb (1:50 dilution in PBS) or an L-specific polyclonal rabbit antiserum (22) (1:10,000 dilution in PBS). The primary antibodies were detected by incubation with dichlorotriazinyl-fluorescein (DTAF)-conjugated anti-mouse immunoglobulin G from goat (1:200 dilution in PBS) or rhodamine-red-ex-conjugated anti-rabbit immunoglobulin G from goat (1:800 dilution in PBS), both purchased from Dianova. Staining was visualized with a fluorescence microscope (Leica DMRBE).
In addition, immunofluorescence studies were done with polyclonal anti-γ2-antisera which were generated in rabbits (Eurogentec) according to the strategy reported by Takatsu et al. (33). Briefly, two animals were immunized with a C-terminal 19-aa peptide, derived from human γ2-adaptin (HQSVQEIFEVNNLPVESWQ), and were conjugated with keyhole limpet hemocyanin (Eurogentec), and the antisera obtained (SA 8329 and SA 8330) proved to be suitable for Western analysis and immunostaining (1:200 dilution in PBS).
RESULTS
Identification of L-binding proteins using the yeast two-hybrid system.
To identify potential host cell proteins that interact with the pre-S1 domain of the HBV L envelope protein, we screened a cDNA library derived from human adult liver by use of the yeast two-hybrid system. Transformation of reporter yeast Y190 cells with a plasmid encoding a fusion between the GAL4 DNA BD and the full-length pre-S1 domain of L protein (BD.L1-108), however, caused transcriptional activation of the β-galactosidase reporter, irrespective of whether the GAL4 transcription AD was present or not (Fig. 1). Addressing this issue, we therefore truncated the pre-S1 domain by constructing bait plasmids that carry either its first 70 (BD.L1-70) or last 65 residues (BD.L44-108). As shown in Fig. 1C, neither of these constructs displayed any transactivator activity and could thus be used for the two-hybrid approach. A further construct, BD.L1-44,70–108, that lacked the amino acid sequence common to BD.L1-70 and BD.L44-108, however, again showed an intrinsic transactivation potential (Fig. 1C). Together these data confirm a recent report that showed transactivator properties of distinct elements of the pre-S1 domain of L protein (16).
For the two-hybrid screens, yeast cells transformed with either the BD.L44-108 or BD.L1-70 bait were next transformed with the prey plasmids containing the liver cDNA library. Out of ∼6 × 106 transformants, several clones were isolated that exhibited a strong and specific interaction between the BD.L44-108 construct and the library construct. To determine the identity of the clones, both restriction analysis and sequencing were carried out. Four of these plasmid clones were found to encode the carboxyterminal region of the recently identified γ2-adaptin protein. Due to its high similarity to γ1-adaptin, γ2-adaptin is thought to constitute a large subunit of heterotetrameric adaptor complexes that are responsible for the sorting of cargo proteins and their directed vesicular traffic (21, 33). The remaining clones encoded elongation factor 2, which was also identified with the screen employing the N-terminal construct BD.L1-70. Among the clones isolated with this N-terminal bait, more than half of them encoded the inter-α-trypsin family heavy chain H4 protein (IHPR), while some other interacting clones were found to carry the cDNA for C1r, a complement component with serine protease activity. The nature of the interactions between pre-S1 and EF2, IHPR, and C1r is under investigation in our laboratory and will not be discussed further in this paper.
Interaction between the pre-S1 domain of L protein and γ2-adaptin in vitro.
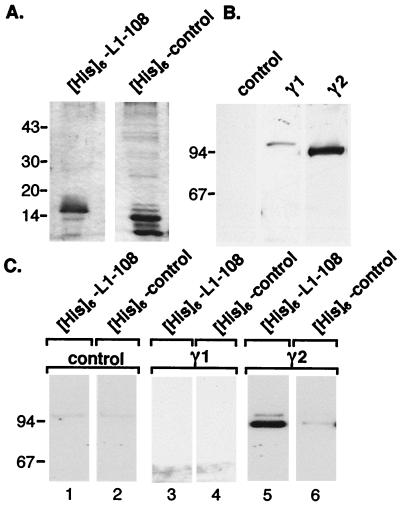
γ2-Adaptin shares the characteristic domain organization of large adaptin molecules, consisting of a large N-terminal head and a C-terminal ear domain that are connected by a flexible hinge region (21, 33). Remarkably, all of the independent library clones isolated with the BD.L44-108 construct encoded the C-terminal third region of γ2-adaptin, i.e., portions of the hinge region and the ear domain. We were therefore interested to demonstrate that the pre-S1–γ2-adaptin interaction, observed in yeast cells, was not confined to these portions of γ2-adaptin nor limited to the two-hybrid system. To measure an interaction of full-length γ2-adaptin with the pre-S1 domain of L protein, we employed an in vitro binding assay. For this purpose, recombinant pre-S1 polypeptide was expressed in bacteria as a fusion protein harboring an N-terminal six-His tag ([His]6-L1-108) (Fig. 2A). A histidine-tagged construct encoding an HBV-unrelated sequence of similar size ([His]6-control) was included as a negative control (Fig. 2A). To obtain native γ2-adaptin protein, transient transfection of COS-7 cells with an expression plasmid for human γ2-adaptin, tagged with an influenza virus HA epitope at its N terminus (pcDNA3-HAγ2), was carried out. For reasons of control, cells were also transfected with an analogous expression plasmid, pcDNA3-HA-γ1, encoding an HA-tagged version of γ1-adaptin protein that is closely related to γ2-adaptin. Synthesis of both the ∼90-kDa γ2-adaptin and the ∼100-kDa γ1-adaptin proteins was verified by immunoblot analysis of cellular lysates with an anti-HA-specific antibody (Fig. 2B). Next, lysates of transfected cells were prepared under nondenaturing conditions and incubated with either [His]6-L1-108 or [His]6-control prebound to Ni-NTA agarose beads. After elution, samples were subjected to immunoblotting with the HA-specific MAb. This analysis clearly identified γ2-adaptin in the presence of the [His]6-L1-108 fusion protein, while the [His]6-control yielded only a very faint background signal (Fig. 2C, lanes 5 and 6, respectively). In contrast, we did not observe binding in samples prepared from either mock-transfected cells (Fig. 2C, lanes 1 and 2) or cells expressing γ1-adaptin (Fig. 2C, lanes 3 and 4). Although the lower yield of γ1-adaptin compared to that of γ2-adaptin might prevent detection of an association with pre-S1 in this experiment, a γ1-adaptin–preS1 interaction altogether appears less likely, because γ1-adaptin also was not discovered in the highly sensitive two-hybrid screen. Therefore, these data confirmed a strong and specific interaction between γ2-adaptin and the pre-S1 domain of L protein.
FIG. 2.
In vitro interaction between the pre-S1 domain of L protein and γ2-adaptin. (A) Analysis of bacterial expression and purification of [His]6-L1-108 and a [His]6-control by SDS-PAGE and staining with Coomassie brilliant blue. (B) Analysis of transient expression of HA-tagged γ1- and γ2-adaptin proteins in transfected COS-7 cells by SDS-PAGE and HA-specific Western blotting. (C) Affinity capture assay. Immobilized His-tagged polypeptides were incubated with lysates of mock-transfected cells (control) or cells expressing γ1- or γ2-adaptin. After elution with imidazole, eluates were analyzed by SDS-PAGE and HA-specific immunoblotting. Numbers to the left of each panel show positions of molecular size standards in kDa.
Interaction between L protein and γ2-adaptin in vivo.
To corroborate the above data, coimmunoprecipitation studies were done to determine if γ2-adaptin also associates with the full-length L protein in vivo. COS-7 cells were (co)transfected with expression vectors encoding HA-tagged versions of either γ2-adaptin or L protein. As a prerequisite of such an approach, the proteins must be expressed efficiently. To investigate this point, cellular lysates of transfected cells were subjected to HA-specific Western blotting. As above, γ2-adaptin appeared in the expected position of 90 kDa, and L protein was obtained in its characteristic doublet of a 39-kDa nonglycosylated and a 42-kDa glycosylated species (Fig. 3A, lanes 1 and 2, respectively). In addition, lysates of L-transfected cells contained both forms of the small S HBV envelope protein in the range of 24 to 27 kDa (Fig. 3A, lane 2) derived from internal initiation of translation. The identity of these bands was confirmed by analyzing extracts of cells transfected with the S gene alone (Fig. 3A, lane 4). Lysates were then subjected to immunoprecipitation with antiserum specific for the HBV envelope proteins, and the immune complexes were analyzed by HA-specific immunoblotting. As shown in Fig. 3B, γ2-adaptin was efficiently coimmunoprecipitated from cells expressing L protein (lane 3), thus demonstrating a specific interaction between the two proteins in mammalian cells. In support, the HBV S protein failed to bring down γ2-adaptin under the same assay conditions (Fig. 3A, lane 5). The specificity of the L–γ2-adaptin interaction in vivo was further assessed by the inability of L protein to coprecipitate γ1-adaptin despite the significant homology between the two γ-adaptins (Fig. 3A, lane 7).
FIG. 3.
In vivo interaction between L protein and γ2-adaptin. COS-7 cells were transiently (co)transfected with the constructs indicated above each lane. (A) Stable synthesis of HA-tagged L and S HBV envelope proteins and γ2- and γ1-adaptins was verified by SDS-PAGE of lysates and HA-specific immunoblotting. (B) For coimmunoprecipitation analysis, lysates of transfected cells were reacted with an antiserum specific for the HBV envelope proteins prior to Western blotting of the immunoprecipitates using the HA-specific MAb. Numbers to the left of each panel show positions of molecular size standards in kDa.
L protein recruits γ2-adaptin in cells.
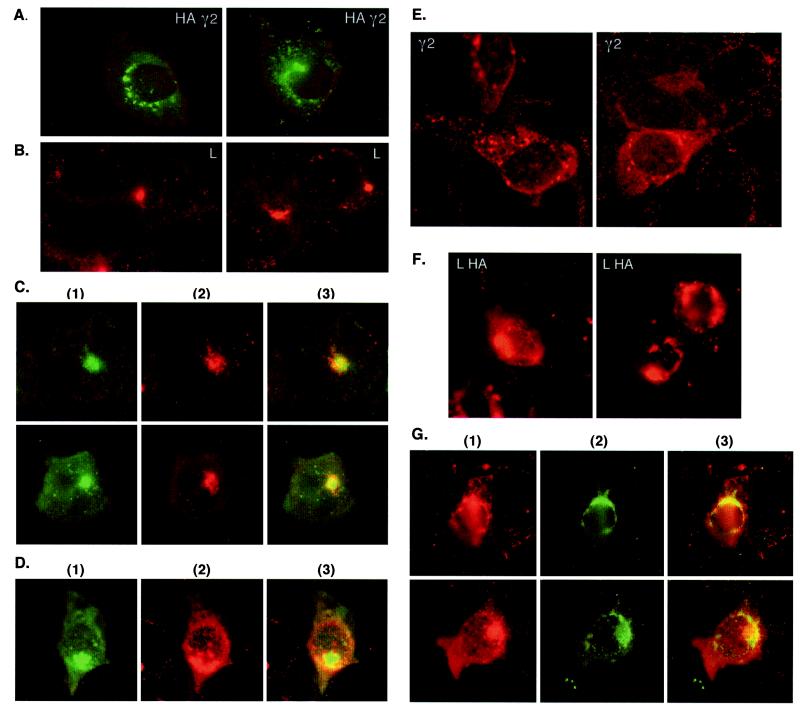
To address whether the interaction between L protein and γ2-adaptin is physiologically relevant, we compared their intracellular localization by performing indirect immunofluorescence analysis. COS-7 cells were transfected with the untagged L wild-type gene and the HA-tagged γ2-adaptin gene, either alone or together, and labeled with anti-L- or anti-HA-specific antibodies. Consistent with previous reports (20, 32), γ2-adaptin yielded a vesicular staining of perinuclear Golgi-like structures (Fig. 4A). By contrast, L protein, expressed at steady state, was localized to a punctate structure within the Golgi complex (Fig. 4B), as defined by costaining with the cis-medial Golgi marker 58K protein (data not shown). Surprisingly, however, we found that upon coexpression of both proteins γ2-adaptin now appeared within the L-characteristic punctate structure, resulting in a striking degree of colocalization of the two proteins, as shown by double labeling of these cells (Fig. 4C). An almost identical pattern of colocalization of the two interacting partners was observed upon analysis of (co)transfected HuH-7 cells (Fig. 4D), a human liver-derived cell line that closely resembles natural host cells of HBV.
FIG. 4.
Colocalization of L protein and γ2-adaptin in transfected COS-7 and HuH-7 cells. (A through C) Indirect immunofluorescence analysis of COS-7 cells expressing HA-tagged γ2-adaptin (A), untagged L protein (B), or both proteins (C). Fixed-permeabilized cells were stained with anti-HA (A; visualized with DTAF), anti-L (B; visualized with rhodamine), or costained with both antibodies (C). In panel C, the staining pattern for γ2-adaptin and L protein are shown in squares 1 and 2, respectively, while square 3 shows the merged dual staining (in yellow-orange). (D) Immunostaining of HuH-7 cells coexpressing HA-tagged γ2-adaptin and untagged L protein was done exactly as for panel C. (E through G) Immunofluorescence localization of γ2-adaptin and L protein in HuH-7 cells. Cells were transfected with the untagged γ2-adaptin gene (E), the HA-tagged L gene (F), or both genes (G). Cells were labeled with anti-γ2-adaptin (SA 8330) (E; visualized with rhodamine), anti-L (F; visualized with rhodamine), or double labeled with anti-γ2-adaptin and anti-HA antibodies (G). In panel G, the staining pattern for γ2-adaptin and L protein are shown in squares 1 and 2, respectively, while square 3 shows the merged dual staining.
To corroborate this finding indicating a recruitment of γ2-adaptin by L protein, immunofluorescence studies were performed with an antiserum raised in rabbits against a synthetic peptide to the C-terminal 19 aa of γ2-adaptin. As shown in Fig. 4E, this antiserum revealed a similar perinuclear Golgi-like staining of γ2-adaptin, expressed without the HA tag in HuH-7 cells. The antiserum even stained the endogenous γ2-adaptin protein, as evident from the perinuclear grainy staining pattern of adjacent nontransfected cells (Fig. 4E), which was undetectable when preimmune serum was used (data not shown). The level of endogenous γ2-adaptin, however, was found to be very low (Fig. 4E) and as such prevented detection of a colocalization of endogenous γ2-adaptin and L protein (data not shown). Nevertheless, colocalization and hence redistribution of expressed γ2-adaptin by coexpressed HA-tagged L protein were again observed when cotransfected HuH-7 cells were double labeled with anti-γ2-adaptin and anti-HA antibodies (Fig. 4G). As γ2-adaptin is seemingly recruited by L protein to that specific location, we consider the L–γ2-adaptin interaction to be functionally important.
The L–γ2-adaptin interaction depends on pre-S domains of L protein oriented to the cytosol.
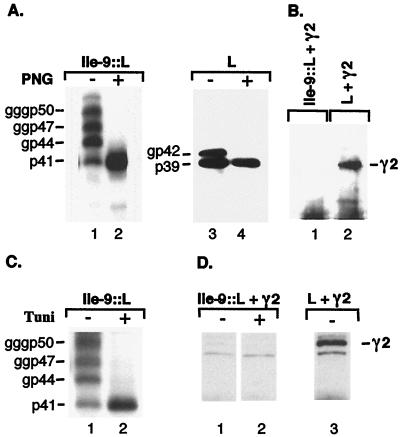
Adaptor proteins are known to decode targeting signals in the cytosolic domains of transmembrane proteins intended for inclusion in transport vesicles (17, 31). We therefore suspected the adaptor-related γ2-adaptin protein to similarly recognize the pre-S1 domain of transmembrane L protein at the cytosolic side of membranes. To test this hypothesis, we took advantage of an L mutant protein that had been shown to be unable to dispose its pre-S domain to the cytosol. Such a uniform e-pre-S topology can be achieved by the addition of a heterologous signal sequence to the N terminus of L protein that enforces cotranslational pre-S translocation into the ER lumen (5, 9). Here, the signal sequence of interleukin-9 was used to induce a uniform luminal orientation of pre-S concomitant with de novo N-glycosylation at two glycan acceptor sites within the pre-S domain. Unlike wild-type L protein, the Ile-9::L mutant hence appeared in non-, single-, double-, and triple-glycosylated forms, as apparent from enzymatic N-deglycosylation with PNGase F (Fig. 5A). Importantly, however, when cotransfected cells were subjected to coimmunoprecipitation as described above, the Ile-9::L mutant failed to bring down γ2-adaptin (Fig. 5B). While these data imply that the L–γ2-adaptin interaction appears to occur in the cytosolic environment, there remained a possibility that the N-glycans attached to the pre-S domain of the Ile-9::L mutant might prevent binding to γ2-adaptin. To address this issue, the Ile-9::L mutant was synthesized in the presence of tunicamycin, which inhibits N-glycosylation. As expected, the Ile-9::L mutant was now made in nonglycosylated form only (Fig. 5C) but remained incapable of interacting with γ2-adaptin (Fig. 5D). From these data we conclude that the luminal pre-S location of the Ile-9::L mutant rather than its pre-S-linked N-glycans interferes with binding to γ2-adaptin.
FIG. 5.
L mutant defective in cytosolic pre-S exposure fails to bind γ2-adaptin. Synthesis, N-linked glycosylation, and γ2-adaptin binding properties of untagged L wild-type or Ile-9::L mutant proteins in transfected COS-7 cells. (A) For analysis of N-linked glycans, lysates of L- or Ile-9::L-transfected cells were divided into two portions and were either mock-treated (−) or digested (+) with PNGase F (PNG) prior to SDS-PAGE and immunoblotting with the envelope-specific MAb H166. Nonglycosylated (p) and glycosylated (gp, ggp, gggp) forms of wild-type and mutant L proteins are indicated on the left of each panel. (B) Coimmunoprecipitation analysis of cotransfected cells was done as for Fig. 3B. (C) Synthesis of Ile-9::L in the absence (−) or presence (+) of tunicamycin (Tuni), an inhibitor of N-linked glycosylation, is shown by H166-specific immunoblotting. (D) Coimmunoprecipitation assay of cotransfected cells, treated without (−) or with (+) tunicamycin, was done as above, except that cells were lysed 24 h after transfection.
The L–γ2-adaptin interaction depends on the ear domain of γ2-adaptin.
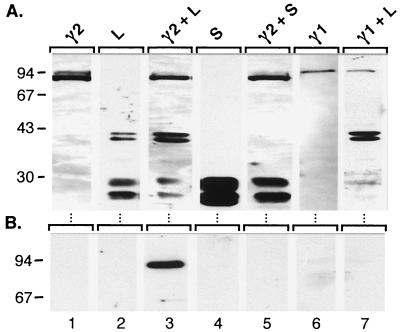
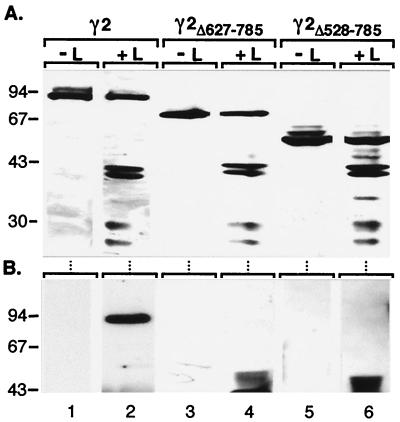
In order to map the structural determinants of γ2-adaptin responsible for L interaction, we suspected its hinge and ear protein portions, isolated in the two-hybrid screen, as candidate binding domains. Accordingly, two γ2-adaptin mutant proteins, γ2Δ627-785 and γ2Δ528-785, were constructed, roughly deleted for either the ear domain or the ear plus hinge domains, respectively. Upon synthesis in transfected COS-7 cells, both γ2Δ627-785 and γ2Δ528-785 mutants yielded stable polypeptides of ∼68 and ∼57 kDa, respectively, in accord with their calculated molecular weights (Fig. 6A, lanes 3 and 5, respectively). Unlike wild-type γ2-adaptin, however, both mutants failed to coimmunoprecipitate with L protein (Fig. 6B). Since even the truncation of the ear domain led to a loss of interaction, we propose the ear domain of γ2-adaptin to be the likely ligand for L protein.
FIG. 6.
C-terminally truncated γ2-adaptin fails to bind L protein. (A) Stable (co)expression of HA-tagged γ2-adaptin wild-type or mutant proteins carrying deletions of the indicated residues and HA-tagged L protein in COS-7 cells is shown by HA-specific immunoblotting of cellular lysates. (B) The coimmunoprecipitation assay was done as for Fig. 3B. Numbers to the left of each panel show positions of molecular mass standards in kDa. Note that the bulky smear in the 43-kDa region present in lanes 4 and 6 is derived from immunoprecipitated HA-tagged L protein.
DISCUSSION
In this study we have used the yeast two-hybrid approach to search for cellular binding proteins of the L envelope protein of HBV, which is pivotal for the outcome of a viral infection. Among different proteins isolated, one prominent cDNA encoded γ2-adaptin, a putative member of clathrin adaptor proteins, which are known to be involved in protein sorting and membrane trafficking. By using different in vitro and in vivo methods, we have confirmed a direct and specific interaction between L protein and γ2-adaptin, thus strongly indicating that γ2-adaptin is a bona fide host cell binding partner of L protein.
γ2-Adaptin that has been recently identified based on its homology to large subunits of heterotetrameric adaptor proteins (21, 33). Despite its similarity to the AP-1 adaptor protein, γ1-adaptin, which mediates TGN-to-endosome transport, γ2-adaptin appears to function independently of AP-1, as evident from the distinct intracellular location of the two γ-adaptins, their differential dependence on the ADP ribosylation factor GTPase for membrane recruitment, and the inability of γ2-adaptin to functionally substitute for the loss of γ1-adaptin in mice (21, 33, 40). In this regard, our experiments showing that the HBV L protein specifically interacts with γ2-adaptin but not with γ1-adaptin add further evidence for γ2-adaptin being a unique entity distinct from γ1-adaptin. In support of and consistent with previous work (21, 33), we found γ2-adaptin to be localized to perinuclear vesicular structures of the Golgi apparatus rather than to TGN structures, where γ1-adaptin is typically found (17, 31). Nevertheless, the distribution of γ2-adaptin suggested its role is played in the secretory rather than in the endocytic pathway (21, 33). Accordingly, we speculate that the L–γ2-adaptin interaction described herein may be functionally important in events guiding the exit of HBV out of the hepatocyte rather than in traffic steps governing virus entry.
Entry of HBV is still poorly understood, but there is increasing evidence that uptake of hepadnaviruses proceeds via receptor-mediated endocytosis concomitant with a viral-host membrane fusion event, presumably occurring at intracellular membranes (1, 18, 36). The pre-S1 domain of L protein has been shown to be absolutely necessary for HBV infectivity, as it mediates receptor binding and probably internalization (20, 24). Although γ2-adaptin specifically associates with the viral receptor, i.e., the pre-S1 domain of L protein, we consider an involvement of this interaction during HBV entry and uncoating to be unlikely, because the pre-S1 domain should be shielded from contacts with γ2-adaptin by the endocytic membrane. In support of this view, here we demonstrated that γ2-adaptin only recognizes the pre-S1 domain of L protein if it is oriented to the cytosolic side of membranes. This is in accordance with the well-characterized mechanisms of cargo recognition by adaptor proteins occurring at the cytosolic face of membranes (17, 31) and thus renders a role for the L–γ-adaptin interaction played during HBV endocytosis largely improbable.
Rather, we would predict a role for the L–γ2-adaptin association in guiding assembly and export of HBV. Hepadnaviruses are thought to bud into intracellular membranes and to leave the cell via the constitutive pathway of secretion (14, 15, 29). Budding sites have been proposed at post-ER–pre-Golgi (intermediate) membranes and/or proximal Golgi membranes, where L protein, the key player of the nucleocapsid envelopment process, accumulates (2, 3, 14, 15, 29, 39). Almost consistently, our immunofluorescence studies revealed a cis-Golgi-like staining pattern for L protein at steady state in transfected COS-7 and HuH-7 cells (Fig. 4) as well as in HBV-replicating human hepatoma cell lines (unpublished data). Interestingly, we observed that L protein selectively recruits γ2-adaptin to its specific location and hence to the presumed HBV budding site. γ2-Adaptin, attracted by L protein, might then in turn recruit other cellular proteins, such as clathrin and additional adaptor protein chains, to the site of assembly to facilitate a membrane fission event or by interacting with the HBV capsid proteins and membrane phospholipids to facilitate assembly of viral particles in a manner similar to that of the native function of adaptor proteins in clathrin-coated pit formation. This is particularly intriguing in view of the fact that the HBV budding process is accompanied by a substantial reorganization of the envelope lipid bilayer (30). Such a membrane deformation could be enabled by assembly of clathrin coats (31) recruited by the L–γ2-adaptin complex. In this scenario, however, proper clathrin coat formation would counteract with nucleocapsid envelopment and virion extrusion into intraluminal cisternae. Possibly, appropriate clathrin growth is impaired by the constitutive heat shock protein, Hsc70, that we had shown previously to bind to the cytosolic pre-S1 domain of L protein (22). Hsc70 is known to regulate clathrin disassembly by acting as an uncoating ATPase (8, 37) but has also been suggested to promote clathrin rearrangements during assembly (13, 38), which would fit our proposal stated above. Such a model, assuming the involvement of the cellular adaptor protein machinery in HBV budding and egress, gained support from a recent report which demonstrated that equine infectious anemic virus utilizes the cellular AP-2 complex to accomplish its assembly and release (28). Alternatively, however, γ2-adaptin, linked to L protein, might facilitate the budding reaction per se, irrespective of whether clathrin is recruited or not. In favor of this interpretation is the recent discovery of a novel family of proteins, termed Golgi-localized, γ-ear-containing, ADP ribosylation factor-binding proteins, or GGAs, that carry a region homologous but not identical to the ear domains of γ1- and γ2-adaptins while being otherwise unrelated to typical large adaptin molecules (7, 12, 34). GGAs are suggested to be components of a novel type of coat that mediates vesicle budding from the TGN but functions independently of clathrin (7, 12, 34). Accordingly, the γ2-adaptin ear domain, engaged by L protein as shown herein, might act in a similar manner, triggering HBV budding even without clathrin support.
It is noteworthy that other roles of the L–γ2-adaptin interaction are possible as well. One likely mechanism could involve disruption of γ2-adaptin function. Our observation that γ2-adaptin is captured by L protein, thereby leaving its physiological location, could have important functional consequences, such as impairing host cell traffic events that are mediated by the putative γ2-adaptin-containing adaptor complex. In this context it is interesting to note that overexpression of the HBV L protein in transgenic mice hepatocytes leads to severe dysfunction of the secretory apparatus (6). By analogy, the E6 oncoprotein of bovine papillomavirus has been shown to interact with AP-1, thereby interfering with the AP-1-dependent trafficking pathway which, in this case, may affect major histocompatibility complex class II-restricted antigen presentation in virus-infected cells and thus may contribute to viral pathogenesis and immune evasion (35).
Apart from compromising γ2-adaptin function, the L–γ2-adaptin interaction could also provide a mechanism by which L protein is intracellularly retained. Unlike the related small and middle envelope proteins of HBV, L protein is not secreted as empty envelope particles due to its pre-S1-specific retention motifs and split mixed topology (5, 9, 19, 26). Possibly, binding of γ2-adaptin to the cytosolic pre-S1 domain of L protein prevents L secretion, thereby simultaneously tethering and concentrating L protein at the budding site where contacts with the nucleocapsid are awaited.
To conclude, we have provided several lines of evidence that the HBV L protein interacts with a host cell adaptor-like structure in a highly specific manner. While the functional role of the L–γ2-adaptin interaction in natural HBV infection still remains unclear and awaits further clarification, our data suggest that a special cell adaptor molecule is used by HBV for benefit.
ACKNOWLEDGMENTS
We are indebted to K. Nakayama for generously providing expression plasmids for γ-adaptins. We thank Tina André and Margaret Werr for expert technical help and R. E. Streeck for helpful discussion and continuous support throughout this work.
This work was supported by the Deutsche Forschungsgemeinschaft (Sonderforschungsbereich 490) and by the Naturwissenschaftlich-Medizinisches Forschungszentrum of the University of Mainz.
REFERENCES
- 1.Breiner K M, Schaller H. Cellular receptor traffic is essential for productive duck hepatitis B virus infection. J Virol. 2000;74:2203–2209. doi: 10.1128/jvi.74.5.2203-2209.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bruss V. A short linear sequence in the pre-S domain of the large hepatitis B virus envelope protein required for virion formation. J Virol. 1997;71:9350–9357. doi: 10.1128/jvi.71.12.9350-9357.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bruss V, Ganem D. The role of envelope proteins in hepatitis B virus assembly. Proc Natl Acad Sci USA. 1991;88:1059–1063. doi: 10.1073/pnas.88.3.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bruss V, Lu X, Thomssen R, Gerlich W H. Post-translational alterations in transmembrane topology of the hepatitis B virus large envelope protein. EMBO J. 1994;13:2273–2279. doi: 10.1002/j.1460-2075.1994.tb06509.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bruss V, Vieluf K. Functions of the internal pre-S domain of the large surface protein in hepatitis B virus particle morphogenesis. J Virol. 1995;69:6652–6657. doi: 10.1128/jvi.69.11.6652-6657.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chisari F V, Fillipi P, Buras J, McLachlan A, Popper H, Pinkert C A, Palmiter R D, Brinster R C. Structural and pathological effects of synthesis of hepatitis B virus large envelope polypeptide in transgenic mice. Proc Natl Acad Sci USA. 1987;84:6909–6913. doi: 10.1073/pnas.84.19.6909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dell'Angelica E C, Puertollano R, Mullins C, Aguilar R C, Vargas J D, Hartnell L M, Bonifacino J S. GGAs: a family of ADP ribosylation factor-binding proteins related to adaptors and associated with the Golgi complex. J Cell Biol. 2000;149:81–93. doi: 10.1083/jcb.149.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DeLuca-Fiaherty C, McKay D B, Parham P, Hill B L. Uncoating protein (hsc70) binds a conformationally labile domain of clathrin light chain LCa to stimulate ATP hydrolysis. Cell. 1990;62:875–887. doi: 10.1016/0092-8674(90)90263-e. [DOI] [PubMed] [Google Scholar]
- 9.Gallina A, Gazina E, Milanesi G. A C-terminal preS1 sequence is sufficient to retain hepatitis B virus L protein in 293 cells. Virology. 1995;213:57–69. doi: 10.1006/viro.1995.1546. [DOI] [PubMed] [Google Scholar]
- 10.Ganem D. Hepadnaviridae and their replication. In: Fields B N, Howley P M, Knipe D M, editors. Virology. 3rd ed. Philadelphia, Pa: Lippincott-Raven Publishers; 1996. p. 2703-2818. [Google Scholar]
- 11.Hildt E, Saher G, Bruss V, Hofschneider P H. The hepatitis B virus large surface protein (LHBs) is a transcriptional activator. Virology. 1996;225:235–239. doi: 10.1006/viro.1996.0594. [DOI] [PubMed] [Google Scholar]
- 12.Hirst J, Lui W W Y, Bright N A, Totty N, Seaman M N J, Robinson M S. A family of proteins with γ-adaptin and VHS domains that facilitate trafficking between the trans-Golgi network and the vacuole/lysosome. J Cell Biol. 2000;149:67–79. doi: 10.1083/jcb.149.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Honing S, Kreimer G, Robenek H, Jockusch B M. Receptor-mediated endocytosis is sensitive to antibodies against the uncoating ATPase (hsc70) J Cell Sci. 1994;107:1185–1196. doi: 10.1242/jcs.107.5.1185. [DOI] [PubMed] [Google Scholar]
- 14.Huovila A J, Eder A M, Fuller S D. Hepatitis B surface antigen assembles in a post-ER, pre-Golgi compartment. J Cell Biol. 1992;118:1305–1320. doi: 10.1083/jcb.118.6.1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kamimura T, Yoshikawa A, Ichida F, Sasaki H. Electron microscopic studies of Dane particles in hepatocytes with special references to intracellular development of Dane particles and their relation with the HBeAg in the serum. Hepatology. 1981;1:392–397. doi: 10.1002/hep.1840010504. [DOI] [PubMed] [Google Scholar]
- 16.Kim H S, Ryu C J, Hong H J. Hepatitis B virus preS1 functions as a transcriptional activation domain. J Gen Virol. 1997;78:1083–1086. doi: 10.1099/0022-1317-78-5-1083. [DOI] [PubMed] [Google Scholar]
- 17.Kirchhausen T. Adaptors for clathrin-mediated traffic. Annu Rev Cell Dev Biol. 1999;15:705–732. doi: 10.1146/annurev.cellbio.15.1.705. [DOI] [PubMed] [Google Scholar]
- 18.Köck J, Borst E-M, Schlicht H-J. Uptake of duck hepatitis B virus into hepatocytes occurs by endocytosis but does not require passage of the virus through an acidic intracellular compartment. J Virol. 1996;70:5827–5831. doi: 10.1128/jvi.70.9.5827-5831.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuroki K, Russnak R, Ganem D. Novel N-terminal amino acid sequence required for retention of a hepatitis B virus glycoprotein in the endoplasmic reticulum. Mol Cell Biol. 1989;9:4459–4466. doi: 10.1128/mcb.9.10.4459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Le Seyec J, Chouteau P, Cannie I, Guguen-Guillouzo C, Gripon P. Infection process of the hepatitis B virus depends on the presence of a defined sequence in the pre-S1 domain. J Virol. 1999;73:2052–2057. doi: 10.1128/jvi.73.3.2052-2057.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lewin D A, Sheff D, Ooi C E, Whitney J A, Yamamoto E, Chicione L M, Webster P, Bonifacino J S, Mellman I. Cloning, expression, and localization of a novel γ-adaptin-like molecule. FEBS Lett. 1998;435:263–268. doi: 10.1016/s0014-5793(98)01083-7. [DOI] [PubMed] [Google Scholar]
- 22.Löffler-Mary H, Werr M, Prange R. Sequence-specific repression of cotranslational translocation of the hepatitis B virus envelope proteins coincides with binding of heat shock protein Hsc70. Virology. 1997;235:144–152. doi: 10.1006/viro.1997.8689. [DOI] [PubMed] [Google Scholar]
- 23.Nassal M. Hepatitis B virus morphogenesis. Curr Top Microbiol Immunol. 1996;214:297–337. doi: 10.1007/978-3-642-80145-7_10. [DOI] [PubMed] [Google Scholar]
- 24.Neurath A R, Kent S B H, Strick N, Parker K. Identification and chemical synthesis of a host cell receptor binding site on hepatitis B virus. Cell. 1986;46:429–436. doi: 10.1016/0092-8674(86)90663-x. [DOI] [PubMed] [Google Scholar]
- 25.Ostapchuk P, Hearing P, Ganem D. A dramatic shift in the transmembrane topology of a viral envelope glycoprotein accompanies hepatitis B viral morphogenesis. EMBO J. 1994;13:1048–1057. doi: 10.1002/j.1460-2075.1994.tb06353.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Prange R, Clemen A, Streeck R E. Myristylation is involved in intracellular retention of hepatitis B virus envelope proteins. J Virol. 1991;65:3919–3923. doi: 10.1128/jvi.65.7.3919-3923.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Prange R, Streeck R E. Novel transmembrane topology of the hepatitis B virus envelope proteins. EMBO J. 1995;14:247–256. doi: 10.1002/j.1460-2075.1995.tb06998.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Puffer B A, Watkins S C, Montelaro R C. Equine infectious anemia virus Gag polyprotein late domain specifically recruits cellular AP-2 adapter protein complexes during virion assembly. J Virol. 1998;72:10218–10221. doi: 10.1128/jvi.72.12.10218-10221.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roingeard P, Lu S, Sureau C, Freschlin M, Arbeille B, Essex M, Romet-Lemonne J. Immunocytochemical and electron microscopic study of hepatitis B virus antigen and complete particle production in hepatitis B virus DNA transfected HepG2 cells. Hepatology. 1990;11:277–285. doi: 10.1002/hep.1840110219. [DOI] [PubMed] [Google Scholar]
- 30.Satoh O, Imai H, Yoneyama T, Utsumi H, Inoue K, Umeda M. Membrane structure of the hepatitis B virus surface antigen particle. J Biochem. 2000;127:543–550. doi: 10.1093/oxfordjournals.jbchem.a022639. [DOI] [PubMed] [Google Scholar]
- 31.Schmid S L. Clathrin-coated vesicle formation and protein sorting: an integrated process. Annu Rev Biochem. 1997;66:511–548. doi: 10.1146/annurev.biochem.66.1.511. [DOI] [PubMed] [Google Scholar]
- 32.Summers J, Smith P M, Horwich A L. Hepadnavirus envelope proteins regulate covalently closed circular DNA amplification. J Virol. 1990;64:2819–2824. doi: 10.1128/jvi.64.6.2819-2824.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Takatsu H, Sakurai M, Shin H-W, Murakami K, Nakayama K. Identification and characterization of novel clathrin adaptor-related proteins. J Biol Chem. 1998;273:24693–24700. doi: 10.1074/jbc.273.38.24693. [DOI] [PubMed] [Google Scholar]
- 34.Takatsu H, Yoshino K, Nakayama K. Adaptor γ ear homology domain conserved in γ-adaptin and GGA proteins that interact with γ-synergin. Biochem Biophys Res Commun. 2000;271:719–725. doi: 10.1006/bbrc.2000.2700. [DOI] [PubMed] [Google Scholar]
- 35.Tong X, Boll W, Kirchhausen T, Howley P M. Interaction of the bovine papillomavirus E6 protein with the clathrin adaptor complex AP-1. J Virol. 1998;72:476–482. doi: 10.1128/jvi.72.1.476-482.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Treichel U, Meyer zum Büschenfelde K-H, Dienes H-P, Gerken G. Receptor-mediated entry of hepatitis B virus particles into liver cells. Arch Virol. 1997;142:493–498. doi: 10.1007/s007050050095. [DOI] [PubMed] [Google Scholar]
- 37.Ungewickell E. The 70-kd mammalian heat shock proteins are structurally and functionally related to the uncoating protein that releases clathrin triskelia from coated vesicles. EMBO J. 1985;4:3385–3391. doi: 10.1002/j.1460-2075.1985.tb04094.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ungewickell E. Wrapping the package. Proc Natl Acad Sci USA. 1999;96:8809–8810. doi: 10.1073/pnas.96.16.8809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xu Z, Bruss V, Yen T S B. Formation of intracellular particles by hepatitis B virus large surface protein. J Virol. 1997;71:5487–5494. doi: 10.1128/jvi.71.7.5487-5494.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zizioli D, Meyer C, Guhde G, Saftig P, von Figura K, Schu P. Early embryonic death of mice deficient in gamma-adaptin. J Biol Chem. 1999;274:5385–5390. doi: 10.1074/jbc.274.9.5385. [DOI] [PubMed] [Google Scholar]